Host–Guest Interaction Study of Olmesartan Medoxomil with β-Cyclodextrin Derivatives
Abstract
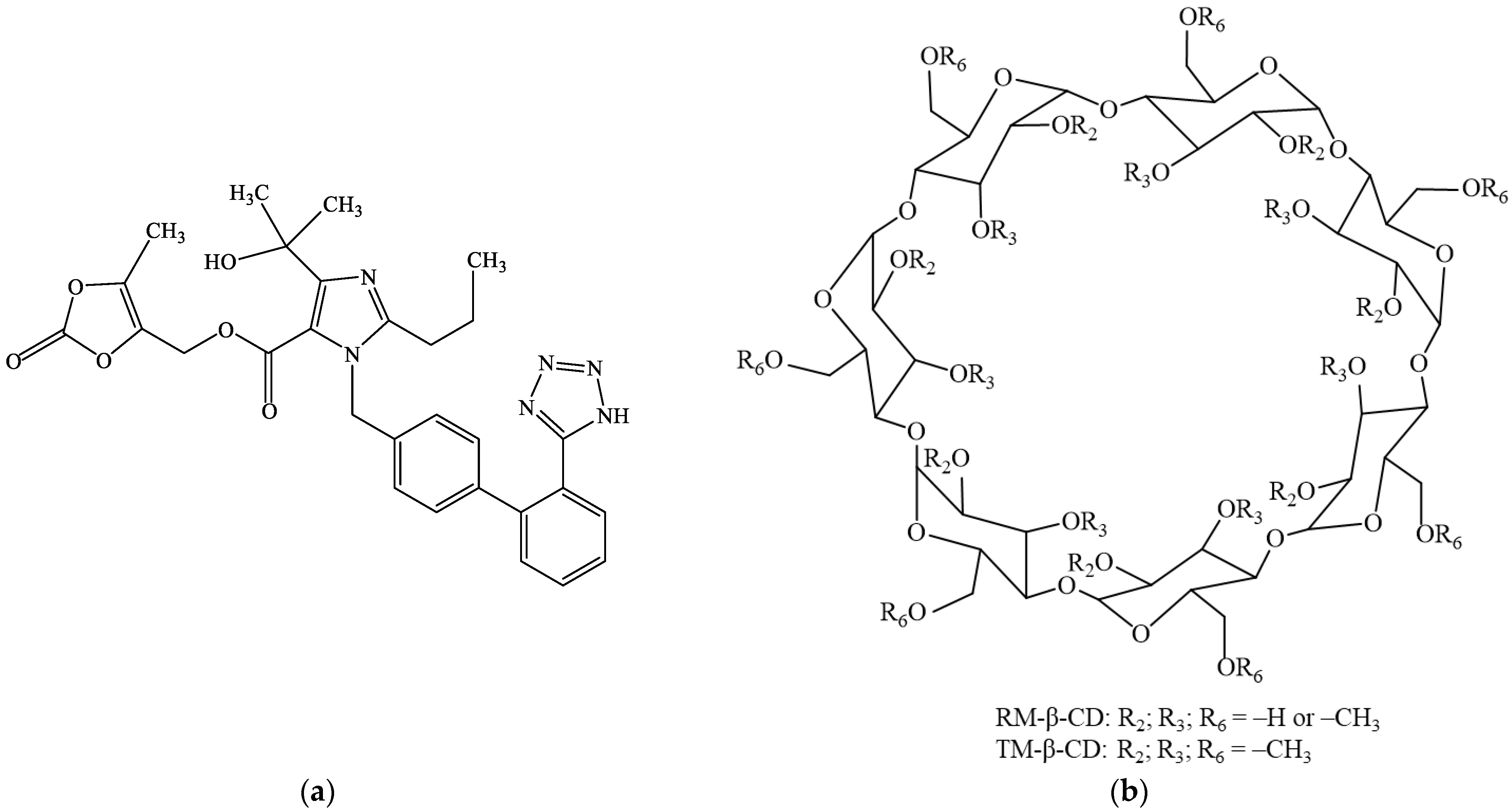
1. Introduction
2. Results and Discussion
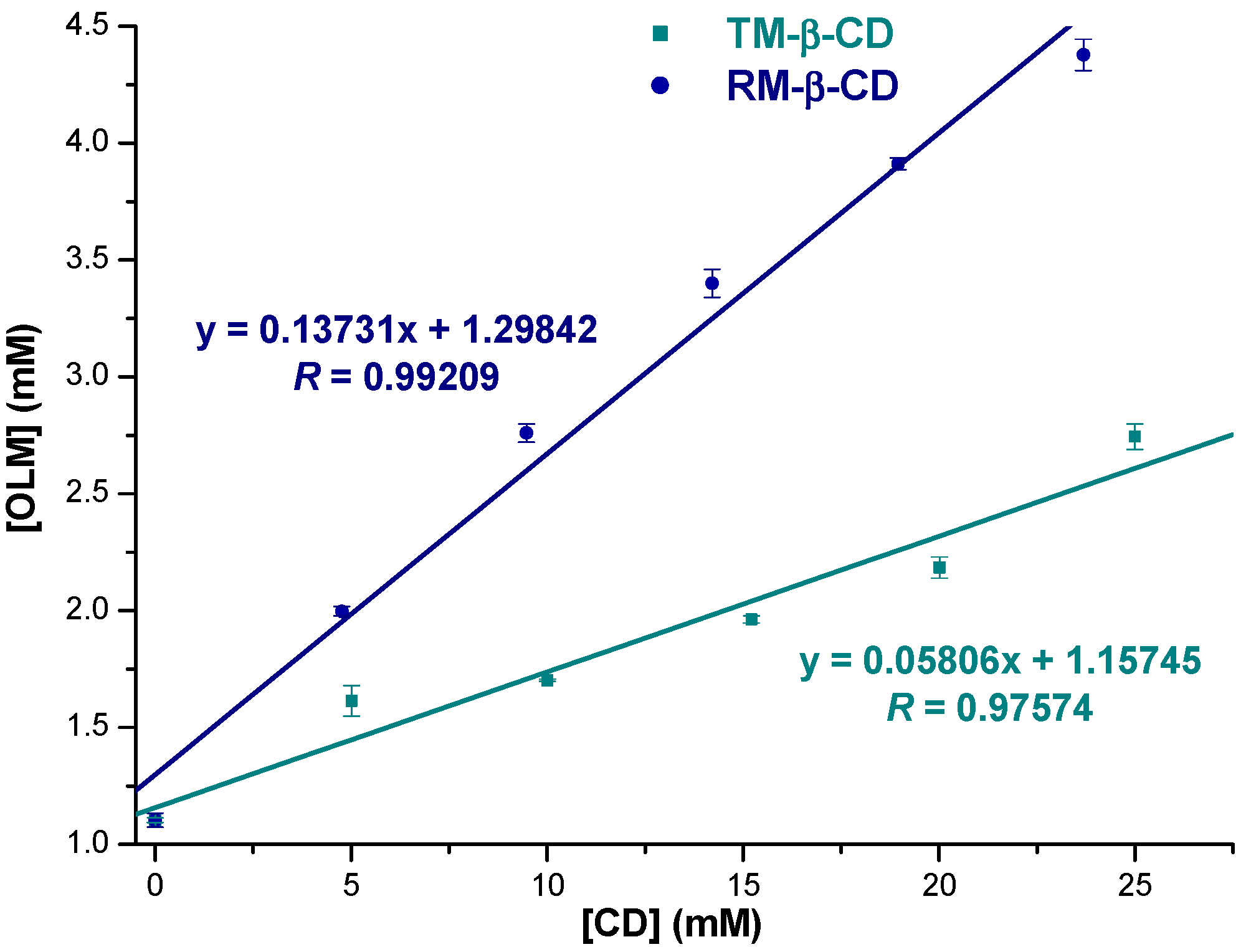
2.1. Phase Solubility Studies
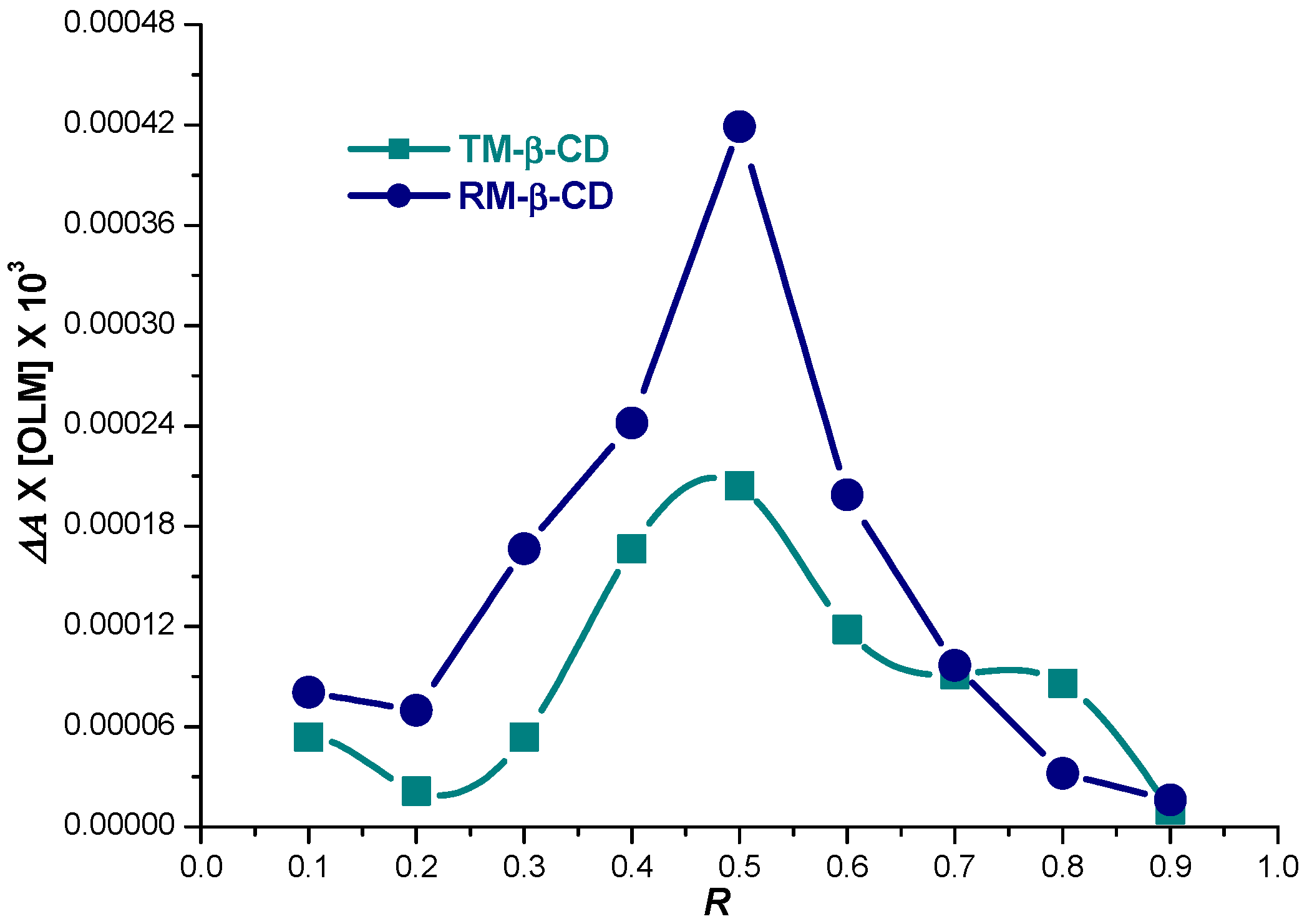
2.2. Job’s Method
2.3. Encapsulation Efficiency and Loading Efficiency Analysis
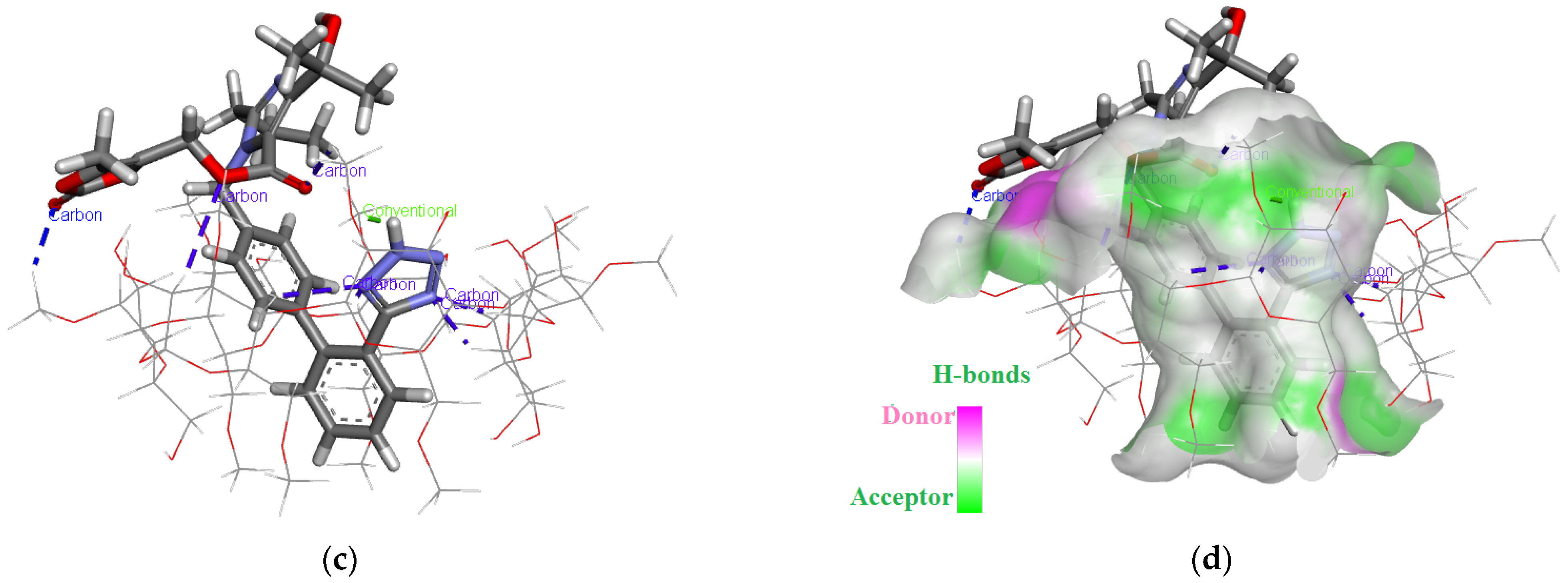
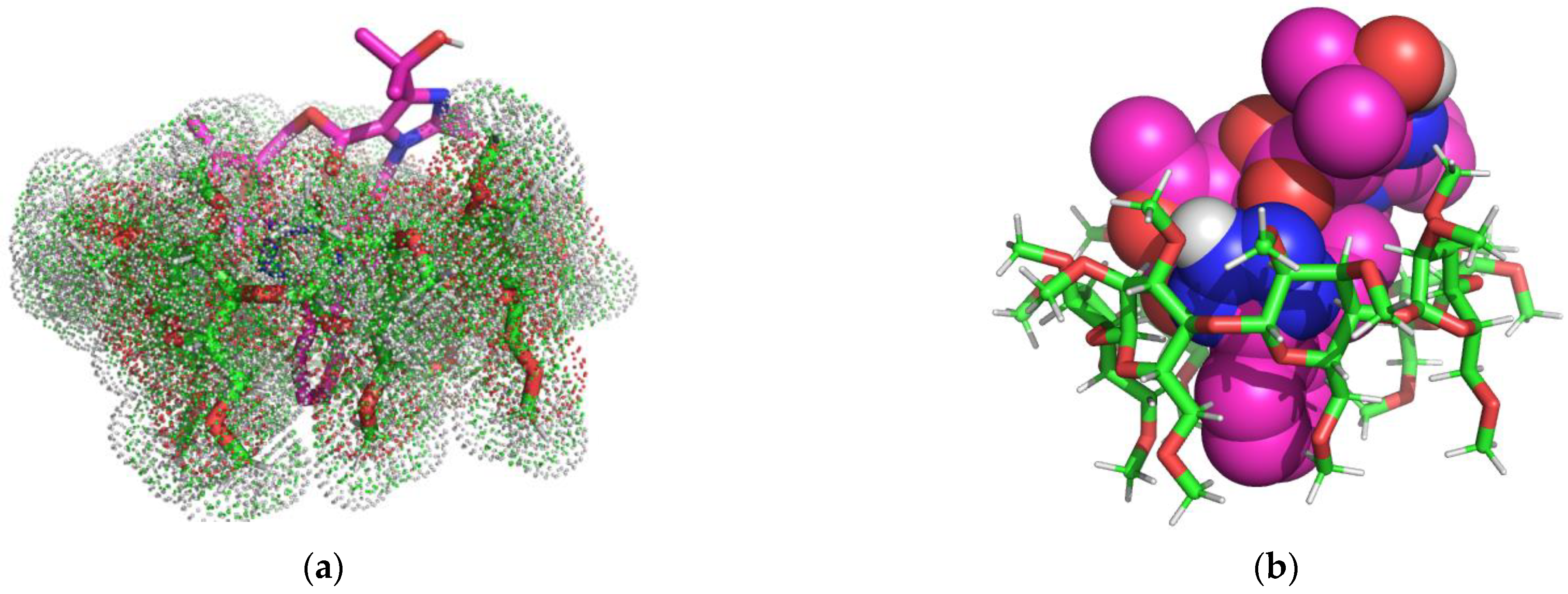
2.4. Molecular Modeling Studies
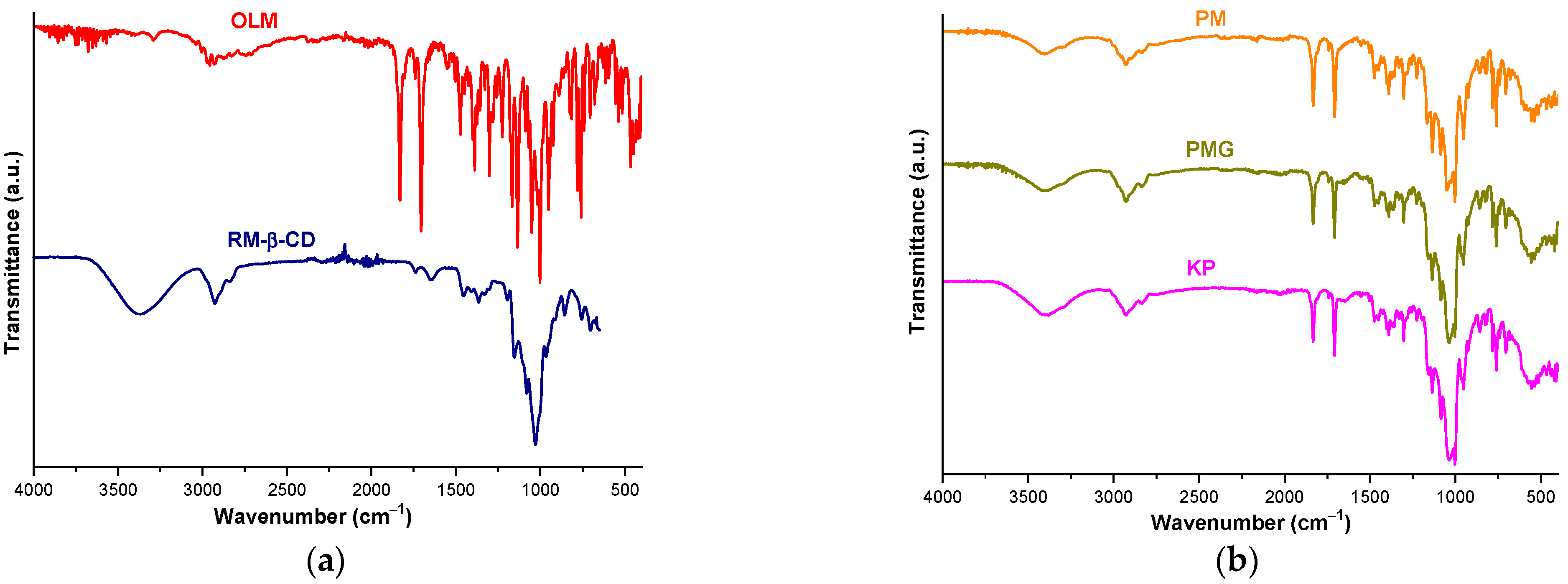
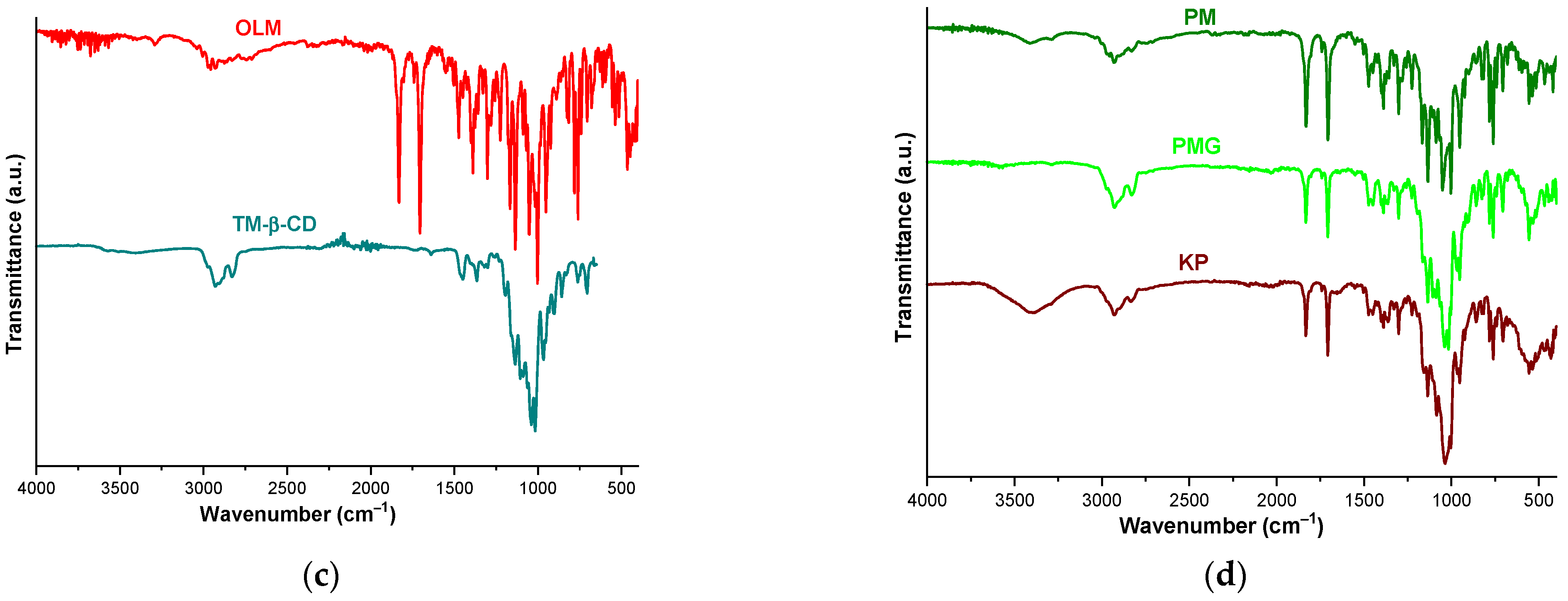
2.5. FTIR Spectroscopy
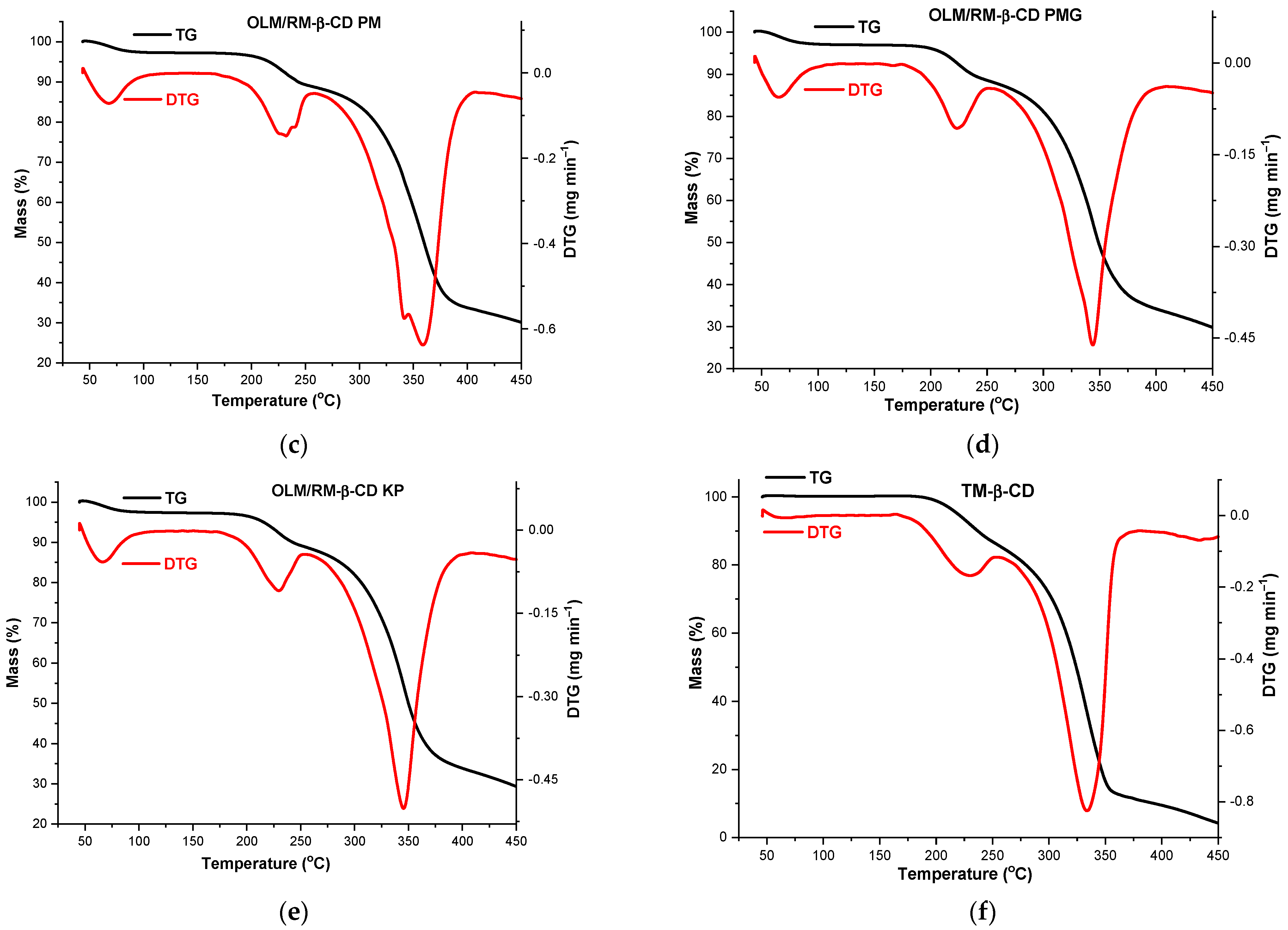
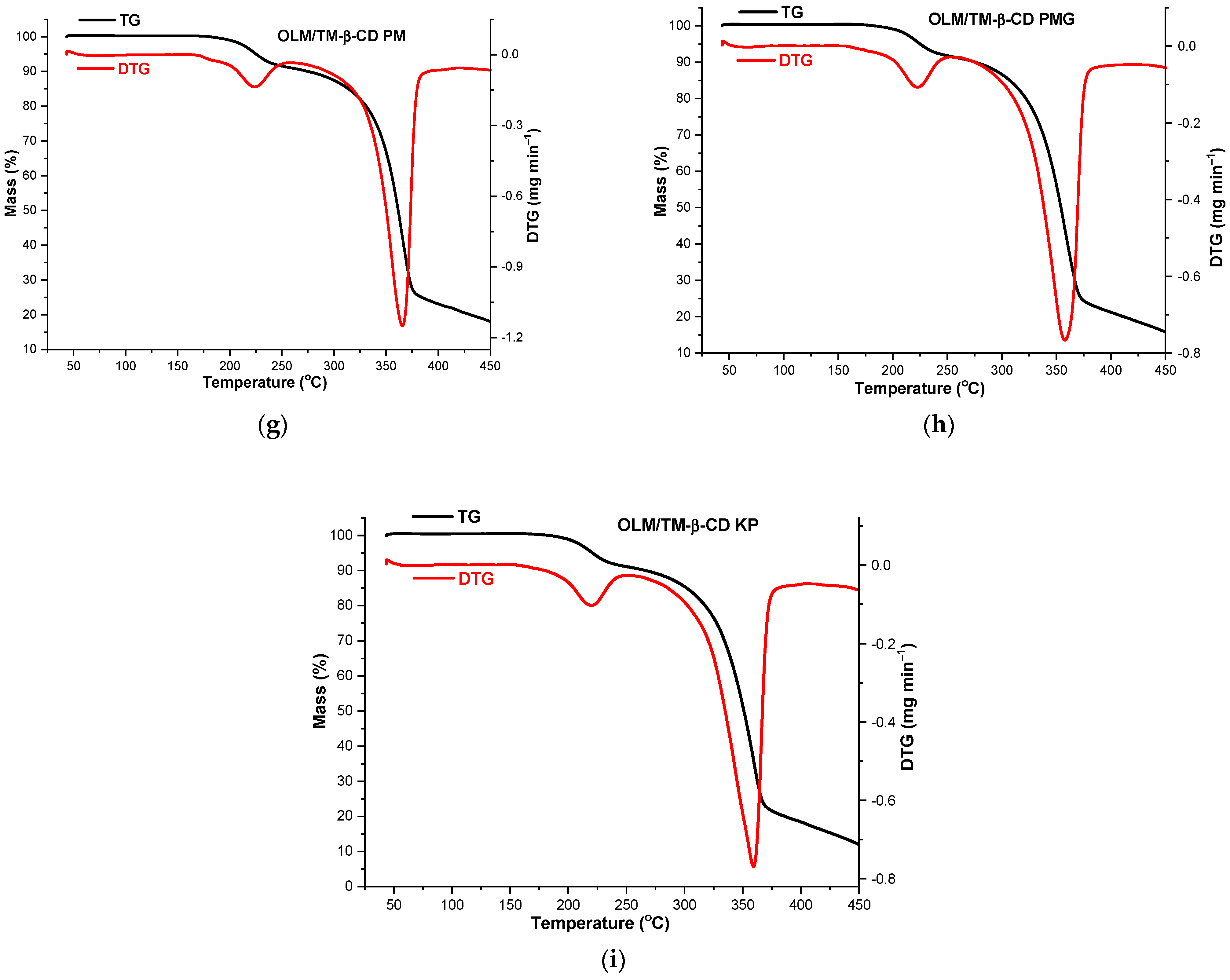
2.6. Thermal Analysis
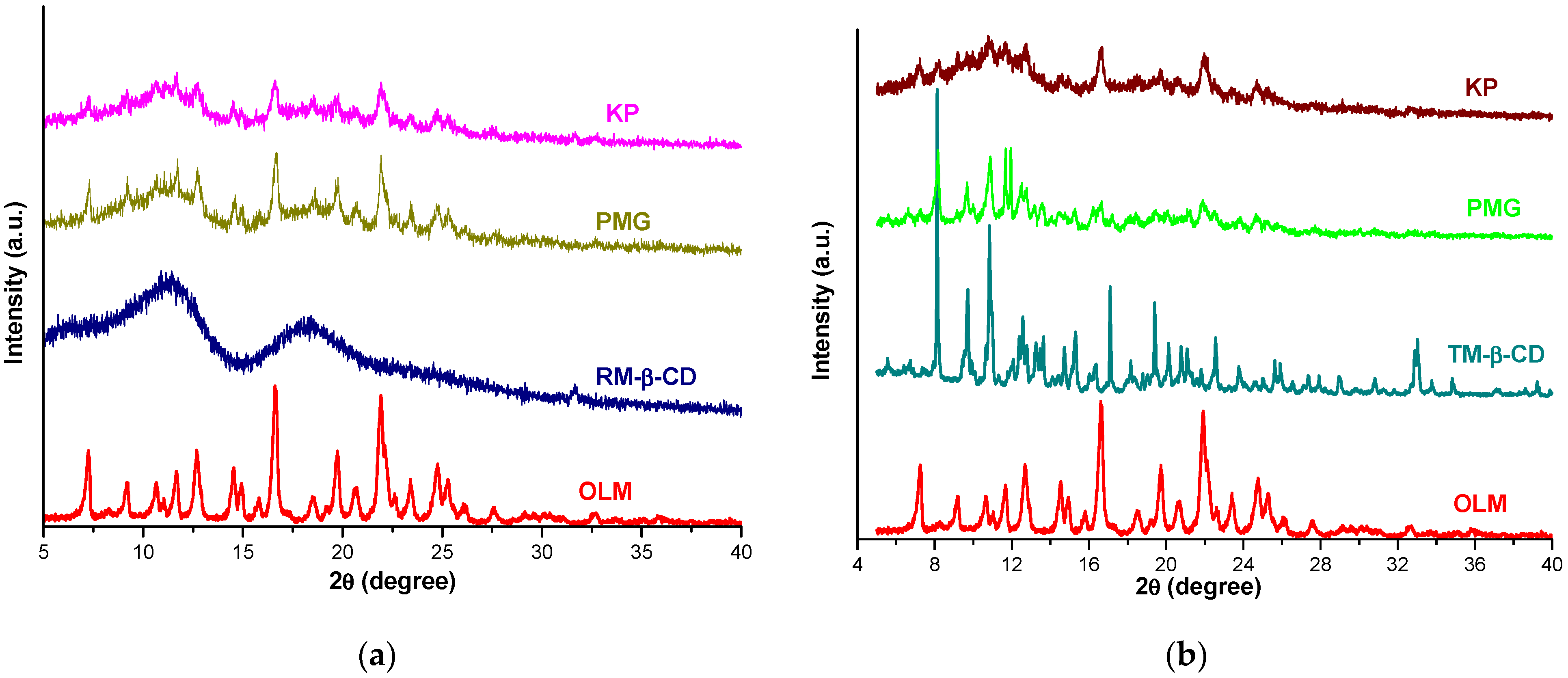
2.7. Powder X-ray Diffraction
2.8. Solubility Profile of OLM/CD Binary Products
3. Materials and Methods
3.1. Materials
3.2. Phase Solubility Studies
3.3. Job’s Method
3.4. Encapsulation Efficiency and Loading Efficiency Analysis
3.5. Molecular Modeling Studies
3.6. Preparation of the Solid Binary Systems
3.7. FTIR Spectroscopy
3.8. Thermal Analysis
3.9. Powder X-ray Diffraction
3.10. Solubility Profile of OLM/CD Kneaded Products
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Han, D.; Han, Z.; Liu, L.; Wang, Y.; Xin, S.; Zhang, H.; Yu, Z. Solubility enhancement of myricetin by inclusion complexation with heptakis-o-(2-hydroxypropyl)-β-cyclodextrin: A joint experimental and theoretical study. Int. J. Mol. Sci. 2020, 21, 766. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Liu, Y. Characterization and stability of beta-acids/hydroxypropyl-β-cyclodextrin inclusion complex. J. Mol. Struct. 2020, 1201, 127159. [Google Scholar] [CrossRef]
- Zhou, J.; Jia, J.; He, J.; Li, J.; Cai, J. Cyclodextrin Inclusion Complexes and Their Application in Food Safety Analysis: Recent Developments and Future Prospects. Foods 2022, 11, 3871. [Google Scholar] [CrossRef]
