Eco-Conscious Approach to Thermoresponsive Star-Comb and Mikto-Arm Polymers via Enzymatically Assisted Atom Transfer Radical Polymerization Followed by Ring-Opening Polymerization
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
4. Instrumentation
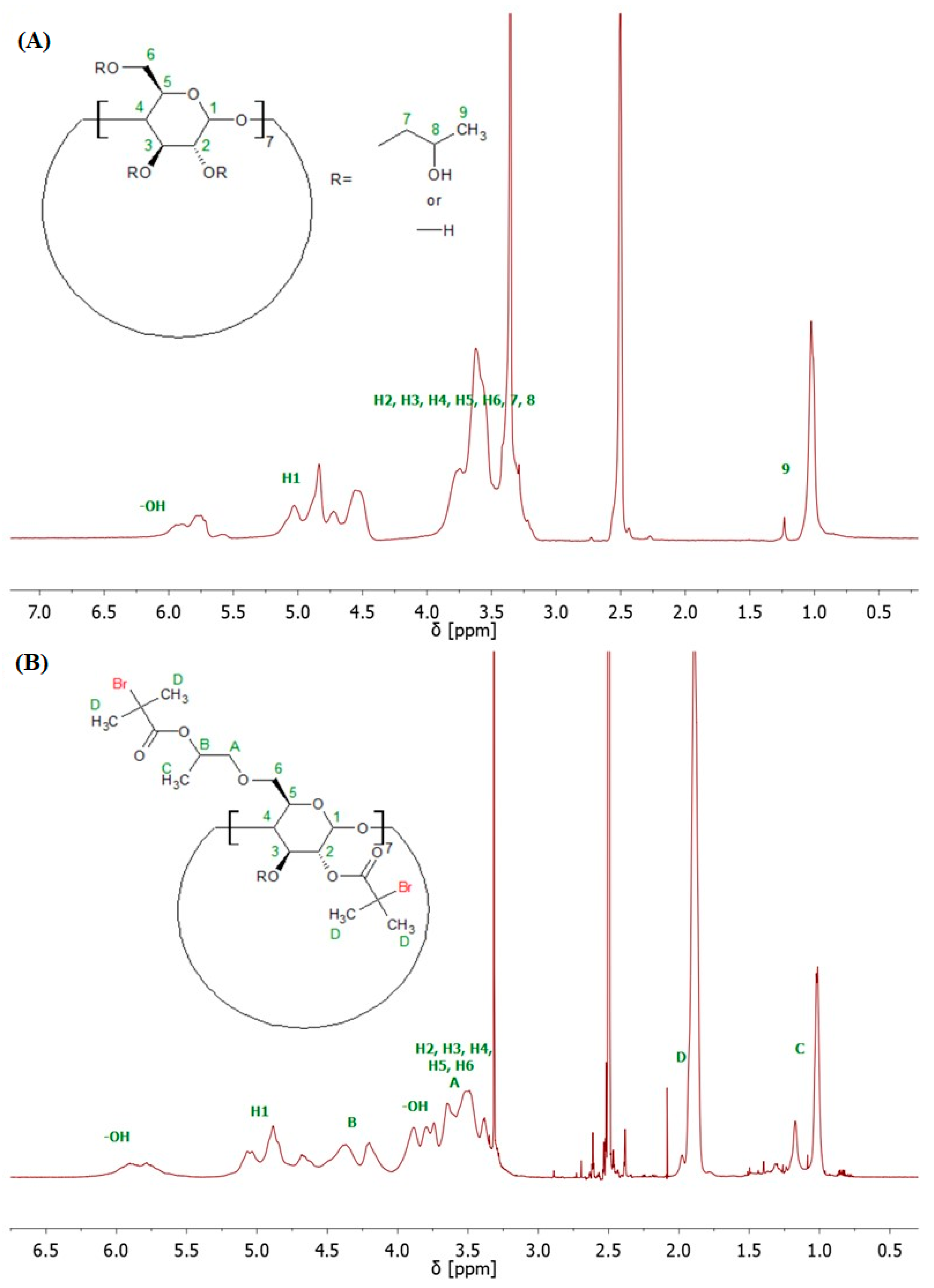
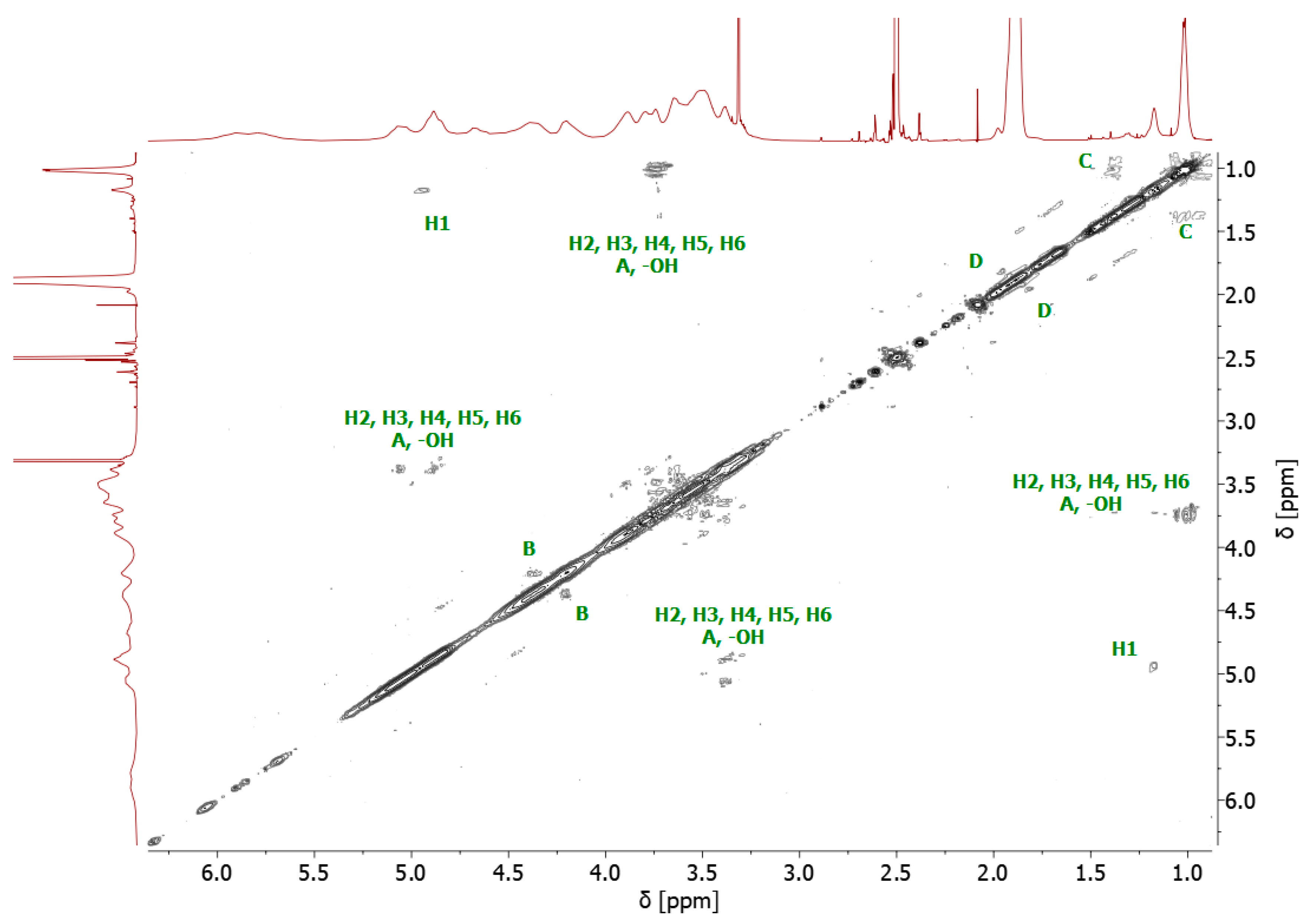
4.1. Nuclear Magnetic Resonance (NMR)
4.2. Attenuated Total Reflectance-Fourier Transform Infrared Spectroscopy (ATR-FTIR)
4.3. High-Resolution Mass Spectrometry (HR-MS)
4.4. UV–Vis Spectroscopy
4.5. Differential Scanning Calorimetry (DSC)
4.6. Size-Exclusion Chromatography (SEC)
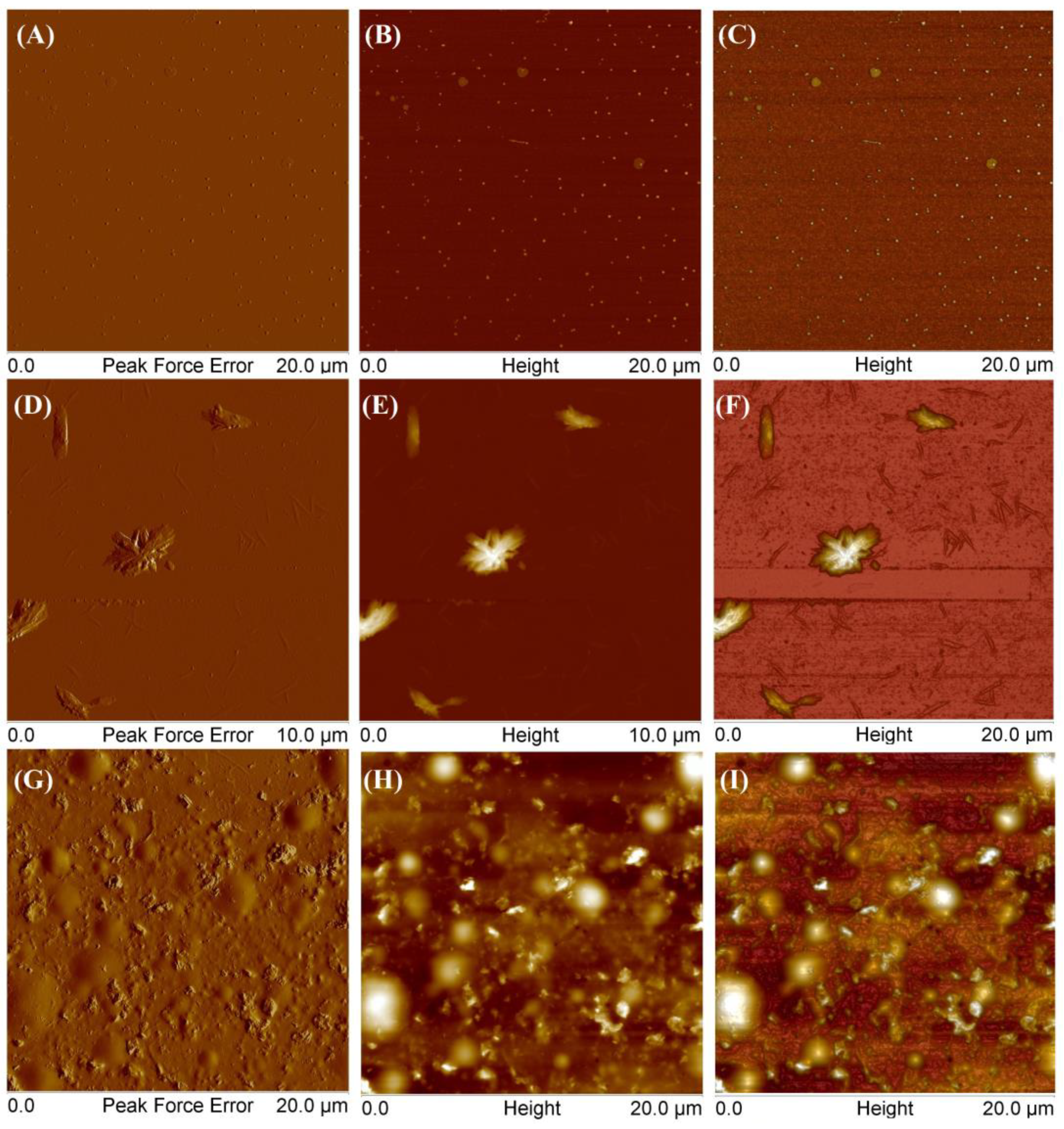
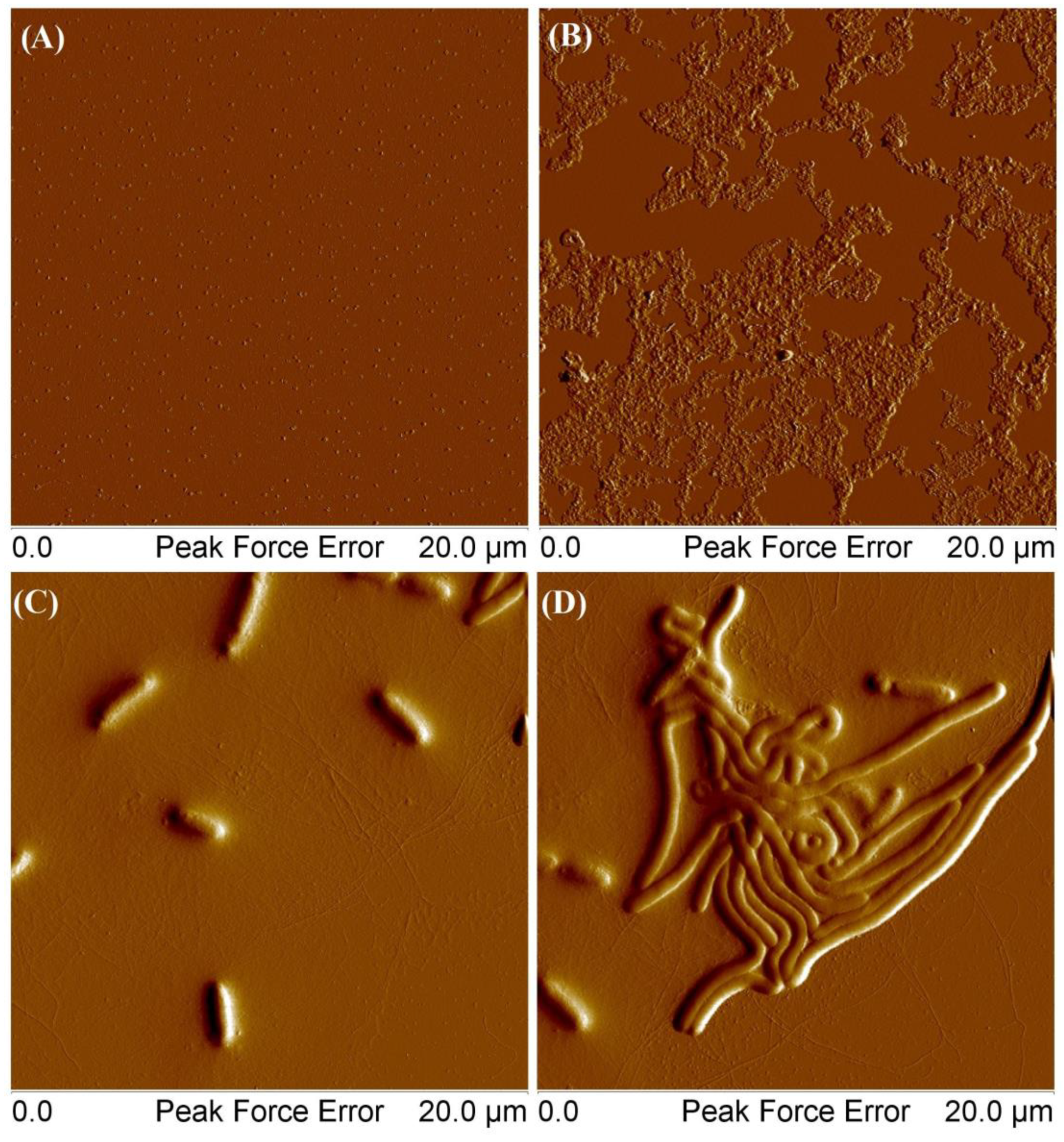
4.7. Atomic Force Microscopy (AFM)
5. Synthetic Methods and Procedures
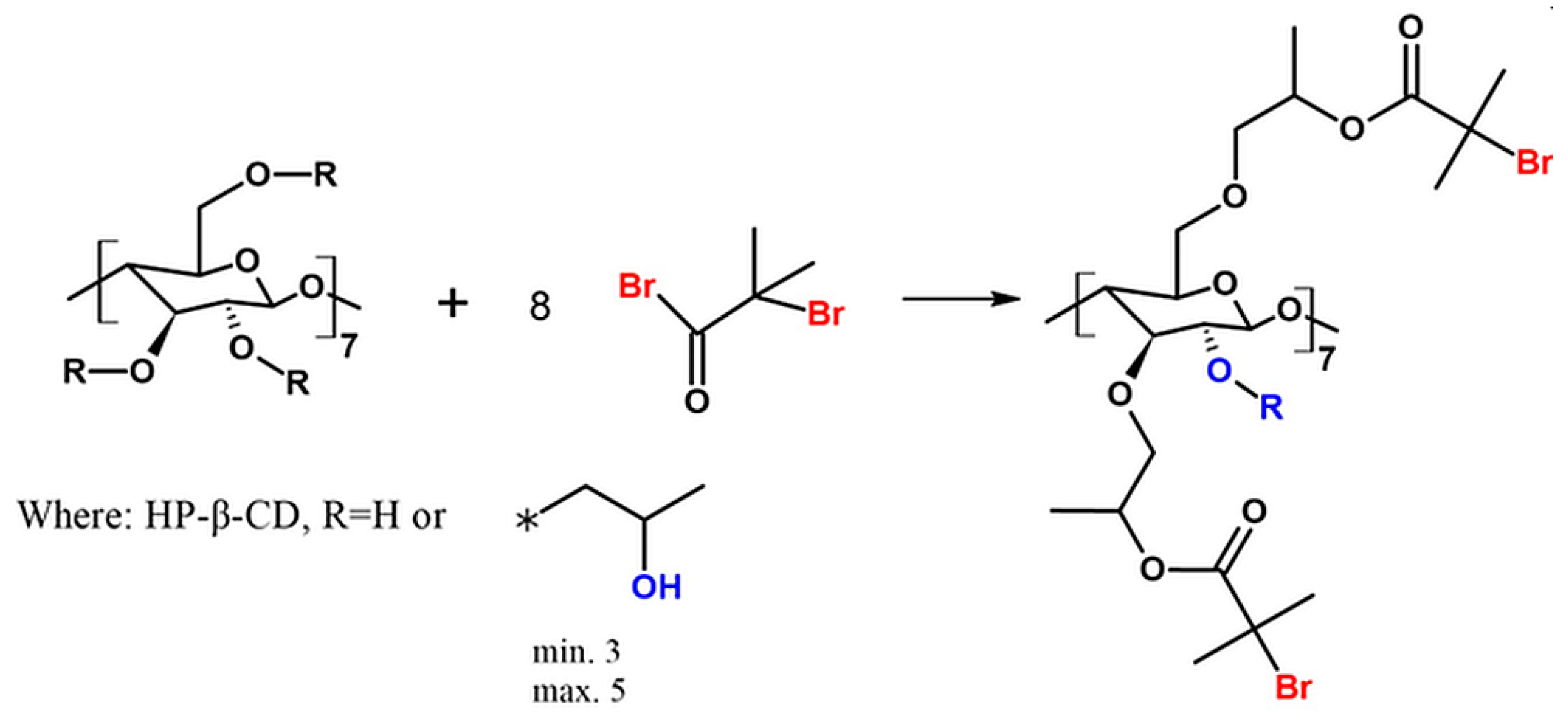
5.1. Synthesis of Bromoisobutyryl-Functionalized CDs (8-Br-HP-β-CD)
5.2. Synthesis of Star-Comb Polymers (Example for OEOMA300, 600:1 Molar Ratio to Initiator)
5.3. Synthesis of Mikto-Arm Star-Comb Polymers Using ROP
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zheng, G.; Pan, C. Reversible addition-fragmentation transfer polymerization in nanosized micelles formed in situ. Macromolecules 2006, 39, 95–102. [Google Scholar] [CrossRef]
- Szczepaniak, G.; Fu, L.; Jafari, H.; Kapil, K.; Matyjaszewski, K. Making ATRP More Practical: Oxygen Tolerance. Acc. Chem. Res. 2021, 54, 1779–1790. [Google Scholar] [CrossRef] [PubMed]
- Kapil, K.; Jazani, A.M.; Szczepaniak, G.; Murata, H.; Olszewski, M.; Matyjaszewski, K. Fully Oxygen-Tolerant Visible-Light-Induced ATRP of Acrylates in Water: Toward Synthesis of Protein-Polymer Hybrids. Macromolecules 2023, 56, 2017–2026. [Google Scholar] [CrossRef] [PubMed]
