Using Magnetic Micelles Combined with Carbon Fiber Ionization Mass Spectrometry for the Screening of Trace Triazine Herbicides from Aqueous Samples
Abstract
1. Introduction
2. Experimental Section
2.1. Chemicals and Materials
2.2. Instrumentation
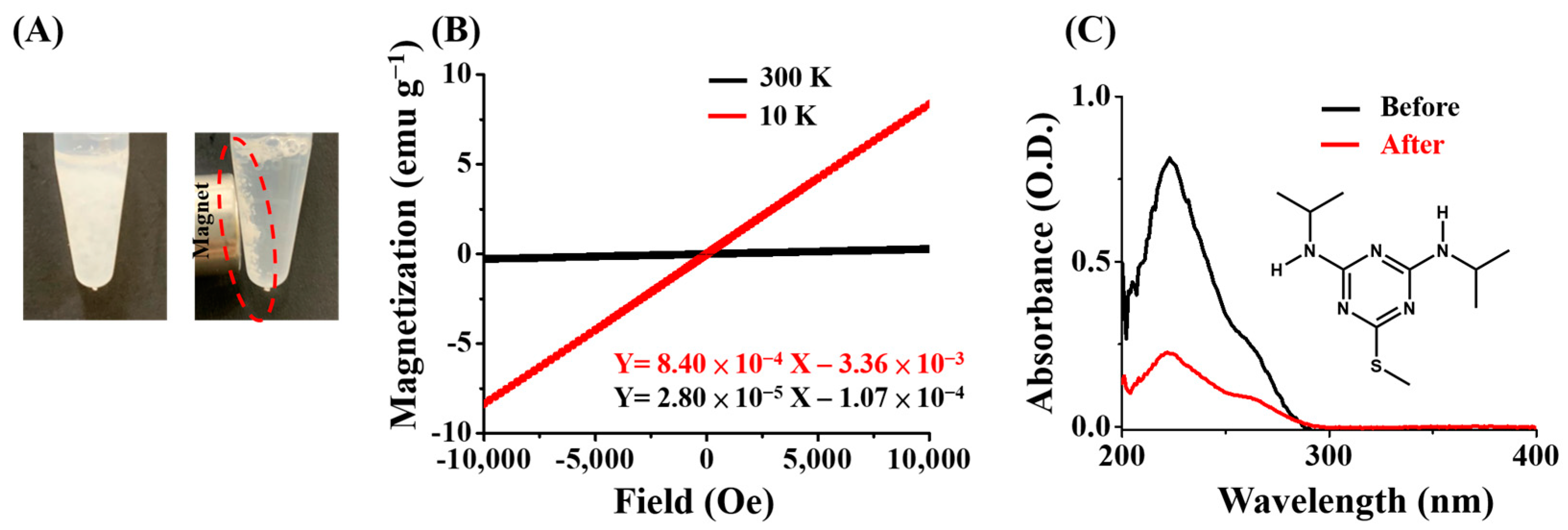
2.3. Synthesis of the Magnetic Gd3+-SDS Conjugates
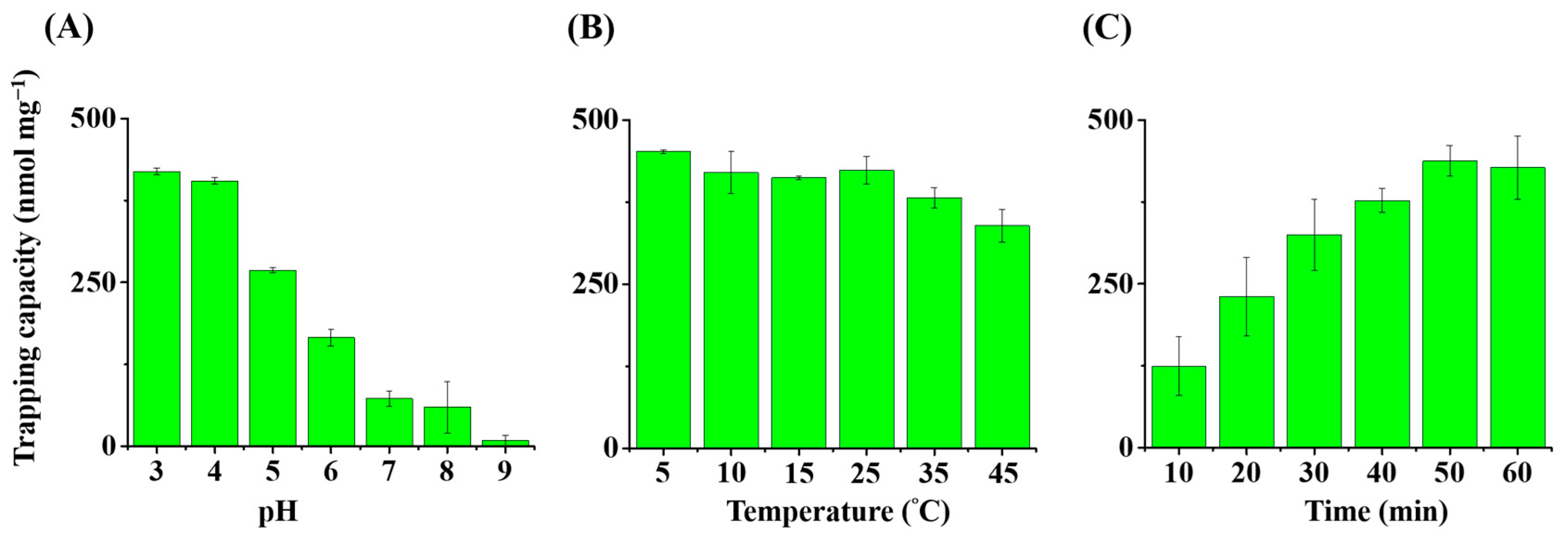
2.4. Optimization of Experimental Parameters
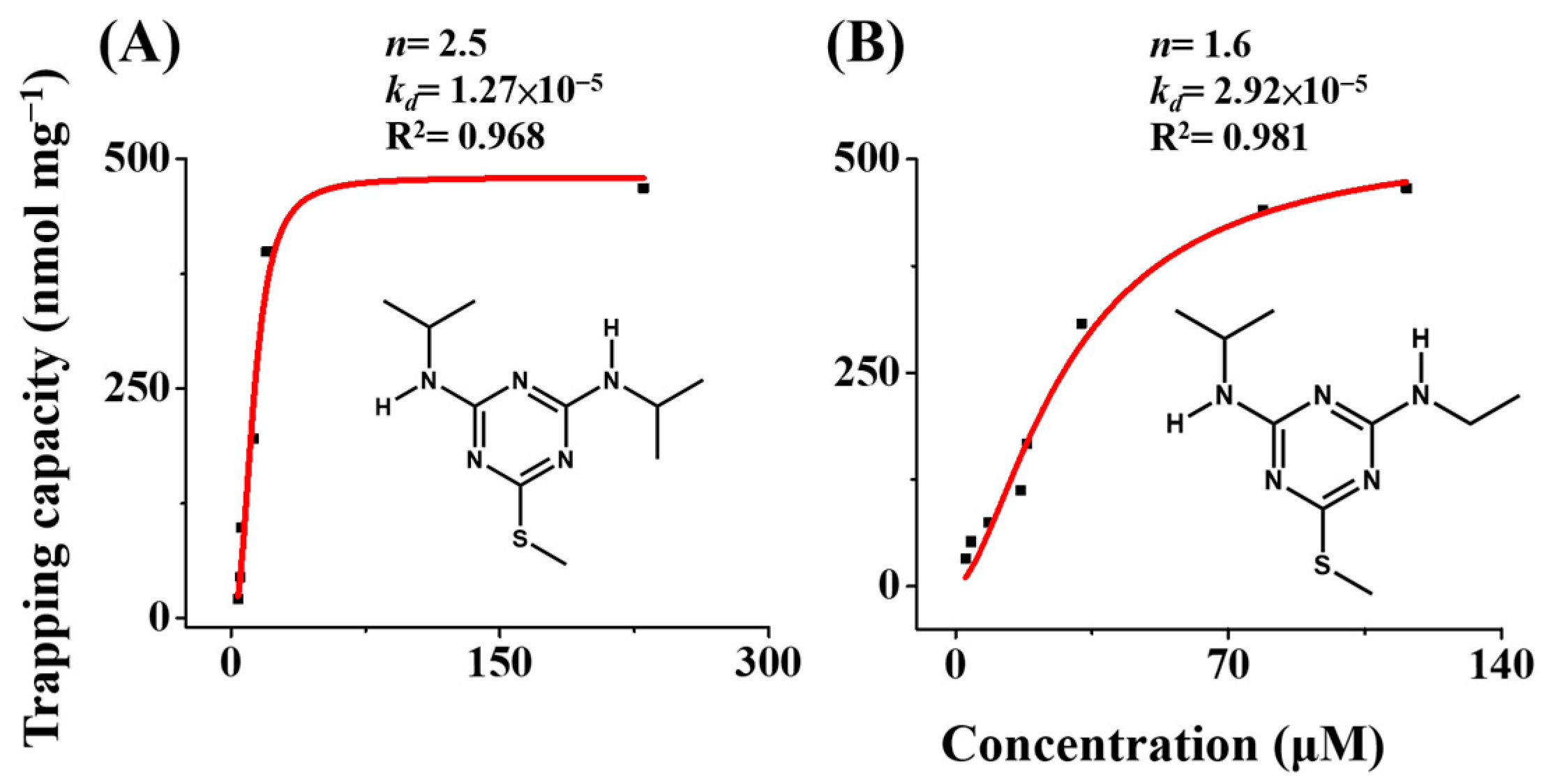
2.5. Examination of Adsorption Isotherms of the Gd3+-SDS Adsorbents against Triazine Herbicides
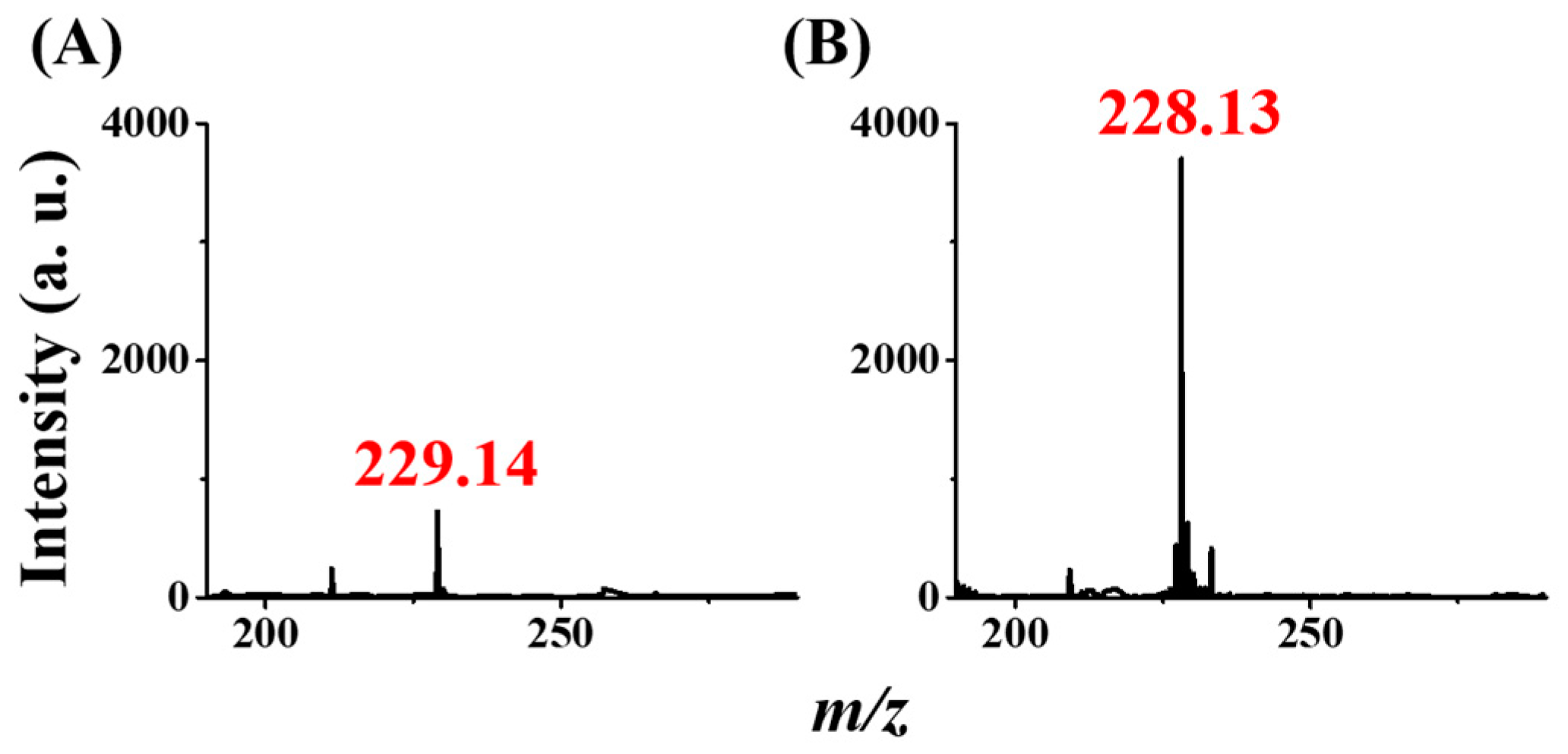
2.6. Using CFI-MS as the Detection Tool
