Theoretical Study on ORR/OER Bifunctional Catalytic Activity of Axial Functionalized Iron Polyphthalocyanine
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structure and Stability
2.2. ORR/OER Mechanism of FePPc and FePPc-L
2.3. Adsorption Properties and OER/ORR Activity Descriptor
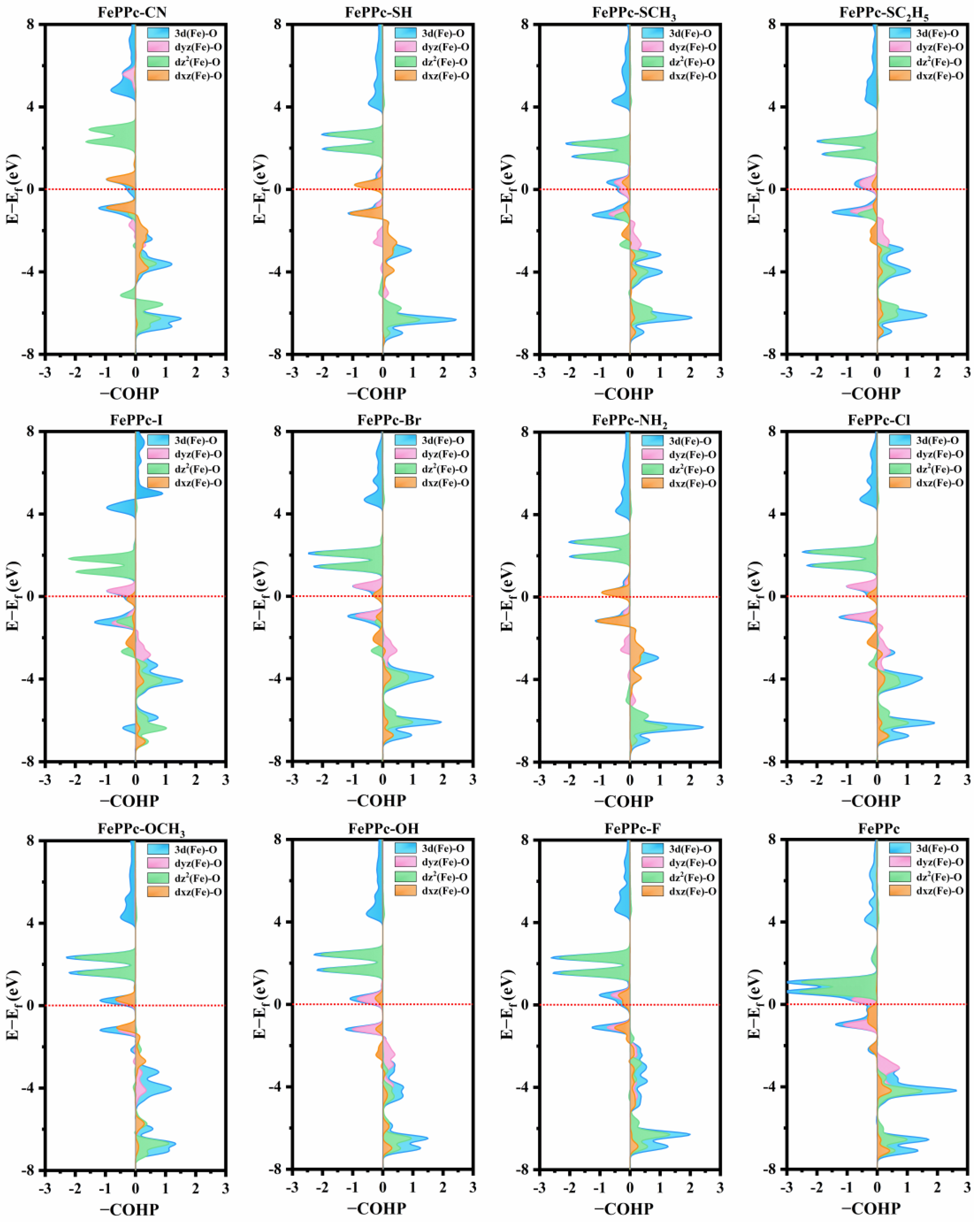
2.4. Electronic Properties Analysis
3. Computational Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Debe, M.K. Electrocatalyst approaches and challenges for automotive fuel cells. Nature 2012, 486, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.V.; White, R.E. A Water and Heat Management Model for Proton-Exchange-Membrane Fuel Cells. J. Electrochem. Soc. 1993, 140, 2178. [Google Scholar] [CrossRef]
- Xu, N.; Zhang, Y.; Zhang, T.; Liu, Y.; Qiao, J. Efficient quantum dots anchored nanocomposite for highly active ORR/OER electrocatalyst of advanced metal-air batteries. Nano Energy 2019, 57, 176–185. [Google Scholar] [CrossRef]
- Zuo, C.; Li, L.; Chen, W.; Zhang, Z. Synergistic effect on the four-electron ORR of the electro-Fenton system to remove micropollutants using an MOF-derived catalyst with carbon black. Appl. Surf. Sci. 2021, 554, 149546. [Google Scholar] [CrossRef]
- Erakulan, E.S.; Thapa, R. Origin of pure and C doped borophene stability and its activity for OER. Appl. Surf. Sci. 2022, 574, 151613. [Google Scholar]
- Lin, R.; Cai, X.; Zeng, H.; Yu, Z. Stability of High-Performance Pt-Based Catalysts for Oxygen Reduction Reactions. Adv. Mater. 2018, 30, 1705332. [Google Scholar] [CrossRef] [PubMed]
- Nie, Y.; Li, L.; Wei, Z. Recent advancements in Pt and Pt-free catalysts for oxygen reduction reaction. Chem. Soc. Rev. 2015, 44, 2168–2201. [Google Scholar] [CrossRef]
- Osgood, H.; Devaguptapu, S.V.; Xu, H.; Cho, J.; Wu, G. Transition metal (Fe, Co, Ni, and Mn) oxides for oxygen reduction and evolution bifunctional catalysts in alkaline media. Nano Today 2016, 11, 601–625. [Google Scholar] [CrossRef]
