Optimization of the Flavonoid Extraction Process from the Stem and Leaves of Epimedium Brevicornum and Its Effects on Cyclophosphamide-Induced Renal Injury
Abstract
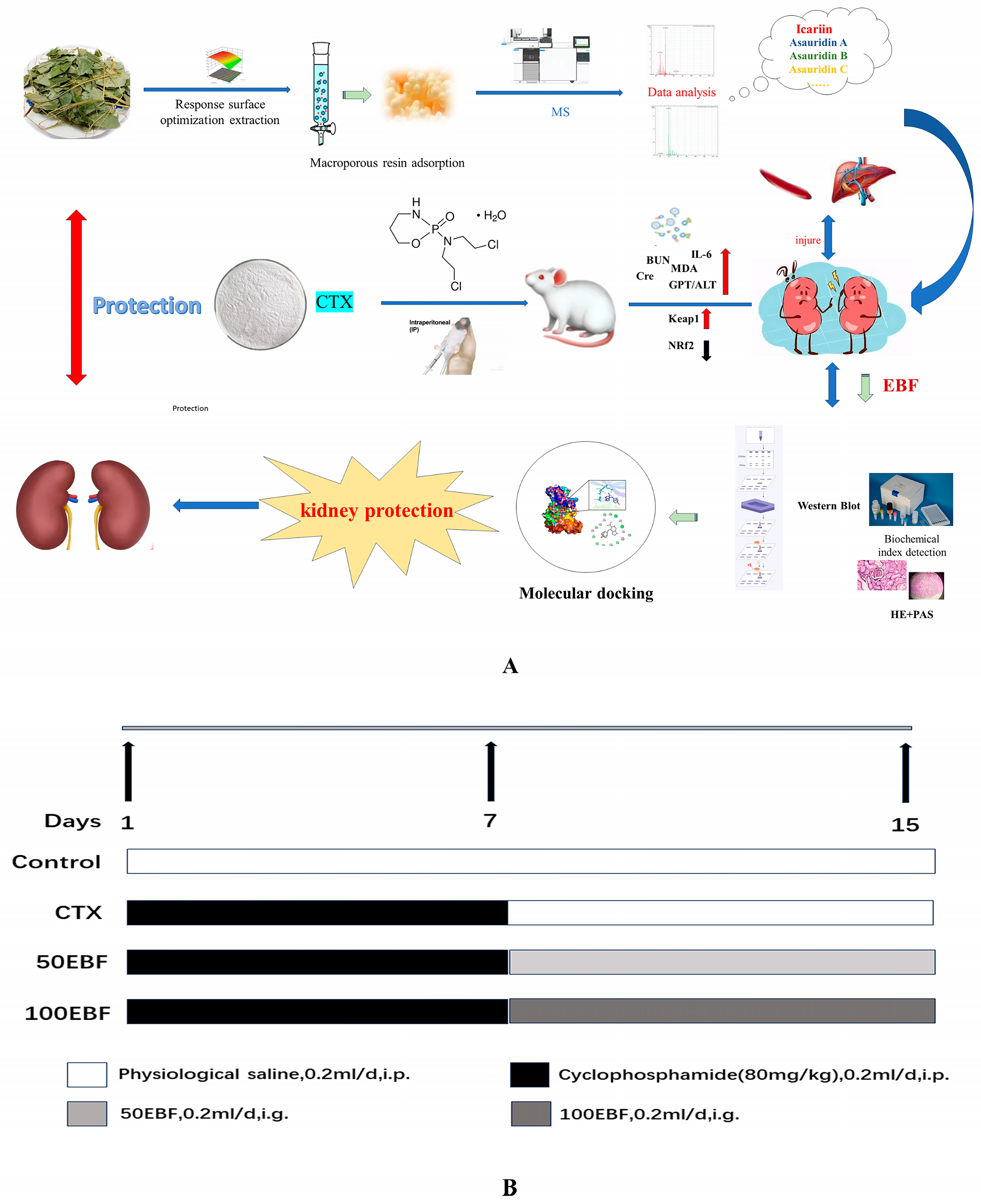
:1. Introduction
2. Results and Discussion
2.1. Compound Preparation Results for Epimedium Stem and Leaf Flavonoids
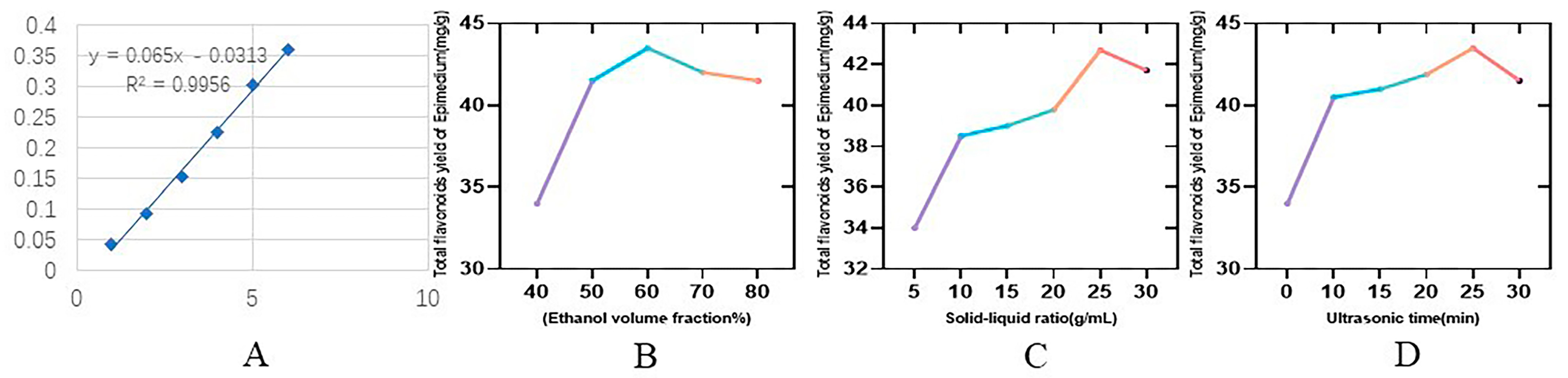
2.1.1. Standard Curves
2.1.2. Results of the One-Way Experiment
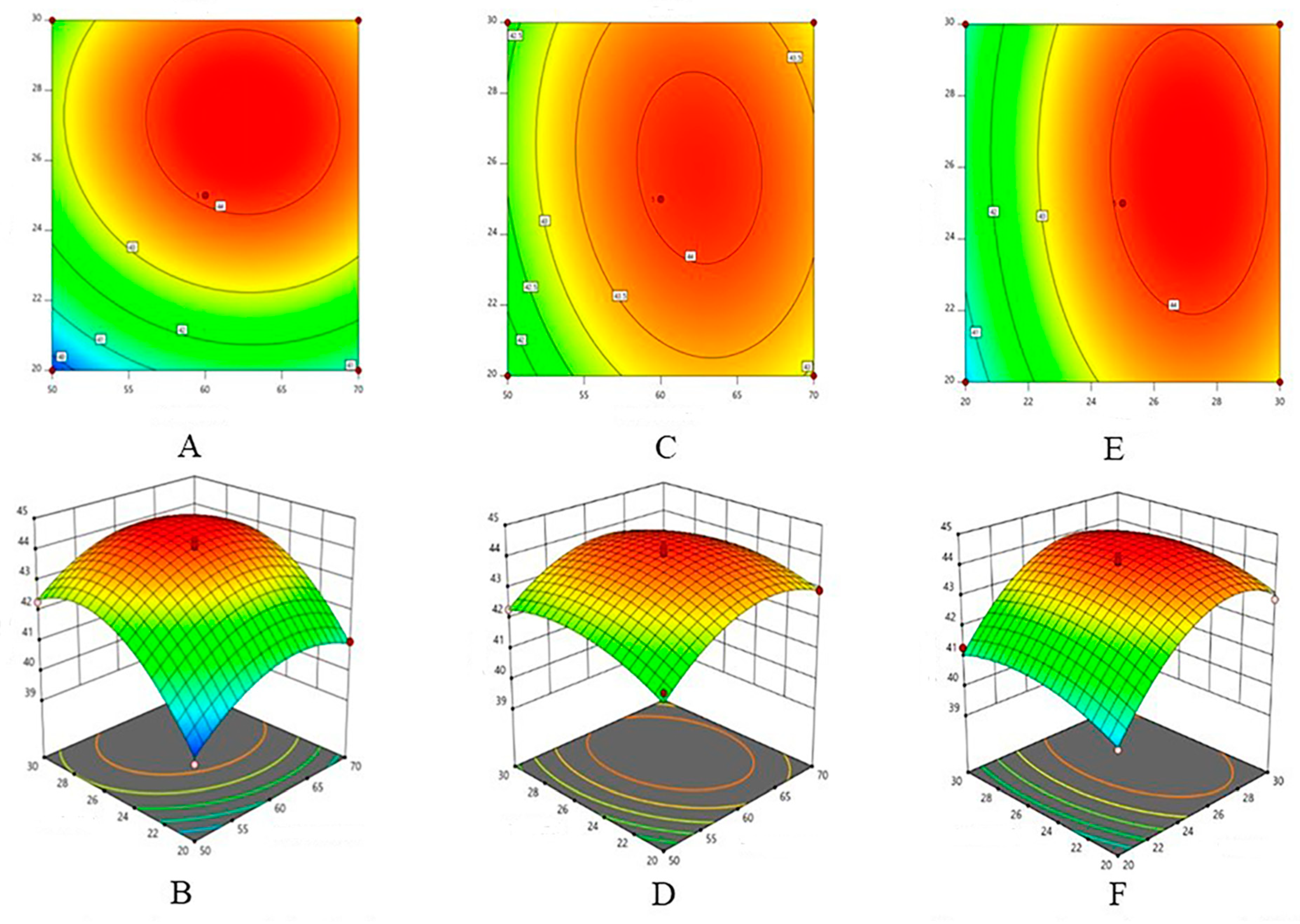
2.1.3. Response Surface Experiment Results
2.1.4. Validation Results of Optimal Extraction Conditions
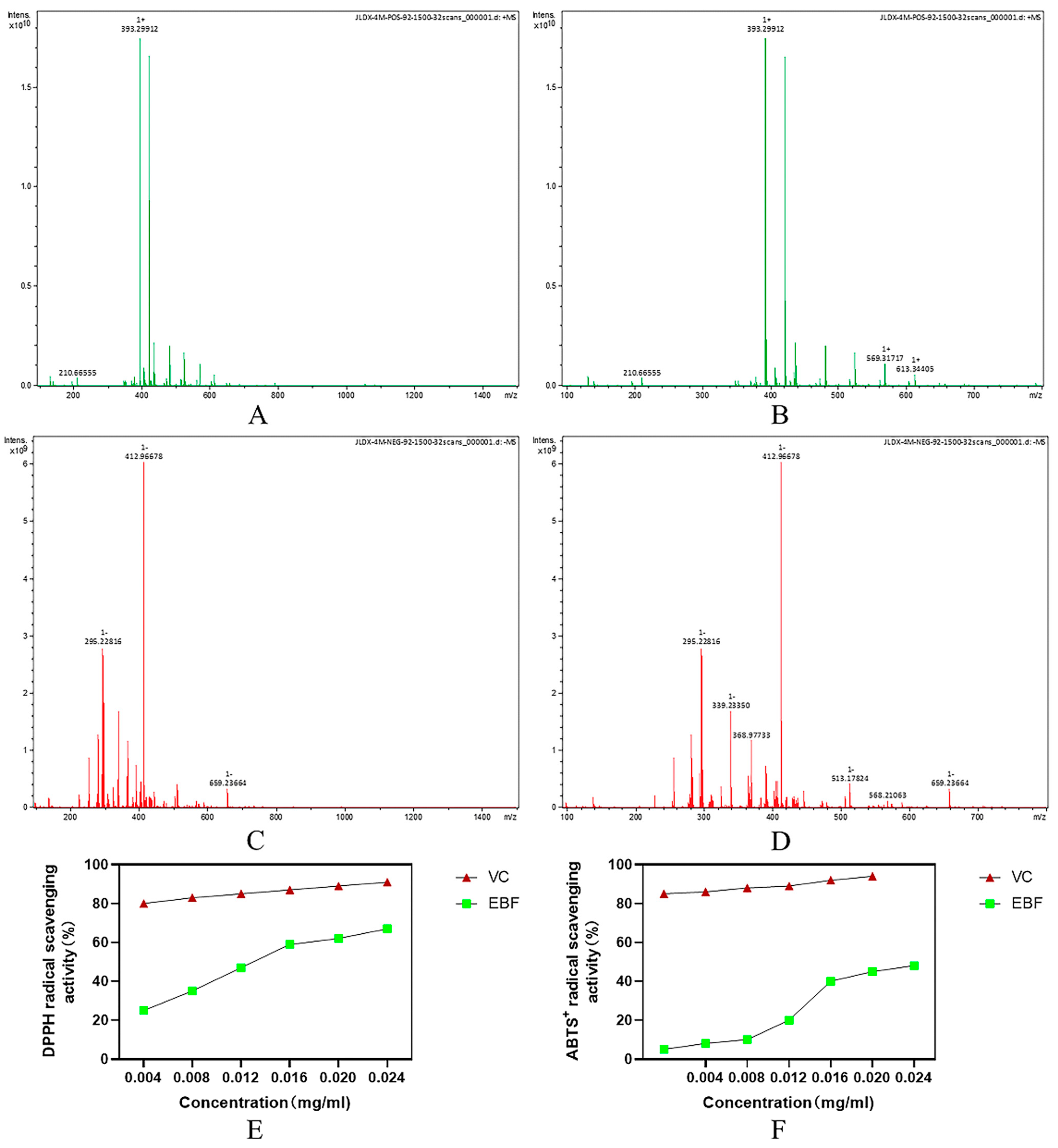
2.2. Mass Spectrometry Analysis of the Flavonoid Composition and Content of Epimedium Stems and Leaves
2.3. In Vitro Antioxidant Activity Assay Results
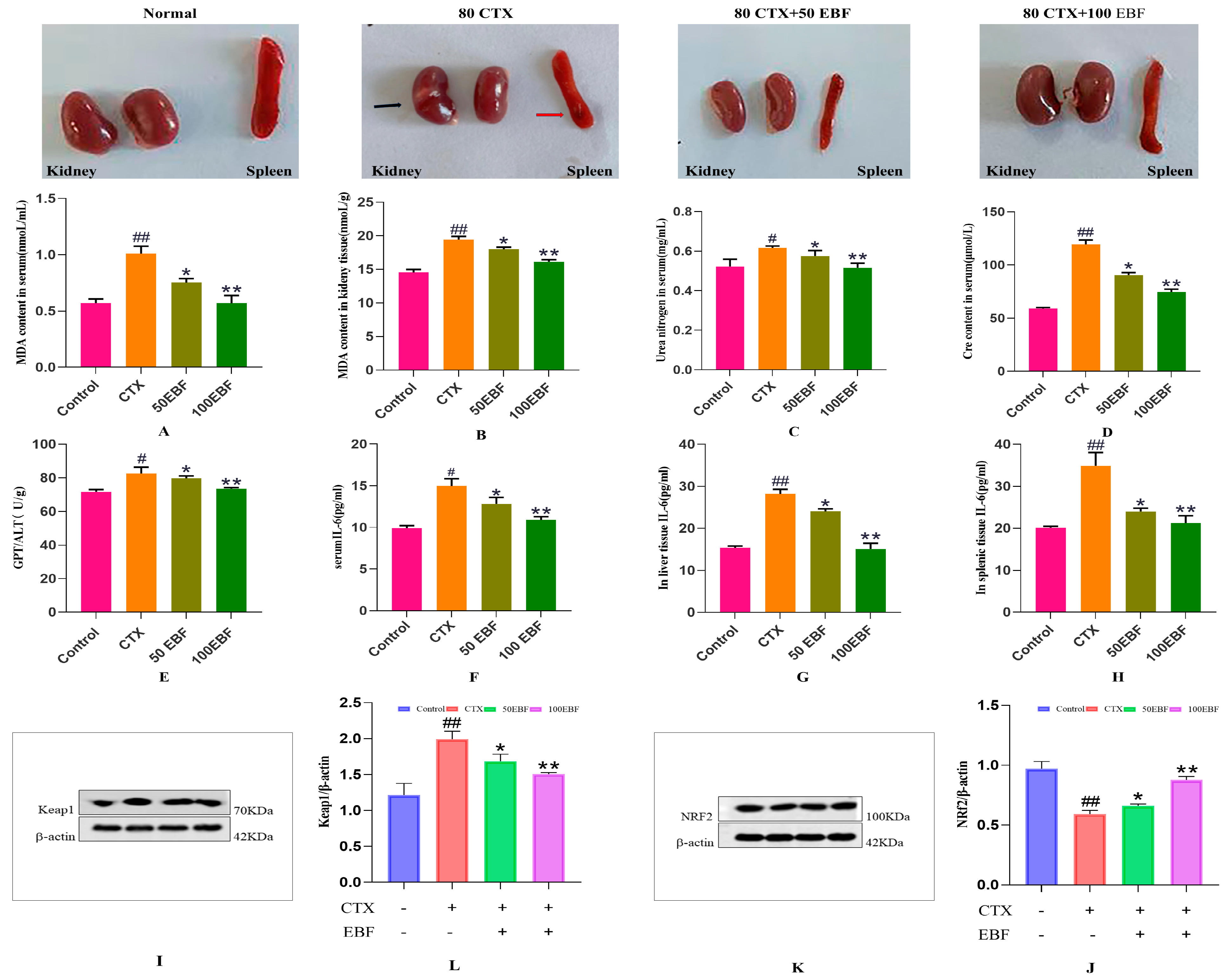
2.4. Effects of Epimedium Stem Flavonoids on Body Weight and Organs in CTX Mice
2.5. Effects of Biochemical Indices of Kidney Injury
2.6. Expression Levels of Keap1 and Nrf2 Proteins in Renal Tissue of Mice in Each Group
2.7. Histopathological Analysis
2.8. Molecular Docking Validation of Core Targets and Active Compounds
3. Materials and Methods
3.1. Materials
3.2. Optimization of the Extraction Process of Total Flavonoids from Epimedium Stems and Leaves
3.2.1. Extraction of Total flavonoids from Epimedium Stems and Leaves
3.2.2. Standard Curve and Sample Determination
3.2.3. One-Way Experiment on the Extraction of Flavonoids from Epimedium Stems and Leaves
3.2.4. Response Surface Experiments on the Extraction of Flavonoids from Epimedium Stems and Leaves
3.2.5. Verification Experiment of Optimal Extraction Conditions
3.3. Purification and Mass Spectrometry (MS) Analysis of Flavonoids from Epimedium Stems and Leaves
3.4. In Vitro Antioxidant Activity Assay
3.5. Animal Research
- blank control group (normal saline)
- model group (80 mg/kg cyclophosphamide intraperitoneal injection)
- total flavonoid low dose (50 mg/kg) group
- total flavonoid high dose (100 mg/kg) group
3.5.1. Sample Collection
3.5.2. Mouse Serum and Kidney Tissue Biochemical Assays
3.5.3. The Protein Levels of Keap1 and Nrf2 in Kidney of Mice Were Detected by Western Blot
3.5.4. Histopathological Observations of the Mouse Kidney
3.6. Molecular Docking
3.7. Statistical Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Jiang, X.; Ren, Z.; Zhao, B.; Zhou, S.; Ying, X.; Tang, Y. Ameliorating Effect of Pentadecapeptide Derived from Cyclina sinensis on Cyclophosphamide-Induced Nephrotoxicity. Mar. Drugs 2020, 18, 462. [Google Scholar] [CrossRef] [PubMed]
- Hwang, M.; Medley, S.; Shakeel, F.; Vanderwerff, B.; Zawistowski, M.; Kidwell, K.M.; Hertz, D.L. Lack of association of CYP2B6 pharmacogenetics with cyclophosphamide toxicity in patients with cancer. Support. Care Cancer 2022, 30, 7355–7363. [Google Scholar] [CrossRef] [PubMed]
- Han, H.-Y.; Choi, M.-S.; Yoon, S.; Ko, J.-W.; Kim, S.-K.; Kim, T.-W. Investigation of Ifosfamide Toxicity Induces Common Upstream Regulator in Liver and Kidney. Int. J. Mol. Sci. 2021, 22, 12201. [Google Scholar] [CrossRef] [PubMed]
- Mao, Z.; Zhong, K.; Liu, X.; Zeng, X. Ferroptosis contributes to cyclophosphamide-induced hemorrhagic cystitis. Chem.-Biol. Interact. 2023, 384, 110701. [Google Scholar] [CrossRef] [PubMed]
- Česen, M.; Eleršek, T.; Novak, M.; Žegura, B.; Kosjek, T.; Filipič, M.; Heath, E. Ecotoxicity and genotoxicity of cyclophosphamide, ifosfamide, their metabolites/transformation products and their mixtures. Environ. Pollut. 2016, 210, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Temel, Y.; Kucukler, S.; Yıldırım, S.; Caglayan, C.; Kandemir, F.M. Protective effect of chrysin on cyclophosphamide-induced hepatotoxicity and nephrotoxicity via the inhibition of oxidative stress, inflammation, and apoptosis. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2020, 393, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.S. Ganoderic acid A against cyclophosphamide-induced hepatic toxicity in mice. J. Biochem. Mol. Toxicol. 2019, 33, e22271. [Google Scholar]
