Thermal Rearrangement of Thiocarbonyl-Stabilised Triphenylphosphonium Ylides Leading to (Z)-1-Diphenylphosphino-2-(phenylsulfenyl)alkenes and Their Coordination Chemistry
Abstract
:1. Introduction
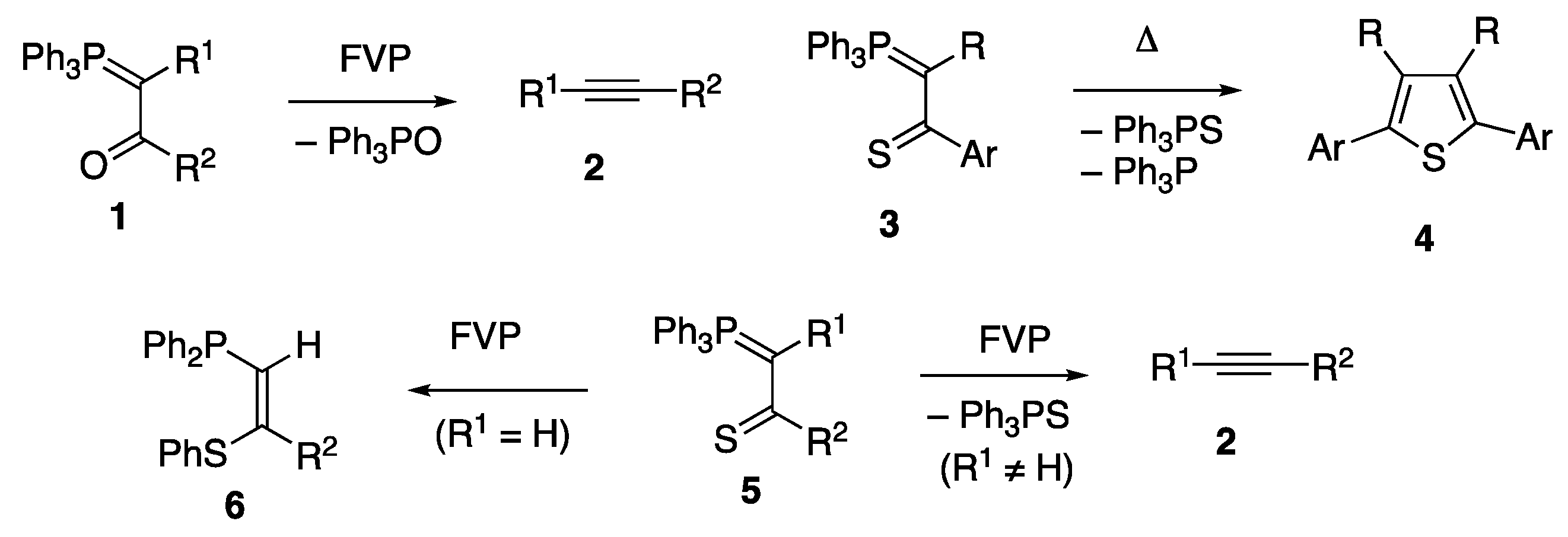
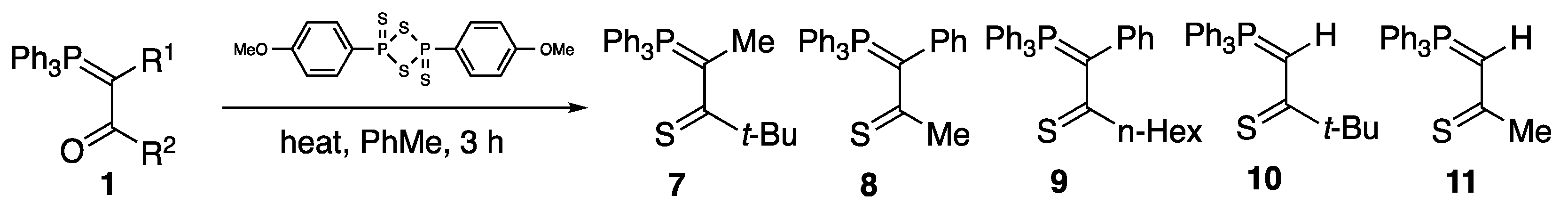
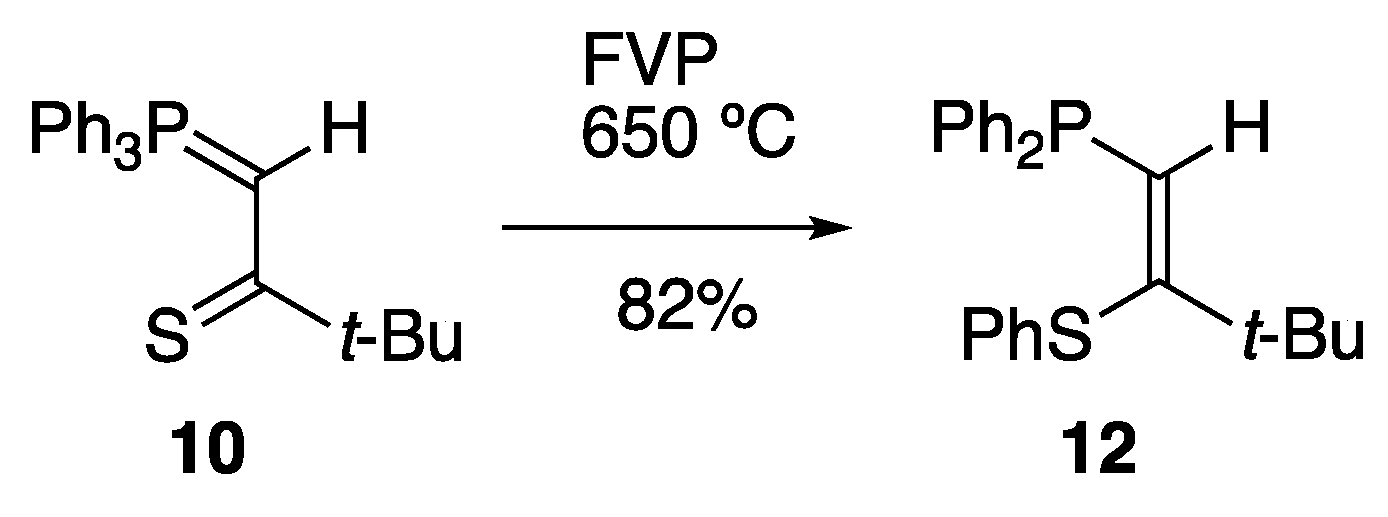
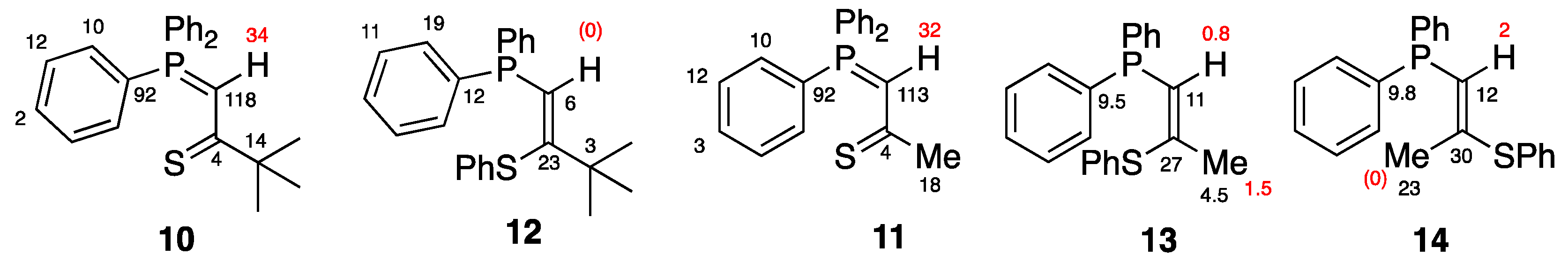
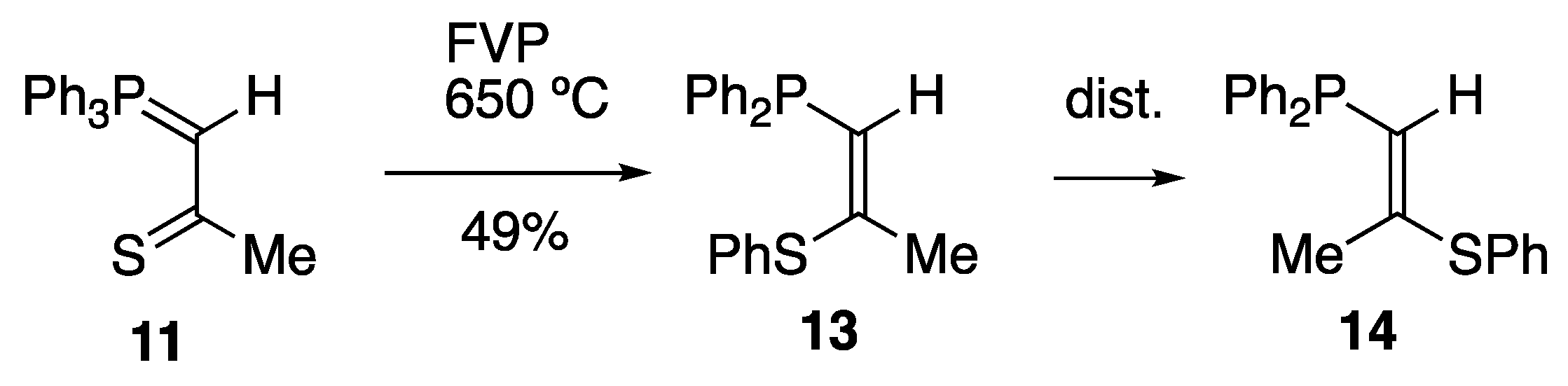
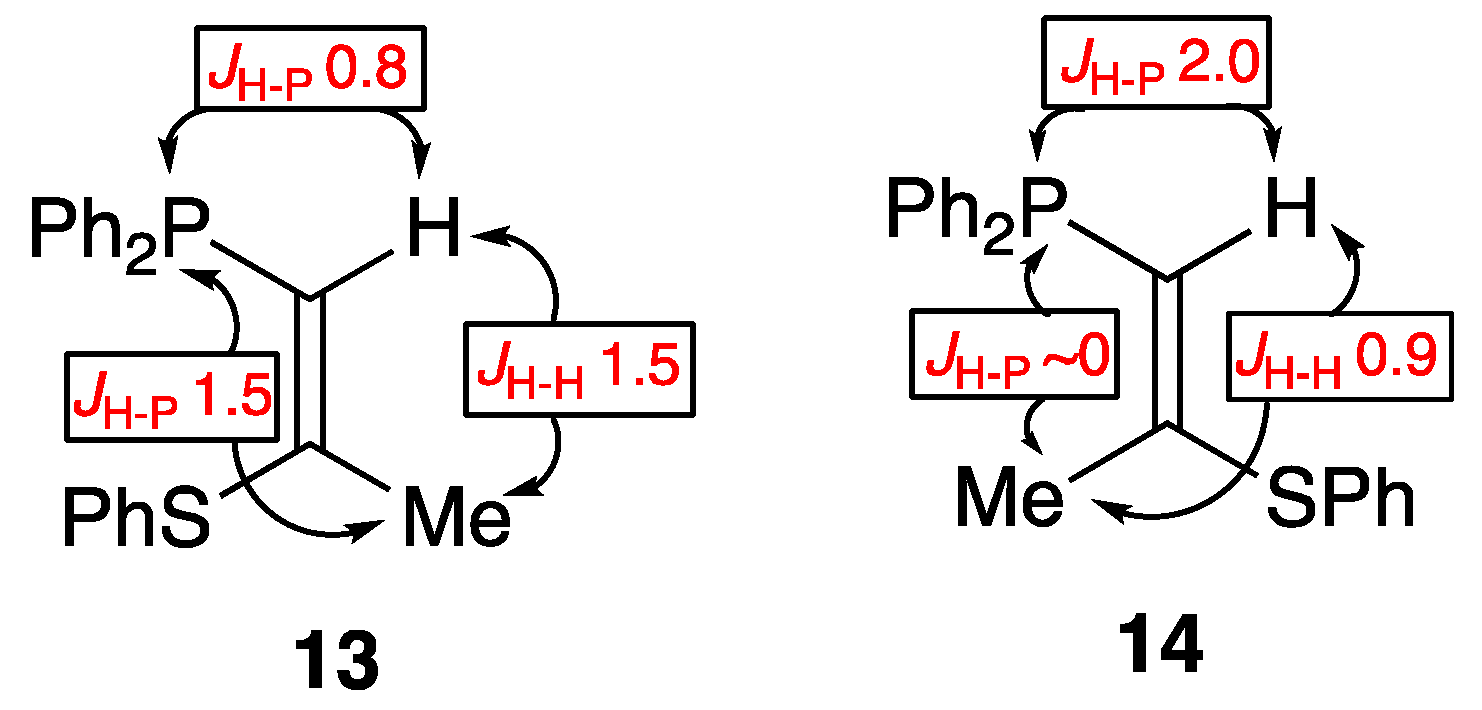
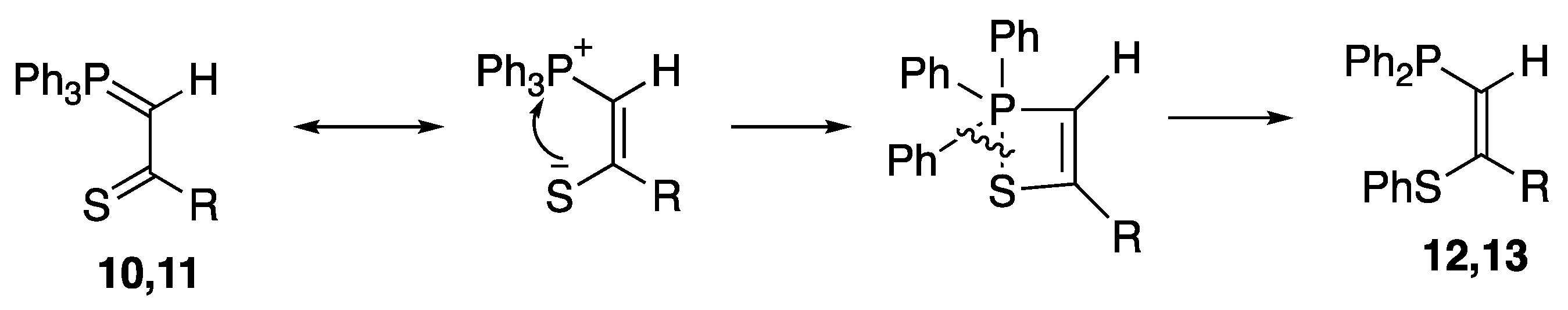
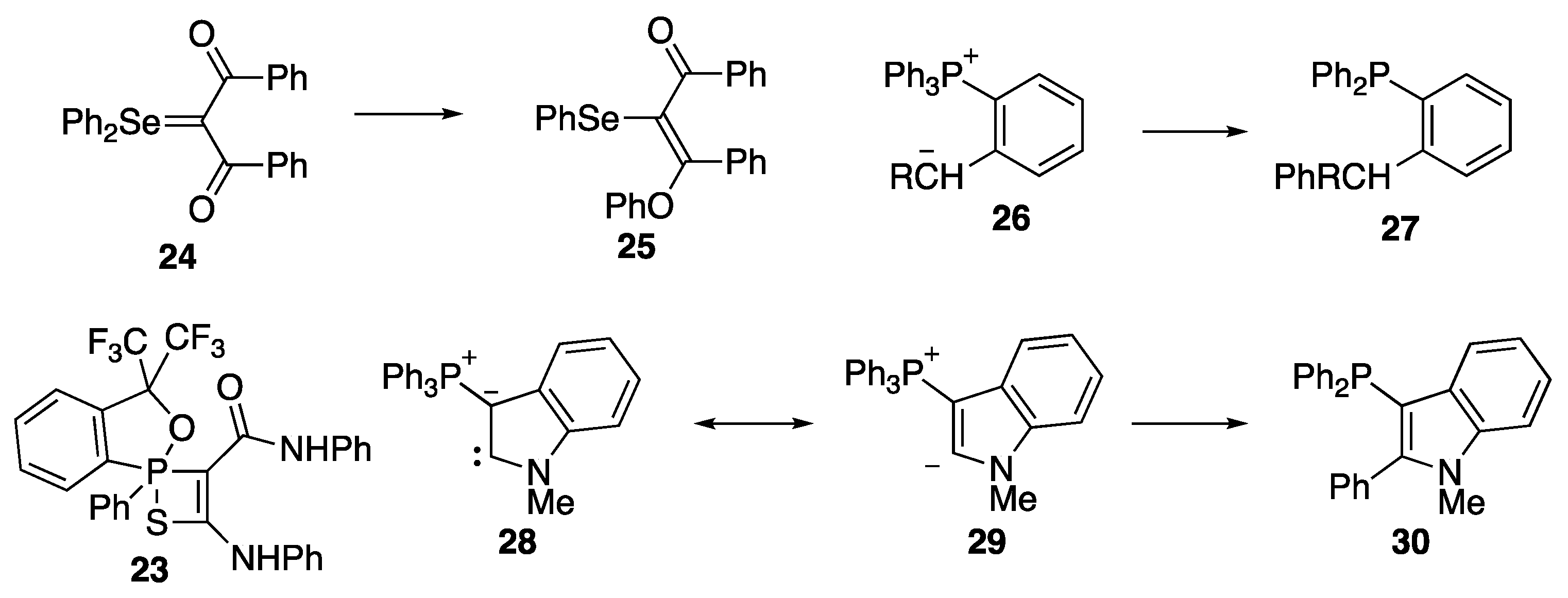
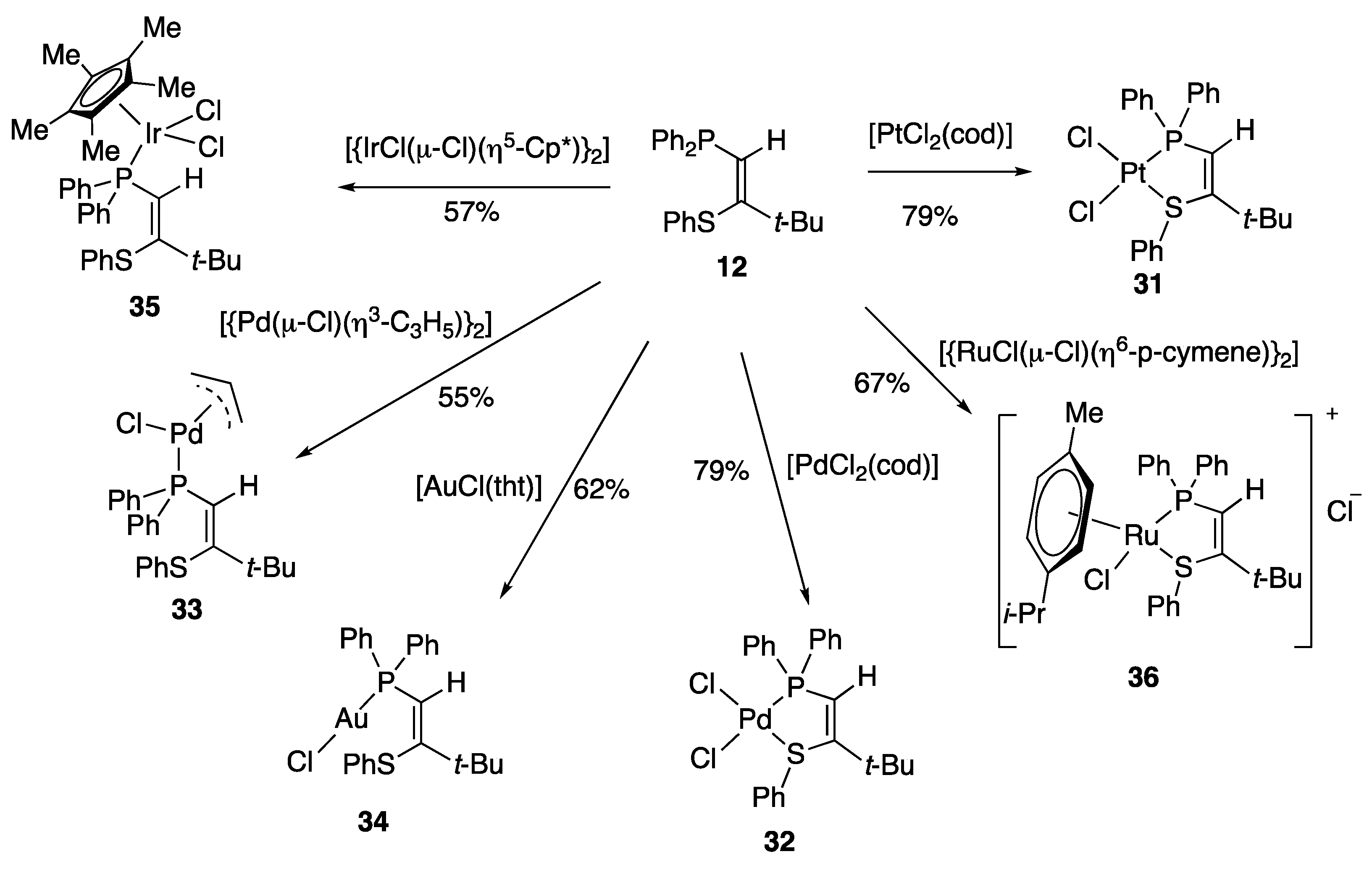
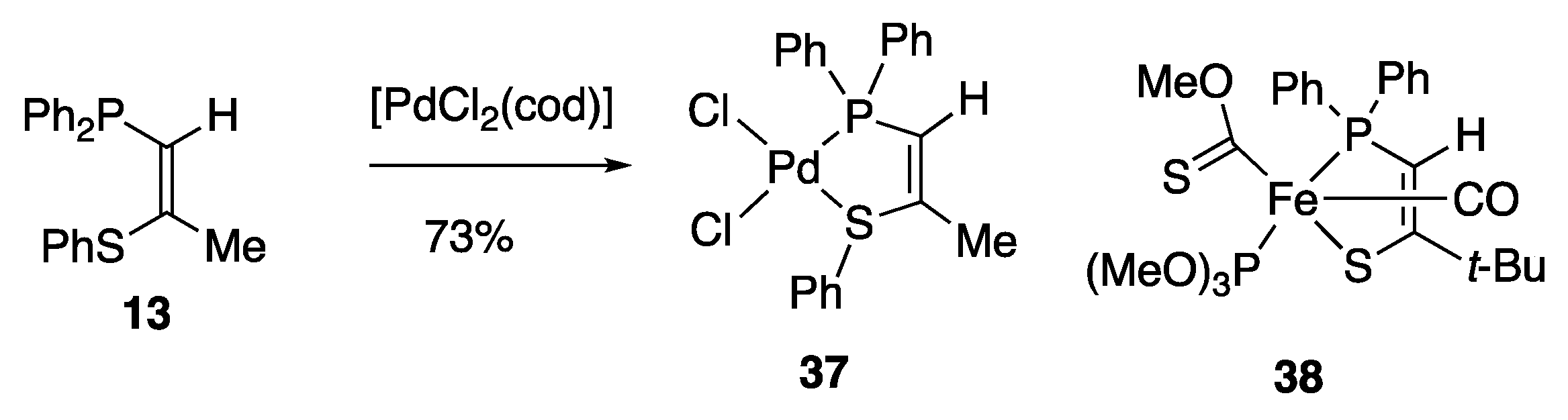
2. Results and Discussion
3. Experimental Section
3.1. General Experimental Details
3.2. Preparation of Thiocarbonyl Ylides
3.2.1. Preparation of Thiopivaloylmethylenetriphenylphosphorane 10
3.2.2. Preparation of Thioacetylmethylenetriphenylphosphorane 11
3.3. Thermal Rearrangement of Thiocarbonyl Ylides
3.3.1. Preparation of (Z)-1-Diphenylphosphino-3,3-dimethyl-2-phenylthiobut-1-ene 12
3.3.2. Preparation of (Z)-1-Diphenylphosphino-2-phenylthiopropene 13
3.4. Formation of Transition Metal Complexes
3.4.1. (Z)-1-Diphenylphosphino-3,3-dimethyl-2-phenylthiobut-1-ene Platinum Dichloride Complex 31
3.4.2. (Z)-1-Diphenylphosphino-3,3-dimethyl-2-phenylthiobut-1-ene Palladium Dichloride Complex 32
3.4.3. (Z)-1-Diphenylphosphino-3,3-dimethyl-2-phenylthiobut-1-ene η3-allyl Palladium Chloride Complex 33
3.4.4. (Z)-1-diphenylphosphino-3,3-dimethyl-2-phenylthiobut-1-ene Gold Chloride Complex 34
3.4.5. (Z)-1-Diphenylphosphino-3,3-dimethyl-2-phenylthiobut-1-ene Pentamethylcyclopentadienyl Iridium Dichloride Complex 35
