Rapid and Simultaneous Determination of Anabolic Andro-Genic Steroids in Livestock and Poultry Meat Using One-Step Solid-Phase Extraction Coupled with UHPLC–MS/MS
Abstract
:1. Introduction
2. Results and Discussion
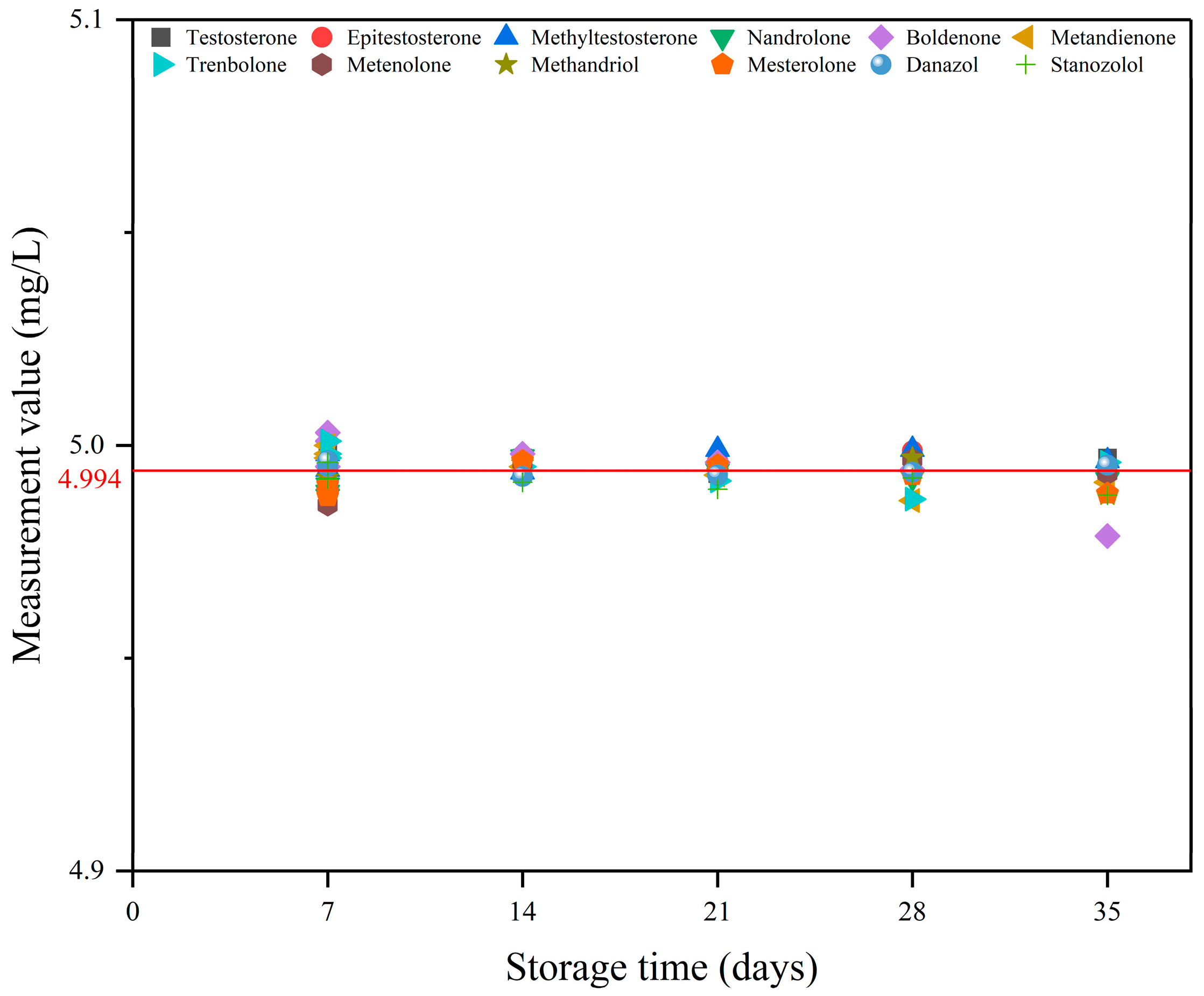
2.1. Stability Testing of Standard Working Solutions
2.2. Optimization of UHPLC–MS/MS Conditions
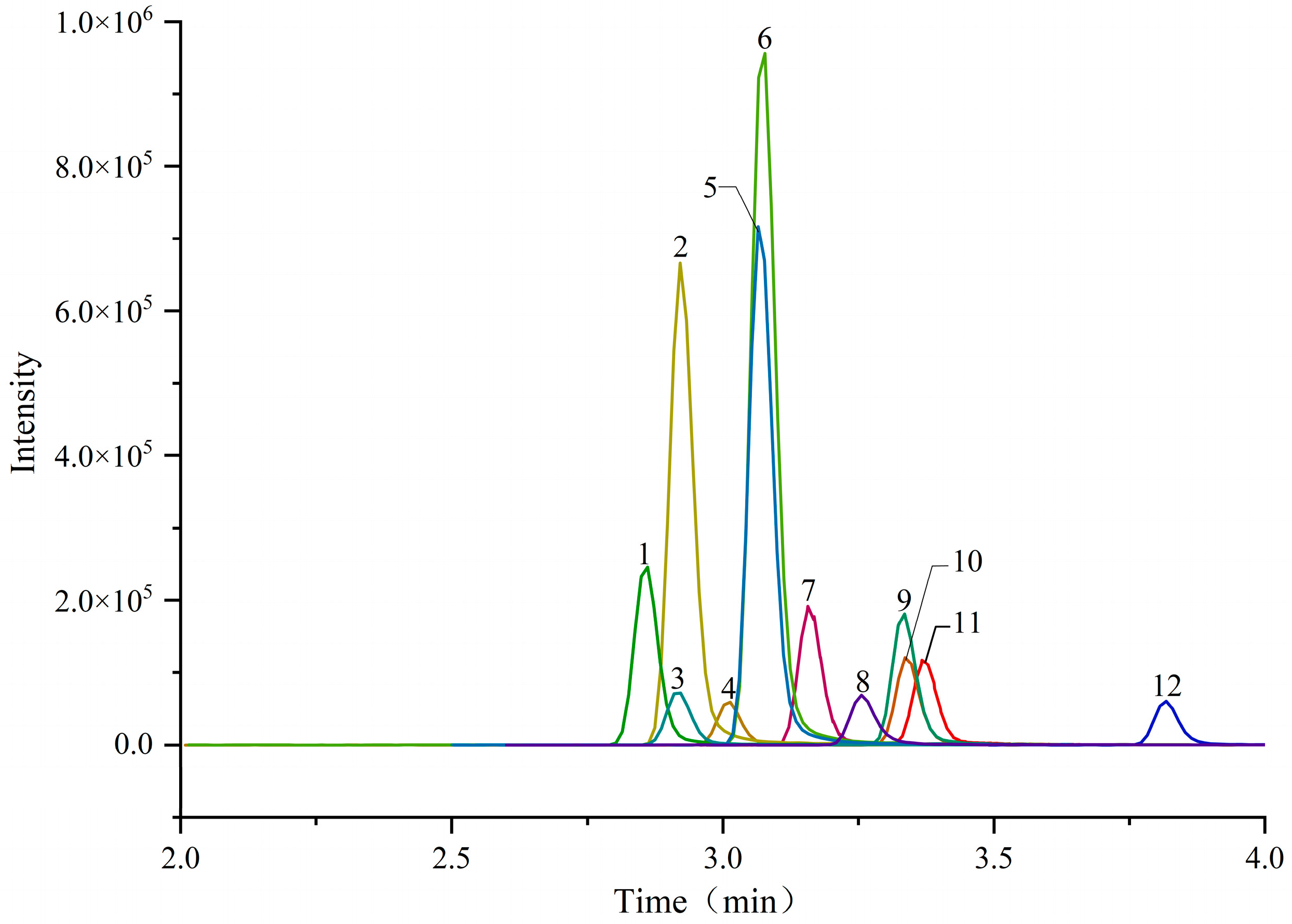
2.2.1. Optimization of Chromatographic Condition
2.2.2. Optimization of Mass Spectrometry Condition
2.3. Optimization of Pretreatment Conditions
2.3.1. Optimization of Extraction Solvent
2.3.2. Optimization of Solid-Phase Extraction Column
2.4. Validation of Bioanalytical Methods
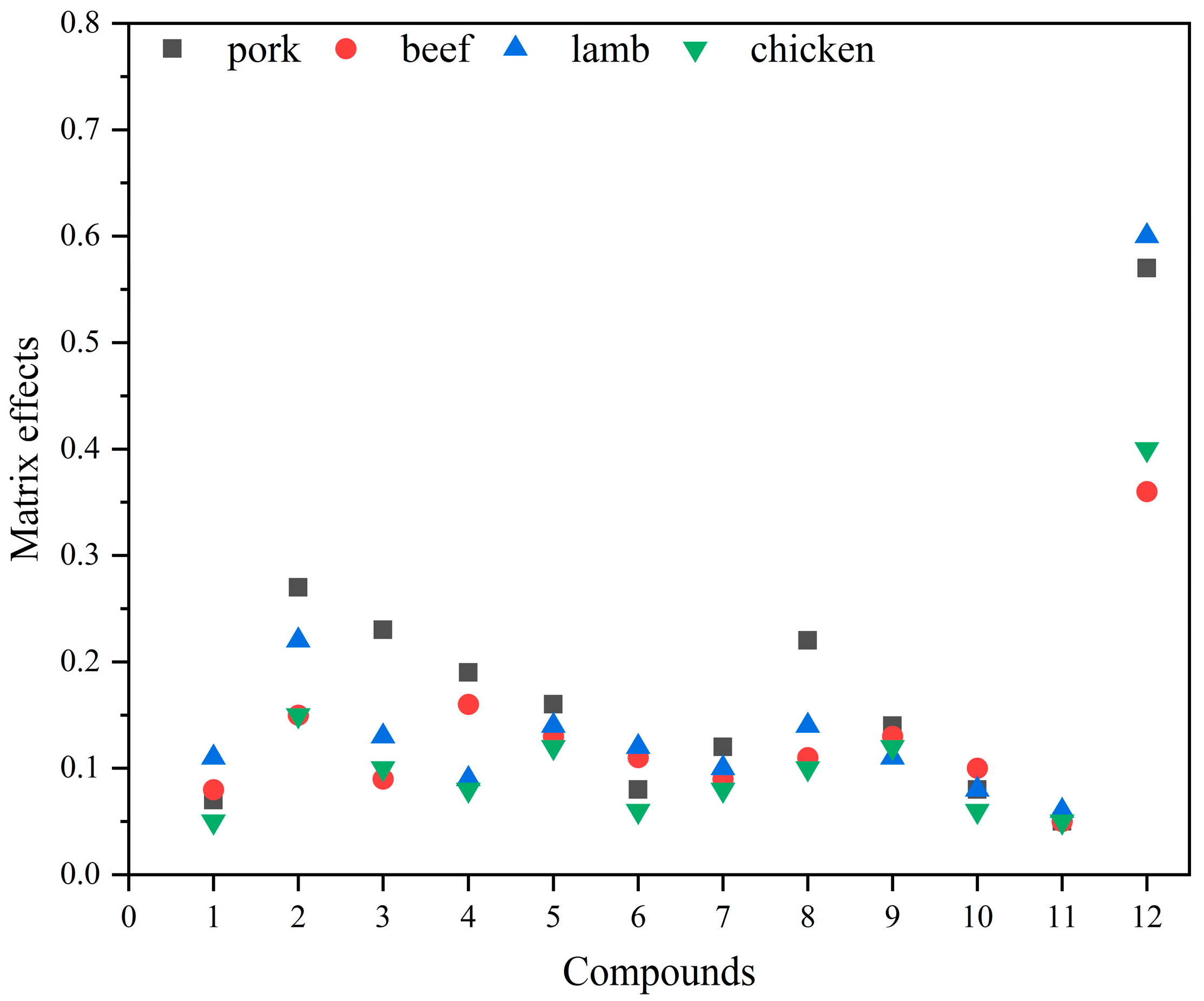
2.4.1. Matrix Effect Evaluation and Elimination
2.4.2. Linearity of the Standards Curves, Limit of Detection, and Limit of Quantification
2.4.3. Recovery and Precision
2.5. Analyses of Commercial Samples
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Sample Collection
3.3. Preparation and Stability Testing of Standard Solutions
3.4. UHPLC–MS/MS Instrumentation and Operating Conditions
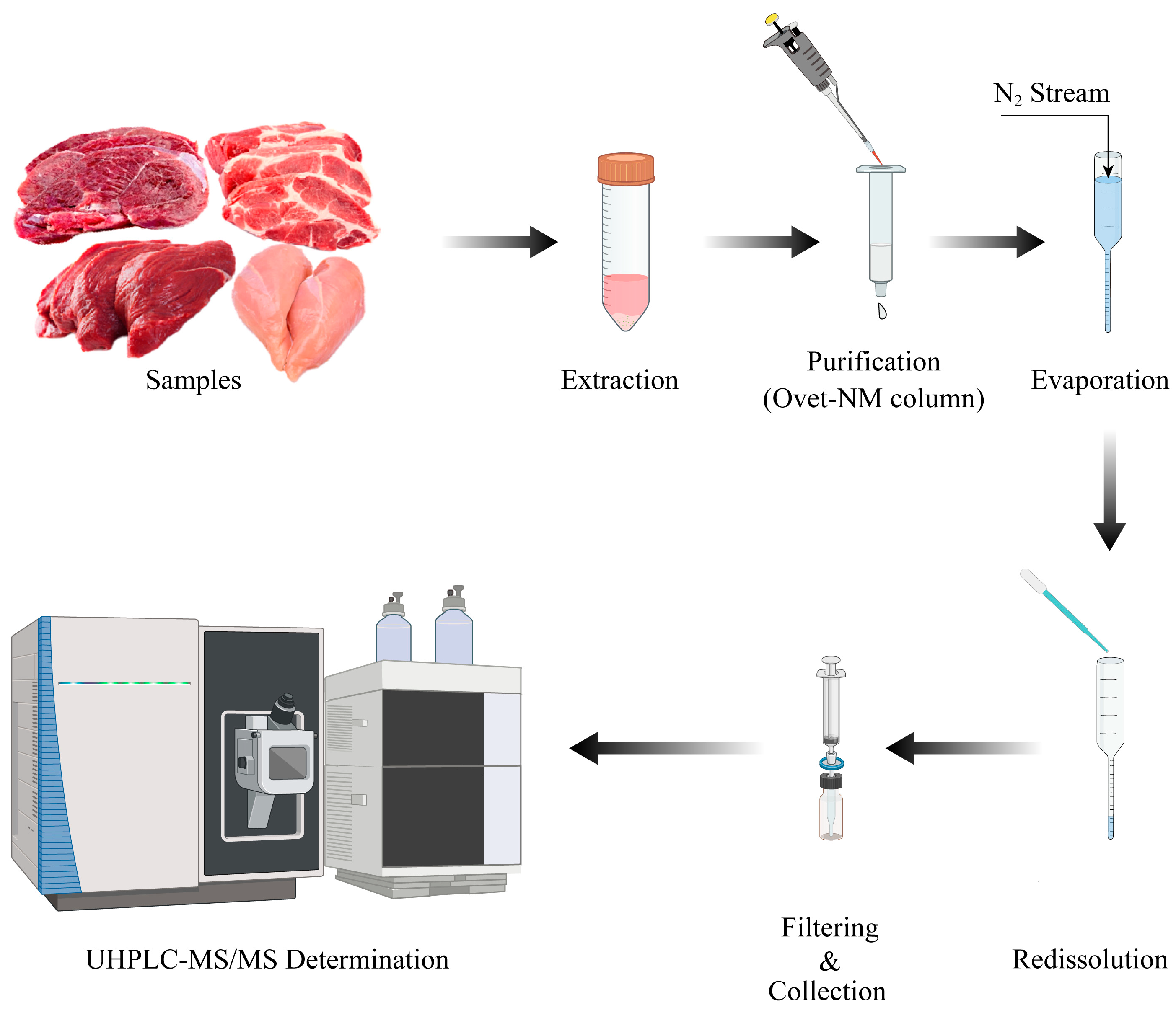
3.5. Sample Preparation
3.6. Method Validation
3.6.1. Matrix Effect Evaluation
3.6.2. Determination of Linearity, LODs, and LOQs
3.6.3. Recovery and Precision Test
3.7. Analysis of Commercial Samples
3.8. Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Compound | Added Level (μg/kg) | Batch | Average Recovery (%) | Intraday RSD (%) | Interday RSD (%) |
|---|---|---|---|---|---|
| Testosterone | 0.5 | 1 | 84.4 | 11.2 | 8.6 |
| 2 | 87.9 | 8.6 | |||
| 3 | 87.8 | 6.5 | |||
| 1 | 1 | 68.3 | 10.6 | 10.0 | |
| 2 | 75.0 | 11.5 | |||
| 3 | 73.1 | 6.2 | |||
| 5 | 1 | 82.9 | 11.6 | 9.2 | |
| 2 | 81.0 | 7.6 | |||
| 3 | 80.7 | 9.5 | |||
| Epitestosterone | 0.5 | 1 | 88.5 | 13.3 | 11.1 |
| 2 | 81.8 | 9.3 | |||
| 3 | 85.9 | 11.9 | |||
| 1 | 1 | 77.6 | 3.8 | 4.5 | |
| 2 | 78.5 | 6.1 | |||
| 3 | 77.8 | 4.0 | |||
| 5 | 1 | 86.0 | 5.5 | 7.0 | |
| 2 | 82.3 | 5.0 | |||
| 3 | 89.1 | 8.5 | |||
| Methyltestosterone | 0.5 | 1 | 83.8 | 13.5 | 11.9 |
| 2 | 81.6 | 10.5 | |||
| 3 | 79.0 | 12.7 | |||
| 1 | 1 | 83.6 | 10.4 | 10.2 | |
| 2 | 86.2 | 11.0 | |||
| 3 | 82.2 | 10.6 | |||
| 5 | 1 | 87.5 | 9.5 | 8.3 | |
| 2 | 85.7 | 5.5 | |||
| 3 | 86.5 | 10.6 | |||
| Nandrolone | 0.5 | 1 | 77.2 | 9.8 | 10.2 |
| 2 | 76.7 | 12.1 | |||
| 3 | 76.9 | 10.6 | |||
| 1 | 1 | 82.5 | 6.8 | 6.5 | |
| 2 | 80.7 | 8.3 | |||
| 3 | 83.5 | 4.7 | |||
| 5 | 1 | 83.3 | 10.4 | 9.1 | |
| 2 | 83.7 | 10.7 | |||
| 3 | 81.6 | 7.3 | |||
| Boldenone | 0.5 | 1 | 80.7 | 10.4 | 9.4 |
| 2 | 82.1 | 6.5 | |||
| 3 | 86.7 | 10.7 | |||
| 1 | 1 | 91.3 | 7.5 | 6.9 | |
| 2 | 88.8 | 7.9 | |||
| 3 | 90.6 | 6.5 | |||
| 5 | 1 | 85.6 | 10.4 | 10.4 | |
| 2 | 83.7 | 10.9 | |||
| 3 | 88.6 | 11.5 | |||
| Metandienone | 0.5 | 1 | 84.6 | 8.9 | 7.9 |
| 2 | 90.3 | 7.7 | |||
| 3 | 85.1 | 6.5 | |||
| 1 | 1 | 85.7 | 6.2 | 7.3 | |
| 2 | 83.5 | 9.2 | |||
| 3 | 86.9 | 7.0 | |||
| 5 | 1 | 83.4 | 12.2 | 10.2 | |
| 2 | 85.2 | 6.8 | |||
| 3 | 83.0 | 12.6 | |||
| Trenbolone | 0.5 | 1 | 76.0 | 9.1 | 10.8 |
| 2 | 87.2 | 8.8 | |||
| 3 | 78.3 | 10.4 | |||
| 1 | 1 | 87.1 | 8.0 | 8.8 | |
| 2 | 87.0 | 10.8 | |||
| 3 | 85.9 | 11.0 | |||
| 5 | 1 | 83.2 | 8.6 | 8.2 | |
| 2 | 77.8 | 9.0 | |||
| 3 | 85.0 | 4.9 | |||
| Metenolone | 0.5 | 1 | 87.2 | 9.2 | 7.2 |
| 2 | 90.0 | 5.1 | |||
| 3 | 83.8 | 6.2 | |||
| 1 | 1 | 86.1 | 8.3 | 6.5 | |
| 2 | 90.2 | 4.4 | |||
| 3 | 93.3 | 4.8 | |||
| 5 | 1 | 84.3 | 10.3 | 9.5 | |
| 2 | 83.5 | 7.3 | |||
| 3 | 84.4 | 12.0 | |||
| Methandriol | 0.5 | 1 | 82.2 | 7.5 | 9.1 |
| 2 | 82.9 | 10.4 | |||
| 3 | 82.7 | 10.6 | |||
| 1 | 1 | 87.7 | 7.7 | 7.2 | |
| 2 | 89.1 | 6.5 | |||
| 3 | 83.7 | 6.2 | |||
| 5 | 1 | 88.1 | 7.5 | 9.7 | |
| 2 | 89.8 | 11.4 | |||
| 3 | 86.3 | 11.0 | |||
| Mesterolone | 0.5 | 1 | 82.1 | 13.0 | 12.5 |
| 2 | 89.6 | 12.4 | |||
| 3 | 85.8 | 13.2 | |||
| 1 | 1 | 81.8 | 11.5 | 8.1 | |
| 2 | 85.7 | 5.0 | |||
| 3 | 83.5 | 7.6 | |||
| 5 | 1 | 83.6 | 10.1 | 9.7 | |