- Dadashi-Silab, S.; Lee, I.H.; Anastasaki, A.; Lorandi, F.; Narupai, B.; Dolinski, N.D.; Allegrezza, M.L.; Fantin, M.; Konkolewicz, D.; Hawker, C.J.; et al. Investigating Temporal Control in Photoinduced Atom Transfer Radical Polymerization. Macromolecules 2020, 53, 5280–5288. [Google Scholar] [CrossRef]
- Konkolewicz, D.; Magenau, A.J.D.; Averick, S.E.; Simakova, A.; He, H.; Matyjaszewski, K. ICAR ATRP with ppm Cu catalyst in water. Macromolecules 2012, 45, 4461–4468. [Google Scholar] [CrossRef]
- Navarro, L.A.; Enciso, A.E.; Matyjaszewski, K.; Zauscher, S. Enzymatically Degassed Surface-Initiated Atom Transfer Radical Polymerization with Real-Time Monitoring. J. Am. Chem. Soc. 2019, 141, 3100–3109. [Google Scholar] [CrossRef]
- Szczepaniak, G.; Łagodzińska, M.; Dadashi-Silab, S.; Gorczyński, A.; Matyjaszewski, K. Fully oxygen-tolerant atom transfer radical polymerization triggered by sodium pyruvate. Chem. Sci. 2020, 11, 8809–8816. [Google Scholar] [CrossRef]
- Enciso, A.E.; Fu, L.; Russell, A.J.; Matyjaszewski, K. A Breathing Atom-Transfer Radical Polymerization: Fully Oxygen-Tolerant Polymerization Inspired by Aerobic Respiration of Cells. Angew. Chem. -Int. Ed. 2018, 57, 933–936. [Google Scholar] [CrossRef]
- Sigg, S.J.; Seidi, F.; Renggli, K.; Silva, T.B.; Kali, G.; Bruns, N. Horseradish peroxidase as a catalyst for atom transfer radical polymerization. Macromol. Rapid Commun. 2011, 32, 1710–1715. [Google Scholar] [CrossRef]
- Neugebauer, D.; Mielańczyk, A.; Waśkiewicz, S.; Biela, T. Epoxy Functionalized Polymethacrylates Based on Various Multifunctional D-Glucopyranoside Acetals. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 2483–2494. [Google Scholar] [CrossRef]
