Thermo-Chemical Modification of Cellulose for the Adsorptive Removal of Titan Yellow from Wastewater
Abstract
1. Introduction
2. Results and Discussion
2.1. Spectroscopic Analysis of TAC, CMC, and UFC
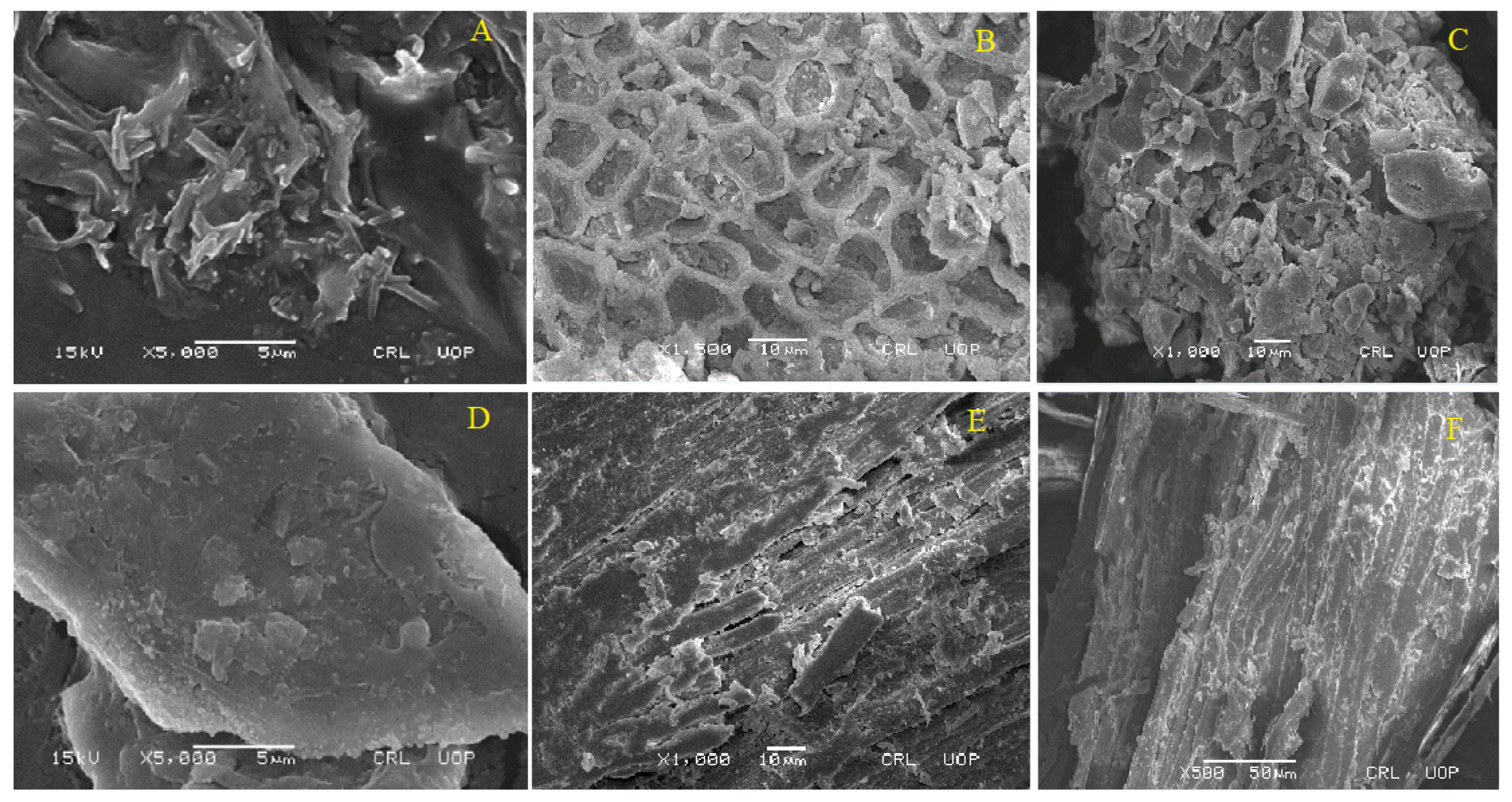
2.2. Morphology of TAC, CMC, and UFC