| 2 | 81.7 | 10.3 | |||
| 3 | 86.0 | 9.9 | |||
| Danazol | 0.5 | 1 | 86.0 | 11.3 | 10.2 |
| 2 | 83.1 | 8.0 | |||
| 3 | 88.3 | 11.6 | |||
| 1 | 1 | 88.3 | 9.3 | 9.5 | |
| 2 | 85.2 | 9.8 | |||
| 3 | 89.2 | 10.4 | |||
| 5 | 1 | 81.6 | 9.3 | 10.8 | |
| 2 | 82.8 | 11.6 | |||
| 3 | 83.5 | 13.1 | |||
| Stanozolol | 0.5 | 1 | 78.6 | 11.0 | 10.2 |
| 2 | 77.9 | 11.0 | |||
| 3 | 80.3 | 10.3 | |||
| 1 | 1 | 75.8 | 9.8 | 9.3 | |
| 2 | 71.7 | 8.3 | |||
| 3 | 75.5 | 10.1 | |||
| 5 | 1 | 76.0 | 7.3 | 8.9 | |
| 2 | 71.8 | 7.2 | |||
| 3 | 75.1 | 10.9 |
| Compound | Added Level (μg/kg) | Batch | Average Recovery (%) | Intraday RSD (%) | Interday RSD (%) |
|---|---|---|---|---|---|
| Testosterone | 0.5 | 1 | 81.8 | 11.6 | 8.3 |
| 2 | 82.9 | 12.6 | |||
| 3 | 83.3 | 7.1 | |||
| 1 | 1 | 91.1 | 7.1 | 6.9 | |
| 2 | 91.5 | 5.3 | |||
| 3 | 85.2 | 4.8 | |||
| 5 | 1 | 94.1 | 10.7 | 10.9 | |
| 2 | 99.4 | 9.8 | |||
| 3 | 97.4 | 13.3 | |||
| Epitestosterone | 0.5 | 1 | 81.4 | 8.8 | 10.0 |
| 2 | 93.4 | 7.6 | |||
| 3 | 87.5 | 9.4 | |||
| 1 | 1 | 79.6 | 8.8 | 8.0 | |
| 2 | 80.7 | 5.1 | |||
| 3 | 74.7 | 6.6 | |||
| 5 | 1 | 96.2 | 6.7 | 7.7 | |
| 2 | 95.1 | 7.9 | |||
| 3 | 87.7 | 5.9 | |||
| Methyltestosterone | 0.5 | 1 | 84.5 | 7.7 | 9.8 |
| 2 | 88.8 | 11.4 | |||
| 3 | 85.7 | 10.7 | |||
| 1 | 1 | 91.7 | 4.7 | 6.6 | |
| 2 | 88.0 | 6.8 | |||
| 3 | 94.0 | 5.5 | |||
| 5 | 1 | 84.8 | 8.2 | 7.0 | |
| 2 | 83.2 | 6.1 | |||
| 3 | 84.2 | 7.9 | |||
| Nandrolone | 0.5 | 1 | 82.1 | 11.3 | 9.0 |
| 2 | 82.5 | 7.4 | |||
| 3 | 83.7 | 9.7 | |||
| 1 | 1 | 86.7 | 7.8 | 7.8 | |
| 2 | 87.7 | 7.4 | |||
| 3 | 87.5 | 9.6 | |||
| 5 | 1 | 86.5 | 8.2 | 8.3 | |
| 2 | 86.5 | 9.0 | |||
| 3 | 87.1 | 9.2 | |||
| Boldenone | 0.5 | 1 | 86.3 | 13.2 | 12.3 |
| 2 | 90.8 | 12.9 | |||
| 3 | 91.5 | 12.4 | |||
| 1 | 1 | 91.8 | 11.9 | 11.1 | |
| 2 | 88.5 | 10.1 | |||
| 3 | 86.7 | 12.4 | |||
| 5 | 1 | 93.6 | 8.6 | 8.0 | |
| 2 | 95.4 | 9.8 | |||
| 3 | 93.2 | 6.6 | |||
| Metandienone | 0.5 | 1 | 88.8 | 6.9 | 7.6 |
| 2 | 83.4 | 6.6 | |||
| 3 | 84.1 | 8.9 | |||
| 1 | 1 | 86.4 | 7.3 | 8.3 | |
| 2 | 87.1 | 8.7 | |||
| 3 | 83.1 | 9.6 | |||
| 5 | 1 | 89.1 | 10.7 | 9.4 | |
| 2 | 86.6 | 9.8 | |||
| 3 | 88.7 | 9.4 | |||
| Trenbolone | 0.5 | 1 | 83.0 | 10.4 | 9.3 |
| 2 | 84.0 | 9.1 | |||
| 3 | 87.0 | 9.5 | |||
| 1 | 1 | 87.0 | 9.8 | 8.1 | |
| 2 | 87.0 | 7.2 | |||
| 3 | 81.2 | 5.9 | |||
| 5 | 1 | 81.8 | 11.4 | 11.2 | |
| 2 | 84.9 | 14.0 | |||
| 3 | 86.7 | 9.2 | |||
| Metenolone | 0.5 | 1 | 76.4 | 11.8 | 9.2 |
| 2 | 77.5 | 7.2 | |||
| 3 | 77.9 | 9.8 | |||
| 1 | 1 | 80.9 | 8.4 | 8.1 | |
| 2 | 79.6 | 6.8 | |||
| 3 | 85.7 | 8.3 | |||
| 5 | 1 | 89.1 | 7.2 | 9.1 | |
| 2 | 93.7 | 9.8 | |||
| 3 | 84.2 | 7.8 | |||
| Methandriol | 0.5 | 1 | 82.2 | 9.3 | 8.0 |
| 2 | 87.7 | 7.2 | |||
| 3 | 81.8 | 6.4 | |||
| 1 | 1 | 87.2 | 8.4 | 7.4 | |
| 2 | 86.7 | 7.6 | |||
| 3 | 86.6 | 7.5 | |||
| 5 | 1 | 84.5 | 5.6 | 7.0 | |
| 2 | 88.0 | 8.3 | |||
| 3 | 86.5 | 7.2 | |||
| Mesterolone | 0.5 | 1 | 88.1 | 7.8 | 8.0 |
| 2 | 82.3 | 7.3 | |||
| 3 | 85.6 | 8.5 | |||
| 1 | 1 | 88.3 | 5.2 | 6.2 | |
| 2 | 92.1 | 6.8 | |||
| 3 | 92.3 | 6.4 | |||
| 5 | 1 | 91.0 | 6.4 | 7.1 | |
| 2 | 85.8 | 5.1 | |||
| 3 | 83.8 | 7.7 | |||
| Danazol | 0.5 | 1 | 87.6 | 11.4 | 8.5 |
| 2 | 88.1 | 7.0 | |||
| 3 | 86.2 | 8.1 | |||
| 1 | 1 | 93.3 | 6.0 | 6.7 | |
| 2 | 94.5 | 6.0 | |||
| 3 | 95.0 | 7.3 | |||
| 5 | 1 | 86.9 | 12.2 | 11.6 | |
| 2 | 89.7 | 11.2 | |||
| 3 | 86.7 | 13.2 | |||
| Stanozolol | 0.5 | 1 | 71.9 | 9.6 | 9.5 |
| 2 | 76.1 | 7.7 | |||
| 3 | 71.2 | 11.3 | |||
| 1 | 1 | 71.6 | 7.6 | 8.7 | |
| 2 | 68.0 | 6.4 | |||
| 3 | 78.6 | 5.2 | |||
| 5 | 1 | 77.8 | 8.9 | 8.1 | |
| 2 | 72.9 | 8.6 | |||
| 3 | 75.5 | 6.8 |
| Compound | Added Level (μg/kg) | Batch | Average Recovery (%) | Intraday RSD (%) | Interday RSD (%) |
|---|---|---|---|---|---|
| Testosterone | 0.5 | 1 | 75.1 | 7.7 | 7.0 |
| 2 | 72.4 | 5.8 | |||
| 3 | 75.6 | 7.7 | |||
| 1 | 1 | 84.5 | 5.5 | 6.8 | |
| 2 | 85.0 | 8.5 | |||
| 3 | 84.0 | 7.4 | |||
| 5 | 1 | 81.5 | 7.3 | 7.9 | |