3.4.6. (Z)-1-Diphenylphosphino-3,3-dimethyl-2-phenylthiobut-1-ene p-Cymene Ruthenium Dichloride Complex 36
3.4.7. (Z)-1-Diphenylphosphino-2-phenylthiopropene Palladium Dichloride Complex 37
3.5. X-ray Structure Determination of Complexes
3.5.1. (Z)-1-Diphenylphosphino-3,3-dimethyl-2-phenylthiobut-1-ene Gold Chloride Complex 34
3.5.2. (Z)-1-Diphenylphosphino-3,3-dimethyl-2-phenylthiobut-1-ene Pentamethylcyclopentadienyl Iridium Dichloride Complex 35
3.5.3. (Z)-1-Diphenylphosphino-2-phenylthiopropene Palladium Dichloride Complex 37
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aitken, R.A.; Atherton, J.I. A new general synthesis of aliphatic and terminal alkynes: Flash vacuum pyrolysis of β-oxoalkylidenetriphenylphosphoranes. J. Chem. Soc. Chem. Commun. 1985, 1140–1141. [Google Scholar] [CrossRef]
- Aitken, R.A.; Thomas, A.W. Pyrolysis involving compounds with C=C, C=O and C=N double bonds. In Chemistry of the Functional Groups; Patai, S., Ed.; Wiley: Chichester, UK, 1997; Suppl. A3; pp. 473–536. [Google Scholar] [CrossRef]
- Eymery, F.; Iorga, B.; Savignac, P. The usefulness of phosphorus compounds in alkyne synthesis. Synthesis 2000, 185–213. [Google Scholar] [CrossRef]
- Aitken, R.A.; Boubalouta, Y.; Chang, D.; Cleghorn, L.P.; Gray, I.P.; Karodia, N.; Reid, E.J.; Slawin, A.M.Z. The Value of 2JP–CO as a Diagnostic Parameter for the Structure and Thermal Reactivity of Carbonyl-Stabilised Phosphonium Ylides. Tetrahedron 2017, 73, 6275–6285. [Google Scholar] [CrossRef]
