Abstract
Oxidative stress is caused by an imbalance between reactive oxygen species and antioxidant levels. Current research suggests that oxidative stress is one of the key factors in the development of many chronic diseases, and it has been a concern for many years. Many natural compounds have been studied for their special free-radical-scavenging properties. The major chemical constituents of the leaves of Diospyros kaki are flavonoids and triterpenoids, both of which are potential antioxidants that can prevent damage caused by reactive oxygen species or reactive nitrogen species and ameliorate diseases associated with oxidative stress. In addition to the major constituents such as flavonoids and triterpenoids, the leaves of Diospyros kaki include compounds such as phenylpropanoids, alkaloids, phenolic acids, and terpenes. Studies have shown these compounds have certain antioxidant and neuroprotective activities. Experiments have shown that flavonoids or the extracts from the leaves of Diospyros kaki have a variety of good pharmacological activities, which could activate oxidative stress and mitochondrial apoptosis, inhibit the proliferation of human prostate cancer cells and induce apoptosis. It also could achieve the effect of anti-cancer cell proliferation and induce apoptosis by regulating oxidative stress. The main chemical substance of the leaves of Diospyros kaki regulating oxidative stress may be these multi-hydroxyl structure compounds. These natural products exhibit significant antioxidant activity and are an important basis for the leaves of Diospyros kaki to treat human diseases by regulating oxidative stress. This review summarizes the structural types of natural products in the leaves of Diospyros kaki and elaborates the mechanism of the leaves of Diospyros kaki in neuroprotection, anti-diabetes, renal protection, retinal degenerative diseases, and anti-cancer from a new perspective of oxidative stress, including how it supplements other pharmacological effects. The chemical constituents and pharmacological effects of the leaves of Diospyros kaki are summarized in this paper. The relationship between the chemical components in the leaves of Diospyros kaki and their pharmacological effects is summarized from the perspective of oxidative stress. This review provides a reference for the study of natural anti-oxidative stress drugs.
1. Introduction
Oxidative stress has attracted a lot of attention since it was proposed in 1985. Oxidative stress refers to a state in which there is an imbalance between oxidation and antioxidant activity in the body, with a tendency toward oxidation. Oxygen radicals are an unavoidable byproduct of many biochemical processes, which are intentionally formed in some cases. As in activated neutrophils, they are produced in the body by environmental electromagnetic radiation and directly as oxidizing pollutants such as ozone and nitrogen dioxide [1]. Oxidative stress causes excessive oxygen free radicals to attack biomolecules such as lipids, proteins, and DNA, which can lead to tissue damage [2]. Oxidative stress has been implicated in the pathogenesis of a variety of common diseases, including stroke, hypertension, diabetes, neurodegenerative diseases, and malignancies [3,4,5]. In the study of a variety of diseases, many natural products have been found to be effective in regulating oxidative stress and thus exert anti-cancer properties [6] and the ability to treat intracerebral hemorrhage [7], diabetes, and neurodegenerative diseases due to an excessive inflammatory response [8]. This may be due to the unique free-radical-scavenging effect of plant-derived natural products [9]. Known studies have shown that medicinal plants are an important source of antioxidants that can help fight oxidative stress and modulate various pharmacological processes, including oxidative stress and inflammation [10].
The persimmon (Diospyros kaki L.) is a plant of the genus Diospyros Linn. in the family Ebenaceae, native to the Yangtze River basin in China. Persimmon leaves are dry or fresh leaves of persimmon trees [11]. As a natural product beneficial to human health, persimmon leaves have always played a key role in the long history of human health development. In China, persimmon leaves have long been used as traditional Chinese medicine. Their application was first recorded in the Diannan Bencao of the Ming Dynasty: Treatment of Eczema with frost and leaves [12]. Persimmon leaves were commonly used to treat cough, hemorrhage, hypertension, stroke, and other diseases [13], and persimmon leaf tea was drunk as a natural dietary supplement in Japan, South Korea, China, and other Asian countries [14]. Persimmon leaves are used in many medical and health-related products, such as cosmetics and the clinical medicine Naoxinqing [15,16]. However, as a natural product with abundant resources, persimmon leaves still have unlimited potential for the healthy development of human beings.
Persimmon has a high research value because it contains rich and diverse compounds and other nutrients [17,18]. Persimmon leaves are reported to be rich in flavonoids, terpenes, lignin, coumarins, alkaloids, polysaccharides, and volatile oils [19] but also contain many nutrients such as vitamin C, choline, several amino acids, calcium, iron, and zinc [20]. The abundance of phenolics in persimmon leaf extract gives it excellent antioxidant activity [21,22], which can also be inferred from the antioxidant activity of other natural products related to persimmon leaves (persimmon peel, persimmon) [23,24]. With the development of modern pharmacological research on persimmon leaves, it is much clearer that persimmon leaves have a wide range of pharmacological effects, including anti-cancer, anti-inflammatory, anti-allergic, hypoglycemic, antihypertensive, neuroprotective, cardiovascular protective, etc. [25]. Many of these pharmacological activities depend on the high antioxidant activity of persimmon leaf extracts. The intake of antioxidants from natural products has extraordinary benefits for human health [25,26].
These natural products from persimmon leaves may help regulate oxidative stress, opening up new possibilities for the treatment of a variety of diseases. Therefore, it is necessary to elaborate and summarize the phytochemistry and pharmacological activity of persimmon leaves, the pharmacological activities of persimmon leaf extracts that work through the regulation of oxidative stress mechanisms, and other biological activities and their mechanisms. In this paper, from the perspective of the structure types of natural products and their pharmacological activities, the types of chemical components in persimmon plants are comprehensively introduced, the particularity of the compound structure is discussed, and the pharmacological activities of persimmon leaves in oxidative stress are emphatically discussed. This paper fully summarizes the pharmacological activities of the chemical components of persimmon leaves, updates the knowledge status in this field, establishes a scientific framework, and provides some new ideas for the study of oxidative stress.
2. Phytochemistry of Persimmon Leaves
2.1. Plant Characteristics and Spread
Persimmons are deciduous or evergreen trees or shrubs with greyish-green or yellowish-brown oval or obovate leaves [27]. Persimmon accessions are very rich, with 450 species, which are widely distributed in Asian countries such as China, Japan, and Korea [28]. There are 57 species of persimmon in China, which are mainly distributed from Southwest to Southeast China, with a cultivation history of more than 3000 years [29]. Persimmon is an economic crop with huge product value and has wide commercial and medical values for derivative products such as persimmon leaf, persimmon stem, persimmon fruit, and persimmon cream [30].
2.2. Chemical Composition of Persimmon Leaves
This article summarizes the structures of all flavonoids, terpenes, phenylpropanoids, steroids, alkaloids, and phenolic acids that have been isolated from persimmon leaves. In addition, there are many nutrients found in persimmon leaves, such as fatty acids, polysaccharides, cellulose, etc., which are only briefly discussed here (Figure 1).
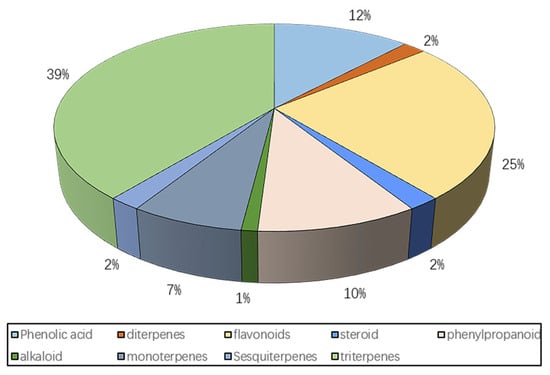
Figure 1.
Chemical composition of persimmon leaves.
2.2.1. Flavonoids
Flavonoids are the main chemical components of persimmon leaves and important active substances. Flavonoids from persimmon leaves possess tyrosinase-inhibitory activity [31]. Persimmon leaf flavonoids (myricetin and its glycosides) inhibit the formation of N-nitrosamines and remove nitrite from the human body [32,33]. These studies have shown that flavonoids in persimmon leaves are important active substances. Flavonoids are one of the major components in persimmon leaves. Sun Huapeng measured the average total flavonoid content of dried persimmon leaves of 15 varieties as 59.77 mL/g [34]. Judging from the process of separating and obtaining flavonoids, persimmon leaves are almost extracted with ethanol, then extracted with ethyl acetate, and flavonoid monomer compounds are obtained by modern separation and enrichment methods. There are 31 flavonoids that have been isolated from persimmon leaves. The flavonoids in persimmon leaves are mainly glycosides or aglycones of quercetin (A), kaempferol (B), myricetin (C), and vitexin (D). Moreover, some flavonoids other than the above four classes were also present (Figure 2, Table 1). Interestingly, the antioxidant activity of galloyl-substituted flavonol glycosides was found to be much higher than that of non-galloyl-substituted flavonoid glycosides [35].
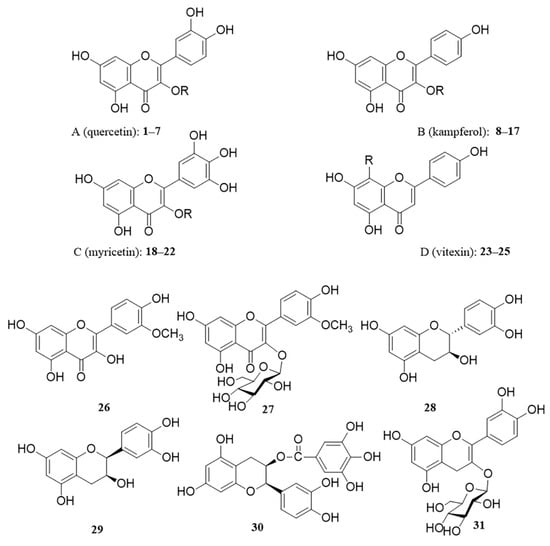
Figure 2.