| 2 | 81.1 | 7.7 | |||
| 3 | 79.6 | 9.9 | |||
| Epitestosterone | 0.5 | 1 | 78.5 | 8.1 | 9.0 |
| 2 | 75.6 | 10.6 | |||
| 3 | 75.9 | 9.5 | |||
| 1 | 1 | 77.0 | 5.7 | 6.6 | |
| 2 | 76.1 | 8.8 | |||
| 3 | 75.7 | 6.2 | |||
| 5 | 1 | 79.2 | 4.7 | 5.5 | |
| 2 | 82.9 | 6.1 | |||
| 3 | 83.8 | 4.6 | |||
| Methyltestosterone | 0.5 | 1 | 89.2 | 10.6 | 9.8 |
| 2 | 82.3 | 8.1 | |||
| 3 | 86.1 | 10.2 | |||
| 1 | 1 | 86.0 | 6.0 | 5.8 | |
| 2 | 84.4 | 5.8 | |||
| 3 | 86.8 | 6.4 | |||
| 5 | 1 | 89.3 | 4.5 | 6.0 | |
| 2 | 87.2 | 5.7 | |||
| 3 | 86.9 | 8.0 | |||
| Nandrolone | 0.5 | 1 | 79.2 | 8.9 | 8.8 |
| 2 | 76.8 | 8.5 | |||
| 3 | 80.4 | 9.8 | |||
| 1 | 1 | 83.8 | 10.6 | 8.5 | |
| 2 | 83.0 | 6.7 | |||
| 3 | 82.3 | 9.4 | |||
| 5 | 1 | 84.8 | 11.1 | 11.0 | |
| 2 | 84.8 | 9.8 | |||
| 3 | 82.9 | 13.9 | |||
| Boldenone | 0.5 | 1 | 81.9 | 8.6 | 8.9 |
| 2 | 81.8 | 11.4 | |||
| 3 | 84.5 | 6.7 | |||
| 1 | 1 | 87.1 | 5.1 | 4.4 | |
| 2 | 89.5 | 4.1 | |||
| 3 | 90.1 | 4.1 | |||
| 5 | 1 | 96.5 | 8.7 | 6.5 | |
| 2 | 100.7 | 4.4 | |||
| 3 | 103.5 | 4.9 | |||
| Metandienone | 0.5 | 1 | 81.1 | 5.3 | 8.2 |
| 2 | 80.1 | 6.8 | |||
| 3 | 85.2 | 11.0 | |||
| 1 | 1 | 85.8 | 10.5 | 8.7 | |
| 2 | 83.8 | 8.3 | |||
| 3 | 85.9 | 8.6 | |||
| 5 | 1 | 84.0 | 5.4 | 6.4 | |
| 2 | 82.2 | 5.8 | |||
| 3 | 82.9 | 8.6 | |||
| Trenbolone | 0.5 | 1 | 87.5 | 9.1 | 8.4 |
| 2 | 89.6 | 9.1 | |||
| 3 | 88.3 | 8.4 | |||
| 1 | 1 | 90.8 | 7.6 | 7.6 | |
| 2 | 84.0 | 4.6 | |||
| 3 | 87.4 | 8.8 | |||
| 5 | 1 | 100.9 | 7.2 | 6.4 | |
| 2 | 101.6 | 4.6 | |||
| 3 | 102.4 | 8.1 | |||
| Metenolone | 0.5 | 1 | 78.2 | 5.2 | 7.2 |
| 2 | 75.3 | 8.7 | |||
| 3 | 79.4 | 7.5 | |||
| 1 | 1 | 87.6 | 5.1 | 5.8 | |
| 2 | 87.5 | 6.1 | |||
| 3 | 89.5 | 6.6 | |||
| 5 | 1 | 86.7 | 8.7 | 7.2 | |
| 2 | 84.8 | 8.6 | |||
| 3 | 86.5 | 4.7 | |||
| Methandriol | 0.5 | 1 | 79.5 | 8.5 | 6.2 |
| 2 | 79.1 | 6.1 | |||
| 3 | 80.9 | 4.3 | |||
| 1 | 1 | 82.3 | 6.0 | 6.4 | |
| 2 | 82.0 | 5.8 | |||
| 3 | 85.2 | 7.5 | |||
| 5 | 1 | 87.3 | 5.3 | 7.8 | |
| 2 | 93.8 | 8.3 | |||
| 3 | 83.9 | 5.4 | |||
| Mesterolone | 0.5 | 1 | 83.6 | 7.0 | 7.9 |
| 2 | 84.2 | 10.3 | |||
| 3 | 88.6 | 5.9 | |||
| 1 | 1 | 92.5 | 7.3 | 6.8 | |
| 2 | 88.9 | 3.7 | |||
| 3 | 91.1 | 7.0 | |||
| 5 | 1 | 91.9 | 4.6 | 6.4 | |
| 2 | 97.0 | 7.7 | |||
| 3 | 95.5 | 6.0 | |||
| Danazol | 0.5 | 1 | 106.1 | 5.6 | 7.8 |
| 2 | 109.8 | 7.3 | |||
| 3 | 101.3 | 10.0 | |||
| 1 | 1 | 94.7 | 8.7 | 6.5 | |
| 2 | 96.7 | 5.9 | |||
| 3 | 98.1 | 5.4 | |||
| 5 | 1 | 104.3 | 5.1 | 5.9 | |
| 2 | 96.6 | 6.6 | |||
| 3 | 100.8 | 3.8 | |||
| Stanozolol | 0.5 | 1 | 77.2 | 10.2 | 10.1 |
| 2 | 73.3 | 10.1 | |||
| 3 | 71.6 | 10.2 | |||
| 1 | 1 | 83.5 | 6.8 | 10.3 | |
| 2 | 75.7 | 12.4 | |||
| 3 | 78.6 | 10.5 | |||
| 5 | 1 | 75.4 | 6.9 | 6.3 | |
| 2 | 76.0 | 7.2 | |||
| 3 | 76.6 | 6.1 |
| Compound | Added Level (μg/kg) | Batch | Average Recovery (%) | Intraday RSD (%) | Interday RSD (%) |
|---|---|---|---|---|---|
| Testosterone | 0.5 | 1 | 80.3 | 13.3 | 11.6 |
| 2 | 84.8 | 12.6 | |||
| 3 | 83.5 | 10.3 | |||
| 1 | 1 | 90.4 | 5.7 | 8.0 | |
| 2 | 86.8 | 10.9 | |||
| 3 | 83.3 | 5.5 | |||
| 5 | 1 | 89.9 | 7.3 | 8.6 | |
| 2 | 85.0 | 10.2 | |||
| 3 | 87.6 | 8.6 | |||
| Epitestosterone | 0.5 | 1 | 86.3 | 8.3 | 7.9 |
| 2 | 87.6 | 7.5 | |||
| 3 | 82.6 | 8.7 | |||
| 1 | 1 | 89.4 | 9.7 | 9.0 | |
| 2 | 83.6 | 8.3 | |||
| 3 | 86.5 | 9.0 | |||
| 5 | 1 | 84.5 | 10.0 | 7.9 | |
| 2 | 83.9 | 6.2 | |||
| 3 | 89.6 | 6.7 | |||
| Methyltestosterone | 0.5 | 1 | 79.1 | 5.6 | 6.9 |
| 2 | 76.1 | 6.7 | |||
| 3 | 79.3 | 8.3 | |||
| 1 | 1 | 81.3 | 10.6 | 9.8 | |
| 2 | 82.1 | 9.4 | |||
| 3 | 86.3 | 9.9 | |||
| 5 | 1 | 83.1 | 10.4 | 8.6 | |
| 2 | 82.9 | 7.7 | |||
| 3 | 81.3 | 9.1 | |||
| Nandrolone | 0.5 | 1 | 81.9 | 9.6 | 9.8 |
| 2 | 86.3 | 12.5 | |||
| 3 | 80.8 | 6.6 | |||
| 1 | 1 | 90.6 | 5.6 | 6.3 | |
| 2 | 93.5 | 3.8 | |||
| 3 | 89.8 | 9.0 | |||
| 5 | 1 | 84.9 | 7.7 | 7.4 | |
| 2 | 88.8 | 7.8 | |||
| 3 | 88.3 | 7.1 | |||
| Boldenone | 0.5 | 1 | 92.9 | 8.9 | 10.6 |
| 2 | 85.8 | 12.2 | |||
| 3 | 84.9 | 9.9 | |||