- Uber, A.M.; Sutherland, S.M. Nephrotoxins and nephrotoxic acute kidney injury. Pediatr. Nephrol. 2020, 35, 1825–1833. [Google Scholar] [CrossRef]
- Cortazar, F.B.; Muhsin, S.A.; Pendergraft, W.F.; Wallace, Z.S.; Dunbar, C.; Laliberte, K.; Niles, J.L. Combination Therapy with Rituximab and Cyclophosphamide for Remission Induction in ANCA Vasculitis. Kidney Int. Rep. 2018, 3, 394–402. [Google Scholar] [CrossRef]
- Zhao, Y.-T.; Yin, H.; Hu, C.; Zeng, J.; Zhang, S.; Chen, S.; Zheng, W.; Li, M.; Jin, L.; Liu, Y.; et al. Tilapia Skin Peptides Ameliorate Cyclophosphamide-Induced Anxiety- and Depression-Like Behavior via Improving Oxidative Stress, Neuroinflammation, Neuron Apoptosis, and Neurogenesis in Mice. Front. Nutr. 2022, 9, 882175. [Google Scholar] [CrossRef]
- Pugh, D.; Farrah, T.E.; Gallacher, P.J.; Kluth, D.C.; Dhaun, N. Cyclophosphamide-Induced Lung Injury. Kidney Int. Rep. 2019, 4, 484–486. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Luo, G.; Etxeberria, J.; Hao, Y. Global Patterns and Trends in Lung Cancer Incidence: A Population-Based Study. J. Thorac. Oncol. 2021, 16, 933–944. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Fang, H.; Chong, Y.; Lin, L.; Xie, T.; Ji, J.; Shen, C.; Shi, C.; Shan, J. Cyclophosphamide Induces Lipid and Metabolite Perturbation in Amniotic Fluid during Rat Embryonic Development. Metabolites 2022, 12, 1105. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Jung, H.A.; Sohn, H.S.; Kim, J.W.; Choi, J.S. Potential of Icariin Metabolites from Epimedium koreanum Nakai as Antidiabetic Therapeutic Agents. Molecules 2017, 22, 986. [Google Scholar] [CrossRef]
- Yin, J.; Jiang, J.; Wang, H.; Lu, G. Protective effects of specneuzhenide on renal injury in rats with diabetic nephropathy. Open Med. 2019, 14, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Bao, H. Icariin reduces mitochondrial oxidative stress injury in diabetic rat hearts. China J. Chin. Mater. Medica 2011, 36, 1503–1507. [Google Scholar]
- Zhu, L.; Luo, C.; Ma, C.; Kong, L.; Huang, Y.; Yang, W.; Huang, C.; Jiang, W.; Yi, J. Inhibition of the NF-κB pathway and ERK-mediated mitochondrial apoptotic pathway takes part in the mitigative effect of betulinic acid on inflammation and oxidative stress in cyclophosphamide-triggered renal damage of mice. Ecotoxicol. Environ. Saf. 2022, 246, 114150. [Google Scholar] [CrossRef]
- Steinbrenner, I.; Sekula, P.; Kotsis, F.; von Cube, M.; Cheng, Y.; Nadal, J.; Schmid, M.; Schneider, M.P.; Krane, V.; Nauck, M.; et al. Association of osteopontin with kidney function and kidney failure in chronic kidney disease patients: The GCKD study. Nephrol. Dial. Transplant. 2022, 38, 1430–1438. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, R.; Mu, L.; Yang, W.; Zhang, X.; Han, L.; Lv, C.; Lu, J. Total flavonoids in Epimedium koreanum Nakai alleviated chronic renal failure via promoting AMPK activation. Food Funct. 2022, 13, 904–919. [Google Scholar] [CrossRef]
- Rodríguez, C.; Muoz, M.; Contreras, C.; Prieto, D. AMPK, Metabolism and Vascular Function. FEBS J. 2021, 288, 3746–3771. [Google Scholar] [CrossRef]
- Zhao, M.-R.; Sui, R.-X.; Yu, M.-Y.; Tian, T.; Zhang, L.; Yang, Y.-B.; Xiao, B.-G. Antagonizing astrocytic platelet activating factor receptor-neuroinflammation for total flavone of epimedium in response to cuprizone demyelination. Int. Immunopharmacol. 2021, 101, 108181. [Google Scholar]