- Usacheva, T.; Kabirov, D.; Beregova, D.; Gamov, G.; Sharnin, V.; Biondi, M.; Mayol, L.; D’Aria, F.; Giancola, C. Thermodynamics of complex formation between hydroxypropyl-β-cyclodextrin and quercetin in water–ethanol solvents at T = 298.15 K. J. Therm. Anal. Calorim. 2019, 138, 417–424. [Google Scholar] [CrossRef]
- Crini, G.; Fourmentin, S.; Fenyvesi, É.; Torri, G.; Fourmentin, M.; Morin-Crini, N. Cyclodextrins, from molecules to applications. Environ. Chem. Lett. 2018, 16, 1361–1375. [Google Scholar] [CrossRef]
- Li, J.; Xu, F.; Dai, Y.; Zhang, J.; Shi, Y.; Lai, D.; Sriboonvorakul, N.; Hu, J. A Review of Cyclodextrin Encapsulation and Intelligent Response for the Release of Curcumin. Polymers 2022, 14, 5421. [Google Scholar] [CrossRef]
- Braga, S.S. Cyclodextrins: Emerging medicines of the new millennium. Biomolecules 2019, 9, 801. [Google Scholar] [CrossRef] [PubMed]
- Braga, S.S.; Lysenko, K.; El-Saleh, F.; Paz, F.A.A. Cyclodextrin-Efavirenz Complexes Investigated by Solid State and Solubility Studies. Proceedings 2021, 78, 15. [Google Scholar] [CrossRef]
- Cid-Samamed, A.; Rakmai, J.; Mejuto, J.C.; Simal-Gandara, J.; Astray, G. Cyclodextrins inclusion complex: Preparation methods, analytical techniques and food industry applications. Food Chem. 2022, 384, 132467. [Google Scholar] [CrossRef]
- Patil, R.B.; Limbhore, D.N.; Vanjari, S.S.; Chavan, M.C. Study of solubility enhancement of quercetin by inclusion complexation with betacyclodextrin. J. Pharm. Sci. Res. 2019, 11, 3102–3107. [Google Scholar]
- Braga, S.S.; El-saleh, F.; Lysenko, K.; Paz, F.A.A. Inclusion Compound of Efavirenz and γ-Cyclodextrin: Solid State Studies and Effect on Solubility. Molecules 2021, 26, 519. [Google Scholar] [CrossRef] [PubMed]
- Tănase, I.M.; Sbârcea, L.; Ledeți, A.; Vlase, G.; Barvinschi, P.; Văruţ, R.M.; Dragomirescu, A.; Axente, C.; Ledeți, I. Physicochemical characterization and molecular modeling study of host–guest systems of aripiprazole and functionalized cyclodextrins. J. Therm. Anal. Calorim. 2020, 141, 1027–1039. [Google Scholar] [CrossRef]