| 1 | 1 | 87.5 | 6.8 | 6.5 | |
| 2 | 93.5 | 7.0 | |||
| 3 | 89.8 | 4.6 | |||
| 5 | 1 | 97.7 | 10.6 | 8.7 | |
| 2 | 92.1 | 7.6 | |||
| 3 | 97.3 | 7.9 | |||
| Metandienone | 0.5 | 1 | 84.5 | 8.0 | 7.3 |
| 2 | 85.3 | 6.8 | |||
| 3 | 87.3 | 7.9 | |||
| 1 | 1 | 88.1 | 6.3 | 6.4 | |
| 2 | 93.4 | 7.5 | |||
| 3 | 92.7 | 4.3 | |||
| 5 | 1 | 85.2 | 8.8 | 9.5 | |
| 2 | 85.2 | 9.0 | |||
| 3 | 85.2 | 8.0 | |||
| Trenbolone | 0.5 | 1 | 87.7 | 7.3 | 7.0 |
| 2 | 84.7 | 4.2 | |||
| 3 | 83.0 | 8.8 | |||
| 1 | 1 | 87.4 | 9.4 | 7.8 | |
| 2 | 85.8 | 7.6 | |||
| 3 | 85.5 | 7.5 | |||
| 5 | 1 | 86.1 | 6.3 | 6.1 | |
| 2 | 87.9 | 6.5 | |||
| 3 | 88.8 | 6.0 | |||
| Metenolone | 0.5 | 1 | 80.9 | 5.9 | 9.2 |
| 2 | 85.0 | 9.8 | |||
| 3 | 83.3 | 11.8 | |||
| 1 | 1 | 92.2 | 5.7 | 7.7 | |
| 2 | 82.5 | 4.6 | |||
| 3 | 89.2 | 8.3 | |||
| 5 | 1 | 88.3 | 9.4 | 10.0 | |
| 2 | 87.5 | 9.5 | |||
| 3 | 84.3 | 12.3 | |||
| Methandriol | 0.5 | 1 | 86.0 | 9.8 | 9.0 |
| 2 | 84.8 | 10.1 | |||
| 3 | 83.4 | 8.5 | |||
| 1 | 1 | 82.9 | 4.5 | 7.1 | |
| 2 | 83.2 | 8.3 | |||
| 3 | 86.3 | 8.3 | |||
| 5 | 1 | 83.2 | 6.2 | 8.1 | |
| 2 | 81.3 | 8.5 | |||
| 3 | 81.5 | 10.4 | |||
| Mesterolone | 0.5 | 1 | 84.6 | 10.5 | 8.4 |
| 2 | 86.8 | 9.6 | |||
| 3 | 86.1 | 5.8 | |||
| 1 | 1 | 86.7 | 10.4 | 8.4 | |
| 2 | 86.8 | 6.6 | |||
| 3 | 84.4 | 9.1 | |||
| 5 | 1 | 90.3 | 5.4 | 8.5 | |
| 2 | 94.6 | 6.0 | |||
| 3 | 85.2 | 11.1 | |||
| Danazol | 0.5 | 1 | 88.1 | 10.2 | 11.6 |
| 2 | 86.7 | 13.8 | |||
| 3 | 84.4 | 12.3 | |||
| 1 | 1 | 95.5 | 9.7 | 9.7 | |
| 2 | 91.0 | 9.4 | |||
| 3 | 87.7 | 9.7 | |||
| 5 | 1 | 90.0 | 12.4 | 12.2 | |
| 2 | 84.1 | 12.3 | |||
| 3 | 86.6 | 13.1 | |||
| Stanozolol | 0.5 | 1 | 75.5 | 11.3 | 11.6 |
| 2 | 74.2 | 13.1 | |||
| 3 | 70.5 | 11.3 | |||
| 1 | 1 | 80.8 | 9.7 | 8.9 | |
| 2 | 83.2 | 10.9 | |||
| 3 | 85.8 | 6.3 | |||
| 5 | 1 | 75.7 | 7.8 | 8.7 | |
| 2 | 72.0 | 8.3 | |||
| 3 | 75.5 | 10.4 |
References
- Keevil, B.G.; Adaway, J. Assessment of Free Testosterone Concentration. J. Steroid Biochem. Mol. Biol. 2019, 190, 207–211. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, H.; Zhou, C.; Yang, L.; Zhai, S.; Yang, P.; Zhao, R.; Li, R. Magnetic Solid Phase Extraction Followed by In-Situ Derivatization with Core–Shell Structured Magnetic Graphene Oxide Nanocomposite for the Accurate Quantification of Free Testosterone and Free Androstenedione in Human Serum. J. Chromatogr. B 2022, 1196, 123188. [Google Scholar] [CrossRef]
- Kanayama, G.; Pope, H.G. History and Epidemiology of Anabolic Androgens in Athletes and Non-Athletes. Mol. Cell. Endocrinol. 2018, 464, 4–13. [Google Scholar] [CrossRef]
- Anadón, A.; Martínez-Larrañaga, M.R.; Castellano, V. Regulatory Aspects for the Drugs and Chemicals Used in Food-Producing Animals in the European Union. In Veterinary Toxicology; Elsevier: Amsterdam, The Netherlands, 2012; pp. 135–155. ISBN 978-0-12-385926-6. [Google Scholar]
- Kunze, M.; Wirthgen, E.; Walz, C.; Spitschak, M.; Brenmoehl, J.; Vanselow, J.; Schwerin, M.; Wimmers, K.; Hoeflich, A. Bioanalytical Validation for Simultaneous Quantification of Non-Aromatic Steroids in Follicular Fluid from Cattle via ESI-LC–MS/MS. J. Chromatogr. B 2015, 1007, 132–139. [Google Scholar] [CrossRef]
- Cha, E.; Jeong, E.S.; Cha, S.; Lee, J. Coupling of Gas Chromatography and Electrospray Ionization High Resolution Mass Spectrometry for the Analysis of Anabolic Steroids as Trimethylsilyl Derivatives in Human Urine. Anal. Chim. Acta 2017, 964, 123–133. [Google Scholar] [CrossRef]
- Young, J. Doping with Testosterone and Androgenic/Anabolic Steroids: Impact on Health, Screening Tools and Medical Care. Ann. Endocrinol. 2023, 84, 401–405. [Google Scholar] [CrossRef]
- Alaedini, S.; Amirahmadi, M.; Kobarfard, F.; Rastegar, H.; Nasirahmadi, S.; Shoeibi, S. Survey of Protein-Based Sport Supplements for Illegally Added Anabolic Steroids Methyltestosterone and 4-Androstenedione by UPLC-MS/MS. Steroids 2021, 165, 108758. [Google Scholar] [CrossRef]
