Effect of Capsaicin Stress on Aroma-Producing Properties of Lactobacillus plantarum CL-01 Based on E-Nose and GC–IMS
Abstract
:1. Introduction
2. Results
2.1. Effect of Different Capsaicin Concentrations on the Aroma-Producing Properties of L. plantarum
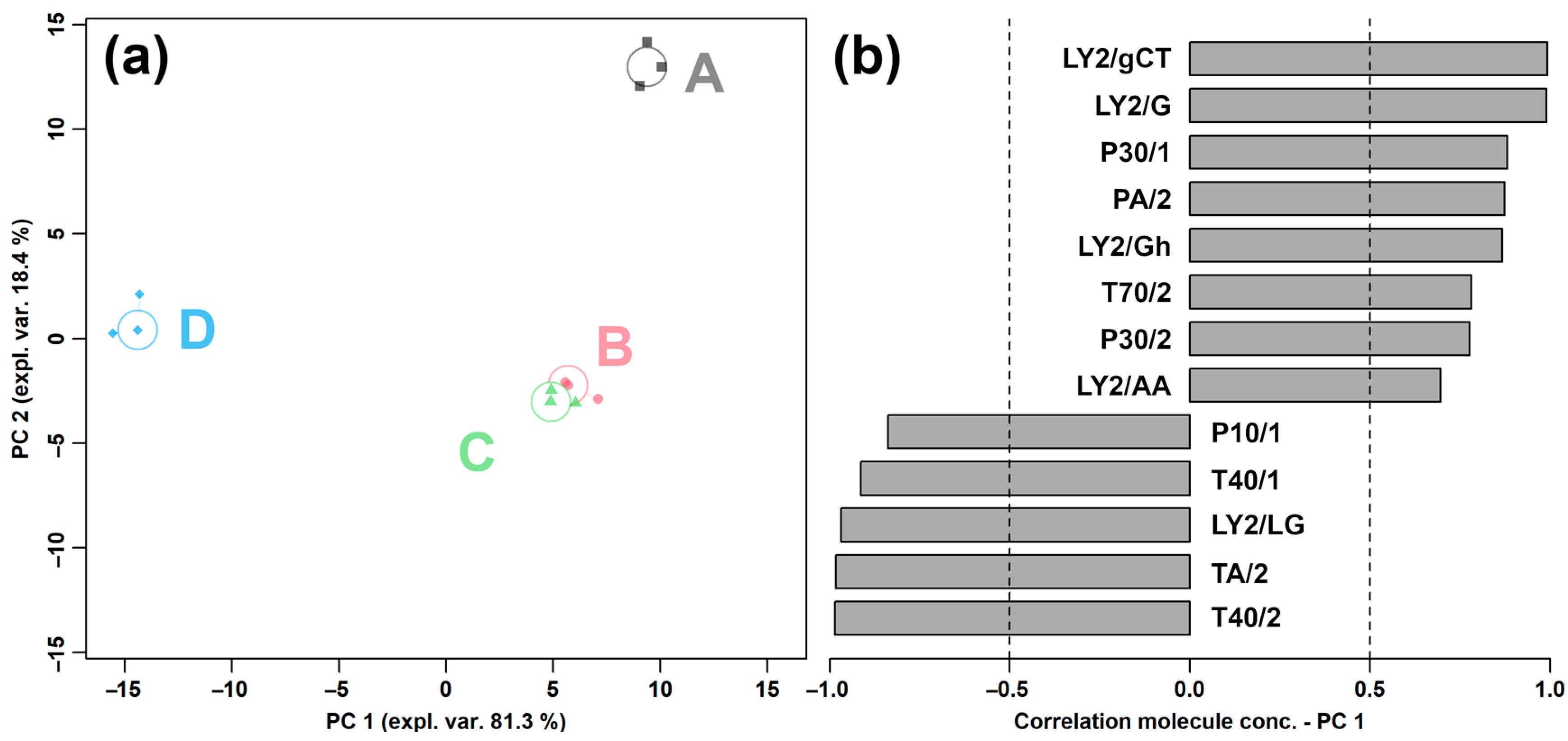
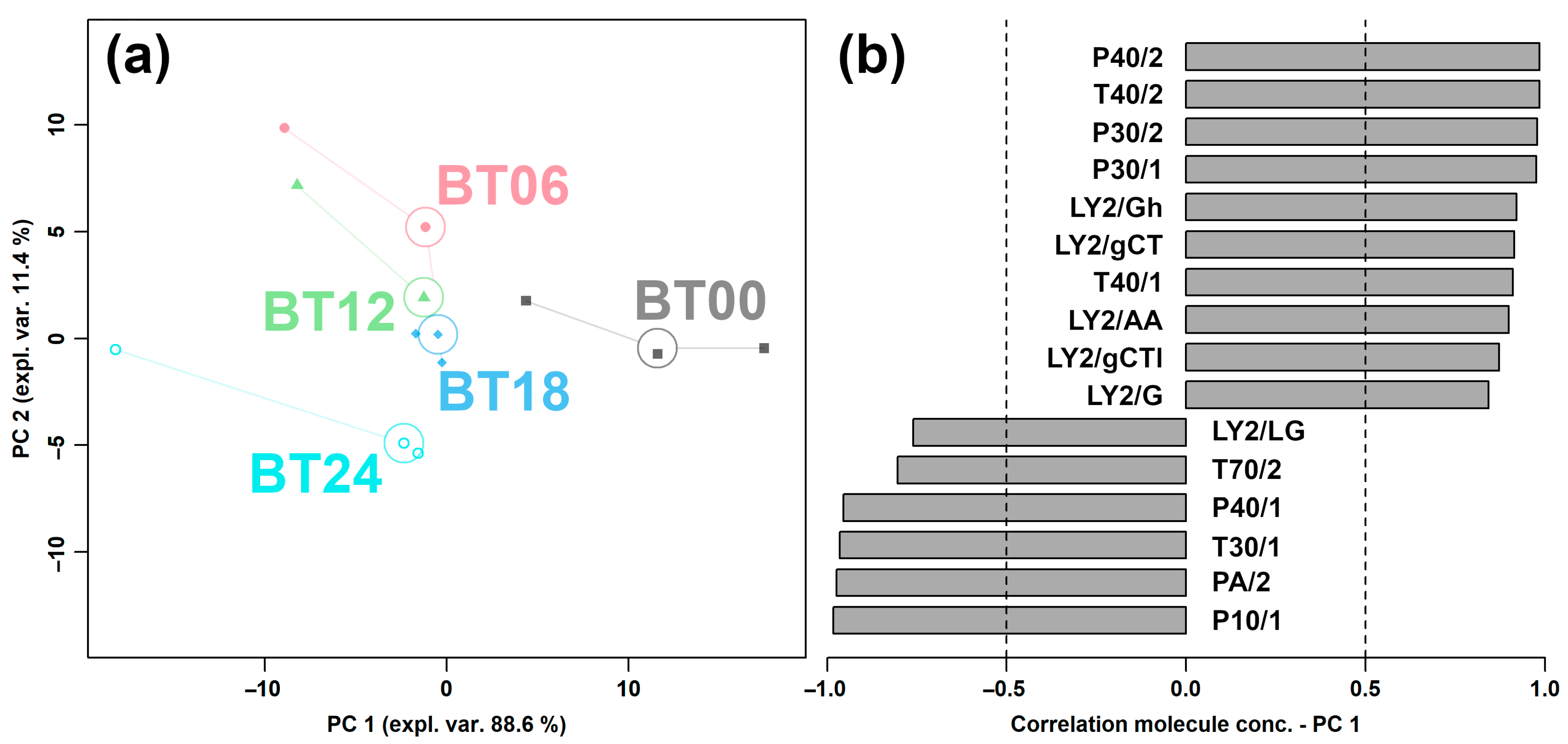
2.1.1. E-Nose Analysis
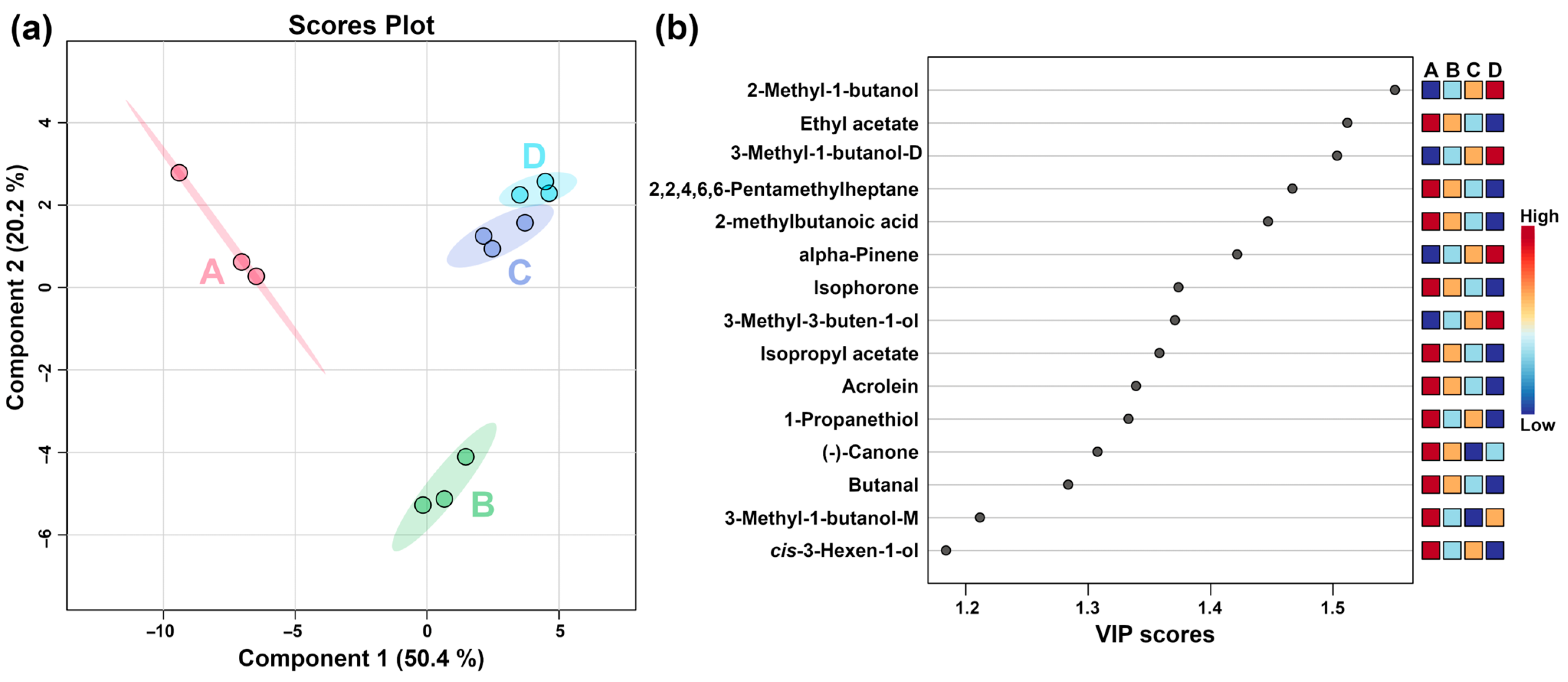
2.1.2. GC–IMS Analysis
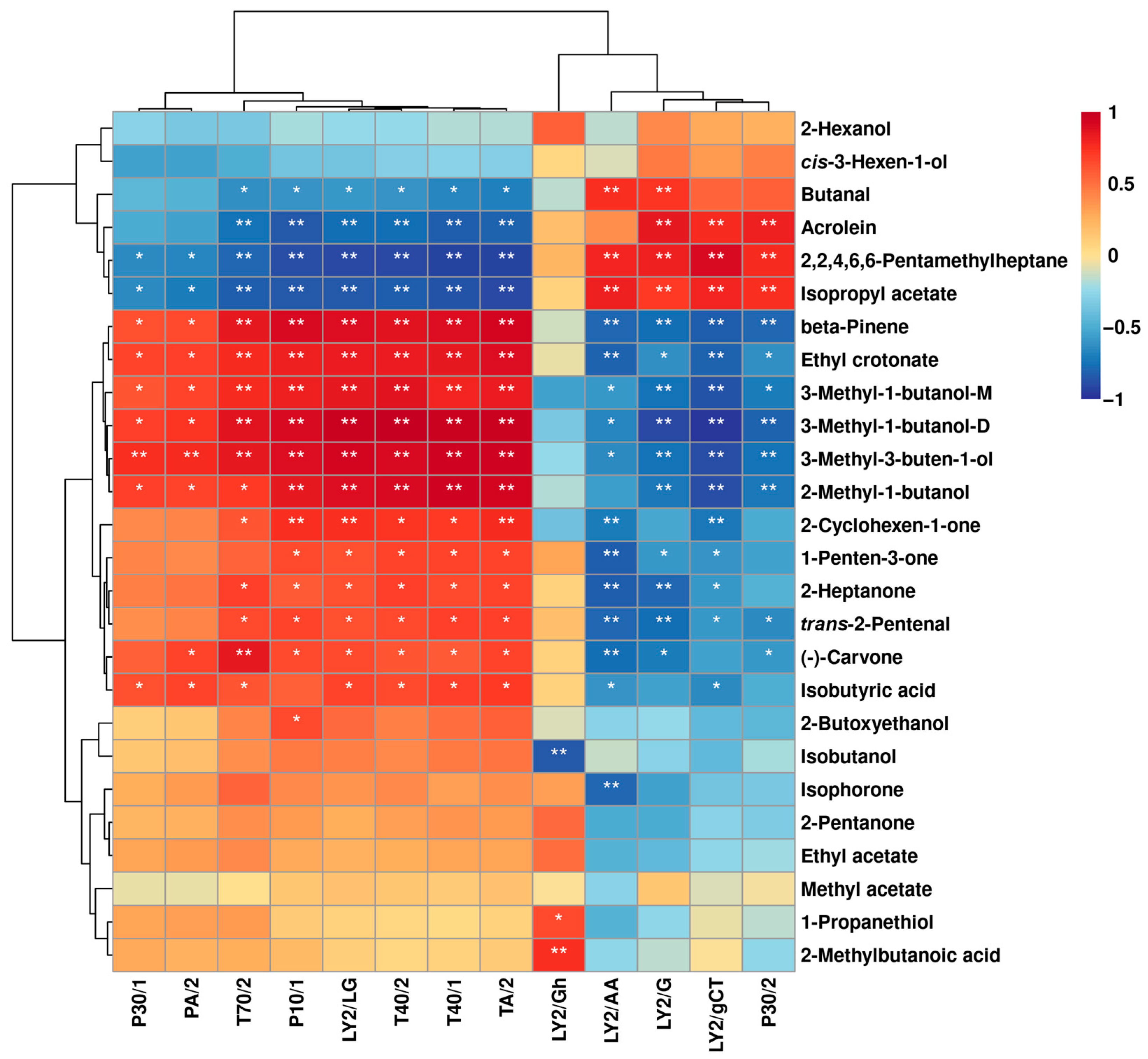
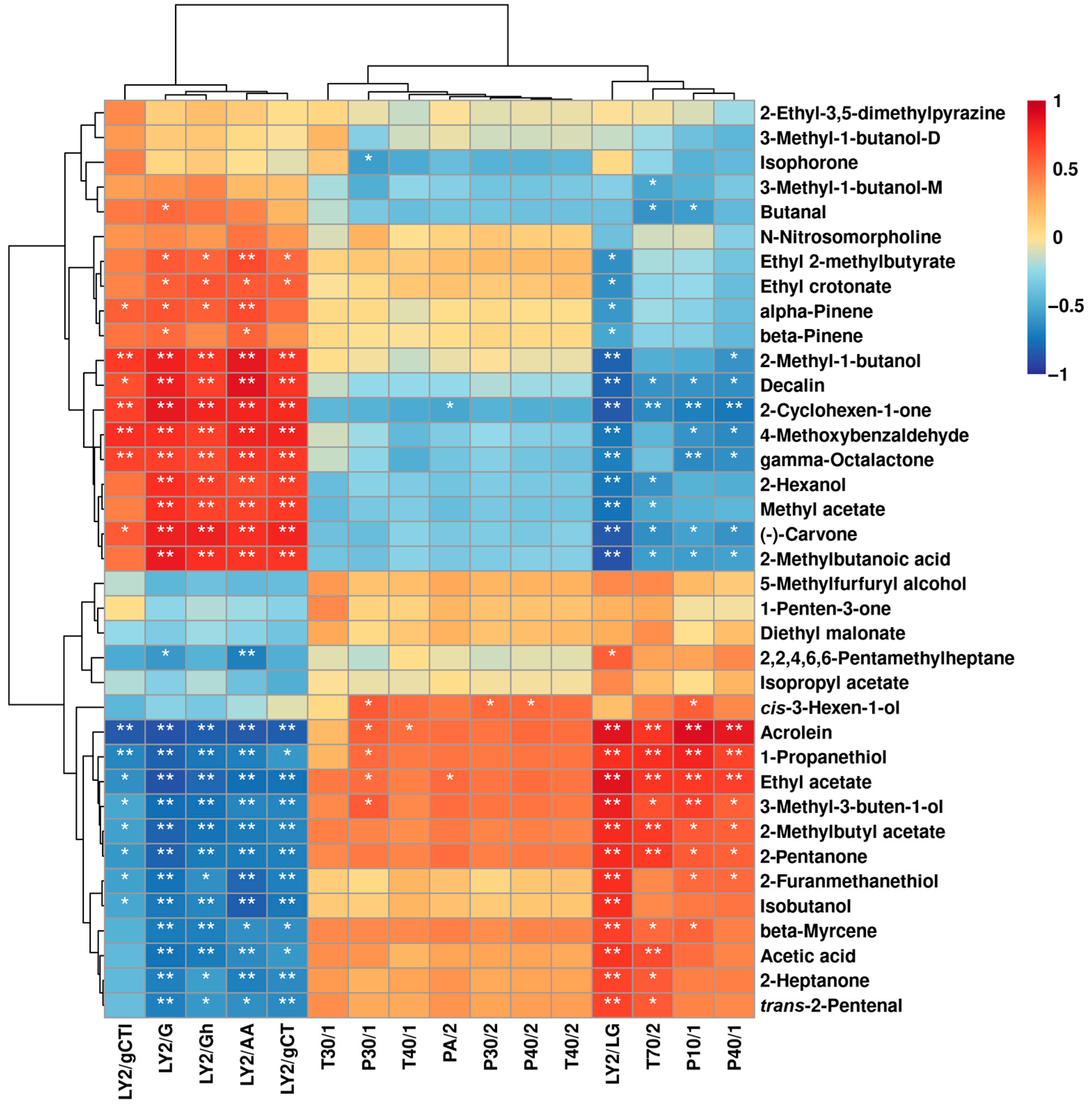
2.1.3. Correlation between E-Nose and GC–IMS
2.2. Effect of Capsaicin Stress on the Aroma-Producing Properties of L. plantarum during Fermentation
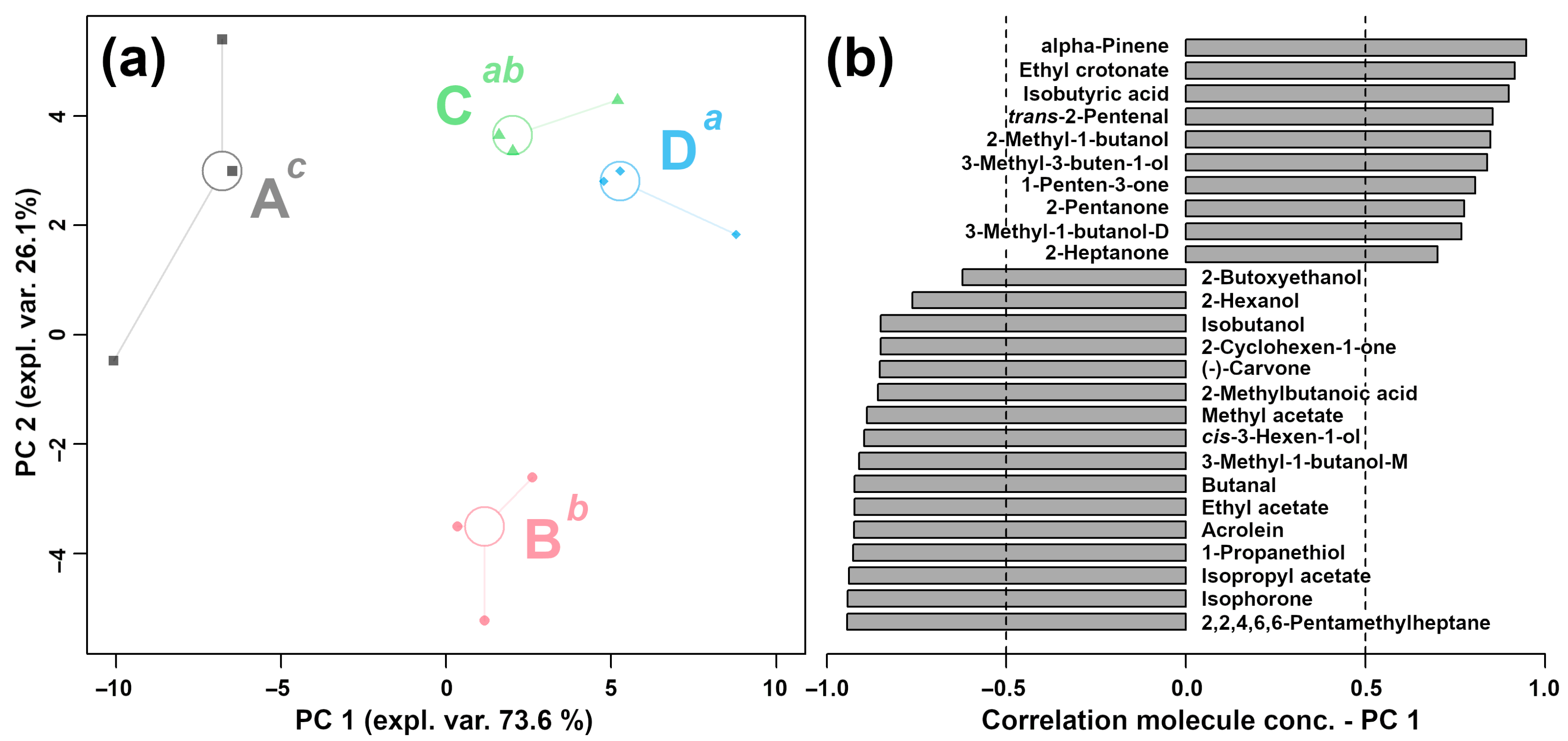
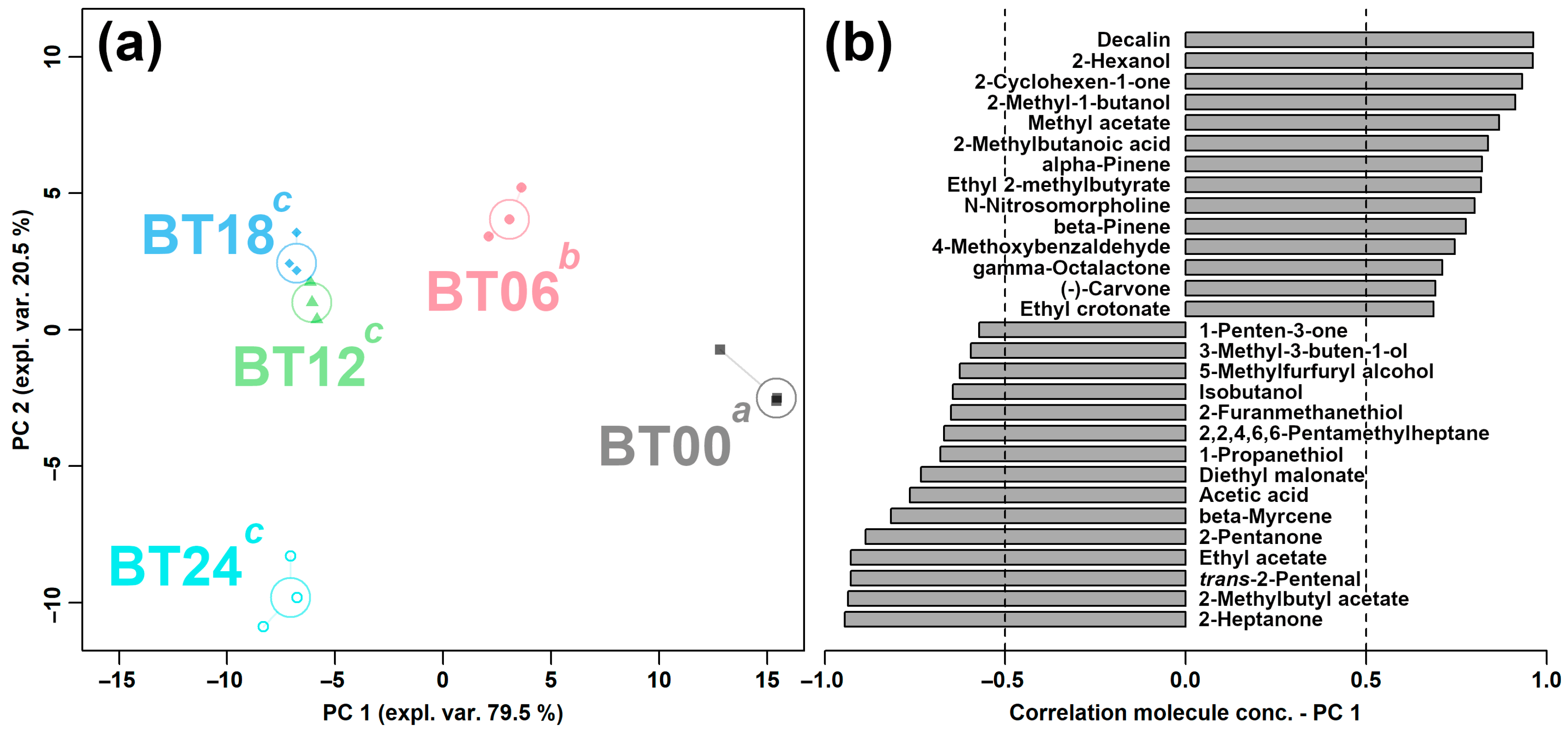
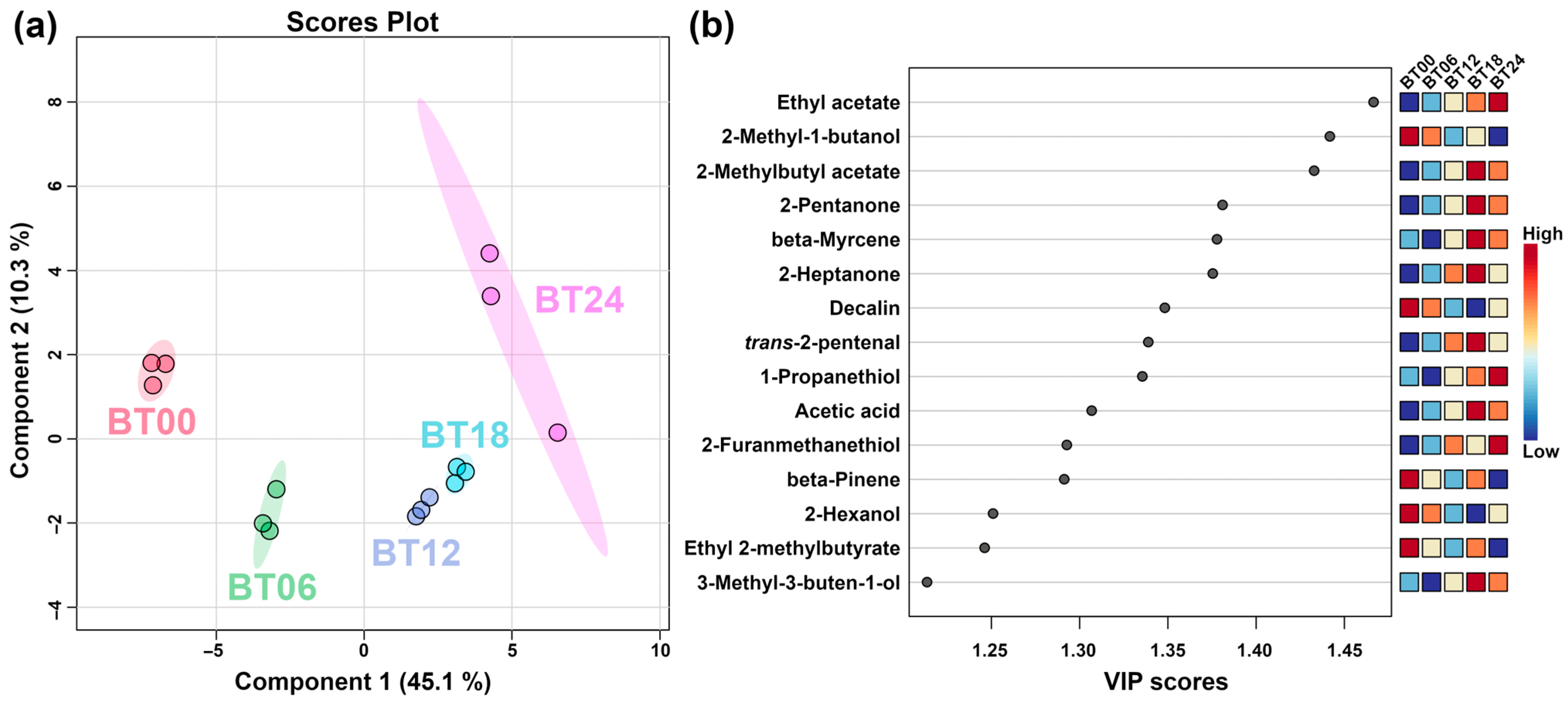
2.2.1. E-Nose Analysis
2.2.2. GC–IMS Analysis
2.2.3. Correlation between E-Nose and GC–IMS
3. Discussion
4. Materials and Methods
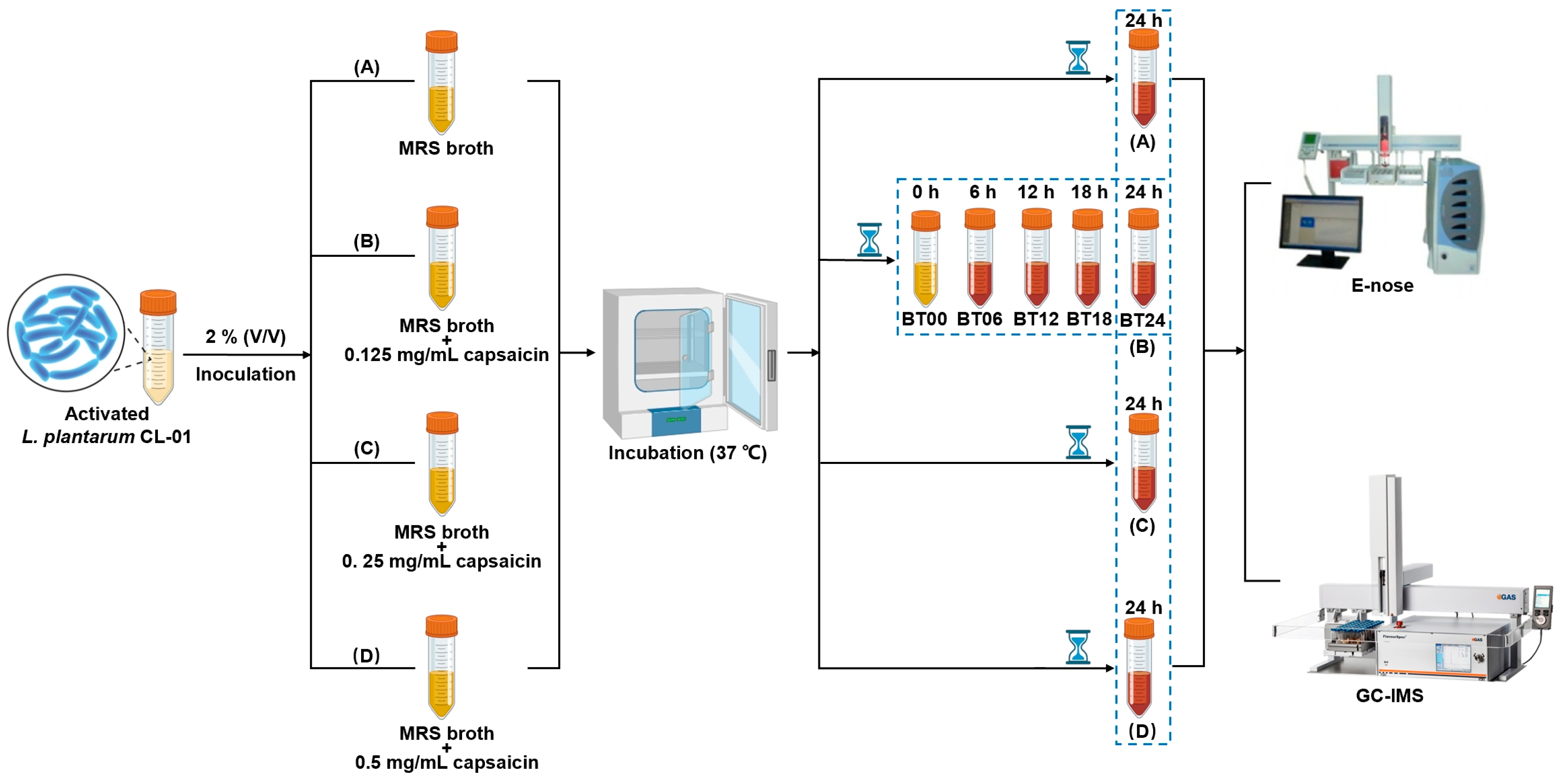
4.1. Activation of Bacteria
4.2. Preparation of Capsaicin Solution
4.3. Fermentation
4.4. E-Nose Analysis
4.5. GC–IMS Analysis
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Heng, Z.; Xu, X.; Xu, X.; Li, Y.; Wang, H.; Huang, W.; Yan, S.; Li, T. Integrated Transcriptomic and Metabolomic Analysis of Chili Pepper Fruits Provides New Insight into the Regulation of the Branched Chain Esters and Capsaicin Biosynthesis. Food Res. Int. 2023, 169, 112856. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Bao, X.; Zhang, X.; Ren, H.; Cai, S.; Hu, X.; Yi, J. Exploring the Phytochemicals and Inhibitory Effects against α-Glucosidase and Dipeptidyl Peptidase-IV in Chinese Pickled Chili Pepper: Insights into Mechanisms by Molecular Docking Analysis. LWT 2022, 162, 113467. [Google Scholar] [CrossRef]
- Sanatombi, K. Antioxidant Potential and Factors Influencing the Content of Antioxidant Compounds of Pepper: A Review with Current Knowledge. Compr. Rev. Food Sci. Food Saf. 2023, 22, 3011–3052. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wu, B.; Zhao, W.; Lao, F.; Chen, F.; Liao, X.; Wu, J. Shifts in Autochthonous Microbial Diversity and Volatile Metabolites during the Fermentation of Chili Pepper (Capsicum frutescens L.). Food Chem. 2021, 335, 127512. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Dong, L.; Jeon, J.; Kwon, S.Y.; Zhao, C.; Baek, H.H. Characterization and Evaluation of Aroma Quality in Doubanjiang, a Chinese Traditional Fermented Red Pepper Paste, Using Aroma Extract Dilution Analysis and a Sensory Profile. Molecules 2019, 24, 3107. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Tang, H.; Mei, Y.; Chen, J.; Wang, X.; Liu, B.; Cai, Y.; Zhao, N.; Yang, M.; Li, H. Effects of Endogenous Capsaicin Stress and Fermentation Time on the Microbial Succession and Flavor Compounds of Chili Paste (a Chinese Fermented Chili Pepper). Food Res. Int. 2023, 168, 112763. [Google Scholar] [CrossRef]
- Ye, Z.; Shang, Z.; Li, M.; Zhang, X.; Ren, H.; Hu, X.; Yi, J. Effect of Ripening and Variety on the Physiochemical Quality and Flavor of Fermented Chinese Chili Pepper (Paojiao). Food Chem. 2022, 368, 130797. [Google Scholar] [CrossRef]
- Li, Y.; Luo, X.; Long, F.; Wu, Y.; Zhong, K.; Bu, Q.; Huang, Y.; Gao, H. Quality Improvement of Fermented Chili Pepper by Inoculation of Pediococcus Ethanolidurans M1117: Insight into Relevance of Bacterial Community Succession and Metabolic Profile. LWT 2023, 179, 114655. [Google Scholar] [CrossRef]
