1. Introduction
The design of cell-membrane-mimicking lipid bilayer platforms is of high interest for understanding the structure and function of cell membranes, as well as for applications such as pharmaceutical drug discovery and biosensor development [
1,
2]. Recently, reconstructing bacterial lipid membranes on sensing devices has become an important area for investigating bacteria–material interactions as well as for scrutinizing biomacromolecular interaction processes like enzymatic and antibiotic activities that occur at bacterial cell membrane interfaces [
3,
4,
5]. As such, the development of well-defined bacterial lipid membrane model systems for different bacterial species is essential because each one has a particular lipid composition, which can even vary among strains [
6]. By utilizing purified lipid extracts derived from bacterial cell membranes, it is possible to incorporate the wide range of naturally occurring lipids, which is advantageous for attempting to mimic membrane properties such as fluidity and rigidity [
7,
8,
9,
10]. Thus far, the lipid extract of
Escherichia coli (
E. coli) bacteria has been the most commonly used model system due to its commercial availability and the importance of
E. coli as an important bacterial species relevant to human and animal health and to food safety [
11]. In combination with biosensing techniques, it is thus possible to study how different types of antimicrobial drug candidates and mitigants interact with
E. coli membranes and to define potencies and mechanisms of action [
3,
12].
The most popular technique for obtaining lipid extracts from
E. coli bacterial cells is solvent extraction, and the total lipid mass can be extracted using a modified Bligh and Dyer method [
13,
14,
15]. These
E. coli lipid extracts contain zwitterionic phosphatidylethanolamine (PE), negatively charged phosphatidylglycerol (PG), and doubly negatively charged cardiolipin (CL) lipids as the major components, and can be used to fabricate bacterial lipid membranes on sensor surfaces. The inclusion of CL, in particular, is an important advantage of working with
E. coli lipid extracts compared to more simplified, binary PE/PG lipid model compositions because the four-chain CL lipid has been reported to have a strong influence on membrane organization [
16,
17]. In addition to studying membrane permeabilization of
E. coli lipid vesicles in bulk solution [
18], different types of solid-supported lipid membrane platforms composed of purified
E. coli lipids have been developed depending on the sensing application and mainly involve the surface deposition of lipid vesicles prepared from
E. coli lipids. For instance, the adsorption and spontaneous rupture of
E. coli lipid vesicles to form supported lipid bilayers (SLBs) on gold, titania, and silica surfaces has been reported by modulating the vesicle–surface interaction strength in different solution environments [
9,
19,
20,
21,
22]. It has also been possible to form an intact adlayer of unruptured
E. coli lipid vesicles on solid surfaces in order to mimic the curved surface of
E. coli bacterial cell membranes [
23].
Another promising platform is the tethered bilayer lipid membrane (tBLM) that can be fabricated on gold electrode surfaces by using
E. coli lipid extracts based on a rapid-solvent-exchange-type process and is compatible with electrochemical impedance spectroscopy (EIS) to characterize the electrochemical properties of
E. coli tBLMs, which are related to membrane integrity. For example, Berry et al. employed
E. coli tBLM platforms in conjunction with EIS measurements to study the interaction kinetics of how a cationic antimicrobial peptide and engineered versions thereof disrupt the bacterial cell membrane mimic [
24]. Notably, a degree of correlation was shown between the ability of a peptide to increase ionic permeability across the
E. coli tBLM and in vitro antibacterial activity level [
24]. It is important to further expand such capabilities to evaluate the membrane-disruptive properties of antimicrobial lipids, especially medium-chain fatty acids and monoglycerides, that are important mitigants for food safety and agricultural applications [
25,
26]. Interestingly, while various fatty acid and monoglyceride mitigants within this class exhibit potent antibacterial properties based on permeabilization-related membrane disruption [
27], only a subset of them inhibits
E. coli bacteria and elucidating the biophysical basis for these different targeting spectrums is an outstanding need that can benefit from
E. coli tBLM platforms.
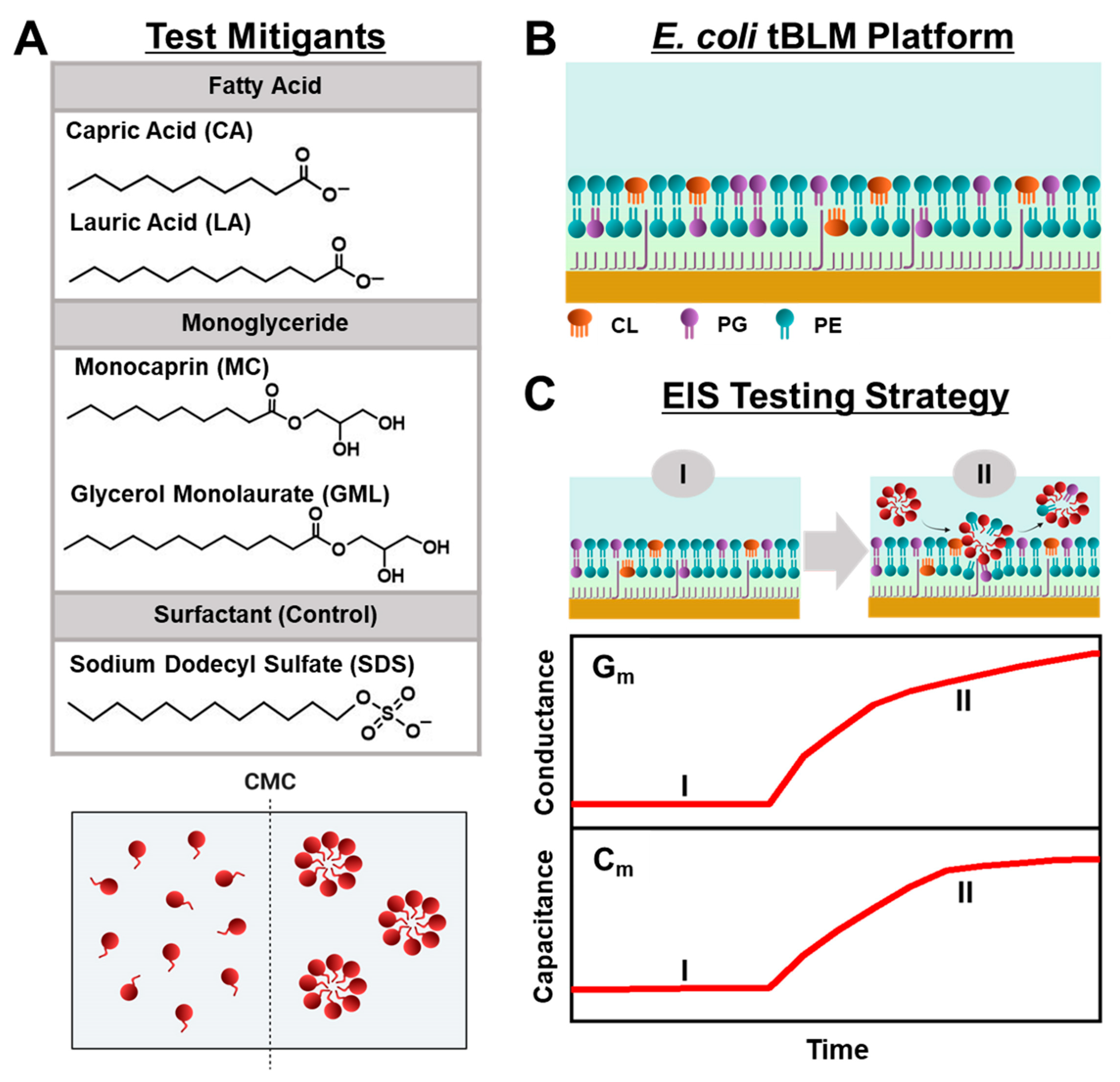
Herein, we conducted EIS measurements to comparatively evaluate the membrane-disruptive effects of medium-chain fatty acid and monoglyceride mitigants on
