Significant Differences in the Effects of Nitrogen Doping on Pristine Biochar and Graphene-like Biochar for the Adsorption of Tetracycline
Abstract
:1. Introduction
2. Results and Discussion
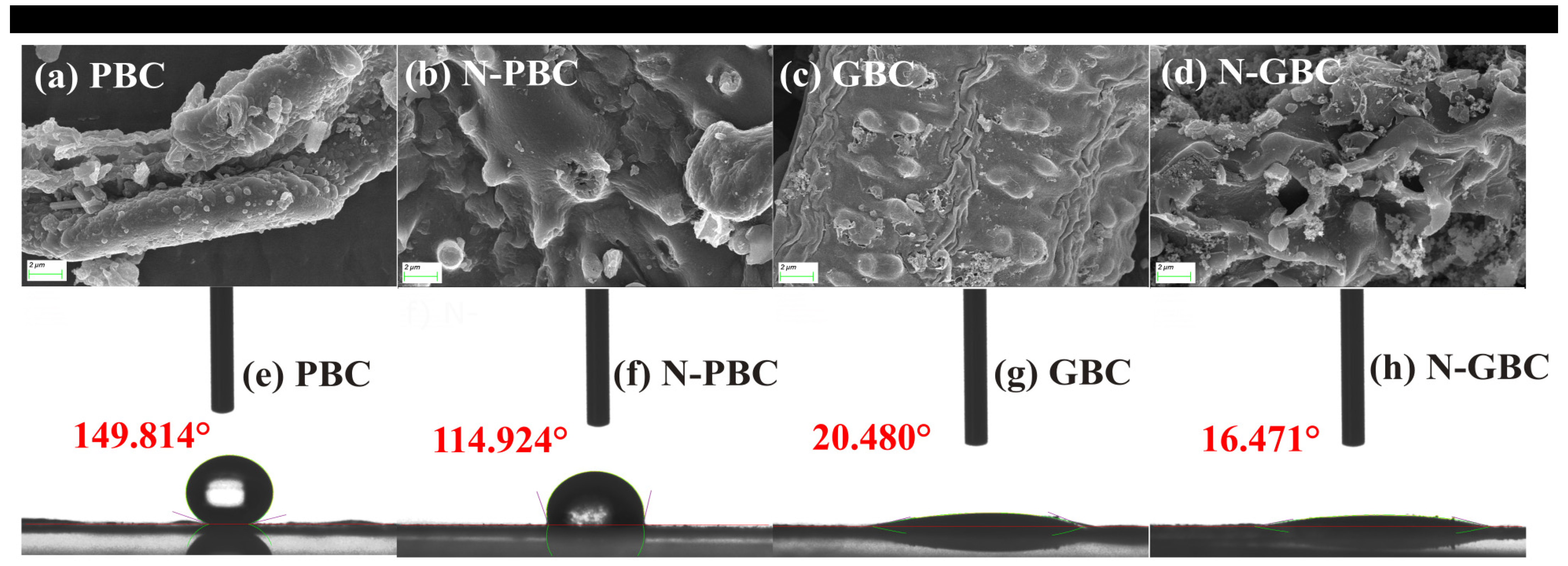
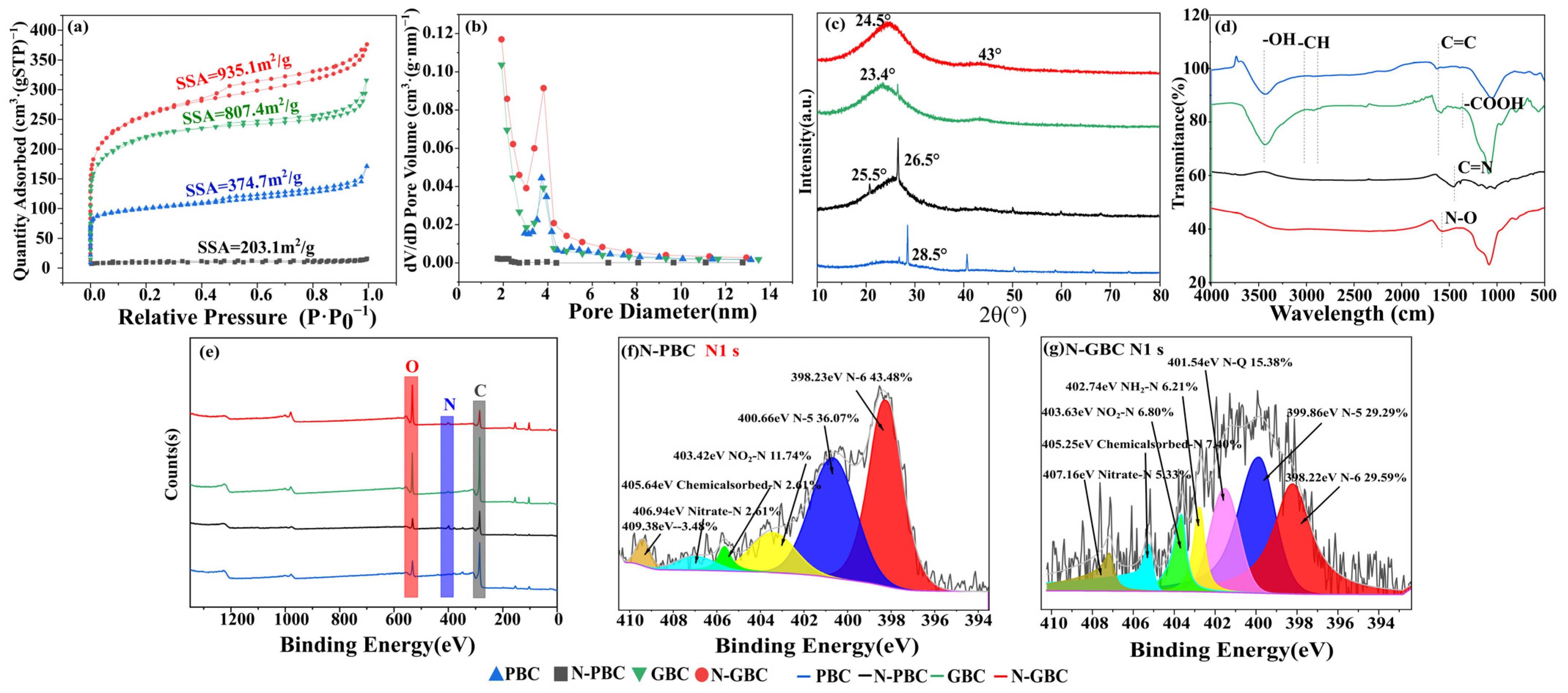
2.1. Characterization of the Tested Biochars
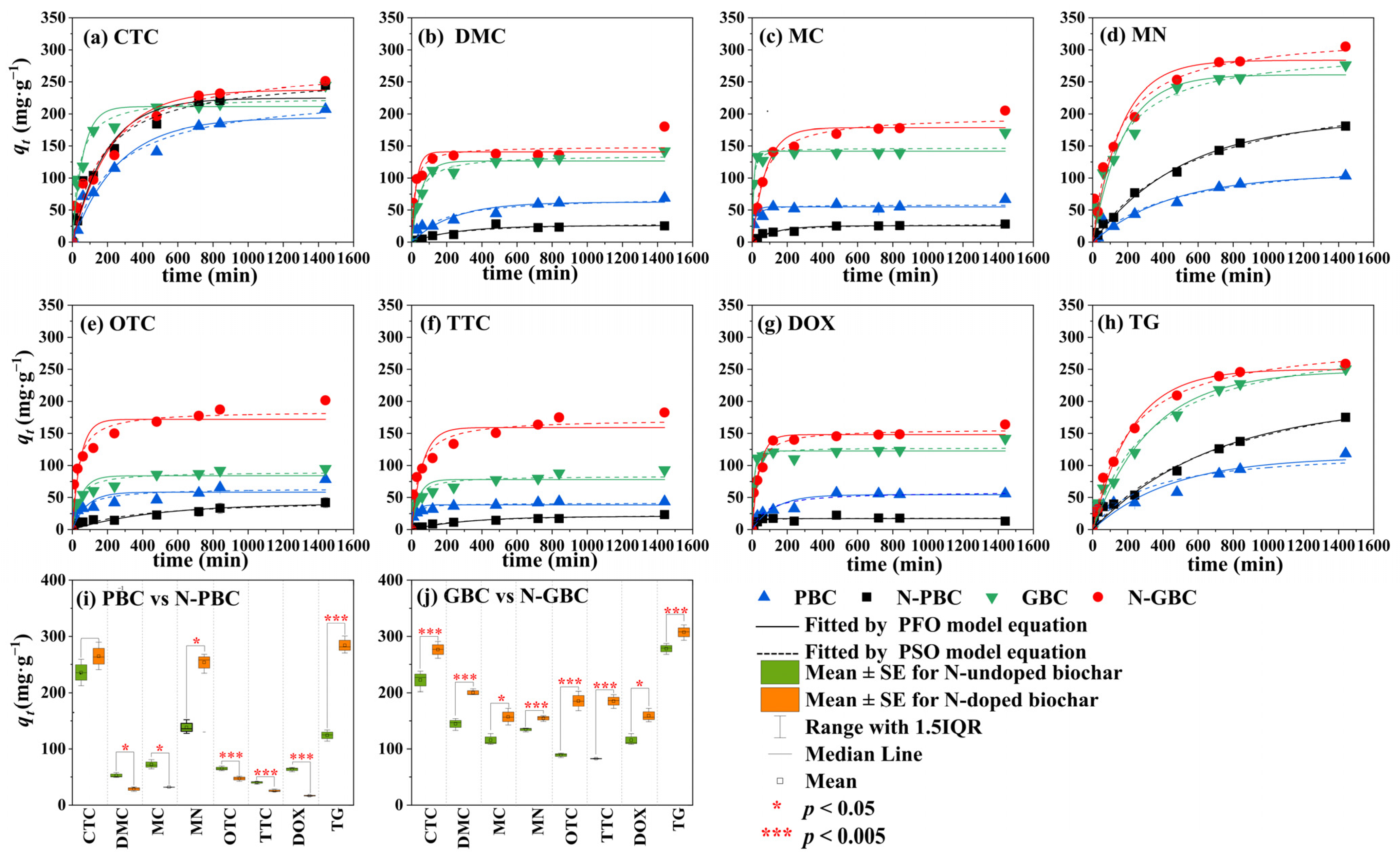
2.2. Adsorption Characteristics
2.2.1. Adsorption Kinetics
2.2.2. Adsorption Isotherm
2.3. Influence of Solution Properties
2.4. Mechanisms for the Effects of N-Doping upon the Adsorption Performance of TCs
2.4.1. Modification of Pore Structure
2.4.2. Modification of Surface Functional Groups
2.4.3. Mechanistic Model Developments and Model Explanation
2.5. The Micro-Mechanisms of N-Doping Effects upon PBC and GBC for the Adsorption of TCs
2.5.1. Effects of N-Doping upon the Chemical Reactivity of Tested Biochars
2.5.2. Effects of N-Doping upon the Interactions of Biochars with TCs
Equilibrium Configurations and Adsorption Sites
Weak Interaction Forces
Key Quantum Chemical Descriptors of TCs for Adsorption Energies
2.6. Application
3. Materials and Methods
3.1. Materials and Tested Biochars
3.2. Characterization of Biochar and TCs Measurements
3.3. Batch Adsorption Experiments
3.4. Adsorption Simulation and Micro-Mechanisms
3.4.1. Adsorption Simulation
3.4.2. Adsorption Micro-Mechanisms
3.5. Mechanistic QSAR Model Development, Validations, and Statistical Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
List of Abbreviations and Definitions
References
- Sabando-Fraile, C.; Corral-Bobadilla, M.; Lostado-Lorza, R.; Somovilla-Gomez, F.J.S. Multiresponse Performance Evaluation and Life Cycle Assessment for the Optimal Elimination of Pb (II) from Industrial Wastewater by Adsorption Using Vine Shoot Activated Carbon. Sustainability 2023, 15, 11007. [Google Scholar] [CrossRef]
- Zu, Z.Y.; Jiang, M.D.; Xu, P.P.; Chen, W.; Ni, Q.Q.; Lu, G.M.; Zhang, L.J. Coronavirus disease 2019 (COVID-19): A perspective from China. Radiology 2020, 296, E15–E25. [Google Scholar] [CrossRef] [PubMed]
- Schnappinger, D.; Hillen, W.J. Tetracyclines: Antibiotic action, uptake, and resistance mechanisms. Arch. Microbiol. 1996, 165, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Rodríguez, M.; Pellerano, R.G.; Pezza, L.; Pezza, H.R.J. An overview of the main foodstuff sample preparation technologies for tetracycline residue determination. Talanta 2018, 182, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, T.J.I. f.E.v.S. In Proceedings of the International Conference on Heavy Metals in the Environment, Toronto, OR, Canada, 21–22 January 1975; M5S1A4; University of Toronto: Toronto, OR, Canada, 1975; Volume 1, p. 3. [Google Scholar]