- Bestmann, H.J.; Schaper, W. Reaktionen von Thioacylalkylidentriphenylphosphoranen-eine neue Thiophensynthese. Tetrahedron Lett. 1979, 20, 243–244. [Google Scholar] [CrossRef]
- Aitken, R.A.; Dawson, G.; Keddie, N.S.; Kraus, H.; Slawin, A.M.Z.; Wheatley, J.; Woollins, J.D. Thermal rearrangement of thiocarbonyl-stabilised triphenylphosphonium ylides leading to (Z)-1-diphenylphosphino-2-phenylsulfenylalkenes. Chem. Commun. 2009, 7381–7383. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Matsuura, H.; Ogata, T.; Inokawa, S. α-Thiocarbonyl-stabilized phosphonium ylides: Preparation, structure, and alkylation reactions. Bull. Chem. Soc. Jpn. 1975, 48, 2907–2910. [Google Scholar] [CrossRef]
- Bestmann, H.J.; Pohlschmidt, A.; Kumar, K. Eine Methode zur Überführung von Acylalkylidentriphenylphosphoranen in Thioacylalkylidentriphenylphosphorane. Tetrahedron Lett. 1992, 33, 5955–5958. [Google Scholar] [CrossRef]
- Pasenok, S.; Appel, W. Process for the Preparation of Novel Stabilised Phosphorus Ylides. European Patent 741138 A2, 6 November 1996. [Google Scholar]
- Capuano, L.; Drescher, S.; Huch, V. Neue Synthesen mit 1,3-ambident-nucleophilen Phosphor-Yliden, VII. Heterocyclische Triphenylphosphonium-chloride, Triphenylphosphonio-olate, acyclische Triphenylphosphonio-thiolate und ihre Wittig-Derivate. Liebigs Ann. Chem. 1993, 1993, 125–129. [Google Scholar] [CrossRef]
- Aitken, R.A.; Atherton, J.I. Flash vacuum pyrolysis of stabilised phosphorus ylides. Part 1. Preparation of aliphatic and terminal alkynes. J. Chem. Soc. Perkin Trans. 1 1994, 1281–1284. [Google Scholar] [CrossRef]