- Chen, Y.; Li, P.; Liao, L.; Qin, Y.; Jiang, L.; Liu, Y. Characteristic Fingerprints and Volatile Flavor Compound Variations in Liuyang Douchi during Fermentation via HS-GC-IMS and HS-SPME-GC-MS. Food Chem. 2021, 361, 130055. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, L.; Wen, R.; Chen, Q.; Kong, B. Role of Lactic Acid Bacteria in Flavor Development in Traditional Chinese Fermented Foods: A Review. Crit. Rev. Food Sci. Nutr. 2020, 62, 2741–2755. [Google Scholar] [CrossRef]
- López-Salas, D.; Oney-Montalvo, J.E.; Ramírez-Rivera, E.; Ramírez-Sucre, M.O.; Rodríguez-Buenfil, I.M. Evaluation of the Volatile Composition and Sensory Behavior of Habanero Pepper during Lactic Acid Fermentation by L. plantarum. Foods 2022, 11, 3618. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Xu, J.; Xu, J.; Wang, J.; Zhang, Y.; Liao, X.; Zhao, L. Microbial Community and Volatile Metabolites Related to the Fermentation Degree of Salted Fermented Chili Peppers. LWT 2023, 181, 114752. [Google Scholar] [CrossRef]
- Duwat, P.; Ehrlich, S.D.; Gruss, A. Effects of Metabolic Flux on Stress Response Pathways in Lactococcus Lactis. Mol. Microbiol. 1999, 31, 845–858. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; He, M.; Wu, C. Cross Protection of Lactic Acid Bacteria during Environmental Stresses: Stress Responses and Underlying Mechanisms. LWT 2021, 144, 111203. [Google Scholar] [CrossRef]
- Gallegos, J.; Arce, C.; Jordano, R.; Arce, L.; Medina, L.M. Target Identification of Volatile Metabolites to Allow the Differentiation of Lactic Acid Bacteria by Gas Chromatography-Ion Mobility Spectrometry. Food Chem. 2017, 220, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wei, B.C.; Wang, X.; Zhang, Y.; Gong, Y.J. Aroma Profiles of Sweet Cherry Juice Fermented by Different Lactic Acid Bacteria Determined through Integrated Analysis of Electronic Nose and Gas Chromatography–Ion Mobility Spectrometry. Front. Microbiol. 2023, 14, 1113594. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Ye, Z.; Mao, L.; Zhang, L.; Zhang, J.; Ding, W.; Han, J.; Mao, K. Analysis of Volatile Organic Compounds and Metabolites of Three Cultivars of Asparagus (Asparagus officinalis L.) Using E-Nose, GC-IMS, and LC-MS/MS. Bioengineered 2022, 13, 8866–8880. [Google Scholar] [CrossRef]
- Gao, Y.; Hou, L.; Gao, J.; Li, D.; Tian, Z.; Fan, B.; Wang, F.; Li, S. Metabolomics Approaches for the Comprehensive Evaluation of Fermented Foods: A Review. Foods 2021, 10, 2294. [Google Scholar] [CrossRef]
- Arnold¤, J.W.; Senter, S.D. Use of Digital Aroma Technology and SPME to Compare Volatile Compounds GC-MS Produced by Bacteria Isolated from Processed Poultry. J. Sci. Food Agric. 1998, 78, 343–348. [Google Scholar] [CrossRef]
- Chen, J.N.; Zhang, Y.Y.; Huang, X.H.; Dong, M.; Dong, X.P.; Zhou, D.Y.; Zhu, B.W.; Qin, L. Integrated Volatolomics and Metabolomics Analysis Reveals the Characteristic Flavor Formation in Chouguiyu, a Traditional Fermented Mandarin Fish of China. Food Chem. 2023, 418, 135874. [Google Scholar] [CrossRef]
- Zhao, X.; Feng, J.; Laghi, L.; Deng, J.; Dao, X.; Tang, J.; Ji, L.; Zhu, C.; Picone, G. Characterization of Flavor Profile of “Nanx Wudl” Sour Meat Fermented from Goose and Pork Using Gas Chromatography–Ion Mobility Spectrometry (GC–IMS) Combined with Electronic Nose and Tongue. Foods 2023, 12, 2194. [Google Scholar] [CrossRef] [PubMed]
- Ricci, A.; Cirlini, M.; Levante, A.; Dall’Asta, C.; Galaverna, G.; Lazzi, C. Volatile Profile of Elderberry Juice: Effect of Lactic Acid Fermentation Using L. plantarum, L. rhamnosus and L. casei strains. Food Res. Int. 2018, 105, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.N.; Chen, C.; Huang, X.N.; Yan, Y.Z.; Chen, J.Y.; Han, B.Z. Influence of Indigenous Lactic Acid Bacteria on the Volatile Flavor Profile of Light-Flavor Baijiu. LWT 2021, 147, 111540. [Google Scholar] [CrossRef]