- Wang, Y.; Han, Y.; Li, L.; Liu, J.; Yan, X.J. Distribution, sources, and potential risk of antibiotic resistance genes in wastewater treatment plant: A review. Environ. Pollut. 2022, 31, 119870. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhang, H.; Xiong, P.; Zhu, Q.; Liao, C.; Jiang, G.J. Occurrence, fate, and risk assessment of typical tetracycline antibiotics in the aquatic environment: A review. Sci. Total Environ. 2021, 753, 141975. [Google Scholar] [CrossRef]
- Xu, L.; Wang, W.; Xu, W.J. Health, Effects of tetracycline antibiotics in chicken manure on soil microbes and antibiotic resistance genes (ARGs). Environ. Geochem. Health 2022, 44, 273–284. [Google Scholar] [CrossRef]
- Wright, D.G. Resisting resistance: New chemical strategies for battling superbugs. Cell Chem. Biol. 2000, 7, R127–R132. [Google Scholar] [CrossRef]
- Gopal, G.; Alex, S.A.; Chandrasekaran, N.; Mukherjee, A. A review on tetracycline removal from aqueous systems by advanced treatment techniques. RSC Adv. 2020, 10, 27081–27095. [Google Scholar] [CrossRef]
- Zhang, Y.; Pei, M.; Zhang, B.; He, Y.; Zhong, Y. Changes of antibiotic resistance genes and bacterial communities in the advanced biological wastewater treatment system under low selective pressure of tetracycline. Water Res. 2021, 207, 117834. [Google Scholar] [CrossRef]
- Minale, M.; Gu, Z.; Guadie, A.; Kabtamu, D.M.; Li, Y.; Wang, X. Application of graphene-based materials for removal of tetracyclines using adsorption and photocatalytic-degradation: A review. J. Environ. Manag. 2020, 276, 111310. [Google Scholar] [CrossRef] [PubMed]
- Scaria, J.; Anupama, K.; Nidheesh, P. Tetracyclines in the environment: An overview on the occurrence, fate, toxicity, detection, removal methods, and sludge management. Sci. Total Environ. 2021, 771, 145291. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.H.; Wang, T.Y.; Chu, H.Y.; Gao, Y.; Wang, C.C.; Li, Y.J.; Chen, L.; Wang, P.; Fu, H.; Zhao, C. Effective elimination of tetracycline antibiotics via photoactivated SR-AOP over vivianite: A new application approach of phosphorus recovery product from WWTP. Chem. Eng. J. 2022, 449, 137784. [Google Scholar] [CrossRef]
