Abstract
This study explores the synthesis, characterization, and application of a heterofunctional initiator derived from 2-hydroxypropyl cyclodextrin (HP-β-CD), having eight bromoester groups and thirteen hydroxyl groups allowing the synthesis of mikto-arm star-shaped polymers. The bromoesterification of HP-β-CD was achieved using α-bromoisobutyryl bromide as the acylation reagent, modifying the cyclodextrin (CD) molecule as confirmed by electrospray ionization mass spectrometry (ESI-MS), nuclear magnetic resonance (NMR), attenuated total reflection-Fourier transform infrared (ATR-FTIR) spectroscopy analysis, and differential scanning calorimetry (DSC) thermograms. The initiator’s effectiveness was further demonstrated by obtaining star-comb and mikto-arm polymers via an enzymatically assisted atom transfer radical polymerization (ATRP) method and subsequent ring-opening polymerization (ROP). The ATR polymerization quality and control depended on the type of monomer and was optimized by the way of introducing the initiator into the reaction mixture. In the case of ATRP, high conversion rates for poly(ethylene oxide) methyl ether methacrylate (OEOMA), with molecular weights (Mn) of 500 g/mol and 300 g/mol, were achieved. The molecular weight distribution of the obtained polymers remained in the range of 1.23–1.75. The obtained star-comb polymers were characterized by different arm lengths. Unreacted hydroxyl groups in the core of exemplary star-comb polymers were utilized in the ROP of ε-caprolactone (CL) to obtain a hydrophilic mikto-arm polymer. Cloud point temperature (TCP) values of the synthesized polymers increased with arm length, indicating the polymers’ reduced hydrophobicity and enhanced solvation by water. Atomic force microscopy (AFM) analysis revealed the ability of the star-comb polymers to create fractals. The study elucidates advancements in the synthesis and utilization of hydrophilic sugar-based initiators for enzymatically assisted ATRP in an aqueous solution for obtaining complex star-comb polymers in a controlled manner.
1. Introduction
Atom transfer radical polymerization (ATRP) is one of the most commonly used methods of reversible-deactivation radical polymerization, which produces a wide range of polymers with strictly defined parameters, such as molecular weight, chain length, topology, and side and end group functionalities. Currently, researchers are seeking environmentally friendly methods of performing ATRP following the principles of green chemistry [1,2,3]. Among them are the ATRP methods that allow the reaction to proceed in the presence of oxygen, including photo-induced ATRP [4], initiators for continuous activator regeneration (ICAR) ATRP [5], and enzymatically assisted ATRP [6,7,8,9]. On the other hand, the shift of ATRP toward green chemistry is also possible due to the usage of initiators based on natural compounds such as carbohydrates, including monosaccharides, disaccharides, oligosaccharides, and polysaccharides [10,11]. Cyclodextrins (CDs) are cyclic oligosaccharides composed of α-D-glucose units linked by α-1,4-acetal bonds. The most widely studied variants of CDs are α-, β-, and γ-CDs, which contain 6, 7, or 8 α-D-glucose units, respectively. CDs are toroidal in shape, with all primary hydroxyl (-OH) groups located on the narrow side and the secondary -OH groups on the wider side. The toroidal shape makes CDs hydrophilic on the outside, though the cavity stays hydrophobic [12,13,14]. CDs are used as excipients in several FDA-approved pharmaceutical products, such as liposomal formulations of doxorubicin, daunorubicin, cytarabine, and amphotericin B [15]. Hydroxypropyl-β-CD is an FDA-approved compound for solubilizing, capturing, and delivering lipophilic drugs in humans, and it has been shown to reverse atherosclerosis in preclinical studies [16]. The use of CD allows the synthesis of star polymers, which are a type of branched polymers. They are characterized by linear or comb-like arms extending from a centrally located core [17]. Star polymers owe their advantageous properties to their unique topological structure. Due to the smaller number of entanglements of the arms, star-shaped polymers have a lower solution viscosity in dilute solutions, compared to analogous linear polymers of the same molecular weight. In addition, polymer viscosity and elasticity change with the length of the arms [18]. Star polymers show remarkable stimuli-responsiveness due to the high density of functional groups that may be present in their structure [19].
Star polymers show great promise in the field of biomedical applications, particularly controlled-release drug delivery systems [20,21,22,23,24]. Moreover, star polymers have been found to play a significant role in the crystallization processes. They can be used to control the nucleation and growth of crystals, which can lead to the formation of unique crystal structures. This has potential applications in various fields, including drug delivery, where the controlled crystallization of therapeutic compounds can affect their bioavailability and efficacy [25]. Star polymers can also remove heavy metals from water, which is related to the star polymer’s spatial arrangement. For example, the tetragonal star-like polyaniline microstructure was applied to selectively and quickly remove Cu(II) and Pb(II) from water [26].
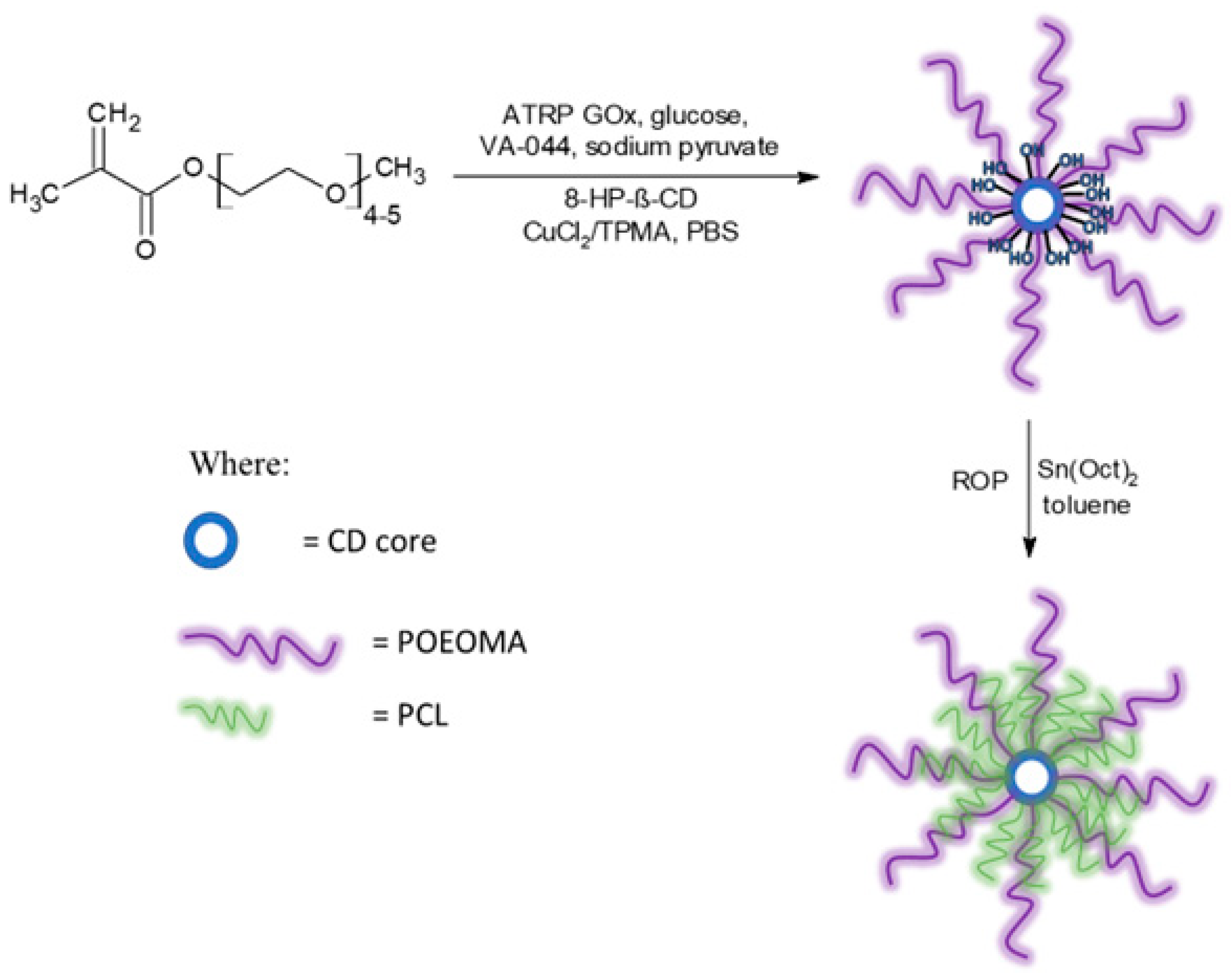
This article presents the results of the synthesis and utilization of bromoisobutyryl-functionalized bio-safe CD derivatives as ATRP initiators, based on (2-hydroxypropyl)-β-CD (HP-β-CD). A heterofunctional initiator with eight bromoester groups (8-Br-HP-β-CD) was used in an enzymatically assisted ATRP (glucose oxidase), also known as breathing ATRP, to obtain 8-arm star-comb poly(oligo ethylene oxide))methyl ether methacrylate (POEOMA) from OEOMA500 and OEOMA300. Subsequently, a mikto-arm star-comb polymer (MSCP1) with additional poly(ε-caprolactone) arms was obtained based on the star-shaped polymer with eight POEOMA500 arms.
2. Results and Discussion
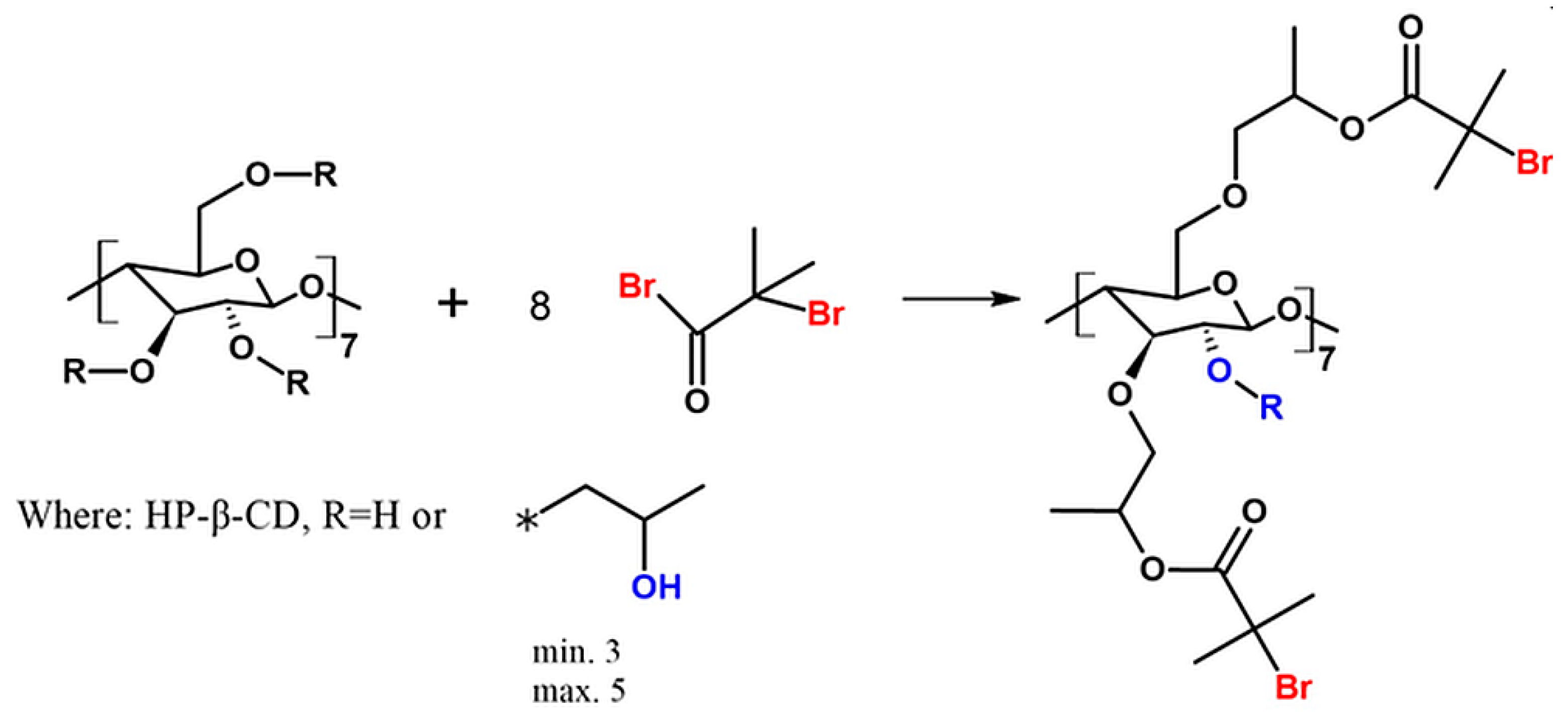
The heterofunctional initiator based on HP-β-CD was synthesized and characterized (Figure 1).