- Aitken, R.A.; Al-Awadi, N.A.; Dawson, G.; El-Dusouqi, O.M.E.; Farrell, D.M.M.; Kaul, K.; Kumar, A. Synthesis, thermal reactivity and kinetics of substituted [(benzoyl)(phenylcarbamoyl)methylene]triphenylphosphoranes and their thiocarbamoyl analogue. Tetrahedron 2005, 61, 129–135. [Google Scholar] [CrossRef]
- Aitken, R.A.; Al-Awadi, N.A.; El-Dusouqi, O.M.E.; Farrell, D.M.M.; Kumar, A. Synthesis, thermal reactivity and kinetics of stabilized phosphorus ylides, part 2: [(arylcarbamoyl)(cyano)methylene]triphenylphosphoranes and their thiocarbamoyl analogues. Int. J. Chem. Kinet. 2006, 38, 496–502. [Google Scholar] [CrossRef]
- Aitken, R.A.; Al-Awadi, N.A.; Dawson, G.; El-Dusouqi, O.M.E.; Kaul, K.; Kumar, A. Kinetic and mechanistic study on the thermal reactivity of stabilized phosphorus ylides, part 3: [(acetyl)(arylcarbamoyl)methylene]triphenylphosphoranes and [(alkoxycarbonyl)(arylcarbamoyl)methylene]triphenylphosphoranes and their thiocarbamoyl analogues. Int. J. Chem. Kinet. 2007, 39, 6–16. [Google Scholar] [CrossRef]
- Hickson, C.L.; McNab, H. E–Z Isomerization of alkenes by flash vacuum pyrolysis. J. Chem. Res. (S) 1989, 176–177. [Google Scholar]
- Seredkina, S.G.; Kolbina, V.E.; Rozinov, V.G.; Mirskova, A.N.; Donskikh, V.I.; Voronkov, M.G. Phosphorylation of bis(organothio)acetylenes with phosphorus pentachloride. J. Gen. Chem. USSR 1982, 52, 2375–2379, Zh. Obshch. Khim.1982, 52, 2694–2698. [Google Scholar]
- Sinyashin, O.G.; Zubanov, V.A.; Musin, R.Z.; Batyeva, E.S.; Pudovik, A.N. Reaction of thioesters of trivalent phosphorus acids with dichloroacetylene. J. Gen. Chem. USSR 1989, 59, 454–458, Zh. Obshch. Khim.1989, 59, 512–516. [Google Scholar]