- Lee, Y.; Suntivich, J.; May, K.J.; Perry, E.E.; Shao-Horn, Y. Synthesis and Activities of Rutile IrO2 and RuO2 Nanoparticles for Oxygen Evolution in Acid and Alkaline Solutions. J. Phys. Chem. Lett. 2012, 3, 399–404. [Google Scholar] [CrossRef]
- Liu, R.; Tang, R.; Feng, J.; Meng, T. Mechanistic insight into the Fe-atom-pairs for breaking the scaling relation in ORR. Chem. Eng. J. 2023, 470, 144261. [Google Scholar] [CrossRef]
- Liu, X.; Fan, L.; Wang, Y.; Zhang, W.; Ai, H.; Wang, Z.; Zhang, D.; Jia, H.; Wang, C. Nanofiber-based Sm0.5Sr0.5Co0.2Fe0.8O3-δ/N-MWCNT composites as an efficient bifunctional electrocatalyst towards OER/ORR. Int. J. Hydrogen Energy 2023, 48, 15555–15565. [Google Scholar] [CrossRef]
- Li, H.; Li, G. Novel palladium-based nanomaterials for multifunctional ORR/OER/HER electrocatalysis. J. Mater. Chem. A 2023, 11, 9383–9400. [Google Scholar] [CrossRef]
- Yu, J.; Jiang, Z.; Huang, T.; Tang, C. BN/Cu/CNT nanoparticles as an efficient tri-functional electrocatalyst for ORR and OER. Int. J. Hydrogen Energy 2023, 48, 20368–20377. [Google Scholar] [CrossRef]
- Gong, L.; Zhu, J.; Xia, F.; Zhang, Y.; Shi, W.; Chen, L.; Yu, J.; Wu, J.; Mu, S. Marriage of Ultralow Platinum and Single-Atom MnN4 Moiety for Augmented ORR and HER Catalysis. ACS Catal. 2023, 13, 4012–4020. [Google Scholar] [CrossRef]
- Miranda-Rojas, S.; Sierra-Rosales, P.; Muñoz-Castro, A.; Arratia-Pérez, R.; Zagal, J.H.; Mendizábal, F. Catalytic aspects of metallophthalocyanines adsorbed on gold-electrode. Theoretical exploration of the binding nature role. Phys. Chem. Chem. Phys. 2016, 18, 29516–29525. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Sokolowski, J.; Song, X.; He, Y.; Mei, Y.; Wu, G. Engineering Local Coordination Environments of Atomically Dispersed and Heteroatom-Coordinated Single Metal Site Electrocatalysts for Clean Energy-Conversion. Adv. Energy Mater. 2020, 10, 1902844. [Google Scholar] [CrossRef]