- De Ronde, W.; Smit, D.L. Anabolic–Androgenic Steroid Abuse and Testicular Function in Men; Recent Insights. Curr. Opin. Pharmacol. 2022, 67, 102318. [Google Scholar] [CrossRef]
- Patil, V.; Jothimani, D.; Harika, K.; Hakeem, A.R.; Sachan, D.; Vij, M.; Rela, M. Versatility of Anabolic Androgenic Steroid–Induced Hepatotoxicity. J. Clin. Exp. Hepatol. 2022, 12, 216–221. [Google Scholar] [CrossRef]
- Jamal, M.; Shakeel, H.A.; Kayani, M.J.; Maqsood, H.; Khawaja, U.A.; Shah, R.N. Anabolic-Androgenic Steroid Use in a Young Body-Builder: A Case Report and Review of the Literature. Ann. Med. Surg. 2022, 83, 104567. [Google Scholar] [CrossRef]
- Shi, P.; Wang, Y.; Wu, W.; Xia, B.; Zhou, Y. A Novel Functionalized Covalent Organic Framework/Carbon Nanotube Composite as an Effective Online Solid-Phase Extraction Sorbent for Simultaneous Detection of 33 Steroid Hormones in Pork. Food Chem. 2022, 379, 132111. [Google Scholar] [CrossRef]
- He, Z.; Shi, X.; Guo, Y.; Guan, F.; Gao, P.; Tang, Y.; Liu, S.; Zhu, Y.; Xie, K.; Chen, H. Simultaneous Quantitative Determination of Residues of Abamectin, Ivermectin, Albendazole and Its Three Metabolites in Beef and Chicken by HPLC-PDA. Food Chem. 2023, 421, 136168. [Google Scholar] [CrossRef]
- Han, L.; Wemm, S.E.; Shen, L.; Spink, D.C.; Wulfert, E.; Cao, Z.T. Noninvasive Detection of Human Dehydroepiandrosterone, Progesterone and Testosterone Using LC-MS/MS Revealed Effects of Birth Control Pills/Devices and Body Weight on Ovulatory Prediction. J. Chromatogr. B 2021, 1174, 122716. [Google Scholar] [CrossRef]
- Mokh, S.; Moussa, F.; Khoury, E.E.L.; Nassar, R.; Bernabò, N.; Al Iskandarani, M. Development of a New Liquid Chromatography-Tandem Mass Spectrometry Method for the Determination of Hormones in Bovine Muscle. J. Pharm. Biomed. Anal. 2020, 190, 113550. [Google Scholar] [CrossRef]
- Ma, M.; Wu, T.; Sun, G.; Zhang, S. Determination of Testosterone in Serum by Magnetic Molecularly Imprinted Polymer-Coupled Nano-ESI-MS. Anal. Biochem. 2022, 653, 114719. [Google Scholar] [CrossRef]
- Guedes-Alonso, R.; Sosa-Ferrera, Z.; Santana-Rodríguez, J.J. Determination of Steroid Hormones in Fish Tissues by Microwave-Assisted Extraction Coupled to Ultra-High Performance Liquid Chromatography Tandem Mass Spectrometry. Food Chem. 2017, 237, 1012–1020. [Google Scholar] [CrossRef]
- Huml, L.; Havlová, D.; Longin, O.; Staňková, E.; Holubová, B.; Kuchař, M.; Prokudina, E.; Rottnerová, Z.; Zimmermann, T.; Drašar, P.; et al. Stanazolol Derived ELISA as a Sensitive Forensic Tool for the Detection of Multiple 17α-Methylated Anabolics. Steroids 2020, 155, 108550. [Google Scholar] [CrossRef]
- Wolecki, D.; Caban, M.; Pazdro, K.; Mulkiewicz, E.; Stepnowski, P.; Kumirska, J. Simultaneous Determination of Non-Steroidal Anti-Inflammatory Drugs and Natural Estrogens in the Mussels Mytilus Edulis Trossulus. Talanta 2019, 200, 316–323. [Google Scholar] [CrossRef]
- Moussa, F.; Mokh, S.; Doumiati, S.; Barboni, B.; Bernabò, N.; Al Iskandarani, M. LC-MS/MS Method for the Determination of Hormones: Validation, Application and Health Risk Assessment in Various Bovine Matrices. Food Chem. Toxicol. 2020, 138, 111204. [Google Scholar] [CrossRef]
- Vanhaecke, L.; Bussche, J.V.; Wille, K.; Bekaert, K.; De Brabander, H.F. Ultra-High Performance Liquid Chromatography–Tandem Mass Spectrometry in High-Throughput Confirmation and Quantification of 34 Anabolic Steroids in Bovine Muscle. Anal. Chim. Acta 2011, 700, 70–77. [Google Scholar] [CrossRef]
- Dasenaki, M.E.; Thomaidis, N.S. Multi-Residue Determination of 115 Veterinary Drugs and Pharmaceutical Residues in Milk Powder, Butter, Fish Tissue and Eggs Using Liquid Chromatography–Tandem Mass Spectrometry. Anal. Chim. Acta 2015, 880, 103–121. [Google Scholar] [CrossRef] [PubMed]