E. coli tBLM platforms. Our approach builds on recent efforts to study antimicrobial lipid and detergent interactions with simplified tBLM platforms [
28,
29,
30] and extends the measurement concept to investigate how various biologically important antimicrobial mitigants interact with reconstituted
E. coli lipid membranes. As depicted in
Figure 1A, we selected two pairs of medium-chain fatty acids and monoglycerides—the 10-carbon long capric acid (CA) fatty acid and its monoglyceride equivalent monocaprin (MC), and the 12-carbon long lauric acid (LA) fatty acid and its monoglyceride equivalent glycerol monolaurate (GML)—for EIS testing because these mitigants are among the most potent, membrane-disruptive ones against Gram-positive bacteria [
31], yet have varying levels of antibacterial activity against Gram-negative bacteria such as
E. coli. From a chemical perspective, all four mitigants are amphipathic molecules that self-assemble into micelles in bulk solution above their respective critical micelle concentration (CMC) values, whereas they exist as monomers at lower concentrations. Using the EIS technique, we conducted concentration-dependent experiments to investigate how different concentrations of each mitigant induce real-time changes in the conductance (G
m) and capacitance (C
m) properties of
E. coli tBLM platforms that are sensitive to membrane ionic permeability and structural integrity, respectively [
32] (
Figure 1B,C). Our findings demonstrate that EIS measurements on
E. coli tBLM platforms are a versatile tool to directly test the membrane-disruptive properties of fatty acid and monoglyceride mitigants and show how ionic permeability changes can be related to the antibacterial activity of different mitigants in terms of both potency and disruption effect magnitude.
2. Results and Discussion
We focused on fabricating tBLM platforms composed of total
E. coli lipid extract, and subsequently investigated the membrane-disruptive effects of 10- and 12-carbon long fatty acids and monoglycerides on these bacterial cell membrane mimics. This approach was based on measuring changes in membrane conductance and capacitance with the EIS technique, and we tested each compound at bulk concentrations corresponding to 4× and 2× of their respective CMC values. In general, shorter-chain fatty acids and monoglycerides have higher CMC values because they are more soluble and have less thermodynamic propensity to self-assemble into micelles [
33].
The tBLM platforms were fabricated on functionalized gold electrode surfaces by using the rapid-solvent-exchange method and possess an ionic reservoir (~4 nm thickness) between the tBLM bottom leaflet and gold electrode surface, as previously described [
34,
35]. Operationally, the frequency-dependent impedance and phase properties of the fabricated tBLM platforms in aqueous buffer solution were measured by the EIS technique (see
Figure S1 for representative Bode and Nyquist plots [
36] of an
E. coli tBLM platform formed using total lipid extract). A frequency sweep was performed to collect impedance and phase data across the full frequency range once every ~3 min cycle and fitted to an equivalent circuit model to monitor time-resolved changes in G
m and C
m signals, which are related to tBLM conductance (i.e., ionic permeability) and capacitance (i.e., structural integrity), respectively. The corresponding values were determined to be in the range of ~1–2 μS and 0.5–1.1 μF/cm
2, respectively, which indicate that the fabricated tethered lipid bilayers had high membrane-integrity/sealing properties [
37,
38]. In more detail,
Table 1 summarizes the full set of electrochemical parameters that were obtained by fitting the EIS data to the equivalent circuit model and confirmed that the fitted parameter values were in good agreement with expected ranges.
More specifically, the fitted G
m signal for the
E. coli tBLM platform was around 1.36 ± 0.45 μS, which agrees well with recently studied tBLM platforms composed of biologically relevant two-chain phospholipids or three-chain triglycerides [
39]. Of note, the fitted C
m signal was 0.83 ± 0.17 μF/cm
2, which is within the range reported for tBLM platforms composed exclusively of two-chain phospholipids (~1.2–1.4 μF/cm
2) or three-chain triglycerides (~0.6 μF/cm
2). Since membrane conductance (G
m) is the inverse of membrane resistance (R
m), the obtained G
m range translates into R
m values of 820 ± 280 kΩ. Compared to tBLMs composed of two-chain phospholipids only, the lower C
m signal for the
E. coli tBLM platform is indicative of a more densely packed membrane. This finding is consistent with the inclusion of four-chain CL lipids in the membrane composition as described above.