- Mei, Z.-Y.; Cai, S.; Zhao, G.; Zou, X.; Fu, Y.; Jiang, J.; An, Q.; Li, M.; Liu, T.; Guo, H. Boosting the ORR active and Zn-air battery performance through ameliorating the coordination environment of iron phthalocyanine. Chem. Eng. J. 2022, 430, 132691. [Google Scholar] [CrossRef]
- Zagal, J.; Páez, M.; Tanaka, A.A.; dos Santos, J.R.; Linkous, C.A. Electrocatalytic activity of metal phthalocyanines for oxygen reduction. J. Electroanal. Chem. 1992, 339, 13–30. [Google Scholar] [CrossRef]
- Mukherjee, M.; Samanta, M.; Das, G.P.; Chattopadhyay, K.K. Investigation of ORR Performances on Graphene/Phthalocyanine Nanocomposite in Neutral Medium. Microsc. Microanal. 2019, 25, 1416–1421. [Google Scholar] [CrossRef]
- Abbaspour, A.; Mirahmadi, E. Electrocatalytic activity of iron and nickel phthalocyanines supported on multi-walled carbon nanotubes towards oxygen evolution reaction. Electrochim. Acta 2013, 105, 92–98. [Google Scholar] [CrossRef]
- Huang, Q.E.; Chen, J.; Luan, P.; Ding, C.; Li, C. Understanding the factors governing the water oxidation reaction pathway of mononuclear and binuclear cobalt phthalocyanine catalysts. Chem. Sci. 2022, 13, 8797–8803. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Niu, H.; Yin, Y.; Wang, X.; Shao, C.; Zhang, Z.; Guo, Y. Enhanced electrochemical oxygen evolution reaction activity on natural single-atom catalysts transition metal phthalocyanines: The substrate effect. Catal. Sci. Technol. 2020, 10, 8339–8346. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, X.; Huang, B.; Wang, P.; Pei, Y. Hydroxyl group modification improves the electrocatalytic ORR and OER activity of graphene supported single and bi-metal atomic catalysts (Ni, Co, and Fe). J. Mater. Chem. A 2019, 7, 24583. [Google Scholar] [CrossRef]
