Electrochemiluminescence Sensor Based on CeO2 Nanocrystalline for Hg2+ Detection in Environmental Samples
Abstract
:1. Introduction
2. Results
2.1. Morphological Characterization
2.2. Optimization of Experimental Conditions
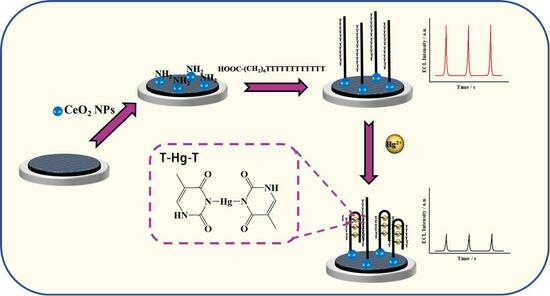
2.3. ECL Mechanism of CeO2
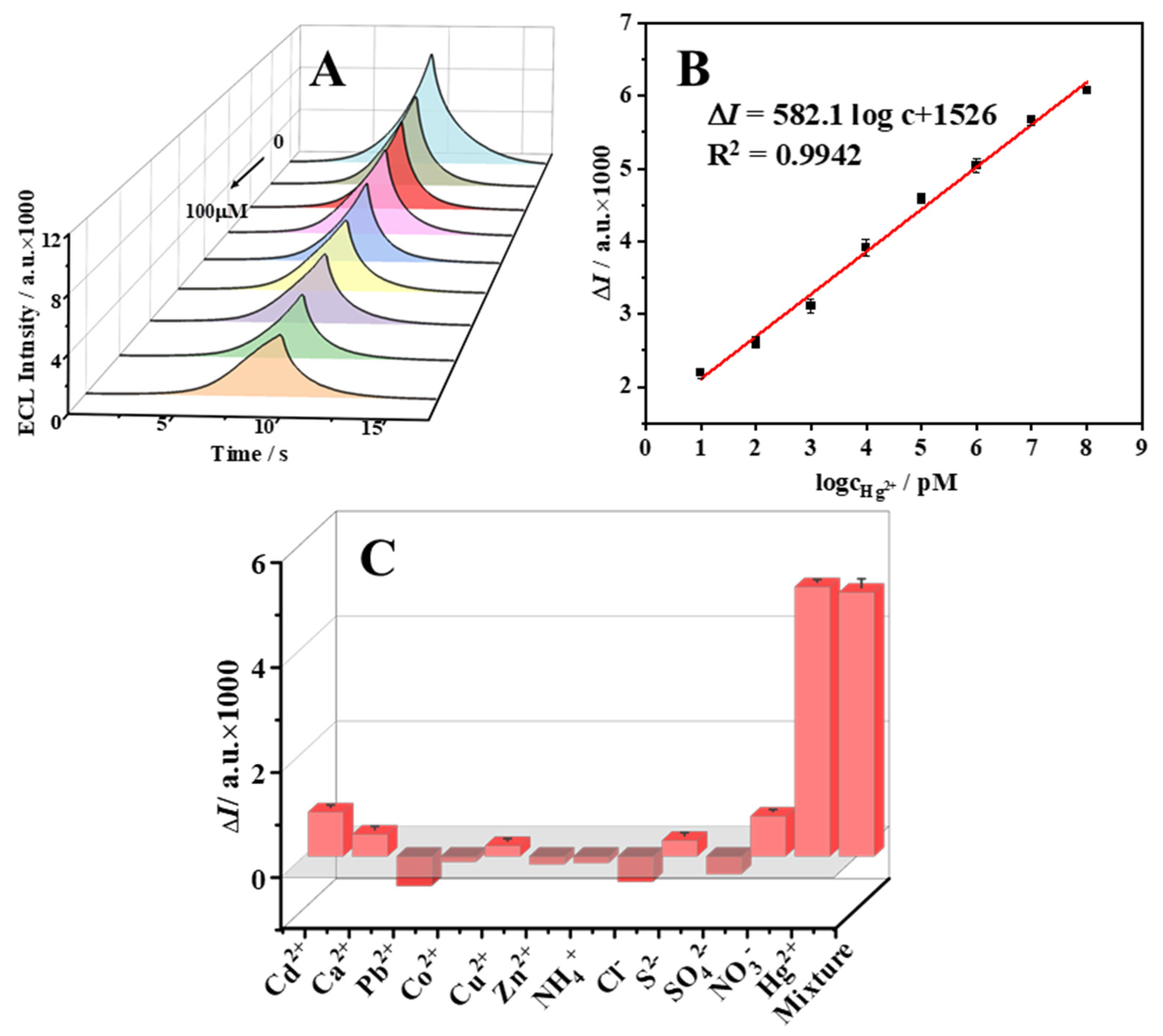
2.4. Response of the ECL Sensor to Hg2+
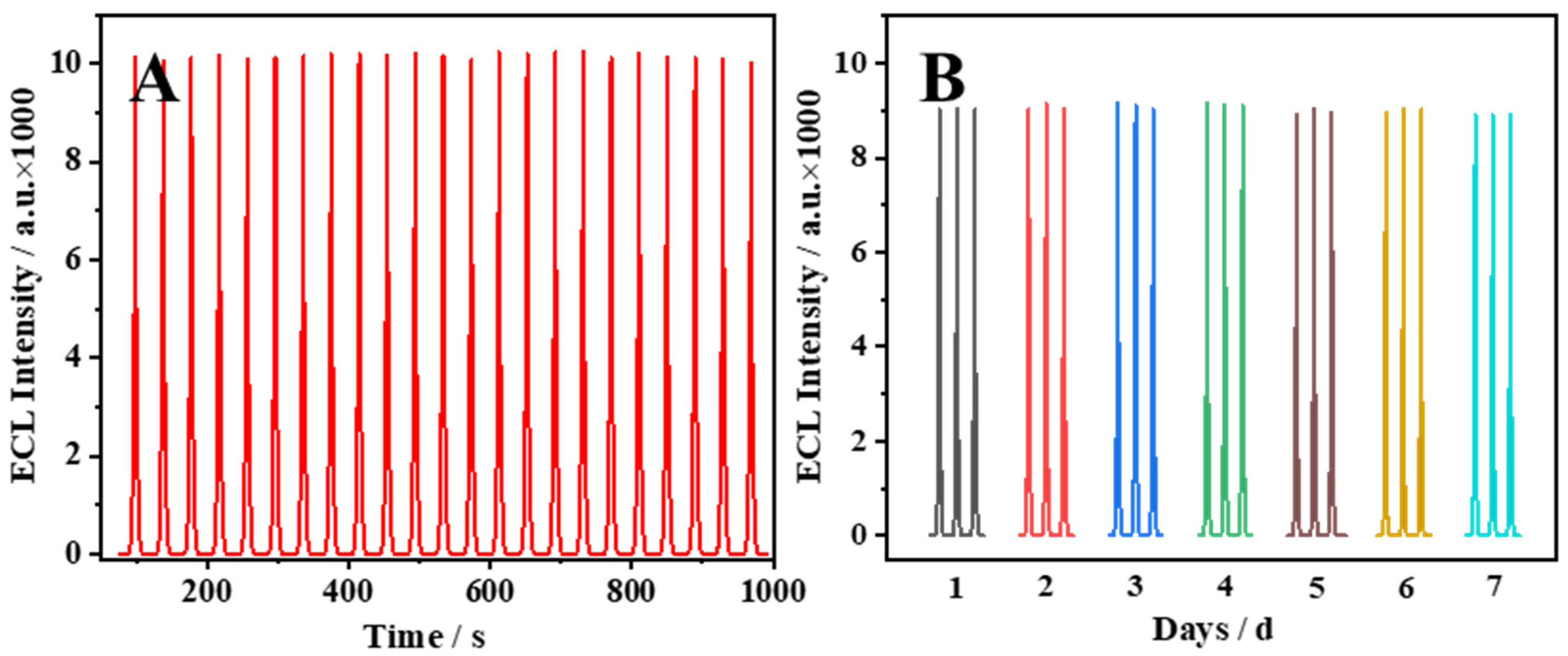
2.5. Stability of the ECL Sensor
2.6. Detection of Hg2+ in Actual Samples
3. Materials and Methods
3.1. Reagents and Chemicals
3.2. Apparatus
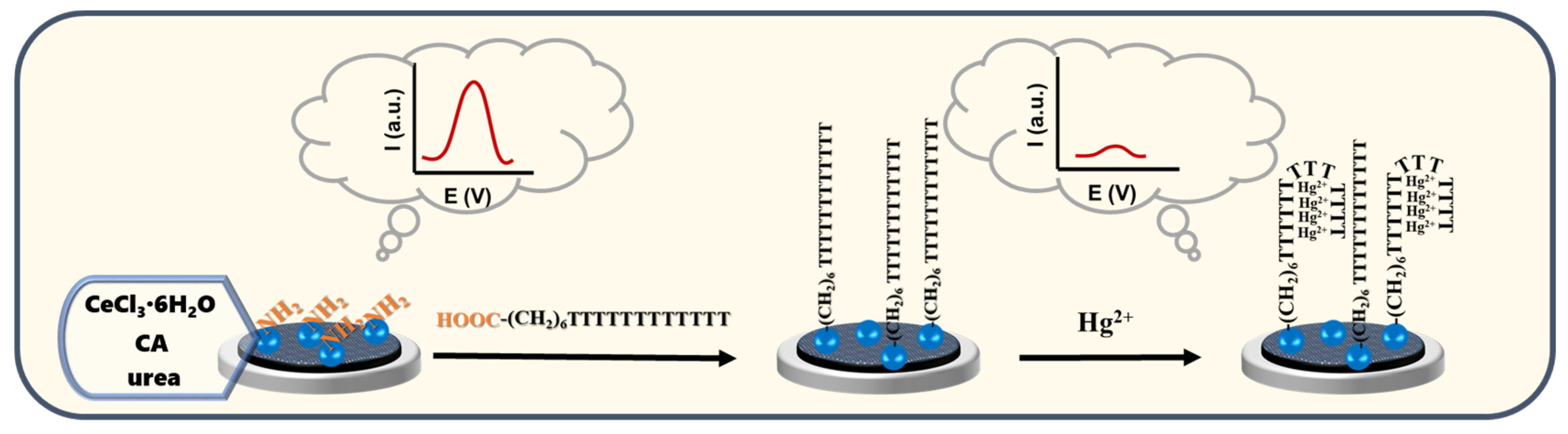
3.3. Synthesis of CeO2 NPs
3.4. Synthesis of CeO2-Apt
3.5. Construction of the Hg2+ ECL Sensor
3.6. Selective Testing
3.7. Actual Sample Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chikhaliwala, P.; Chandra, S. Dendrimers: New tool for enhancement of electrochemiluminescent signal. J. Organomet. Chem. 2016, 821, 78–90. [Google Scholar] [CrossRef]
- Hiramoto, K.; Villani, E.; Iwama, T.; Komatsu, K.; Inagi, S.; Inoue, K.Y.; Nashimoto, Y.; Ino, K.; Shiku, H. Recent Advances in Electrochemiluminescence-Based Systems for Mammalian Cell Analysis. Micromachines 2020, 11, 530. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, P.L.; Nie, Y.X.; Ma, Q. Recent development of organic nanoemitter-based ECL sensing application. Trends Anal. Chem. 2021, 143, 116410. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Pan, J.B.; Li, Q.; Zhou, Y.; Yang, S.; Xu, J.J.; Hua, D.B. Improved AIE-Active Probe with High Sensitivity for Accurate Uranyl Ion Monitoring in the Wild Using Portable Electrochemiluminescence System for Environmental Applications. Adv. Funct. Mater. 2020, 30, 2000220. [Google Scholar] [CrossRef]
- Chen, D.L.; Zhang, M.; Zhou, F.Y.; Hai, H.; Li, J.P. Ultrasensitive electroluminescence biosensor for a breast cancer marker microRNA based on target cyclic regeneration and multi-labeled magnetized nanoparticles. Microchim. Acta 2019, 186, 628. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Zhang, H.X.; Wang, Z.H.; Liu, Q.Y.; Liu, Y. A novel ECL method for histone acetyltransferases (HATs) activity analysis by integrating HCR signal amplification and ECL silver clusters. Talanta 2019, 198, 39–44. [Google Scholar] [CrossRef]