- Dutta, J.; Mala, A.A. Removal of antibiotic from the water environment by the adsorption technologies: A review. Water Sci. Technol. 2020, 82, 401–426. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Li, X.; Wang, J.; Lin, P.; Chen, C.; Zhang, X.; Suffet, I.M. Activated carbon adsorption of quinolone antibiotics in water: Performance, mechanism, and modeling. J. Environ. Sci. 2017, 56, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Ötker, H.M.; Akmehmet-Balcıoğlu, I. Adsorption and degradation of enrofloxacin, a veterinary antibiotic on natural zeolite. J. Hazard. Mater. 2005, 122, 251–258. [Google Scholar] [CrossRef]
- Fan, Y.; Huang, L.; Wu, L.; Zhang, C.; Zhu, S.; Xiao, X.; Li, M.; Zou, X. Adsorption of sulfonamides on biochars derived from waste residues and its mechanism. J. Hazard. Mater. 2021, 406, 124291. [Google Scholar] [CrossRef]
- Xiang, W.; Zhang, X.; Chen, J.; Zou, W.; He, F.; Hu, X.; Tsang, D.C.; Ok, Y.S.; Gao, B. Biochar technology in wastewater treatment: A critical review. Chemosphere 2020, 252, 126539. [Google Scholar] [CrossRef]
- Jang, H.M.; Kan, E. A novel hay-derived biochar for removal of tetracyclines in water. Bioresour. Technol. 2019, 274, 162–172. [Google Scholar] [CrossRef]
- Cheng, N.; Wang, B.; Wu, P.; Lee, X.; Xing, Y.; Chen, M.; Gao, B. Adsorption of emerging contaminants from water and wastewater by modified biochar: A review. Environ. Pollut. 2021, 273, 116448. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Preparation, modification and environmental application of biochar: A review. J. Clean. Prod. 2019, 227, 1002–1022. [Google Scholar] [CrossRef]
- Godwin, P.M.; Pan, Y.; Xiao, H.; Afzal, M.T. Progress in preparation and application of modified biochar for improving heavy metal ion removal from wastewater. J. Bioresour. Bioprod. 2019, 4, 31–42. [Google Scholar] [CrossRef]
- Qi, Y.; Ge, B.; Zhang, Y.; Jiang, B.; Wang, C.; Akram, M.; Xu, X. Three-dimensional porous graphene-like biochar derived from Enteromorpha as a persulfate activator for sulfamethoxazole degradation: Role of graphitic N and radicals transformation. J. Hazard. Mater. 2020, 399, 123039. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Xiao, J.; Yılmaz, M.; Zhang, T.C.; Yuan, S. N, P Co-doped porous biochar derived from cornstalk for high performance CO2 adsorption and electrochemical energy storage. Sep. Purif. Technol. 2022, 299, 121719. [Google Scholar] [CrossRef]
- Chen, Q.; Zheng, J.; Xu, J.; Dang, Z.; Zhang, L. Insights into sulfamethazine adsorption interfacial interaction mechanism on mesoporous cellulose biochar: Coupling DFT/FOT simulations with experiments. Chem. Eng. J. 2019, 356, 341–349. [Google Scholar] [CrossRef]