Structure of flavonoids in persimmon leaves.

Table 1.
Structure names and numbers of flavonoids in persimmon leaves.
2.2.2. Triterpenes
Triterpenes are structurally diverse organic compounds characterized by polycyclic skeletons modified in various ways. Saponins are triterpenes combined with natural sugars. These natural products are of great importance in chronic diseases associated with oxidative stress, such as diabetes and neurodegenerative diseases, as well as in anti-inflammatory, hepatoprotective, antibacterial, antiviral, immunosuppressive, and other aspects [43,44]. It is worth noting that most of the triterpenoids in persimmon leaves are distributed in the ethyl acetate extraction layer of the ethanol extract. At present, 32 ursane-type triterpenes have been isolated from persimmon leaves—21 compounds with feature A and 11 other ursane-type triterpenes. It is interesting to note that many triterpenes isolated from persimmon leaves have the structure of E-ring cracking at positions 18 and 19, which are different from common triterpene skeletons (Figure 3, Table 2). These 18 and 19 secoursane triterpenoids are also characteristic compounds in persimmon leaves. There are other types of triterpenes in persimmon leaves, including oleanane and lupinane (Figure 4, Table 3).
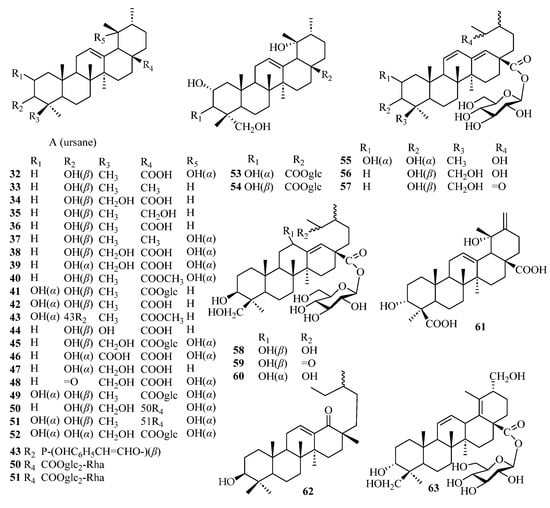
Figure 3.
Structures of ursane-type triterpenes.

Table 2.
Structure names and numbers of ursane-type triterpenes in persimmon leaves.
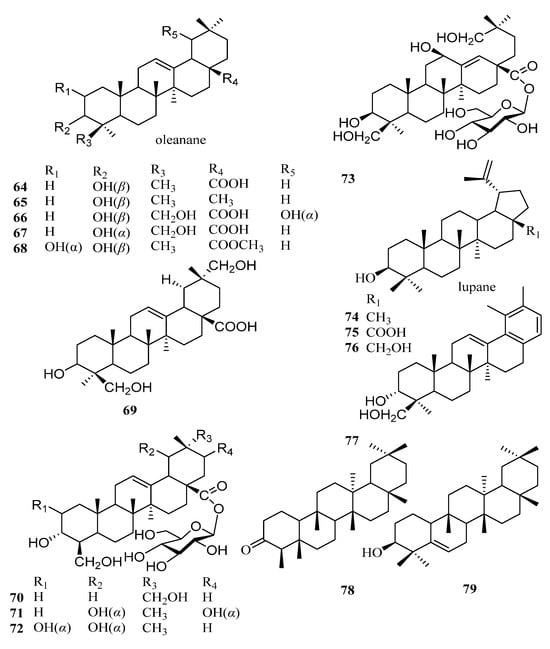
Figure 4.
Structures of other types of triterpenoids in persimmon leaves.

Table 3.
Names and numbers of other types of triterpenoid structures in persimmon leaves.
2.2.3. Other Natural Products in Persimmon Leaves
In addition to the above triterpenoid structure, there are also some monoterpenes, sesquiterpenes and diterpenoids in persimmon leaves (Figure 5, Table 4). Phenylpropanoids (94–106), steroids (107, 108), alkaloids (109), and a large number of phenolic acids (110–124) are also found in persimmon leaves (Figure 6, Table 5) (Figure 7, Table 6). These natural products show certain antioxidant and neuroprotective activities, and more phenolic hydroxyl groups will improve their antioxidant capacity [55,56]. In particular, studies have shown that vomifoliol 9-O-α-arabinofuranosyl (1→6)-β-d-glucopyranoside (84) isolated from persimmon leaves can inhibit α-glucosidase activity and has some therapeutic significance in type 2 diabetes [57]. Polysaccharides in persimmon leaves are also important nutrients that play a significant role in anti-cancer, anti-osteoporosis, and immune regulation [58,59,60].
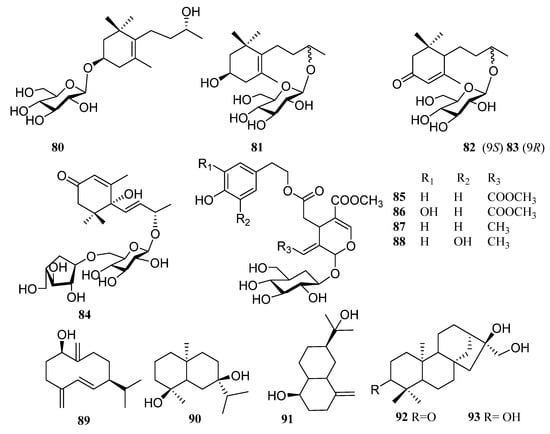
Figure 5.
Structures of monoterpenes, sesquiterpenes, and diterpenes in persimmon leaves.

Table 4.
Structure names and numbers of monoterpenes, sesquiterpenes and diterpenes in persimmon leaves.
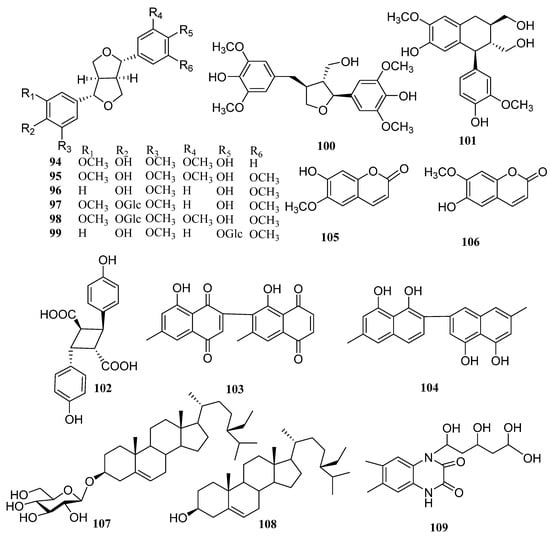
Figure 6.
Structures of phenylpropanoids, steroids and alkaloids in persimmon leaves.

Table 5.
Structure names and numbers of phenylpropanoids, steroids and alkaloids in persimmon leaves.
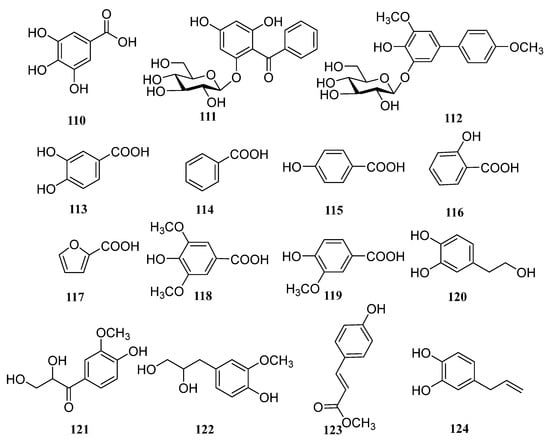
Figure 7.
Phenolic acid structures in persimmon leaves.

Table 6.
Structure names and numbers of phenolic acids in persimmon leaves.
3. Diseases Related to Oxidative Stress
It is well known that the excessive accumulation of reactive oxygen species (ROS) is detrimental to human health when the ROS are produced in excess and the antioxidant system is unable to correct the imbalance between the ROS [66]. ROS include superoxide anions, hydroxyl radicals, and hydrogen peroxide [67]. ROS production is dominated by mitochondrial oxidative phosphorylation and the nicotinamide adenine dinucleotide phosphate oxidase systems [68]. Mitochondria are the main source of ROS in cells. Mitochondrial reactive oxygen species have been implicated in the pathogenesis of many diseases and are involved in important physiological processes such as cell proliferation, differentiation, aging, and apoptosis [69]. The excessive accumulation of ROS leads to oxidative stress, which has been implicated in the pathogenesis of many diseases, including diabetes, cardiovascular diseases, cancer, and neurodegenerative diseases [70,71,72]. The above diseases related to oxidative stress are also the focus of research on persimmon leaves due to their antioxidant activity (Figure 8).
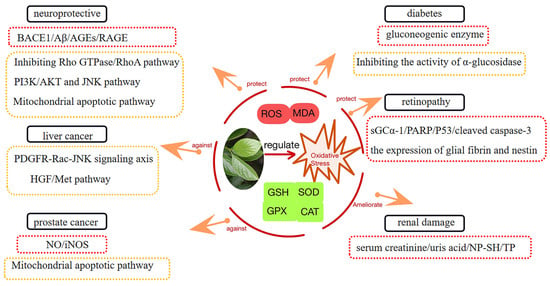
Figure 8.
Persimmon leaf is involved in the regulation of oxidative stress to exert therapeutic effects in some diseases.