- Tu, L.P. Gold nanomaterials for biochemical sensing. Gold Bull. 2022, 55, 169–185. [Google Scholar] [CrossRef]
- Lv, X.H.; Ma, H.M.; Wu, D.; Yan, T.; Ji, L.; Liu, Y.X.; Pang, X.H.; Du, B.; Wei, Q. Novel gold nanocluster electrochemiluminescence immunosensors based on nanoporous NiGd-Ni2O3-Gd2O3 alloys. Biosens. Bioelectron. 2016, 75, 142–147. [Google Scholar] [CrossRef]
- Zhang, H.H.; Zhang, C.Y.; Qu, H.; Xi, F.N. Immunosensor with Enhanced Electrochemiluminescence Signal Using Platinum Nanoparticles Confined within Nanochannels for Highly Sensitive Detection of Carcinoembryonic Antigen. Molecules 2023, 28, 6559. [Google Scholar] [CrossRef]
- Wei, H.; Wang, E. Electrochemiluminescence of tris(2,2′-bipyridyl)ruthenium and its applications in bioanalysis: A review. Luminescence 2011, 26, 77–85. [Google Scholar] [CrossRef]
- Kerr, E.; Doeven, E.H.; Francis, P.S. Recent advances in mechanistic understanding and analytical methodologies of the electrochemiluminescence of tris(2,2′-bipyridine) ruthenium(II) and tri-n-propylamine. Curr. Opin. Electrochem. 2022, 35, 101034. [Google Scholar] [CrossRef]
- Hua, Q.; Tang, F.Y.; Wang, X.B.; Li, M.; Gu, X.W.; Sun, W.J.; Luan, F.; Tian, C.Y.; Zhuang, X.M. Electrochemiluminescence sensor based on EuS nanocrystals for ultrasensitive detection of mercury ions in seafood. Sens. Actuators B Chem. 2022, 352, 131075. [Google Scholar] [CrossRef]
- Tian, C.Y.; Wang, L.; Luan, F.; Fu, X.L.; Zhuang, X.M.; Chen, L.X. A novel electrochemiluminescent emitter of europium hydroxide nanorods and its application in bioanalysis. Chem. Commun. 2019, 55, 12479–12482. [Google Scholar] [CrossRef] [PubMed]
- Moghaddam, M.R.; Ganjali, M.R.; Hosseini, M.; Faridbod, F.; Pur, M.R.K. A novel electrochemiluminescnece sensor based on an Ru(bpy)32+−Eu2O3—Nafion nanocomposite and its application in the detection of diphenhydramine. Int. J. Electrochem. Sci. 2017, 12, 5220–5232. [Google Scholar] [CrossRef]