2.7. Examination of Interference Species
2.8. Analysis of Simulated Real Samples
3. Results and Discussion
3.1. Using the Gd3+-SDS Conjugates as the Adsorbents for Trapping Herbicides
3.2. Optimization of Experimental Parameters
3.3. Examination of the Adsorption Isotherms of Triazine Herbicides on the Gd3+-SDS Adsorbents
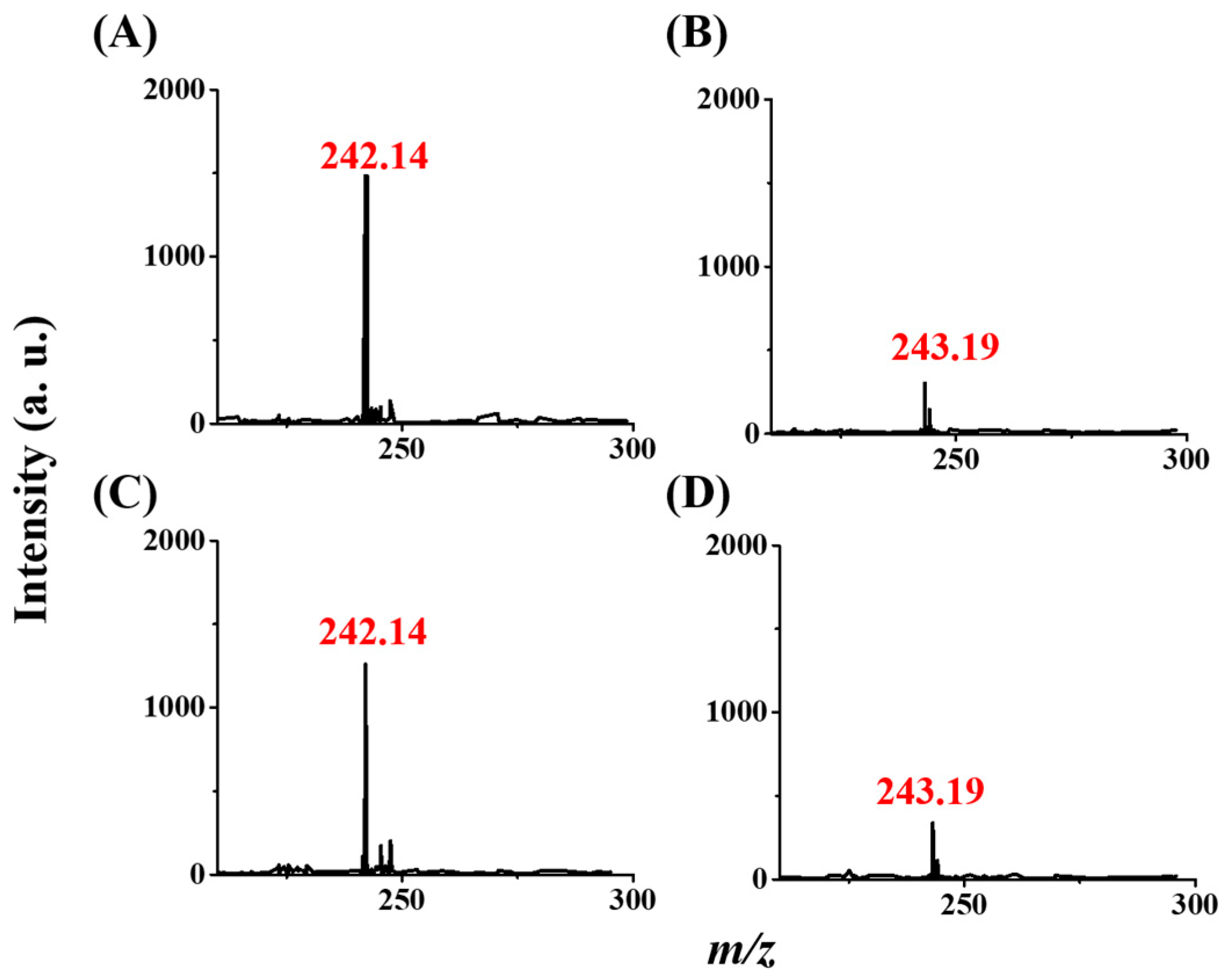
3.4. Using CFI-MS as the Detection Tool
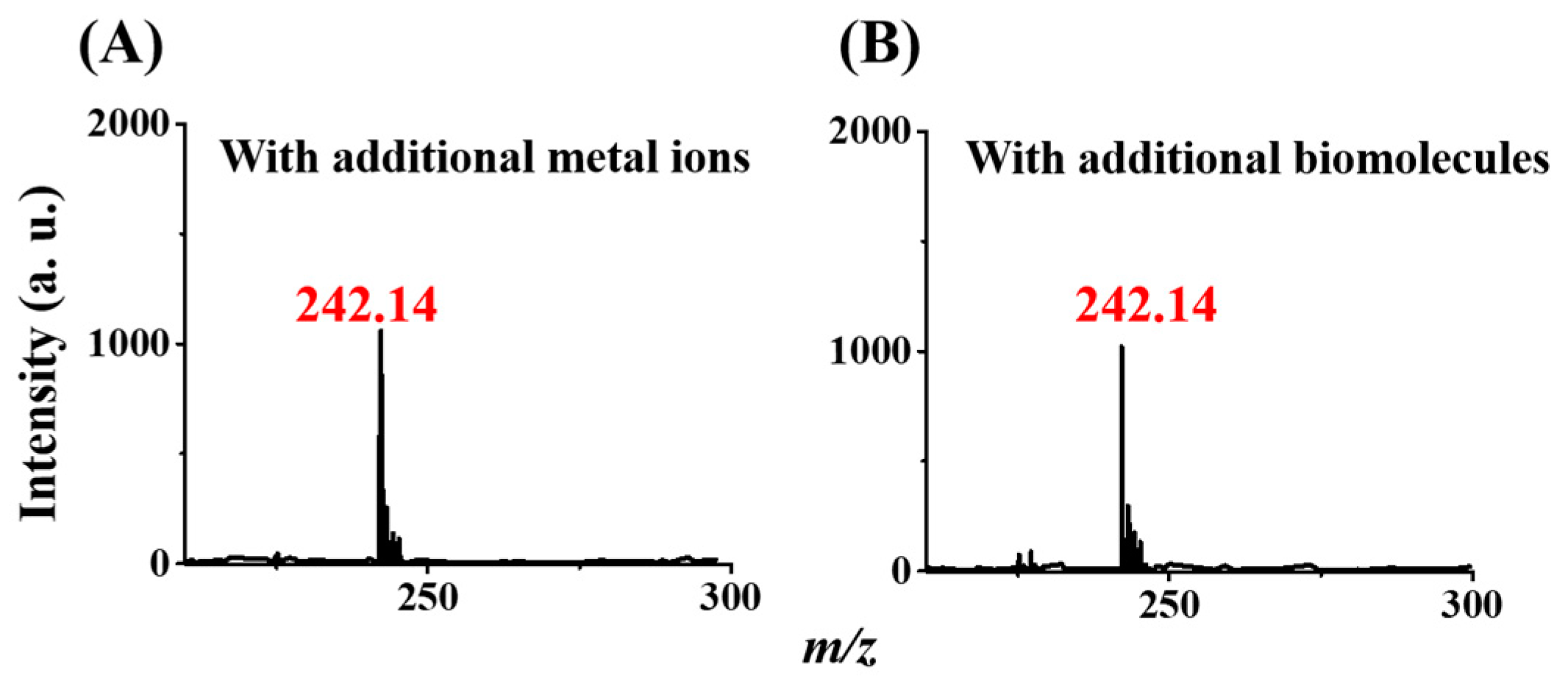
3.5. Examination of the Effects of Interference Species
3.6. Analysis of Simulated Real Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pimentel, D. Pesticides and Pest Control, Integrated Pest Management: Innovation-Development Process; Springer: Berlin/Heidelberg, Germany, 2009; pp. 83–87. [Google Scholar]
- Tiryaki, O.; Temur, C. The fate of pesticide in the environment. J. Biol. Environ. Sci. 2010, 4, 29–38. [Google Scholar]
- Semren, T.Ž.; Žunec, S.; Pizent, A. Oxidative stress in triazine pesticide toxicity: A review of the main biomarker findings. Arch. Ind. Hyg. Toxicol. 2018, 69, 109–125. [Google Scholar] [CrossRef] [PubMed]
- de Campos Ventura, B.; de Angelis, D.D.F.; Marin-Morales, M.A. Mutagenic and genotoxic effects of the Atrazine herbicide in Oreochromis niloticus (Perciformes, Cichlidae) detected by the micronuclei test and the comet assay. Pestic. Biochem. Physiol. 2008, 90, 42–51. [Google Scholar] [CrossRef]
- Wiegand, C.; Krause, E.; Steinberg, C.; Pflugmacher, S. Toxicokinetics of atrazine in embryos of the zebrafish (Danio rerio). Ecotoxicol. Environ. Saf. 2001, 49, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Santos, T.G.; Martinez, C.B. Atrazine promotes biochemical changes and DNA damage in a Neotropical fish species. Chemosphere 2012, 89, 1118–1125. [Google Scholar] [CrossRef] [PubMed]
- Ackerman, F. The Economics of Atrazine. Int. J. Occup. Environ. Health 2007, 13, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Swan, S.H.; Kruse, R.L.; Liu, F.; Barr, D.B.; Drobnis, E.Z.; Redmon, J.B.; Wang, C.; Brazil, C.; Overstreet, J.W. Study for Future Families Research Group. Semen quality in relation to biomarkers of pesticide exposure. Environ. Health Perspect. 2003, 111, 1478–1484. [Google Scholar] [CrossRef]
- Navaratna, D.; Elliman, J.; Cooper, A.; Shu, L.; Baskaran, K.; Jegatheesan, V. Impact of herbicide Ametryn on microbial communities in mixed liquor of a membrane bioreactor (MBR). Bioresour. Technol. 2012, 113, 181–190. [Google Scholar] [CrossRef]
- Ali, I.; Al-Othman, Z.A.; Alwarthan, A. Green synthesis of functionalized iron nano particles and molecular liquid phase adsorption of ametryn from water. J. Mol. Liq. 2016, 221, 1168–1174. [Google Scholar] [CrossRef]
- Farré, M.; Fernandez, J.; Paez, M.; Granada, L.; Barba, L.; Gutierrez, H.; Pulgarin, C.; Barceló, D. Analysis and toxicity of methomyl and ametryn after biodegradation. Anal. Bioanal. Chem. 2002, 373, 704–709. [Google Scholar] [CrossRef]
- Guidelines for Canadian Drinking Water Quality (Table 2. Chemical and Physical Parameters). 2022. Available online: https://www.canada.ca/en/health-canada/services/environmental-workplace-health/reports-publications/water-quality/guidelines-canadian-drinking-water-quality-summary-table.html (accessed on 10 July 2023).