- Yi, L.; Zuo, L.; Wei, C.; Fu, H.; Qu, X.; Zheng, S.; Xu, Z.; Guo, Y.; Li, H.; Zhu, D. Enhanced adsorption of bisphenol A, tylosin, and tetracycline from aqueous solution to nitrogen-doped multiwall carbon nanotubes via cation-π and π-π electron-donor-acceptor (EDA) interactions. Sci. Total Environ. 2020, 719, 137389. [Google Scholar] [CrossRef]
- Li, Y.; Xing, B.; Wang, X.; Wang, K.; Zhu, L.; Wang, S. Nitrogen-doped hierarchical porous biochar derived from corn stalks for phenol-enhanced adsorption. Energy Fuel 2019, 33, 12459–12468. [Google Scholar] [CrossRef]
- Zhou, H.; Li, X.; Jin, H.; She, D. Mechanism of a double-channel nitrogen-doped lignin-based carbon on the highly selective removal of tetracycline from water. Bioresour. Technol. 2022, 346, 126652. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, Z.; Xu, G.; Li, G. Pyrolysis of penicillin fermentation residue and sludge to produce biochar: Antibiotic resistance genes destruction and biochar application in the adsorption of penicillin in water. J. Hazard. Mater. 2021, 413, 125385. [Google Scholar] [CrossRef]
- Zhang, X.; Lin, X.; He, Y.; Chen, Y.; Luo, X.; Shang, R. Study on adsorption of tetracycline by Cu-immobilized alginate adsorbent from water environment. Int. J. Biol. Macromol. 2019, 124, 418–428. [Google Scholar] [CrossRef]
- Parolo, M.E.; Savini, M.C.; Valles, J.M.; Baschini, M.T.; Avena, M.J. Tetracycline adsorption on montmorillonite: pH and ionic strength effects. Appl. Clay. Sci. 2008, 40, 179–186. [Google Scholar] [CrossRef]
- Leng, L.; Li, J.; Yuan, X.; Li, J.; Han, P.; Hong, Y.; Wei, F.; Zhou, W. Beneficial synergistic effect on bio-oil production from co-liquefaction of sewage sludge and lignocellulosic biomass. Bioresour. Technol. 2018, 251, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Revellame, E.D.; Fortela, D.L.; Sharp, W.; Hernandez, R.; Zappi, M.E. Adsorption kinetic modeling using pseudo-first order and pseudo-second order rate laws: A review. Clean. Eng. Technol. 2020, 1, 100032. [Google Scholar] [CrossRef]
- Kajjumba, G.W.; Emik, S.; Öngen, A.; Özcan, H.K.; Aydın, S. Modelling of adsorption kinetic processes—Errors, theory and application. In Advanced Sorption Process Applications; IntechOpen: London, UK, 2018; pp. 1–19. [Google Scholar]
- Al-Ghouti, M.A.; Da’ana, D.A. Guidelines for the use and interpretation of adsorption isotherm models: A review. J. Hazard. Mater. 2020, 393, 122383. [Google Scholar] [CrossRef]