- Ma, G.Y.; Wu, P.P.A.; Wu, K.; Deng, A.P.; Li, J.G. A novel electrochemiluminescence immunoassay based on highly efficient resonance energy transfer for florfenicol detection. Talanta 2021, 235, 122732. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.G.; Zhao, M.; Hu, X.J.; Wang, X.F.; Wang, L. Electrogenerated chemiluminescence behavior of Tb complex and its application in sensitive sensing Cd2+. Electrochim. Acta 2017, 228, 1–8. [Google Scholar] [CrossRef]
- Yang, G.M.; He, Y.; Zhao, J.W.; Chen, S.H.; Yuan, R. Ratiometric electrochemiluminescence biosensor based on Ir nanorods and CdS quantum dots for the detection of organophosphorus pesticides. Sens. Actuators B Chem. 2021, 341, 130008. [Google Scholar] [CrossRef]
- Babamiri, B.; Salimi, A.; Hallaj, R. A molecularly imprinted electrochemiluminescence sensor for ultrasensitive HIV-1 gene detection using EuS nanocrystals as luminophore. Biosens. Bioelectron. 2018, 117, 332–339. [Google Scholar] [CrossRef]
- Yang, L.; Zhu, W.J.; Ren, X.; Khan, M.S.; Zhang, Y.; Du, B.; Wei, Q. Macroporous graphene capped Fe3O4 for amplified electrochemiluminescence immunosensing of carcinoembryonic antigen detection based on CeO2@TiO2. Biosens. Bioelectron. 2017, 91, 842–848. [Google Scholar] [CrossRef]
- Cheng, W.Q.; Lin, Z.; Zhao, L.A.; Fan, N.K.; Bai, H.J.; Cheng, W.; Zhao, M.; Ding, S.J. CeO2/MXene heterojunction-based ultrasensitive electrochemiluminescence biosensing for BCR-ABL fusion gene detection combined with dual-toehold strand displacement reaction for signal amplification. Biosens. Bioelectron. 2022, 210, 114287. [Google Scholar] [CrossRef]
- Malone, A.; Figueroa, L.; Wang, W.S.; Smith, N.M.; Ranville, J.F.; Vuono, D.C.; Zapata, F.D.A.; Paredes, L.M.; Sharp, J.O.; Bellona, C. Transitional dynamics from mercury to cyanide-based processing in artisanal and small-scale gold mining: Social, economic, geochemical, and environmental considerations. Sci. Total Environ. 2023, 898, 165492. [Google Scholar] [CrossRef] [PubMed]