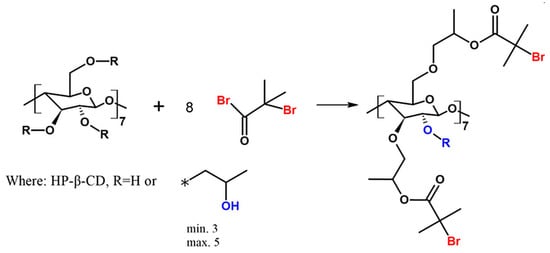
Figure 1.
Scheme of the synthesis of heterofunctional initiator based on CD. The asterisk (*) indicates the point of connection of the 2-hydroxypropyl group instead of R to the main molecule.
Multifunctional initiators based on β-cyclodextrin are described in the literature [27,28,29]. Admittedly, an ATRP initiator with one bromoester group based on HP-β-CD was previously described by Zhu et al. [30]. However, this is the first time when HP-β-CD initiator with eight bromoester groups has been synthesized according to the slightly changed procedure described by Pan et al. (utilized for β-CD) [31]. In this paper, we describe for the first time the utilization of CD-based initiator in enzymatically assisted ATRP as an effective route to obtain star-comb and mikto-arm star-comb polymers.
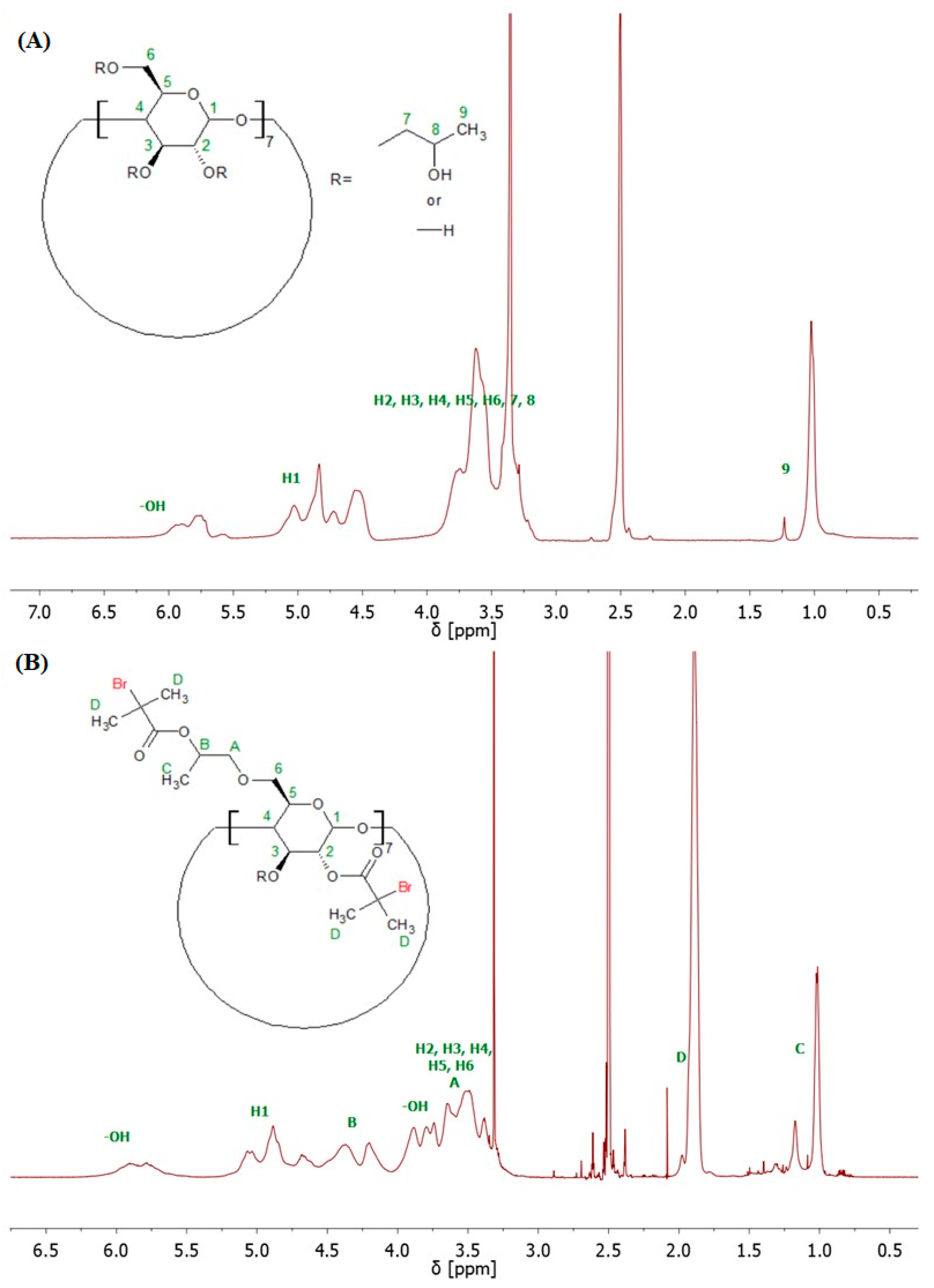
The structure of 8-Br-HP-β-CD was confirmed by spectroscopic and spectrometric analyses. ATR-FTIR analysis confirmed the bromoesterification reaction (Supplementary Figure S2). The peak corresponding to the stretching vibrations of the -OH group decreased its intensity after the reaction, which indicates partial substitution of the -OH groups in the molecule. The peak responsible for the –C=O stretching vibrations present in the spectrum of the initiator proves that the CD was successfully modified. Analysis of the 1H NMR spectrum confirmed the presence of eight bromoester groups in the CD molecule (Figure 2). The degree of -OH groups substitution in HP-β-CD was calculated from the integrals of the peaks assigned to the acetal proton (H1) and six protons from the bromoester group.
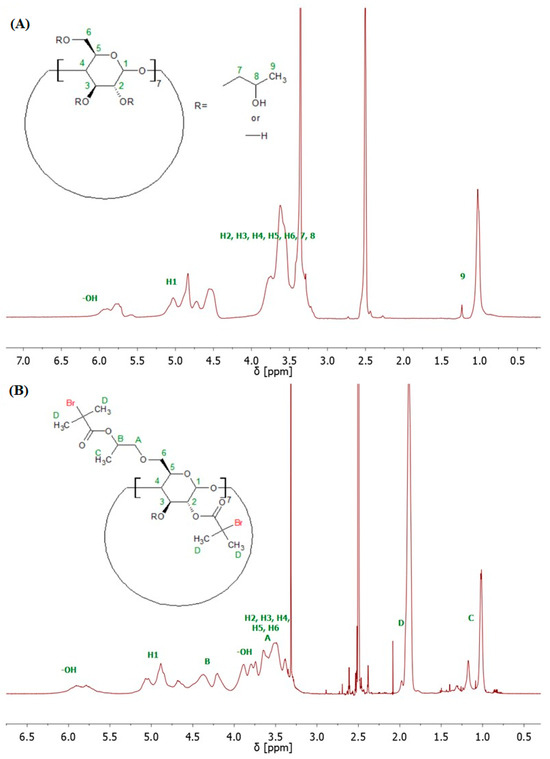
Figure 2.
1H NMR (600 MHz, DMSO-d6) spectra of HP-β-CD (A), and 8-Br-HP-β-CD (B).
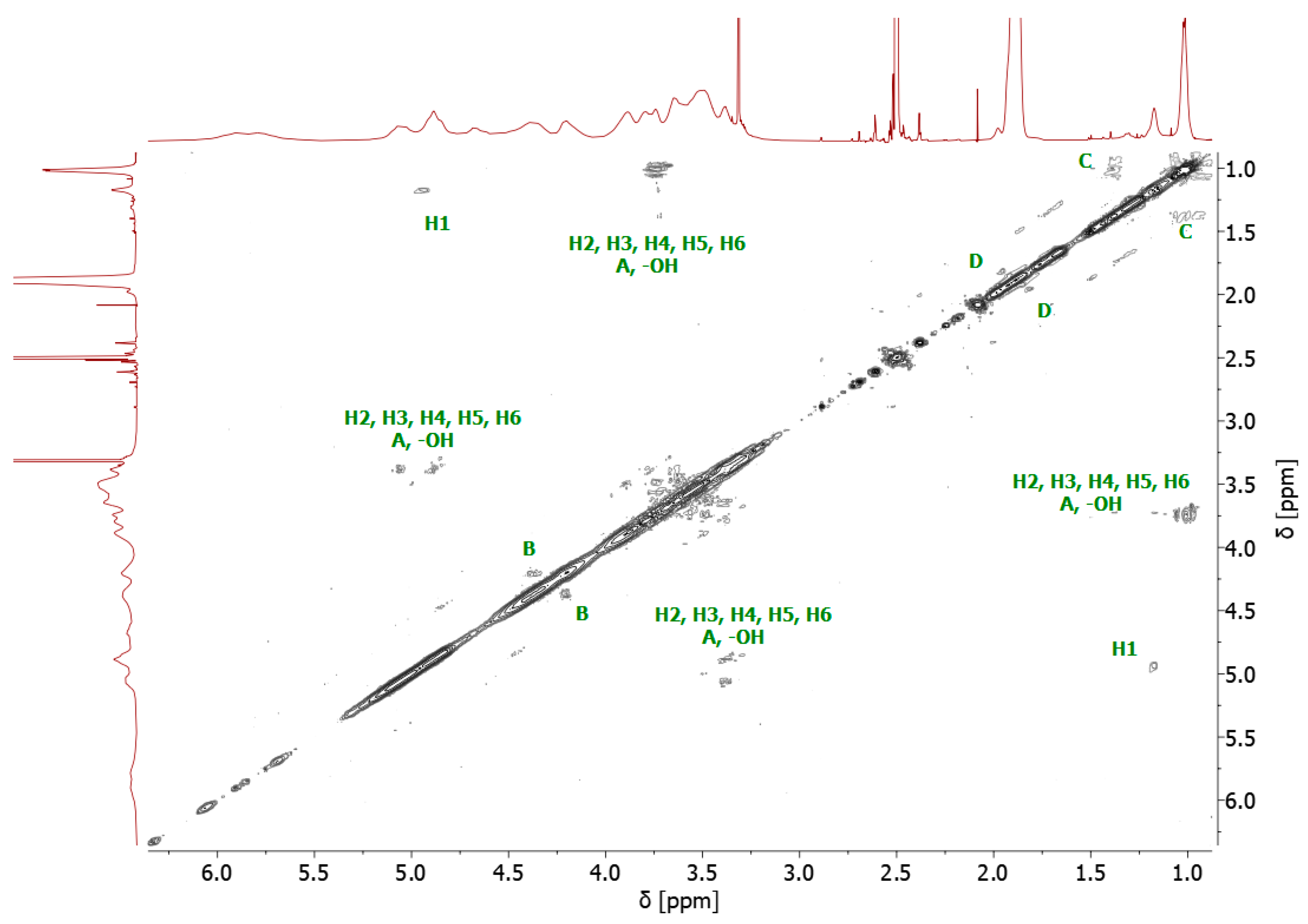
The bromoesterification of the initiator with the -OH groups was also verified by 13C NMR spectroscopy. The 13C NMR spectrum of the bromoesterified initiator exhibited a signal at 171 ppm, corresponding to the carbonyl carbon of the ester group, and a signal at 31 ppm, corresponding to the carbon attached to the bromine atom (Supplementary Figure S3). Two-dimensional (2D) gradient-selected correlation spectroscopy (gCOSY) NMR was also performed to confirm the structure and corresponding signals (Figure 3).
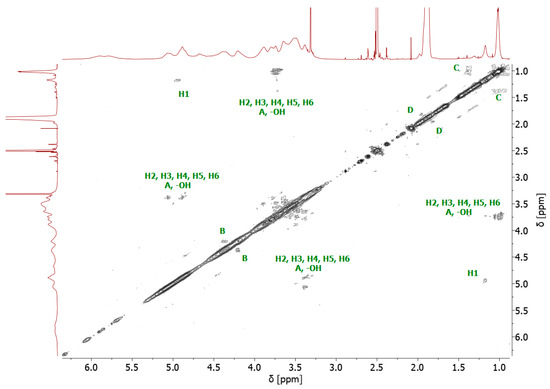
Figure 3.
1H-1H homonuclear gCOSY 2D NMR spectrum of 8-Br-HP-β-CD.
DSC thermograms showed that the esterification of CDs affects the thermal properties of the obtained bromoester derivatives, changing the melting temperature from 219.67 °C (HP-β-CD) to 210.23 °C (8-Br-HP-β-CD) (Supplementary Figure S4).
Next, 8-Br-HP-β-CD was used to obtain star-comb polymers via an enzymatically assisted ATRP method using a CuCl2/TPMA catalyst complex in phosphate-buffered saline, pH 7.4. Each reaction was carried out for 2 h at 45 °C (Figure 4).
