Synthesis, Structure, Biological Activity, and Luminescence Properties of a “Butterfly”-Type Silver Cluster with 3-Benzyl-4-phenyl-1,2,4-triazol-5-thiol
Abstract
1. Introduction
2. Results and Discussion
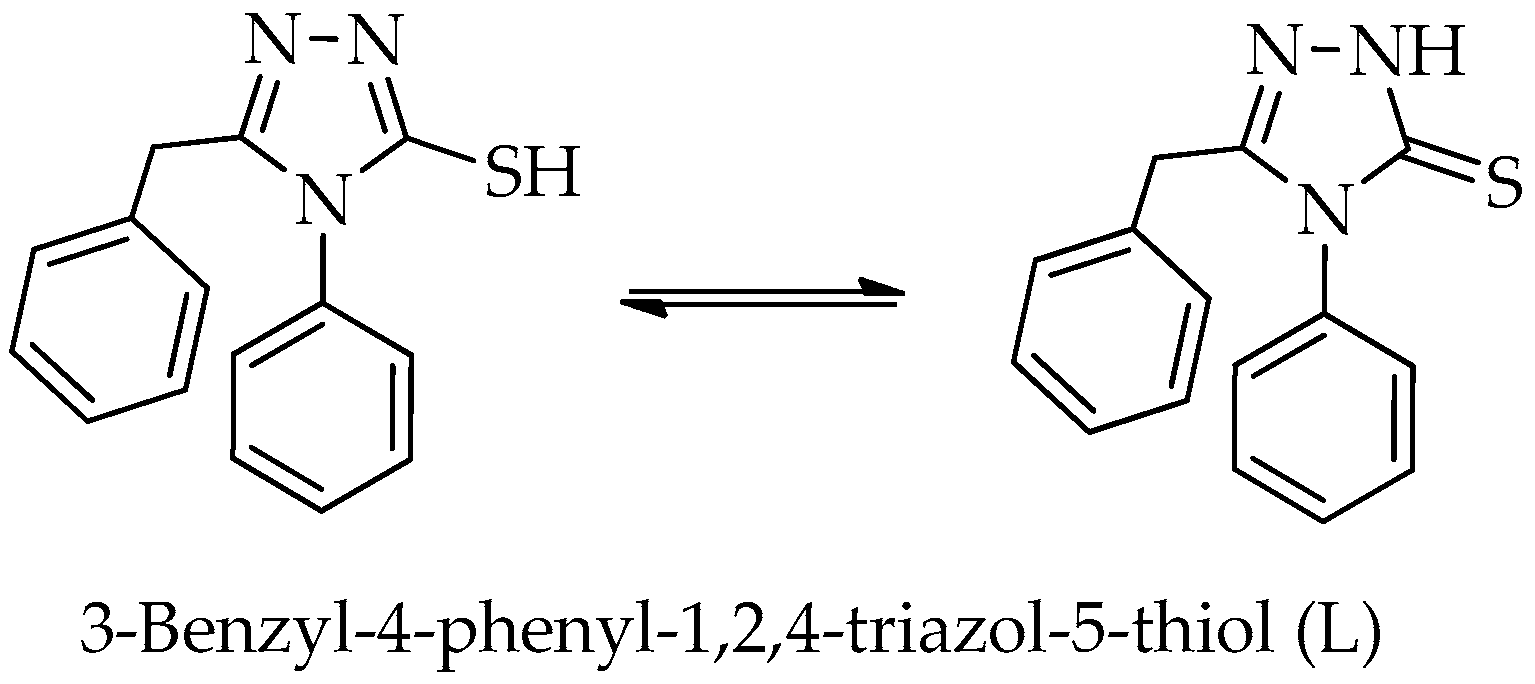
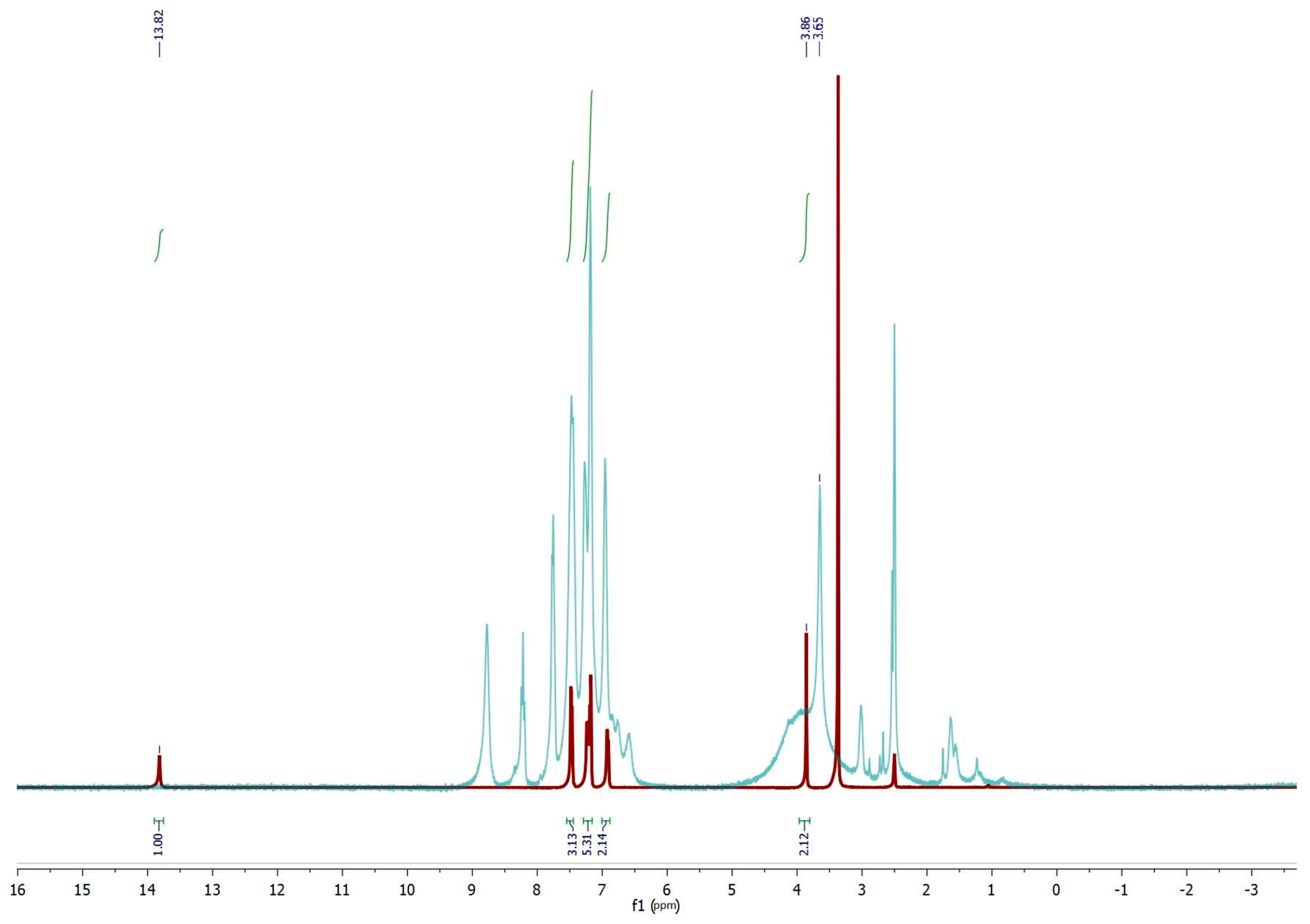
2.1. Synthesis and Characterization
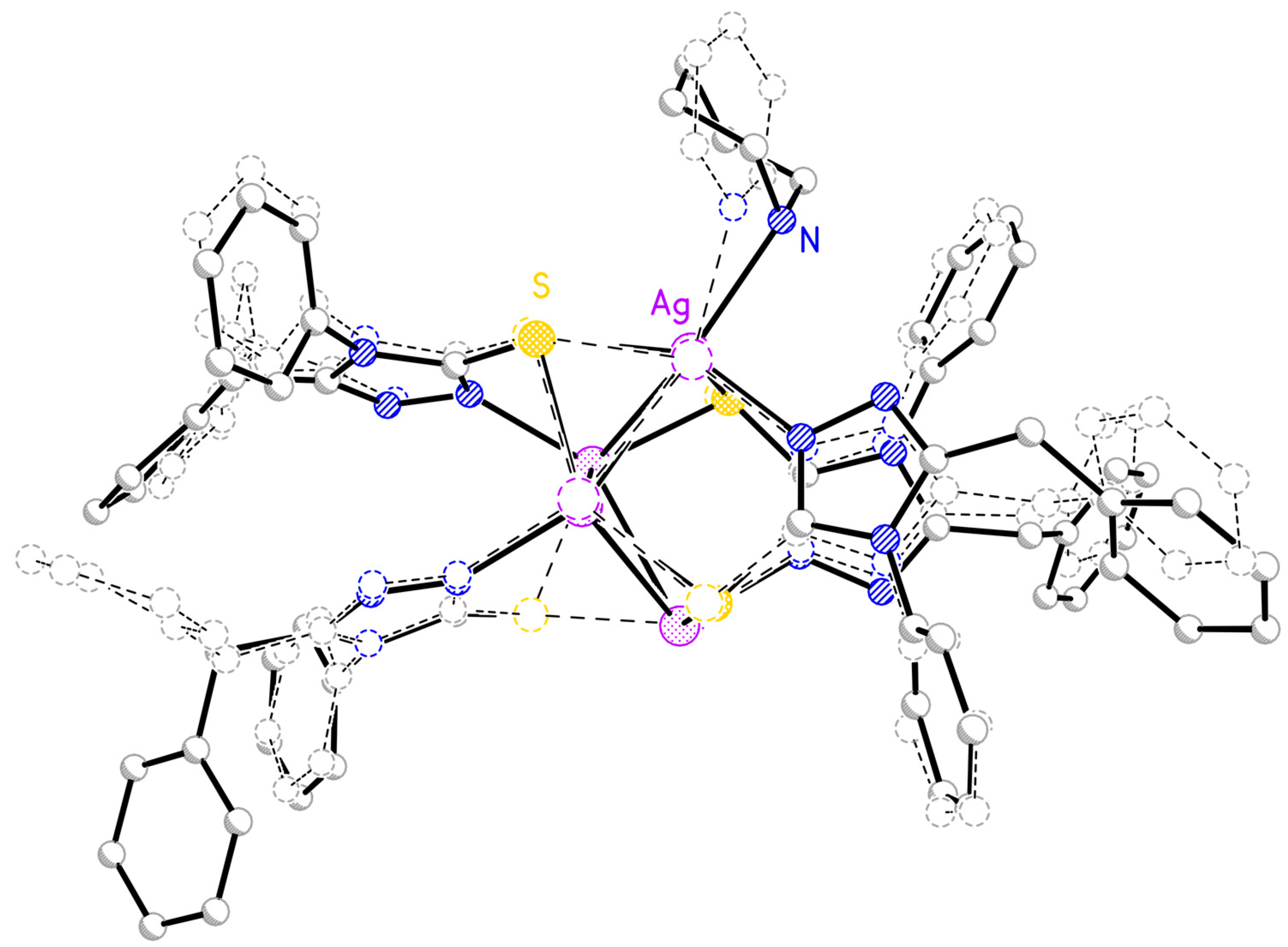
2.2. Molecular Structure of I
2.3. Luminescent Properties
2.4. Biological Activity
3. Materials and Methods
3.1. General Remarks
3.2. Synthesis of I
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hernández-Romero, D.; Rosete-Luna, S.; López-Monteon, A.; Chávez-Piña, A.; Pérez-Hernández, N.; Marroquín-Flores, J.; Cruz-Navarro, A.; Pesado-Gómez, G.; Morales-Morales, D.; Colorado-Peralta, R. First-Row Transition Metal Compounds Containing Benzimidazole Ligands: An Overview of Their Anticancer and Antitumor Activity. Coord. Chem. Rev. 2021, 439, 213930. [Google Scholar] [CrossRef]
- Suárez-Moreno, G.V.; Hernández-Romero, D.; García-Barradas, Ó.; Vázquez-Vera, Ó.; Rosete-Luna, S.; Cruz-Cruz, C.A.; López-Monteon, A.; Carrillo-Ahumada, J.; Morales-Morales, D.; Colorado-Peralta, R. Second and Third-Row Transition Metal Compounds Containing Benzimidazole Ligands: An Overview of Their Anticancer and Antitumour Activity. Coord. Chem. Rev. 2022, 472, 214790. [Google Scholar] [CrossRef]
- Bland, B.R.A.; Gilfoy, H.J.; Vamvounis, G.; Robertson, K.N.; Cameron, T.S.; Aquino, M.A.S. Hydrogen Bonding in Diruthenium(II,III) Tetraacetate Complexes with Biologically Relevant Axial Ligands. Inorg. Chim. Acta 2005, 358, 3927–3936. [Google Scholar] [CrossRef]
- Melník, M.; Sprusansky, O.; Musil, P. Bio-Medical Aspects of Purine Alkaloids. Adv. Biol. Chem. 2014, 4, 274–280. [Google Scholar] [CrossRef][Green Version]
- Rukk, N.S.; Kuz’mina, L.G.; Davydova, G.A.; Buzanov, G.A.; Retivov, V.M.; Belus, S.K.; Kozhukhova, E.I.; Barmashov, A.E.; Khrulev, A.A.; Simonova, M.A.; et al. Synthesis, Structure and Cytotoxicity of a Zinc(II) Bromide Complex with Caffeine. Mendeleev Commun. 2019, 29, 640–642. [Google Scholar] [CrossRef]
- Trommenschlager, A.; Chotard, F.; Bertrand, B.; Amor, S.; Richard, P.; Bettaïeb, A.; Paul, C.; Connat, J.-L.; Le Gendre, P.; Bodio, E. Gold(I)–Coumarin–Caffeine-Based Complexes as New Potential Anti-Inflammatory and Anticancer Trackable Agents. ChemMedChem 2018, 13, 2408–2414. [Google Scholar] [CrossRef]
- Ma, Z.; Moulton, B. Supramolecular Medicinal Chemistry: Mixed-Ligand Coordination Complexes. Mol. Pharm. 2007, 4, 373–385. [Google Scholar] [CrossRef]
- Rukk, N.S.; Kuzmina, L.G.; Shamsiev, R.S.; Davydova, G.A.; Mironova, E.A.; Ermakov, A.M.; Buzanov, G.A.; Skryabina, A.Y.; Streletskii, A.N.; Vorob’eva, G.A.; et al. Zinc(II) and Cadmium(II) Halide Complexes with Caffeine: Synthesis, X-Ray Crystal Structure, Cytotoxicity and Genotoxicity Studies. Inorg. Chim. Acta 2019, 487, 184–200. [Google Scholar] [CrossRef]