- Seredkina, S.G.; Mirskova, A.N.; Bannikova, O.B.; Dolgushin, G.V. Phosphorylation of organylthiochloroacetylenes by phosphorus pentachloride. J. Gen. Chem. USSR 1991, 61, 983–988, Zh. Obshch. Khim.1991, 61, 1084–1090. [Google Scholar]
- Voskuil, W.; Arens, J.F. Chemistry of acetylenic ethers LXII. Tertiary phosphines with an acetylene-phosphorus bond. Recl. Trav. Chim. Pays-Bas 1962, 81, 993–1008. [Google Scholar] [CrossRef]
- Kolomiets, A.F.; Fokin, A.V.; Rudnitskaya, L.S.; Krolevets, A.A. Alkenyldichlorophosphines. Bull. Acad. Sci. USSR Div. Chem. Sci. 1976, 25, 171–173, Izv. Akad. Nauk. SSSR Ser. Khim.1976, 181–183. [Google Scholar] [CrossRef]
- Kolomiets, A.F.; Fokin, A.V.; Krolevets, A.A.; Bronnyi, O.V. Reactions of alkenes with phosphorus pentachloride and trichlorosilane. Bull. Acad. Sci. USSR Div. Chem. Sci. 1976, 25, 200–201, Izv. Akad. Nauk. SSSR, Ser. Khim.1976, 207–209. [Google Scholar] [CrossRef]
- Braga, A.L.; Alves, E.F.; Silveira, C.C.; de Andrade, L.H. Stereoselective addition of sodium organyl chalcogenolates to alkynylphosphonates: Synthesis of diethyl 2-(organyl)-2-(organochalcogenyl)vinylphosphonates. Tetrahedron Lett. 2000, 41, 161–163. [Google Scholar] [CrossRef]
- Braga, A.L.; Vargas, F.; Zeni, G.; Silveira, C.C.; de Andrade, L.H. Synthesis of β-organotelluro vinylphosphine oxides by hydrotelluration of 1-alkynylphosphine oxides and their palladium-catalyzed cross-coupling with alkynes. Tetrahedron Lett. 2002, 43, 4399–4402. [Google Scholar] [CrossRef]