- Li, J.; Tang, Y.; Wang, H.; Wang, C.; Tian, J.; Liu, D.; Li, C.M.; Guo, C. Oxygen plasma induced interfacial CoOx/Phthalocyanine Cobalt as bifunctional electrocatalyst towards oxygen-involving reactions. Int. J. Hydrogen Energy 2022, 47, 9905–9914. [Google Scholar] [CrossRef]
- Morais, R.G.; Rey-Raap, N.; Figueiredo, J.L.; Pereira, M.F.R. Optimization of cobalt on CNT towards the oxygen evolution reaction and its synergy with iron (II) phthalocyanine as bifunctional oxygen electrocatalyst. Catal. Today 2023, 418, 114057. [Google Scholar] [CrossRef]
- Helsel, N.; Choudhury, P. Investigation of bifunctionality of FePc-functionalized graphene for enhanced ORR/OER activity. Mol. Catal. 2023, 545, 113213. [Google Scholar] [CrossRef]
- Yang, S.; Yu, Y.; Dou, M.; Zhang, Z.; Dai, L.; Wang, F. Two-Dimensional Conjugated Aromatic Networks as High-Site-Density and Single-Atom Electrocatalysts for the Oxygen Reduction Reaction. Angew. Chem. Int. Ed. 2019, 58, 14724–14730. [Google Scholar] [CrossRef]
- Yang, S.; Yu, Y.; Dou, M.; Zhang, Z.; Wang, F. Edge-Functionalized Polyphthalocyanine Networks with High Oxygen Reduction Reaction Activity. J. Am. Chem. Soc. 2020, 142, 17524–17530. [Google Scholar] [CrossRef]
- Mukherjee, B. Solvothermally Synthesized Iron Phthalocyanine Nanostructure for High ORR Response: A Joint Experimental Investigation and DFT Analysis. J. Electrochem. Soc. 2020, 167, 116501. [Google Scholar] [CrossRef]
- Mukherjee, M.; Samanta, M.; Banerjee, P.; Chattopadhyay, K.K.; Das, G.P. Endorsement of Manganese Phthalocyanine microstructures as electrocatalyst in ORR: Experimental and computational study. Electrochim. Acta 2019, 296, 528–534. [Google Scholar] [CrossRef]
- Aralekallu, S.; Sajjan, V.A.; Palanna, M.; Prabhu, C.P.K.; Hojamberdiev, M.; Sannegowda, L.K. Ni foam-supported azo linkage cobalt phthalocyanine as an efficient electrocatalyst for oxygen evolution reaction. J. Power Sources 2020, 449, 227516. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, D.; Lee, J.; Lee, L.Y.S.; Ng, D.K.P. Tuning the Electrochemical Properties of Polymeric Cobalt Phthalocyanines for Efficient Water Splitting. Adv. Funct. Mater. 2021, 31, 2103290. [Google Scholar] [CrossRef]