- Sahakijpijarn, S.; Moon, C.; Koleng, J.J.; Christensen, D.J.; Williams, R.O. Development of remdesivir as a dry powder for inhalation by thin film freezing. Pharmaceutics 2020, 12, 1002. [Google Scholar] [CrossRef] [PubMed]
- Ilyich, T.V.; Lapshina, E.A.; Maskevich, A.A.; Veiko, A.G.; Lavysh, A.V.; Palecz, B.; Stępniak, A.; Buko, V.U.; Zavodnik, I.B. Inclusion Complexes of Quercetin with β-Cyclodextrins: Ultraviolet and Infrared Spectroscopy and Quantum Chemical Modeling. Biophysics 2020, 65, 381–389. [Google Scholar] [CrossRef]
- Vakali, V.; Papadourakis, M.; Georgiou, N.; Zoupanou, N.; Diamantis, D.A.; Javornik, U.; Papakyriakopoulou, P.; Plavec, J.; Valsami, G.; Tzakos, A.G.; et al. Comparative Interaction Studies of Quercetin with 2-Hydroxyl-propyl-β-cyclodextrin and 2,6-Methylated-β-cyclodextrin. Molecules 2022, 27, 5490. [Google Scholar] [CrossRef] [PubMed]
- De Gaetano, F.; Scala, A.; Celesti, C.; Lambertsen Larsen, K.; Genovese, F.; Bongiorno, C.; Leggio, L.; Iraci, N.; Iraci, N.; Mazzaglia, A.; et al. Amphiphilic Cyclodextrin Nanoparticles as Delivery System for Idebenone: A Preformulation Study. Molecules 2023, 28, 3023. [Google Scholar] [CrossRef]
- Du, F.; Pan, T.; Ji, X.; Hu, J.; Ren, T. Study on the preparation of geranyl acetone and β-cyclodextrin inclusion complex and its application in cigarette flavoring. Sci. Rep. 2020, 10, 12375. [Google Scholar] [CrossRef]
- Sbârcea, L.; Ledeţi, I.; Drəgan, L.; Kurunczi, L.; Fuliaş, A.; Udrescu, L. Fosinopril sodium-hydroxypropyl-β-cyclodextrin inclusion complex: Thermal decomposition kinetics and compatibility studies. J. Therm. Anal. Calorim. 2015, 120, 981–990. [Google Scholar] [CrossRef]
- Sbârcea, L.; Tănase, I.M.; Ledeți, A.; Cîrcioban, D.; Vlase, G.; Barvinschi, P.; Miclău, M.; Văruţ, R.M.; Suciu, O.; Ledeți, I. Risperidone/Randomly Methylated β-Cyclodextrin Inclusion Complex-Compatibility Study with Pharmaceutical Excipients. Molecules 2021, 26, 1690. [Google Scholar] [CrossRef]