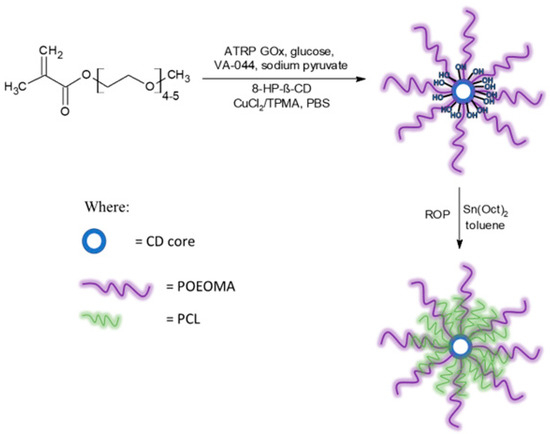
Figure 4.
Reaction scheme for the synthesis of star-comb polymers and mikto-arm star-comb polymers.
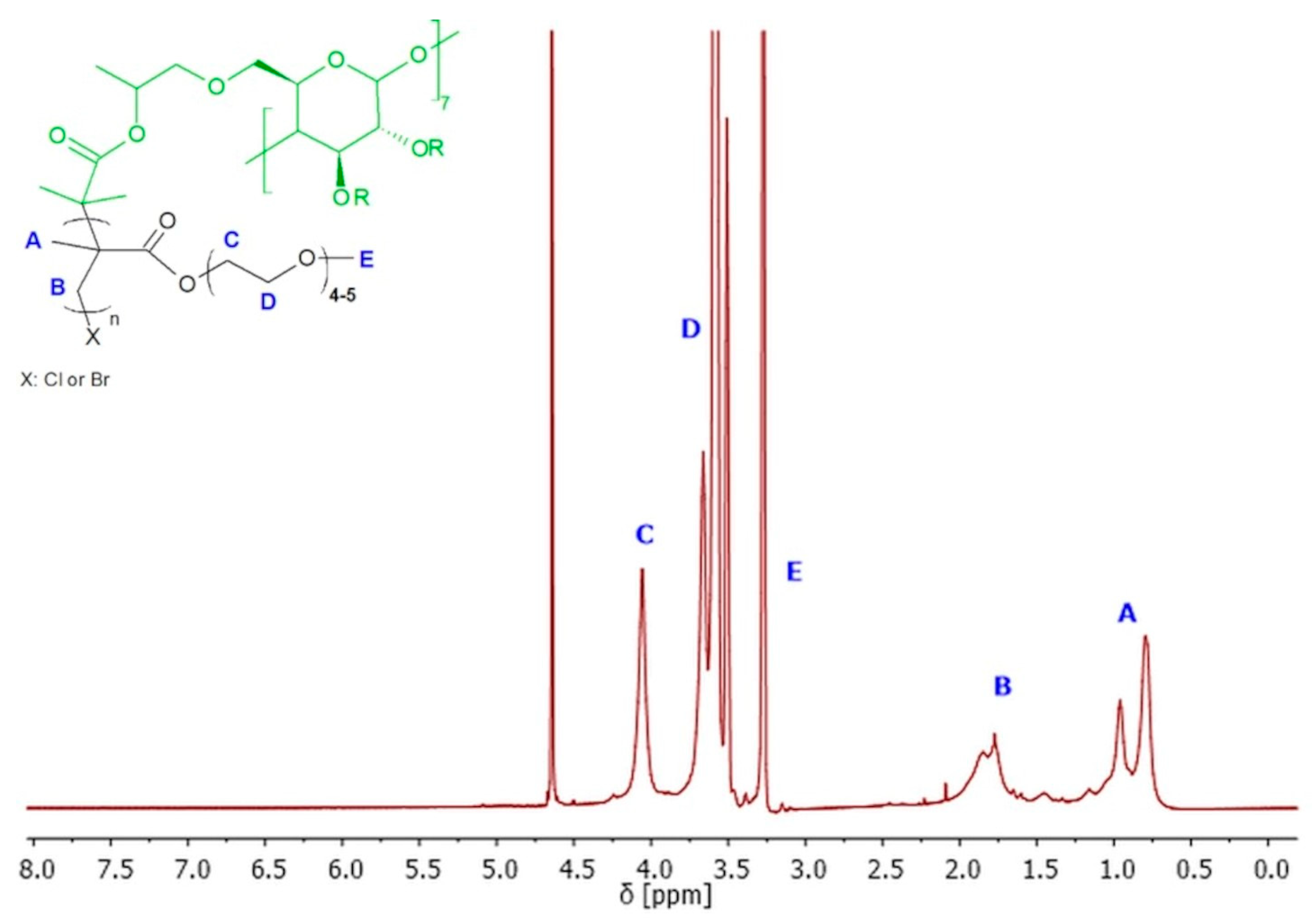
Polymers SCP1–SCP3 were obtained with 91–94% of monomer conversion, while polymers SCP4–SCP9 were obtained with 65–89% of monomer conversion (Table 1). The 1H NMR spectroscopy was used to confirm the successful synthesis and purification of the polymer, as well as to determine the conversions of monomers, the average degree of polymerization (DP), and finally theoretical number average molecular weight (Mn). The 1H NMR spectrum of the polymer showed characteristic signals for the repeating units, and no signals for the impurities or the unreacted monomer (Figure 5).

Table 1.
Characteristics of the obtained polymers.
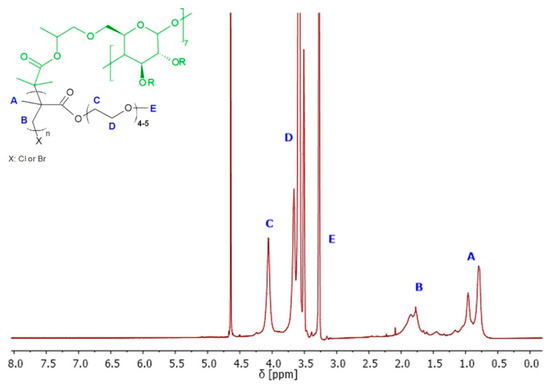
Figure 5.
1H NMR (600 MHz, D2O) spectra of exemplary 8-arm star-comb polymer SCP7.
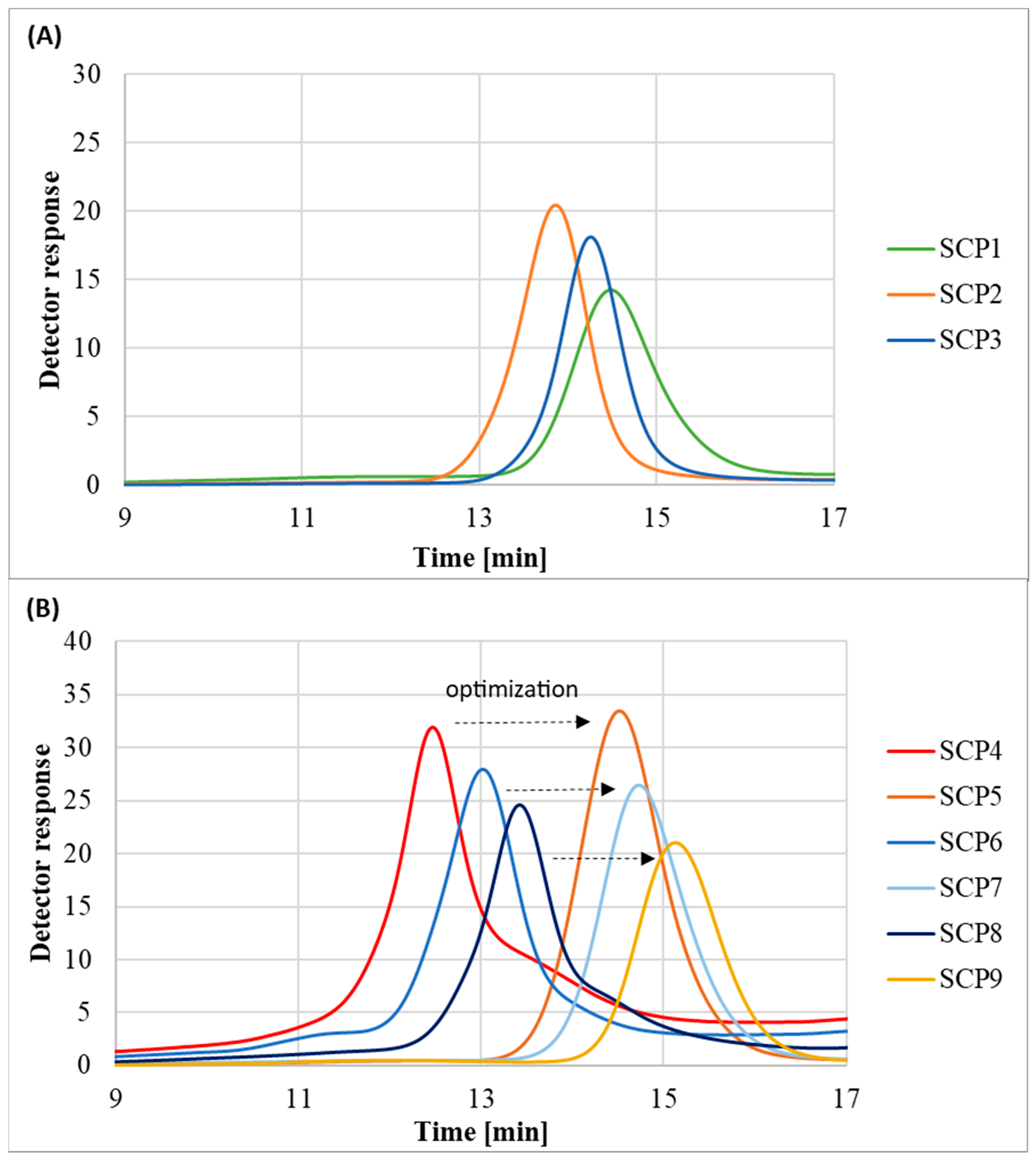
The molecular weight distribution values of the obtained polymers SCP1-SCP3 stayed in the range of 1.23–1.38 and chromatograms showed monomodal peaks, which indicates that the OEOMA500-based polymers were synthesized in a controlled manner (Figure 6A). However, in the case of OEOMA300-based polymers synthesized without the addition of methanol, it was observed that besides the main signal, there were also peaks corresponding to the high and low molecular weight species. In order to regain control over the polymerization process, the reactions were repeated with the exception that the initiator was dissolved in a small amount of methanol prior to addition to the reaction mixture. Lack of control over the polymerization process in the case of the OEOMA300 monomer was probably linked with its lower hydrophilicity in comparison to the OEOMA500 (Figure 6B) [32].
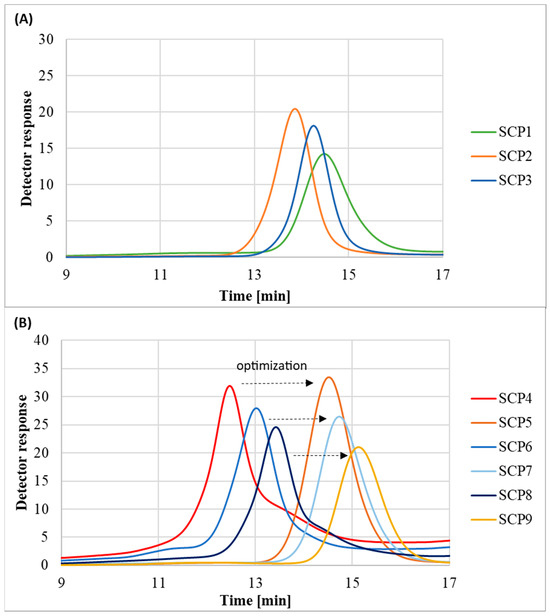
Figure 6.
SEC traces of eight-arm POEOMA star-comb polymers: (A) POEOMA500 polymers; (B) POEOMA300 polymers.
The polymers prepared with the initiator dissolved in methanol had lower Đ than the polymers prepared with the solid initiator, indicating a more homogeneous and efficient initiation process (Figure 6B). However, the dissolution of the initiator in methanol also slightly reduced the monomer conversion, suggesting a trade-off between dispersity and yield. This can be explained by the fact that the activity of enzymes like glucose oxidase can be significantly influenced by the solvent environment [33]. Enzymes, being proteins, have a specific three-dimensional structure crucial for their activity. For instance, organic solvents, including alcohols like ethanol or methanol, can cause changes in the protein structure, often leading to decreasing activity, denaturation, or inactivation of the enzyme [34].