- Dastoor, A.; Angot, H.; Bieser, J.; Christensen, J.H.; Douglas, T.A.; Heimburger-Boavida, L.E.; Jiskra, M.; Mason, R.P.; McLagan, D.S.; Obrist, D.; et al. Arctic mercury cycling. Nat. Rev. Earth Environ. 2022, 3, 270–286. [Google Scholar] [CrossRef]
- Okereafor, U.; Makhatha, M.; Mekuto, L.; Uche-Okereafor, N.; Sebola, T.; Mavumengwana, V. Toxic Metal Implications on Agricultural Soils, Plants, Animals, Aquatic life and Human Health. Int. J. Environ. Res. Public Health 2020, 17, 2204. [Google Scholar] [CrossRef] [PubMed]
- Hirai, T.; Abe, O.; Nakamura, M.; Inui, S.; Uetani, H.; Ueda, M.; Azuma, M. Brain structural changes in patients with chronic methylmercury poisoning in Minamata. Brain Res. 2023, 1805, 148278. [Google Scholar] [CrossRef] [PubMed]
- Marumoto, M.; Sakamoto, M.; Marumoto, K.; Tsuruta, S.; Komohara, Y. Mercury and Selenium Localization in the Cerebrum, Cerebellum, Liver, and Kidney of a Minamata Disease Case. Acta Histochem. Cytochem. 2020, 53, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Liang, P.; Yu, J.; Yang, E.J.; Mo, Y.J. Determination of Mercury in Food and Water Samples by Displacement-Dispersive Liquid-Liquid Microextraction Coupled with Graphite Furnace Atomic Absorption Spectrometry. Food Anal. Methods 2015, 8, 236–242. [Google Scholar] [CrossRef]
- Khalizov, A.F.; Guzman, F.J.; Cooper, M.; Mao, N.; Antley, J.; Bozzelli, J. Direct detection of gas-phase mercuric chloride by ion drift—Chemical ionization mass spectrometry. Atmos. Environ. 2020, 238, 117687. [Google Scholar] [CrossRef]
- Chen, X.P.; Han, C.; Cheng, H.Y.; Liu, J.H.; Xu, Z.G.; Yin, X.F. Determination of mercurial species in fish by inductively coupled plasma mass spectrometry with anion exchange chromatographic separation. Anal. Chim. Acta 2013, 796, 7–13. [Google Scholar] [CrossRef]
- Miao, P.; Tang, Y.G.; Wang, L. DNA Modified Fe3O4@Au Magnetic Nanoparticles as Selective Probes for Simultaneous Detection of Heavy Metal Ions. ACS Appl. Mater. Interfaces 2017, 9, 3940–3947. [Google Scholar] [CrossRef]