- Lin, L.; Jiang, W.B.; Xu, P. Comparative study on pharmaceuticals adsorption in reclaimed water desalination concentrate using biochar: Impact of salts and organic matter. Sci. Total Environ. 2017, 601, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Zhang, P.; Wang, C.; Tang, J.; Sun, H. Enhancement of persulfate activation by Fe-biochar composites: Synergism of Fe and N-doped biochar. Appl. Catal. B Environ. 2022, 303, 120926. [Google Scholar] [CrossRef]
- Diao, Y.; Shan, R.; Li, M.; Gu, J.; Yuan, H.; Chen, Y. Efficient Adsorption of a Sulfonamide Antibiotic in Aqueous Solutions with N-doped Magnetic Biochar: Performance, Mechanism, and Reusability. ACS Omega 2022, 8, 879–892. [Google Scholar] [CrossRef]
- Zhong, Q.; Lin, Q.; He, W.; Fu, H.; Huang, Z.; Wang, Y.; Wu, L. Study on the nonradical pathways of nitrogen-doped biochar activating persulfate for tetracycline degradation. Sep. Purif. Technol. 2021, 276, 119354. [Google Scholar] [CrossRef]
- Singh, B.; Raven, M.D. 21 X-ray diffraction analysis of biochar. In Biochar: A Guide to Analytical Methods; CRC Press: Boca Raton, FL, USA, 2017; p. 245. [Google Scholar]
- Kang, J.; Duan, X.; Wang, C.; Sun, H.; Tan, X.; Tade, M.O.; Wang, S. Nitrogen-doped bamboo-like carbon nanotubes with Ni encapsulation for persulfate activation to remove emerging contaminants with excellent catalytic stability. Chem. Eng. J. 2018, 332, 398–408. [Google Scholar] [CrossRef]
- Li, R.; Hu, D.; Hu, K.; Deng, H.; Zhang, M.; Wang, A.; Qiu, R.; Yan, K.J. Coupling adsorption-photocatalytic reduction of Cr (VI) by metal-free N-doped carbon. Sci. Total Environ. 2020, 704, 135284. [Google Scholar] [CrossRef] [PubMed]
- Leng, L.; Xu, S.; Liu, R.; Yu, T.; Zhuo, X.; Leng, S.; Xiong, Q.; Huang, H. Nitrogen containing functional groups of biochar: An overview. Bioresour. Technol. 2020, 298, 122286. [Google Scholar] [CrossRef] [PubMed]
- Kasera, N.; Kolar, P.; Hall, S.G. Nitrogen-doped biochars as adsorbents for mitigation of heavy metals and organics from water: A review. Biochar 2022, 4, 17. [Google Scholar] [CrossRef]
- Qian, L.; Chen, B. Interactions of aluminum with biochars and oxidized biochars: Implications for the biochar aging process. J. Agr. Food Chem. 2014, 62, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, J.; Sun, M.; Li, W.; Hu, X. Adsorption of CO2 by nitrogen doped corn straw based biochar. Arab. J. Geosci. 2021, 14, 1875. [Google Scholar] [CrossRef]