- Gao, T.; Chen, J.; Xu, J.; Gu, H.; Zhao, P.; Wang, W.; Pan, S.; Tao, Y.; Wang, H.; Yang, J. Screening of a Novel Lactiplantibacillus plantarum MMB-05 and Lacticaseibacillus casei Fermented Sandwich Seaweed Scraps: Chemical Composition, In Vitro Antioxidant, and Volatile Compounds Analysis by GC-IMS. Foods 2022, 11, 2875. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Cheng, X.; Yang, J.; Wang, X.; Lü, X. Unraveling the Difference in Physicochemical Properties, Sensory, and Volatile Profiles of Dry Chili Sauce and Traditional Fresh Dry Chili Sauce Fermented by Lactobacillus plantarum PC8 Using Electronic Nose and HS-SPME-GC-MS. Food Biosci. 2022, 50, 102057. [Google Scholar] [CrossRef]
- Lu, Y.; Tan, X.; Lv, Y.; Yang, G.; Chi, Y.; He, Q. Flavor Volatiles Evolution of Chinese Horse Bean-Chili-Paste during Ripening, Accessed by GC×GC-TOF/MS and GC-MS-Olfactometry. Int. J. Food Prop. 2020, 23, 570–581. [Google Scholar] [CrossRef]
- Chen, C.; Lu, Y.; Yu, H.; Chen, Z.; Tian, H. Influence of 4 Lactic Acid Bacteria on the Flavor Profile of Fermented Apple Juice. Food Biosci. 2019, 27, 30–36. [Google Scholar] [CrossRef]
- Wang, Z.; Dou, R.; Yang, R.; Cai, K.; Li, C.; Li, W. Changes in Phenols, Polysaccharides and Volatile Profiles of Noni (Morinda citrifolia L.) Juice during Fermentation. Molecules 2021, 26, 2604. [Google Scholar] [CrossRef]
- Chen, H.; Nie, X.; Peng, T.; Xiang, L.; Liu, D.; Luo, H.; Zhao, Z. Effects of Low-Temperature and Low-Salt Fermentation on the Physicochemical Properties and Volatile Flavor Substances of Chinese Kohlrabi Using Gas Chromatography–Ion Mobility Spectrometry. Fermentation 2023, 9, 146. [Google Scholar] [CrossRef]
- Wang, J.; Wang, R.; Xiao, Q.; Liu, C.; Deng, F.; Zhou, H. SPME/GC-MS Characterization of Volatile Compounds of Chinese Traditional-Chopped Pepper during Fermentation. Int. J. Food Prop. 2019, 22, 1863–1872. [Google Scholar] [CrossRef]
- Gou, Y.; Ma, X.; Niu, X.; Ren, X.; Muhatai, G.; Xu, Q. Exploring the Characteristic Aroma Components of Traditional Fermented Koumiss of Kazakh Ethnicity in Different Regions of Xinjiang by Combining Modern Instrumental Detection Technology with Multivariate Statistical Analysis Methods for Odor Activity Value and Sensory Analysis. Foods 2023, 12, 2223. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Al-Dalali, S.; Wang, Z.; Xu, B.; Zhou, H. Investigation of Volatile Flavor Compounds and Characterization of Aroma-Active Compounds of Water-Boiled Salted Duck Using GC–MS–O, GC–IMS, and E-Nose. Food Chem. 2022, 386, 132728. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xu, H.; Ding, S.; Zhou, H.; Qin, D.; Deng, F.; Wang, R. Changes in Volatile Compounds of Fermented Minced Pepper during Natural and Inoculated Fermentation Process Based on Headspace–Gas Chromatography–Ion Mobility Spectrometry. Food Sci. Nutr. 2020, 8, 3362–3379. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhang, L.; Liu, Q.; Wang, Y.; Chen, Q.; Kong, B. The Potential Correlation between Bacterial Diversity and the Characteristic Volatile Flavour of Traditional Dry Sausages from Northeast China. Food Microbiol. 2020, 91, 103505. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wei, W.; Tang, L.; Wang, D.; Wang, Y.; Wu, Z.; Zhang, W. Characterization of Aroma and Bacteria Profiles of Sichuan Industrial Paocai by HS-SPME-GC-O-MS and 16S RRNA Amplicon Sequencing. Food Res. Int. 2021, 149, 110667. [Google Scholar] [CrossRef] [PubMed]