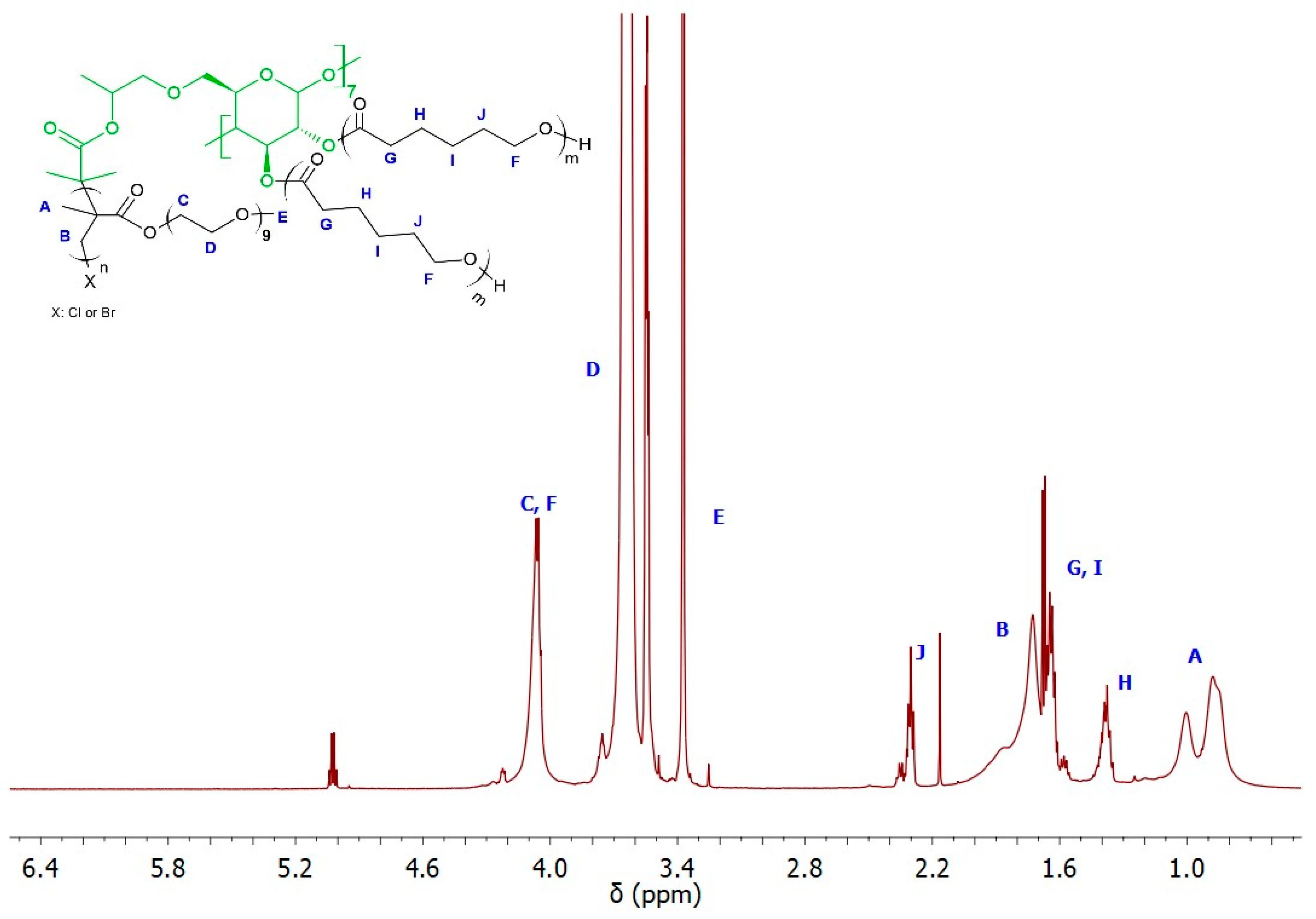
The ability of the remaining hydroxyl groups in the core of the star-comb macromolecules to initiate polymerization was examined by performing ROP of ε-CL. As model macroinitiators, SCP3 and SCP9 were selected due to the lowest molecular weight from both groups of star-comb polymers with POEOMA300 and POEOMA500 arms. However, only a mikto-arm star-comb polymer based on SCP3 retained solubility in water, which had a crucial impact on its further directions of application. Figure 7 shows the spectrum of MSCP1 where characteristic signals from polymethacrylate and polyester arms can be seen. The mole fractions of PCL arms in MSCP1 and MSCP2 were calculated from the NMR spectra, based on the integral values from the signal at 1.4 ppm (marked on the spectrum as H) and methyl groups in the range from 0.5 to 1.2 ppm (marked on the spectrum as A), and were equal to 21% and 36%, respectively.
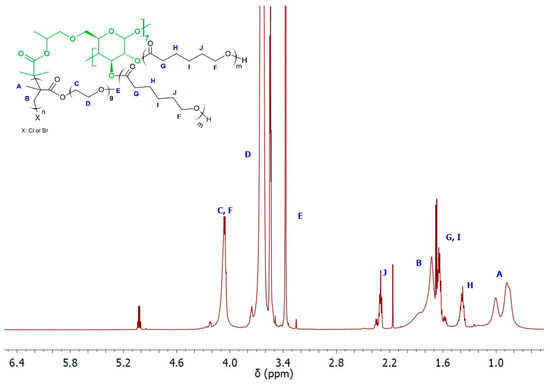
Figure 7.
1H NMR (600 MHz, CDCl3) spectra of MSCP1.
The conversion of the ε-CL was equal to 36% for MSCP1 and 69% for MSCP2, and the obtained mikto-arm polymer exhibited decreased Mn, SEC value in comparison to the macroinitiator (for MSCP1 51,300 g/mol vs. 103,100 g/mol and for MSCP2 29,100 g/mol vs. 34,400 g/mol). However, in the case of the dispersity index, comparing the macroinitiator to the polymer, the results are as follows: ĐMSCP1 = 1.20 vs. ĐSCP3 = 1.23 and for MSCP2 ĐMSCP2 = 1.31 vs. ĐSCP3 = 1.27 (Supplementary Figure S5 and Table S1).
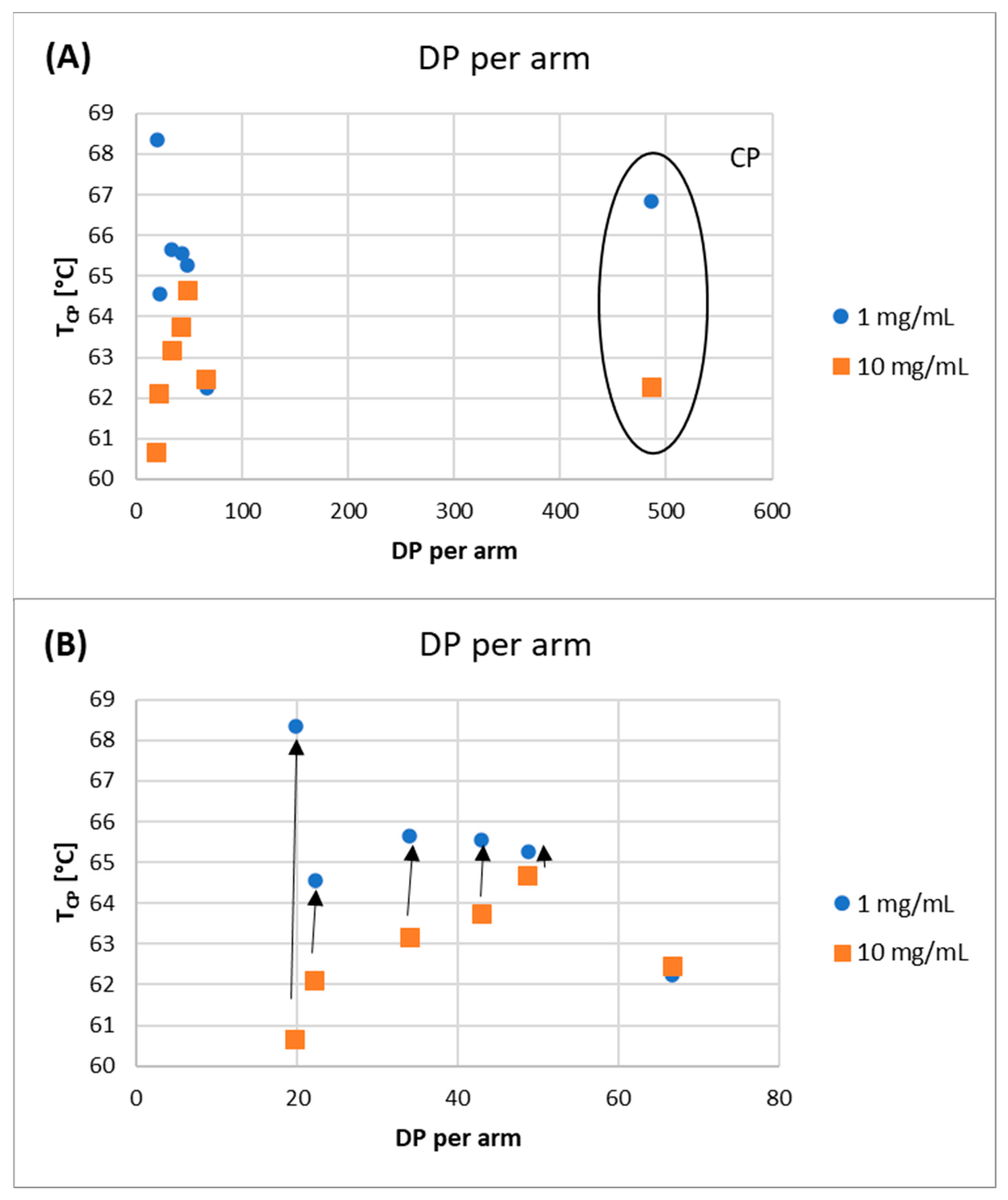
The studies of the phase transition of the polymer solutions showed good reproducibility and reversibility, as evidenced by the almost identical TCP values on the first and second heating curves. The TCP values of the polymer solutions decreased with increasing polymer concentration (Figure 8).
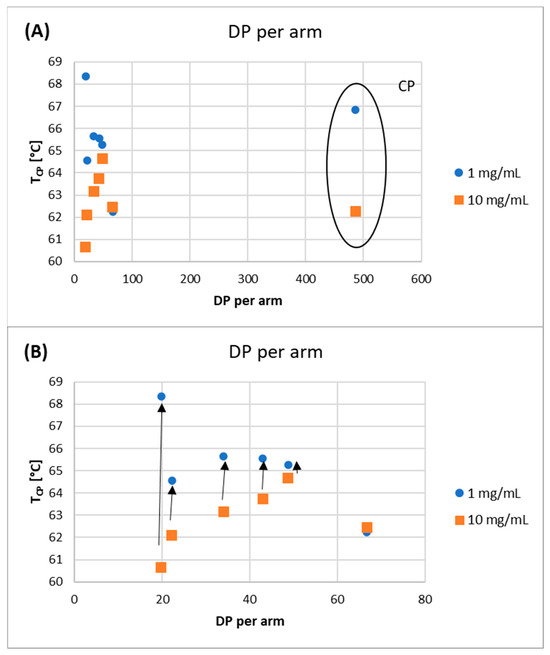
Figure 8.
TCP as a function of DP per arm of obtained polymers: (A) All polymers, (B) a subset of the top graph showing only star-comb polymers.