In the study of the antioxidant activity of the total flavone extract of persimmon leaves (TFPL), it was found that the TFPL was able to significantly reduce the levels of ROS and malondialdehyde in mouse osteoblast mouse embryonic osteoblasts cells, while the activities of catalase (CAT), superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) were enhanced. The TFPL has better reducing ability and free-radical-scavenging ability and is dose-dependent, even more significantly than rutin [73]. Rats, after gamma irradiation, develop a liver injury and increased levels of oxidative stress and metabolic abnormalities; treatment with persimmon leaf extract (PL) resulted in reduced levels of oxidative stress, indicated not only by decreased malondialdehyde (MDA) levels and xanthine oxidase (XO) activity but also by increased glutathione (GSH) levels and SOD, CAT and xanthine dehydrogenase (XDH) activities. In addition to reducing liver damage, PL (1000mg/kg BW/day) can inhibit glucose concentration, increase insulin levels, improve dyslipidemia, and significantly reduce atherosclerosis indicators compared with the control group (Table 7) [74].

Table 7.
Effect of persimmon leaf extract complex or combination of persimmon leaf and other drugs in treating diseases.
3.1. Diabetes and Its Complications
The water extract of persimmon leaf (PLE) was administered to alloxan-induced hyperglycemic rats for two weeks. The experimental group showed a significant hypoglycemic effect; this was reflected in the decrease in fasting blood glucose (p < 0.01) and the increase in liver glycogen content (p < 0.01) [75]. After the administration of PLE, the serum levels of total cholesterol (TC), triglyceride (TG) and low-density lipoprotein cholesterol (LDL-C) in hyperlipidemia rats decreased, the levels of high-density protein cholesterol (HDL-C) increased, and the activities of SOD, GSH-Px and hepatic lipase (HL) increased significantly, indicating that PLE had a significant effect on lowering blood lipids. This study indicated that the mechanism of blood lipid-lowering by PLE may be related to eliminating oxygen free radicals in the body and improving HL levels [76]. To investigate the underlying mechanisms by which PL ameliorates hyperglycemia, hyperlipidemia, and hepatic steatosis in type 2 diabetes, Un Ju Jung observed that PL ameliorated plasma and hepatic oxidative stress, resulting in reduced hepatic fatty acid oxidation, hyperlipidemia, and hepatic steatosis, after adding PL (5%, w/w) to the normal diet of C57BL/KsJ-db/db mice for 5 consecutive weeks. Gluconeogenic enzyme activity was suppressed in the liver, while glycogen content and glucokinase activity and its mRNA expression levels were increased, which may shed light on the underlying mechanism of PL against hyperglycemia [77]. PLE is also able to exert hypoglycemic effects by inhibiting α-glucosidase activity, increasing antioxidant capacity, and maintaining β-cell function [78].
Renal damage caused by oxidative stress is often associated with the onset of diabetes [79]. The carbon tetrachloride (CCl4)-induced generation of ROS and toxic free radicals causes nephrotoxicity [80]. However, the treatment of Swiss albino rats with CCl4-induced nephrotoxicity treated with PLE resulted in a significant decrease in serum creatinine, MDA, and uric acid levels, but this increased total protein (TP) and nonprotein sulfhydryl (NP-SH) levels, showing renoprotective effects. The most effective natural products include kaempferol, quercetin, astragaloside, and rutin [81]. In treating renal oxidative damage in type 2 diabetic mice, Myung-Sook Choi found that persimmon leaf powder could reduce the levels of oxidative stress markers, improve antioxidant enzyme (SOD, CAT, glutathione peroxidase (GPX)) activities and mRNA expression, alleviate oxidative stress, and thereby improve renal protection [82].
Retinopathy is a microvascular complication of diabetes, and oxidative stress is a key factor closely associated with the disease [5]. The retina is one of the tissues with the highest oxygen consumption [83]. The excessive accumulation of reactive oxygen species leads to retinal damage and even blindness, which is also the reason why retinal damage is often caused by oxidative stress [84]. Supplementation with antioxidants from natural products is important in the fight against retinal degeneration [85]. During the treatment of mice with microbead-induced ocular hypertension, the ethanol extract of persimmon leaves showed activity in treating retinal degenerative diseases by upregulating soluble guanylate cyclase (sGCα-1), reducing retinal ganglion cell loss and optic nerve damage [86]. In the mouse model of N-Methyl-N-nitrosourea-induced retinal degeneration, the retinal thickness of mice increased after treatment with an oral ethanol extract of Diospyros kaki (EEDK); based on the antioxidant properties of EEDK, the expression of endogenous antioxidant enzymes (SOD, GPX) was upregulated, and the expression of glial fibrin and nestin in Müller and astrocyte cells was inhibited, showing a protective effect against oxidative stress-induced cell death. It is worth noting that quercetin played an important role in this process [87]. The treatment of retinal ganglion cells (RGC-5) cells with EEDK significantly increased cell viability and inhibited the upregulation of poly (ADP-ribose) polymerase (PARP), and P53 and cleaved caspase-3 proteins were inhibited, reducing oxidative stress and apoptosis. Moreover, EEDK also has a certain protective effect on retinal degeneration caused by mechanical injury [88].
3.2. Neuroprotective Activity
Flavonoids from the leaves of Diospyros kaki (FLDK-P70) can reduce hypoxia reoxygenation-induced neuronal death and apoptosis in a dose-dependent manner, and the underlying mechanism may be related to the antioxidant activity of the flavone [89]. For H2O2-induced apoptosis-like injury in mouse neuroblastoma–rat glioma hybrid cells (NG108-15 cells), treatment with FLDK-P70 could improve redox imbalance, reduce MDA and ROS levels, and alleviate the damage of oxidative stress to nerve cells by upregulating the expression of B-cell lymphoma-2 (Bcl-2), a suppressor protein of apoptosis [90]. After the oral administration of FLDK to amyloid precursor protein/presenilin1 (APP/PS1) transgenic mice, Amyloid-β peptide (Aβ) production was reduced, the expression of β-site amyloid precursor protein cleavage enzyme 1 (BACE1) was downregulated, antioxidant enzyme activities were increased, and lipid peroxidation products were decreased. MDA and inflammatory mediators suggest that FLDK ameliorates cognitive deficits in mice by regulating oxidative stress and anti-inflammatory activities, as well as by removing Aβ deposits [91]. In addition, FLDK has a synaptic protective function that may be mediated by regulating the synapse-associated protein Rho guanosine triphosphatase (Rho GTPase), thereby inhibiting the expression of the downstream protein Ras homolog gene family member A (RhoA) and improving synaptic dysfunction and reversing memory impairment [92]. D-galactose-induced oxidative stress and neuroinflammation-mediated brain senescence in mice can be inhibited by FLDK, depending on the ability of FLDK to reduce the level of oxidative stress and inhibit the expression of advanced glycation end products (AGEs) and AGEs receptors (RAGE) and D-galactose-induced neuroinflammation. FLDK also ameliorates synapse-associated protein damage by inhibiting the phosphatidylinositol 3-kinase (PI3K/AkT) and C-Jun N-terminal kinase (JNK) apoptotic signaling pathways [92]. In addition to flavonoids, triterpenoids and other compounds in persimmon leaves also show excellent neuroprotective activity [46,55]. Persimmon leaf ethyl acetate extract (EAPL) alleviates the apoptosis of hippocampal neurons by regulating oxidative stress and mitochondrial-mediated apoptosis-associated proteins; this includes a decrease in phospho-C-Jun N-terminal kinases and capase-3 expression and a decrease in the relative ratio of Bcl-2-associated X protein. The natural products analyzed for their effect on Alzheimer’s were mainly flavones and triterpenes [93].
3.3. Anti-Liver Cancer
Studies have shown that different polar parts of persimmon leaves (ethyl acetate part, n-butanol part and water extraction part) all have tumor-inhibitory effects on mice with mouse hepatoma cell (H22) liver cancer [94]. The compounds of persimmon water extract (PWE) can improve the liver dysfunction caused by lipotoxicity, which is linked to PWE’s ability to regulate oxidative stress, improve mitochondrial dysfunction, and reduce phosphatidylcholine (PCs) and lysophosphatidylcholine (lysoPCs) [95]. Flavonoids isolated from persimmon leaves (PLF) have a strong free-radical-scavenging capacity and can increase the ROS levels in cancer cells (HCT116 (colorectal cancer) and HepG2 (liver cancer cells) and promote apoptosis, indicating that PLF’s anti-proliferative and apoptotic effects on cancer cells are related to oxidative stress [96]. Compared with cyclophosphamide, PLF has fewer side effects and shows anti-cachexia activity. PLF can enhance the immunity of mice and inhibit the growth of liver tumors. The inhibition rate was 49.35% [97]. Synergistic effects with significantly higher tumor-inhibition rates were observed when H22 tumor-bearing mice were treated with a combination of cyclophosphamide and persimmon leaf ethyl acetate (PE). PE was able to enhance the antioxidant capacity of H22 tumor-bearing mice bodies so that the SOD level and pro-apoptotic protein Bax expression in tumor tissues were obviously upregulated, while the MDA level and the expression of the inhibitor apoptotic protein Bcl-2 were downregulated [98]. Of course, in addition to regulating oxidative stress, persimmon leaf extract can also act on other signaling pathways to show therapeutic potential against liver cancer. In cancer cells (HepG2 and Human embryonic kidney 293Acells with high basal JNK (C-Jun N-terminal kinase) activity), EEDK leads to JNK-AP-1/p53-mediated cancer cell death by activating the PDGFR-Rac-JNK signaling axis [99]. EEDK can also inhibit hepatocyte growth factor (HGF)-mediated cell migration and invasion, weaken HGF-mediated JNK/C-Jun activation, and reduce HGF receptor Met activity, suggesting that EEDK may treat hepatocellular carcinoma by inhibiting the HGF/Mesenchymal-epithelial transition factor signaling pathway [100].