- Sabela, M.I.; Mpanza, T.; Kanchi, S.; Sharma, D.; Bisetty, K. Electrochemical Sensing Platform Amplified with a Nanobiocomposite of L-Phenylalanine Ammonia-Lyase Enzyme for the Detection of Capsaicin. Biosens. Bioelectron. 2016, 83, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Wen, R.; Kong, B.; Yin, X.; Zhang, H.; Chen, Q. Characterisation of Flavour Profile of Beef Jerky Inoculated with Different Autochthonous Lactic Acid Bacteria Using Electronic Nose and Gas Chromatography–Ion Mobility Spectrometry. Meat Sci. 2022, 183, 108658. [Google Scholar] [CrossRef]
- Zhang, Q.; Ma, J.; Yang, Y.; Deng, J.; Zhu, K.; Yi, Y.; Tang, J.; Jiang, X.; Zhu, C.; Laghi, L. Effects of S. Cerevisiae Strains on the Sensory Characteristics and Flavor Profile of Kiwi Wine Based on E-Tongue, GC-IMS and 1H-NMR. LWT 2023, 185, 115193. [Google Scholar] [CrossRef]
- Liu, H.; Yu, Y.; Zou, B.; Yu, Y.; Yang, J.; Xu, Y.; Chen, X.; Yang, F. Evaluation of Dynamic Changes and Regularity of Volatile Flavor Compounds for Different Green Plum (Prunus mume Sieb. et Zucc) Varieties during the Ripening Process by HS-GC–IMS with PLS-DA. Foods 2023, 12, 551. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, J.; Zhu, Z.; Lei, Y.; Huang, S.; Huang, M. Effect of Ageing Time on the Flavour Compounds in Nanjing Water-Boiled Salted Duck Detected by HS-GC-IMS. LWT 2022, 155, 112870. [Google Scholar] [CrossRef]
- Box, G.E.P.; Cox, D.R. An Analysis of Transformations. J. R. Stat. Soc. Ser. B (Methodol.) 1964, 26, 211–243. [Google Scholar] [CrossRef]


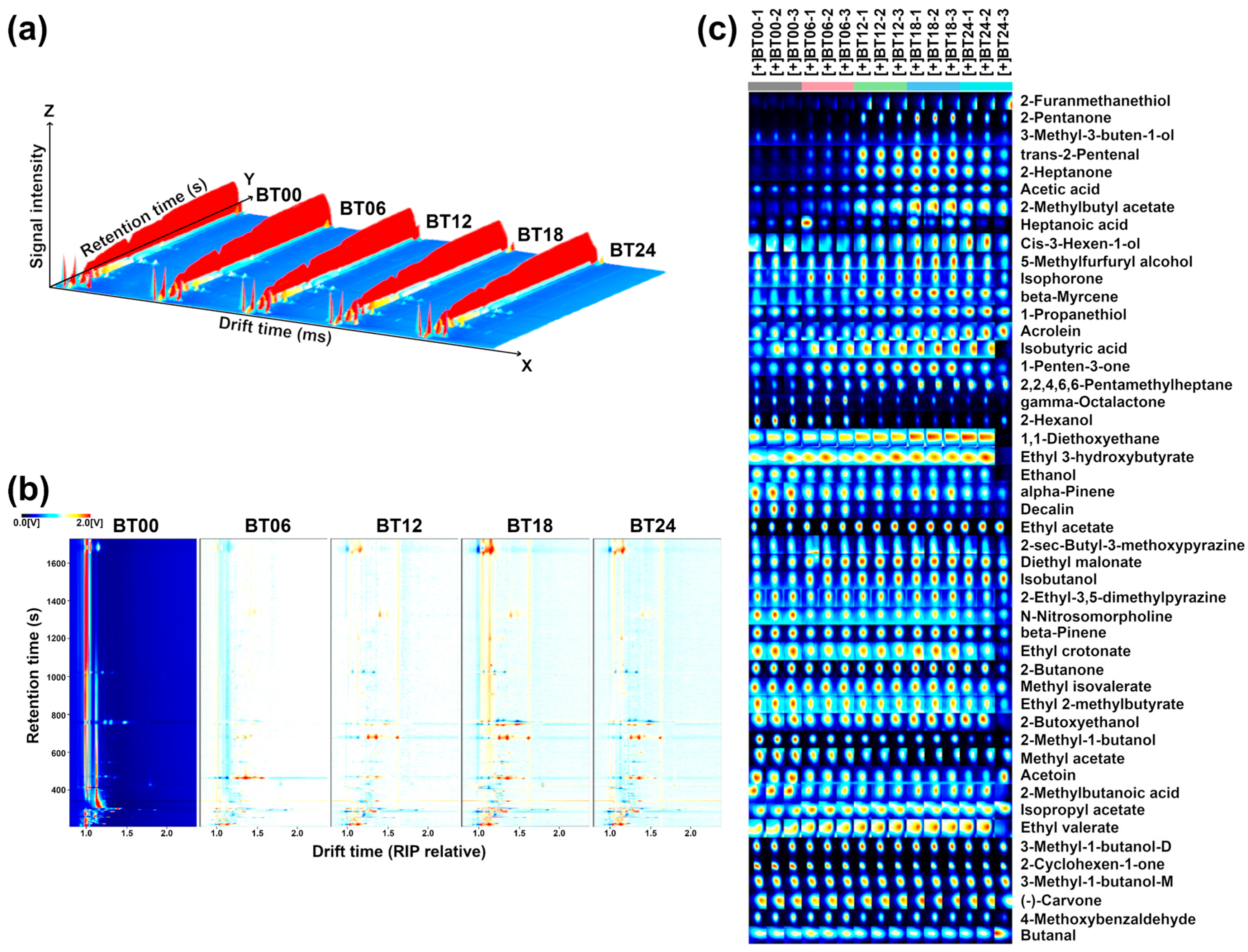
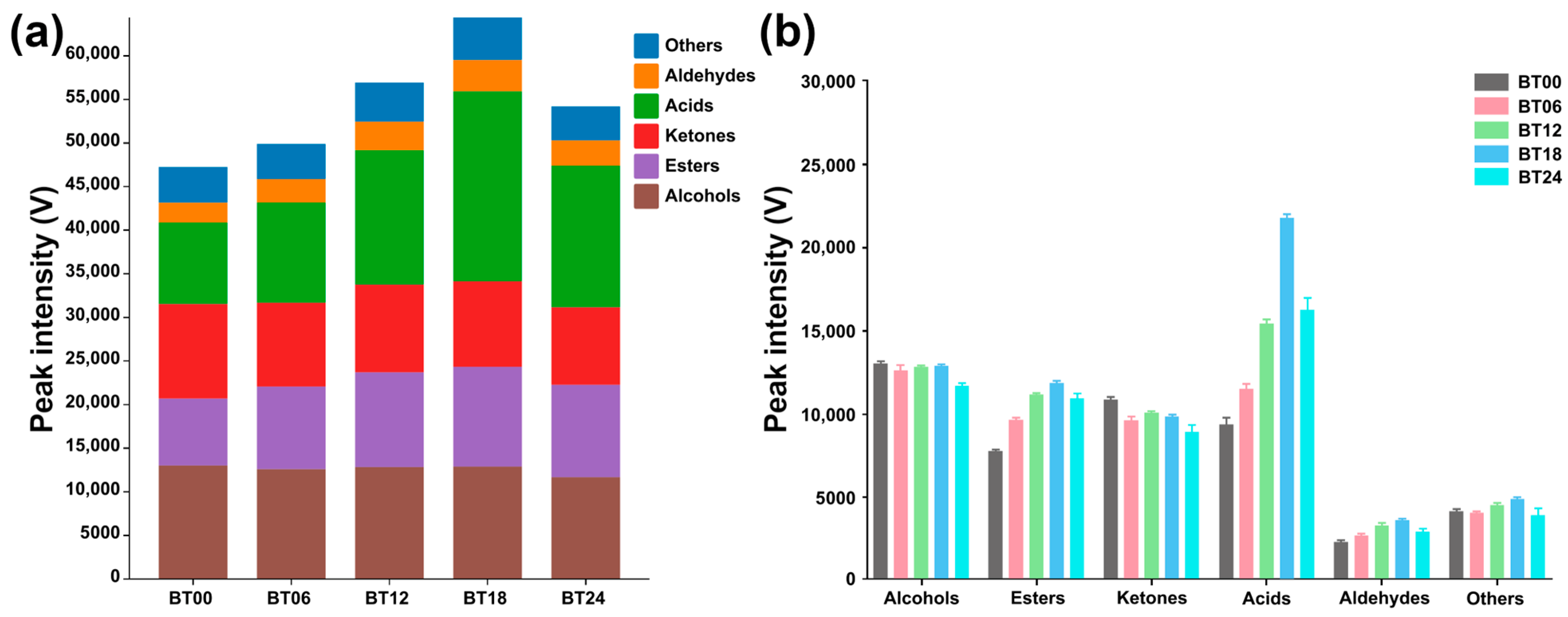
| Compounds | CAS | Formula | RI * | RT [s] | DT [ms] | Peak Intensity (V) | |||
|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | ||||||
| Alcohols (12) | |||||||||
| 1-Propanethiol | 107-03-9 | C3H8S | 823.2 | 264.886 | 1.17068 | 5.61 × 102 ± 0.75 a # | 3.50 × 102 ± 8.97 c | 3.88 × 102 ± 10.80 b | 3.18 × 102 ± 7.57 d |
| 2-Butoxyethanol | 111-76-2 | C6H14O2 | 885.3 | 290.249 | 1.20111 | 1.80 × 102 ± 34.80 a | 1.20 × 102 ± 15.60 b | 1.37 × 102 ± 5.08 ab | 1.32 × 102 ± 7.13 ab |
| 2-Furanmethanethiol | 98-02-2 | C5H6OS | 895.6 | 294.418 | 1.34532 | 1.70 × 102 ± 38.60 ab | 2.01 × 102 ± 22.50 a | 1.38 × 102 ± 8.64 ab | 1.21 × 102 ± 16.00 b |
| 2-Hexanol | 626-93-7 | C6H14O | 808.7 | 258.979 | 1.28001 | 2.21 × 102 ± 31.80 a | 1.21 × 102 ± 3.22 c | 1.69 × 102 ± 2.43 b | 1.44 × 102 ± 3.62 b |
| 2-Methyl-1-butanol | 137-32-6 | C5H12O | 724.9 | 224.785 | 1.22777 | 1.93 × 102 ± 15.90 c | 2.62 × 102 ± 16.60 b | 4.22 × 102 ± 10.50 a | 4.64 × 102 ± 31.10 a |
| 3-Methyl-1-butanol-D | 123-51-3 | C5H12O | 1178.6 | 760.516 | 1.48999 | 2.70 × 103 ± 25.00 b | 2.73 × 103 ± 47.50 b | 3.03 × 103 ± 22.70 a | 3.32 × 103 ± 1.38 × 102 a |
| 3-Methyl-1-butanol-M | 123-51-3 | C5H12O | 1178 | 757.997 | 1.24036 | 4.02 × 103 ± 2.49 × 102 a | 2.61 × 103 ± 88.30 b | 2.61 × 103 ± 50.10 b | 2.63 × 103 ± 1.24 × 102 b |
| 3-Methyl-3-buten-1-ol | 763-32-6 | C5H10O | 1258.7 | 1067.888 | 1.15898 | 2.74 × 102 ± 8.59 b | 3.00 × 102 ± 28.80 ab | 3.36 × 102 ± 34.00 ab | 3.61 × 102 ± 22.10 a |
| 5-Methylfurfuryl alcohol | 3857-25-8 | C6H8O2 | 953.8 | 326.696 | 1.2638 | 1.36 × 102 ± 8.00 a | 1.57 × 102 ± 9.41 a | 1.52 × 102 ± 5.46 a | 1.49 × 102 ± 7.48 a |
| cis-3-Hexen-1-ol | 928-96-1 | C6H12O | 822.6 | 264.628 | 1.22689 | 1.63 × 102 ± 5.99 a | 75.60 ± 1.41 c | 1.01 × 102 ± 2.67 b | 73.00 ± 1.15 c |
| Ethanol | 64-17-5 | C2H6O | 887.7 | 291.195 | 1.13063 | 1.27 × 102 ± 13.10 a | 79.80 ± 13.30 b | 1.11 × 102 ± 1.37 a | 1.12 × 102 ± 12.40 a |
| Isobutanol | 78-83-1 | C4H10O | 1077.9 | 475.544 | 1.17179 | 7.75 × 102 ± 52.10 a | 4.78 × 102 ± 11.70 bc | 4.63 × 102 ± 17.40 c | 5.14 × 102 ± 16.80 b |
| Esters (11) | |||||||||