- Zhou, Y.; Gao, G.; Chu, W.; Wang, L.-W. Computational screening of transition metal-doped phthalocyanine monolayers for oxygen evolution and reduction. Nanoscale Adv. 2020, 2, 710–716. [Google Scholar] [CrossRef]
- Wang, Y.; Yuan, H.; Li, Y.; Chen, Z. Two-dimensional iron-phthalocyanine (Fe-Pc) monolayer as a promising single-atom-catalyst for oxygen reduction reaction: A computational study. Nanoscale 2015, 7, 11633–11641. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.-M.; Liu, S.; Li, Y.-Y.; Wei, X.; Ye, G.; Zhu, W.; Su, Y.; Wang, J.; Liu, H.; He, Z.; et al. Insight into the Mechanism of Axial Ligands Regulating the Catalytic Activity of Fe–N4 Sites for Oxygen Reduction Reaction. Adv. Energy Mater. 2022, 12, 2103588. [Google Scholar] [CrossRef]
- Huang, Z.; Tang, Q. Axial Coordination Effect on the Oxygen Reduction Reaction of FeN4 Electrocatalysts Based on Grand Canonical Density Functional Theory. J. Phys. Chem. C 2022, 126, 21606–21615. [Google Scholar] [CrossRef]
- Cao, R.; Thapa, R.; Kim, H.; Xu, X.; Gyu Kim, M.; Li, Q.; Park, N.; Liu, M.; Cho, J. Promotion of oxygen reduction by a bio-inspired tethered iron phthalocyanine carbon nanotube-based catalyst. Nat. Commun. 2013, 4, 2076. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Li, Z.; Xia, D.; Zheng, L.; Liao, Y.; Li, K.; Zuo, X. Probing the influence of the center atom coordination structure in iron phthalocyanine multi-walled carbon nanotube-based oxygen reduction reaction catalysts by X-ray absorption fine structure spectroscopy. J. Power Sources 2015, 291, 20–28. [Google Scholar] [CrossRef]
- Yang, X.; Xia, D.; Kang, Y.; Du, H.; Kang, F.; Gan, L.; Li, J. Unveiling the Axial Hydroxyl Ligand on Fe-N4-C Electrocatalysts and Its Impact on the pH-Dependent Oxygen Reduction Activities and Poisoning Kinetics. Adv. Sci. 2020, 7, 2000176. [Google Scholar] [CrossRef]