- Tănase, I.M.; Sbârcea, L.; Ledeţi, A.; Barvinschi, P.; Cîrcioban, D.; Vlase, G.; Văruţ, R.M.; Ledeţi, I. Compatibility studies with pharmaceutical excipients for aripiprazole–heptakis (2,6-di-O-methyl)-β-cyclodextrin supramolecular adduct. J. Therm. Anal. Calorim. 2020, 142, 1963–1976. [Google Scholar] [CrossRef]
- Simsek, T.; Rasulev, B.; Mayer, C.; Simsek, S. Preparation and characterization of inclusion complexes of β-cyclodextrin and phenolics from wheat bran by combination of experimental and computational techniques. Molecules 2020, 25, 4275. [Google Scholar] [CrossRef] [PubMed]
- Saokham, P.; Muankaew, C.; Jansook, P.; Loftsson, T. Solubility of cyclodextrins and drug/cyclodextrin complexes. Molecules 2018, 23, 1161. [Google Scholar] [CrossRef]
- D’Aria, F.; Serri, C.; Niccoli, M.; Mayol, L.; Quagliariello, V.; Iaffaioli, R.V.; Biondi, M.; Giancola, C. Host–guest inclusion complex of quercetin and hydroxypropyl-β-cyclodextrin: A calorimetric study. J. Therm. Anal. Calorim. 2017, 130, 451–456. [Google Scholar] [CrossRef]
- Wangsawangrung, N.; Choipang, C.; Chaiarwut, S.; Ekabutr, P.; Suwantong, O.; Chuysinuan, P.; Techasakul, S.; Supaphol, P. Quercetin/Hydroxypropyl-β-Cyclodextrin Inclusion Complex-Loaded Hydrogels for Accelerated Wound Healing. Gels 2022, 8, 573. [Google Scholar] [CrossRef]
- Lima Nascimento, J.; Coelho, A.G.; Oliveira Barros, Y.S.; Sousa Oliveira, I.; Vieira da Silva, F.; Custódio Viana, A.F.S.; Araújo, B.Q.; dos Santos Rocha, M.; das Chagas Pereira de Andrade, F.; de Oliveira Barbosa, C.; et al. Production and Characterization of a β-Cyclodextrin Inclusion Complex with Platonia insignis Seed Extract as a Proposal for a Gastroprotective System. Appl. Sci. 2023, 13, 58. [Google Scholar] [CrossRef]
- Moulahcene, L.; Skiba, M.; Milon, N.; Fadila, H.; Bounoure, F.; Lahiani-Skiba, M. Removal Efficiency of Insoluble β-Cyclodextrin Polymer from Water–Soluble Carcinogenic Direct Azo Dyes. Polymers 2023, 15, 732. [Google Scholar] [CrossRef] [PubMed]