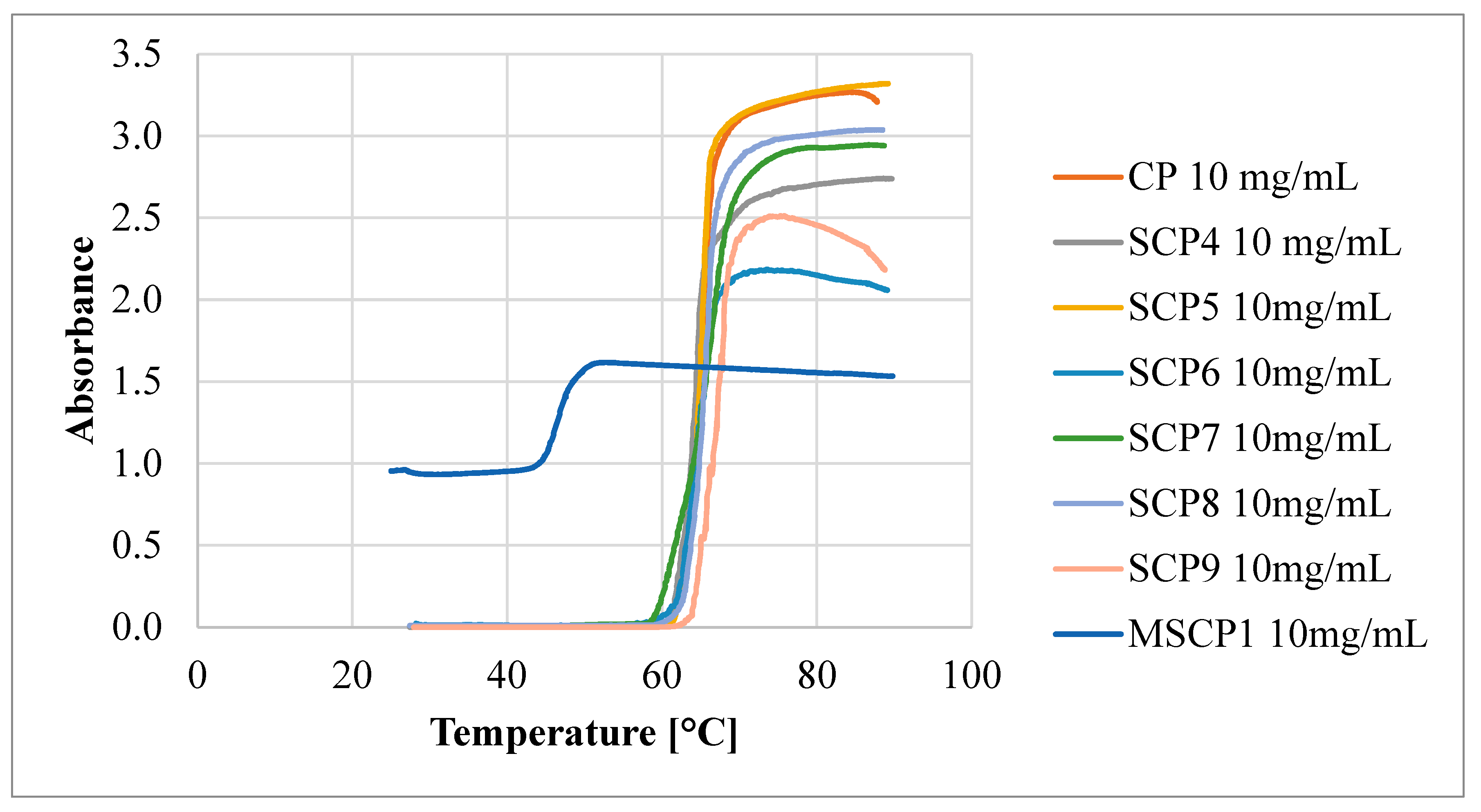
This indicates that the polymer–polymer interactions are stronger than the polymer–water interactions at higher polymer concentrations. However, as the DParm values increase, the differences between the TCP values decrease, until the DParm > 60 repeating units, where this difference disappears completely. To verify the effect of polymer topology on the TCP value, a comb-like polymer (CP) was synthesized via the utilization of HO-EBiB initiator and OEOMA300 as the monomer. The CP obtained using the enzymatically assisted ATRP method displayed TCP values comparable to those reported in the literature. Namely, TCP of P(OEOMA300) solution in water at a concentration of 2 mg/mL was equal to 66.5 °C, and for P(OEOMA300) solution in water at a concentration of 5 mg/mL TCP was equal to 66 °C [35,36]. In our studies, TCP values for CP solution in water were equal to 66.85 °C at a concentration of 1 mg/mL and 62.25 °C at a concentration of 10 mg/mL These results slightly differed from those obtained for the star-comb polymers (TCP in the range of 60.65–64.65 °C for 10 mg/mL), confirming that the polymer architecture influences the TCP. This evidence further supports the notion that the star-comb architecture does indeed affect the thermosensitivity of the polymers. In general, the TCP values of the star-comb polymers showed a positive correlation with the arm length, i.e., the degree of polymerization per arm. It can be explained by the reduced hydrophobicity and enhanced solvation of the polymers with longer arms. The absorbance plots of the tested polymers differ in terms of maximum end values (Figure 9).
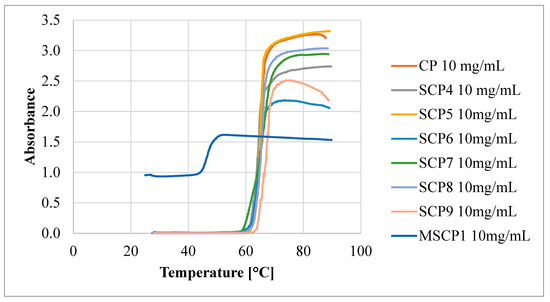
Figure 9.
Absorbance as a function of the temperature of obtained polymers.
This may be related to the topology of the tested compounds, which makes it easier to form clusters of polymers. Different absorbance values may also be related to different DP per arm of the tested polymers. A star-comb polymer with short arms may adopt a more “tight” conformation where the arms are close together. On the other hand, a polymer with longer arms may adopt a more “spread out” conformation where the arms are far apart. These different conformations can affect the availability and exposure of the functional groups that can absorb light.
In the experimental evaluation of polymers designated as SCP1-SCP3, the absence of a TCP was observed, corroborating their hydrophilic characteristics. Specifically, the absorbance levels measured via UV–Vis spectroscopy demonstrated negligible variations, thereby substantiating the hydrophilic nature of these polymers. Conversely, the mikto-arm star-comb polymer synthesized from poly(oligo(ethylene glycol) methyl ether methacrylate) (POEOMA500) and ε-caprolactone (SCP3) exhibited TCP in water. At a concentration of 1 mg/mL, the TCP was equal to 47.65 °C, while at a 10 mg/mL concentration, the TCP decreased to 45.35 °C. These data suggest that the incorporation of poly(ε-caprolactone) (PCL) arms changed the hydrophilic/hydrophobic balance, enabling the phase transition of mikto-arm star-comb polymer in water solution.
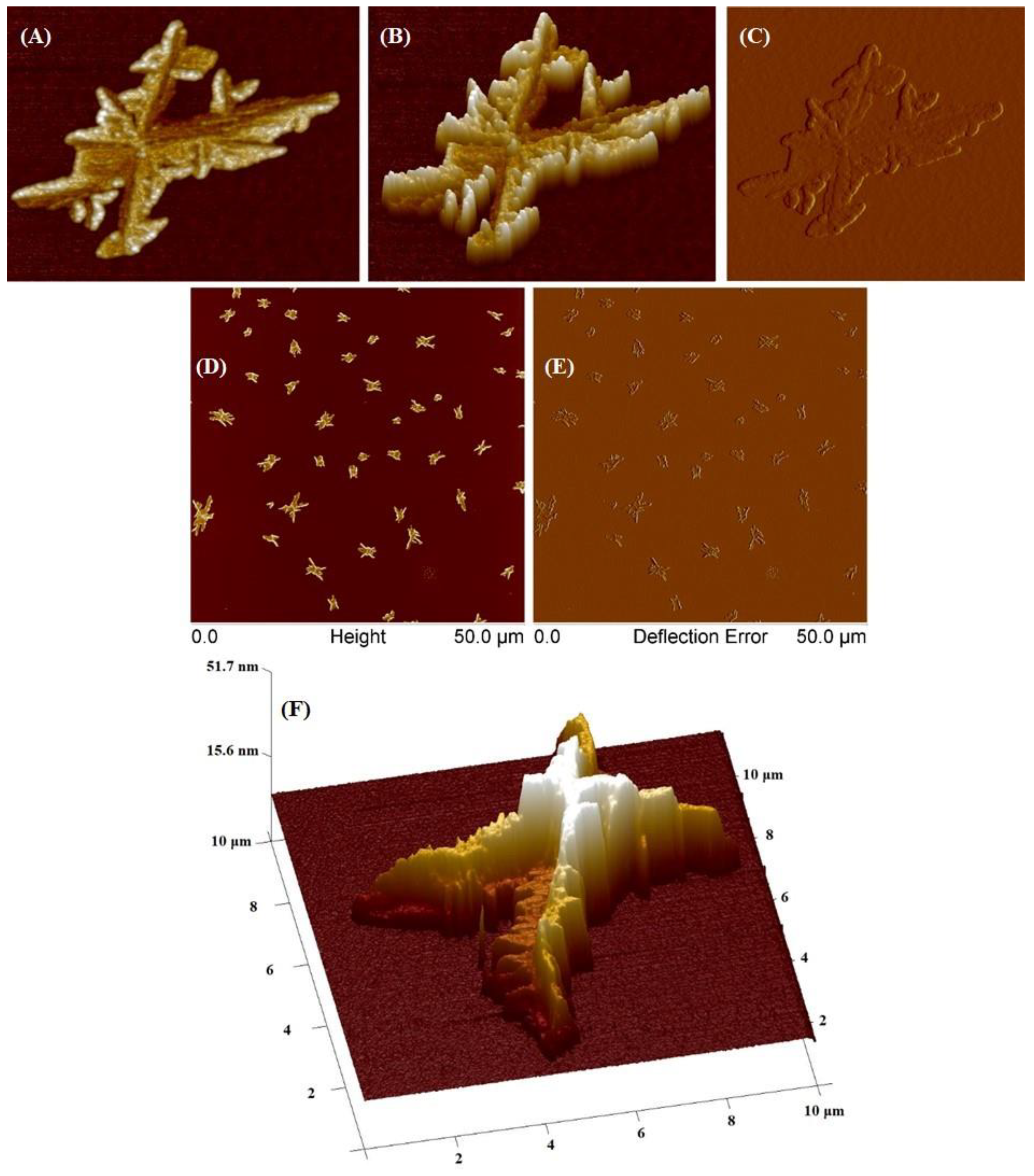
The SCP2 polymer was investigated utilizing AFM (Figure 10). At a concentration of 0.01 mg/mL, SCP2 formed large aggregates and individual fractal-like elements upon drying of the substrate. The average height of these individual objects was 19 nm, with a minimum height of 10 nm and a maximum of 50 nm. The average surface area of stars in the sample is 2.3 μm² (with a range of 0.5–8 μm²), and the average diameter was 1.7 μm (with a range of 0.81–3.3 μm). Moreover, large aggregates with a diameter of ~10 μm were also detected, but these were isolated cases. The formation of aggregates with sizes around 10 μm is most likely related to the stronger intermolecular interaction between macromolecules than the macromolecule–substrate interaction. It is worth noticing that this phenomenon was also observed and described previously in the literature by other researchers who studied crystal patterns formed by polyethylene oxide (PEO) [37].
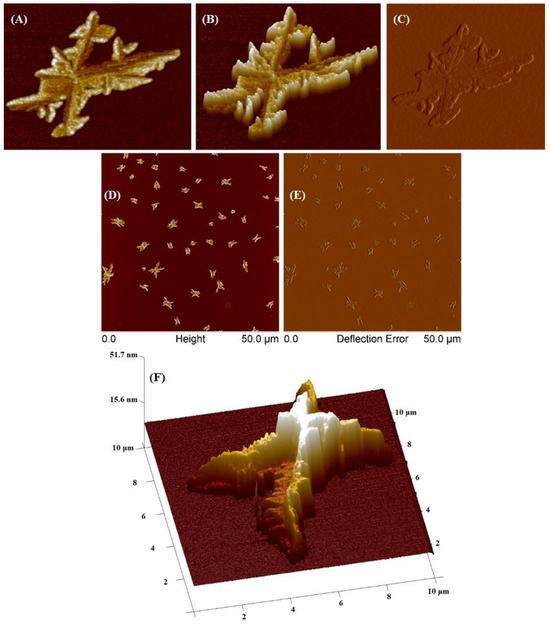
Figure 10.
AFM images of obtained star-comb-polymer SCP2 at a concentration of 0.01 g/mL. (A–C) images of a single structure, (D,E) images of various structures, (F) 3D image of a single structure.
Despite its low concentration of 0.001, the SCP2 sample formed single aggregates of various geometric shapes on the surface of the polymer mica (Supplementary Figure S6). These aggregates had diameters ranging from 50 to 60 μm and heights between 300 and 500 nm.
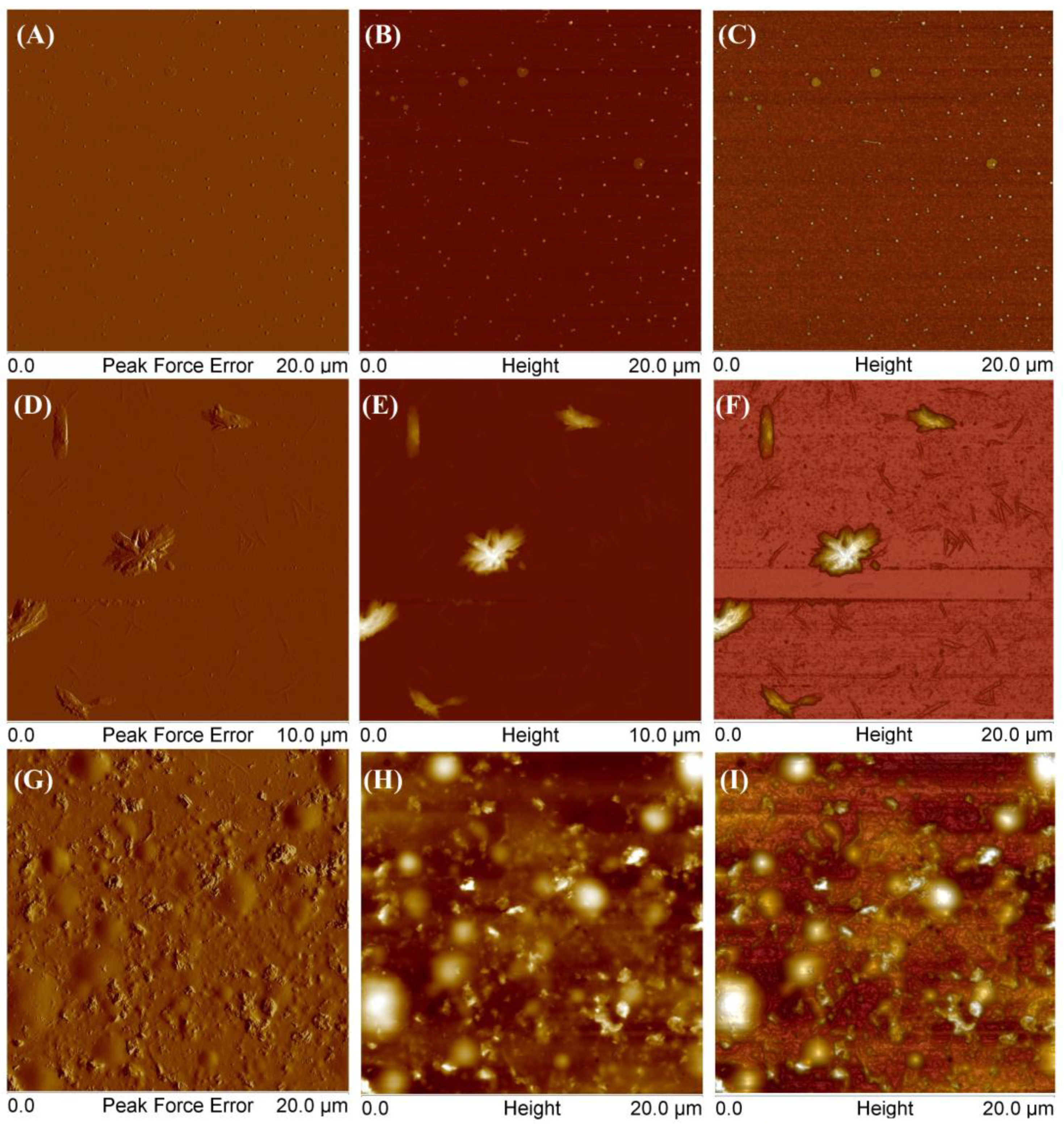
To compare polymers obtained from different monomers, SCP3 was also examined (Figure 11).