- Przybyla, M.A.; Yilmaz, G.; Remzi Becer, C. Natural cyclodextrins and their derivatives for polymer synthesis. Polym. Chem. 2020, 11, 7582–7602. [Google Scholar] [CrossRef]
- Crini, G. Review: A history of cyclodextrins. Chem. Rev. 2014, 114, 10940–10975. [Google Scholar] [CrossRef] [PubMed]
- Szejtli, J. Introduction and General Overview of Cyclodextrin Chemistry. Chem. Rev. 1998, 98, 1743–1753. [Google Scholar] [CrossRef] [PubMed]
- Morin-Crini, N.; Fourmentin, S.; Fenyvesi, É.; Lichtfouse, E.; Torri, G.; Fourmentin, M.; Crini, G. 130 Years of Cyclodextrin Discovery for Health, Food, Agriculture, and the Industry: A Review; Springer International Publishing: New York, NY, USA, 2021; Volume 19, ISBN 0123456789. [Google Scholar]
- Gidwani, B.; Vyas, A. A Comprehensive Review on Cyclodextrin-Based Carriers for Delivery of Chemotherapeutic Cytotoxic Anticancer Drugs. BioMed Res. Int. 2015, 2015, 198268. [Google Scholar] [CrossRef] [PubMed]
- Mendelsohn, A.R.; Larrick, J.W. Preclinical Reversal of Atherosclerosis by FDA-Approved Compound that Transforms Cholesterol into an Anti-Inflammatory “prodrug”. Rejuvenation Res. 2016, 19, 252–255. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.M.; McKenzie, T.G.; Fu, Q.; Wong, E.H.H.; Xu, J.; An, Z.; Shanmugam, S.; Davis, T.P.; Boyer, C.; Qiao, G.G. Star Polymers. Chem. Rev. 2016, 116, 6743–6836. [Google Scholar] [CrossRef] [PubMed]
- Roovers, J. Concentration Dependence of the Relative Viscosity of Star Polymers. Macromolecules 1994, 27, 5359–5364. [Google Scholar] [CrossRef]
- Wu, W.; Wang, W.; Li, J. Star polymers: Advances in biomedical applications. Prog. Polym. Sci. 2015, 46, 55–85. [Google Scholar] [CrossRef]