In addition, the Q
s parameter represents the imperfect capacitance in the reservoir region, whereas the ∝
s parameter is defined as the CPE dimensional constant and describes the contribution of restricted ion diffusion in the reservoir region. The fitted values of both parameters agree well with those obtained for tBLM platforms composed of biologically relevant phospholipids [
39], while the electrolyte resistance (R
e) is also consistent with the ionic strength of the buffer composition used in this study. We also fabricated
E. coli tBLM platforms from more purified polar lipid extracts, which had a similar lipid composition to the total extract and had been prepared by further precipitating the total lipid extract with acetone, followed by extraction with diethyl ether. In this case, similar electrochemical parameters were obtained, confirming that
E. coli tBLM platforms could be fabricated from total or polar lipid extracts.
Since we were interested in characterizing how the tested mitigants affect tBLM properties, we focused on tracking quantitative changes in the G
m and C
m signals upon compound addition because these parameters are directly related to the membrane properties. Conversely, other fitted parameters are mainly related to the ionic reservoir space or bulk solution properties and are less directly affected by membrane properties. Practically, changes in the G
m and C
m signals were measured relative to their baseline values prior to compound addition in order to assess the interaction kinetics and corresponding degree of membrane disruption. Bode plot representations of the EIS frequency vs. phase were also analyzed in order to detect qualitative changes in the frequency-at-minimum-phase and phase-at-minimum-phase signatures before and after compound addition for ~30 min. Such information provides insight into changes in membrane ionic transport properties and membrane densification/thinning, respectively [
40].
Therefore, after tBLM fabrication, the appropriate test compound at a defined concentration was added to the measurement chamber by pipette injection. The compound was incubated with the tBLM platform for 30 min during this treatment stage and then a buffer washing step was performed to remove the test compound from the bulk solution. The time resolution of the data collection was around 3 min per data point. To confirm the sensing capabilities of the tBLM platform composed of the total
E. coli lipid extract, we first tested the membrane-disruptive effects of sodium dodecyl sulfate (SDS) at concentrations above and below CMC (
Figure S2). SDS was active only above its CMC, and the interaction kinetics and membrane-solubilizing behavior showed general agreement with past EIS measurements conducted on tBLM platforms composed of diphytanoyl lipids as well as with results obtained using other techniques like electron microscopy [
38,
41]. We proceeded to test the membrane-disruptive effects of the different medium-chain fatty acids and monoglycerides on the tBLM platform composed of the total
E. coli lipid extract.
2.1. EIS Measurements with Medium-Chain Fatty Acids
We compared the membrane-disruptive effects of 10-carbon long CA and 12-carbon long LA at different bulk concentrations (approximately 4×, 2×, and 0.5×) relative to their respective CMC values. The reported CMC values of CA and LA in equivalent buffer conditions are 3500 μM and 900 μM, respectively, and were used as guides to define the specific test concentrations [
42]. The EIS data for each test compound are presented below.
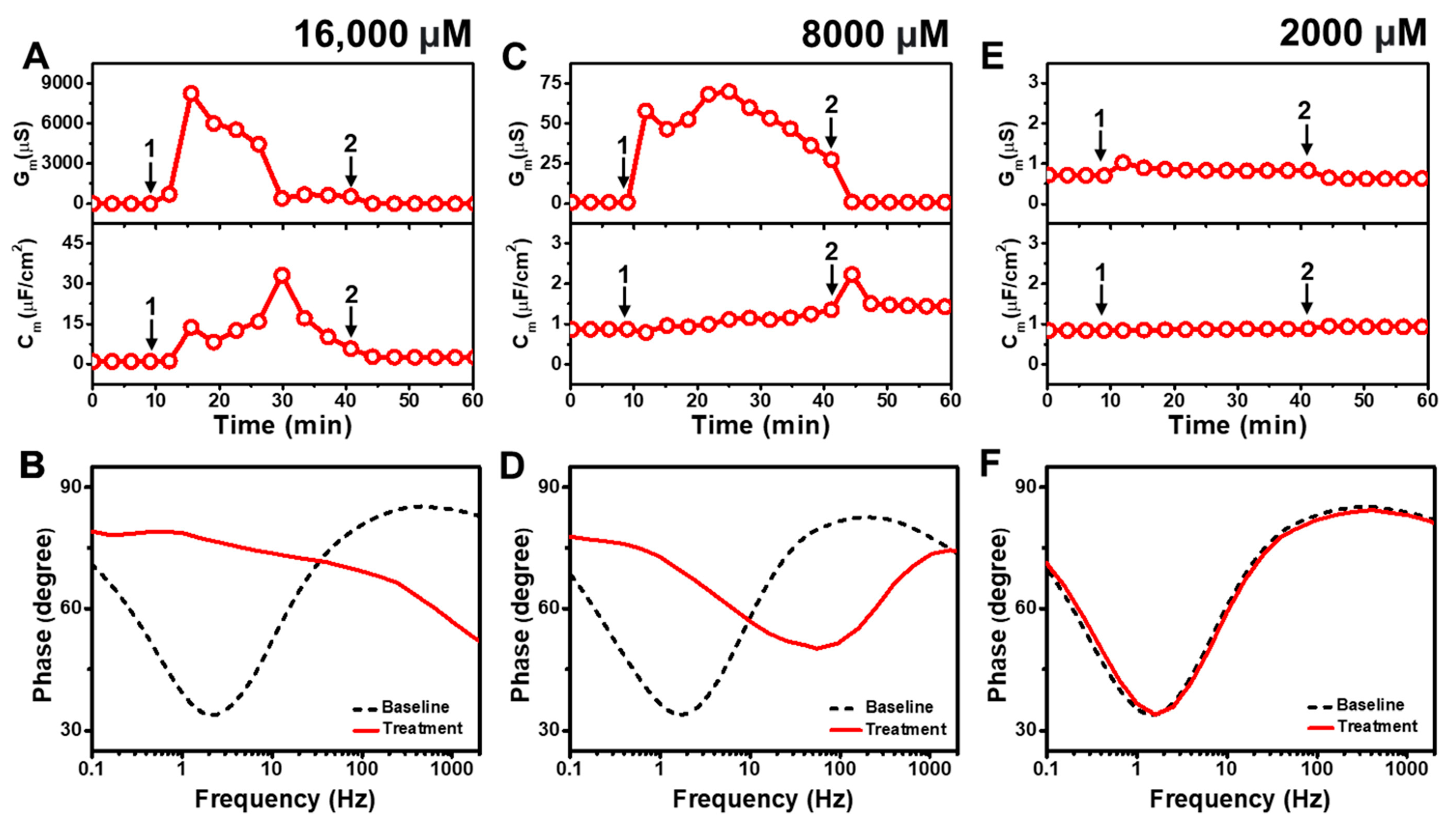
2.1.1. Capric Acid
Figure 2 presents the EIS results of CA addition to
E. coli tBLMs at around 4×, 2×, and 0.5× CMC concentrations. Upon 16,000 μM CA addition to the tBLM (~4× CMC), the G
m and C
m signals initially spiked and reached maximum values around 8250 μS and 14 μF/cm
2 (
Figure 2A). Then, the G
m signal gradually decreased and eventually stabilized at around 675 μS, whereas the C
m signal increased transiently to a peak of ~33 μF/cm
2 before gradually decreasing to ~17 μF/cm
2. This dynamic interaction behavior indicates that CA addition causes a large increase in membrane conductance (and, thus, a decrease in membrane resistance) that is similar to the SDS effect (cf.
