Plumeriapropionics A–E, Carboxyl-Substituted Phenylpropionic Acid Derivatives with Anti-Inflammatory Activity from Plumeria rubra L.
Abstract
:1. Introduction
2. Results and Discussion
2.1. Phytochemical Investigation
2.2. Anti-Inflammatory Activity
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Anti-Inflammatory Bioassays
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bihani, T.; Tandel, P.; Wadekar, J. Plumeria obtusa L.: A systematic review of its traditional uses, morphology, phytochemistry and pharmacology. Phytomed. Plus 2021, 1, 100052. [Google Scholar] [CrossRef]
- Bihani, T. Plumeria rubra L.—A review on its ethnopharmacological, morphological, phytochemical, pharmacological and toxicological studies. J. Ethnopharmacol. 2021, 264, 113291. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.Y.; Lin, C.Z.; Lu, X.J.; Liu, F.L.; Wu, A.Z.; Zhang, L.; Zhang, L.; Zhu, C.C. New iridoids from the flowers of Plumeria rubra “Acutifolia”. Phytochem. Lett. 2018, 25, 81–85. [Google Scholar] [CrossRef]
- Zhang, S.N.; Song, H.Z.; Ma, R.J.; Liang, C.Q.; Wang, H.S.; Tan, Q.G. Potential anti-diabetic isoprenoids and a long-chain δ-lactone from frangipani (Plumeria rubra). Fitoterapia 2020, 146, 104684. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, N.; Saleem, M.; Riaz, N.; Ali, M.S.; Yaqoob, A.; Nasim, F.H.; Jabbar, A. Isolation and characterization of the chemical constituents from Plumeria rubra. Phytochem. Lett. 2013, 6, 291–298. [Google Scholar] [CrossRef]
- Siddiqui, B.S.; Firdous Begum, S. Two triterpenoids from the leaves of Plumeria obtusa. Phytochemistry 1999, 52, 1111–1115. [Google Scholar] [CrossRef]
- Akhtar, N.; Malik, A. Oleanene type triterpenes from Plumeria rubra. Phytochemistry 1993, 32, 1523–1525. [Google Scholar] [CrossRef]