3.4. Prostate Cancer
The total flavonoids extracted from persimmon leaves (FPL) could inhibit the proliferation, migration and induce the apoptosis of human prostate cancer cells (PC-3). By detecting the activities of ROS, MDA, nitrite and inducible nitric oxide synthase (iNOS), it was found that FPL could activate oxidative stress and change mitochondrial membrane permeability, thereby inhibiting cell proliferation, migration and inducing apoptosis and have a certain therapeutic effect on prostate cancer [101]. The anti-prostate cancer activity of flavonoids in persimmon leaves has been supported by few studies [102]. Relevant studies have shown that some flavonoid derivatives from persimmon have significant anti-prostate cancer activities [103,104]. Fisetin has good anti-prostate cancer activity. The treatment of LNCaP (human prostate cancer cells) with fisetin found that fisetin can inhibit LNCaP cells by arresting the cell cycle in the G1 phase, regulating the CKI-cyclin-cdk network, and inducing apoptosis [105]. There are also research findings that fisetin inhibits the Akt signaling pathway, leading to a decrease in the expression of PI3-K (Phosphatidylinositol 3-kinase protein) and the phosphorylation of Thr308 and Ser473 sites. It also inhibits the growth of prostate cancer cells, promotes apoptosis, inhibits the PI3-K/Akt and JNK signaling pathways, reduces the expression of matrix metalloproteinases (−2 and −9), and inhibits the metastatic ability of PC-3 [106]. In addition to diosquinone, a naphthoquinone epoxide previously isolated from the root bark of Diospyros mespiliformis (Hostch) and D. tricolor [Ebenaceae] has shown anti-prostate cancer activity [107].
3.5. Cardio Cerebral Vascular and Myocardial Protection
Many studies have shown that oxidative stress is a key factor in the pathogenesis and subsequent evolution of many diseases. Enhanced oxidative stress on cellular components and alterations in the molecular pathways that support the pathophysiology of cardiovascular disorders are caused by abnormal free radical production. Ang-II promotes the generation of ROS, which leads to the activation of a variety of signaling kinases, which are mostly regulated by ROS. The enhanced expression of procollagen I and III is apparent at the molecular level, as well as significant contractile dysfunction, both of which are closely correlated with increased NADPH-oxidase activation. ROS may damage myofibrillar proteins, causing contractile dysfunction in HF. Changes in ROS levels can also influence the functionality of ion channels and transporters, including calcium channels. P66Shc suppresses the fork head box O (FOXO) transcription factors in the nucleus, resulting in a reduction in the expression of ROS-scavenging enzymes [108]. The prevalence and incidence of cardiovascular disease are currently increasing, so the correlations between oxidative stress and cardiovascular disease had been intensively studied. Persimmon leaves proved to have good activities in cardiovascular diseases, so the mechanism of persimmon leaves in cardio cerebral vascular has been studied.
Ri Ryu found that ethanolic extracts of persimmon (EPL) leaf could prevent and improve thrombosis by inhibiting coagulation and the production of serotonin, thromboxane A2, and soluble P-selectin [42]. Likewise, a mixture of ethanolic extracts of persimmon leaves and Citrus junos Sieb (CJS) can significantly improve coagulation parameters and lipid metabolism disorders in C57BL/6J mice [109]. The regulation of lipid parameters is of great significance for the prevention and treatment of atherosclerosis, but at the same dose, the ability of the phospholipid complexes of total flavonoids from persimmon leaves (PLF-PC) to regulate the levels of total cholesterol (TC), triglycerides (TG), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein-cholesterol (HDL-C), APOB/APOA1 (apolipoprotein A1, apolipoprotein B) in serum is better than that of PLF, because of the higher bioavailability of PLF-PC [110]. In addition, proanthocyanidins in persimmon leaves can dilate blood vessels through the endothelium-dependent nitric oxide/cGMP pathway and exert antihypertensive effects [111]. EPL shows cardioprotective effects in a rat model of acute myocardial ischemia [112]. Ouyang Ping found that persimmon leaf flavonoids can significantly inhibit the apoptosis of neonatal rat cardiomyocytes, which were induced by hypoxia and reoxygenation and advanced glycation end products [113]. In vitro and in vivo studies have shown that persimmon leaf flavonoids can improve cerebral ischemia tolerance and alleviate cerebral ischemia/reperfusion injury in mice [114].
Based on the above description, we also summarized the mechanism of other biological activities of persimmon leaves (Figure 9)
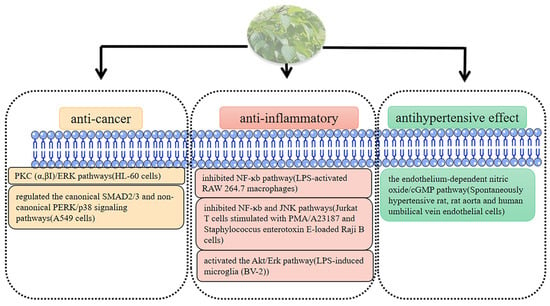
Figure 9.
Mechanism of other biological activities in persimmon leave.
4. Other Human Diseases
4.1. Anti-Lung Cancer
PLF can enhance the cytotoxicity of heavy ion irradiation on lung adenocarcinoma (A549) cells and reduce the phosphorylation of the ataxia telangiectasia-mutated (ATM)-dependent pathway checkpoints during DNA damage, and combination therapy can also reduce tumor volume [115]. Kayoko KAWAKAMI. found that flavonols with the 2″-galloly moiety of PLE can enhance the cytotoxicity of doxorubicin (DOX) to A549 cells and inhibit the phosphorylation of the ATM pathway and protein phosphorylation of related checkpoints in a dose-dependent manner. More significant, however, is that G2/M checkpoints are canceled; the results show that the effect may be related to the presence of gallic acid flavonoid glycosides [116]. Persimmon leaf polysaccharides regulate the canonical Recombinant Mothers Against Decapentaplegic Homolog2/3 and non-canonical phosphorylated extracellular-signal-regulated kinase/p38 signaling pathways and regulate the expression of epithelial marker E-cadherin, mesenchymal markers, N-cadherin and vimentin by inhibiting the transforming growth factor-β1(TGF-β1) pathway, thereby inhibiting the EMT (Epithelial-mesenchymal transition) and migration of A549 cells, as well as invasion and anoikis resistance [58].
4.2. Acute Promyelocytic Leukemia
Through different pathways of protein kinase C (α, βI)/ERK, an acetone extract of D. kaki leaves (KV-1) in combination with low-dose 1,25-dihydroxyvitamin D3 [1,25-(OH)2D3] can induce human promyelocytic leukemia (HL-60) cells to differentiate along the monocyte pathway, whereas stimulation with all-trans retinoic acid (ATRA) induces HL-60 cells to differentiate along the granulocyte pathway, significantly increasing the differentiation level of HL-60 cells. KV-1 not only has the potential to work synergistically with 1,25-(OH)2D3 or ATRA in the treatment of human promyelocytic leukemia but can also reduce the side effects of both drugs [117].
4.3. Anti-Inflammatory
Jung Keun Cho studied the anti-inflammatory effect of PLE and found that PLE could attenuate ultraviolet B (UVB)-induced inflammation in HacaT keratinocytes and mice [118]. Oral administration of PLE resulted in reduced contact dermatitis and ear swelling and decreased lymph node weight in phthalic anhydride (PA)-allergic mice [119]. Supplementation of the diet of ulcerative colitis (UC) models with persimmon-derived tannins significantly reduced disease activity and colonic inflammatory responses, owing to their ability to alter microbiota composition (inhibition of Enterobacteriaceae and Enterococcus expansion) and immune responses, which may make them promising drug candidates for the treatment of chronic inflammatory bowel disease (IBD) [120]. Kyoung-Su Kim isolated two triterpenoids, coussaric acid (CA) and betulinic acid (BA), from persimmon leaves, and their studies showed that CA and BA could inhibit the nuclear factor kappa B pathway in the inflammatory mouse leukemia cells of monocyte macrophage macrophages induced by lipopolysaccharide (LPS), thereby reducing the production of pro-inflammatory cytokines and pro-inflammatory mediators, showing anti-inflammatory potential [121]. In a skin allergy and atopic dermatitis (NC/Nga) mouse model, PLE can alleviate the behavioral response of dermatitis mice, increase serum IgE levels, and significantly inhibit the development of a dermatitis response [122]. Similarly, after oral administration of PLE to Def-sensitized (NC/Nga) mice, the expression of T helper 2(Th2) chemokines (chemokine C-C motif chemokine 17, chemokine C-C motif chemokine 22, chemokine C-C motif chemokine 27) in ear tissue was inhibited, and serum IgE levels were reduced [123]. The anti-inflammatory mechanism of PLE was studied by Hyun-Su Lee, and PLE exhibited inhibitory effects on NF-ĸB and JNK pathways, thereby blocking the activation of T cells in ear tissue and lymph nodes at a non-toxic concentration of 50 μm/mL. PLE can effectively reduce the mRNA level of Interleukin-2 in Junkat T cells, in addition to controlling the infiltration of effector cytokines and mast cells produced by activated T cells [124]. Naoxinqing can regulate the expression of inflammatory factors and activate the Akt/Erk pathway to exert anti-inflammatory and anti-apoptotic effects, which play an important role in the treatment of stroke [125].
5. Experimental and Clinical Studies
To evaluate the effect of persimmon leaves, experimental and clinical studies on anti-diabetics, anti-tumor and neuroprotective activity have been carried out, which are summarized in Table 8.

Table 8.
Experimental and clinical studies regarding the use of persimmon leaves.
6. Conclusions
In recent years, many experimental and clinical studies of traditional Chinese medicine have been conducted, indicating that the pharmacodynamic mechanisms of many natural ingredients are related to oxidative stress. The main compounds of persimmon leaves are flavonoids and triterpenoids, which are potential antioxidants with polyhydroxyl structures. In the follow-up research, it is worth studying the pharmacological activity of triterpenoids with more novel structures. These natural products from persimmon leaves may regulate oxidative stress and treat a variety of diseases. This review has shown that persimmon leaves are important source of antioxidants that can help fight oxidative stress and modulate various pharmacological processes. This provides a new direction for the research on drugs for oxidative stress-related diseases.