Figure S2). However, in contrast to SDS, the CA interaction cannot lead to permanent membrane solubilization so the tBLM remodels itself over time to partially reorganize its sealing properties, albeit in a still highly damaged state. The final G
m and C
m values were reduced to around 1.6 μS and 2.5 μF/cm
2 after buffer rinsing, pointing to a degree of membrane thinning that persisted even after CA was removed from the bulk solution. According to Bode plot analysis, 16,000 μM CA addition caused a shift in the EIS phase profile that indicated a surfactant-type interaction, which is consistent with the appreciable G
m shift that signified extensive membrane disruption during the interaction stage [
43] (
Figure 2B).
Upon 8000 μM CA addition (~2× CMC), the G
m signal increased to ~70 μS before gradually decreasing to roughly 27 μS (
Figure 2C). A corresponding increase in the C
m signal of around ~1.2 μF/cm
2 was also recorded. Subsequent buffer rinsing caused the G
m signal to decrease back to ~0.9 μS, while the C
m signal transiently spiked before remaining steady at around ~1.4 μF/cm
2. The corresponding Bode plot showed that 8000 μM CA addition caused the phase minimum to shift to an appreciably higher frequency and phase, which corresponds to membrane damage and thinning (
Figure 2D). By contrast, when 2000 μM CA was added (~0.5× CMC), there was only a slight rise in the G
m signal by around ~0.4 μS and negligible change in the C
m signal (
Figure 2E). After buffer washing, the G
m signal returned to almost its baseline value and the C
m shift was insignificant. The Bode plot also showed negligible change in the phase minimum due to 2000 μM CA addition, supporting the idea that CA was inactive below its CMC (
Figure 2F).
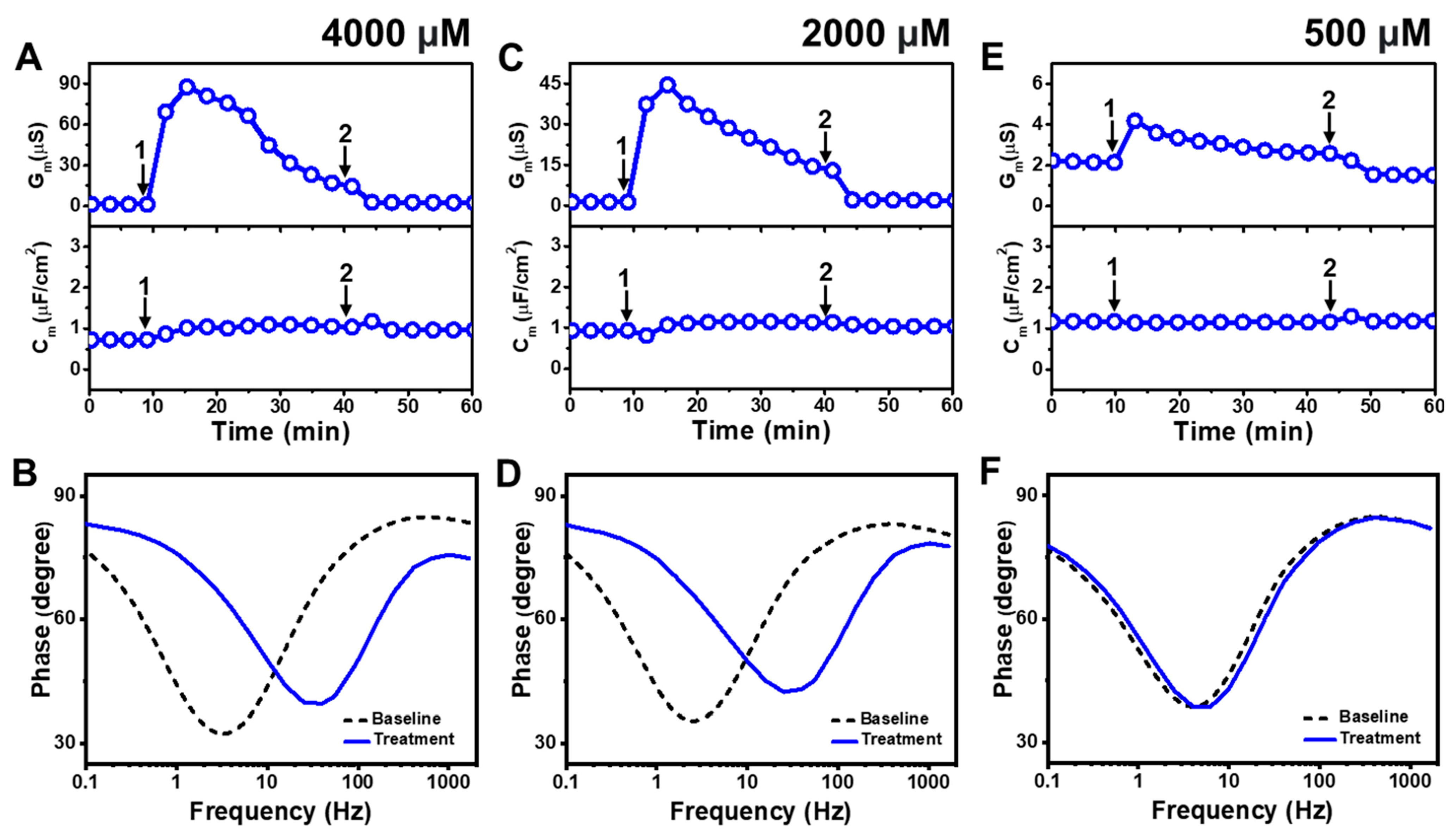
2.1.2. Lauric Acid
Figure 3 presents the EIS results of LA addition to
E. coli tBLMs at around 4×, 2×, and 0.5× CMC concentrations. Upon 4000 μM LA addition to the tBLM (~4× CMC), the G
m and C
m signals transiently increased to ~88 μS and ~1 μF/cm
2, respectively, while the G
m signal then gradually decreased to ~23 μS (
Figure 3A). After buffer rinsing, the final G
m and C
m signals were reduced to around ~2.5 μS and ~1 μF/cm
2, respectively. The corresponding Bode plots showed that the phase minimum transitioned to a modestly higher frequency and phase due to 4000 μM LA addition, which signified membrane damage (
Figure 3B).