- Lima, P.S.S.; Lucchese, A.M.; Araújo-Filho, H.G.; Menezes, P.P.; Araújo, A.A.S.; Quintans-Júnior, L.J.; Quintans, J.S.S. Inclusion of terpenes in cyclodextrins: Preparation, characterization and pharmacological approaches. Carbohydr. Polym. 2016, 151, 965–987. [Google Scholar] [CrossRef] [PubMed]
- Anraku, M.; Tabuchi, R.; Goto, M.; Iohara, D.; Mizukai, Y.; Maezaki, Y.; Michihara, A.; Kadowaki, D.; Otagiri, M.; Hirayama, F. Design and evaluation of an extended-release olmesartan tablet using chitosan/cyclodextrin composites. Pharmaceutics 2019, 11, 82. [Google Scholar] [CrossRef]
- Arun, B.; Narendar, D.; Veerabrahma, K. Development of olmesartan medoxomil lipid-based nanoparticles and nanosuspension: Preparation, characterization and comparative pharmacokinetic evaluation. Artif. Cells Nanomed. Biotechnol. 2018, 46, 126–137. [Google Scholar] [CrossRef]
- Kadowaki, D.; Anraku, M.; Tasaki, Y.; Taguchi, K.; Shimoishi, K.; Seo, H.; Hirata, S.; Maruyama, T.; Otagiri, M. Evaluation for antioxidant and renoprotective activity of olmesartan using nephrectomy rats. Biol. Pharm. Bull. 2009, 32, 2041–2045. [Google Scholar] [CrossRef]
- Prajapati, S.T.; Joshi, H.A.; Patel, C.N. Preparation and Characterization of Self-Microemulsifying Drug Delivery System of Olmesartan Medoxomil for Bioavailability Improvement. J. Pharm. 2013, 2013, 728425. [Google Scholar] [CrossRef] [PubMed]
- Al-Majed, A.A.; Bakheit, A.H.H.; Abdel Aziz, H.A.; Al-Jallal, A.A.M. Olmesartan. Profiles Drug Subst. Excip. Relat. Methodol. 2017, 42, 241–286. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Ren, W.; Dushkin, A.V.; Su, W. Preparation, characterization, in vitro and in vivo studies of olmesartan medoxomil in a ternary solid dispersion with N-methyl-D-glucamine and hydroxypropyl-β-cyclodextrin. J. Drug Deliv. Sci. Technol. 2020, 56, 101546. [Google Scholar] [CrossRef]
- Censi, R.; Di Martino, P. Polymorph impact on the bioavailability and stability of poorly soluble drugs. Molecules 2015, 20, 18759–18776. [Google Scholar] [CrossRef]