- Fraissinet, F.; Pereira, T.; Violin, A.; Feugray, G.; Bach-Ngohou, K.; Brunel, V. A Novel Fully-Automated Method to Measure Steroids in Serum by Liquid Chromatography-Tandem Mass Spectrometry. J. Mass Spectrom. Adv. Clin. Lab 2023, 27, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Gervasoni, J.; Schiattarella, A.; Primiano, A.; D’Addurno, I.; Cocci, A.; Zuppi, C.; Persichilli, S. Simultaneous Quantification of 17-Hydroxyprogesterone, Androstenedione, Testosterone and Cortisol in Human Serum by LC-MS/MS Using TurboFlow Online Sample Extraction. Clin. Biochem. 2016, 49, 998–1003. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Swezey, R.R.; Green, C.E.; Lee, M.S.; Bunin, D.I.; Parman, T. A Tandem Liquid Chromatography and Tandem Mass Spectrometry (LC/LC–MS/MS) Technique to Separate and Quantify Steroid Isomers 11β-Methyl-19-Nortestosterone and Testosterone. J. Chromatogr. B 2022, 1193, 123165. [Google Scholar] [CrossRef] [PubMed]
- Koloka, O.; Koulama, M.; Hela, D.; Albanis, T.; Konstantinou, I. Determination of Multiclass Pharmaceutical Residues in Milk Using Modified QuEChERS and Liquid-Chromatography-Hybrid Linear Ion Trap/Orbitrap Mass Spectrometry: Comparison of Clean-Up Approaches and Validation Studies. Molecules 2023, 28, 6130. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Ning, J.; Cheng, X.; Lv, Q.; Teng, S.; Wang, W. Rapid and High-Throughput Determination of Sixteen β-Agonists in Livestock Meat Using One-Step Solid-Phase Extraction Coupled with UHPLC-MS/MS. Foods 2022, 12, 76. [Google Scholar] [CrossRef] [PubMed]
- Kaklamanos, G.; Theodoridis, G. Rapid Multi-Method for the Determination of Growth Promoters in Bovine Milk by Liquid Chromatography–Tandem Mass Spectrometry. J. Chromatogr. B 2013, 930, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Foodmate. Available online: http://down.foodmate.net/standard/yulan.php?itemid=16931 (accessed on 24 October 2023).
- Zhang, X.; Fang, C.; Lou, X.; Yang, G.; Kong, C.; Shi, Y.; Huang, D. Determination of 17α-Methyltestosterone in Aquatic Products Using High-Performance Liquid Chromatography-Tandem Mass Spectrometry. Microchem. J. 2022, 183, 108119. [Google Scholar] [CrossRef]
- Domenech-Coca, C.; Mariné-Casadó, R.; Caimari, A.; Arola, L.; Del Bas, J.M.; Bladé, C.; Rodriguez-Naranjo, M.I. Dual Liquid-Liquid Extraction Followed by LC-MS/MS Method for the Simultaneous Quantification of Melatonin, Cortisol, Triiodothyronine, Thyroxine and Testosterone Levels in Serum: Applications to a Photoperiod Study in Rats. J. Chromatogr. B 2019, 1108, 11–16. [Google Scholar] [CrossRef]
- Shahbazi, Y.; Malekinejad, H.; Tajik, H. Determination of Naturally Occurring Estrogenic Hormones in Cow’s and River Buffalo’s Meat by HPLC-FLD Method. J. Food Drug Anal. 2016, 24, 457–463. [Google Scholar] [CrossRef]
- López-García, M.; Romero-González, R.; Garrido Frenich, A. Determination of Steroid Hormones and Their Metabolite in Several Types of Meat Samples by Ultra High Performance Liquid Chromatography—Orbitrap High Resolution Mass Spectrometry. J. Chromatogr. A 2018, 1540, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, M.R.; Pudale, A.; Raut, P.; Utture, S.; Ahammed Shabeer, T.P.; Banerjee, K. A Unified Approach for High-Throughput Quantitative Analysis of the Residues of Multi-Class Veterinary Drugs and Pesticides in Bovine Milk Using LC-MS/MS and GC–MS/MS. Food Chem. 2019, 272, 292–305. [Google Scholar] [CrossRef] [PubMed]
- Weng, Y.; Xie, F.; Xu, L.; Zagorevski, D.; Spink, D.C.; Ding, X. Analysis of Testosterone and Dihydrotestosterone in Mouse Tissues by Liquid Chromatography–Electrospray Ionization–Tandem Mass Spectrometry. Anal. Biochem. 2010, 402, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Li, X.; Ding, S.; Zhang, S.; Jiang, H.; Li, J.; Shen, J. Ultra-High-Pressure Liquid Chromatography–Tandem Mass Spectrometry for the Analysis of Six Resorcylic Acid Lactones in Bovine Milk. J. Chromatogr. A 2009, 1216, 2587–2591. [Google Scholar] [CrossRef] [PubMed]
- Foodmate. Available online: http://down.foodmate.net/standard/yulan.php?itemid=17441 (accessed on 24 October 2023).
- Foodmate. Available online: http://down.foodmate.net/standard/yulan.php?itemid=15544 (accessed on 19 September 2023).