Author Contributions
Writing—original draft preparation, C.H.; writing—review and editing, Y.Z., X.W., J.X., J.G. and H.P.; C.H. and X.W. contributed equally to this paper. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Natural Science Foundation of Liaoning [2021-MS-214], Scientific Research Fund Project of Education Department of Liaoning Province [LJKZ0917], Natural Science Foundation of Liaoning Province 2022-MS-221.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
Author J.X. was employed by the company Lonch Group Wanrong Pharmaceutical Co. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Betteridge, D.J. What is oxidative stress? Metabolism 2000, 49, 3–8. [Google Scholar] [CrossRef] [PubMed]
- WA, T.Y. What Is Oxidative Stress? Jpn Med. Assoc. 2000, 124, 1549–1553. [Google Scholar]
- Facchinetti, F.; Dawson, V.L.; Dawson, T.M. Free radicals as mediators of neuronal injury. Cell. Mol. Neurobiol. 1998, 18, 667–682. [Google Scholar] [CrossRef] [PubMed]
- Barinaga, M. Stroke-damaged neurons may commit cellular suicide. Science 1998, 281, 1302–1303. [Google Scholar] [CrossRef] [PubMed]
- Kang, Q.Z.; Yang, C.X. Oxidative stress and diabetic retinopathy: Molecular mechanisms, pathogenetic role and therapeutic implications. Redox Biol. 2020, 37, 101799. [Google Scholar] [CrossRef] [PubMed]
- Shiau, J.-P.; Chuang, Y.-T.; Tang, J.-Y.; Yang, K.-H.; Chang, F.-R.; Hou, M.-F.; Yen, C.-Y.; Chang, H.-W. The Impact of Oxidative Stress and AKT Pathway on Cancer Cell Functions and Its Application to Natural Products. Antioxidants 2022, 11, 1845. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Li, R.; Fan, X. Targeting Oxidative Stress in Intracerebral Hemorrhage: Prospects of the Natural Products Approach. Antioxidants 2022, 11, 1811. [Google Scholar] [CrossRef]
- Jia, Z.; Babu, P.V.A.; Chen, W.; Sun, X. Natural Products Targeting on Oxidative Stress and Inflammation: Mechanisms, Therapies, and Safety Assessment. Oxidative Med. Cell. Longev. 2018, 2018, 6576093. [Google Scholar] [CrossRef]
- Dwivedi, S.; Kushalan, S.; Paithankar, J.G.; D’souza, L.C.; Hegde, S.; Sharma, A. Environmental toxicants, oxidative stress and health adversities: Interventions of phytochemicals. J. Pharm. Pharmacol. 2022, 74, 516–536. [Google Scholar] [CrossRef]
- Agbor, G.A.; Dell’Agli, M.; Kuiate, J.-R.; Ojo, O. Editorial: The Role of Medicinal Plants and Natural Products in Modulating Oxidative Stress and Inflammatory Related Disorders. Front. Pharmacol. 2022, 13, 957296. [Google Scholar] [CrossRef]
- Editorial Committee of the State Administration of Traditional Chinese Medicine. Zhonghua Ben Cao; Shanghai Science and Technology Press: Shanghai, China, 1998. [Google Scholar]
- Deng, H.; Wen, Q.W.; Luo, Y.L.; Huang, Y.H.; Huang, R.B. Effect of different solvent extracts of persimmon leaf on antioxidant capacity in diabetic mice. J. Cent. South Univ. Sci. 2012, 37, 469–473. [Google Scholar] [CrossRef]
- Lu, X.; Li, X.L. Chemical constituents and their pharmacological effects in persimmon leaves. Chin. J. Chem. Educ. 2016, 37, 5. [Google Scholar] [CrossRef]
- Tsurunaga, Y.; Takabayashi, Y.; Nishi, M.; Suzuki, Y. Differences in the ascorbic acid, astragalin, and polyphenol contents, and the DPPH radical scavenging activity of 22 commercial persimmon leaf tea products. J. Home Econ. Jpn 2011, 62, 437–444. [Google Scholar] [CrossRef]
- Hanamura, T.; Uchida, E.; Aoki, H. Skin-Lightening Effect of a Polyphenol Extract from Acerola (Malpighia emarginata DC.) Fruit on UV-Induced Pigmentation. Biosci. Biotechnol. Biochem. 2008, 72, 3211–3218. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.B.; Lin, J.; Liu, M.; Wang, D.Q.; Li, C.Y. Chemical composition, pharmacological effects and clinical application research progress of Naoxinqing tablets. Cent. South Pharm. 2021, 19, 137–139. [Google Scholar]
- Kul, R. Integrated application of plant growth promoting rhizobacteria and biochar improves salt tolerance in eggplant seedlings. Turk. J. Agric. For. 2022, 46, 677–702. [Google Scholar] [CrossRef]
- Saleem, M.H.; Afzal, J.; Rizwan, M.; Shah, Z.-U.-H.; Depar, N.; Usman, K. Chromium toxicity in plants: Consequences on growth, chromosomal behavior andmineral nutrient status. Turk. J. Agric. For. 2022, 46, 371–389. [Google Scholar] [CrossRef]
- Lin, J.F.; Lin, H.T.; Xie, L.H.; Lin, Q.Y.; Chen, S.J.; Zhao, Y.F. Chemical composition, pharmacological effects, clinical application and development and utilization of persimmon leaf. Food Ferment. Ind. 2005, 31, 7. [Google Scholar] [CrossRef]
- Clark, C.J.; Smith, G.S. Seasonal changes in the mineral nutrient content of persimmon leaves. Sci. Hortic. 1990, 42, 85–97. [Google Scholar] [CrossRef]
- An, B.J.; Kwak, J.H.; Park, J.M.; Lee, J.Y.; Park, T.S.; Lee, J.T.; Son, J.H.; Jo, C.; Byun, M.W. Inhibition of enzyme activities and the antiwrinkle effect of polyphenol isolated from the persimmon leaf (Diospyros kaki folium) on human skin. Dermatol. Surg. 2005, 31, 848–854; discussion 854. [Google Scholar] [CrossRef]
- Nisar, M.; Shah, S.M.M.; Khan, I.; Sheema; Sadiq, A.; Khan, S.; Shah, S.M.H. Larvicidal, insecticidal, brine shrimp cytotoxicity and anti-oxidant activities of Diospyros kaki (L.) reported from Pakistan. Pak. J. Pharm. Sci. 2015, 28, 1239–1243. [Google Scholar] [PubMed]
- Fukai, S.; Tanimoto, S.; Maeda, A.; Fukuda, H.; Okada, Y.; Nomura, M. Pharmacological Activity of Compounds Extracted from Persimmon Peel (Diospyros kaki THUNB.). J. Oleo Sci. 2009, 58, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, Y.; Ito, T.; Yano, H.; Kita, E.; Mikasa, K.; Okada, M.; Furutani, A.; Murono, Y.; Shibata, M.; Nishii, Y.; et al. Antioxidant potential in non-extractable fractions of dried persimmon (Diospyros kaki Thunb.). Food Chem. 2016, 202, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Xie, Z.; Xu, X.; Yang, D. Persimmon (Diospyros kaki L.) leaves: A review on traditional uses, phytochemistry and pharmacological properties. J. Ethnopharmacol. 2015, 163, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.M.; Tran, B.Q.; Jang, Y.J.; Park, S.H.; Fondrie, W.E.; Chowdhury, K.; Yoon, S.H.; Goodlett, D.R.; Chae, S.W.; Chae, H.J.; et al. Assessment of the Therapeutic Potential of Persimmon Leaf Extract on Prediabetic Subjects. Mol. Cells 2017, 40, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Zhen, H.S.; Zhang, S.P.; Tang, W.H. Experimental study of persimmon leaf discrimination. Chin. Tradit. Herb. Drugs 1998, 9, 627–629. [Google Scholar]
- Mallavadhani, U.V.; Panda, A.K.; Rao, Y.R. Pharmacology and chemotaxonomy of Diospyros. Phytochemistry 1998, 49, 901–951. [Google Scholar] [CrossRef]
- Li, N.; Zhou, Z.H. General situation of pharmaceutical research on persimmon. Chin. J. Ethnomedicine Ethnopharmacy 2015, 24, 40–43. [Google Scholar]
- Yang, H.; Zhao, H.; Liu, Y.H.; Li, Y.M.; Ren, S.F.; Lian, X.F.; Zhang, Z.H. Status of persimmon resource development and utilization. Biot. Resour. 2019, 41, 402–410. [Google Scholar] [CrossRef]
- Xue, Y.L.; Miyakawa, T.; Hayashi, Y.; Okamoto, K.; Hu, F.; Mitani, N.; Furihata, K.; Sawano, Y.; Tanokura, M. Isolation and tyrosinase inhibitory effects of polyphenols from the leaves of persimmon, Diospyros kaki. J. Agric. Food Chem. 2011, 59, 6011–6017. [Google Scholar] [CrossRef]
- Chen, G.; Xue, J.; Feng, X.Z. Inhibitory effect of water extract and its main contents of persimmon leaves on stimulus-induced superoxide generation in human neutrophils. J. Food Biochem. 2009, 33, 113–121. [Google Scholar] [CrossRef]