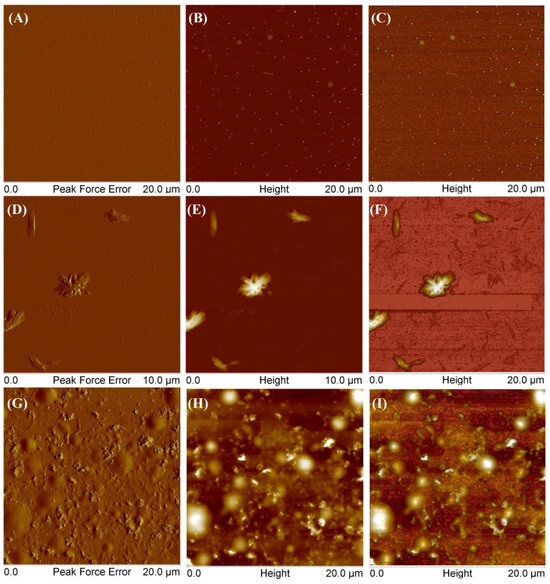
Figure 11.
AFM images of obtained star-comb-polymer SCP3 at a concentration of 0.001 g/mL (A–F) and at a concentration of 0.01 g/mL (G–I).
At the lowest tested concentration, the polymer exhibited several distinct forms. Figure 11A–C show round aggregates with a height of 10 to 17 nm and a diameter of 120 to 250 nm. Additionally, the polymer also formed larger aggregates with thicknesses equal to 4–6 nm and the diameter in the range 300–610 nm. Other scans (D–F) showed flat, elongated objects with a thickness of 5–7 nm and a length of 95–260 nm and higher branched ones with a thickness of 500–900 nm and a length of 1.39–2.21 μm. As the concentration increased, the polymer tended to form a coating on the mica surface with round convexes with a height of 180–300 nm (G–I). Figure 12 presents AFM images of mikto-arm polymers MSCP1.
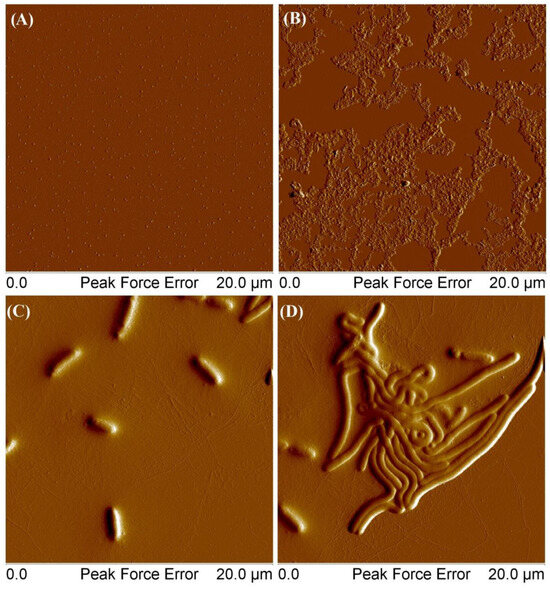
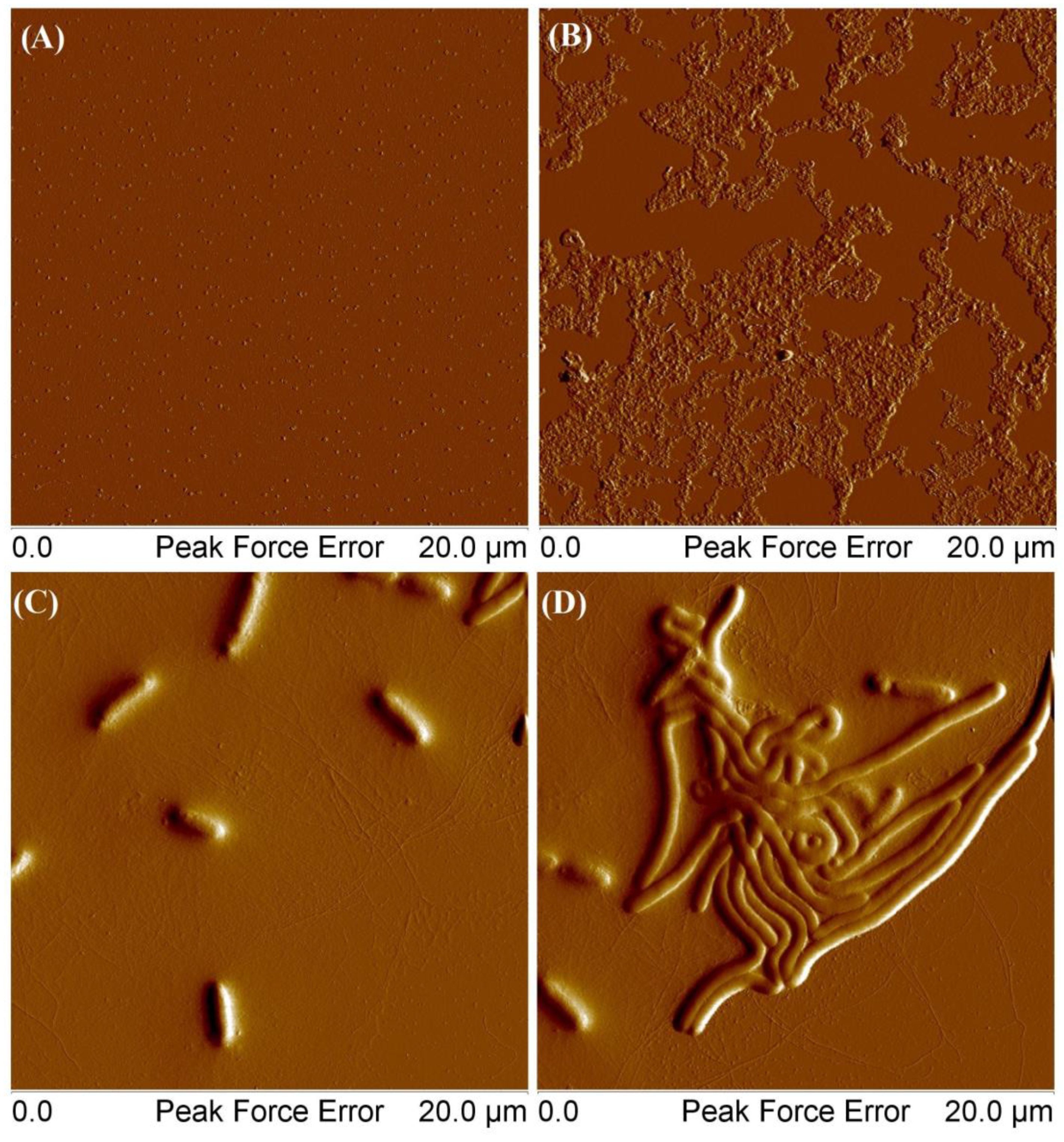
Figure 12.
AFM images of obtained MSCP1: At a concentration of 0.001 g/mL (A), at a concentration of 0.01 g/mL (B), and at a concentration of 0.001 g/mL from a solution that was set aside for a week (C,D).
MSCP1 at a concentration of 0.001 mg/mL formed single clusters with an average height of 8 nm. For a concentration of 0.01 mg/mL, the thickness of the deposited layer ranged from 30 to 70 nm. However, in the case of polymers deposited from solutions that were left to stand for a week (picture C), the average height of the structures was 270 nm, the length ranged from 1.85 μm to 3.31 μm, and the height of thin fibrils stayed within the range 1–3 nm. For picture D, the height of large elements was 130–170 nm, and the height of thin filaments was between 1 and 3 nm. The self-organization of the star polymer comprising a cyclodextrin core, 8 arms of POEOMA500, and 13 arms of polycaprolactone in an aqueous environment could be influenced by the amphiphilic nature of the polymer and the specific properties of its components. The hydrophilic POEOMA500 arms could possibly extend into the aqueous medium, while the hydrophobic polycaprolactone arms might avoid water, potentially leading to an asymmetric structure that could facilitate self-organization over time. The formation of wormlike micelles depends on the ratio between the hydrophilic and hydrophobic segment of the mikto-arm polymer, and it is initiated by the spontaneous collapse of the water-insoluble phase, large enough to form a micellar core. Presumably, the entanglements of hydrophobic chains facilitated the connection of many individual macromolecules to form a cylindrical core, while hydrophilic chains surrounding the core stabilized the cylindrycal structure. Our observation aligns with the documented behaviors of amphiphilic block copolymers possessing different macromolecular architectures [38,39]. In such systems, the interplay between hydrophilic and hydrophobic interactions is finely balanced, leading to the self-assembly of various morphologies, including spheres, rods, and vesicles, depending on the copolymer’s composition and the surrounding conditions. This process is significantly influenced by the thermodynamics of the system, where factors like temperature, solvent quality, and polymer concentration play decisive roles in determining the final structure of the self-assembled micelles.
Based on the AFM images, polymeric aggregates exhibit an irregular, fractal-like morphology, suggesting a semi-crystalline polymer, which was confirmed by DSC analysis (Supplementary Figure S7) [40,41]. They are not flat, exhibiting variations in height across their entire surface, which indicates a complex three-dimensional structure. The surface topology of the imaged aggregates is characterized by numerous irregularities and protrusions, possibly due to the unique molecular structure of the individual macromolecules.
3. Materials and Methods
HP-β-CD (ThermoFisher, 97%, Waltham, MA, USA), 4-(N, N-dimethylamino)-pyridine (DMAP, Sigma-Aldrich, ≥99%, Poznan, Poland), triethylamine (TEA, Sigma-Aldrich, ≥99.5%, Poznan, Poland), anhydrous N-methyl-2-pyrrolidione (NMP, Sigma-Aldrich, 99.5%, Poznan, Poland), 2-bromoisobutyryl bromide (BiBB, Sigma-Aldrich, 98%, Poznan, Poland), 2-hydroxyethyl 2-bromoisobutyrate (HO-EBiB, Sigma-Aldrich, 95%, Poznan, Poland), tin(II) 2-ethylhexanoate (Alfa Aesar, 96%, Warsaw, Poland ), poly(ethylene glycol) methyl ether methacrylate (OEOMA300, Sigma-Aldrich, Poznan, Poland), poly(ethylene oxide) methyl ether methacrylate (OEOMA500, Sigma-Aldrich, Poznan, Poland), ε-caprolactone (Alfa Aesar, 99%, Warsaw, Poland), 2,2′-azobis [2-(2-imidazolin-2-yl)propane] dihydrochloride (VA-044, TCI, >98%, Eschborn, Germany), glucose (TCI, 98%, Eschborn, Germany), glucose oxidase (GOx) (Type XS, Sigma-Aldrich, Poznan, Poland), copper (II) chloride CuCl2 (Sigma-Aldrich, 99.995%, Poznan, Poland), tris(2-pyridylmethyl)amine (TPMA, Sigma-Aldrich, >98%, Poznan, Poland), sodium pyruvate (TCI, >97%, Eschborn, Germany), cyclohexane (Chempur, 99.5%, Piekary Slaskie, Poland), tetrahydrofuran (THF) (EUROCHEM BGD, p.a., Tarnow, Poland), methanol (Chempur, p.a., Piekary Slaskie, Poland), dimethylformamide (Chempur, p.a., Piekary Slaskie, Poland), n-heptane (Chempur, p.a., Piekary Slaskie, Poland), toluene (STANLAB, p.a., Lublin, Poland), chlorotrimethylsilane (Sigma-Aldrich, ≥98%, Poznan, Poland). All chemicals were used as received.