| 2-Methylbutyl acetate | 624-41-9 | C7H14O2 | 895.2 | 294.269 | 1.29296 | 1.56 × 102 ± 8.78 c | 2.44 × 102 ± 6.28 a | 1.97 × 102 ± 3.12 b | 1.77 × 102 ± 11.00 bc |
| Diethyl malonate | 105-53-3 | C7H12O4 | 1077.9 | 475.544 | 1.25078 | 1.22 × 103 ± 46.50 a | 1.02 × 103 ± 22.20 a | 1.07 × 103 ± 97.60 a | 1.04 × 103 ± 33.60 a |
| Ethyl 2-methylbutyrate | 7452-79-1 | C7H14O2 | 1014.3 | 383.166 | 1.228 | 1.79 × 102 ± 18.30 a | 2.06 × 102 ± 7.20 a | 1.87 × 102 ± 5.33 a | 1.86 × 102 ± 8.86 a |
| Ethyl 3-hydroxybutyrate | 5405-41-4 | C6H12O3 | 901.6 | 297.442 | 1.16822 | 82.40 ± 50.90 a | 92.90 ± 20.10 a | 1.10 × 102 ± 4.76 a | 99.80 ± 24.80 a |
| Ethyl acetate | 141-78-6 | C4H8O2 | 880.9 | 288.431 | 1.33717 | 4.36 × 103 ± 2.24 × 102 a | 3.92 × 103 ± 48.00 ab | 3.59 × 103 ± 64.00 bc | 3.47 × 103 ± 93.50 c |
| Ethyl crotonate | 623-70-1 | C6H10O2 | 1140.8 | 632.689 | 1.1866 | 96.70 ± 6.44 b | 1.09 × 102 ± 2.09 ab | 1.14 × 102 ± 6.65 a | 1.13 × 102 ± 6.94 a |
| Ethyl pentanoate | 539-82-2 | C7H14O2 | 907.7 | 300.837 | 1.26713 | 3.10 × 102 ± 94.20 a | 3.27 × 102 ± 38.80 a | 3.07 × 102 ± 19.20 a | 3.00 × 102 ± 19.10 a |
| Isopropyl acetate | 108-21-4 | C5H10O2 | 907 | 300.471 | 1.15882 | 6.87 × 102 ± 1.15 × 102 a | 3.55 × 102 ± 25.30 b | 3.13 × 102 ± 7.05 b | 3.03 × 102 ± 26.70 b |
| Methyl acetate | 79-20-9 | C3H6O2 | 810.2 | 259.584 | 1.19708 | 2.30 × 102 ± 2.32 a | 1.30 × 102 ± 7.11 c | 1.53 × 102 ± 3.91 b | 1.35 × 102 ± 1.91 c |
| Methyl isovalerate | 556-24-1 | C6H12O2 | 1028 | 400.946 | 1.1944 | 3.51 × 102 ± 16.00 b | 3.88 × 102 ± 5.63 a | 3.61 × 102 ± 3.68 ab | 3.52 × 102 ± 7.84 ab |
| gamma-Octalactone | 104-50-7 | C8H14O2 | 1245.8 | 1023.798 | 1.33273 | 4.06 × 102 ± 5.65 a | 2.93 × 102 ± 16.60 b | 4.08 × 102 ± 78.30 a | 3.70 × 102 ± 15.00 a |
| Ketones (8) | |||||||||
| 1-Penten-3-one | 1629-58-9 | C5H8O | 1026.1 | 398.551 | 1.31696 | 94.90 ± 6.23 c | 1.42 × 102 ± 6.94 ab | 1.55 × 102 ± 4.33 a | 1.38 × 102 ± 6.71 b |
| 2-Butanone | 78-93-3 | C4H8O | 898.5 | 295.719 | 1.24091 | 7.44 × 102 ± 53.70 b | 9.15 × 102 ± 38.90 a | 7.70 × 102 ± 5.81 ab | 7.96 × 102 ± 61.40 ab |
| 2-Cyclohexen-1-one | 930-68-7 | C6H8O | 914.4 | 304.625 | 1.40015 | 3.34 × 103 ± 2.33 × 102 a | 2.18 × 103 ± 21.80 c | 2.43 × 103 ± 27.50 b | 2.39 × 103 ± 50.10 b |
| 2-Heptanone | 110-43-0 | C7H14O | 1158.5 | 681.077 | 1.26393 | 5.60 × 102 ± 28.7 b | 6.66 × 102 ± 19.80 a | 6.28 × 102 ± 6.66 a | 6.37 × 102 ± 18.80 a |
| 2-Pentanone | 107-87-9 | C5H10O | 982.7 | 342.913 | 1.36883 | 1.49 × 102 ± 7.48 c | 3.09 × 102 ± 18.90 a | 2.75 × 102 ± 11.90 ab | 2.62 × 102 ± 13.80 b |
| Acetoin | 513-86-0 | C4H8O2 | 724.8 | 224.762 | 1.0636 | 4.10 × 102 ± 10.60 a | 3.08 × 102 ± 61.10 a | 4.00 × 102 ± 17.60 a | 3.81 × 102 ± 54.10 a |
| (-)-Carvone | 6485-40-1 | C10H14O | 1179.8 | 765.243 | 1.3148 | 1.17 × 103 ± 52.20 a | 1.08 × 103 ± 17.50 ab | 1.00 × 103 ± 51.30 b | 1.01 × 103 ± 33.30 b |
| Isophorone | 78-59-1 | C9H14O | 1119.9 | 580.59 | 1.25872 | 2.63 × 102 ± 25.20 a | 1.95 × 102 ± 7.17 b | 1.88 × 102 ± 10.60 b | 1.75 × 102 ± 12.20 b |
| Acids (4) | |||||||||
| 2-Methylbutanoic acid | 116-53-0 | C5H10O2 | 872.8 | 285.138 | 1.20659 | 1.38 × 102 ± 14.50 a | 1.11 × 102 ± 5.56 ab | 1.01 × 102 ± 7.51 bc | 92.80 ± 3.27 c |
| Acetic acid | 64-19-7 | C2H4O2 | 1439.7 | 1686.517 | 1.15796 | 7.13 × 103 ± 1.81 × 102 a | 7.22 × 103 ± 4.79 × 102 a | 7.73 × 103 ± 1.07 × 103 a | 7.65 × 103 ± 5.08 × 102 a |
| Heptanoic acid | 111-14-8 | C7H14O2 | 1073.3 | 464.167 | 1.36185 | 4.64 × 102 ± 2.04 × 102 a | 2.96 × 102 ± 74.00 a | 1.05 × 103 ± 6.92 × 102 a | 3.28 × 102 ± 8.83 a |
| Isobutyric acid | 79-31-2 | C4H8O2 | 729 | 226.443 | 1.15617 | 59.20 ± 25.90 b | 1.42 × 102 ± 39.70 a | 1.36 × 102 ± 9.65 ab | 1.56 × 102 ± 42.00 a |