- Tian, Y.H.; Du, H.Z.; Wang, L.; Li, S.F.; Zhang, L.; Zhang, L.W. Nitrite Scavenging and Inhibition of N-Nitrosamines Formation by Phenolic Extracts From Diospyros lotus L. Leaves and Active Ingredients. Nat. Prod. Commun. 2020, 15. [Google Scholar] [CrossRef]
- Sun, H.P. Optimization of persimmon resources in Hunan Province and research on the isolation and identification of flavonoids from persimmon leaves; Hunan Agricultural University: Changsha, China, 2010. [Google Scholar]
- Kawakami, K.; Shibukura, Y.; Kanno, T.; Furuki, T.; Aketa, S.; Hirayama, M. Identification of 2″-Galloylated Flavonol 3-O-Glycosides Accumulating in Developing Leaves of Persimmon. Phytochem. Anal. 2011, 22, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Lu, H.; Wang, C.; Yamashita, K.; Manabe, M.; Meng, Z.; Xu, S.; Kodama, H. Effect of five flavonoid compounds isolated from leaves of Diospyros kaki on stimulus-induced superoxide generation and tyrosyl phosphorylation of proteins in human neutrophils. Clin. Chim. Acta Int. J. Clin. Chem. 2002, 326, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.; Park, J.E.; Lee, J.S.; Lee, J.H.; Hwang, H.; Jung, S.H.; Kwon, H.C.; Jang, D.S. Chemical Constituents of the Leaves of Diospyros kaki (Persimmon). Plants 2021, 10, 2032. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Xue, J.; Xu, S.X.; Zhang, R.Q. Chemical constituents of the leaves of Diospyros kaki and their cytotoxic effects. J. Asian Nat. Prod. Res. 2007, 9, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Wei, S.H.; Huang, J.; Sun, J. A novel C-glycosylflavone from the leaves of Diospyros kaki. J. Asian Nat. Prod. Res. 2009, 11, 503–507. [Google Scholar] [CrossRef]
- Zhou, Z.; Li, N.; Zhang, H.F.; Wang, Q.Q.; Yu, Q.; Wang, F.; Dai, Y.H.; Wang, D.; Liu, D.C. Simultaneous quantitative analysis of 11 flavonoid derivatives with a single marker in persimmon leaf extraction and evaluation of their myocardium protection activity. J. Nat. Med. 2019, 73, 404–418. [Google Scholar] [CrossRef]
- Zhang, S.H.; Wang, Y.Z.; Meng, F.Y.; Li, Y.L.; Li, C.X.; Duan, F.P.; Wang, Q.; Zhang, X.T.; Zhang, C.N. Studies of the microbial metabolism of flavonoids extracted from the leaves of Diospyros kaki by intestinal bacteria. Arch. Pharmacal Res. 2015, 38, 614–619. [Google Scholar] [CrossRef]
- Ryu, R.; Jung, U.J.; Seo, Y.R.; Kim, H.J.; Moon, B.S.; Bae, J.S.; Lee, D.G.; Choi, M.S. Beneficial effect of persimmon leaves and bioactive compounds on thrombosis. Food Sci. Biotechnol. 2015, 24, 233–240. [Google Scholar] [CrossRef]
- Dinda, B.; Debnath, S.; Mohanta, B.C.; Harigaya, Y. Naturally Occurring Triterpenoid Saponins. Chem. Biodivers. 2010, 7, 2327–2580. [Google Scholar] [CrossRef] [PubMed]
- Dzubak, P.; Hajduch, M.; Vydra, D.; Hustova, A.; Kvasnica, M.; Biedermann, D.; Markova, L.; Urban, M.; Sarek, J. Pharmacological activities of natural triterpenoids and their therapeutic implications. Nat. Prod. Rep. 2006, 23, 394–411. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.P.; He, C.H. Simultaneous quantification of three major bioactive triterpene acids in the leaves of Diospyros kaki by high-performance liquid chromatography method. J. Pharm. Biomed. Anal. 2006, 41, 950–956. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Xu, S.; Sha, Y. Studies on the Constituents of Diospyros kaki Leaves (I). Chin. J. Med. Chem. 2000, 9, 347–353. [Google Scholar]
- Mallavadhani, U.V.; And, A.; Rao, Y.R. Diospyros melanoxylon Leaves: A Rich Source of Pentacyclic Triterpenes. Pharm. Biol. 2001, 39, 20–24. [Google Scholar] [CrossRef]
- Chen, G.; Jia, P.Y.; Xu, S.X.; Zhang, R.Q. Studies on the Constituents of Diospyros kaki Leaves (II). Chin. Tradit. Herb. Drugs 2005, 36, 26–28. [Google Scholar]
- Zhang, Y.; Zhao, L.; Huang, S.W.; Wang, W.; Song, S.J. Triterpene saponins with neuroprotective effects from the leaves of Diospyros kaki Thunb. Fitoterapia 2018, 129, 138–144. [Google Scholar] [CrossRef]
- Chen, G.; Wang, Z.Q.; Jia, J.M. Three Minor Novel Triterpenoids from the Leaves of Diospyros kaki. Chem. Pharm. Bull. 2009, 57, 532–535. [Google Scholar] [CrossRef]
- Chen, G.; Ren, H.M.; Yu, C.Y. A new 18,19-secoursane triterpene from the leaves of Diospyros kaki. Chem. Nat. Compd. 2012, 47, 918–920. [Google Scholar] [CrossRef]
- Thuong, P.T.; Lee, C.H.; Dao, T.T.; Nguyen, P.H.; Kim, W.G.; Lee, S.J.; Oh, W.K. Triterpenoids from the Leaves of Diospyros kaki (Persimmon) and Their Inhibitory Effects on Protein Tyrosine Phosphatase 1B. J. Nat. Prod. 2008, 71, 1775–1778. [Google Scholar] [CrossRef]
- Zhou, F.X.; Liang, P.Y.; Wen, J.; Ma, Y.; Zhang, K.S.; Wu, J.M.; Liang, C. Chemical composition of persimmon leaf. China J. Chin. Mater. Medica 1987, 12, 38. [Google Scholar]
- Higa, M.; Ogihara, K.; Yogi, S. Bioactive naphthoquinone derivatives from Diospyros maritima BLUME. Chem. Pharm. Bull. 1998, 46, 1189–1193. [Google Scholar] [CrossRef]
- Huang, S.W.; Qiao, J.W.; Sun, X.; Gao, P.Y.; Li, L.Z.; Liu, Q.B.; Sun, B.; Wu, D.L.; Song, S.J. Secoiridoids and lignans from the leaves of Diospyros kaki Thunb. with antioxidant and neuroprotective activities. J. Funct. Foods 2016, 24, 183–195. [Google Scholar] [CrossRef]
- Qiao, J.W.; Huang, S.W.; Song, S.J.; Xu, F.Q.; Huang, X.X.; Zhang, W.; Wu, D.L. Study on the Chemical Constituents of Persimmon Leaves. J. Chin. Med. Mater. 2016, 39, 2513–2517. [Google Scholar] [CrossRef]
- Wang, L.; Xu, M.L.; Rasmussen, S.K.; Wang, M.H. Vomifoliol 9-O-α-arabinofuranosyl (1→6)-β-d-glucopyranoside from the leaves of Diospyros kaki stimulates the glucose uptake in HepG2 and 3T3-L1 cells. Carbohydr. Res. 2011, 346, 1212–1216. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.C.; Choi, J.W.; Song, N.E.; Cho, C.W.; Rhee, Y.K.; Hong, H.D. Polysaccharide isolated from persimmon leaves (Diospyros kaki Thunb.) suppresses TGF-β1-induced epithelial-to-mesenchymal transition in A549 cells. Int. J. Biol. Macromol. 2020, 164, 3835–3845. [Google Scholar] [CrossRef]
- Hwang, Y.H.; Ha, H.; Kim, R.; Cho, C.W.; Song, Y.R.; Hong, H.D.; Kim, T. Anti-Osteoporotic Effects of Polysaccharides Isolated from Persimmon Leaves via Osteoclastogenesis Inhibition. Nutrients 2018, 10, 901. [Google Scholar] [CrossRef]
- Shin, M.S.; Lee, H.; Hong, H.D.; Shin, K.S. Characterization of immunostimulatory pectic polysaccharide isolated from leaves of Diospyros kaki Thumb. (Persimmon). J. Funct. Foods 2016, 26, 319–329. [Google Scholar] [CrossRef]
- Ue-Cachon, A.H.; Molina-Salinas, G.M.; Said-Fernandez, S.; Mendez-Gonzalez, M.; Caceres-Farfan, M.; Borges-Argaez, R. A new dimeric naphthoquinone from Diospyros anisandra. Nat. Prod. Res. 2013, 27, 1174–1178. [Google Scholar] [CrossRef]
- Row, L.R.; Rao, C.S. Chemical examination of diospyros species-part V: A novel aromatisation of ring B and other reactions of Bauerenol. Tetrahedron Lett. 1967, 8, 4845–4852. [Google Scholar] [CrossRef]
- ZHOU, X.-T. Research progress on chemical constituents and pharmacological effects of Diospyros kaki leaves. Chin. Tradit. Herb. Drugs 2014, 45, 3195–3203. [Google Scholar] [CrossRef]
- Fan, J.-P.; Zhang, R.-F.; Zhang, X.-H.; Zhu, J.-H.; Huang, J.-Z. Separation of three triterpene acids in leaves of Diospyros kaki by high performance liquid chromatography using hydroxypropyl-β-cyclodextrin as mobile phase modifier. J. Liq. Chromatogr. Relat. Technol. 2011, 34, 1340–1355. [Google Scholar] [CrossRef]
- Chen, G.; Xu, S.X.; Wang, H.Z.; Zhang, R.Q. Kakispyrol, a new biphenyl derivative from the leaves of Diospyros kaki. J. Asian Nat. Prod. Res. 2005, 7, 265–268. [Google Scholar] [CrossRef]