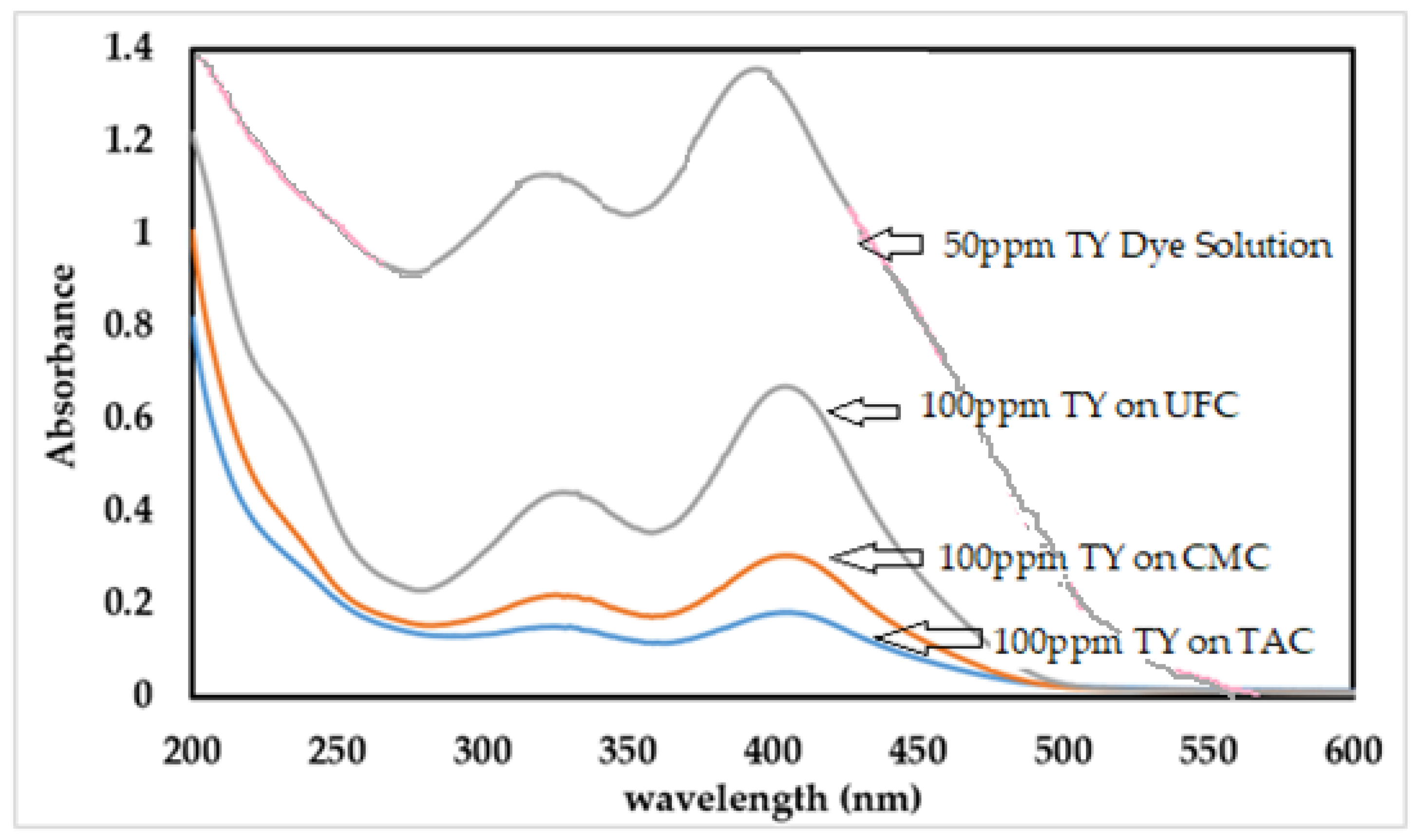
2.3. Absorption Spectroscopic Analysis of TAC, CMC, and UFC
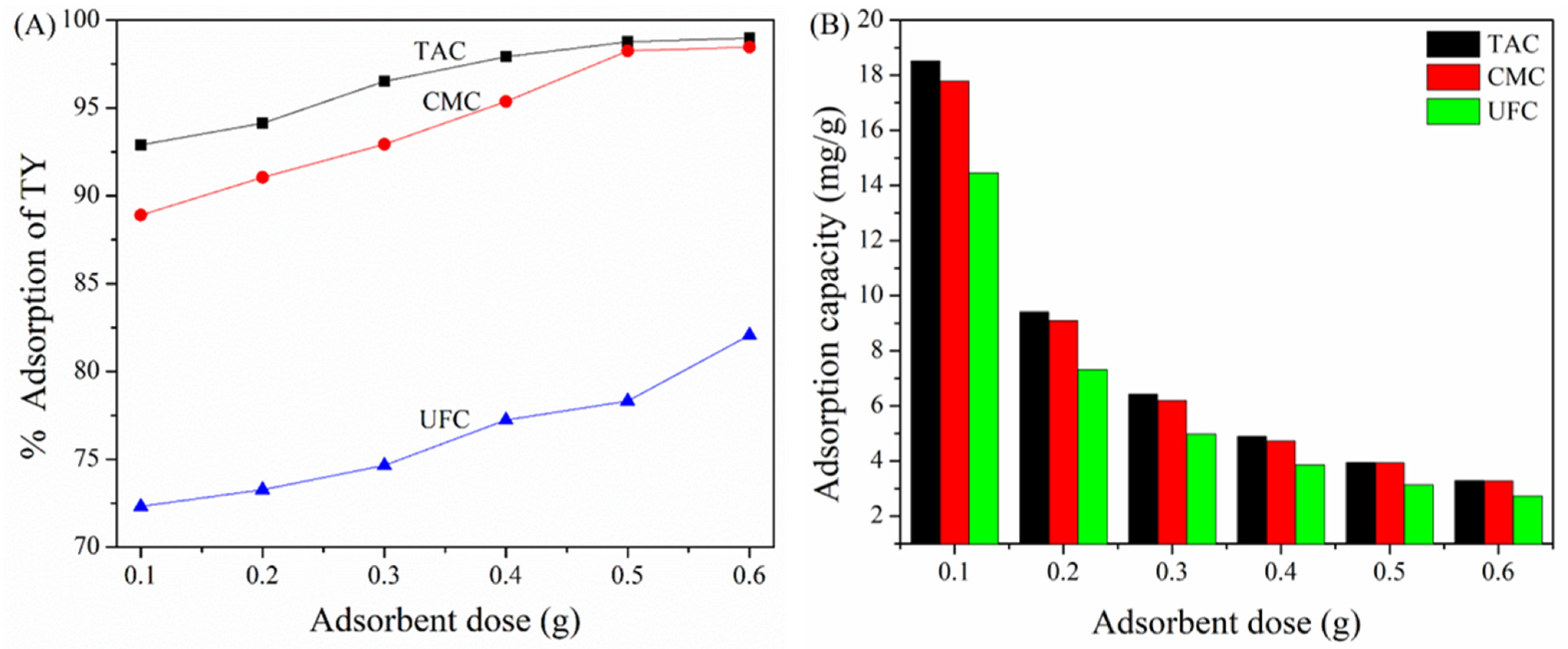
2.4. Effect of Adsorbent