- Preedy, V.R. Caffeine Chemistry, Analysis, Function and Effects; The Royal Society of Chemistry: London, UK, 2012; ISBN 978-1-84973-367-0. [Google Scholar]
- Viganor, L.; Howe, O.; McCarron, P.; McCann, M.; Devereux, M. The Antibacterial Activity of Metal Complexes Containing 1, 10-Phenanthroline: Potential as Alternative Therapeutics in the Era of Antibiotic Resistance. Curr. Top. Med. Chem. 2017, 17, 1280–1302. [Google Scholar] [CrossRef]
- Nikolić, M.A.; Szécsényi, K.M.; Dražić, B.; Rodić, M.V.; Stanić, V.; Tanasković, S. Binuclear Co(II) Complexes with Macrocycle and Carboxylato Ligands: Structure, Cytotoxicity and Thermal Behavior. J. Mol. Struct. 2021, 1236, 130133. [Google Scholar] [CrossRef]
- Olmo, A.; Calzada, J.; Nuñez, M. Benzoic Acid and Its Derivatives as Naturally Occurring Compounds in Foods and as Additives: Uses, Exposure, and Controversy. Crit. Rev. Food Sci. Nutr. 2017, 57, 3084–3103. [Google Scholar] [CrossRef] [PubMed]
- Melnic, S.; Prodius, D.; Simmons, C.; Zosim, L.; Chiriac, T.; Bulimaga, V.; Rudic, V.; Turta, C. Biotechnological Application of Homo- and Heterotrinuclear Iron(III) Furoates for Cultivation of Iron-Enriched Spirulina. Inorg. Chim. Acta 2011, 373, 167–172. [Google Scholar] [CrossRef]
- Melnic, S.; Prodius, D.; Stoeckli-Evans, H.; Shova, S.; Turta, C. Synthesis and Anti-Tuberculosis Activity of New Hetero(Mn, Co, Ni)Trinuclear Iron(III) Furoates. Eur. J. Med. Chem. 2010, 45, 1465–1469. [Google Scholar] [CrossRef] [PubMed]
- Odularu, A.T.; Ajibade, P.A. Dithiocarbamates: Challenges, Control, and Approaches to Excellent Yield, Characterization, and Their Biological Applications. Bioinorg. Chem. Appl. 2019, 2019, 8260496. [Google Scholar] [CrossRef] [PubMed]
- Hogarth, G. Metal-Dithiocarbamate Complexes: Chemistry and Biological Activity. Mini-Rev. Med. Chem. 2012, 12, 1202–1215. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Luo, M.; Zhang, Y.; Fang, F.; Li, M.; An, F.; Zhao, D.; Zhang, J. Free Radical as a Double-Edged Sword in Disease: Deriving Strategic Opportunities for Nanotherapeutics. Coord. Chem. Rev. 2023, 475, 214875. [Google Scholar] [CrossRef]
- Zhang, P.; Sadler, P.J. Redox-Active Metal Complexes for Anticancer Therapy. Eur. J. Inorg. Chem. 2017, 2017, 1541–1548. [Google Scholar] [CrossRef]
- Kaim, W.; Schwederski, B. Non-Innocent Ligands in Bioinorganic Chemistry—An Overview. Coord. Chem. Rev. 2010, 254, 1580–1588. [Google Scholar] [CrossRef]
- Kaim, W.; Schwederski, B. Cooperation of Metals with Electroactive Ligands of Biochemical Relevance: Beyond Metalloporphyrins. Pure Appl. Chem. 2004, 76, 351–364. [Google Scholar] [CrossRef]