- Trachootham, D.; Lu, W.Q.; Ogasawara, M.A.; Valle, N.R.D.; Huang, P. Redox regulation of cell survival. Antioxid. Redox Signal. 2008, 10, 1343–1374. [Google Scholar] [CrossRef] [PubMed]
- Poprac, P.; Jomova, K.; Simunkova, M.; Kollar, V.; Rhodes, C.J.; Valko, M. Targeting Free Radicals in Oxidative Stress-Related Human Diseases. Trends Pharmacol. Sci. 2017, 38, 592–607. [Google Scholar] [CrossRef] [PubMed]
- Dunn, J.D.; Alvarez, L.A.J.; Zhang, X.Z.; Soldati, T. Reactive oxygen species and mitochondria: A nexus of cellular homeostasis. Redox Biol. 2015, 6, 472–485. [Google Scholar] [CrossRef]
- Zhang, B.; Pan, C.; Feng, C.; Yan, C.; Yu, Y.; Chen, Z.; Guo, C.; Wang, X. Role of mitochondrial reactive oxygen species in homeostasis regulation. Redox Rep. Commun. Free Radic. Res. 2022, 27, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Rains, J.L.; Jain, S.K. Oxidative stress, insulin signaling, and diabetes. Free Radic. Biol. Med. 2011, 50, 567–575. [Google Scholar] [CrossRef]
- He, F.; Zuo, L. Redox Roles of Reactive Oxygen Species in Cardiovascular Diseases. Int. J. Mol. Sci. 2015, 16, 27770–27780. [Google Scholar] [CrossRef]
- Hadrava Vanova, K.; Kraus, M.; Neuzil, J.; Rohlena, J. Mitochondrial complex II and reactive oxygen species in disease and therapy. Redox Rep. 2020, 25, 26–32. [Google Scholar] [CrossRef]
- Sun, L.J.; Zhang, J.B.; Lu, X.Y.; Zhang, L.Y.; Zhang, Y.L. Evaluation to the antioxidant activity of total flavonoids extract from persimmon (Diospyros kaki L.) leaves. Food Chem. Toxicol. 2011, 49, 2689–2696. [Google Scholar] [CrossRef] [PubMed]
- Ashry, O.M.; Hussein, E.M.; Abd El-Azime, A.S. Restorative role of persimmon leaf (Diospyros kaki) to gamma irradiation-induced oxidative stress and tissue injury in rats. Int. J. Radiat. Biol. 2017, 93, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.L.; Wang, Y.Q.; Xu, J.L.; Zhang, R.S. Effect of persimmon leaf extract on blood glucose and liver glycogen content in hyperglycemic rats. Chin. Arch. Tradit. Chin. Med. 2010, 2, 413–415. [Google Scholar]
- Pan, C.W.; Wang, W.; Xie, Y.F.; Qi, X.Z.; Jiang, L.X. Effect of persimmon leaves on oxygen free radicals and lipid metabolizing enzymes in hyperlipidemic rats. Guid. J. Tradit. Chin. Med. Pharm. 2016, 22, 3. [Google Scholar]
- Jung, U.J.; Park, Y.B.; Kim, S.R.; Choi, M.S. Supplementation of Persimmon Leaf Ameliorates Hyperglycemia, Dyslipidemia and Hepatic Fat Accumulation in Type 2 Diabetic Mice. PLoS ONE 2012, 7, e49030. [Google Scholar] [CrossRef]
- Bae, U.-J.; Park, S.-H.; Jung, S.-Y.; Park, B.-H.; Chae, S.-W. Hypoglycemic effects of aqueous persimmon leaf extract in a murine model of diabetes. Mol. Med. Rep. 2015, 12, 2547–2554. [Google Scholar] [CrossRef]
- Ozbek, E. Induction of oxidative stress in kidney. Int. J. Nephrol. 2012, 2012, 465897. [Google Scholar] [CrossRef]
- Yoshioka, H.; Usuda, H.; Fukuishi, N.; Nonogaki, T.; Onosaka, S. Carbon Tetrachloride-Induced Nephrotoxicity in Mice Is Prevented by Pretreatment with Zinc Sulfate. Biol. Pharm. Bull. 2016, 39, 1042–1046. [Google Scholar] [CrossRef]
- Shahat, A.A.; Ullah, R.; Alqahtani, A.S.; Hassanein, H.M.; Husseiny, H.A.; Mohammed, N.M.; Herqash, R.N. Nephroprotective effect of persimmon leaves (Diospyros kaki L.f.) against CCl(4)-induced renal toxicity in Swiss Albino rats. Drug Chem. Toxicol. 2022, 45, 1578–1586. [Google Scholar] [CrossRef]
- Choi, M.-S.; Jeong, M.J.; Park, Y.B.; Kim, S.R.; Jung, U.J. The leaf of Diospyros kaki Thumb ameliorates renal oxidative damage in mice with type 2 diabetes. Prev. Nutr. Food Sci. 2016, 21, 378. [Google Scholar] [CrossRef][Green Version]
- Yu, D.Y.; Cringle, S.J. Oxygen distribution and consumption within the retina in vascularised and avascular retinas and in animal models of retinal disease. Prog. Retin. Eye Res. 2001, 20, 175–208. [Google Scholar] [CrossRef] [PubMed]
- Brennan, L.A.; Kantorow, M. Mitochondrial function and redox control in the aging eye: Role of MsrA and other repair systems in cataract and macular degenerations. Exp. Eye Res. 2009, 88, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Usui, S.; Oveson, B.C.; Lee, S.Y.; Jo, Y.J.; Yoshida, T.; Miki, A.; Miki, K.; Iwase, T.; Lu, L.L.; Campochiaro, P.A. NADPH oxidase plays a central role in cone cell death in retinitis pigmentosa. J. Neurochem. 2009, 110, 1028–1037. [Google Scholar] [CrossRef] [PubMed]
- Prater, M.R.; Zimmerman, K.L.; Pinn, L.C.; Keay, J.M.; Laudermilch, C.L.; Holladay, S.D. Role of maternal dietary antioxidant supplementation in murine placental and fetal limb development. Placenta 2006, 27, 502–509. [Google Scholar] [CrossRef]
- Ahn, H.R.; Yang, J.W.; Kim, J.Y.; Lee, C.Y.; Kim, T.J.; Jung, S.H. The Intraocular Pressure-Lowering Effect of Persimmon leaves (Diospyros kaki) in a Mouse Model of Glaucoma. Int. J. Mol. Sci. 2019, 20, 5268. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.R.; Kim, K.A.; Kang, S.W.; Lee, J.Y.; Kim, T.J.; Jung, S.H. Persimmon Leaves (Diospyros kaki) Extract Protects Optic Nerve Crush-Induced Retinal Degeneration. Sci. Rep. 2017, 7, 46449. [Google Scholar] [CrossRef]
- Bei, W.; Zang, L.; Guo, J.; Peng, W.; Xu, A.; Good, D.A.; Hu, Y.; Wu, W.; Hu, D.; Zhu, X.; et al. Neuroprotective effects of a standardized flavonoid extract from Diospyros kaki leaves. J. Ethnopharmacol. 2009, 126, 134–142. [Google Scholar] [CrossRef]
- Bei, W.; Peng, W.; Ma, Y.; Xu, A. Flavonoids from the leaves of Diospyros kaki reduce hydrogen peroxide-induced injury of NG108-15 cells. Life Sci. 2005, 76, 1975–1988. [Google Scholar] [CrossRef]
- Ma, Y.J.; Ma, B.; Shang, Y.Y.; Yin, Q.Q.; Hong, Y.; Xu, S.; Shen, C.; Hou, X.Y.; Liu, X.P. Flavonoid-rich ethanol extract from the leaves of &ITDiospyros kaki &ITattenuates cognitive deficits, amyloid-beta production, oxidative stress, and neuroinflammation in APP/PS1 transgenic mice. Brain Res. 2018, 1678, 85–93. [Google Scholar] [CrossRef]
- Ma, Y.; Ma, B.; Shang, Y.; Yin, Q.; Wang, D.; Xu, S.; Hong, Y.; Hou, X.; Liu, X. Flavonoid-Rich Ethanol Extract from the Leaves of Diospyros kaki Attenuates D-Galactose-Induced Oxidative Stress and Neuroinflammation-Mediated Brain Aging in Mice. Oxidative Med. Cell. Longev. 2018, 2018, 8938207. [Google Scholar] [CrossRef]
- Huang, S.W.; Wang, W.; Zhang, M.Y.; Liu, Q.B.; Luo, S.Y.; Peng, Y.; Sun, B.; Wu, D.L.; Song, S.J. The effect of ethyl acetate extract from persimmon leaves on Alzheimer’s disease and its underlying mechanism. Phytomedicine Int. J. Phytother. Phytopharm. 2016, 23, 694–704. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhang, A.W.; Luo, Y.; Feng, Y.P.; Liang, Y.H. Tumor suppressive effects of different polar parts of persimmon leaf in mice with H22 ascites tumor and H22, S180 solid tumor. Chin. J. Exp. Tradit. Med. Formulae 2015, 21, 167–173. [Google Scholar] [CrossRef]
- Kang, J.Y.; Lee, U.; Park, S.K.; Kim, J.M.; Kim, M.J.; Moon, J.H.; Lee, H.L.; Jeong, H.R.; Park, H.W.; Kim, C.W.; et al. Persimmon Water Extract Suppresses Hepatic Lipotoxicity by Regulating Lipid Metabolites. J. Med. Food 2022, 25, 710–721. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Guo, Y.; Alsaif, G.; Gao, Y. Total Flavonoids Isolated from Diospyros kaki L. f. Leaves Induced Apoptosis and Oxidative Stress in Human Cancer Cells. Anticancer Res. 2020, 40, 5201–5210. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wei, Y.; Zhao, S.; Zhang, M.; Yan, X.; Gao, X.; Li, J.; Gao, Y.; Zhang, A.; Gao, Y. Antitumor and immunomodulatory activities of total flavonoids extract from persimmon leaves in H-22 liver tumor-bearing mice. Sci. Rep. 2018, 8, 10523. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhang, B.; Zhang, Y.; Zhang, A.W.; Luo, Y.; Ma, X.B. Effect of cyclophosphamide combined with ethyl acetate site of persimmon leaf on antioxidant capacity and protein expression of Bcl-2 and Bax in tumor tissues of H22 mice. Chin. J. Exp. Tradit. Med. Formulae 2015, 21, 120–123. [Google Scholar]