- Thakkar, H.; Parmar, M.; Patel, A.; Patel, B.; Chauhan, N. Studies on inclusion complex as potential systems for enhancement of oral bioavailability of olmesartan medoxomil. Chron. Young Sci. 2012, 3, 129. [Google Scholar] [CrossRef]
- Higuchi, T.K.; Connors, A. Phase-solubility techniques. Adv. Anal. Chem. Instrum. 1965, 4, 117–211. [Google Scholar]
- Sbârcea, L.; Tănase, I.M.; Ledeți, A.; Cîrcioban, D.; Vlase, G.; Barvinschi, P.; Miclău, M.; Văruţ, R.M.; Trandafirescu, C.; Ledeți, I. Encapsulation of Risperidone by Methylated β-Cyclodextrins: Physicochemical and Molecular Modeling Studies. Molecules 2020, 25, 5694. [Google Scholar] [CrossRef]
- Xiao, Z.; Yu, P.; Sun, P.; Kang, Y.; Niu, Y.; She, Y.; Zhao, D. Inclusion complexes of β-cyclodextrin with isomeric ester aroma compounds: Preparation, characterization, mechanism study, and controlled release. Carbohydr. Polym. 2024, 333, 121977. [Google Scholar] [CrossRef] [PubMed]
- Kou, X.; Zhang, Y.; Su, D.; Wang, H.; Huang, X.; Niu, Y.; Ke, Q.; Xiao, Z.; Meng, Q. Study on host-guest interaction of aroma compounds/γ-cyclodextrin inclusion complexes. LWT 2023, 178, 114589. [Google Scholar] [CrossRef]
- Morris, G.M.; Ruth, H.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. Software news and updates AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- DeLano, W.L. Pymol: An open-source molecular graphics tool. CCP4 Newsl. Protein Crystallogr. 2002, 40, 82–92. [Google Scholar]
- Abdelquader, M.M.; Essa, E.A.; El Maghraby, G.M. Inhibition of Co-Crystallization of Olmesartan Medoxomil and Hydrochlorothiazide for Enhanced Dissolution Rate in Their Fixed Dose Combination. AAPS PharmSciTech 2019, 20, 3. [Google Scholar] [CrossRef]
- Murillo-Fernández, M.A.; Montero-Zeledón, E.; Abdala-Saiz, A.; Vega-Baudrit, J.R.; Araya-Sibaja, A.M. Interaction and Compatibility Studies in the Development of Olmesartan Medoxomil and Hydrochlorothiazide Formulations under a Real Manufacturing Process. Pharmaceutics 2022, 14, 424. [Google Scholar] [CrossRef] [PubMed]