Dose on TAC, CMC, and UFC
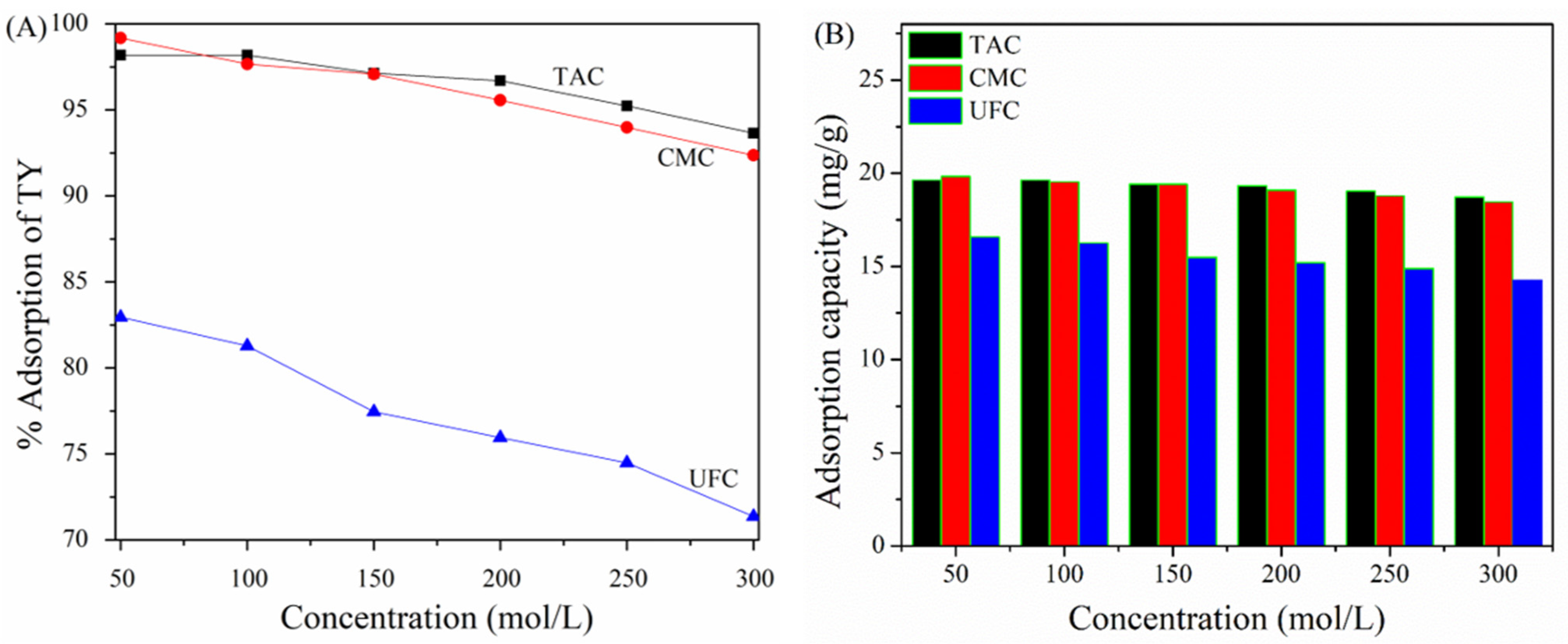
2.5. Effect of Initial TY Concentration on TAC, CMC, and UFC
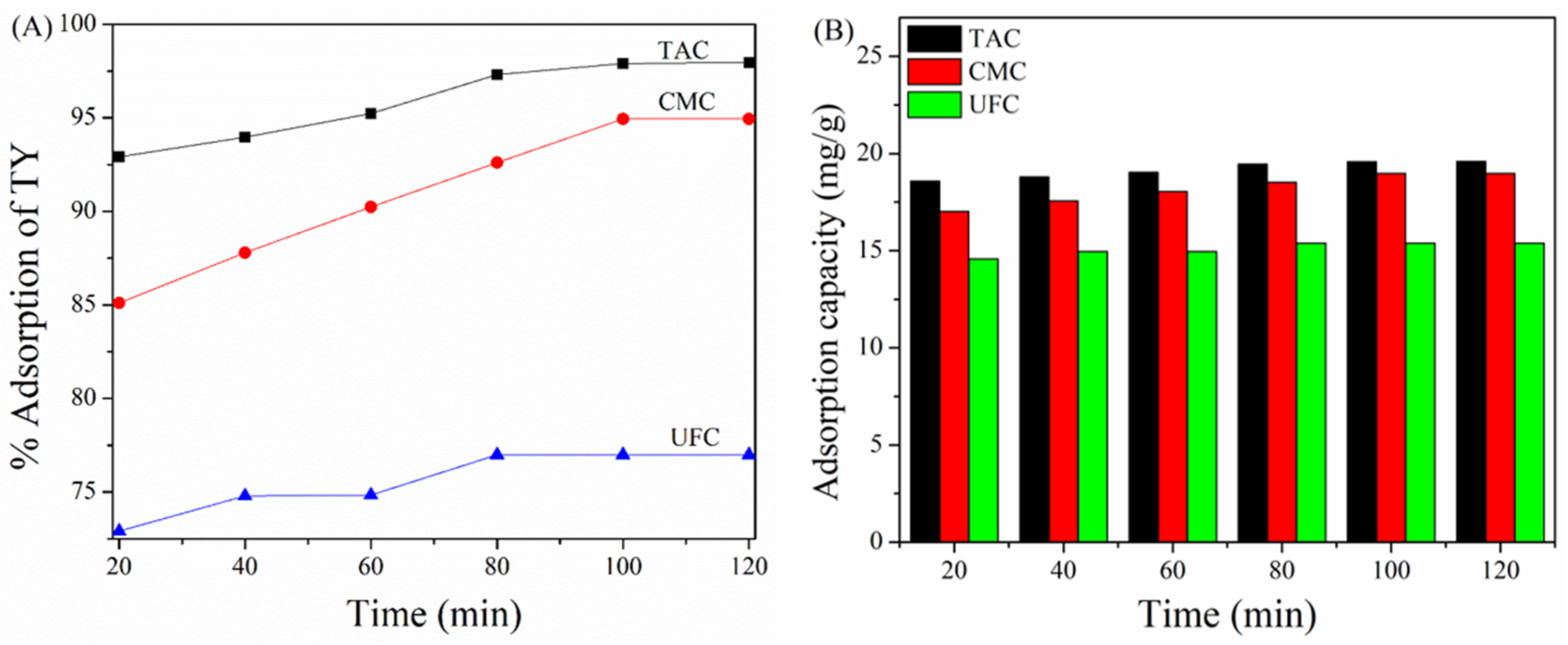