- Kim, H.S.; Suh, J.S.; Jang, Y.K.; Ahn, S.H.; Raja, G.; Kim, J.C.; Jung, Y.; Jung, S.H.; Kim, T.J. Anti-cancer potential of persimmon (Diospyros kaki) leaves via the PDGFR-Rac-JNK pathway. Sci. Rep. 2020, 10, 18119. [Google Scholar] [CrossRef]
- Ko, H.; Huh, G.; Jung, S.H.; Kwon, H.; Jeon, Y.; Park, Y.N.; Kim, Y.J. Diospyros kaki leaves inhibit HGF/Met signaling-mediated EMT and stemness features in hepatocellular carcinoma. Food Chem. Toxicol. 2020, 142, 111475. [Google Scholar] [CrossRef]
- Ding, Y.; Ren, K.; Dong, H.H.; Song, F.; Chen, J.; Guo, Y.T.; Liu, Y.S.; Tao, W.J.; Zhang, Y.L. Flavonoids from persimmon (Diospyros kaki L.) leaves inhibit proliferation and induce apoptosis in PC-3 cells by activation of oxidative stress and mitochondrial apoptosis. Chem. Biol. Interact. 2017, 275, 210–217. [Google Scholar] [CrossRef]
- Adhami, V.M.; Syed, D.N.; Khan, N.; Mukhtar, H. Dietary flavonoid fisetin: A novel dual inhibitor of PI3K/Akt and mTOR for prostate cancer management. Biochem. Pharmacol. 2012, 84, 1277–1281. [Google Scholar] [CrossRef]
- Naito, R.; Kano, H.; Shimada, T.; Makino, T.; Kadomoto, S.; Iwamoto, H.; Yaegashi, H.; Izumi, K.; Kadono, Y.; Nakata, H.; et al. A new flavonoid derivative exerts antitumor effects against androgen-sensitive to cabazitaxel-resistant prostate cancer cells. Prostate 2021, 81, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.; Ma, Q.; Zhong, G.; He, J.; Sang, Z. Isolation and characterization of flavonoid derivatives with anti-prostate cancer and hepatoprotective activities from the flowers of Hosta plantaginea (Lam.) Aschers. J. Ethnopharmacol. 2020, 253, 112685. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Afaq, F.; Syed, D.N.; Mukhtar, H. Fisetin, a novel dietary flavonoid, causes apoptosis and cell cycle arrest in human prostate cancer LNCaP cells. Carcinogenesis 2008, 29, 1049–1056. [Google Scholar] [CrossRef] [PubMed]
- Syed, D.N.; Adhami, V.M.; Khan, M.I.; Mukhtar, H. Inhibition of Akt/mTOR signaling by the dietary flavonoid fisetin. Anticancer Agents Med. Chem. 2013, 13, 995–1001. [Google Scholar] [CrossRef]
- Adeniyi, B.A.; Robert, M.F.; Chai, H.; Fong, H.H.S. In vitro cytotoxicity activity of diosquinone, a naphthoquinone epoxide. Phytother. Res. 2003, 17, 282–284. [Google Scholar] [CrossRef]
- Rotariu, D.; Babes, E.E.; Tit, D.M.; Moisi, M.; Bustea, C.; Stoicescu, M.; Radu, A.-F.; Vesa, C.M.; Behl, T.; Bungau, A.F.; et al. Oxidative stress—Complex pathological issues concerning the hallmark of cardiovascular and metabolic disorders. Biomed. Pharmacother. 2022, 152, 113238. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.H.; Kim, H.J.; Ryu, R.; Han, H.J.; Han, Y.J.; Lee, M.K.; Choi, M.S.; Park, Y.B. A Mixture of Ethanol Extracts of Persimmon Leaf and Citrus junos Sieb Improves Blood Coagulation Parameters and Ameliorates Lipid Metabolism Disturbances Caused by Diet-Induced Obesity in C57BL/6J Mice. J. Microbiol. Biotechnol. 2016, 26, 295–308. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, Y.; Zhang, M.; Gu, L.; Liu, Z.; Jia, J.; Chen, X. Effects of phospholipid complexes of total flavonoids from Persimmon (Diospyros kaki L.) leaves on experimental atherosclerosis rats. J. Ethnopharmacol. 2016, 191, 245–253. [Google Scholar] [CrossRef]
- Kawakami, K.; Aketa, S.; Sakai, H.; Watanabe, Y.; Nishida, H.; Hirayama, M. Antihypertensive and vasorelaxant effects of water-soluble proanthocyanidins from persimmon leaf tea in spontaneously hypertensive rats. Biosci. Biotechnol. Biochem. 2011, 75, 1435–1439. [Google Scholar] [CrossRef]
- Deng, R.C.; Zhang, W.S.; Yang, H.J.; Meng, F.Y.; Du, S.S.; Hua, Y.; Sun, L.P.; Wang, Y.Y. Anti ischemic effect of ethanolic extract of persimmon leaf in rats. Chin. J. Inf. Tradit. Chin. Med. 2004, 11, 2. [Google Scholar] [CrossRef]
- Ou, Y.P.; Bei, W.J.; Lai, W.Y.; Xu, D.L.; Peng, W.L. Effect of persimmon leaf flavone on hypoxia reoxygenation as well as advanced glycation end products induced apoptosis in neonatal rat cardiomyocytes. J. South. Med. Univ. 2003, 23, 680–682. [Google Scholar] [CrossRef]
- Miao, M.S.; Zhang, X.X.; Wang, L.A. Persimmon leaf flavonoid induces brain ischemic tolerance in mice. Neural Regen. Res. 2013, 8, 1376–1382. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, K.; Nishida, H.; Tatewaki, N.; Eguchi-Kasai, K.; Hirayama, M. Persimmon Leaf Flavonols Enhance the Anti-Cancer Effect of Heavy Ion Radiotherapy on Murine Xenograft Tumors. J. Cancer Ther. 2013, 04, 1150–1157. [Google Scholar] [CrossRef][Green Version]
- Kawakami, K.; Nishida, H.; Tatewaki, N.; Nakajima, Y.; Konishi, T.; Hirayama, M. Persimmon Leaf Extract Inhibits the ATM Activity during DNA Damage Response Induced by Doxorubicin in A549 Lung Adenocarcinoma Cells. Biosci. Biotechnol. Biochem. 2011, 75, 650–655. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Cho, S.S.; Simkhada, J.R.; Park, S.J.; Lee, H.J.; Kim, T.S.; Yoo, J.C. Effects and action mechanism of Diospyros kaki on the differentiation of human leukemia HL-60 cells. Oncol. Rep. 2010, 23, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.K.; Park, J.M.; Jeon, I.H.; Kim, H.S.; Jang, S.I. Effect of Persimmon Leaf Extract on Utraviolet B-induced Inflammation in HaCaT Keratinocytes and Mice. J. Korean Soc. Appl. Biol. Chem. 2011, 54, 583–590. [Google Scholar] [CrossRef]
- Mok, J.Y.; Jeon, I.H.; Cho, J.-K.; Park, J.M.; Kim, H.S.; Kang, H.J.; Kim, H.S.; Jang, S.I. Effect of Persimmon Leaf Extract on Phthalic Anhydride-induced Allergic Response in Mice. Prev. Nutr. Food Sci. 2012, 17, 14–21. [Google Scholar] [CrossRef][Green Version]
- Kitabatake, M.; Matsumura, Y.; Ouji-Sageshima, N.; Nishioka, T.; Hara, A.; Kayano, S.; Ito, T. Persimmon-derived tannin ameliorates the pathogenesis of ulcerative colitis in a murine model through inhibition of the inflammatory response and alteration of microbiota. Sci. Rep. 2021, 11, 7286. [Google Scholar] [CrossRef]
- Kim, K.S.; Lee, D.S.; Kim, D.C.; Yoon, C.S.; Ko, W.; Oh, H.; Kim, Y.C. Anti-Inflammatory Effects and Mechanisms of Action of Coussaric and Betulinic Acids Isolated from Diospyros kaki in Lipopolysaccharide-Stimulated RAW 264.7 Macrophages. Molecules 2016, 21, 1206. [Google Scholar] [CrossRef]
- Kotani, M.; Matsumoto, M.; Fujita, A.; Higa, S.; Wang, W.; Suemura, M.; Kishimoto, T.; Tanaka, T. Persimmon leaf extract and astragalin inhibit development of dermatitis and IgE elevation in NC/Nga mice. J. Allergy Clin. Immunol. 2000, 106, 159–166. [Google Scholar] [CrossRef]
- Cho, J.K.; Jeon, I.H.; Park, J.M.; Kim, H.S.; Jang, S.I. Inhibitory Effect of Persimmon Leaf Extract on Development of Atopic Dermatitis-Like Skin Lesions. J. Korean Soc. Appl. Biol. Chem. 2011, 54, 653–657. [Google Scholar] [CrossRef]
- Lee, H.S.; Kim, E.N.; Kim, G.R.; Jeong, G.S. Persimmon leaf extract protects mice from atopic dermatitis by inhibiting T cell activation via regulation of the JNK pathway. Phytother. Res. 2021, 35, 2545–2556. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.H.; Lin, J.; Guo, H.B.; Li, C.Y. Anti inflammatory and anti apoptotic effects of S1-12 Naoxinqing Tablet on BV-2 cells induced by lipopolysaccharide. Acta Neuropharmacol. 2018, 8, 35. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).