- Dobhal, M.P.; Hasan, A.M.; Sharma, M.C.; Joshi, B.C. Ferulic acid esters from Plumeria bicolor. Phytochemistry 1999, 51, 319–321. [Google Scholar] [CrossRef]
- Siddiqui, B.S.; Ilyas, F.; Rasheed, M.; Begum, S. Chemical constituents of leaves and stem bark of Plumeria obtusa. Phytochemistry 2004, 65, 2077–2084. [Google Scholar] [CrossRef] [PubMed]
- Kardono, L.B.S.; Tsauri, S.; Padmawinata, K.; Kinghorn, A.D. A flavan-3-ol glycoside from bark of Plumeria rubra. Phytochemistry 1990, 29, 2995–2997. [Google Scholar] [CrossRef]
- Fernandes, H.B.; Machado, D.L.; Dias, J.M.; Brito, T.V.; Batista, J.A.; Silva, R.O.; Pereira, A.C.T.C.; Ferreira, G.P.; Ramos, M.V.; Mediros, J.V.R. Laticifer proteins from Plumeria pudica inhibit the inflammatory and nociceptive responses by decreasing the action of inflammatory mediators and pro-inflammatory cytokines. Rev. Bras. de Farmacogn. 2015, 25, 269–277. [Google Scholar] [CrossRef]
- Vijayalakshimi, A.; Ravichandiran, V.; Velraj, M.; Hemalatha, S.; Sudharani, G.; Jayakumari, S. Anti–anaphylactic and anti–inflammatory activities of a bioactive alkaloid from the root bark of Plumeria acutifolia Poir. Asian Pac. J. Trop. Biomed. 2011, 1, 401–405. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.P.; Liu, M.S.; Pei, Y.H.; Zhang, J.Q.; Kang, S.L. Phenylpropionic acid derivates from the bark of Cerbera manghas. Fitoterapia 2010, 81, 852–854. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.S. Natural Medicinal Chemistry; People’s Medical Publishing House: Beijing, China, 2001; p. 109. [Google Scholar]
- Wang, S.K.; Chen, T.X.; Wang, W.; Xu, L.L.; Zhang, Y.Q.; Jin, Z.; Liu, Y.B.; Tang, Y.Z. Aesculetin exhibited anti-inflammatory activities through inhibiting NF-кB and MAPKs pathway In Vitro and In Vivo. J. Ethnopharmacol. 2022, 296, 115489. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Wu, Q.L.; Chen, D.N.; Jiang, M.H.; Chen, B.; Lu, Y.J.; Li, J.; Liu, L.; Chen, S.H. Absolute configuration of polypropionate derivatives: Decempyrones A-J and their MptpA inhibition and anti-inflammatory activities. Bioorg. Chem. 2021, 115, 105156. [Google Scholar] [CrossRef] [PubMed]