- Fennimore, S.A.; Smith, R.F.; Tourte, L.; LeStrange, M.; Rachuy, J.S. Evaluation and economics of a rotating cultivator in bok choy, celery, lettuce, and radicchio. Weed Technol. 2014, 28, 176–188. [Google Scholar] [CrossRef]
- Rubira, R.J.G.; Camacho, S.A.; Aoki, P.H.B.; Paulovich, F.V.; Oliveira, O.N.; Constantino, C.J.L. Probing trace levels of prometryn solutions: From test samples in the lab toward real samples with tap water. J. Mater. Sci. 2016, 51, 3182–3190. [Google Scholar] [CrossRef]
- Zhou, Q.; Xiao, J.; Ding, Y. Sensitive determination of fungicides and prometryn in environmental water samples using multiwalled carbon nanotubes solid-phase extraction cartridge. Anal. Chim. Acta 2007, 602, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Likas, D.T.; Tsiropoulos, N.G.; Miliadis, G.E. Rapid gas chromatographic method for the determination of famoxadone, trifloxystrobin and fenhexamid residues in tomato, grape and wine samples. J. Chromatogr. A 2007, 1150, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.-J.; Ho, W.-H. Determination of fungicides in water using liquid phase microextraction and gas chromatography with electron capture detection. Anal. Chim. Acta 2004, 527, 61–67. [Google Scholar] [CrossRef]
- Angioni, A.; Garau, A.; Caboni, P.; Russo, M.T.; Farris, G.A.; Zara, S.; Cabras, P. Gas chromatographic ion trap mass spectrometry determination of zoxamide residues in grape, grape processing, and in the fermentation process. J. Chromatogr. A 2005, 1097, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Baggiani, C.; Baravalle, P.; Giraudi, G.; Tozzi, C. Molecularly imprinted solid-phase extraction method for the high-performance liquid chromatographic analysis of fungicide pyrimethanil in wine. J. Chromatogr. A 2007, 1141, 158–164. [Google Scholar] [CrossRef]
- Singh, S.B.; Foster, G.D.; Khan, S.U. Determination of thiophanate methyl and carbendazim residues in vegetable samples using microwave-assisted extraction. J. Chromatogr. A 2007, 1148, 152–157. [Google Scholar] [CrossRef]
- Bonora, S.; Benassi, E.; Maris, A.; Tugnoli, V.; Ottani, S.; Di Foggia, M. Raman and SERS study on atrazine, prometryn and simetryn triazine herbicides. J. Mol. Struct. 2013, 1040, 139–148. [Google Scholar] [CrossRef]
- Chen, J.; Duan, C.; Guan, Y. Sorptive extraction techniques in sample preparation for organophosphorus pesticides in complex matrices. J. Chromatogr. B 2010, 878, 1216–1225. [Google Scholar] [CrossRef]
- Li, Q.; Wang, X.; Yuan, D. Solid-phase extraction of polar organophosphorous pesticides from aqueous samples with oxidized carbon nanotubes. J. Environ. Monit. 2009, 11, 439–444. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yang, H.; Zhang, J.; Liu, Y.; Wang, L.; Bai, L.; Yang, L.; Wei, D.; Wang, W.; Niu, Y.; Chen, H. Rapid removal of anionic dye from water by poly(ionic liquid)-modified magnetic nanoparticles. J. Mol. Liq. 2019, 284, 383–392. [Google Scholar] [CrossRef]
- Wei, Y.; Han, B.; Hu, X.; Lin, Y.; Wang, X.; Deng, X. Synthesis of Fe3O4 nanoparticles and their magnetic properties. Procedia Eng. 2012, 27, 632–637. [Google Scholar] [CrossRef]
- Bagheri, A.R.; Arabi, M.; Ghaedi, M.; Ostovan, A.; Wang, X.; Li, J.; Chen, L. Dummy molecularly imprinted polymers based on a green synthesis strategy for magnetic solid-phase extraction of acrylamide in food samples. Talanta 2019, 195, 390–400. [Google Scholar] [CrossRef] [PubMed]
- Arabi, M.; Ghaedi, M.; Ostovan, A. Development of a lower toxic approach based on green synthesis of water-compatible molecularly imprinted nanoparticles for the extraction of hydrochlorothiazide from human urine. ACS Sustain. Chem. Eng. 2017, 5, 3775–3785. [Google Scholar] [CrossRef]
- Boruah, P.K.; Darabdhara, G.; Das, M.R. Polydopamine functionalized graphene sheets decorated with magnetic metal oxide nanoparticles as efficient nanozyme for the detection and degradation of harmful triazine pesticides. Chemosphere 2021, 268, 129328. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Wang, P.; Liu, D.; Zhou, Z. Hydrophilic–lipophilic balanced magnetic nanoparticles: Preparation and application in magnetic solid-phase extraction of organochlorine pesticides and triazine herbicides in environmental water samples. Talanta 2014, 127, 1–8. [Google Scholar] [CrossRef]