| Aldehydes (4) | |||||||||
| 4-Methoxybenzaldehyde | 123-11-5 | C8H8O2 | 1245.4 | 1022.538 | 1.20737 | 6.61 × 102 ± 21.60 a | 5.73 × 102 ± 15.80 b | 7.55 × 102 ± 65.80 a | 6.80 × 102 ± 56.90 a |
| Acrolein | 107-02-8 | C3H4O | 823.8 | 265.156 | 1.06297 | 3.55 × 102 ± 77.30 a | 1.33 × 102 ± 6.91 b | 1.31 × 102 ± 4.85 × 10−1 b | 1.12 × 102 ± 7.26 b |
| Butanal | 123-72-8 | C4H8O | 882.4 | 289.035 | 1.0965 | 6.75 × 102 ± 1.16 × 102 a | 3.35 × 102 ± 3.37 b | 3.20 × 102 ± 6.24 b | 3.14 × 102 ± 19.70 b |
| trans-2-Pentenal | 1576-87-0 | C5H8O | 1157.6 | 677.582 | 1.35994 | 4.87 × 102 ± 18.70 b | 9.03 × 102 ± 31.50 a | 8.63 × 102 ± 28.80 a | 8.42 × 102 ± 40.30 a |
| Others (9) | |||||||||
| 1,1-Diethoxyethane | 105-57-7 | C6H14O2 | 738.4 | 230.277 | 1.13101 | 1.95 × 102 ± 1.07 × 102 a | 3.20 × 102 ± 82.00 a | 3.89 × 102 ± 11.70 a | 3.91 × 102 ± 22.30 a |
| 2,2,4,6,6-Pentamethylheptane | 13475-82-6 | C12H26 | 917.4 | 306.324 | 1.0399 | 1.26 × 102 ± 7.43 a | 72.00 ± 3.01 b | 59.00 ± 3.10 bc | 55.10 ± 4.93 c |
| 2-Ethyl-3,5-dimethylpyrazine | 13925-07-0 | C8H12N2 | 1083.3 | 489.054 | 1.21129 | 1.66 × 102 ± 3.07 a | 1.76 × 102 ± 2.54 a | 1.27 × 102 ± 4.79 c | 1.43 × 102 ± 5.97 b |
| 2-sec-Butyl-3-methoxypyrazine | 24168-70-5 | C9H14N2O | 1077.9 | 475.467 | 1.28604 | 2.78 × 102 ± 41.80 a | 2.98 × 102 ± 4.14 a | 3.57 × 102 ± 72.70 a | 3.16 × 102 ± 7.87 a |
| alpha-Pinene | 80-56-8 | C10H16 | 1003.7 | 369.402 | 1.28369 | 80.00 ± 9.55 a | 85.70 ± 4.26 a | 80.10 ± 2.57 a | 89.00 ± 4.30 a |
| beta-Myrcene | 123-35-3 | C10H16 | 985 | 345.005 | 1.21594 | 9.39 × 102 ± 41.60 ab | 9.80 × 102 ± 69.40 a | 8.96 × 102 ± 9.06 ab | 8.58 × 102 ± 26.00 b |
| beta-Pinene | 127-91-3 | C10H16 | 1107.2 | 548.783 | 1.29583 | 2.42 × 102 ± 15.40 b | 2.88 × 102 ± 10.10 a | 3.00 × 102 ± 5.83 a | 3.16 × 102 ± 13.20 a |
| Decalin | 91-17-8 | C10H18 | 1052.4 | 432.721 | 1.26618 | 72.50 ± 5.85 ab | 62.50 ± 4.89 b | 78.50 ± 6.92 a | 80.20 ± 6.37 a |
| N-Nitrosomorpholine | 59-89-2 | C4H8N2O2 | 1106.9 | 548.072 | 1.19339 | 2.13 × 102 ± 7.97 ab | 2.21 × 102 ± 4.52 ab | 2.08 × 102 ± 4.96 b | 2.31 × 102 ± 5.36 a |
| Compounds | Peak Intensity | ||||
|---|---|---|---|---|---|
| BT00 | BT06 | BT12 | BT18 | BT24 | |
| Alcohols (12) | |||||
| 1-Propanethiol | 4.36 × 102 ± 4.74 c * | 3.79 × 102 ± 9.26 d | 5.03 × 102 ± 5.39 b | 5.19 × 102 ± 8.35 b | 6.15 × 102 ± 16.40 a |
| 2-Butoxyethanol | 3.35 × 102 ± 6.14 a | 2.66 × 102 ± 14.60 a | 2.70 × 102 ± 4.12 a | 2.71 × 102 ± 5.34 a | 2.63 × 102 ± 1.00 × 102 a |
| 2-Furanmethanethiol | 95.50 ± 3.04 c | 1.08 × 102 ± 3.51 bc | 2.32 × 102 ± 19.00 a | 1.78 × 102 ± 3.53 ab | 3.19 × 102 ± 1.66 × 102 a |
| 2-Hexanol | 7.23 × 102 ± 10.50 a | 5.05 × 102 ± 19.10 b | 2.14 × 102 ± 2.08 cd | 1.84 × 102 ± 1.48 d | 2.68 × 102 ± 76.00 c |
| 2-Methyl-1-butanol | 1.32 × 103 ± 41.30 a | 8.77 × 102 ± 24.60 b | 7.35 × 102 ± 11.80 c | 7.58 × 102 ± 14.10 c | 4.09 × 102 ± 41.40 d |
| 3-Methyl-1-butanol-D | 4.29 × 103 ± 18.10 ab | 4.46 × 103 ± 87.90 a | 4.35 × 103 ± 29.40 ab | 4.20 × 103 ± 45.70 ab | 4.12 × 103 ± 2.35 × 102 b |
| 3-Methyl-1-butanol-M | 3.94 × 103 ± 37.40 ab | 4.05 × 103 ± 1.84×102 a | 3.72 × 103 ± 59.40 ab | 3.48 × 103 ± 63.10 b | 4.18 × 103 ± 8.06 × 102 a |
| 3-Methyl-3-buten-1-ol | 6.17 × 102 ± 44.50 ab | 5.75 × 102 ± 73.80 b | 6.44 × 102 ± 35.70 ab | 8.53 × 102 ± 59.20 a | 8.44 × 102 ± 1.13 × 102 a |
| 5-Methylfurfuryl alcohol | 2.20 × 102 ± 11.40 c | 2.26 × 102 ± 2.21 bc | 2.54 × 102 ± 5.41 ab | 2.74 × 102 ± 2.80 a | 2.31 × 102 ± 13.90 bc |
| cis-3-Hexen-1-ol | 1.26 × 102 ± 3.88 a | 88.40 ± 5.13 c | 1.02 × 102 ± 2.14 b | 1.12 × 102 ± 1.86 ab | 1.30 × 102 ± 12.60 a |