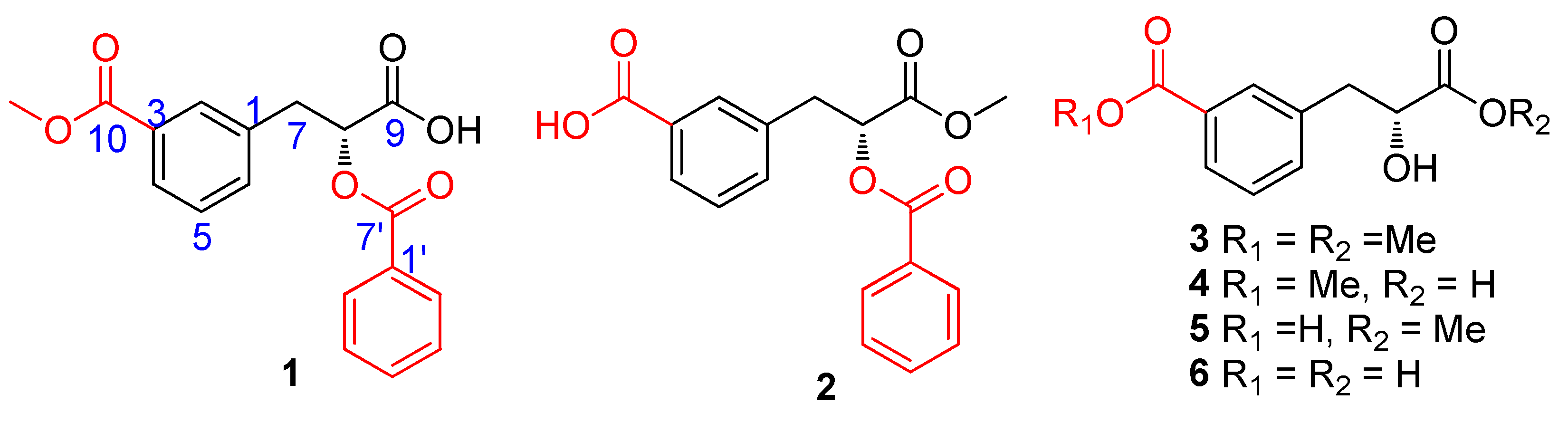
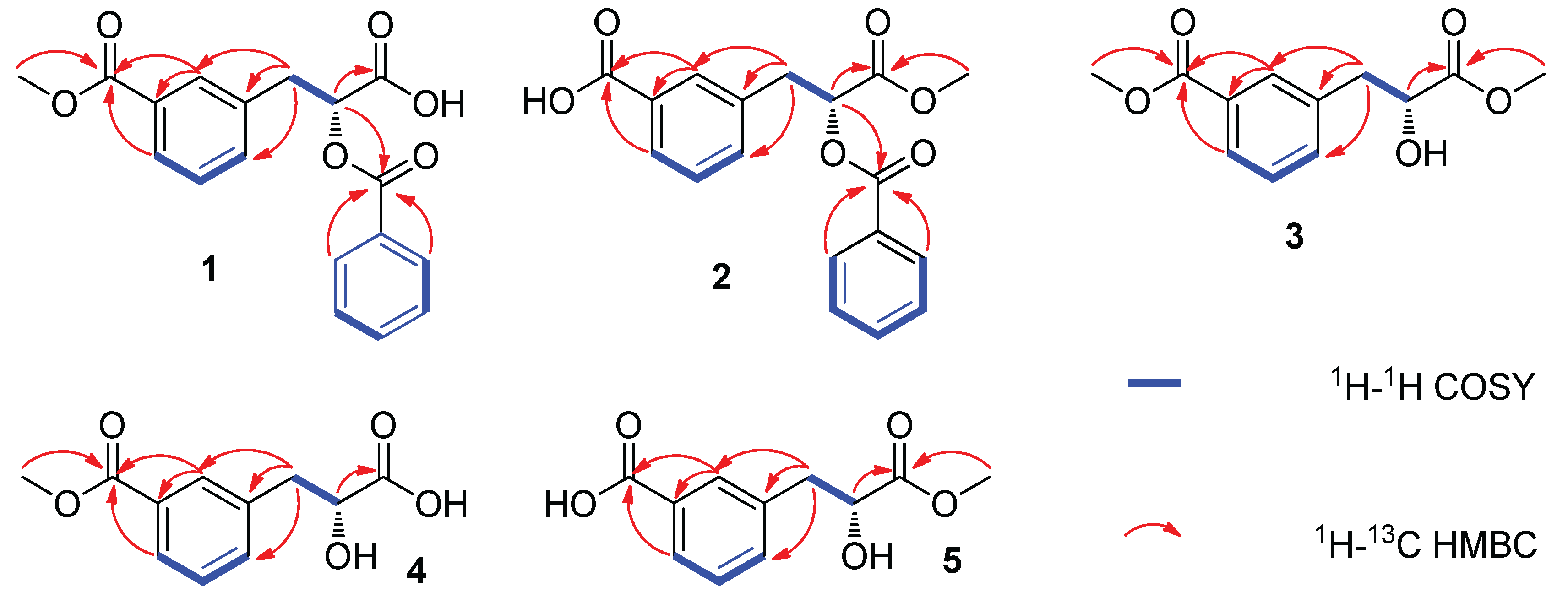
| Position | 1 a | 2 a | ||
|---|---|---|---|---|
| δH, Mult, (J in Hz) | δC | δH, Mult, (J in Hz) | δC | |
| 1 | - | 137.3 | - | 136.5 |
| 2 | 8.00 (br, s) | 130.4 | 7.96 (s) | 130.6 |
| 3 | - | 129.7 | - | 128.7 |
| 4 | 7.82 (dd, 7.8, 1.2) | 127.8 | 7.83 (d, 7.8) | 128.0 |
| 5 | 7.45 (dd, 7.8, 7.2) | 128.9 | 7.44 (dd, 7.8, 7.8) | 128.7 |
| 6 | 7.64 (d, 7.2) | 134.5 | 7.57 (d, 7.8) | 134.0 |
| 7 | 3.38 (dd, 14.4, 4.2) 3.30 (dd, 14.4, 7.8) | 36.4 | 3.37 (dd, 14.4, 4.8) 3.31 (dd, 14.4, 7.8) | 36.4 |
| 8 | 5.36 (dd, 7.8, 4.2) | 73.1 | 5.46 (dd, 7.8, 4.8) | 72.9 |
| 9 | - | 170.4 | - | 169.5 |
| 10 | - | 165.2 | - | 165.1 |
| 9-OMe | - | - | 3.66 | 52.3 |
| 10-OMe | 3.83 (s) | 52.2 | - | - |
| 1′ | - | 130.9 | - | 130.9 |
| 2′,6′ | 7.94 (m) | 129.4 | 7.95 (m) | 129.4 |
| 3′,5′ | 7.48 (m) | 128.9 | 7.49 (m) | 129.0 |
| 4′ | 7.65 (m) | 133.8 | 7.67 (m) | 134.0 |
| 7′ | - | 165.2 | - | 165.1 |
| Position | 3 a | 4 a | 5 a | |||
|---|---|---|---|---|---|---|
| δH, Mult, (J in Hz) | δC | δH, Mult, (J in Hz) | δC | δH, Mult, (J in Hz) | δC | |
| 1 | - | 138.5 | - | 139.9 | - | 138.3 |
| 2 | 7.83 (s) | 130.2 | 7.84 (s) | 130.7 | 7.81 (s) | 130.5 |
| 3 | - | 129.5 | - | 129.8 | - | 130.7 |
| 4 | 7.81 (d, 8.0) | 127.2 | 7.79 (d, 7.6) | 127.4 | 7.80 (d, 8.0) | 127.5 |
| 5 | 7.42 (dd, 8.0, 8.0) | 128.5 | 7.41 (dd, 7.6, 7.6) | 128.8 | 7.39 (dd, 8.0, 7.6) | 128.4 |
| 6 | 7.49 (d, 8.0) | 134.4 | 7.51 (d, 7.6) | 134.9 | 7.45 (d, 7.6) | 134.0 |
| 7 | 3.03 (dd, 14.0, 4.8) 2.90 (dd, 14.0, 8.4) | 39.7 | 3.04 (dd, 13.6, 4.0) 2.81 (dd, 13.6, 8.4) | 40.3 | 3.02 (dd, 13.6, 4.8) 2.90 (dd, 13.6, 8.0) | 39.9 |