- Yıldırım, S.; Fikarová, K.; Pilařová, V.; Nováková, L.; Solich, P.; Horstkotte, B. Lab-in-Syringe Automated Protein Precipitation and Salting-out Homogenous Liquid-Liquid Extraction Coupled Online to UHPLC-MS/MS for the Determination of Beta-Blockers in Serum. Anal. Chim. Acta 2023, 1251, 340966. [Google Scholar] [CrossRef] [PubMed]
- Leite, M.; Freitas, A.; Barbosa, J.; Ramos, F. Comprehensive Assessment of Different Extraction Methodologies for Optimization and Validation of an Analytical Multi-Method for Determination of Emerging and Regulated Mycotoxins in Maize by UHPLC-MS/MS. Food Chem. Adv. 2023, 2, 100145. [Google Scholar] [CrossRef]
- Dong, H.; Guo, X.; Xian, Y.; Luo, H.; Wang, B.; Wu, Y. A Salting Out-Acetonitrile Homogeneous Extraction Coupled with Gas Chromatography–Mass Spectrometry Method for the Simultaneous Determination of Thirteen N-Nitrosamines in Skin Care Cosmetics. J. Chromatogr. A 2015, 1422, 82–88. [Google Scholar] [CrossRef]
- Xiong, X.; Li, D.; Du, Z.; Xiong, C.; Jiang, H. Magnetic Solid-Phase Extraction Modified Quick, Easy, Cheap, Effective, Rugged and Safe Method Combined with Pre-Column Derivatization and Ultra-High Performance Liquid Chromatography-Tandem Mass Spectrometry for Determination of Estrogens and Estrogen Mimics in Pork and Chicken Samples. J. Chromatogr. A 2020, 1622, 461137. [Google Scholar] [CrossRef]
- Temerdashev, A.; Dmitrieva, E.; Azaryan, A.; Gashimova, E. Determination of Oxprenolol, Methandienone and Testosterone in Meat Samples by UHPLC-Q-ToF. Heliyon 2023, 9, e13260. [Google Scholar] [CrossRef]
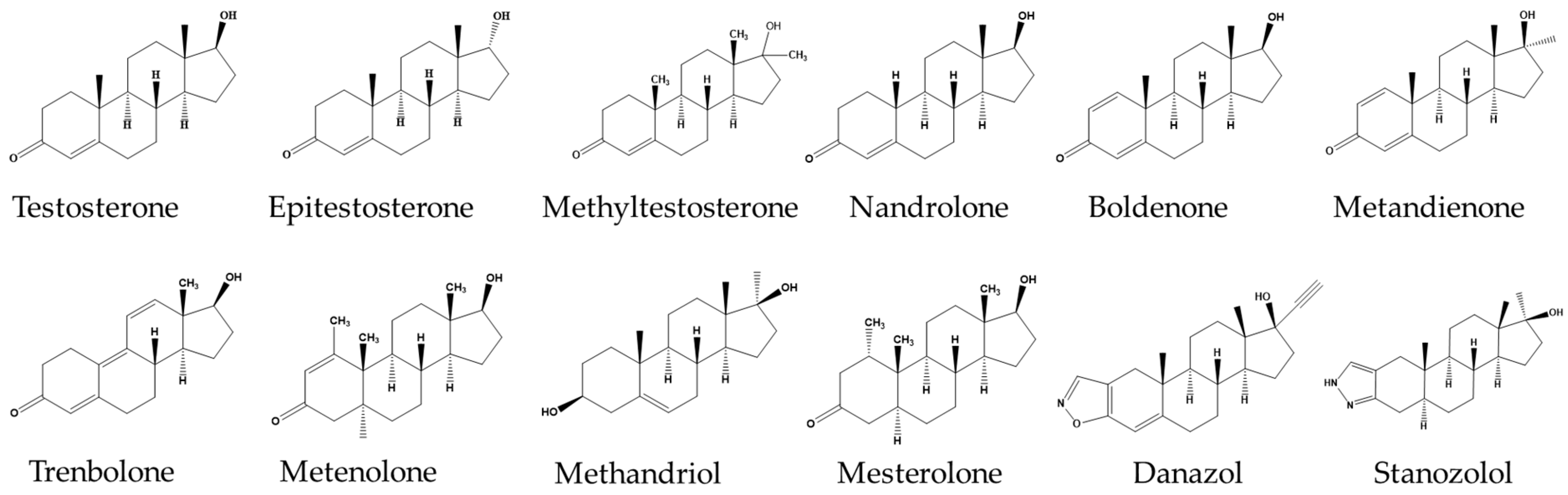
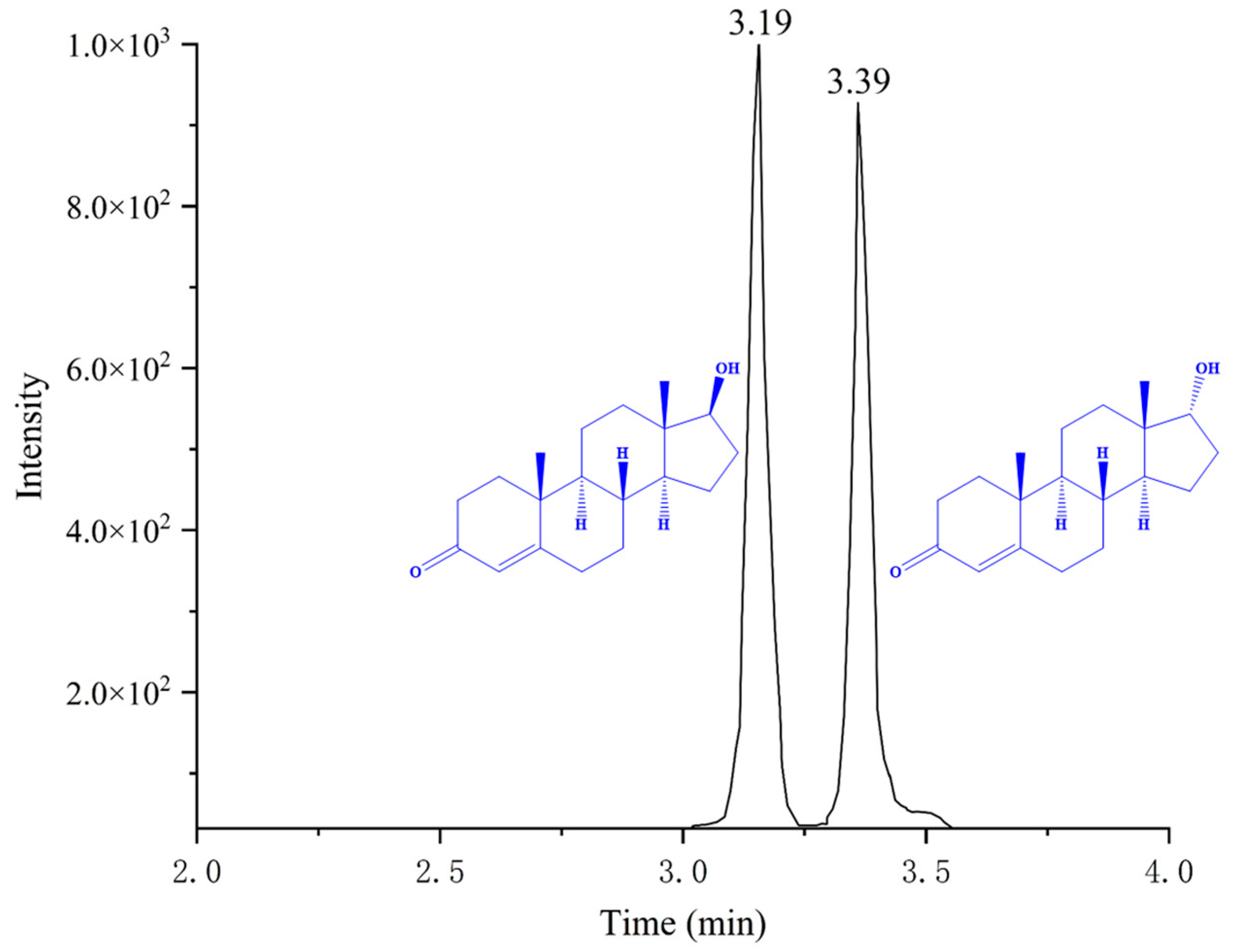
| Compound | Precursor (m/z) | Product (m/z) | Collision Energy (V) | RF Lens (V) |
|---|---|---|---|---|
| Testosterone | 289.212 | 97.000 * | 21.68 | 120 |
| 289.212 | 109.000 | 24.41 | 120 | |
| Epitestosterone | 289.212 | 97.000 * | 21.60 | 120 |
| 289.212 | 109.000 | 24.37 | 120 | |
| Methyltestosterone | 303.212 | 187.054 * | 21.00 | 124 |
| 303.212 | 285.137 | 16.00 | 124 | |
| Nandrolone | 275.175 | 109.000 * | 25.70 | 119 |
| 275.175 | 257.208 | 15.50 | 119 | |
| Boldenone | 287.175 | 121.071 * | 22.85 | 95 |
| 287.175 | 269.208 | 10.23 | 95 | |
| Metandienone | 301.175 | 121.071 * | 24.82 | 93 |
| 301.175 | 149.155 | 14.32 | 93 | |
| Trenbolone | 271.175 | 199.054 * | 22.89 | 144 |
| 271.175 | 253.137 | 19.52 | 144 | |
| Metenolone | 303.212 | 109.000 * | 26.14 | 124 |
| 303.212 | 285.137 | 15.76 | 124 | |
| Methandriol | 287.400 | 269.100 * | 11.10 | 120 |
| 287.400 | 159.100 | 21.10 | 120 | |
| Mesterolone | 301.000 | 121.000 * | 26.00 | 101 |
| 301.000 | 149.000 | 15.00 | 101 | |
| Danazol | 338.300 | 120.000 * | 35.00 | 120 |
| 338.300 | 148.200 | 15.00 | 120 | |
| Stanozolol | 329.200 | 121.000 * | 36.90 | 120 |
| 329.200 | 107.100 | 42.00 | 120 |
| Extraction Solvent | Number of AASs | ||
|---|---|---|---|
| Recovery < 60% | Recovery 60% to 120% | Recovery > 120% | |
| Methanol | 5 | 6 | 1 |
| Acetonitrile | 1 | 8 | 3 |
| Ethyl acetate | 3 | 9 | 0 |
| Acetonitrile (containing 1% acetic acid, v/v) | 0 | 12 | 0 |
| SPE Columns | Number of AASs | ||
|---|---|---|---|
| Recovery < 60% | Recovery 60% to 120% | Recovery > 120% | |
| HLB | 1 | 11 | 0 |
| C18 | 2 | 10 | 0 |
| MAX | 4 | 8 | 0 |
| QVet-NM | 0 | 12 | 0 |
| Matrix | Compound | Regression Equation | R2 | Linear Range (μg/L) | LOD (μg/kg) | LOQ (μg/kg) |
|---|---|---|---|---|---|---|
| Pork | Testosterone | y = 0.001690x − 0.001235 | 0.9957 | 1–100 | 0.08 | 0.24 |
| Epitestosterone | y = 0.002980x − 0.02382 | 0.9982 | 1–100 | 0.12 | 0.37 | |
| Methyltestosterone | y = 0.002109x + 0.5309 | 0.9968 | 1–100 | 0.13 | 0.40 | |
| Nandrolone | y = 0.09590x + 0.002411 | 0.9980 | 1–100 | 0.08 | 0.60 | |
| Boldenone | y = 0.0001184x − 0.0001063 | 0.9966 | 1–100 | 0.06 | 0.18 | |
| Metandienone | y = 0.007545x + 0.0001471 | 0.9995 | 1–100 | 0.03 | 0.09 | |
| Trenbolone | y = 0.003224x + 0.006057 | 0.9984 | 1–100 | 0.12 | 0.36 | |
| Metenolone | y = 0.002862x + 0.08988 | 0.9956 | 1–100 | 0.19 | 0.57 | |
| Methandriol | y = 0.001485x − 0.3889 | 0.9968 | 1–100 | 0.24 | 0.72 | |
| Mesterolone | y = 0.005878x + 0.006874 | 0.9929 | 1–100 | 0.03 | 0.09 | |