The interaction kinetics of the G
m signal response were similar in response to 2000 μM LA addition (~2× CMC), in which case there was an initial, transient increase to ~45 μS before gradually decreasing to ~18 μS (
Figure 3C). After buffer rinsing, the G
m signal dropped to ~2 μS while the C
m signal shift was minimal throughout the interaction process. A similar change in the position of the phase minimum in the Bode plot was observed upon 2000 μM LA addition as in the 4000 μM LA addition case described above (
Figure 3D). This finding indicates that 2000 μM LA addition also causes membrane damage, while the absence of a C
m signal shift supports that membrane disruption mainly stems from ionic permeability changes rather than from the loss of membrane integrity. On the other hand, the addition of 500 μM LA (~0.5× CMC) caused a much slighter and reversible change in the G
m signal to around ~4 μS and there was no change in the C
m signal (
Figure 3E). The corresponding Bode plot revealed negligible membrane disruption upon 500 μM LA addition, with no change in the phase minimum that confirmed LA was inactive below its CMC (
Figure 3F).
2.2. EIS Measurements with Medium-Chain Monoglycerides
Similar EIS experiments were performed to test the membrane-disruptive effects of 10-carbon long MC and 12-carbon long GML at different bulk concentrations (approximately 4×, 2×, and 0.5×) relative to their respective CMC values. The reported CMC values of MC and GML in equivalent buffer conditions are 600 μM and 60 μM, respectively, and were used as guides to define the specific test concentrations [
42]. The EIS data for each test compound are presented below.
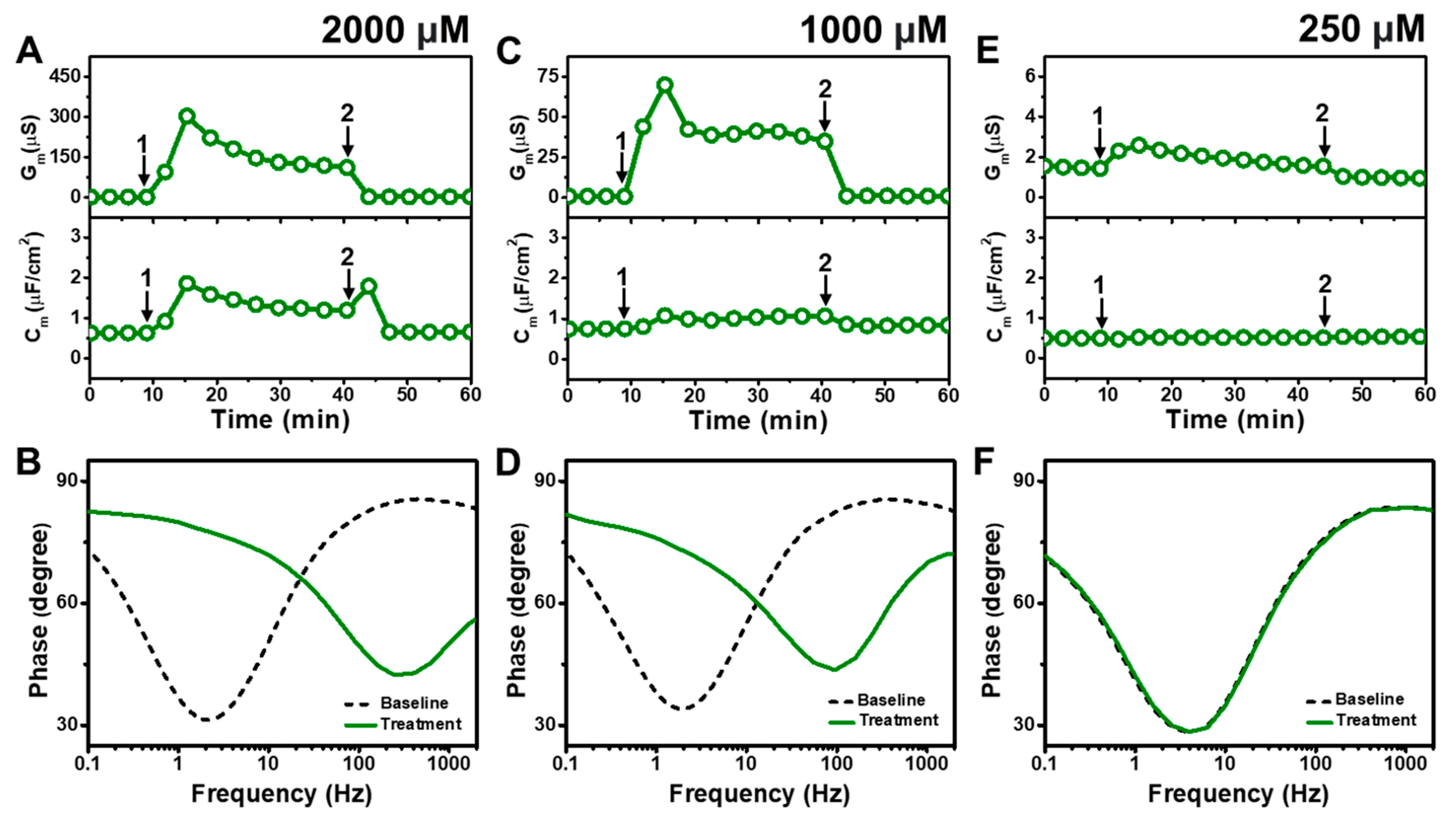
2.2.1. Monocaprin
Figure 4 presents the EIS results of MC addition to
E. coli tBLMs at around 4×, 2×, and 0.5× CMC concentrations. Upon 2000 μM MC addition to the tBLM (~4× CMC), the G
m and C
m signals rose to ~302 μS and ~1.9 μF/cm
2, respectively, before stabilizing at around 123 μS and 1.2 μF/cm
2, respectively (
Figure 4A). The G
m and C
m signals then decreased to around 1.8 μS and 0.7 μF/cm
2, respectively, after buffer rinsing. According to Bode plot analysis, extensive membrane damage occurred due to 2000 μM MC addition, as indicated by shifting of the phase minimum to a much higher frequency and phase (
Figure 4B).