- Zhang, T.; Huang, L.Z.; Wu, J.; Lu, D.; Ma, B.L.; Du, Z.T. Microwave-Assisted Synthesis of 2-Substituted 1h-Benzo[d]Imidazoles and Their Antifungal Activities in Vitro. Heterocycles 2013, 87, 1545–1552. [Google Scholar] [CrossRef]
- Nikiforova, M.E.; Yambulatov, D.S.; Nelyubina, Y.V.; Primakov, P.V.; Bekker, O.B.; Majorov, K.B.; Shmelev, M.A.; Khoroshilov, A.V.; Eremenko, I.L.; Lutsenko, I.A. Current Design of Mixed-Ligand Complexes of Magnesium(II): Synthesis, Crystal Structure, Thermal Properties and Biological Activity against Mycolicibacterium Smegmatis and Bacillus Kochii. Crystals 2023, 13, 1306. [Google Scholar] [CrossRef]
- Yambulatov, D.S.; Lutsenko, I.A.; Nikolaevskii, S.A.; Petrov, P.A.; Smolyaninov, I.V.; Malyants, I.K.; Shender, V.O.; Kiskin, M.A.; Sidorov, A.A.; Berberova, N.T.; et al. Diimine Cisplatin Derivatives: Synthesis, Structure, Cyclic Voltammetry and Cytotoxicity. Molecules 2022, 27, 8565. [Google Scholar] [CrossRef] [PubMed]
- Lutsenko, I.A.; Yambulatov, D.S.; Kiskin, M.A.; Nelyubina, Y.V.; Primakov, P.V.; Bekker, O.B.; Levitskiy, O.A.; Magdesieva, T.V.; Imshennik, V.K.; Maksimov, Y.V.; et al. Improved In Vitro Antimycobacterial Activity of Trinuclear Complexes Cobalt(II,III) and Iron(III) with 2-Furoic Acid against Mycolicibacterium Smegmatis. ChemistrySelect 2020, 5, 11837–11842. [Google Scholar] [CrossRef]
- Lutsenko, I.A.; Yambulatov, D.S.; Kiskin, M.A.; Nelyubina, Y.V.; Primakov, P.V.; Bekker, O.B.; Sidorov, A.A.; Eremenko, I.L. Mononuclear Cu(II), Zn(II), and Co(II) Complexes with 2-Furoate Anions and 2,2′-Bpy: Synthesis, Structure, and Biological Activity. Russ. J. Coord. Chem. 2020, 46, 787–794. [Google Scholar] [CrossRef]
- Yambulatov, D.S.; Nikolaevskii, S.A.; Lutsenko, I.A.; Kiskin, M.A.; Shmelev, M.A.; Bekker, O.B.; Efimov, N.N.; Ugolkova, E.A.; Minin, V.V.; Sidorov, A.A.; et al. Copper(II) Trimethylacetate Complex with Caffeine: Synthesis, Structure, and Biological Activity. Russ. J. Coord. Chem. 2020, 46, 772–778. [Google Scholar] [CrossRef]
- Potts, K.T. The Chemistry of 1,2,4-Triazoles. Chem. Rev. 1961, 61, 87–127. [Google Scholar] [CrossRef]
- Council of Europe. European Pharmacopoeia, 8.0; European Treaty Series; Council of Europe: Strasbourg, France, 2007; ISBN 9789287160591. [Google Scholar]
- Kazeminejad, Z.; Marzi, M.; Shiroudi, A.; Kouhpayeh, S.A.; Farjam, M.; Zarenezhad, E. Novel 1, 2, 4-Triazoles as Antifungal Agents. Biomed Res. Int. 2022, 2022, 4584846. [Google Scholar] [CrossRef] [PubMed]
- Paroni, R.; Del Puppo, M.; Borghi, C.; Sirtori, C.R.; Galli Kienle, M. Pharmacokinetics of Ribavirin and Urinary Excretion of the Major Metabolite 1,2,4-Triazole-3-Carboxamide in Normal Volunteers. Int. J. Clin. Pharmacol. Ther. Toxicol. 1989, 27, 302–307. [Google Scholar]