- Nørskov, J.K.; Rossmeisl, J.; Logadottir, A.; Lindqvist, L.; Kitchin, J.R.; Bligaard, T.; Jonsson, H. Origin of the overpotential for oxygen reduction at a fuel-cell cathode. J. Phys. Chem. B 2004, 108, 17886–17892. [Google Scholar] [CrossRef]
- Liang, Z.; Luo, M.; Chen, M.; Liu, C.; Peera, S.G.; Qi, X.; Liu, J.; Kumar, U.P.; Liang, T.L.T. Evaluating the catalytic activity of transition metal dimers for the oxygen reduction reaction. J. Colloid Interface Sci. 2020, 568, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Mathew, K.; Sundararaman, R.; Letchworth-Weaver, K.; Arias, T.A.; Hennig, R.G. Implicit solvation model for density-functional study of nanocrystal surfaces and reaction pathways. J. Chem. Phys. 2014, 140, 084106. [Google Scholar] [CrossRef] [PubMed]
- Briquet, L.G.V.; Sarwar, M.; Mugo, J.; Jones, G.; Calle-Vallejo, F. A New Type of Scaling Relations to Assess the Accuracy of Computational Predictions of Catalytic Activities Applied to the Oxygen Evolution Reaction. ChemCatChem 2017, 9, 1261–1268. [Google Scholar] [CrossRef]
- Wang, J.; Fan, Y.; Qi, S.; Li, W.; Zhao, M. Bifunctional HER/OER or OER/ORR Catalytic Activity of Two-Dimensional TM3(HITP)2 with TM = Fe–Zn. J. Phys. Chem. C 2020, 124, 9350–9359. [Google Scholar] [CrossRef]
- Xu, H.; Cheng, D.; Cao, D.; Zeng, X.C. A universal principle for a rational design of single-atom electrocatalysts. Nat. Catal. 2018, 1, 339–348. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, A.; Zhang, Z.; Jiao, M.; Zhou, Z. Transition metal anchored C2N monolayers as efficient bifunctional electrocatalysts for hydrogen and oxygen evolution reactions. J. Mater. Chem. A 2018, 6, 11446–11452. [Google Scholar] [CrossRef]
- Meng, Y.; Yin, C.; Li, K.; Tang, H.; Wang, Y.; Wu, Z. Design of high efficient oxygen reduction catalyst from the transition metal dimer phthalocyanine monolayer. Appl. Surf. Sci. 2019, 480, 905–911, 2095–2108. [Google Scholar] [CrossRef]