- Yuan, D.; Wang, H.; He, H.; Jia, L.; He, Y.; Wang, T.; Zeng, X.; Li, Y.; Li, S.; Zhang, C. Protective Effects of Total Flavonoids fromEpimediumon the Male Mouse Reproductive System Against Cyclophosphamide-Induced Oxidative Injury by Up-Regulating the Expressions of SOD3 and GPX1. Phytother. Res. 2013, 28, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Yang, Z.; Shi, J.; Yang, J.; Zhao, J.; He, Y.; Qi, M. Total flavonoids of Epimedium ameliorates testicular damage in streptozotocin-induced diabetic rats by suppressing inflammation and oxidative stress. Trends Ecol. Evol. 2020, 35, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Abraham, P.; Rabi, S. Protective effect of aminoguanidine against cyclophosphamide-induced oxidative stress and renal damage in rats. Redox Rep. Commun. Free Radic. Res. 2011, 16, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Sugumar, E.; Kanakasabapathy, I.; Abraham, P. Normal plasma creatinine level despite histological evidence of damage and increased oxidative stress in the kidneys of cyclophosphamide treated rats. Clin. Chim. Acta 2007, 376, 244–245. [Google Scholar] [CrossRef] [PubMed]
- Aboubakr, M.; Farag, A.; Elfadadny, A.; Alkafafy, M.; Soliman, A.; Elbadawy, M. Antioxidant and anti-apoptotic potency of allicin and lycopene against methotrexate-induced cardiac injury in rats. Environ. Sci. Pollut. Res. 2023, 30, 88724–88733. [Google Scholar] [CrossRef]
- Sallam, A.O.; Rizk, H.A.; Emam, M.A.; Fadl, S.E.; Abdelhiee, E.Y.; Khater, H.; Elkomy, A.; Aboubakr, M. The Ameliorative Effects of L-Carnitine against Cisplatin-Induced Gonadal Toxicity in Rats. Pak. Vet. J. 2021, 41, 147–151. [Google Scholar]
- Liu, W.; Wang, J.; Zhang, Z.; Xu, J.; Xie, Z.; Slavin, M.; Gao, X. In vitro and in vivo antioxidant activity of a fructan from the roots of Arctium lappa L. Int. J. Biol. Macromol. 2014, 65, 446–453. [Google Scholar] [CrossRef]
- Zhao, X.; Yang, Z.B.; Yang, W.R.; Wang, Y.; Jiang, S.Z.; Zhang, G.G. Effects of ginger root (Zingiber officinale) on laying performance and antioxidant status of laying hens and on dietary oxidation stability. Poult. Sci. 2011, 90, 1720. [Google Scholar] [CrossRef]
- Gschwend, D.A.; Good, A.C.; Kuntz, I.D. Molecular docking towards drug discovery. J. Mol. Recognit. 2015, 9, 175–186. [Google Scholar] [CrossRef]
- Meng, X.Y.; Zhang, H.X.; Mezei, M.; Cui, M. Molecular Docking: A Powerful Approach for Structure-Based Drug Discovery. Curr. Comput. Aided Drug Des. 2016, 7, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Luo, A.; Ge, Z.; Fan, Y.; Luo, A.; Chun, Z.; He, X. In Vitro and In Vivo Antioxidant Activity of a Water-Soluble Polysaccharide from Dendrobium denneanum. Molecules 2011, 16, 1579–1592. [Google Scholar] [CrossRef] [PubMed]
- Pinzi, L.; Caporuscio, F.; Rastelli, G. Selection of protein conformations for structure-based polypharmacology studies. Drug Discov. Today 2018, 23, 1889–1896. [Google Scholar] [CrossRef]
- Chen, L.; Ma, R.; Luo, P.; Shi, D.; Shi, X.; Nian, H.; Chang, S.-X.; Yuan, W.; Li, G.-W. Effects of Total Flavonoids of Epimedium on Bone Marrow Adipose Tissue in Ovariectomized Rats. Front. Endocrinol. 2022, 13, 900816. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Chen, H.; Wang, Z.; Lan, G.; Zhang, L. Comparative studies on antioxidant activities of extracts and fractions from the leaves and stem of Epimedium koreanum Nakai. J. Food Sci. Technol. 2013, 50, 1122–1129. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Rijia, C.; Chunyan, Y.; Pharmacy, S.O.; Journal of Guangdong Pharmaceutical University. Optimization of ultrasonic assisted extraction of total flavonoids from leaves of Psidium guajava by response surface methodology. J. Guangdong Pharm. Univ. 2014, 5, 417–421. [Google Scholar]
- Zhang, X.; Tang, B.; Wen, S.; Wang, Y.; Pan, C.; Qu, L.; Yin, Y.; Wei, Y. Advancements in the Biotransformation and Biosynthesis of the Primary Active Flavonoids Derived from Epimedium. Molecules 2023, 28, 7173. [Google Scholar] [CrossRef]
- Abramovič, H.; Grobin, B.; Poklar Ulrih, N.; Cigić, B. Relevance and Standardization of In Vitro Antioxidant Assays: ABTS, DPPH, and Folin–Ciocalteu. J. Chem. 2018, 2018, 4608405. [Google Scholar] [CrossRef]
- Kotora, P.; Ereň, F.; Filo, J.; Loos, D.; Gregáň, F. The Scavenging of DPPH, Galvinoxyl and ABTS Radicals by Imine Analogs of Resveratrol. Molecules 2016, 21, 127. [Google Scholar] [CrossRef]