- Amin, N.H.; El-Saadi, M.T.; Ibrahim, A.A.; Abdel-Rahman, H.M. Design, Synthesis and Mechanistic Study of New 1,2,4-Triazole Derivatives as Antimicrobial Agents. Bioorg. Chem. 2021, 111, 104841. [Google Scholar] [CrossRef]
- Hasegawa, T.; Eiki, J.; Chiba, M. Interindividual Variations in Metabolism and Pharmacokinetics of 3-(6-Methylpyridine-3-Yl-Sulfanyl)-6-(4H-[1,2,4]Triazole-3-Yl-Sulfanyl)-N-(1,3-Thiazole-2-Yl)-2-Pyridine Carboxamide, a Glucokinase Activator, in Rats Caused by the Genetic. Drug Metab. Dispos. 2014, 42, 1548–1555. [Google Scholar] [CrossRef]
- Sumrra, S.H.; Habiba, U.; Zafar, W.; Imran, M.; Chohan, Z.H. A Review on the Efficacy and Medicinal Applications of Metal-Based Triazole Derivatives. J. Coord. Chem. 2020, 73, 2838–2877. [Google Scholar] [CrossRef]
- Zafar, W.; Sumrra, S.H.; Chohan, Z.H. A Review: Pharmacological Aspects of Metal Based 1,2,4-Triazole Derived Schiff Bases. Eur. J. Med. Chem. 2021, 222, 113602. [Google Scholar] [CrossRef] [PubMed]
- Chohan, Z.H.; Hanif, M. Antibacterial and Antifungal Metal Based Triazole Schiff Bases. J. Enzyme Inhib. Med. Chem. 2013, 28, 944–953. [Google Scholar] [CrossRef] [PubMed]
- Hanif, M.; Chohan, Z.H. Design, Spectral Characterization and Biological Studies of Transition Metal(II) Complexes with Triazole Schiff Bases. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 104, 468–476. [Google Scholar] [CrossRef] [PubMed]
- Chohan, Z.H.; Sumrra, S.H. Some Biologically Active Oxovanadium(IV) Complexes of Triazole Derived Schiff Bases: Their Synthesis, Characterization and Biological Properties. J. Enzyme Inhib. Med. Chem. 2010, 25, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Sumrra, S.H.; Hanif, M.; Chohan, Z.H. Design, Synthesis and in Vitro Bactericidal/Fungicidal Screening of Some Vanadyl(IV)Complexes with Mono- and Di-Substituted ONS Donor Triazoles. J. Enzyme Inhib. Med. Chem. 2015, 30, 800–808. [Google Scholar] [CrossRef] [PubMed]
- Bormio Nunes, J.H.; Hideki Nakahata, D.; Corbi, P.P.; Ferraz de Paiva, R.E. Beyond Silver Sulfadiazine: A Dive into More than 50 Years of Research and Development on Metal Complexes of Sulfonamides in Medicinal Inorganic Chemistry. Coord. Chem. Rev. 2023, 490, 215228. [Google Scholar] [CrossRef]
- Bharathi, S.; Mahendiran, D.; Kumar, R.S.; Choi, H.J.; Gajendiran, M.; Kim, K.; Rahiman, A.K. Silver(I) Metallodrugs of Thiosemicarbazones and Naproxen: Biocompatibility, in Vitro Anti-Proliferative Activity and in Silico Interaction Studies with EGFR, VEGFR2 and LOX Receptors. Toxicol. Res. 2020, 9, 28–44. [Google Scholar] [CrossRef]
- de Souza, C.C.; de Azevedo-França, J.A.; Barrias, E.; Cavalcante, S.C.F.; Vieira, E.G.; Ferreira, A.M.D.C.; de Souza, W.; Navarro, M. Silver and Copper-Benznidazole Derivatives as Potential Antiparasitic Metallodrugs: Synthesis, Characterization, and Biological Evaluation. J. Inorg. Biochem. 2023, 239, 112047. [Google Scholar] [CrossRef]