- Lin, L.; Long, X.; Yang, X.; Shi, P.; Su, L. Theoretical study of Mo2N supported transition metal single-atom catalyst for OER/ORR bifunctional electrocatalysis. Phys. Chem. Chem. Phys. 2023, 25, 24721–24732. [Google Scholar] [CrossRef]
- Allred, A.L. Electronegativity values from thermochemical data. J. Inorg. Nucl. Chem. 1961, 17, 215–221. [Google Scholar] [CrossRef]
- Seo, M.H.; Higgins, D.; Jiang, G.; Choi, S.M.; Han, B.; Chen, Z. Theoretical insight into highly durable iron phthalocyanine derived non-precious catalysts for oxygen reduction reactions. J. Mater. Chem. A 2014, 2, 19707–19716. [Google Scholar] [CrossRef]
- Deringer, V.L.; Tchougréeff, A.L.; Dronskowski, R. Crystal orbital Hamilton population (COHP) analysis as projected from plane-wave basis sets. J. Phys. Chem. A 2011, 115, 5461–5466. [Google Scholar] [CrossRef] [PubMed]
- Maintz, S.; Deringer, V.L.; Tchougréeff, A.L.; Dronskowski, R. Analytic projection from plane-wave and PAW wavefunctions and application to chemical-bonding analysis in solids. J. Comput. Chem. 2013, 34, 2557–2567. [Google Scholar] [CrossRef] [PubMed]
- Bridgeman, A.J.; Cavigliasso, G.; Ireland, L.R.; Rothery, J. The Mayer bond order as a tool in inorganic chemistry. J. Chem. Soc. Dalton Trans. 2001, 14, 2095–2108. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188. [Google Scholar] [CrossRef]

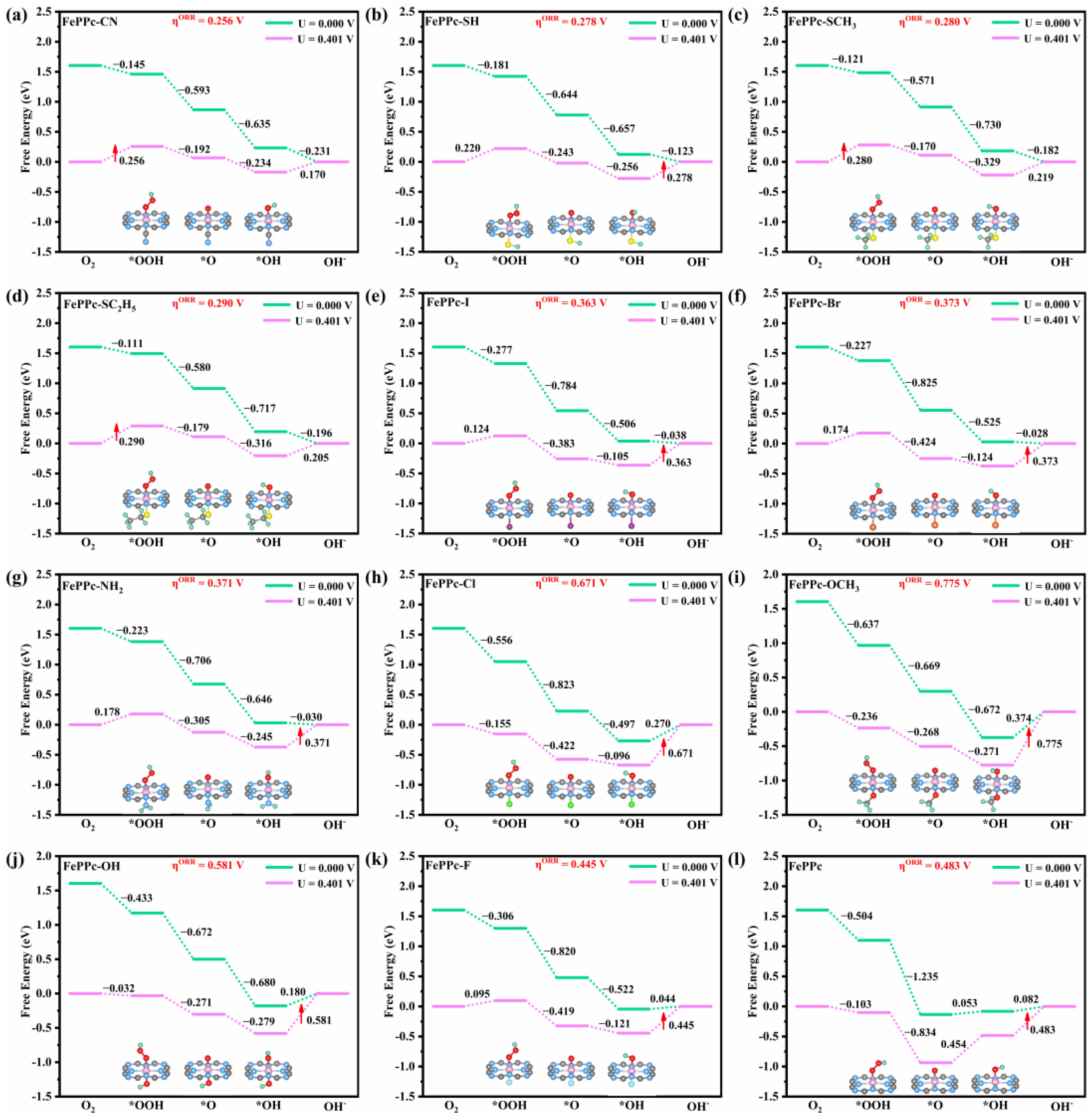
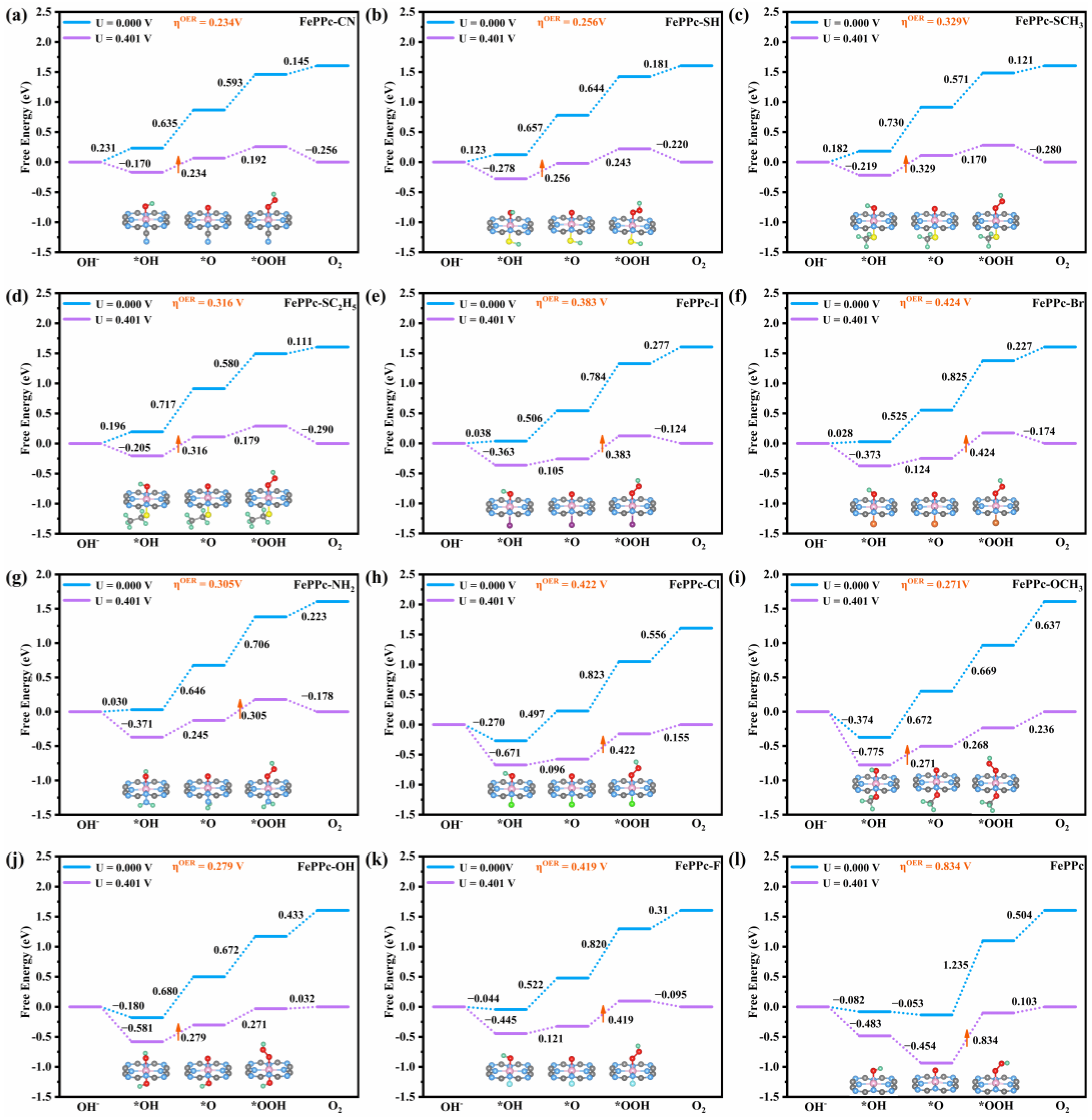
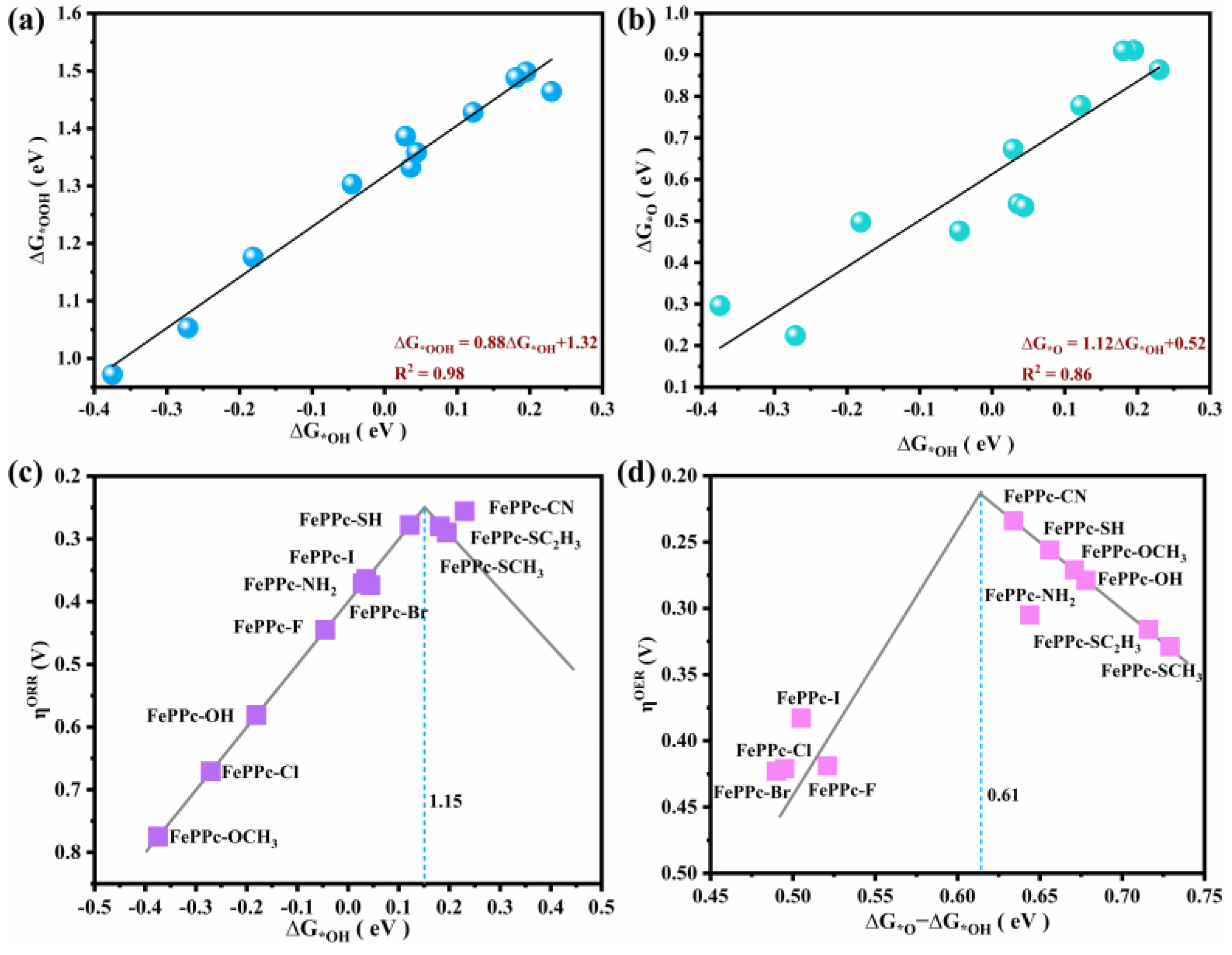
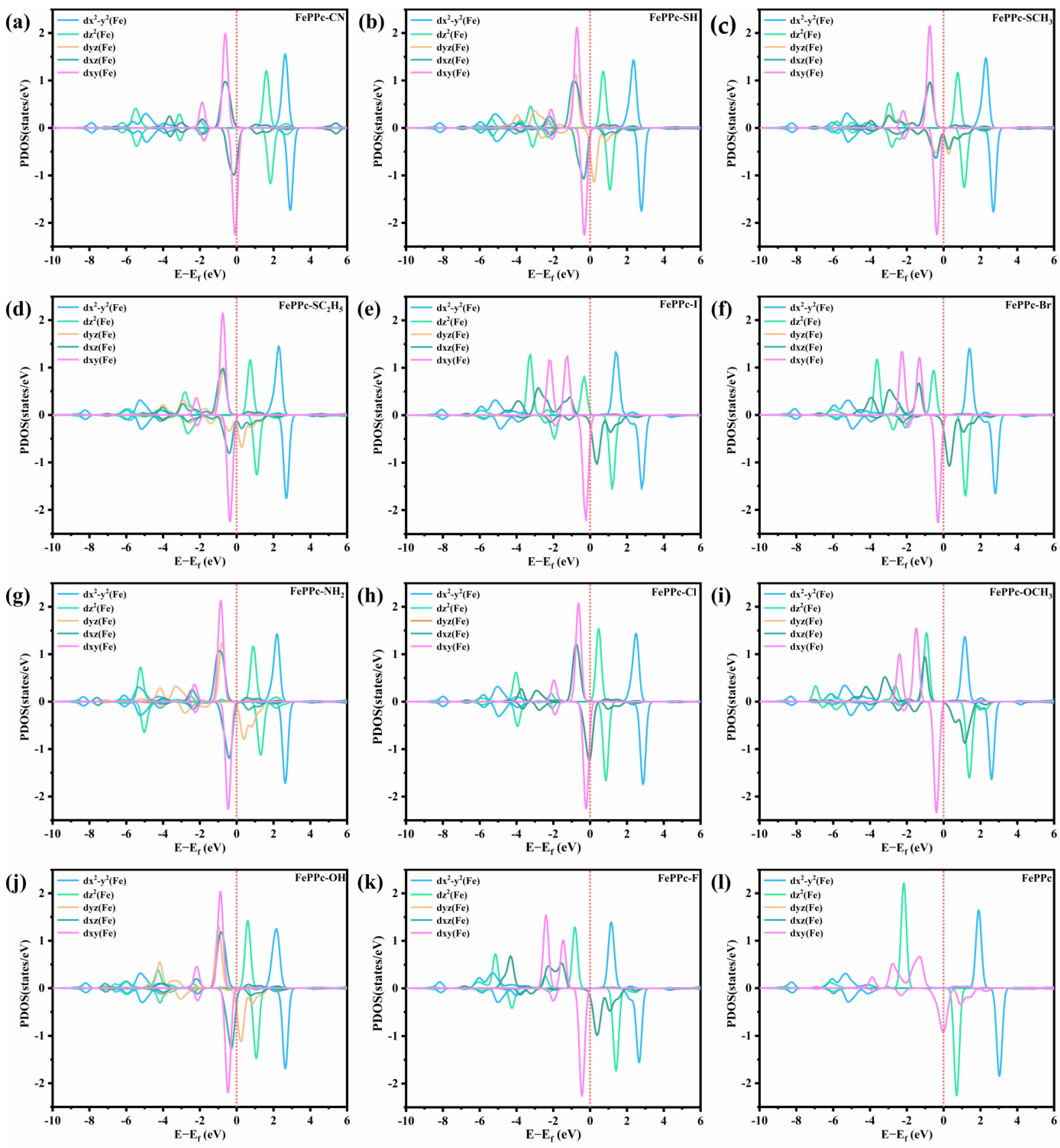
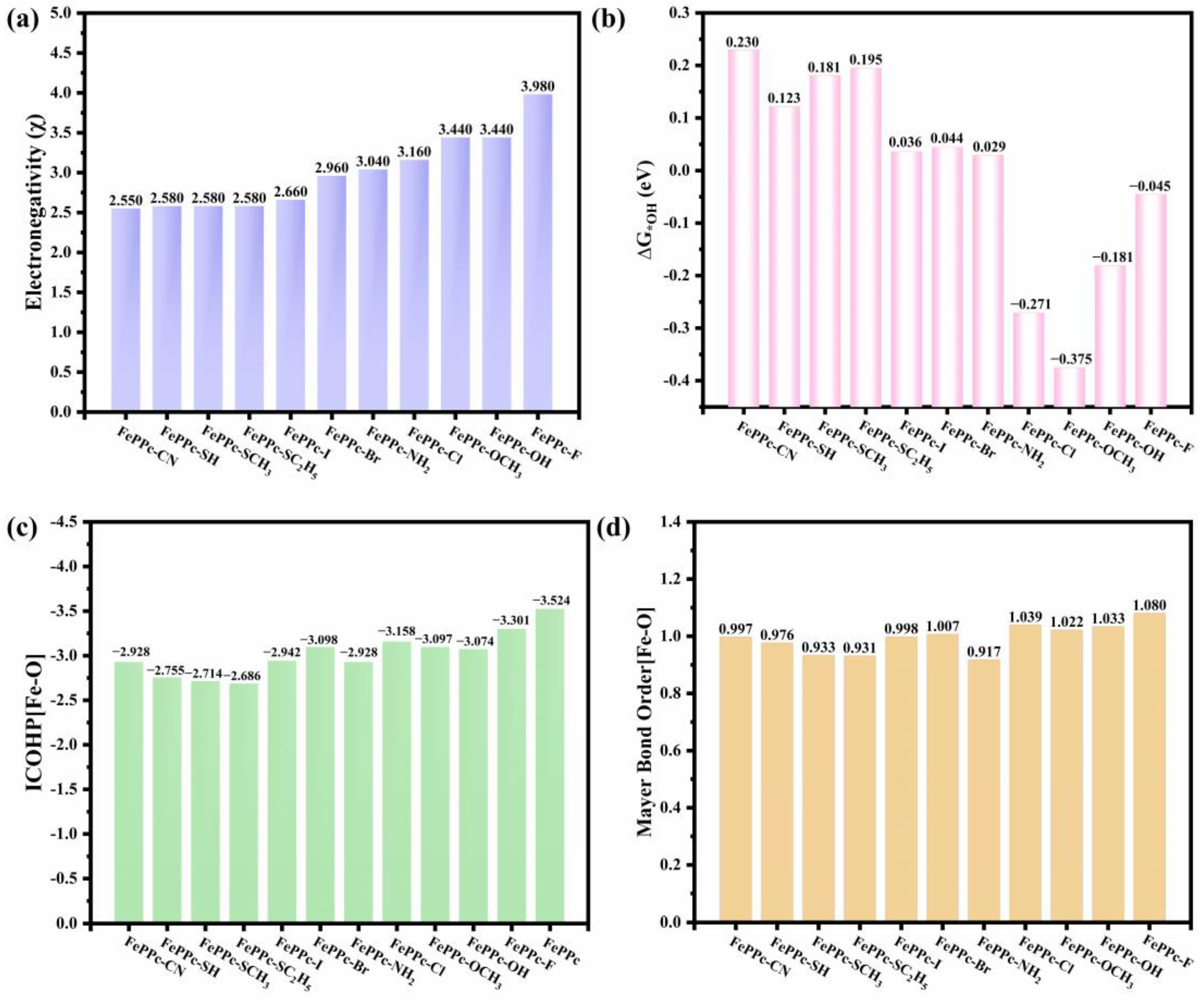
| Structure | ΔG*OOH | ΔG*O | ΔG*OH |
|---|---|---|---|
| FePPc-CN | 1.464 | 0.864 | 0.230 |
| FePPc-SH | 1.428 | 0.778 | 0.122 |
| FePPc-SCH3 | 1.488 | 0.910 | 0.181 |
| FePPc-SC2H5 | 1.498 | 0.911 | 0.195 |
| FePPc-I | 1.332 | 0.541 | 0.036 |
| FePPc-Br | 1.358 | 0.534 | 0.044 |