- Galal, S.M.; Mansour, H.H.; Elkhoely, A.A. Diallyl sulfide alleviates cyclophosphamide-induced nephropathic encephalopathy in rats. Toxicol. Mech. Methods 2020, 30, 208. [Google Scholar] [CrossRef]
- Bedir, F.; Kocatürk, H.; Yapanoğlu, T.; Gürsul, C.; Arslan, R.; Mammadov, R.; Çoban, A.; Altuner, D.; Suleyman, H. Protective effect of taxifolin against prooxidant and proinflammatory kidney damage associated with acrylamide in rats. Biomed. Pharmacother. 2021, 139, 111660. [Google Scholar] [CrossRef] [PubMed]
- Diao, H.-Y.; Zhu, W.; Liu, J.; Yin, S.; Wang, J.-H.; Li, C.-L. Salvianolic Acid A Improves Rat Kidney Injury by Regulating MAPKs and TGFβ1/Smads Signaling Pathways. Molecules 2023, 28, 3630. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.-l.; Liu, S.-h.; Shen, S.-h.; Jian, L.-y.; Yuan, Q.; Guo, H.-h.; Huang, J.-s.; Chen, P.-h.; Huang, R.-f. Protective Mechanism of Cordyceps sinensis Treatment on Acute Kidney Injury-Induced Acute Lung Injury through AMPK/mTOR Signaling Pathway. Chin. J. Integr. Med. 2023, 29, 875–884. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, L.; Zhou, Y.; Feng, X.; Ye, C.; Wang, C. Icariin attenuates renal fibrosis in chronic kidney disease by inhibiting interleukin-1β/transforming growth factor-β-mediated activation of renal fibroblasts. Phytother. Res. 2021, 35, 6204–6215. [Google Scholar] [CrossRef] [PubMed]
- Caglayan, C.; Temel, Y.; Kandemir, F.M.; Yildirim, S.; Kucukler, S. Naringin protects against cyclophosphamide-induced hepatotoxicity and nephrotoxicity through modulation of oxidative stress, inflammation, apoptosis, autophagy, and DNA damage. Environ. Sci. Pollut. Res. 2018, 25, 20968–20984. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Lin, X.; Li, H.; Yuan, J.; Peng, Y.; Dong, L.; Dai, S. Paeoniflorin ameliorates renal function in cyclophosphamide-induced mice via AMPK suppressed inflammation and apoptosis. Biomed. Pharmacother. Biomed. Pharmacother. 2016, 84, 1899–1905. [Google Scholar] [CrossRef] [PubMed]
- Sawie, H.G.; Khadrawy, Y.A.; El-Gizawy, M.M.; Mourad, H.H.; Omara, E.A.; Hosny, E.N. Effect of alpha-lipoic acid and caffeine-loaded chitosan nanoparticles on obesity and its complications in liver and kidney in rats. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2023, 396, 3017–3031. [Google Scholar] [CrossRef]
- Hu, J.F.; Wang, H.X.; Li, H.H.; Hu, J.; Yu, Y.; Gao, Q. Inhibition of ALDH2 expression aggravates renal injury in a rat sepsis syndrome model. Exp. Ther. Med. 2017, 14, 2249–2254. [Google Scholar] [CrossRef]
- Rehman, M.U.; Tahir, M.; Ali, F.; Qamar, W.; Lateef, A.; Khan, R.; Quaiyoom, A.; Oday-O-Hamiza; Sultana, S. Cyclophosphamide-induced nephrotoxicity, genotoxicity, and damage in kidney genomic DNA of Swiss albino mice: The protective effect of Ellagic acid. Mol. Cell. Biochem. 2012, 365, 119–127. [Google Scholar] [CrossRef]
- Vaara, S.T.; Glassford, N.; Eastwood, G.M.; Canet, E.; Mrtensson, J.; Bellomo, R. Point-of-care creatinine measurements to predict acute kidney injury. Acta Anaesthesiol. Scand. 2020, 64, 766–773. [Google Scholar] [CrossRef]
- Xie, C.; Liu, L.; Wang, Z.; Xie, H.; Feng, Y.; Suo, J.; Feng, G. Icariin Improves Sepsis-Induced Mortality and Acute Kidney Injury. Pharmacol. Int. J. Exp. Clin. Pharmacol. 2018, 102, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, M.; Najafi, H.; Mohamadi Yarijani, Z.; Vaezi, G.; Hojati, V. Piperine pretreatment attenuates renal ischemia-reperfusion induced liver injury. Heliyon 2019, 5, e02180. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, A.; Prabakarakrishnan, R.; Govindaraju, K.; Sugumar, V.; Sathiskumar, K.; Narenkumar, J.; Ramanan, A.; Kumar, B.S. Hepato and renoprotective activity of Kappaphycus alvarezii ethanolic extract in cisplatin causes hepatic and kidney harm in Albino Wistar rats. Aquac. Int. 2023, 31, 1925–1940. [Google Scholar] [CrossRef]