- Mielańczyk, A.; Neugebauer, D. Designing drug conjugates based on sugar decorated V-shape and star polymethacrylates: Influence of composition and architecture of polymeric carrier. Bioconjug. Chem. 2015, 26, 2303–2310. [Google Scholar] [CrossRef]
- Yang, D.P.; Oo, M.N.N.L.; Deen, G.R.; Li, Z.; Loh, X.J. Nano-Star-Shaped Polymers for Drug Delivery Applications. Macromol. Rapid Commun. 2017, 38, 1700410. [Google Scholar] [CrossRef]
- Lotocki, V.; Kakkar, A. Miktoarm star polymers: Branched architectures in drug delivery. Pharmaceutics 2020, 12, 827. [Google Scholar] [CrossRef] [PubMed]
- Wiltshire, J.T.; Qiao, G.G. Recent advances in star polymer design: Degradability and the potential for drug delivery. Aust. J. Chem. 2007, 60, 699–705. [Google Scholar] [CrossRef]
- Chong, Y.K.; Zainol, I.; Ng, C.H.; Ooi, I.H. Miktoarm star polymers nanocarrier: Synthesis, characterisation, and in-vitro drug release study. J. Polym. Res. 2019, 26, 79. [Google Scholar] [CrossRef]
- Lemanowicz, M.; Mielańczyk, A.; Walica, T.; Kotek, M.; Gierczycki, A. Application of polymers as a tool in crystallization—A review. Polymers 2021, 13, 2695. [Google Scholar] [CrossRef] [PubMed]
- Behbahani, M.; Bide, Y.; Salarian, M.; Niknezhad, M.; Bagheri, S.; Bagheri, A.; Nabid, M.R. The use of tetragonal star-like polyaniline nanostructures for efficient solid phase extraction and trace detection of Pb(II) and Cu(II) in agricultural products, sea foods, and water samples. Food Chem. 2014, 158, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Ohno, K.; Wong, B.; Haddleton, D.M. Synthesis of well-defined cyclodextrin-core star polymers. J. Polym. Sci. Part A Polym. Chem. 2001, 39, 2206–2214. [Google Scholar] [CrossRef]