- Raju, S.K.; Karunakaran, A.; Kumar, S.; Sekar, P.; Murugesan, M.; Karthikeyan, M. Silver Complexes as Anticancer Agents: A Perspective Review. Ger. J. Pharm. Biomater. 2022, 1, 6–28. [Google Scholar] [CrossRef]
- Ji, D.; Wang, Y. An Optimized Protocol of Protargol Staining for Ciliated Protozoa. J. Eukaryot. Microbiol. 2018, 65, 705–708. [Google Scholar] [CrossRef] [PubMed]
- Sekito, T.; Sadahira, T.; Watanabe, T.; Maruyama, Y.; Watanabe, T.; Iwata, T.; Wada, K.; Edamura, K.; Araki, M.; Watanabe, M. Medical Uses for Silver Nitrate in the Urinary Tract (Review). World Acad. Sci. J. 2022, 4, 6. [Google Scholar] [CrossRef]
- Antsiferova, A.A.; Kashkarov, P.K.; Koval’chuk, M.V. Effect of Different Forms of Silver on Biological Objects. Nanobiotechnol. Rep. 2022, 17, 155–164. [Google Scholar] [CrossRef]
- Suthar, J.K.; Vaidya, A.; Ravindran, S. Toxic Implications of Silver Nanoparticles on the Central Nervous System: A Systematic Literature Review. J. Appl. Toxicol. 2023, 43, 4–21. [Google Scholar] [CrossRef] [PubMed]
- McGhie, B.S.; Aldrich-Wright, J.R. Photoactive and Luminescent Transition Metal Complexes as Anticancer Agents: A Guiding Light in the Search for New and Improved Cancer Treatments. Biomedicines 2022, 10, 578. [Google Scholar] [CrossRef]
- Luengo, A.; Fernández-Moreira, V.; Marzo, I.; Gimeno, M.C. Trackable Metallodrugs Combining Luminescent Re(I) and Bioactive Au(I) Fragments. Inorg. Chem. 2017, 56, 15159–15170. [Google Scholar] [CrossRef]
- Dasari, S.; Singh, S.; Sivakumar, S.; Patra, A.K. Dual-Sensitized Luminescent Europium(ΙΙΙ) and Terbium(ΙΙΙ) Complexes as Bioimaging and Light-Responsive Therapeutic Agents. Chem.—A Eur. J. 2016, 22, 17387–17396. [Google Scholar] [CrossRef]
- Bünzli, J.-C.G. Lanthanide Luminescence for Biomedical Analyses and Imaging. Chem. Rev. 2010, 110, 2729–2755. [Google Scholar] [CrossRef]
- Visbal, R.; Fernández-Moreira, V.; Marzo, I.; Laguna, A.; Gimeno, M.C. Cytotoxicity and Biodistribution Studies of Luminescent Au(I) and Ag(I) N-Heterocyclic Carbenes. Searching for New Biological Targets. Dalton Trans. 2016, 45, 15026–15033. [Google Scholar] [CrossRef]
- Ling, Y.; Chen, Z.-X.; Zhou, Y.-M.; Weng, L.-H.; Zhao, D.-Y. A Novel Green Phosphorescent Silver(i) Coordination Polymer with Three-Fold Interpenetrated CdSO4-Type Net Generated via in Situ Reaction. CrystEngComm 2011, 13, 1504–1508. [Google Scholar] [CrossRef]
- Lin, S.; Cui, Y.-Z.; Qiu, Q.-M.; Han, H.-L.; Li, Z.-F.; Liu, M.; Xin, X.-L.; Jin, Q.-H. Synthesis, Characterization, Luminescent Properties of Silver (I) Complexes Based on Organic P-Donor Ligands and Mercaptan Ligands. Polyhedron 2017, 134, 319–329. [Google Scholar] [CrossRef]