| FePPc-NH2 | 1.386 | 0.673 | 0.029 |
| FePPc-Cl | 1.053 | 0.224 | −0.271 |
| FePPc-OCH3 | 0.972 | 0.296 | −0.375 |
| FePPc-OH | 1.176 | 0.497 | −0.181 |
| FePPc-F | 1.303 | 0.476 | −0.045 |
| FePPc | 1.105 | −0.137 | −0.083 |
| Structure | QFe(|e|) | QN(|e|) | QL(|e|) | MFe(μB) |
|---|---|---|---|---|
| FePPc-CN | 1.223 | −4.515 | −0.537 | 0.532 |
| FePPc-SH | 1.179 | −4.420 | −0.203 | 0.779 |
| FePPc-SCH3 | 1.130 | −4.399 | −0.089 | 0.753 |
| FePPc-SC2H5 | 1.079 | −4.368 | −0.039 | 0.756 |
| FePPc-I | 1.228 | −4.578 | −0.436 | 2.267 |
| FePPc-Br | 1.235 | −4.546 | −0.541 | 2.323 |
| FePPc-NH2 | 1.248 | −4.442 | −0.128 | 0.789 |
| FePPc-Cl | 1.250 | −4.516 | −0.445 | 0.833 |
| FePPc-OCH3 | 1.340 | −4.537 | −0.403 | 2.303 |
| FePPc-OH | 1.362 | −4.462 | −0.391 | 0.833 |
| FePPc-F | 1.417 | −4.544 | −0.702 | 2.695 |
| FePPc | 1.345 | −4.748 | -- | 1.866 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, G.; Feng, X.; Ren, R.; Wang, Y.; Meng, J.; Jia, J. Theoretical Study on ORR/OER Bifunctional Catalytic Activity of Axial Functionalized Iron Polyphthalocyanine. Molecules 2024, 29, 210. https://doi.org/10.3390/molecules29010210
Wang G, Feng X, Ren R, Wang Y, Meng J, Jia J. Theoretical Study on ORR/OER Bifunctional Catalytic Activity of Axial Functionalized Iron Polyphthalocyanine. Molecules. 2024; 29(1):210. https://doi.org/10.3390/molecules29010210
Chicago/Turabian StyleWang, Guilin, Xiaoqin Feng, Rongrong Ren, Yuxin Wang, Jie Meng, and Jianfeng Jia. 2024. "Theoretical Study on ORR/OER Bifunctional Catalytic Activity of Axial Functionalized Iron Polyphthalocyanine" Molecules 29, no. 1: 210. https://doi.org/10.3390/molecules29010210
APA StyleWang, G., Feng, X., Ren, R., Wang, Y., Meng, J., & Jia, J. (2024). Theoretical Study on ORR/OER Bifunctional Catalytic Activity of Axial Functionalized Iron Polyphthalocyanine. Molecules, 29(1), 210. https://doi.org/10.3390/molecules29010210