| Ethanol | 3.67 × 102 ± 18.60 a | 3.01 × 102 ± 16.00 a | 2.53 × 102 ± 1.91 a | 2.31 × 102 ± 11.10 a | 2.27 × 102 ± 1.18 × 102 a |
| Isobutanol | 5.71 × 102 ± 11.90 b | 6.73 × 102 ± 6.40 a | 6.89 × 102 ± 15.20 a | 6.63 × 102 ± 20.50 a | 8.00 × 102 ± 1.52 × 102 a |
| Esters (11) | |||||
| 2-Methylbutyl acetate | 99.50 ± 4.49 e | 1.53 × 102 ± 2.90 d | 2.94 × 102 ± 5.46 c | 3.71 × 102 ± 6.05 a | 3.39 × 102 ± 9.33 b |
| Diethyl malonate | 1.20 × 103 ± 24.30 b | 1.65 × 103 ± 2.31×102 a | 1.62 × 103 ± 29.60 a | 1.59 × 103 ± 37.00 a | 1.57 × 103 ± 64.10 a |
| Ethyl 2-methylbutyrate | 3.56 × 102 ± 5.43 a | 3.10 × 102 ± 5.20 b | 3.06 × 102 ± 18.70 bc | 3.14 × 102 ± 5.72 ab | 2.81 × 102 ± 8.99 c |
| Ethyl 3-hydroxybutyrate | 1.86 × 102 ± 22.00 a | 2.04 × 102 ± 19.10 a | 1.95 × 102 ± 9.19 a | 1.96 × 102 ± 1.45 a | 1.64 × 102 ± 1.07 × 102 a |
| Ethyl acetate | 3.01 × 103 ± 18.20 d | 4.16 × 103 ± 60.70 c | 5.46 × 103 ± 53.40 b | 5.94 × 103 ± 45.70 ab | 6.66 × 103 ± 1.02 × 103 a |
| Ethyl crotonate | 2.27 × 102 ± 3.85 a | 1.89 × 102 ± 3.33 a | 1.99 × 102 ± 19.40 a | 1.95 × 102 ± 8.02 a | 1.61 × 102 ± 13.20 b |
| Ethyl pentanoate | 5.82 × 102 ± 24.40 a | 5.89 × 102 ± 30.70 a | 5.83 × 102 ± 2.94 a | 4.52 × 102 ± 16.70 a | 5.17 × 102 ± 1.81 × 102 a |
| Isopropyl acetate | 3.94 × 102 ± 9.07 b | 4.85 × 102 ± 40.50 a | 4.51 × 102 ± 4.14 ab | 4.42 × 102 ± 5.51 ab | 5.30 × 102 ± 1.26 × 102 a |
| Methyl acetate | 4.22 × 102 ± 3.88 a | 3.54 × 102 ± 19.50 b | 2.38 × 102 ± 6.10 c | 1.57 × 102 ± 4.21 d | 2.74 × 102 ± 35.40 c |
| Methyl isovalerate | 6.45 × 102 ± 1.81 a | 6.52 × 102 ± 17.70 a | 6.49 × 102 ± 5.72 a | 6.63 × 102 ± 8.82 a | 6.32 × 102 ± 48.60 a |
| gamma-Octalactone | 6.73 × 102 ± 19.20 a | 8.14 × 102 ± 53.80 a | 4.18 × 102 ± 18.90 b | 4.58 × 102 ± 32.50 b | 4.51 × 102 ± 69.60 b |
| Ketones (8) | |||||
| 1-Penten-3-one | 1.77 × 102 ± 4.43 c | 2.47 × 102 ± 1.43 a | 2.55 × 102 ± 4.85 a | 2.70 × 102 ± 9.97 a | 1.95 × 102 ± 9.25 b |
| 2-Butanone | 1.69 × 103 ± 82.20 a | 1.63 × 103 ± 74.30 a | 1.55 × 103 ± 26.40 a | 1.57 × 103 ± 15.10 a | 1.26 × 103 ± 3.08 × 102 a |
| 2-Cyclohexen-1-one | 5.83 × 103 ± 54.10 a | 4.57 × 103 ± 1.72 × 102 b | 3.77 × 103 ± 24.00 c | 3.55 × 103 ± 21.40 d | 4.16 × 103 ± 3.55 × 102 bc |
| 2-Heptanone | 1.89 × 102 ± 5.28 d | 3.49 × 102 ± 11.50 c | 1.02 × 103 ± 11.90 ab | 1.04 × 103 ± 19.20 a | 9.86 × 102 ± 32.50 b |
| 2-Pentanone | 78.90 ± 5.19 d | 1.14 × 102 ± 17.30 c | 3.75 × 102 ± 8.37 b | 5.17 × 102 ± 7.64 a | 4.01 × 102 ± 34.50 b |
| Acetoin | 7.22 × 102 ± 1.20 × 102 a | 5.34 × 102 ± 28.00 a | 4.46 × 102 ± 7.64 a | 4.02 × 102 ± 9.21 a | 4.80 × 102 ± 2.91 × 102 a |
| (-)-Carvone | 1.92 × 103 ± 14.90 a | 1.80 × 103 ± 44.90 a | 1.67 × 103 ± 64.70 a | 1.32 × 103 ± 59.60 b | 1.66 × 103 ± 1.96 × 102 a |
| Isophorone | 2.59 × 102 ± 5.55 a | 3.10 × 102 ± 15.90 a | 2.87 × 102 ± 10.30 a | 2.65 × 102 ± 13.40 a | 2.76 × 102 ± 35.90 a |
| Acids (4) | |||||
| 2-Methylbutanoic acid | 2.26 × 102 ± 3.97 a | 1.84 × 102 ± 12.00 b | 1.66 × 102 ± 8.23 b | 1.18 × 102 ± 1.07 c | 1.68 × 102 ± 8.39 b |
| Acetic acid | 8.18 × 103 ± 1.21 × ×103 c | 9.50 × 103 ± 1.32 × 103 bc | 1.30 × 104 ± 4.87 × 102 ab | 1.79 × 104 ± 1.67 × 103 a | 1.56 × 104 ± 4.01 × 103 ab |
| Heptanoic acid | 6.40 × 102 ± 1.37 × 102 a | 1.31 × 103 ± 1.19 × 103 a | 8.24 × 102 ± 1.48 × 102 a | 1.38 × 103 ± 4.92 × 102 a | 8.44 × 102 ± 5.76 × 102 a |
| Isobutyric acid | 3.24 × 102 ± 1.08 × 102 a | 4.06 × 102 ± 12.50 a | 4.18 × 102 ± 9.14 a | 4.22 × 102 ± 12.00 a | 2.95 × 102 ± 1.83 × 102 a |
| Aldehydes (4) | |||||
| 4-Methoxybenzaldehyde | 1.36 × 103 ± 13.30 a | 1.47 × 103 ± 86.10 a | 9.64 × 102 ± 5.35 b | 1.11 × 103 ± 79.50 ab | 9.68 × 102 ± 1.91 × 102 b |