- Ferreira, N.; Viana, T.; Henriques, B.; Tavares, D.S.; Jacinto, J.; Colónia, J.; Pinto, J.; Pereira, E. Application of response surface methodology and box-behnken design for the optimization of mercury removal by Ulva sp. J. Hazard. Mater. 2023, 445, 130405. [Google Scholar] [CrossRef]
- Tan, T.; Zhang, C.; Han, Y.; Chu, R.J.; Xi, W.Y.; Chen, X.L.; Sun, J.Y.; Huang, H.; Hu, Y.J.; Huang, X.H. Fine-tuning bromide AIE probes for Hg2+ detection in mitochondria with wash-free staining. J. Hazard. Mater. 2024, 464, 132999. [Google Scholar] [CrossRef] [PubMed]
- Li, D.L.; Liu, L.Y.; Huang, Q.L.; Tong, T.; Zhou, Y.; Li, Z.Y.; Bai, Q.Q.; Liang, H.; Chen, L.L. Recent advances on aptamer-based biosensors for detection of pathogenic bacteria. World J. Microbiol. Biotechnol. 2021, 27, 45. [Google Scholar] [CrossRef] [PubMed]
- Alkhamis, O.; Canoura, J.; Yu, H.X.; Liu, Y.Z.; Xiao, Y. Innovative engineering and sensing strategies for aptamer-based small-molecule detection. TrAC Trends Anal. Chem. 2019, 121, 115699. [Google Scholar] [CrossRef] [PubMed]
- Ono, A.; Togashi, H. Highly Selective Oligonucleotide-Based Sensor for Mercury (II) in Aqueous Solutions. Angew. Chem. 2004, 116, 4300–4302. [Google Scholar] [CrossRef] [PubMed]
- Guzman, M.; Estrada, M.; Miridonov, S. Synthesis of cerium oxide (IV) hollow nanospheres with tunable structure and their performance in the 4-nitrophenol adsorption. Microporous Mesoporous Mater. 2019, 278, 241–250. [Google Scholar] [CrossRef]
- Zhu, X.; Tang, L.; Wang, J.J.; Peng, B.; Ouyang, X.L.; Tan, J.S.; Yu, J.F.; Feng, H.P.; Tang, J.L. Enhanced peroxidase-like activity of boron nitride quantum dots anchored porous CeO2 nanorods by aptamer for highly sensitive colorimetric detection of kanamycin. Sens. Actuators B Chem. 2021, 330, 129318. [Google Scholar] [CrossRef]
- Liu, C.; Tuffour, A.; Liao, J.H.; Li, M.L.; Lv, Q.X.; Zhou, D.R.; Gao, L. Highly sensitive detection of Hg2+ using molybdenum disulfide-DNA sensors. Chem. Phys. 2020, 534, 110758. [Google Scholar] [CrossRef]