- Pan, J.; Jiang, F.-L.; Wu, M.-Y.; Chen, L.; Gai, Y.-L.; Bawaked, S.M.; Mokhtar, M.; AL-Thabaiti, S.A.; Hong, M.-C. A Series of D10 Metal Clusters Constructed by 2,6-Bis[3-(Pyrazin-2-Yl)-1,2,4-Triazolyl]Pyridine: Crystal Structures and Unusual Luminescences. Cryst. Growth Des. 2014, 14, 5011–5018. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Jin, S.; Muhammad, N.; Guo, Z. Stimuli-Responsive Therapeutic Metallodrugs. Chem. Rev. 2019, 119, 1138–1192. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Liu, L.-Y.; Mao, Z.-W.; Zou, T. Approaches towards Understanding the Mechanism-of-Action of Metallodrugs. Coord. Chem. Rev. 2022, 453, 214311. [Google Scholar] [CrossRef]
- Stingaci, E.; Zveaghinteva, M.; Pogrebnoi, S.; Lupascu, L.; Valica, V.; Uncu, L.; Smetanscaia, A.; Drumea, M.; Petrou, A.; Ciric, A.; et al. New Vinyl-1,2,4-Triazole Derivatives as Antimicrobial Agents: Synthesis, Biological Evaluation and Molecular Docking Studies. Bioorg. Med. Chem. Lett. 2020, 30, 127368. [Google Scholar] [CrossRef] [PubMed]
- Patra, S.A.; Sahu, G.; Das, S.; Dinda, R. Recent Advances in Mitochondria-Localized Luminescent Ruthenium(II) Metallodrugs as Anticancer Agents. ChemMedChem 2023, 18, e202300397. [Google Scholar] [CrossRef] [PubMed]
- Bell, R.A.; Kramer, J.R. Structural Chemistry and Geochemistry of Silver-Sulfur Compounds: Critical Review. Environ. Toxicol. Chem. 1999, 18, 9–22. [Google Scholar] [CrossRef]
- Huang, X.; Li, H.; Tu, Z.; Liu, L.; Wu, X.; Chen, J.; Liang, Y.; Zou, Y.; Yi, Y.; Sun, J.; et al. Highly Conducting Neutral Coordination Polymer with Infinite Two-Dimensional Silver–Sulfur Networks. J. Am. Chem. Soc. 2018, 140, 15153–15156. [Google Scholar] [CrossRef]
- Silva, R.M.; Smith, M.D.; Gardinier, J.R. Anion- and Solvent-Directed Assembly in Silver Bis(Thioimidazolyl)Methane Chemistry and the Silver−Sulfur Interaction. Inorg. Chem. 2006, 45, 2132–2142. [Google Scholar] [CrossRef]
- Koshenskova, K.A.; Baravikov, D.E.; Khoroshilov, A.V.; Nelyubina, Y.V.; Primakov, P.V.; Bekker, O.B.; Dokuchaeva, K.S.; Dolgushin, F.M.; Kiskin, M.A.; Eremenko, I.L.; et al. Polymer Cu2+ and Ag+ Furancarboxylate Complexes with 4,4′-Bipyridine: Synthetic Approaches, Structure, Thermal Behavior, and Biological Activity. Russ. Chem. Bull. 2023, 72, 1894–1904. [Google Scholar] [CrossRef]
- Fackler, J.P.; López, C.A.; Staples, R.J.; Wang, S.; Winpenny, R.E.P.; Lattimer, R.P. Self Assembly of Isostructural Copper(I)-Silver(I) Butterfly Clusters with 2-Mercaptothiazoline; Syntheses and Structures of (PPh3)2Cu4(C3H4NS2)4, [(C5H5N)Cu4(C3H4NS2)4], (PPh3)2Ag4(C3H4NS2)4 and (PPh3)2Ag2Cu2(C3H4NS2)4. J. Chem. Soc. Chem. Commun. 1992, 2, 146–148. [Google Scholar] [CrossRef]
- Spek, A. PLATON SQUEEZE: A Tool for the Calculation of the Disordered Solvent Contribution to the Calculated Structure Factors. Acta Crystallogr. Sect. C 2015, 71, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Pyykkö, P. Strong Closed-Shell Interactions in Inorganic Chemistry. Chem. Rev. 1997, 97, 597–636. [Google Scholar] [CrossRef] [PubMed]