| Acrolein | 1.58 × 102 ± 2.13 ab | 1.39 × 102 ± 14.60 b | 1.52 × 102 ± 3.96 ab | 1.52 × 102 ± 1.42 ab | 2.34 × 102 ± 95.90 a |
| Butanal | 5.14 × 102 ± 3.89 a | 5.57 × 102 ± 36.30 a | 4.70 × 102 ± 8.23 ab | 4.53 × 102 ± 1.89 b | 5.78 × 102 ± 1.59 × 102 a |
| trans-2-Pentenal | 2.45 × 102 ± 7.56 d | 4.98 × 102 ± 12.80 c | 1.45 × 103 ± 14.10 a | 1.56 × 103 ± 34.20 a | 1.28 × 103 ± 1.11 × 102 b |
| Others (9) | |||||
| 1,1-Diethoxyethane | 6.60 × 102 ± 39.00 a | 7.59 × 102 ± 46.30 a | 8.17 × 102 ± 17.00 a | 9.00 × 102 ± 29.80 a | 7.05 × 102 ± 5.52 × 102 a |
| 2,2,4,6,6-Pentamethylheptane | 57.30 ± 2.16 c | 86.20 ± 6.63 ab | 95.70 ± 5.15 a | 70.30 ± 3.35 b | 1.10 × 102 ± 15.90 a |
| 2-Ethyl-3,5-dimethylpyrazine | 3.05 × 102 ± 11.90 a | 2.95 × 102 ± 5.52 a | 2.87 × 102 ± 6.83 a | 3.03 × 102 ± 2.63 a | 2.97 × 102 ± 39.60 a |
| 2-sec-Butyl-3-methoxypyrazine | 4.86 × 102 ± 9.69 a | 5.62 × 102 ± 1.32 × 102 a | 5.23 × 102 ± 11.50 a | 5.45 × 102 ± 33.70 a | 4.58 × 102 ± 72.40 a |
| alpha-Pinene | 1.86 × 102 ± 2.32 a | 1.50 × 102 ± 5.47 b | 1.42 × 102 ± 2.67 bc | 1.57 × 102 ± 9.29 b | 1.27 × 102 ± 7.95 c |
| beta-Myrcene | 1.16 × 103 ± 74.90 b | 1.12 × 103 ± 24.70 b | 1.36 × 103 ± 18.60 a | 1.47 × 103 ± 20.60 a | 1.47 × 103 ± 15.30 a |
| beta-Pinene | 5.81 × 102 ± 11.00 a | 5.04 × 102 ± 13.80 b | 4.94 × 102 ± 6.52 b | 5.17 × 102 ± 15.40 b | 4.24 × 102 ± 4.26 c |
| Decalin | 2.03 × 102 ± 3.87 a | 1.65 × 102 ± 10.40 b | 96.90 ± 3.18 c | 95.30 ± 2.61 c | 99.80 ± 12.70 c |
| N-Nitrosomorpholine | 4.72 × 102 ± 7.53 a | 3.78 × 102 ± 15.50 b | 3.62 × 102 ± 12.90 b | 3.80 × 102 ± 2.35 b | 3.91 × 102 ± 24.50 b |
| Sensor Number | Sensor Name | Performance Description |
|---|---|---|
| 1 | LY2/LG | Sensitive to oxidizing gas |
| 2 | LY2/G | Sensitive to ammonia, carbon monoxide |
| 3 | LY2/AA | Sensitive to ethanol |
| 4 | LY2/Gh | Sensitive to ammonia/organic amines |
| 5 | LY2/gCT1 | Sensitive to hydrogen sulfide |
| 6 | LY2/gCT | Sensitive to propane/butane |
| 7 | T30/1 | Sensitive to organic solvents |
| 8 | P10/1 | Sensitive to hydrocarbons |
| 9 | P10/2 | Sensitive to methane |
| 10 | P40/1 | Sensitive to fluorine |
| 11 | T70/2 | Sensitive to aromatic compounds |
| 12 | PA/2 | Sensitive to ethanol, ammonia/organic amines |
| 13 | P30/1 | Sensitive to polar compounds (ethanol) |
| 14 | P40/2 | Sensitive to heteroatom/chloride/aldehydes |
| 15 | P30/2 | Sensitive to alcohol |
| 16 | T40/2 | Sensitive to aldehydes |
| 17 | T40/1 | Sensitive to chlorinated compounds |
| 18 | TA/2 | Sensitive to air quality |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Tang, J.; Deng, J.; Cai, Z.; Jiang, X.; Zhu, C. Effect of Capsaicin Stress on Aroma-Producing Properties of Lactobacillus plantarum CL-01 Based on E-Nose and GC–IMS. Molecules 2024, 29, 107. https://doi.org/10.3390/molecules29010107
Zhang Q, Tang J, Deng J, Cai Z, Jiang X, Zhu C. Effect of Capsaicin Stress on Aroma-Producing Properties of Lactobacillus plantarum CL-01 Based on E-Nose and GC–IMS. Molecules. 2024; 29(1):107. https://doi.org/10.3390/molecules29010107
Chicago/Turabian StyleZhang, Qian, Junni Tang, Jing Deng, Zijian Cai, Xiaole Jiang, and Chenglin Zhu. 2024. "Effect of Capsaicin Stress on Aroma-Producing Properties of Lactobacillus plantarum CL-01 Based on E-Nose and GC–IMS" Molecules 29, no. 1: 107. https://doi.org/10.3390/molecules29010107
APA StyleZhang, Q., Tang, J., Deng, J., Cai, Z., Jiang, X., & Zhu, C. (2024). Effect of Capsaicin Stress on Aroma-Producing Properties of Lactobacillus plantarum CL-01 Based on E-Nose and GC–IMS. Molecules, 29(1), 107. https://doi.org/10.3390/molecules29010107