- İşeri, S.; Ercan, F.; Gedik, N.; Yüksel, M.; Alican, İ. Simvastatin attenuates cisplatin-induced kidney and liver damage in rats. Toxicology 2007, 230, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, P.; Sharma, H.; Kumar, U.; Mayachari, A.; Sangli, G.; Singh, S. Protective effects of Glycyrrhiza glabra supplementation against methotrexate-induced hepato-renal damage in rats: An experimental approach. J. Ethnopharmacol. 2020, 263, 113209. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Jiang, W.; Zhang, L.; Abasubong, K.; Zhang, D.; Li, X.; Jiang, G.; Chi, C.; Liu, W. Protective effects of dietary icariin on lipopolysaccharide-induced acute oxidative stress and hepatopancreas injury in Chinese mitten crab, Eriocheir sinensis. Comp. Biochem. Physiol. Toxicol. Pharmacol. CBP 2022, 251, 109192. [Google Scholar] [CrossRef]
- Xia, H.; Shanshan, X.; Sumeng, L.; Fang, X.; Tao, Z.; Cheng, C. LncRNA RMRP aggravates LPS-induced HK-2 cell injury and AKI mice kidney injury by upregulating COX2 protein via targeting ELAVL1. Int. Immunopharmacol. 2023, 116, 109676. [Google Scholar] [CrossRef]
- Zhao, P.; Zhang, L.; Gao, L.; Ding, Q.; Yang, Q.; Kuai, J. Ulinastatin attenuates lipopolysaccharide-induced cardiac dysfunction by inhibiting inflammation and regulating autophagy. Exp. Ther. Med. 2020, 20, 1064–1072. [Google Scholar] [CrossRef]
- Kang, X.; Jing, M.; Zhang, G.; He, L.; Hong, P.; Deng, C. The Ameliorating Effect of Plasma Protein from Tachypleus tridentatus on Cyclophosphamide-Induced Acute Kidney Injury in Mice. Mar. Drugs 2019, 17, 227. [Google Scholar] [CrossRef]
- Smith, E.; Al-Abadi, E.; Armon, K.; Bailey, K.; Hedrich, C.M. Outcomes following mycophenolate mofetil versus cyclophosphamide induction treatment for proliferative juvenile-onset lupus nephritis. Lupus 2019, 28, 096120331983671. [Google Scholar] [CrossRef]
- Uribe-Carretero, E.; Martinez-Chacón, G.; Yakhine-Diop, S.M.S.; Duque-González, G.; Rodríguez-Arribas, M.; Alegre-Cortés, E.; Paredes-Barquero, M.; Canales-Cortés, S.; Pizarro-Estrella, E.; Cuadrado, A.; et al. Loss of KEAP1 Causes an Accumulation of Nondegradative Organelles. Antioxidants 2022, 11, 1398. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Cho, M.S.; Thiagarajan, P.; Aung, F.M.; Sood, A.K.; Afshar-Kharghan, V. A small amount of cyclooxygenase 2 (COX2) is constitutively expressed in platelets. Platelets 2016, 28, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Guan, N.; Zhang, M.; Gong, W.Y.; Mao, X.Y.; Yang, S.S.; Hao, C.M. The hypoxia-inducible factor prolyl hydroxylase inhibitor FG4592 promotes natriuresis through upregulation of COX2 in the renal medulla. Hypertens. Res. 2022, 45, 814–823. [Google Scholar] [CrossRef] [PubMed]
- Amanat, S.; Shal, B.; Seo, E.K.; Ali, H.; Khan, S. Icariin attenuates cyclophosphamide-induced cystitis via down-regulation of NF-кB and up-regulation of Nrf-2/HO-1 signaling pathways in mice model. Int. Immunopharmacol. 2022, 106, 108604. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.M.; Germoush, M.O.; Al-Anazi, K.M.; Mahmoud, A.H.; Allam, A.A. Commiphora molmol protects against methotrexate-induced nephrotoxicity by up-regulating Nrf2/ARE/HO-1 signaling. Biomed. Pharmacother. Biomed. Pharmacother. 2018, 106, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Zhao, H.; Qiao, C. Icariin protects podocytes from NLRP3 activation by Sesn2-induced mitophagy through the Keap1-Nrf2/HO-1 axis in diabetic nephropathy. Phytomedicine Int. J. Phytother. Phytopharm. 2022, 99, 154005. [Google Scholar] [CrossRef] [PubMed]
- Shraim, A.M.; Ahmed, T.A.; Rahman, M.M.; Hijji, Y.M. Determination of total flavonoid content by aluminum chloride assay: A critical evaluation. LWT 2021, 150, 111932. [Google Scholar] [CrossRef]
- Jia, G.; Zhao, H.; Hou, D.; Sun, T.; Lin, W. Quantitative Determination of Total Flavonoids from Polygonatum sibiricum by Spectrophotometry. IOP Conf. Ser. Mater. Sci. Eng. 2019, 677, 022126. [Google Scholar] [CrossRef]