- Li, J.; Xiao, H.; Kim, Y.S.; Lowe, T.L. Synthesis of water-soluble cationic polymers with star-like structure based on cyclodextrin core via ATRP. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 6345–6354. [Google Scholar] [CrossRef]
- Seidi, F.; Shamsabadi, A.A.; Amini, M.; Shabanian, M.; Crespy, D. Functional materials generated by allying cyclodextrin-based supramolecular chemistry with living polymerization. Polym. Chem. 2019, 10, 3674–3711. [Google Scholar] [CrossRef]
- Zhu, Y.; Zheng, X.; Yu, B.; Yang, W.; Zhao, N.; Xu, F. Efficient gene carriers composed of 2-hydroxypropyl-β-cyclodextrin, ethanolamine-functionalized poly(glycidyl methacrylate), and poly((2-dimethyl amino)ethyl methacrylate) by combination of ATRP and click chemistry. Macromol. Biosci. 2014, 14, 1135–1148. [Google Scholar] [CrossRef]
- Pan, Y.; Xue, Y.; Snow, J.; Xiao, H. Tailor-made antimicrobial/antiviral star polymer via ATRP of cyclodextrin and guanidine-based macromonomer. Macromol. Chem. Phys. 2015, 216, 511–518. [Google Scholar] [CrossRef]
- Constantinou, A.P.; Tall, A.; Li, Q.; Georgiou, T.K. Liquid–liquid phase separation in aqueous solutions of poly(ethylene glycol) methacrylate homopolymers. J. Polym. Sci. 2022, 60, 188–198. [Google Scholar] [CrossRef]
- Dudkaitė, V.; Kairys, V.; Bagdžiūnas, G. Understanding the activity of glucose oxidase after exposure to organic solvents. J. Mater. Chem. B 2023, 11, 2409–2416. [Google Scholar] [CrossRef] [PubMed]