- Circioban, D.; Ledeţi, I.; Vlase, G.; Ledeţi, A.; Axente, C.; Vlase, T.; Dehelean, C. Kinetics of heterogeneous-induced degradation for artesunate and artemether. J. Therm. Anal. Calorim. 2018, 134, 749–756. [Google Scholar] [CrossRef]
- Lavorgna, M.; Iacovino, R.; Russo, C.; Di Donato, C.; Piscitelli, C.; Isidori, M. A new approach for improving the antibacterial and tumor cytotoxic activities of pipemidic acid by including it in trimethyl-β-cyclodextrin. Int. J. Mol. Sci. 2019, 20, 416. [Google Scholar] [CrossRef]
- Cova, T.F.; Milne, B.F.; Pais, A.A.C.C. Host flexibility and space filling in supramolecular complexation of cyclodextrins: A free-energy-oriented approach. Carbohydr. Polym. 2019, 205, 42–54. [Google Scholar] [CrossRef]
- Sbârcea, L.; Udrescu, L.; Drǎgan, L.; Trandafirescu, C.; Szabadai, Z.; Bojiţǎ, M. Fosinopril-cyclodextrin inclusion complexes: Phase solubility and physicochemical analysis. Pharmazie 2011, 66, 584–589. [Google Scholar] [CrossRef] [PubMed]
- Detrich, Á.; Dömötör, K.J.; Katona, M.T.; Markovits, I.; Vargáné Láng, J. Polymorphic forms of bisoprolol fumarate: Preparation and characterization. J. Therm. Anal. Calorim. 2019, 135, 3043–3055. [Google Scholar] [CrossRef]
- Hu, Z.; Li, S.; Wang, S.; Zhang, B.; Huang, Q. Encapsulation of menthol into cyclodextrin metal-organic frameworks: Preparation, structure characterization and evaluation of complexing capacity. Food Chem. 2021, 338, 127839. [Google Scholar] [CrossRef]
- Protein DATA Bank. Available online: http://www.pdb.org/pdb/home/home.do (accessed on 10 June 2023).
- Jambhekar, S.; Breen, P. Cyclodextrins in pharmaceutical formulations II: Solubilization, binding constant, and complexation efficiency. Drug Discov. Today 2016, 21, 363–368. [Google Scholar] [CrossRef]