- Schmidbaur, H.; Schier, A. Argentophilic Interactions. Angew. Chem. Int. Ed. 2015, 54, 746–784. [Google Scholar] [CrossRef] [PubMed]
- Tsyba, I.; Mui, B.B.; Bau, R.; Noguchi, R.; Nomiya, K. Synthesis and Structure of a Water-Soluble Hexanuclear Silver(I) Nicotinate Cluster Comprised of a “Cyclohexane-Chair”-Type of Framework, Showing Effective Antibacterial and Antifungal Activities: Use of “Sparse Matrix” Techniques for Growing Crystals of Water-Soluble Inorganic Complexes. Inorg. Chem. 2003, 42, 8028–8032. [Google Scholar] [CrossRef] [PubMed]
- Whitcomb, D.R.; Swatloski, R.P.; Rogers, R.D. Mode of Complex Formation between Thiones and Silver Ion within a Photothermographic Formulation: The Crystal and Molecular Structure of Hexa(Silver-5-Methyl-2-Mercaptobenzimidazole THF). J. Imaging Sci. Technol. 2007, 51, 547–551. [Google Scholar] [CrossRef]
- Artem’ev, A.V.; Ryzhikov, M.R.; Berezin, A.S.; Kolesnikov, I.E.; Samsonenko, D.G.; Bagryanskaya, I.Y. Photoluminescence of Ag(I) Complexes with a Square-Planar Coordination Geometry: The First Observation. Inorg. Chem. Front. 2019, 6, 2855–2864. [Google Scholar] [CrossRef]
- Ramón-García, S.; Ng, C.; Anderson, H.; Chao, J.D.; Zheng, X.; Pfeifer, T.; Av-Gay, Y.; Roberge, M.; Thompson, C.J. Synergistic Drug Combinations for Tuberculosis Therapy Identified by a Novel High-Throughput Screen. Antimicrob. Agents Chemother. 2011, 55, 3861–3869. [Google Scholar] [CrossRef]
- Bekker, O.B.; Sokolov, D.N.; Luzina, O.A.; Komarova, N.I.; Gatilov, Y.V.; Andreevskaya, S.N.; Smirnova, T.G.; Maslov, D.A.; Chernousova, L.N.; Salakhutdinov, N.F.; et al. Synthesis and Activity of (+)-Usnic Acid and (−)-Usnic Acid Derivatives Containing 1,3-Thiazole Cycle against Mycobacterium Tuberculosis. Med. Chem. Res. 2015, 24, 2926–2938. [Google Scholar] [CrossRef]
- Lutsenko, I.A.; Baravikov, D.E.; Koshenskova, K.A.; Kiskin, M.A.; Nelyubina, Y.V.; Primakov, P.V.; Voronina, Y.K.; Garaeva, V.V.; Aleshin, D.A.; Aliev, T.M.; et al. What Are the Prospects for Using Complexes of Copper(II) and Zinc(II) to Suppress the Vital Activity of Mycolicibacterium Smegmatis? RSC Adv. 2022, 12, 5173–5183. [Google Scholar] [CrossRef]
- Polucci, P.; Magnaghi, P.; Angiolini, M.; Asa, D.; Avanzi, N.; Badari, A.; Bertrand, J.; Casale, E.; Cauteruccio, S.; Cirla, A.; et al. Alkylsulfanyl-1,2,4-Triazoles, a New Class of Allosteric Valosine Containing Protein Inhibitors. Synthesis and Structure–Activity Relationships. J. Med. Chem. 2013, 56, 437–450. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. SHELXT Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. Sect. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A Short History of SHELX. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef]