- Qu, S.; Yuan, Y.; Yang, X.; Xu, H.; Mohamed, A.K.; Zhang, J.; Zhao, C.; Liu, L.; Wang, B.; Wang, X.J.C.E.J. Carbon defects in biochar facilitated nitrogen doping: The significant role of pyridinic nitrogen in peroxymonosulfate activation and ciprofloxacin degradation. Chem. Eng. J. 2022, 441, 135864. [Google Scholar] [CrossRef]
- Ortiz-Medina, J.; Wang, Z.; Cruz-Silva, R.; Morelos-Gomez, A.; Wang, F.; Yao, X.; Terrones, M.; Endo, M.J. Defect engineering and surface functionalization of nanocarbons for metal-free catalysis. Adv. Mater. 2019, 31, 1805717. [Google Scholar] [CrossRef]
- Du, L.Q.; Ahmad, S.; Liu, L.A.; Wang, L.; Tang, J.C. A review of antibiotics and antibiotic resistance genes (ARGs) adsorption by biochar and modified biochar in water. Sci. Total Environ. 2023, 858, 14. [Google Scholar] [CrossRef]
- Jiang, Z.; Huang, X.; Wu, Q.; Li, M.; Xie, Q.; Liu, Z.; Zou, X. Adsorption of sulfonamides on polyamide microplastics in an aqueous solution: Behavior, structural effects, and its mechanism. Chem. Eng. J. 2023, 454, 140452. [Google Scholar] [CrossRef]
- Yang, S.S.; Lu, W.C.; Gu, T.H.; Yan, L.M.; Li, G.Z. QSPR Study of n-Octanol/Water Partition Coefficient of Some Aromatic Compounds Using Support Vector Regression. QSAR Comb. Sci. 2009, 28, 175–182. [Google Scholar] [CrossRef]
- Chen, Q.; Zheng, J.; Yang, Q.; Dang, Z.; Zhang, L. Effect of carbon chain structure on the phthalic acid esters (PAEs) adsorption mechanism by mesoporous cellulose biochar. Chem. Eng. J. 2019, 362, 383–391. [Google Scholar] [CrossRef]
- Jiang, Z.; Huang, L.; Fan, Y.; Zhou, S.; Zou, X. Contrasting effects of microplastic aging upon the adsorption of sulfonamides and its mechanism. Chem. Eng. J. 2022, 430, 132939. [Google Scholar] [CrossRef]
- Eriksson, L.; Jaworska, J.; Worth, A.P.; Cronin, M.T.; McDowell, R.M.; Gramatica, P. Methods for reliability and uncertainty assessment and for applicability evaluations of classification-and regression-based QSARs. Environ. Health Persp. 2003, 111, 1361–1375. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Xiao, X.; Zhou, H.; Chen, F.; Zeng, J.; Wang, W.; Feng, G.; Huang, X. Effects of soil acidification on the toxicity of organophosphorus pesticide on Eisenia fetida and its mechanism. J. Hazard. Mater. 2018, 359, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Liu, L.; Zhang, S.; Tang, J. Nitrogen-doped biochar (N-doped BC) and iron/nitrogen co-doped biochar (Fe/N co-doped BC) for removal of refractory organic pollutants. J. Hazard. Mater. 2023, 446, 130727. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Wang, B.; Shen, J.; Yan, P.; Kang, J.; Wang, W.; Bi, L.; Zhu, X.; Li, Y.; Wang, S. Preparation of novel N-doped biochar and its high adsorption capacity for atrazine based on π–π electron donor-acceptor interaction. J. Hazard. Mater. 2022, 432, 128757. [Google Scholar] [CrossRef]