2.6. Effect of Contact Time on Adsorption of TY on TAC, CMC, and UFC
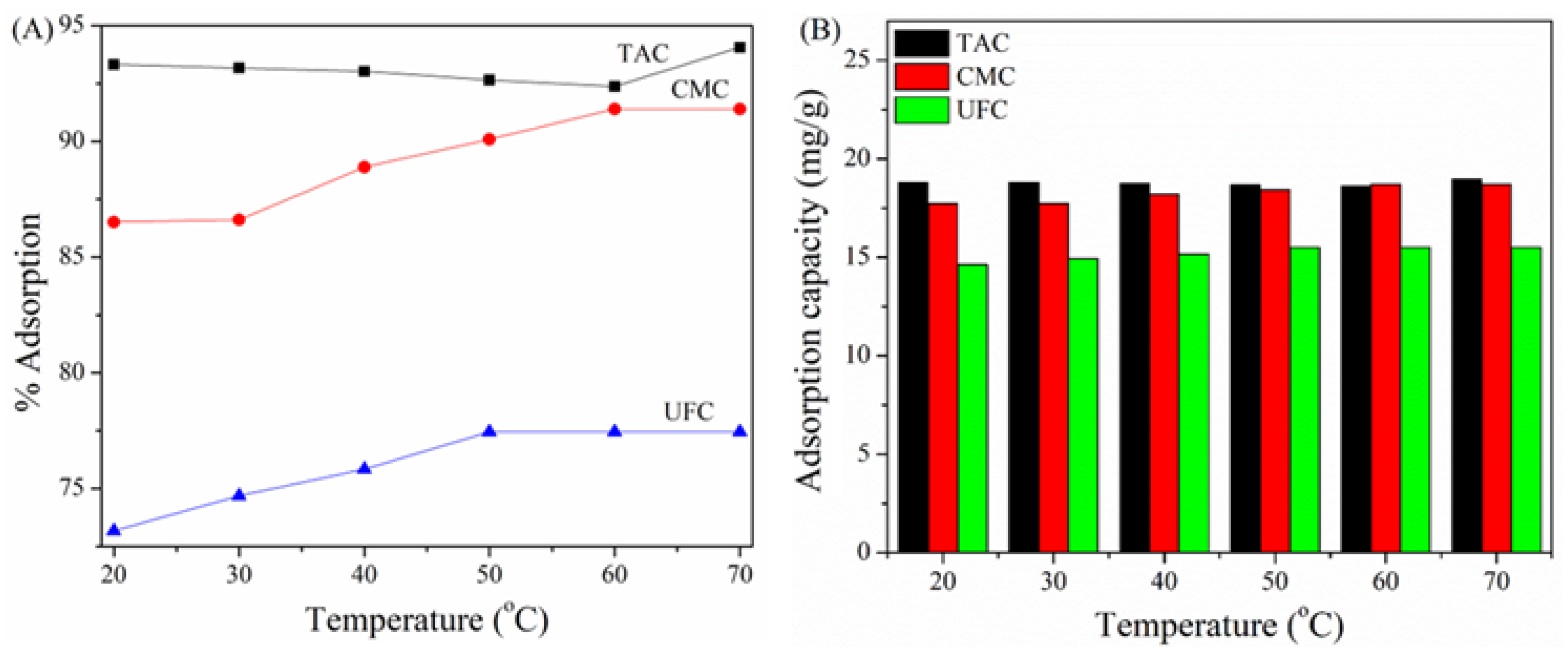
2.7. Effect of Temperature