| 8 | 4.29 (dd, 8.4, 4.8) | 71.0 | 4.06 (m) | 71.6 | 4.28 (dd, 8.0, 4.8) | 71.1 |
| 9 | - | 173.8 | - | 175.6 | - | 173.9 |
| 10 | - | 166.4 | - | 166.9 | - | 167.6 |
| 9-OMe | 3.62 (s) | 51.5 | - | - | 3.61 (s) | 51.5 |
| 10-OMe | 3.84 (s) | 52.1 | 3.84 (s) | 52.5 | - | - |
| Compound | IC50 (µM) | Compound | IC50 (µM) |
|---|---|---|---|
| 1 | 28.26 ± 0.15 | 4 | 33.16 ± 0.18 |
| 2 | 6.52 ± 0.23 | 5 | 7.13 ± 0.16 |
| 3 | 35.68 ± 0.17 | 6 | 6.68 ± 0.22 |
| Hydrocortisone a | 5.61 ± 0.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, X.; Gan, M.; Wu, M.; Zheng, T.; Enkhchimeg, C.; Li, H.; Feng, S.; Zhou, J.; Song, X. Plumeriapropionics A–E, Carboxyl-Substituted Phenylpropionic Acid Derivatives with Anti-Inflammatory Activity from Plumeria rubra L. Molecules 2024, 29, 115. https://doi.org/10.3390/molecules29010115
Zhou X, Gan M, Wu M, Zheng T, Enkhchimeg C, Li H, Feng S, Zhou J, Song X. Plumeriapropionics A–E, Carboxyl-Substituted Phenylpropionic Acid Derivatives with Anti-Inflammatory Activity from Plumeria rubra L. Molecules. 2024; 29(1):115. https://doi.org/10.3390/molecules29010115
Chicago/Turabian StyleZhou, Xueming, Minlin Gan, Meizhu Wu, Ting Zheng, Chuluunbaatar Enkhchimeg, Haixiang Li, Shuo Feng, Jingqi Zhou, and Xinming Song. 2024. "Plumeriapropionics A–E, Carboxyl-Substituted Phenylpropionic Acid Derivatives with Anti-Inflammatory Activity from Plumeria rubra L." Molecules 29, no. 1: 115. https://doi.org/10.3390/molecules29010115
APA StyleZhou, X., Gan, M., Wu, M., Zheng, T., Enkhchimeg, C., Li, H., Feng, S., Zhou, J., & Song, X. (2024). Plumeriapropionics A–E, Carboxyl-Substituted Phenylpropionic Acid Derivatives with Anti-Inflammatory Activity from Plumeria rubra L. Molecules, 29(1), 115. https://doi.org/10.3390/molecules29010115