- Wei, J.-L.; Chen, Y.-C. Using magnetic ions to pobe and induce magnetism of pyrophosphates, bacteria, and mammalian cells. ACS Appl. Mater. Interfaces 2018, 10, 30837–30843. [Google Scholar] [CrossRef]
- Wei, J.-L.; Huang, D.-Y.; Chen, Y.-C. Using gadolinium ions as affinity probes to selectively enrich and magnetically isolate bacteria from complex samples. Anal. Chim. Acta 2020, 1113, 18–25. [Google Scholar] [CrossRef]
- Mukerjee, P.; Mysels, K.J. Critical Micelle Concentrations of Aqueous Surfactant Systems. In National Standard Reference Data System; United States of Commerce: Washington, DC, USA, 1971. [Google Scholar]
- Chen, C.-W.; Yang, T.-L.; Chen, Y.-C. Sing magnetic micelles as adsorbents to remove dyes from aqueous solutions. J. Environ. Chem. Eng. 2023, 11, 109457. [Google Scholar] [CrossRef]
- Best, J.; Weber, J.; Monaco, T. Influence of soil pH on s-triazine availability to plants. Weed Sci. 1975, 23, 378–382. [Google Scholar] [CrossRef]
- Wu, M.-L.; Chen, T.-Y.; Chen, W.-J.; Baig, M.M.F.; Wu, Y.-C.; Chen, Y.-C. Carbon fiber ionization mass spectrometry coupled with solid phase microextraction for analysis of Benzo[a]pyrene. Anal. Chim. Acta 2019, 1049, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.-L.; Chen, T.-Y.; Chen, Y.-C.; Chen, Y.-C. Carbon fiber ionization mass spectrometry for the analysis of analytes in vapor, liquid, and solid phases. Anal. Chem. 2017, 89, 13458–13465. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.-L.; Wu, Y.-C.; Chen, Y.-C. Detection of pesticide residues on intact tomatoes by carbon fiber ionization mass spectrometry. Anal. Bioanal. Chem. 2019, 411, 1095–1105. [Google Scholar] [CrossRef] [PubMed]
- Weiss, J.N.; Valdez, D.J.; Nieto, P.S.; Garbarino-Pico, E.; Avalle, L.B.; Díaz-Fajreldines, H.; Schurrer, C.; Cheng, K.M.; Guido, M.E. The Hill equation revisited: Uses and misuses. FASEB J. 1997, 11, 835–841. [Google Scholar] [CrossRef] [PubMed]
- Gaggi, C.; Sbrilli, G.; El Naby, A.H.; Bucci, M.; Duccini, M.; Bacci, E. Toxicity and hazard ranking of s-triazine herbicides using microtox®, two green algal species and a marine crustacean. Environ. Toxicol. Chem. 1995, 14, 1065–1069. [Google Scholar] [CrossRef]
- Lee, S.B.; Martin, C.R. pH-Switchable, ion-permselective gold nanotubule membrane based on chemisorbed cysteine. Anal. Chem. 2001, 73, 768–775. [Google Scholar] [CrossRef]
- Salis, A.; Boström, M.; Medda, L.; Cugia, F.; Barse, B.; Parsons, D.F.; Ninham, B.W.; Monduzzi, M. Measurements and theoretical interpretation of points of zero charge/potential of BSA protein. Langmuir 2011, 27, 11597–11604. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.-W.; Yang, T.-L.; Chen, Y.-C. Using Magnetic Micelles Combined with Carbon Fiber Ionization Mass Spectrometry for the Screening of Trace Triazine Herbicides from Aqueous Samples. Molecules 2024, 29, 137. https://doi.org/10.3390/molecules29010137
Chen C-W, Yang T-L, Chen Y-C. Using Magnetic Micelles Combined with Carbon Fiber Ionization Mass Spectrometry for the Screening of Trace Triazine Herbicides from Aqueous Samples. Molecules. 2024; 29(1):137. https://doi.org/10.3390/molecules29010137
Chicago/Turabian StyleChen, Chih-Wei, Tzu-Ling Yang, and Yu-Chie Chen. 2024. "Using Magnetic Micelles Combined with Carbon Fiber Ionization Mass Spectrometry for the Screening of Trace Triazine Herbicides from Aqueous Samples" Molecules 29, no. 1: 137. https://doi.org/10.3390/molecules29010137
APA StyleChen, C.-W., Yang, T.-L., & Chen, Y.-C. (2024). Using Magnetic Micelles Combined with Carbon Fiber Ionization Mass Spectrometry for the Screening of Trace Triazine Herbicides from Aqueous Samples. Molecules, 29(1), 137. https://doi.org/10.3390/molecules29010137