- Milčić, N.; Švaco, P.; Sudar, M.; Tang, L.; Findrik Blažević, Z.; Majerić Elenkov, M. Impact of organic solvents on the catalytic performance of halohydrin dehalogenase. Appl. Microbiol. Biotechnol. 2023, 107, 2351–2361. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Jiménez, A.; Montoya-Villegas, K.A.; Licea-Claverie, A.; Gónzalez-Ayón, M.A. Tunable thermo-responsive copolymers from DEGMA and OEGMA synthesized by RAFT polymerization and the effect of the concentration and saline phosphate buffer on its phase transition. Polymers 2019, 11, 1657. [Google Scholar] [CrossRef] [PubMed]
- Szweda, D.; Szweda, R.; Dworak, A.; Trzebicka, B. Thermoresponsive poly[oligo(ethylene glycol) methacrylate]s and their bioconjugates—Synthesis and solution behavior. Polimery/Polymers 2017, 62, 298–310. [Google Scholar] [CrossRef]
- Jin, L.; Zhang, G.; Zhai, X.; Ma, Z.; Zheng, P.; Wang, W. Macromolecular effect on crystal pattern formation in ultra-thin films: Molecular segregation in a binary blend of PEO fractions. Polymer 2009, 50, 6157–6165. [Google Scholar] [CrossRef]
- Liu, X.; Gitsov, I. Nonionic amphiphilic linear dendritic block copolymers. solvent-induced self-assembly and morphology tuning. Macromolecules 2019, 52, 5563–5573. [Google Scholar] [CrossRef]
- Nazemi, A.; Boott, C.E.; Lunn, D.J.; Gwyther, J.; Hayward, D.W.; Richardson, R.M.; Winnik, M.A.; Manners, I. Monodisperse cylindrical micelles and block comicelles of controlled length in aqueous media. J. Am. Chem. Soc. 2016, 138, 4484–4493. [Google Scholar] [CrossRef]
- Wu, X.; Shi, S.; Yu, Z.; Russell, T.P.; Wang, D. AFM nanomechanical mapping and nanothermal analysis reveal enhanced crystallization at the surface of a semicrystalline polymer. Polymer 2018, 146, 188–195. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, X.B.; Zhang, H.L.; Zhu, D.S.; Sun, Y.J.; Yan, S.K.; Wang, J.; Chen, X.F.; Wan, X.H.; Chen, E.Q.; et al. AFM study of crystallization and melting of a poly(ethylene oxide) diblock copolymer containing a tablet-like block of poly{2,5-bis[(4-methoxyphenyl) oxycarbonyl]styrene} in ultrathin films. Polymer 2006, 47, 1217–1225. [Google Scholar] [CrossRef]





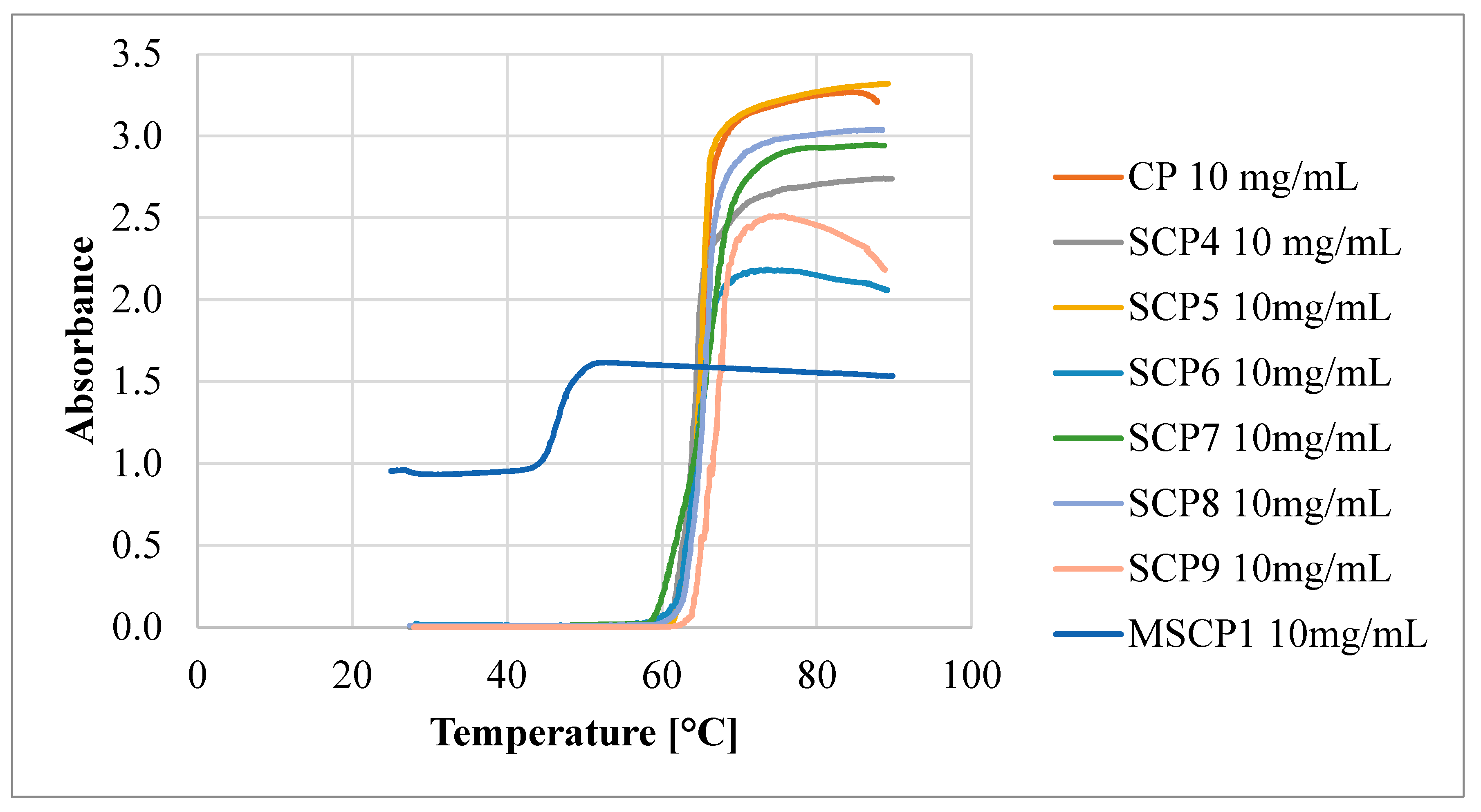

| Entry | Reaction Conditions | OEOMA Conversion [%] | DPOEOMA | DP per Arm | Mn NMR [g/mol] | Mn, SEC [g/mol] | Mw, MALLS [g/mol] | Ð SEC |
|---|---|---|---|---|---|---|---|---|
| SCP1 | [OEOMA500]0:[8-Br-HP-β-CD]0:[VA-044]0:[CuCl2]0:[TPMA]0 = 600:1:0.9:0.06:0.18 | 94% | 564 | 71 | 282,000 | 65,900 | 325,500 | 1.38 |
| SCP2 | [OEOMA500]0:[8-Br-HP-β-CD]0:[VA-044]0:[CuCl2]0:[TPMA]0 = 400:1:0.9:0.06:0.18 | 95% | 380 | 48 | 190,000 | 175,700 | 214,400 | 1.26 |
| SCP3 | [OEOMA500]0:[8-Br-HP-β-CD]0:[VA-044]0:[CuCl2]0:[TPMA]0 = 200:1:0.9:0.06:0.18 | 91% | 182 | 23 | 91,000 | 103,100 | 111,900 | 1.23 |
| CP | [OEOMA300]0:[HO-EBiB]0:[VA-044]0:[CuCl2]0:[TPMA]0 = 600:1:0.9:0.06:0.18 | 81% | 486 | 486 | 145,800 | 76,000 | 124,900 | 1.61 |
| SCP4 | [OEOMA300]0:[8-Br-HP-β-CD]0:[VA-044]0:[CuCl2]0:[TPMA]0 = 600:1:0.9:0.06:0.18 | 89% | 534 | 67 | 160,200 | 380,700 | - | 1.75 |
| SCP5 | [OEOMA300]0:[8-Br-HP-β-CD]0:[VA-044]0:[CuCl2]0:[TPMA]0 = 600:1:0.9:0.06:0.18 (+1.5 mL MeOH with initiator) | 65% | 390 | 49 | 117,000 | 686,00 | 98,500 | 1.34 |
| SCP6 | [OEOMA300]0:[8-Br-HP-β-CD]0:[VA-044]0:[CuCl2]0:[TPMA]0 = 00:1:0.9:0.06:0.18 | 86% | 344 | 43 | 103,200 | 294,000 | - | 1.44 |
| SCP7 | [OEOMA300]0:[8-Br-HP-β-CD]0:[VA-044]0:[CuCl2]0:[TPMA]0 = 400:1:0.9:0.06:0.18 (+ 1.5 mL MeOH with initiator) | 68% | 272 | 34 | 81,600 | 56,800 | 76,800 | 1.26 |