- Miar, M.; Shiroudi, A.; Pourshamsian, K.; Oliaey, A.R.; Hatamjafari, F. Theoretical investigations on the HOMO–LUMO gap and global reactivity descriptor studies, natural bond orbital, and nucleus-independent chemical shifts analyses of 3-phenylbenzo [d] thiazole-2 (3 H)-imine and its para-substituted derivatives: Solvent and substituent effects. J. Chem. Res. 2021, 45, 147–158. [Google Scholar]
- Mei, Y.; Xu, J.; Zhang, Y.; Li, B.; Fan, S.; Xu, H. Effect of Fe–N modification on the properties of biochars and their adsorption behavior on tetracycline removal from aqueous solution. Bioresour. Technol. 2021, 325, 124732. [Google Scholar] [CrossRef]
- Fuster, F.; Silvi, B. Does the topological approach characterize the hydrogen bond? Theor. Chem. Acc. 2000, 104, 13–21. [Google Scholar] [CrossRef]
- Han, C.-H.; Park, H.-D.; Kim, S.-B.; Yargeau, V.; Choi, J.-W.; Lee, S.-H.; Park, J.-A. Oxidation of tetracycline and oxytetracycline for the photo-Fenton process: Their transformation products and toxicity assessment. Water Res. 2020, 172, 115514. [Google Scholar] [CrossRef] [PubMed]
- Leichtweis, J.; Vieira, Y.; Welter, N.; Silvestri, S.; Dotto, G.L.; Carissimi, E. A review of the occurrence, disposal, determination, toxicity and remediation technologies of the tetracycline antibiotic. Process Saf. Environ. 2022, 160, 25–40. [Google Scholar] [CrossRef]
- Yu, W.; Lian, F.; Cui, G.; Liu, Z. N-doping effectively enhances the adsorption capacity of biochar for heavy metal ions from aqueous solution. Chemosphere 2018, 193, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Lian, F.; Cui, G.; Liu, Z.; Duo, L.; Zhang, G.; Xing, B. One-step synthesis of a novel N-doped microporous biochar derived from crop straws with high dye adsorption capacity. J. Environ. Manag. 2016, 176, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.S.; Wang, Y.; Li, Y.; Wang, Z.; Xie, Z.X.; Tang, J. Removal of tetracycline from aqueous solution by biochar derived from rice straw. Environ. Sci. Pollut. R 2018, 25, 29529–29540. [Google Scholar] [CrossRef] [PubMed]
- Sun, H. COMPASS: An ab initio force-field optimized for condensed-phase applications overview with details on alkane and benzene compounds. J. Phys. Chem. B 1998, 102, 7338–7364. [Google Scholar] [CrossRef]