- Hou, M.; Hu, W.; Xiu, Z.; Jiang, A.; Men, L.; Hao, K.; Sun, X.; Cao, D. Preparative Purification of Total Flavonoids from Sophora tonkinensis Gagnep. by Macroporous Resin Column Chromatography and Comparative Analysis of Flavonoid Profiles by HPLC-PAD. Molecules 2019, 24, 3200. [Google Scholar] [CrossRef]
- Xie, Y.; Guo, Q.S.; Wang, G.S. Preparative Separation and Purification of the Total Flavonoids in Scorzonera austriaca with Macroporous Resins. Molecules 2016, 21, 768. [Google Scholar] [CrossRef]
- Zheng, Y.; Duan, W.; Sun, J.; Zhao, C.; Cheng, Q.; Li, C.; Peng, G. Structural Identification and Conversion Analysis of Malonyl Isoflavonoid Glycosides in Astragali Radix by HPLC Coupled with ESI-Q TOF/MS. Molecules 2019, 24, 3929. [Google Scholar] [CrossRef] [PubMed]
- Compton, D.L.; Evans, K.O.; Appell, M.; Goodell, J.R. Protection of Antioxidants, Vitamins E and C, from Ultraviolet Degradation using Feruloylated Vegetable Oil. J. Am. Oil Chem. Soc. 2019, 96, 999–1009. [Google Scholar] [CrossRef]
- Sirivibulkovit, K.; Nouanthavong, S.; Sameenoi, Y. Paper-based DPPH Assay for Antioxidant Activity Analysis. Anal. Sci. 2018, 34, 795–800. [Google Scholar] [CrossRef]
- Oei, A.T.T.; Garnett, J.L. The surface reaction between manganese dioxide and 1,1-diphenyl-2-picrylhydrazine. Two type EPR methods for the estimation of active surface areas of oxides. J. Catal. 1970, 19, 176–194. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, Y.-F.; Zhou, Z.-Q. Phenolic and flavonoid contents of mandarin (Citrus reticulata Blanco) fruit tissues and their antioxidant capacity as evaluated by DPPH and ABTS methods. J. Integr. Agric. 2018, 17, 256–263. [Google Scholar] [CrossRef]
- Tang, X.; Jiao, C.; Wei, Y.; Zhuang, X.-Y.; Xiao, Q.; Chen, J.; Chen, F.-Q.; Yang, Q.-M.; Weng, H.-F.; Fang, B.-S.; et al. Biochemical Characterization and Cold-Adaption Mechanism of a PL-17 Family Alginate Lyase Aly23 from Marine Bacterium Pseudoalteromonas sp. ASY5 and Its Application for Oligosaccharides Production. Mar. Drugs 2022, 20, 126. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhao, X.; Xu, M. Chemical Composition, Antimicrobial and Antioxidant Activity of Essential Oil from Allium tenuissimum L. Flowers. Foods 2022, 11, 3876. [Google Scholar] [CrossRef]
- Yu, B. Effect of total flavone of Epimedium on gene expression of androgen receptor in kidney and gonad of kidney-yang deficiency mice. Shanxi Norm. Univ. 2008, 55, 02. [Google Scholar]
- Xu, Y.; Lu, X.; Zhang, L.; Wang, L.; Sun, C. Icaritin activates Nrf2/Keap1 signaling to protect neuronal cells from oxidative stress. Chem. Biol. Drug Des. 2020, 97, 111–120. [Google Scholar] [CrossRef]
- Li, Y.; Li, Z.; Ye, T.; Hao, F.; Wang, Y.; Li, W.; Yan, Q.; Shi, H.; Han, W. Mechanism of Erzhiwan in treating osteoporosis based on molecular docking technology and molecular dynamics simulation. J. Mol. Model. 2022, 29, 21. [Google Scholar] [CrossRef]
- Sokalingam, S.; Munussami, G.; Kim, J.-R.; Lee, S.-G. Validation on the molecular docking efficiency of lipocalin family of proteins. J. Ind. Eng. Chem. 2018, 67, 293–300. [Google Scholar] [CrossRef]
- Rehan, M.; Shafiullah; Sultanat. Cytotoxicity of oleanane type triterpene from leaf extract of Pterospermum acerifolium (in vitro) and theoretical investigation of inhibitory signaling pathway. Chin. Herb. Med. 2021, 13, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Pertiwi, K.O.; Fatchiyah, F. The Two Peptides Fragments of Goat Milk CSN1S2 Protein Blocked Insulin Receptor to Interact Ligand: In Silico Study. J. Phys. Conf. Ser. 2020, 1665, 012018. [Google Scholar] [CrossRef]


| Test Number | X1 Ethanol Volume Fraction (%) | X2 Ratio (g/mL) | X3 Ultrasound Time (min) | Total Flavonoid Yield of Epimedium (mg/g) |
|---|---|---|---|---|
| 1 | −1 | −1 | 0 | 39.4 |