With similar interaction kinetics, the addition of 1000 μM MC (~2× CMC) caused the G
m signal to increase to around 75 μS before stabilizing at around 41 μS (
Figure 4C). However, there was only a slight and nearly negligible increase in the C
m signal. After buffer washing, the G
m and C
m signals returned to near-baseline values of around 1.0 μS and 0.8 μF/cm
2, respectively. The Bode plots showed that 1000 μM MC addition caused the phase minimum to shift to a higher frequency and phase, albeit with a lower frequency shift than in the case of 2000 μM MC addition (
Figure 4D). This finding supports that 1000 μM MC treatment still caused membrane damage, but to a lesser extent than 2000 μM MC treatment. By contrast, the addition of 250 μM MC (~0.5× CMC) caused only a slight increase in the G
m signal to around 1.8 μS, which returned to the baseline value following buffer washing (
Figure 4E). No change in the C
m signal was observed as well. In addition, the Bode plots showed no change in the phase minimum before and after 250 μM MC addition, indicating negligible membrane-disruptive effects of MC below its CMC (
Figure 4F).
2.2.2. Glycerol Monolaurate
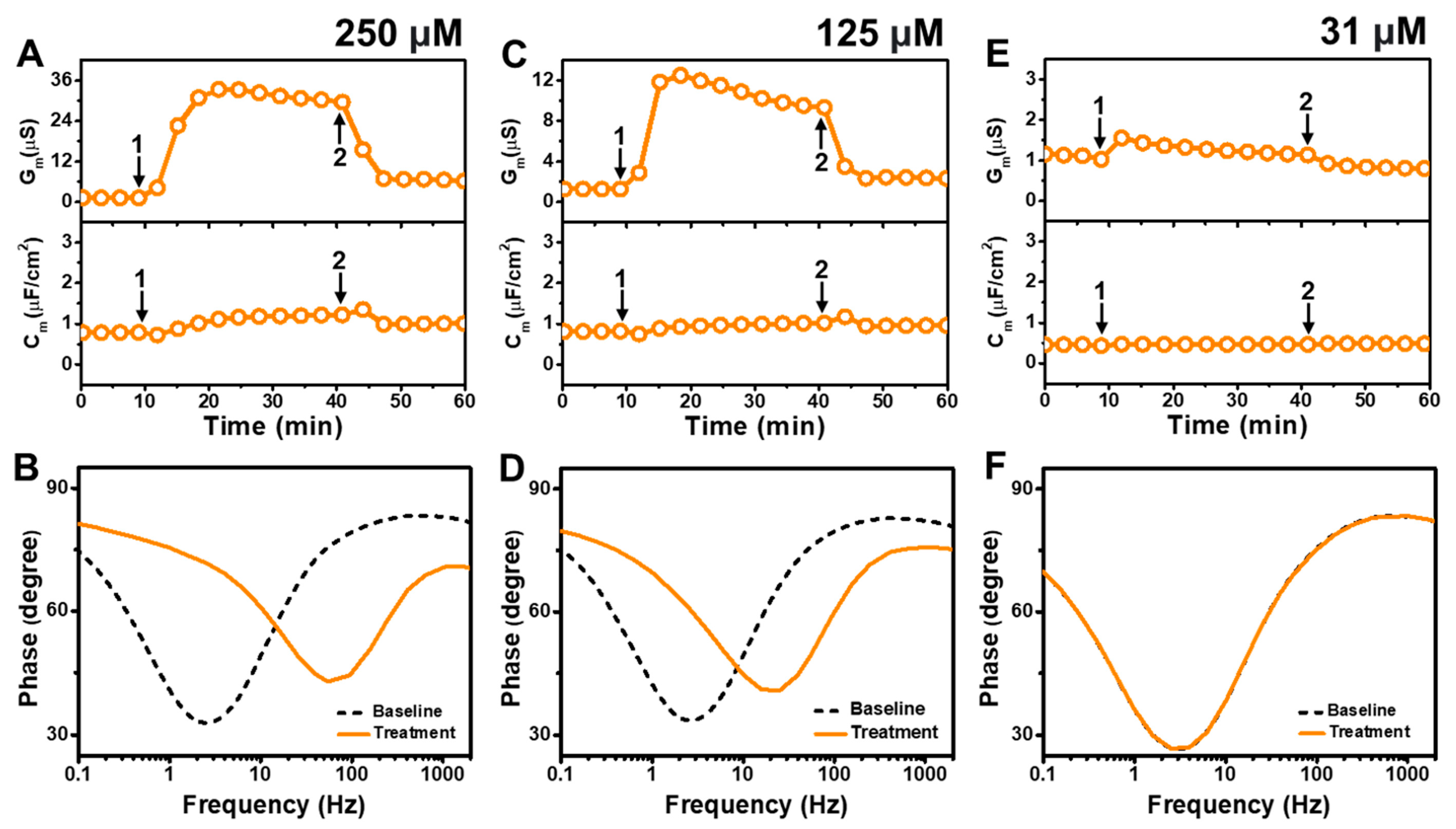
Figure 5 presents the EIS results of GML addition to
E. coli tBLMs at around 4×, 2×, and 0.5× CMC concentrations. The addition of 250 μM GML (~4× CMC) caused the G
m signal to increase appreciably to ~31 μS while there was only a slight increase in the C
m signal to 1.2 μF/cm
2 (
Figure 5A). After buffer washing, the G
m and C
m signals decreased to 6.2 μS and ~1.0 μF/cm
2, respectively. The Bode plots indicated that 250 μM GML caused the phase minimum to shift to a higher frequency and phase, which provided additional evidence of membrane damage (
Figure 5B).