- Mennini, N.; Maestrelli, F.; Cirri, M.; Mura, P. Analysis of physicochemical properties of ternary systems of oxaprozin with randomly methylated-ß-cyclodextrin and L-arginine aimed to improve the drug solubility. J. Pharm. Biomed. Anal. 2016, 129, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Poulson, B.G.; Alsulami, Q.A.; Sharfalddin, A.; El Agammy, E.F.; Mouffouk, F.; Emwas, A.H.; Jaremko, L.; Jaremko, M. Cyclodextrins: Structural, Chemical, and Physical Properties, and Applications. Polysaccharides 2022, 3, 1–31. [Google Scholar] [CrossRef]
- Kaczmarek-Klinowska, M.; Łudzik, K.; Jażdżewska, M.; Jóźwiak, M.; Hornowski, T.; Bilski, P. Characterization of behavior of the inclusion complex between methyl-β-cyclodextrin and nimodipine in water-ethanol mixed media. J. Mol. Liq. 2024, 395, 123762. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andor, M.; Temereancă, C.; Sbârcea, L.; Ledeți, A.; Man, D.E.; Mornoș, C.; Ridichie, A.; Cîrcioban, D.; Vlase, G.; Barvinschi, P.; et al. Host–Guest Interaction Study of Olmesartan Medoxomil with β-Cyclodextrin Derivatives. Molecules 2024, 29, 2209. https://doi.org/10.3390/molecules29102209
Andor M, Temereancă C, Sbârcea L, Ledeți A, Man DE, Mornoș C, Ridichie A, Cîrcioban D, Vlase G, Barvinschi P, et al. Host–Guest Interaction Study of Olmesartan Medoxomil with β-Cyclodextrin Derivatives. Molecules. 2024; 29(10):2209. https://doi.org/10.3390/molecules29102209
Chicago/Turabian StyleAndor, Minodora, Claudia Temereancă, Laura Sbârcea, Adriana Ledeți, Dana Emilia Man, Cristian Mornoș, Amalia Ridichie, Denisa Cîrcioban, Gabriela Vlase, Paul Barvinschi, and et al. 2024. "Host–Guest Interaction Study of Olmesartan Medoxomil with β-Cyclodextrin Derivatives" Molecules 29, no. 10: 2209. https://doi.org/10.3390/molecules29102209
APA StyleAndor, M., Temereancă, C., Sbârcea, L., Ledeți, A., Man, D. E., Mornoș, C., Ridichie, A., Cîrcioban, D., Vlase, G., Barvinschi, P., Caunii, A., Văruţ, R.-M., Trandafirescu, C. M., Buda, V., Ledeți, I., & Rădulescu, M. (2024). Host–Guest Interaction Study of Olmesartan Medoxomil with β-Cyclodextrin Derivatives. Molecules, 29(10), 2209. https://doi.org/10.3390/molecules29102209