| 2 | 1 | −1 | 0 | 40.99 |
| 3 | −1 | 1 | 0 | 42.3 |
| 4 | 1 | 1 | 0 | 43.5 |
| 5 | −1 | 0 | −1 | 41.8 |
| 6 | 1 | 0 | −1 | 42.92 |
| 7 | −1 | 0 | 1 | 42.3 |
| 8 | 1 | 0 | 1 | 42.8 |
| 9 | 0 | −1 | −1 | 40.3 |
| 10 | 0 | 1 | −1 | 42.9 |
| 11 | 0 | −1 | 1 | 41.3 |
| 12 | 0 | −1 | 1 | 43.5 |
| 13 | 0 | 0 | 0 | 44.1 |
| 14 | 0 | 0 | 0 | 44.3 |
| 15 | 0 | 0 | 0 | 44.12 |
| 16 | 0 | 0 | 0 | 43.72 |
| 17 | 0 | 0 | 0 | 44.16 |
| Source (of Information etc.) | Square Sum (e.g., Equation of Squares) | (Number of) Degrees of Freedom (Physics) | Mean Square | F-Value | PR > F |
|---|---|---|---|---|---|
| modelling | 33.03 | 9 | 3.67 | 43.54 | <0.0001 |
| X1 | 2.43 | 1 | 2.43 | 28.84 | 0.0010 |
| X2 | 13.03 | 1 | 13.03 | 154.58 | <0.0001 |
| X3 | 0.4900 | 1 | 0.4900 | 5.81 | 0.0467 |
| X X12 | 0.0380 | 1 | 0.0380 | 0.4511 | 0.5234 |
| X X13 | 0.0961 | 1 | 0.0961 | 1.14 | 0.3211 |
| X X23 | 0.0400 | 1 | 0.0400 | 0.4745 | 0.5131 |
| X12 | 4.54 | 1 | 4.54 | 53.90 | 0.0002 |
| X22 | 9.39 | 1 | 9.39 | 111.45 | <0.0001 |
| X32 | 1.45 | 1 | 1.45 | 17.17 | 0.0043 |
| residual | 0.5901 | 7 | 0.0843 | ||
| F-value of the mismatch ratio | 0.4037 | 3 | 0.1346 | 2.89 | 0.1661 |
| pure error | 0.1864 | 4 | 0.0466 | ||
| aggregate | 33.62 | 16 |
| m/z | Search | PPM | Ion Types | Molecular Formula | Calculate Mass | Intensity | Chinese Name |
|---|---|---|---|---|---|---|---|
| 367.1193 | 367.1187 | 1.63 | [M-H]- | C21H20O6 | 368.1260 | 360345120 | Isodehydro Icaritin or Anhydrocaritin or Anhydrocicaritin |
| 403.0962 | 403.0954 | 2.01 | [M+Cl]- | C21H20O6 | 368.1260 | 280661824 | Isodehydro Icaritin or Anhydrocaritin or Anhydrocicaritin |
| 407.0713 | 407.0722 | −2.12 | [M-2H+41K]- | C21H20O6 | 368.1260 | 912871 | Isodehydro Icaritin or Anhydrocaritin or Anhydrocicaritin |
| 413.1245 | 413.1242 | 0.84 | [M+HCOOH-H]- | C21H20O6 | 368.1260 | 2518385 | Isodehydro Icaritin or Anhydrocaritin or Anhydrocicaritin |
| 427.1399 | 427.1398 | 0.16 | [M+CH3COOH-H]- | C21H20O6 | 368.1260 | 5993696 | Isodehydro Icaritin or Anhydrocaritin or Anhydrocicaritin |
| 735.2453 | 735.2447 | 0.76 | [2M-H]- | C21H20O6 | 368.1260 | 3180218 | Isodehydro Icaritin or Anhydrocaritin or Anhydrocicaritin |
| 535.1381 | 535.1376 | 0.81 | [M+Cl]- | C26H28O10 | 500.1682 | 1107085 | Icariin II |
| 545.1666 | 545.1665 | 0.24 | [M+HCOOH-H]- | C26H28O10 | 500.1682 | 1046836 | Icariin II |
| 493.1502 | 493.1504 | −0.44 | [M-H20-H]- | C27H28O10 | 512.1682 | 12936170 | Icariin |
| 557.1674 | 557.1665 | 1.69 | [M+HCOOH-H]- | C27H28O10 | 512.1682 | 1417257 | Icariin |
| 571.1828 | 571.1821 | 1.24 | [M+CH3COOH-H]- | C27H28O10 | 512.1682 | 2500022 | Icariin |
| 495.1662 | 495.1661 | 0.25 | [M-H20-H]- | C27H30O10 | 514.1839 | 1322234 | Icariin II |
| 549.1534 | 549.1533 | 0.26 | [M+Cl]- | C27H30O10 | 514.1839 | 2988083 | Icariin II |
| 573.1985 | 573.1978 | 1.33 | [M+CH3COOH-H]- | C27H30O10 | 514.1839 | 1115969 | Icariin II |
| 511.1612 | 511.1610 | 0.39 | [M-H20-H]- | C27H30O11 | 530.1788 | 1931379 | Icariin I |
| 565.1486 | 565.1482 | 0.63 | [M+Cl]- | C27H30O11 | 530.1788 | 3307556 | Icariin I |
| 613.1927 | 613.1927 | 0.11 | [M-H20-H]- | C31H36O14 | 632.2105 | 1870759 | Icariin F |
| 631.2031 | 631.2032 | −0.19 | [M-H]- | C31H36O14 | 632.2105 | 1578115 | Icariin F |
| 677.2100 | 677.2087 | 1.88 | [M+HCOOH-H]- | C31H36O14 | 632.2105 | 1261814 | Icariin F |
| 645.2198 | 645.2189 | 1.47 | [M-H]- | C32H38O14 | 646.2262 | 24605194 | Arrowleaf Icariin B |
| 681.1960 | 681.1956 | 0.59 | [M+Cl]- | C32H38O14 | 646.2262 | 1442420 | Arrowleaf Icariin B |
| 691.2253 | 691.2244 | 1.29 | [M+HCOOH-H]- | C32H38O14 | 646.2262 | 6129730 | Arrowleaf Icariin B |
| 695.2119 | 695.2112 | 1.01 | [M+Cl]- | C33H40O14 | 660.2418 | 13050456 | 2″-Rhamnosylpastramonoside II |
| 643.2039 | 643.2032 | 1.09 | [M-H20-H]- | C32H38O15 | 662.2211 | 10209444 | Icariin A |
| 661.2145 | 661.2138 | 1.10 | [M-H]- | C32H38O15 | 662.2211 | 4458815 | Icariin A |