An amount of 0.01 M Phosphate buffer saline (PBS, pH 7.4, Sigma-Aldrich, Poznan, Poland) was prepared according to the following procedure: one tablet was dissolved in 200 mL of deionized water yielding 0.01 M phosphate buffer saline (0.0027 M potassium chloride and 0.137 M sodium chloride).
4. Instrumentation
4.1. Nuclear Magnetic Resonance (NMR)
1H NMR and 13C NMR spectra of the synthesized initiator, polymers, and reaction mixtures were collected on a Varian Inova 600 MHz spectrometer (Palo Alto, CA, USA) at 25 °C using DMSO-d6 as a solvent and tetramethylsilane (TMS) as an internal standard for initiator; deuterium oxide (D2O) for OEOMA-based homopolymers and corresponding reaction mixtures; deuterated chloroform (CDCl3) for MSCP1 and MSCP2, and corresponding reaction mixtures. The samples were prepared by dissolving ~7 mg of the solid samples in 0.7 cm3 of deuterated solvent, and in the case of reaction mixtures 0.12 cm3 (0.01 cm3 for MSCP1-2) of the reaction mixture in 0.58 cm3 of deuterated solvent.
4.2. Attenuated Total Reflectance-Fourier Transform Infrared Spectroscopy (ATR-FTIR)
ATR-FTIR spectra were recorded on a Perkin-Elmer Spectrum Two 1000 FT-IR Infrared Spectrometer with an option of attenuated total reflection (ATR) (Perkin Elmer, Waltham, MA, USA). Spectra were collected at eight scans per spectrum and 2 cm−1 resolution in the range of 650–4000 cm−1 at 25 °C.
4.3. High-Resolution Mass Spectrometry (HR-MS)
HR-MS analyses were performed on a Waters Xevo G2 Q-TOF mass spectrometer (Waters Corporation, Milford, MA, USA) equipped with an electrospray ionization (ESI) source operating in positive ion modes. Full-scan MS data were collected from 100–5000 Da in positive ion mode with a scan time of 0.1 s. To ensure accurate mass measurements, data were collected in centroid mode, and the mass was corrected during acquisition using leucine enkephalin solution as an external reference (Lock-SprayTM, Waters Corporation, Milford, MA, USA), which generated a reference ion at m/z 556.2771 Da ([M+H]+) in the positive ESI mode. The accurate mass and composition for the molecular ion adducts were calculated using the MassLynx 4.1 software (Waters) incorporated with the instrument.
4.4. UV–Vis Spectroscopy
UV–Vis spectroscopy (Evolution 300 Spectrophotometer, Thermo Scientific, Waltham, MA, USA) was performed to determine the TCP of the star-comb polymers and their linear counterpart. The polymers were dissolved in distilled water at concentrations of 1 mg/mL, and 10 mg/mL. TCP of the polymer solutions was determined by a heating–cooling–heating cycle. The polymer solutions were heated from 25 °C to 90 °C, then cooled to 25 °C, and finally heated again to 90 °C at a rate of 2 °C/min. The transmittance of the polymer solutions was measured at 542 nm. The TCP was defined as the temperature at which an extremum of the first derivation is located by differentiating the transmittance vs. temperature curve.
4.5. Differential Scanning Calorimetry (DSC)
Thermal analyses were performed using a Mettler Toledo DSC 3 differential scanning calorimeter equipped with an XS105DU analytical balance (Greifensee, Switzerland). A total of ~1.5 mg (4.34 mg for SCP2) of the sample was used for measurements. The samples were positioned in 40 μL aluminum standard crucibles with a lid and a pin. Samples were tested in temperatures ranging from −20 °C to 280 °C (0–230 °C for SCP2) at a heating rate of 10 °C/min.
4.6. Size-Exclusion Chromatography (SEC)
Molecular weights (Mn, SEC) and dispersity indices (Ð) were determined by a size-exclusion chromatograph (Ultimate 3000, Waltham, MA, USA) equipped with an isocratic pump, autosampler, degasser, thermostatic box for columns, and differential refractometer RefractoMax 521 Detector and DAWN® (Waltham, MA, USA), and a multi-angle laser light scattering (MALLS) detector. ASTRA 7.3.2.17 data analysis software was used for data collecting and processing. The refractive index detection (RID) calculated molecular weight was based on calibration using linear polystyrene standards (Mp = 580–3,000,000 g/mol). A pre-column guard 5 μm 50 × 7.5 mm and double PLGel 5 μm MIXED-C and MIXED-D 300 × 7.5 mm column were used for separation. The measurements were carried out in THF (high-performance liquid chromatography (HPLC) grade) with the solvent at 35 °C with a flow rate of 1 mL/min.
4.7. Atomic Force Microscopy (AFM)
The analysis of topography was conducted using a BioScope Catalyst atomic force microscope from Veeco/Digital Instruments, which is equipped with a NanoScope V controller. The preparation of samples involved the following steps. First, a predetermined concentration (0.01 mg mL−1 and 0.001 mg mL−1) of the polymer solution under study was prepared in water. A volume of 5 μL of this solution was then dropped onto discharged mica and allowed to dry at room temperature. The BioScope Catalyst from Bruker was used to perform measurements in contact mode. A ScanAsyst-Air cantilever with a spring constant of 0.4 N m−1 was utilized for star-comb polymer SCP2 measurements, and a ScanAsyst-Fluid cantilever with a spring constant of 0.7 N m−1 was utilized for SCP3 and mikto-arm star-comb polymer MSCP1. The scanning speed was 0.65 μm/s, and the scan resolution was 512 × 512. All images were captured under ambient conditions. For the analysis of data, NanoScope version 1.80 and ImageJ Fiji, an open source software (1.54d) were employed.
5. Synthetic Methods and Procedures
5.1. Synthesis of Bromoisobutyryl-Functionalized CDs (8-Br-HP-β-CD)
HP-β-CD (2 g, 1.465 mmol) was placed in the jacketed reaction vessel and dissolved with an anhydrous NMP (20.6 mL) at 0 °C, resulting in a clear solution. TEA (1.305 g, 1.89 mmol) (deacid reagent) and a small amount (48 mg, 0.392 mmol) of DMAP (catalyst) were added to the solution. BiBB (2.695 g, 11.72 mmol) was diluted with anhydrous NMP (7.33 mL). The resulting solution was added dropwise to the HP-β-CD solution under an inert atmosphere (Ar). The mixture was stirred at 0 °C for 2 h, and then at room temperature for 24 h. The mixture containing the crude product was filtered to remove the triethylammonium bromide byproduct. The residual brown solution was precipitated in 800 cm3 water. Then, it was filtered under vacuum, and the white precipitate was washed with 2 L of water. Subsequently, the filter with the precipitate was left for 20 h to dry. Dichloromethane (20 mL) was used to dissolve the crude white solid, and the solution was recrystallized in cold n-hexane. The isolated white product (1.522 g, yield 40.63%) was dried under vacuum and kept in a sealed vial until required. The structure of 8-Br-HP-β-CD was confirmed by spectroscopic (1H NMR and 13C NMR (DMSO- d6, 600 MHz)) analyses. HR-MS (time of flight (TOF-ESI)) was calculated for 8-Br-HP-β-CD [M+K]+ 2289 (Supplementary Figure S1).
5.2. Synthesis of Star-Comb Polymers (Example for OEOMA300, 600:1 Molar Ratio to Initiator)
Star-comb polymer synthesis was performed by enzyme-assisted ATRP in the presence of air in phosphate-buffered saline (PBS), pH 7.4 at 45 °C for 2 h. The reaction was carried out in a glass vial, and the volume of the reaction mixture was 10 mL. First, OEOMA300 monomer (0.5 mL, 1.73 mmol) was added to the reaction vial. Then, glucose (0.18 g, 1 mmol) and GOx (0.0016 g, 10 nmol) were added to the vial. GOx catalyzed the oxidation of β-D-glucose to β-D-glucono-1,5-lactone and hydrogen peroxide (H2O2) using molecular oxygen as the electron acceptor. Subsequently, the previously synthesized initiator 8-HP-β-CD (7.39 mg, 2.89 µmol) (2-Hydroxyethyl 2-bromoisobutyrate for linear polymer CP (0.838 μL, 5.78 μmol)) was introduced (as a solid, entries SCP4, SCP6, SCP8, and SCP1-SCP3 or dissolved in 1.5 mL of methanol, entries SCP5, SCP7, and SCP9). Then, VA-044 (0.84 mg, 2.6 μmol) and CuCl2/TPMA (4.66 μL, 0.173 μmol CuCl2 and 0.519 μmol TPMA) (1:3 molar ratio in DMF, CuCl2 5 mg/mL) were added. VA-044 was used as the reducing agent. CuCl2 was used as a catalyst, and TPMA was used as a ligand. Sodium pyruvate (55.02 mg, 0.5 mol) was added sequentially, followed by 9.5 mL of PBS (8 mL for entries with initiator dissolved in methanol). Sodium pyruvate eliminates H2O2 from the reaction mixture. The whole mixture was then thoroughly mixed and placed in an oil bath. After the reaction, 20 mL of methanol was added to the reaction mixture, and the reaction mixture was concentrated using a rotary evaporator to get rid of the water and methanol. Subsequently, 20 mL of THF was added to the residual reaction mixture to dissolve the polymer, which was then passed through a neutral aluminum oxide (Al2O3) column to get rid of the catalyst. The polymer was then precipitated three times in cyclohexane to dispose of the monomer and dried under a vacuum. The structures of the synthesized polymers were confirmed by 1H NMR spectra. Number average molecular weights (Mn, NMR) were calculated based on 1H NMR spectra recorded for reaction mixtures. First, the conversion of monomer was calculated based on signals’ integrals from the vinyl group in unreacted monomer (average calculated from two signals at 6.04–6.01 ppm and 5.62–5.59 ppm) and methyl group from repeating units in the polymer (signals at 1.21–0.50 ppm). The degree of polymerization (DP) was determined by multiplying the monomer conversion by the initial ratio of monomer to initiator. DP per arm was determined by dividing the total DP by 8 referring to the number of initiation sites of the initiator. Mn from NMR was calculated by multiplying the DP value times the molar weight of the monomer.
5.3. Synthesis of Mikto-Arm Star-Comb Polymers Using ROP
The Schlenk reactor was subjected to a silanization process 24 h before carrying out the ROP. This pre-treatment involved the introduction of a 6% v/v solution of trimethylchlorosilane [(CH3)3SiCl] in toluene into the reactor. Following the required time, the silanizing solution was decanted, and the reactor underwent a drying process under vacuum conditions at a temperature of 140 °C for a duration of two hours. Subsequently, a macroinitiator (0.2 g, 2.17 μmol), solubilized in toluene (1 mL), along with the ε-caprolactone (ε-CL, 31.3 mL, 283 μmol), was introduced into the silanized Schlenk reactor. The reaction mixture was purged with inert gas. Thereafter, the catalyst, tin(II) 2-ethylhexanoate [Sn(Oct)2] (0.7 μL, 2.17 μmol), was incorporated into the reaction mixture. The Schlenk reactor was then positioned within an oil bath calibrated to a temperature of 100 °C. Upon completion of the reaction, the reactor was extricated from the oil bath and subjected to a cooling period of 15 min. The resultant product was isolated through a dual precipitation process using n-heptane as the precipitating agent. The isolated product was then subjected to a drying process at ambient conditions under a vacuum until a constant mass was achieved. 1H NMR spectra analysis confirmed the final structure of MSCP1.
6. Conclusions
In this work, star-comb polymers with OEOMA arms and CD-based cores were synthesized by enzymatically assisted ATRP in the presence of air. The polymers were characterized by ATR-FTIR, 1H NMR, 13C NMR, UV–Vis, and SEC/MALLS analyses. The thermosensitivity of the polymers was evaluated by measuring their cloud point temperatures in water. The well-defined star-comb POEOMA300s obtained using a solution of initiator in methanol were characterized by narrow molecular weight distributions (Mw/Mn < 1.35). The various lengths of arms and cores based on CD can be useful for future applications as a polymeric absorbent of metals in wastewater treatment and their subsequent recovery, in controlled drug release systems, biomedicine, cosmetics, the food industry, and in agriculture. Due to the relatively high TCP of OEOMA300-based polymers, they are not suitable for use as potential drug delivery systems throughout the body. However, they can be applied topically, using methods like laser heating above the TCP. Nevertheless, they are suitable for potential industrial applications, particularly in the removal of pollutants, such as heavy metals, from water. Thus, these polymers contribute to an environmentally friendly approach, providing a novel strategy for the synthesis of well-defined star-comb polymers following the principles of green chemistry.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules29010055/s1, Figure S1. HR-MS spectra of the obtained 8-Br-HP-β-CD initiator; Figure S2. IR spectra of HP-β-CD and 8-Br-HP-β-CD initiator; Figure S3. 13C NMR (600 MHz, DMSO) spectra of 8Br-HP-β-CD; Figure S4. 2 DSC thermograms for HP-β-CD and 8-Br-HP-β-CD; Figure S5. SEC traces of MSCP1 and SCP3; Figure S6. AFM images of obtained star-comb-polymer SCP8 at 0.001 mg/mL; Figure S7. DSC thermograms for SCP2; Table S1. Characteristics of the obtained mikto-arm polymers. Supplementary data associated with this article can be found in the online version.