- Kawashima, T.; Iijima, T.; Kikuchi, H.; Okazaki, R. Synthesis of the first stable pentacoordinate 1,2-thiaphosphetene. Phosphorus Sulfur Silicon Relat. Elem. 1999, 144–146, 149–152. [Google Scholar] [CrossRef]
- Magdesieva, N.N.; Kyandzhetsian, R.A.; Rakitin, O.A. Rearrangements of selenonium ylides with two electron withdrawing groups. J. Org. Chem. USSR 1975, 11, 2636–2641, Zh. Org. Khim.1975, 11, 2562–2567. [Google Scholar]
- Zbiral, E. Phosphororganische Verbindungen III. Zum Mechanismus der durch Arine an Alkylenphosphoranen ausgelösten Umlagerung. Tetrahedron Lett. 1964, 5, 3963–3967. [Google Scholar] [CrossRef]
- Nakafuji, S.; Kobayashi, J.; Kawashima, T. Generation and coordinating properties of a carbene bearing a phosphorus ylide: An intensely electron-donating ligand. Angew. Chem. Int. Ed. 2008, 47, 1141–1144. [Google Scholar] [CrossRef] [PubMed]
- Robert, P.; Le Bozec, H.; Dixneuf, P.H.; Hartstock, F.; Taylor, N.J.; Carty, A.J. Chemistry of η2-CS2 complexes. Mononuclear iron compounds containing alkoxythiocarbonyl and chelating Ph2PCH=C(R)S ligands vis coupling of coordinated CS2 and phosphinoacetylenes: X-ray structure of Fe(CO)[P(OMe)3][Ph2PCH=C(t-Bu)S][CS(OMe)]. Organometallics 1982, 1, 1148–1154. [Google Scholar] [CrossRef]
- Uson, R.; Laguna, A.; Laguna, M.; Briggs, D.A.; Murray, H.H.; Fackler, J.P., Jr. (Tetrahydrothiophene)gold(I) or gold(II) complexes. Inorg. Synth. 1989, 26, 85–91. [Google Scholar] [CrossRef]
- Drew, D.; Doyle, J.R.; Shaver, A.G. Cyclic diolefin complexes of platinum and palladium. Inorg. Synth. 1991, 28, 346–349. [Google Scholar] [CrossRef]
- McDermott, J.X.; White, J.F.; Whitesides, G.M. Thermal decomposition of bis(phosphone)platinum(II) metallocycles. J. Am. Chem. Soc. 1976, 60, 6521–6528. [Google Scholar] [CrossRef]