| 697.1909 | 697.1905 | 0.63 | [M+Cl]- | C32H38O15 | 662.2211 | 1738067 | Icariin A |
| 657.2198 | 657.2189 | 1.37 | [M-H20-H]- | C33H40O15 | 676.2367 | 26417782 | Icariin |
| 675.2303 | 675.2294 | 1.24 | [M-H]- | C33H40O15 | 676.2367 | 12396209 | Icariin |
| 697.2090 | 697.2108 | −2.67 | [M-2H+Na]- | C33H40O15 | 676.2367 | 4287317 | Icariin |
| 711.2068 | 711.2061 | 1.00 | [M+Cl]- | C33H40O15 | 676.2367 | 14240036 | Icariin |
| 721.2360 | 721.2349 | 1.51 | [M+HCOOH-H]- | C33H40O15 | 676.2367 | 1058330 | Icariin |
| 659.1989 | 659.1981 | 1.19 | [M-H20-H]- | C32H38O16 | 678.2160 | 5541848 | Hexandraside E |
| 673.2147 | 673.2138 | 1.29 | [M-H20-H]- | C33H40O16 | 692.2316 | 14542177 | Icariin |
| 713.2040 | 713.2058 | −2.53 | [M-2H+Na]- | C33H40O16 | 692.2316 | 5589455 | Icariin |
| 727.2019 | 727.2010 | 1.16 | [M+Cl]- | C33H40O16 | 692.2316 | 10505580 | Icariin |
| 807.2720 | 807.2717 | 0.38 | [M-H]- | C38H48O19 | 808.2790 | 1672456 | Baohuoside V or Chaohuodin B |
| 843.2497 | 843.2484 | 1.59 | [M+Cl]- | C38H48O19 | 808.2790 | 2725050 | Baohuoside V or Chaohuodin B |
| 853.2779 | 853.2772 | 0.79 | [M+HCOOH-H]- | C38H48O19 | 808.2790 | 961657 | Baohuoside V or Chaohuodin B |
| 821.2876 | 821.2874 | 0.28 | [M-H]- | C39H50O19 | 822.2946 | 1491425 | Chao Hao Ding C |
| 857.2652 | 857.2640 | 1.40 | [M+Cl]- | C39H50O19 | 822.2946 | 13225374 | Chao Hao Ding C |
| 859.2431 | 859.2427 | 0.49 | [M-2H+K]- | C39H50O19 | 822.2946 | 1830197 | Chao Hao Ding C |
| 823.2677 | 823.2666 | 1.32 | [M-H]- | C38H48O20 | 824.2739 | 3252587 | Epimedium, genus of herbaceous flowering plant, cultivated in the Far East as aphrodisiac |
| 819.2734 | 819.2717 | 2.08 | [M-H20-H]- | C39H50O20 | 838.2895 | 1129049 | Chao Hao Ding A |
| 837.2841 | 837.2823 | 2.18 | [M-H]- | C39H50O20 | 838.2895 | 2038814 | Chao Hao Ding A |
| 859.2618 | 859.2637 | −2.12 | [M-2H+Na]- | C39H50O20 | 838.2895 | 4975924 | Chao Hao Ding A |
| 873.2600 | 873.2589 | 1.20 | [M+Cl]- | C39H50O20 | 838.2895 | 4745870 | Chao Hao Ding A |
| Groups | Initial Weight (g) | Final Weight (g) | Kidney Weight (g) | Renal Index (mg/g) |
|---|---|---|---|---|
| Control | 19.70 ± 1.23 | 27.32 ± 1.34 | 0.33 ± 0.05 | 1.50 ± 0.04 |
| CTX | 20.27 ± 1.01 | 23.23 ± 2.18 ## | 0.30 ± 0.04 | 1.47 ± 0.03 |
| 50EBF | 20.28 ± 1.56 | 25.18 ± 1.87 | 0.32 ± 0.04 * | 1.48 ± 0.02 * |
| 100EBF | 20.70 ± 1.03 | 26.67 ± 1.01 | 0.33 ± 0.03 * | 1.49 ± 0.02 * |
| Level (of Achievement etc.) | X1 Ethanol Volume Fraction (%) | X2 Liquid Dose Ratio (g/mL) | X3 Ultrasound Time (min) |
|---|---|---|---|
| −1 | 50 | 1:20 | 20 |
| 0 | 60 | 1:25 | 25 |
| 1 | 70 | 1:30 | 30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, M.; Pei, H.; Sun, L.; Chen, W.; Zong, Y.; Zhao, Y.; Du, R.; He, Z. Optimization of the Flavonoid Extraction Process from the Stem and Leaves of Epimedium Brevicornum and Its Effects on Cyclophosphamide-Induced Renal Injury. Molecules 2024, 29, 207. https://doi.org/10.3390/molecules29010207
Shi M, Pei H, Sun L, Chen W, Zong Y, Zhao Y, Du R, He Z. Optimization of the Flavonoid Extraction Process from the Stem and Leaves of Epimedium Brevicornum and Its Effects on Cyclophosphamide-Induced Renal Injury. Molecules. 2024; 29(1):207. https://doi.org/10.3390/molecules29010207
Chicago/Turabian StyleShi, Meiling, Hongyan Pei, Li Sun, Weijia Chen, Ying Zong, Yan Zhao, Rui Du, and Zhongmei He. 2024. "Optimization of the Flavonoid Extraction Process from the Stem and Leaves of Epimedium Brevicornum and Its Effects on Cyclophosphamide-Induced Renal Injury" Molecules 29, no. 1: 207. https://doi.org/10.3390/molecules29010207
APA StyleShi, M., Pei, H., Sun, L., Chen, W., Zong, Y., Zhao, Y., Du, R., & He, Z. (2024). Optimization of the Flavonoid Extraction Process from the Stem and Leaves of Epimedium Brevicornum and Its Effects on Cyclophosphamide-Induced Renal Injury. Molecules, 29(1), 207. https://doi.org/10.3390/molecules29010207