| Compound | MIC, (nmol/disc) | Zone of Inhibition, mm | |
|---|---|---|---|
| 24 h | 24 h | 120 h | |
| L | >1000 | 0 | 0 |
| I | 50 | 6.5 ± 0.1 | 6.4 ± 0 * |
| [Ag3(fur)(bpy)3]n [62] | 15 | 6.4 ± 0.12 | 6.2 ± 0 ** |
| Rif | 5 | 7.1 ± 0.76 | 7.0 ± 0.4 * |
| Parameter | Value |
|---|---|
| Empirical formula | C150H154N30O11S8Ag8 |
| Formula weight | 3316.2 |
| Crystal system | Monoclinic |
| Space group | Pc |
| Z | 2 |
| T, K | 150 |
| a (Å) | 23.729(3) |
| b (Å) | 25.401(3) |
| c (Å) | 12.617(1) |
| α (°) | 90 |
| β (°) | 93.340(4) |
| γ (°) | 90 |
| V (Å3) | 7591.9(14) |
| Dcalc (g/cm3) | 1.451 |
| μ(Mo-Kα) (mm−1) | 1.17 |
| F(000) | 3324 |
| 2θmax (deg.) | 50 |
| Rint | 0.0988 |
| Reflections measured | 137,347 |
| Independent reflections | 118,888 |
| Observed reflections [I > 2σ(I)] | 18,266 |
| Parameters/restraints | 1447/305 |
| R1 | 0.0561 |
| wR2 | 0.1470 |
| GooF | 1.002 |
| Flack | 0.18(4) |
| Δρmax/Δρmin (e/Å−3) | 1.34/−0.96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yambulatov, D.S.; Lutsenko, I.A.; Baravikov, D.E.; Dolgushin, F.M.; Astaf’eva, T.V.; Bekker, O.B.; Nersisyan, L.G.; Samvelyan, M.A.; Ghochikyan, T.V.; Kiskin, M.A.; et al. Synthesis, Structure, Biological Activity, and Luminescence Properties of a “Butterfly”-Type Silver Cluster with 3-Benzyl-4-phenyl-1,2,4-triazol-5-thiol. Molecules 2024, 29, 105. https://doi.org/10.3390/molecules29010105
Yambulatov DS, Lutsenko IA, Baravikov DE, Dolgushin FM, Astaf’eva TV, Bekker OB, Nersisyan LG, Samvelyan MA, Ghochikyan TV, Kiskin MA, et al. Synthesis, Structure, Biological Activity, and Luminescence Properties of a “Butterfly”-Type Silver Cluster with 3-Benzyl-4-phenyl-1,2,4-triazol-5-thiol. Molecules. 2024; 29(1):105. https://doi.org/10.3390/molecules29010105
Chicago/Turabian StyleYambulatov, Dmitriy S., Irina A. Lutsenko, Dmitry E. Baravikov, Fedor M. Dolgushin, Tatiana V. Astaf’eva, Olga B. Bekker, Lusik G. Nersisyan, Melanya A. Samvelyan, Tariel V. Ghochikyan, Mikhail A. Kiskin, and et al. 2024. "Synthesis, Structure, Biological Activity, and Luminescence Properties of a “Butterfly”-Type Silver Cluster with 3-Benzyl-4-phenyl-1,2,4-triazol-5-thiol" Molecules 29, no. 1: 105. https://doi.org/10.3390/molecules29010105
APA StyleYambulatov, D. S., Lutsenko, I. A., Baravikov, D. E., Dolgushin, F. M., Astaf’eva, T. V., Bekker, O. B., Nersisyan, L. G., Samvelyan, M. A., Ghochikyan, T. V., Kiskin, M. A., Eremenko, I. L., & Ivanov, V. K. (2024). Synthesis, Structure, Biological Activity, and Luminescence Properties of a “Butterfly”-Type Silver Cluster with 3-Benzyl-4-phenyl-1,2,4-triazol-5-thiol. Molecules, 29(1), 105. https://doi.org/10.3390/molecules29010105