In the case of 125 μM GML addition (~2× CMC), the G
m signal increased to around 10 μS, while the C
m signal only increased marginally to ~1 μF/cm
2 (
Figure 5C). After buffer washing, the G
m and C
m signals were reduced to around 2.3 μS and ~0.8 μF/cm
2, respectively. The Bode plots before and after 125 μM GML addition showed a shift of the phase minimum to higher frequency and phase that indicated membrane damage, while the shift magnitudes were smaller than in the 250 μM GML case and, thus, demonstrate that the extent of membrane disruption was smaller in the 125 μM GML case (
Figure 5D). We also tested the effects of 31 μM GML addition (~0.5× CMC) and observed only a modest increase in the G
m signal to around 1.2 μS, which returned to the baseline following buffer washing (
Figure 5E). There was no change in the C
m signal as well. Furthermore, the Bode plot analysis showed that 31 μM GML addition caused no change in the position of the phase minimum, indicating that GML does not cause membrane disruption below its CMC (
Figure 5F).
2.3. Comparison of Membrane-Disruptive Effects
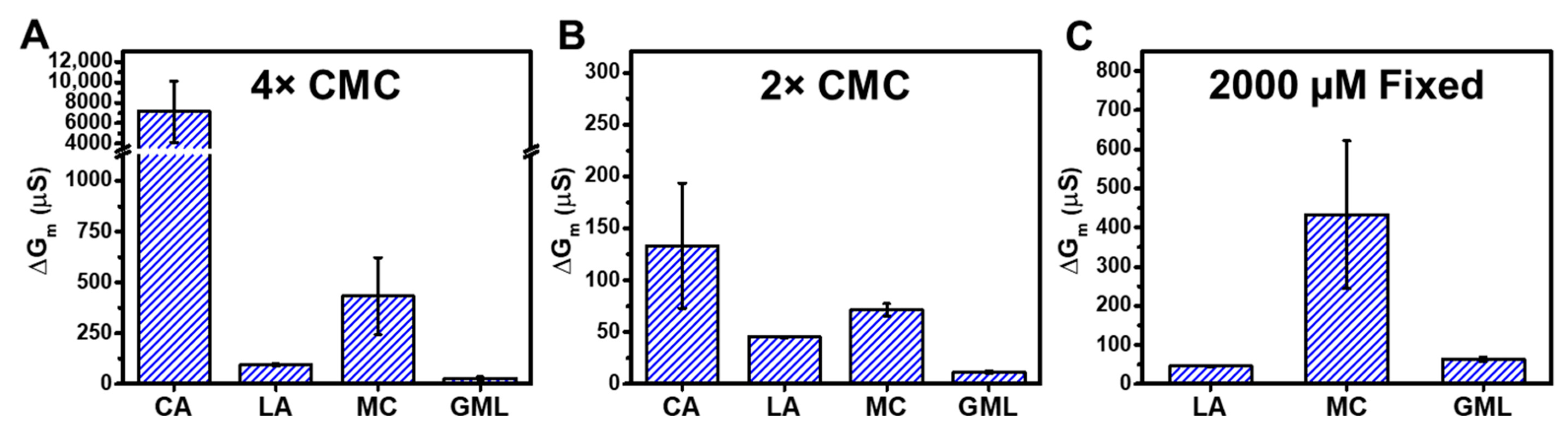
Figure 6 summarizes the trend in ΔG
m shifts that occurred when tBLM platforms composed of
E. coli total lipid extracts were treated with different fatty acid and monoglyceride mitigants at 4× and 2× concentration levels relative to their respective CMCs. At 4× CMC, CA caused the largest ΔG
m shifts around 7100 μS and MC caused the second largest ΔG
m shifts around 420 μS. By contrast, LA and GML caused ΔG
m shifts of around 90 μS and 30 μS, respectively (
Figure 6A). Thus, the membrane-permeabilizing effects of the four mitigants occurred in the following sequence: CA > MC > LA > GML. The same trend was observed when comparing EIS data obtained at 2× CMC (
Figure 6B). In that case, CA demonstrated strong membrane-disruptive effects, with ΔG
m shifts around 130 μS. MC also exhibited extensive membrane disruption and caused ΔG
m shifts around 65 μS. On the other hand, LA and GML caused appreciably smaller ΔG
m shifts of around 45 μS and 10 μS, respectively (see also
Table S1 for a detailed summary).
Since all four tested mitigants principally exhibited membrane-disruptive activity above their respective CMC values, this comparative approach supports that CA and MC with 10-carbon long saturated chains cause more extensive membrane disruption—as indicated by ionic permeability changes—than LA and GML with 12-carbon long saturated chains. In terms of comparing EIS data at equivalent molar concentrations, we may further note that 2000 µM MC caused ΔG
m shifts around 420 μS, whereas 2000 µM LA and 2000 µM GML caused smaller ΔG
m shifts around 45 μS and 60 μS, respectively (
Figure 6C; see also
Figure S3 for 2000 µM GML data). At this concentration, CA is inactive since its CMC is around 3500 µM, whereas it is difficult to work with 12-carbon long LA and GML at higher concentrations due to solubility considerations.