| SCP8 | [OEOMA300]0:[8-Br-HP-β-CD]0:[VA-044]0:[CuCl2]0:[TPMA]0 = 200:1:0.9:0.06:0.18 | 89% | 178 | 22 | 53,400 | 150,600 | - | 1.69 |
| SCP9 | [OEOMA300]0:[8-Br-HP-β-CD]0:[VA-044]0:[CuCl2]0:[TPMA]0 = 200:1:0.9:0.06:0.18 (+ 1.5 mL MeOH with initiator) | 79% | 158 | 20 | 47,400 | 34,400 | 43,300 | 1.27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fronczyk, T.; Mielańczyk, A.; Klymenko, O.; Erfurt, K.; Neugebauer, D. Eco-Conscious Approach to Thermoresponsive Star-Comb and Mikto-Arm Polymers via Enzymatically Assisted Atom Transfer Radical Polymerization Followed by Ring-Opening Polymerization. Molecules 2024, 29, 55. https://doi.org/10.3390/molecules29010055
Fronczyk T, Mielańczyk A, Klymenko O, Erfurt K, Neugebauer D. Eco-Conscious Approach to Thermoresponsive Star-Comb and Mikto-Arm Polymers via Enzymatically Assisted Atom Transfer Radical Polymerization Followed by Ring-Opening Polymerization. Molecules. 2024; 29(1):55. https://doi.org/10.3390/molecules29010055
Chicago/Turabian StyleFronczyk, Tomasz, Anna Mielańczyk, Olesya Klymenko, Karol Erfurt, and Dorota Neugebauer. 2024. "Eco-Conscious Approach to Thermoresponsive Star-Comb and Mikto-Arm Polymers via Enzymatically Assisted Atom Transfer Radical Polymerization Followed by Ring-Opening Polymerization" Molecules 29, no. 1: 55. https://doi.org/10.3390/molecules29010055
APA StyleFronczyk, T., Mielańczyk, A., Klymenko, O., Erfurt, K., & Neugebauer, D. (2024). Eco-Conscious Approach to Thermoresponsive Star-Comb and Mikto-Arm Polymers via Enzymatically Assisted Atom Transfer Radical Polymerization Followed by Ring-Opening Polymerization. Molecules, 29(1), 55. https://doi.org/10.3390/molecules29010055