- Bu, Y.W.; Wang, K.; Yang, X.Y.; Nie, G.M. Photoelectrochemical sensor for detection Hg2+ based on in situ generated MOFs-like structures. Anal. Chim. Acta 2022, 1233, 340496. [Google Scholar] [CrossRef]
- Fang, Y.M.; Zhang, Y.; Cao, L.G.; Yang, J.Z.; Hu, M.H.; Pang, Z.L.; He, J.H. Portable Hg2+ Nanosensor with ppt Level Sensitivity Using Nanozyme as the Recognition Unit, Enrichment Carrier, and Signal Amplifier. ACS Appl. Mater. Interfaces 2020, 12, 11761–11768. [Google Scholar] [CrossRef]
- Zhou, J.; Wei, D.; Hu, C.C.; Li, S.; Ma, L.; Chen, Z.; Deng, Y. A simple label-free electrochemical aptamer sensor for detection of Hg2+ based on black phosphorus. Mater. Express 2022, 12, 802–809. [Google Scholar] [CrossRef]

| Samples | Added (pM) | Found (pM) | Recovery (%) | RSD (%) |
|---|---|---|---|---|
| Fish | 1 × 102 | 0.8299 × 102 | 82.99 | 0.81 |
| 1 × 105 | 1.050 × 105 | 105.0 | 1.4 | |
| 1 × 108 | 0.9258 × 108 | 92.58 | 1.7 | |
| Shrimp | 1 × 102 | 0.8831 × 102 | 88.31 | 0.7 |
| 1 × 105 | 0.8730 × 105 | 87.30 | 2.5 | |
| 1 × 108 | 0.8551 × 108 | 85.51 | 0.73 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, C.; Tang, F.; Guo, W.; Wei, M.; Wang, L.; Zhuang, X.; Luan, F. Electrochemiluminescence Sensor Based on CeO2 Nanocrystalline for Hg2+ Detection in Environmental Samples. Molecules 2024, 29, 1. https://doi.org/10.3390/molecules29010001
Tian C, Tang F, Guo W, Wei M, Wang L, Zhuang X, Luan F. Electrochemiluminescence Sensor Based on CeO2 Nanocrystalline for Hg2+ Detection in Environmental Samples. Molecules. 2024; 29(1):1. https://doi.org/10.3390/molecules29010001
Chicago/Turabian StyleTian, Chunyuan, Feiyan Tang, Wei Guo, Minggang Wei, Li Wang, Xuming Zhuang, and Feng Luan. 2024. "Electrochemiluminescence Sensor Based on CeO2 Nanocrystalline for Hg2+ Detection in Environmental Samples" Molecules 29, no. 1: 1. https://doi.org/10.3390/molecules29010001
APA StyleTian, C., Tang, F., Guo, W., Wei, M., Wang, L., Zhuang, X., & Luan, F. (2024). Electrochemiluminescence Sensor Based on CeO2 Nanocrystalline for Hg2+ Detection in Environmental Samples. Molecules, 29(1), 1. https://doi.org/10.3390/molecules29010001