- Bennett, M.A.; Huang, T.-N.; Matheson, T.W.; Smith, A.K.; Ittel, S.; Nickerson, W. (η6-Hexamethylbenzene)ruthenium complexes. Inorg. Synth. 1982, 21, 74–78. [Google Scholar] [CrossRef]
- White, C.; Yates, A.; Maitlis, P.M.; Heinekey, D.M. (η5-Pentamethylcyclopentadienyl)rhodium and -iridium compounds. Inorg. Synth. 1992, 29, 228–234. [Google Scholar] [CrossRef]
- Tatsuno, Y.; Yoshida, T.; Seiotsuka; Al-Salem, N.; Shaw, B.L. (η3-Allyl)Palladium(II) complexes. Inorg. Synth. 1979, 19, 220–223. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELXL. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
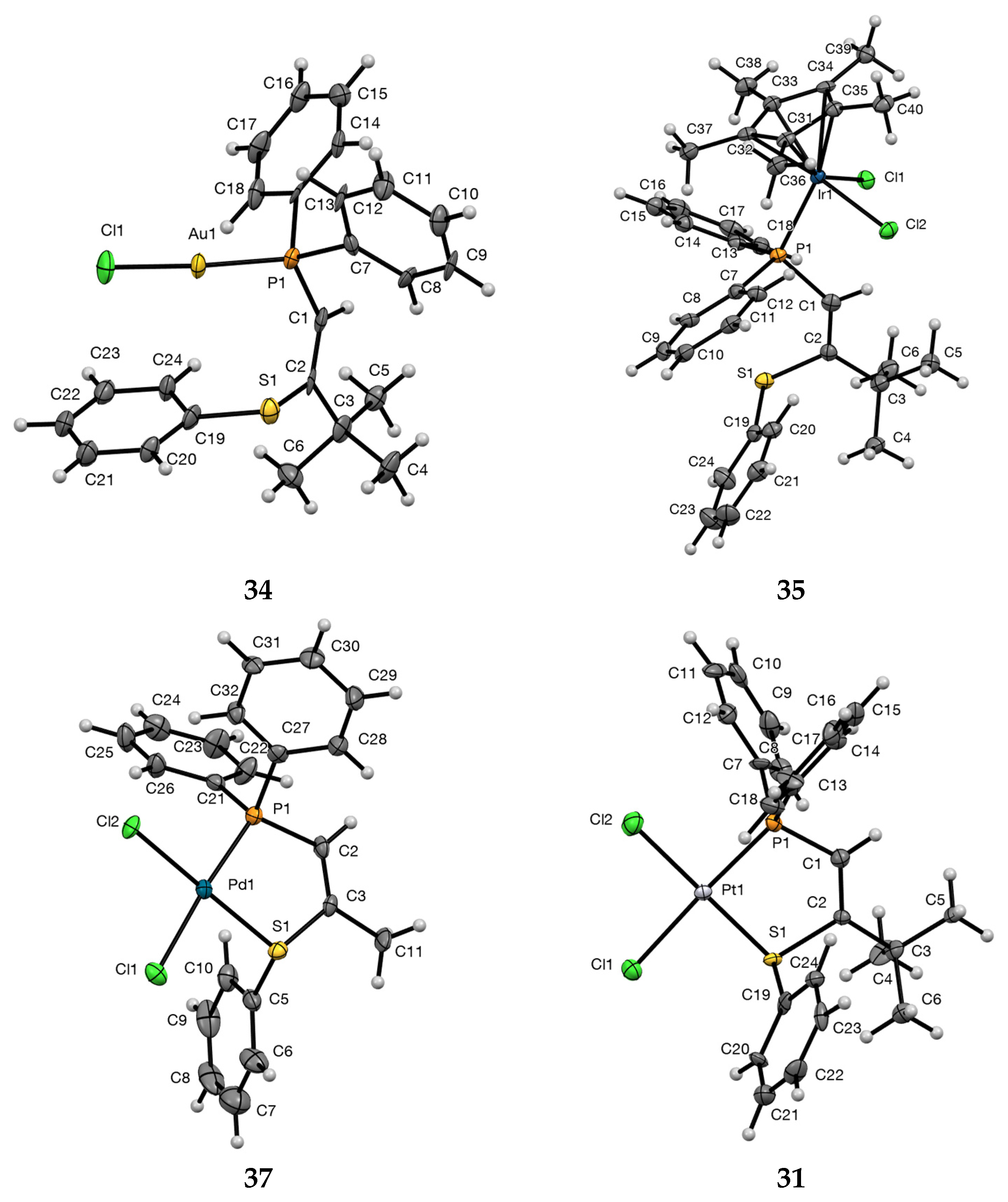

| Bond lengths (Å) | |||||
|---|---|---|---|---|---|
| Compound | P–CH | CH=C | =C–S | P–M | M–S |
| 10 | 1.739(2) | 1.373(3) | 1.708(2) | — | — |
| 12 | 1.818(4) | 1.316(6) | 1.788(4) | — | — |
| 31 | 1.773(14) | 1.34(2) | 1.825(14) | 2.216(6) | 2.259(5) |
| 34 | 1.795(12) | 1.332(17) | 1.782(13) | 2.229(3) | — |
| 35 | 1.824(5) | 1.318(7) | 1.787(5) | 2.3076(12) | — |
| 37 | 1.798(8) | 1.322(11) | 1.796(8) | 2.227(2) | 2.252(2) |
| 38 | 1.770(6) | 1.346(8) | 1.764(5) | 2.256(1) | 2.307(2) |
| Angles (°) | |||||
| Compound | P–C=C | C=C–S | =C–P–M | P–M–S | M–S–C= |
| 10 | 124.05(16) | 122.46(16) | — | — | — |
| 12 | 125.5(3) | 115.1(3) | — | — | — |
| 31 | 121.4(11) | 118.0(10) | 106.6(6) | 88.59(19) | 105.3(5) |
| 34 | 128.7(10) | 118.9(9) | 121.2(4) | — | — |
| 35 | 131.7(4) | 119.8(4) | 109.27(15) | — | — |
| 37 | 119.6(6) | 119.1(6) | 107.2(3) | 87.07(8) | 106.8(3) |