- Cheng, L.; Ji, Y.H.; Liu, X.M. Insights into interfacial interaction mechanism of dyes sorption on a novel hydrochar: Experimental and DFT study. Chem. Eng. Sci. 2021, 233, 116432. [Google Scholar] [CrossRef]
- Anderson, P.D.; Weber, L.J. In Proceedings of the International Conference on Heavy Metals in the Environment, Toronto, OR, Canada, 21–22 January 1975; p. 933.
- Pal, R.; Chattaraj, P.K. Chemical reactivity from a conceptual density functional theory perspective. J. Indian Chem. Soc. 2021, 98, 100008. [Google Scholar] [CrossRef]
- Mahmood, T.; Saddique, M.T.; Naeem, A.; Westerhoff, P.; Mustafa, S.; Alum, A. Comparison of different methods for the point of zero charge determination of NiO. Ind. Eng. Chem. Res. 2011, 50, 10017–10023. [Google Scholar] [CrossRef]
- Liu, Z.; Feng, F.; Li, Y.; Sun, Y.; Tagawa, K. A corncob biochar-based superhydrophobic photothermal coating with micro-nano-porous rough-structure for ice-phobic properties. Surf. Coat. Technol. 2023, 457, 129299. [Google Scholar] [CrossRef]
- Sergio, M.; Lu, T. The geometry and electronic structure of Aristolochic acid: Possible implications for a frozen resonance. J. Phys. Org. Chem. 2013, 26, 473–483. [Google Scholar]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rong, L.; Wu, L.; Zhang, T.; Hu, C.; Tang, H.; Pan, H.; Zou, X. Significant Differences in the Effects of Nitrogen Doping on Pristine Biochar and Graphene-like Biochar for the Adsorption of Tetracycline. Molecules 2024, 29, 173. https://doi.org/10.3390/molecules29010173
Rong L, Wu L, Zhang T, Hu C, Tang H, Pan H, Zou X. Significant Differences in the Effects of Nitrogen Doping on Pristine Biochar and Graphene-like Biochar for the Adsorption of Tetracycline. Molecules. 2024; 29(1):173. https://doi.org/10.3390/molecules29010173
Chicago/Turabian StyleRong, Lingling, Ligui Wu, Tiao Zhang, Cui Hu, Haihui Tang, Hongcheng Pan, and Xiaoming Zou. 2024. "Significant Differences in the Effects of Nitrogen Doping on Pristine Biochar and Graphene-like Biochar for the Adsorption of Tetracycline" Molecules 29, no. 1: 173. https://doi.org/10.3390/molecules29010173
APA StyleRong, L., Wu, L., Zhang, T., Hu, C., Tang, H., Pan, H., & Zou, X. (2024). Significant Differences in the Effects of Nitrogen Doping on Pristine Biochar and Graphene-like Biochar for the Adsorption of Tetracycline. Molecules, 29(1), 173. https://doi.org/10.3390/molecules29010173