In addition to the ΔG
m shifts that provide insight into ionic permeability changes, we may also briefly comment on the trend in ΔC
m shifts that reflect the degree of membrane integrity. In general, 10-carbon long CA and MC induced discernible ΔC
m shifts that pointed to a loss of
E. coli membrane integrity due to membrane disruption and/or thinning [
38]. By contrast, 12-carbon long LA and GML had a largely negligible effect on the ΔC
m shifts, indicating that membrane integrity was preserved even when there were modest permeability changes.
We also proceeded to test the effects of the medium-chain fatty acids and monoglycerides on the tBLM platform derived from the polar
E. coli lipid extract. Similar trends in ΔG
m and ΔC
m shifts were obtained compared to the data from the total
E. coli tBLM platform, reinforcing that CA and MC with 10-carbon long saturated chains caused more extensive disruption of reconstituted
E. coli membranes than LA and GML with 12-carbon long saturated chains (
Figure S4).
As such, the overall difference in the membrane-disruptive effects of the tested mitigants with 10- vs. 12-carbon long, saturated hydrocarbon chains is consistent across the different EIS readouts and the variation in disruption extent may relate to the chain length-dependent packing parameters of the different mitigants. In general, the insertion of single-chain fatty acids and monoglycerides into phospholipid membranes can trigger spontaneous bilayer curvature [
44]. With decreasing chain length, the hydrophobic part of the inserting, single-chain molecule is smaller and can, consequently, increase the degree of positive spontaneous curvature due to the membrane bending outward [
44]. This bending affects inter-leaflet coupling and causes the bilayer core to become more disordered as compensation, which, in turn, leads to membrane thinning [
44]. Conceptually, the induction of positive spontaneous curvature and membrane thinning would occur to a greater extent for CA compared to LA and for MC compared to GML, which matches with the experimental data trendwise and can be rationalized by the shorter chain lengths of CA and MC.
From a biological perspective, the EIS findings also agree well with the known antimicrobial spectrums of the different compounds; i.e., antimicrobial fatty acids and monoglycerides that cause greater membrane disruption are more likely to inhibit
E. coli, and vice versa. Indeed, LA and GML are known to be among the most potent antimicrobial fatty acids and monoglycerides to inhibit Gram-positive bacteria, respectively, but are largely inactive against Gram-negative bacteria, especially
E. coli [
45,
46,
47,
48,
49,
50]. In the EIS measurements, we observed that they cause smaller changes in membrane ionic permeability and do not affect the structural integrity of the
E. coli membranes overall. In marked contrast, CA and MC have been reported to exhibit antibacterial activity against
E. coli and cause bacterial cell damage [
46,
47,
49,
51,
52], which are consistent with the appreciably larger changes in membrane ionic permeability as well as with the loss of membrane integrity and membrane thinning effects detected in the EIS measurements. Notably, it has been reported that CA causes greater in vitro membrane permeabilization of
E. coli cell membranes than LA [
49], which directly matches our results obtained with reconstituted
E. coli membranes in the tBLM platform. Together, our findings support that studying the membrane-disruptive effects of fatty acid and monoglyceride mitigants with this bacterial lipid-derived EIS measurement approach can provide predictive insight into the antibacterial activity and potency of different test compounds to inhibit bacteria.
4. Conclusions
In this study, we have investigated the membrane-disruptive effects of various medium-chain fatty acid and monoglyceride mitigants using E. coli tBLM platforms. The label-free EIS measurement approach enabled us to determine that all tested mitigants are mainly active above their respective CMC values while key differences in the extent of membrane disruption were identified. CA and MC with 10-carbon long hydrocarbon chains caused appreciably larger changes in ionic permeability than LA and GML with 12-carbon long hydrocarbon chains, and also caused more extensive structural damage to the tethered lipid bilayers, as indicated by the time-resolved tracking of the tBLM platform’s electrical conductance and capacitance properties. Since changes in ionic permeability and membrane integrity directly contribute to the antibacterial activity of mitigants in this class, the EIS readouts provided mechanistic insight into the potential utility of these mitigants for disrupting E. coli membranes. This capability is an important advantage of the EIS approach compared to other measurement options like the quartz crystal microbalance-dissipation (QCM-D) technique, which is sensitive to changes in biomacromolecular mass and hydrodynamically coupled solvent mass due to three-dimensional membrane remodeling processes when these mitigants interact with supported lipid bilayers, for example, but QCM-D does not directly probe permeability changes or membrane integrity.
Notably, both CA and MC are known to inhibit
E. coli, whereas GML and LA are inactive against
E. coli, and the EIS results obtained in this study agree well with those previously reported antibacterial activities, because CA and MC caused greater disruption of
E. coli membranes, whereas LA and GML were appreciably less disruptive. These findings establish that the EIS technique is sensitive not only for the detection of the concentration-dependent onset of membrane disruption by a particular compound but also for the evaluation of the relative magnitudes of membrane disruption comparatively across a panel of compounds. The latter insights are particularly valuable because they support that the degree of membrane permeability change caused by an interacting amphiphilic molecule is an important factor rather than merely whether permeabilization occurs. While additional factors like the complex architectural properties of bacterial cell walls (e.g., peptidoglycan layer) might also influence the degree of antibacterial activity, our findings suggest that direct testing of antimicrobial mitigants with reconstituted bacterial lipid membranes is an advantageous measurement option for the real-time tracking of biologically relevant, membrane permeability changes. As lipid extracts from different bacterial species become available, it will be useful to further test the membrane-disruptive properties of antimicrobial drug and mitigant candidates against various types of bacteria, especially within a broader framework of correlating biophysical insights with microbiological evaluation and to design tailored mitigant formulations with enhanced activities for targeted applications (e.g., for food safety or cellular agriculture). Building on these capabilities, in the future, we may further explore how other electrochemical biosensing techniques based on voltametric methods like ramped/pulsed amperometry and cyclic voltammetry can be integrated with bacterial lipid-derived tBLM platforms to study fundamental mechanistic aspects of membrane disruption, including how electroporation-related membrane defects might modulate membrane-disruptive behaviors [
37].