Author Contributions
Conceptualization, A.M. and D.N.; Methodology, A.M., O.K. and K.E.; Software, O.K.; Validation, A.M. and D.N.; Formal analysis, T.F., O.K. and K.E.; Investigation, T.F., O.K. and K.E.; Data curation, T.F., A.M., O.K. and K.E.; Writing—original draft, T.F. and A.M.; Visualization, T.F., A.M. and O.K.; Supervision, A.M. and D.N.; Funding acquisition, T.F. and A.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Polish Budget Funds for Scientific Research in 2022 and 2023 as core funding for R&D activities at the Silesian University of Technology-funding for young scientists, grant number BKM-606/RCH4/2022 (04/040/BKM22/0218) and BKM-545/RCH4/2023 (04/040/BKM23/0259) and the grant for starting research on a new subject no. 32/014/SDU/10-22-68 of the Rector of Silesian University of Technology.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article and supplementary materials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zheng, G.; Pan, C. Reversible addition-fragmentation transfer polymerization in nanosized micelles formed in situ. Macromolecules 2006, 39, 95–102. [Google Scholar] [CrossRef]
- Szczepaniak, G.; Fu, L.; Jafari, H.; Kapil, K.; Matyjaszewski, K. Making ATRP More Practical: Oxygen Tolerance. Acc. Chem. Res. 2021, 54, 1779–1790. [Google Scholar] [CrossRef] [PubMed]
- Kapil, K.; Jazani, A.M.; Szczepaniak, G.; Murata, H.; Olszewski, M.; Matyjaszewski, K. Fully Oxygen-Tolerant Visible-Light-Induced ATRP of Acrylates in Water: Toward Synthesis of Protein-Polymer Hybrids. Macromolecules 2023, 56, 2017–2026. [Google Scholar] [CrossRef] [PubMed]
- Dadashi-Silab, S.; Lee, I.H.; Anastasaki, A.; Lorandi, F.; Narupai, B.; Dolinski, N.D.; Allegrezza, M.L.; Fantin, M.; Konkolewicz, D.; Hawker, C.J.; et al. Investigating Temporal Control in Photoinduced Atom Transfer Radical Polymerization. Macromolecules 2020, 53, 5280–5288. [Google Scholar] [CrossRef]
- Konkolewicz, D.; Magenau, A.J.D.; Averick, S.E.; Simakova, A.; He, H.; Matyjaszewski, K. ICAR ATRP with ppm Cu catalyst in water. Macromolecules 2012, 45, 4461–4468. [Google Scholar] [CrossRef]
- Navarro, L.A.; Enciso, A.E.; Matyjaszewski, K.; Zauscher, S. Enzymatically Degassed Surface-Initiated Atom Transfer Radical Polymerization with Real-Time Monitoring. J. Am. Chem. Soc. 2019, 141, 3100–3109. [Google Scholar] [CrossRef]
- Szczepaniak, G.; Łagodzińska, M.; Dadashi-Silab, S.; Gorczyński, A.; Matyjaszewski, K. Fully oxygen-tolerant atom transfer radical polymerization triggered by sodium pyruvate. Chem. Sci. 2020, 11, 8809–8816. [Google Scholar] [CrossRef]
- Enciso, A.E.; Fu, L.; Russell, A.J.; Matyjaszewski, K. A Breathing Atom-Transfer Radical Polymerization: Fully Oxygen-Tolerant Polymerization Inspired by Aerobic Respiration of Cells. Angew. Chem. -Int. Ed. 2018, 57, 933–936. [Google Scholar] [CrossRef]
- Sigg, S.J.; Seidi, F.; Renggli, K.; Silva, T.B.; Kali, G.; Bruns, N. Horseradish peroxidase as a catalyst for atom transfer radical polymerization. Macromol. Rapid Commun. 2011, 32, 1710–1715. [Google Scholar] [CrossRef]
- Neugebauer, D.; Mielańczyk, A.; Waśkiewicz, S.; Biela, T. Epoxy Functionalized Polymethacrylates Based on Various Multifunctional D-Glucopyranoside Acetals. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 2483–2494. [Google Scholar] [CrossRef]
- Przybyla, M.A.; Yilmaz, G.; Remzi Becer, C. Natural cyclodextrins and their derivatives for polymer synthesis. Polym. Chem. 2020, 11, 7582–7602. [Google Scholar] [CrossRef]
- Crini, G. Review: A history of cyclodextrins. Chem. Rev. 2014, 114, 10940–10975. [Google Scholar] [CrossRef] [PubMed]
- Szejtli, J. Introduction and General Overview of Cyclodextrin Chemistry. Chem. Rev. 1998, 98, 1743–1753. [Google Scholar] [CrossRef] [PubMed]
- Morin-Crini, N.; Fourmentin, S.; Fenyvesi, É.; Lichtfouse, E.; Torri, G.; Fourmentin, M.; Crini, G. 130 Years of Cyclodextrin Discovery for Health, Food, Agriculture, and the Industry: A Review; Springer International Publishing: New York, NY, USA, 2021; Volume 19, ISBN 0123456789. [Google Scholar]
- Gidwani, B.; Vyas, A. A Comprehensive Review on Cyclodextrin-Based Carriers for Delivery of Chemotherapeutic Cytotoxic Anticancer Drugs. BioMed Res. Int. 2015, 2015, 198268. [Google Scholar] [CrossRef] [PubMed]
- Mendelsohn, A.R.; Larrick, J.W. Preclinical Reversal of Atherosclerosis by FDA-Approved Compound that Transforms Cholesterol into an Anti-Inflammatory “prodrug”. Rejuvenation Res. 2016, 19, 252–255. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.M.; McKenzie, T.G.; Fu, Q.; Wong, E.H.H.; Xu, J.; An, Z.; Shanmugam, S.; Davis, T.P.; Boyer, C.; Qiao, G.G. Star Polymers. Chem. Rev. 2016, 116, 6743–6836. [Google Scholar] [CrossRef] [PubMed]
- Roovers, J. Concentration Dependence of the Relative Viscosity of Star Polymers. Macromolecules 1994, 27, 5359–5364. [Google Scholar] [CrossRef]
- Wu, W.; Wang, W.; Li, J. Star polymers: Advances in biomedical applications. Prog. Polym. Sci. 2015, 46, 55–85. [Google Scholar] [CrossRef]
- Mielańczyk, A.; Neugebauer, D. Designing drug conjugates based on sugar decorated V-shape and star polymethacrylates: Influence of composition and architecture of polymeric carrier. Bioconjug. Chem. 2015, 26, 2303–2310. [Google Scholar] [CrossRef]
- Yang, D.P.; Oo, M.N.N.L.; Deen, G.R.; Li, Z.; Loh, X.J. Nano-Star-Shaped Polymers for Drug Delivery Applications. Macromol. Rapid Commun. 2017, 38, 1700410. [Google Scholar] [CrossRef]
- Lotocki, V.; Kakkar, A. Miktoarm star polymers: Branched architectures in drug delivery. Pharmaceutics 2020, 12, 827. [Google Scholar] [CrossRef] [PubMed]
- Wiltshire, J.T.; Qiao, G.G. Recent advances in star polymer design: Degradability and the potential for drug delivery. Aust. J. Chem. 2007, 60, 699–705. [Google Scholar] [CrossRef]
- Chong, Y.K.; Zainol, I.; Ng, C.H.; Ooi, I.H. Miktoarm star polymers nanocarrier: Synthesis, characterisation, and in-vitro drug release study. J. Polym. Res. 2019, 26, 79. [Google Scholar] [CrossRef]
- Lemanowicz, M.; Mielańczyk, A.; Walica, T.; Kotek, M.; Gierczycki, A. Application of polymers as a tool in crystallization—A review. Polymers 2021, 13, 2695. [Google Scholar] [CrossRef] [PubMed]
- Behbahani, M.; Bide, Y.; Salarian, M.; Niknezhad, M.; Bagheri, S.; Bagheri, A.; Nabid, M.R. The use of tetragonal star-like polyaniline nanostructures for efficient solid phase extraction and trace detection of Pb(II) and Cu(II) in agricultural products, sea foods, and water samples. Food Chem. 2014, 158, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Ohno, K.; Wong, B.; Haddleton, D.M. Synthesis of well-defined cyclodextrin-core star polymers. J. Polym. Sci. Part A Polym. Chem. 2001, 39, 2206–2214. [Google Scholar] [CrossRef]
- Li, J.; Xiao, H.; Kim, Y.S.; Lowe, T.L. Synthesis of water-soluble cationic polymers with star-like structure based on cyclodextrin core via ATRP. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 6345–6354. [Google Scholar] [CrossRef]
- Seidi, F.; Shamsabadi, A.A.; Amini, M.; Shabanian, M.; Crespy, D. Functional materials generated by allying cyclodextrin-based supramolecular chemistry with living polymerization. Polym. Chem. 2019, 10, 3674–3711. [Google Scholar] [CrossRef]
- Zhu, Y.; Zheng, X.; Yu, B.; Yang, W.; Zhao, N.; Xu, F. Efficient gene carriers composed of 2-hydroxypropyl-β-cyclodextrin, ethanolamine-functionalized poly(glycidyl methacrylate), and poly((2-dimethyl amino)ethyl methacrylate) by combination of ATRP and click chemistry. Macromol. Biosci. 2014, 14, 1135–1148. [Google Scholar] [CrossRef]
- Pan, Y.; Xue, Y.; Snow, J.; Xiao, H. Tailor-made antimicrobial/antiviral star polymer via ATRP of cyclodextrin and guanidine-based macromonomer. Macromol. Chem. Phys. 2015, 216, 511–518. [Google Scholar] [CrossRef]
- Constantinou, A.P.; Tall, A.; Li, Q.; Georgiou, T.K. Liquid–liquid phase separation in aqueous solutions of poly(ethylene glycol) methacrylate homopolymers. J. Polym. Sci. 2022, 60, 188–198. [Google Scholar] [CrossRef]
- Dudkaitė, V.; Kairys, V.; Bagdžiūnas, G. Understanding the activity of glucose oxidase after exposure to organic solvents. J. Mater. Chem. B 2023, 11, 2409–2416. [Google Scholar] [CrossRef] [PubMed]
- Milčić, N.; Švaco, P.; Sudar, M.; Tang, L.; Findrik Blažević, Z.; Majerić Elenkov, M. Impact of organic solvents on the catalytic performance of halohydrin dehalogenase. Appl. Microbiol. Biotechnol. 2023, 107, 2351–2361. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Jiménez, A.; Montoya-Villegas, K.A.; Licea-Claverie, A.; Gónzalez-Ayón, M.A. Tunable thermo-responsive copolymers from DEGMA and OEGMA synthesized by RAFT polymerization and the effect of the concentration and saline phosphate buffer on its phase transition. Polymers 2019, 11, 1657. [Google Scholar] [CrossRef] [PubMed]
- Szweda, D.; Szweda, R.; Dworak, A.; Trzebicka, B. Thermoresponsive poly[oligo(ethylene glycol) methacrylate]s and their bioconjugates—Synthesis and solution behavior. Polimery/Polymers 2017, 62, 298–310. [Google Scholar] [CrossRef]
- Jin, L.; Zhang, G.; Zhai, X.; Ma, Z.; Zheng, P.; Wang, W. Macromolecular effect on crystal pattern formation in ultra-thin films: Molecular segregation in a binary blend of PEO fractions. Polymer 2009, 50, 6157–6165. [Google Scholar] [CrossRef]
- Liu, X.; Gitsov, I. Nonionic amphiphilic linear dendritic block copolymers. solvent-induced self-assembly and morphology tuning. Macromolecules 2019, 52, 5563–5573. [Google Scholar] [CrossRef]
- Nazemi, A.; Boott, C.E.; Lunn, D.J.; Gwyther, J.; Hayward, D.W.; Richardson, R.M.; Winnik, M.A.; Manners, I. Monodisperse cylindrical micelles and block comicelles of controlled length in aqueous media. J. Am. Chem. Soc. 2016, 138, 4484–4493. [Google Scholar] [CrossRef]
- Wu, X.; Shi, S.; Yu, Z.; Russell, T.P.; Wang, D. AFM nanomechanical mapping and nanothermal analysis reveal enhanced crystallization at the surface of a semicrystalline polymer. Polymer 2018, 146, 188–195. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, X.B.; Zhang, H.L.; Zhu, D.S.; Sun, Y.J.; Yan, S.K.; Wang, J.; Chen, X.F.; Wan, X.H.; Chen, E.Q.; et al. AFM study of crystallization and melting of a poly(ethylene oxide) diblock copolymer containing a tablet-like block of poly{2,5-bis[(4-methoxyphenyl) oxycarbonyl]styrene} in ultrathin films. Polymer 2006, 47, 1217–1225. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).