| 38 | 118.3(2) | 120.7(2) | 108.4(1) | 85.1(0) | 106.3(1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aitken, R.A.; Dawson, G.; Keddie, N.S.; Kraus, H.; Milton, H.L.; Slawin, A.M.Z.; Wheatley, J.; Woollins, J.D. Thermal Rearrangement of Thiocarbonyl-Stabilised Triphenylphosphonium Ylides Leading to (Z)-1-Diphenylphosphino-2-(phenylsulfenyl)alkenes and Their Coordination Chemistry. Molecules 2024, 29, 221. https://doi.org/10.3390/molecules29010221
Aitken RA, Dawson G, Keddie NS, Kraus H, Milton HL, Slawin AMZ, Wheatley J, Woollins JD. Thermal Rearrangement of Thiocarbonyl-Stabilised Triphenylphosphonium Ylides Leading to (Z)-1-Diphenylphosphino-2-(phenylsulfenyl)alkenes and Their Coordination Chemistry. Molecules. 2024; 29(1):221. https://doi.org/10.3390/molecules29010221
Chicago/Turabian StyleAitken, R. Alan, Graham Dawson, Neil S. Keddie, Helmut Kraus, Heather L. Milton, Alexandra M. Z. Slawin, Joanne Wheatley, and J. Derek Woollins. 2024. "Thermal Rearrangement of Thiocarbonyl-Stabilised Triphenylphosphonium Ylides Leading to (Z)-1-Diphenylphosphino-2-(phenylsulfenyl)alkenes and Their Coordination Chemistry" Molecules 29, no. 1: 221. https://doi.org/10.3390/molecules29010221
APA StyleAitken, R. A., Dawson, G., Keddie, N. S., Kraus, H., Milton, H. L., Slawin, A. M. Z., Wheatley, J., & Woollins, J. D. (2024). Thermal Rearrangement of Thiocarbonyl-Stabilised Triphenylphosphonium Ylides Leading to (Z)-1-Diphenylphosphino-2-(phenylsulfenyl)alkenes and Their Coordination Chemistry. Molecules, 29(1), 221. https://doi.org/10.3390/molecules29010221