| Danazol | y = 0.03880x + 0.07963 | 0.9977 | 1–100 | 0.30 | 0.90 | |
| Stanozolol | y = 0.005689x + 0.0002292 | 0.9974 | 1–100 | 0.30 | 0.90 | |
| Beef | Testosterone | y = 0.001100x + 0.09015 | 0.9936 | 1–100 | 0.06 | 0.18 |
| Epitestosterone | y = 0.001678x + 0.003171 | 0.9979 | 1–100 | 0.06 | 0.18 | |
| Methyltestosterone | y = 0.08854x + 0.004840 | 0.9962 | 1–100 | 0.07 | 0.23 | |
| Nandrolone | y = 0.07633x + 0.004184 | 0.9986 | 1–100 | 0.09 | 0.30 | |
| Boldenone | y = 0.009562x + 0.001751 | 0.9995 | 1–100 | 0.05 | 0.15 | |
| Metandienone | y = 0.009745x + 0.01987 | 0.9915 | 1–100 | 0.03 | 0.09 | |
| Trenbolone | y = 0.002319x + 0.09887 | 0.9985 | 1–100 | 0.09 | 0.30 | |
| Metenolone | y = 0.001380x + 0.004296 | 0.9971 | 1–100 | 0.19 | 0.60 | |
| Methandriol | y = 0.001413x + 0.07204 | 0.9968 | 1–100 | 0.27 | 0.80 | |
| Mesterolone | y = 0.007392x + 0.004931 | 0.9934 | 1–100 | 0.03 | 0.09 | |
| Danazol | y = 0.03512x + 0.09421 | 0.9985 | 1–100 | 0.17 | 0.50 | |
| Stanozolol | y = 0.003588x + 0.0001100 | 0.9961 | 1–100 | 0.24 | 0.73 | |
| Lamb | Testosterone | y = 0.001527x + 0.006818 | 0.9995 | 1–100 | 0.05 | 0.15 |
| Epitestosterone | y = 0.002364x + 0.05624 | 0.9931 | 1–100 | 0.06 | 0.18 | |
| Methyltestosterone | y = 0.001200x + 0.005994 | 0.9917 | 1–100 | 0.10 | 0.30 | |
| Nandrolone | y = 0.04545x + 0.002813 | 0.9988 | 1–100 | 0.20 | 0.60 | |
| Boldenone | y = 0.009871x + 0.006202 | 0.9957 | 1–100 | 0.05 | 0.15 | |
| Metandienone | y = 0.0001146x − 0.006042 | 0.9992 | 1–100 | 0.29 | 0.90 | |
| Trenbolone | y = 0.002577x + 0.002403 | 0.9987 | 1–100 | 0.03 | 0.09 | |
| Metenolone | y = 0.001835x + 0.002530 | 0.9917 | 1–100 | 0.13 | 0.40 | |
| Methandriol | y = 0.001440x + 0.006457 | 0.9977 | 1–100 | 0.26 | 0.80 | |
| Mesterolone | y = 0.006078x + 0.001785 | 0.9979 | 1–100 | 0.03 | 0.09 | |
| Danazol | y = 0.04350x − 0.08949 | 0.9903 | 1–100 | 0.33 | 0.90 | |
| Stanozolol | y = 0.006099x − 0.002605 | 0.9972 | 1–100 | 0.09 | 0.30 | |
| Chicken | Testosterone | y = 0.07008x + 0.04804 | 0.9934 | 1–100 | 0.06 | 0.18 |
| Epitestosterone | y = 0.001597x − 0.002645 | 0.9970 | 1–100 | 0.08 | 0.24 | |
| Methyltestosterone | y = 0.09237x − 0.02228 | 0.9964 | 1–100 | 0.13 | 0.40 | |
| Nandrolone | y = 0.03718x + 0.001061 | 0.9912 | 1–100 | 0.19 | 0.60 | |
| Boldenone | y = 0.008562x − 0.009533 | 0.9938 | 1–100 | 0.06 | 0.18 | |
| Metandienone | y = 0.005442x − 0.0001624 | 0.9934 | 1–100 | 0.05 | 0.15 | |
| Trenbolone | y = 0.002110x − 0.001296 | 0.9922 | 1–100 | 0.07 | 0.21 | |
| Metenolone | y = 0.001328x − 0.09989 | 0.9965 | 1–100 | 0.09 | 0.30 | |
| Methandriol | y = 0.001276x + 0.005650 | 0.9943 | 1–100 | 0.20 | 0.60 | |
| Mesterolone | y = 0.003602x − 0.007355 | 0.9926 | 1–100 | 0.05 | 0.15 | |
| Danazol | y = 0.03519x − 0.04157 | 0.9942 | 1–100 | 0.30 | 0.90 | |
| Stanozolol | y = 0.003903x − 0.0001216 | 0.9929 | 1–100 | 0.20 | 0.60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Yan, Y.; Wang, Y.; Lv, Q.; Teng, S.; Wang, W. Rapid and Simultaneous Determination of Anabolic Andro-Genic Steroids in Livestock and Poultry Meat Using One-Step Solid-Phase Extraction Coupled with UHPLC–MS/MS. Molecules 2024, 29, 84. https://doi.org/10.3390/molecules29010084
Wang L, Yan Y, Wang Y, Lv Q, Teng S, Wang W. Rapid and Simultaneous Determination of Anabolic Andro-Genic Steroids in Livestock and Poultry Meat Using One-Step Solid-Phase Extraction Coupled with UHPLC–MS/MS. Molecules. 2024; 29(1):84. https://doi.org/10.3390/molecules29010084
Chicago/Turabian StyleWang, Liqun, Yonghong Yan, Yan Wang, Qingqin Lv, Shuang Teng, and Wei Wang. 2024. "Rapid and Simultaneous Determination of Anabolic Andro-Genic Steroids in Livestock and Poultry Meat Using One-Step Solid-Phase Extraction Coupled with UHPLC–MS/MS" Molecules 29, no. 1: 84. https://doi.org/10.3390/molecules29010084
APA StyleWang, L., Yan, Y., Wang, Y., Lv, Q., Teng, S., & Wang, W. (2024). Rapid and Simultaneous Determination of Anabolic Andro-Genic Steroids in Livestock and Poultry Meat Using One-Step Solid-Phase Extraction Coupled with UHPLC–MS/MS. Molecules, 29(1), 84. https://doi.org/10.3390/molecules29010084