on Adsorption of TY on TAC, CMC, and UFC
2.8. Adsorption Kinetic Studies
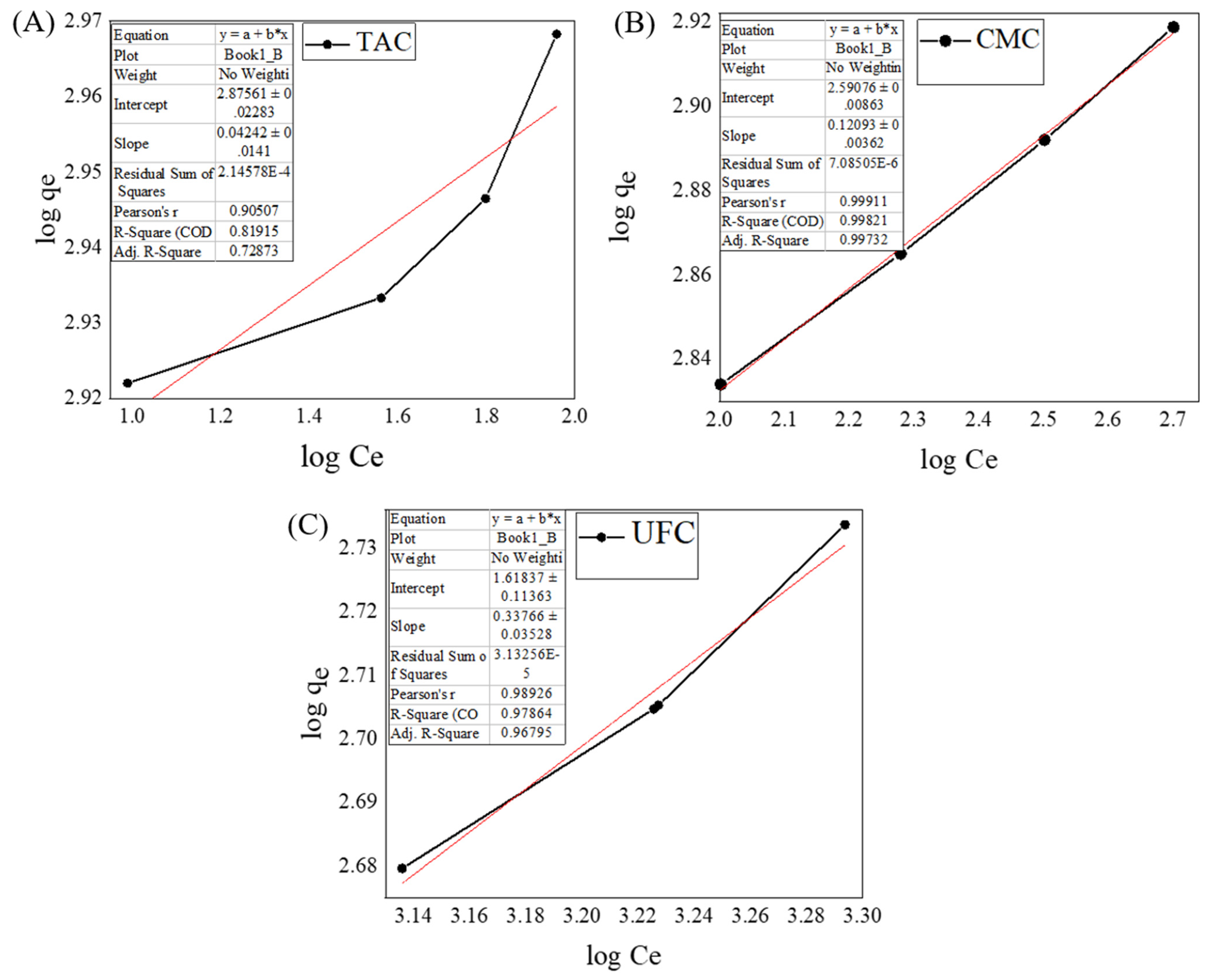
2.9. Adsorption Isotherm Model
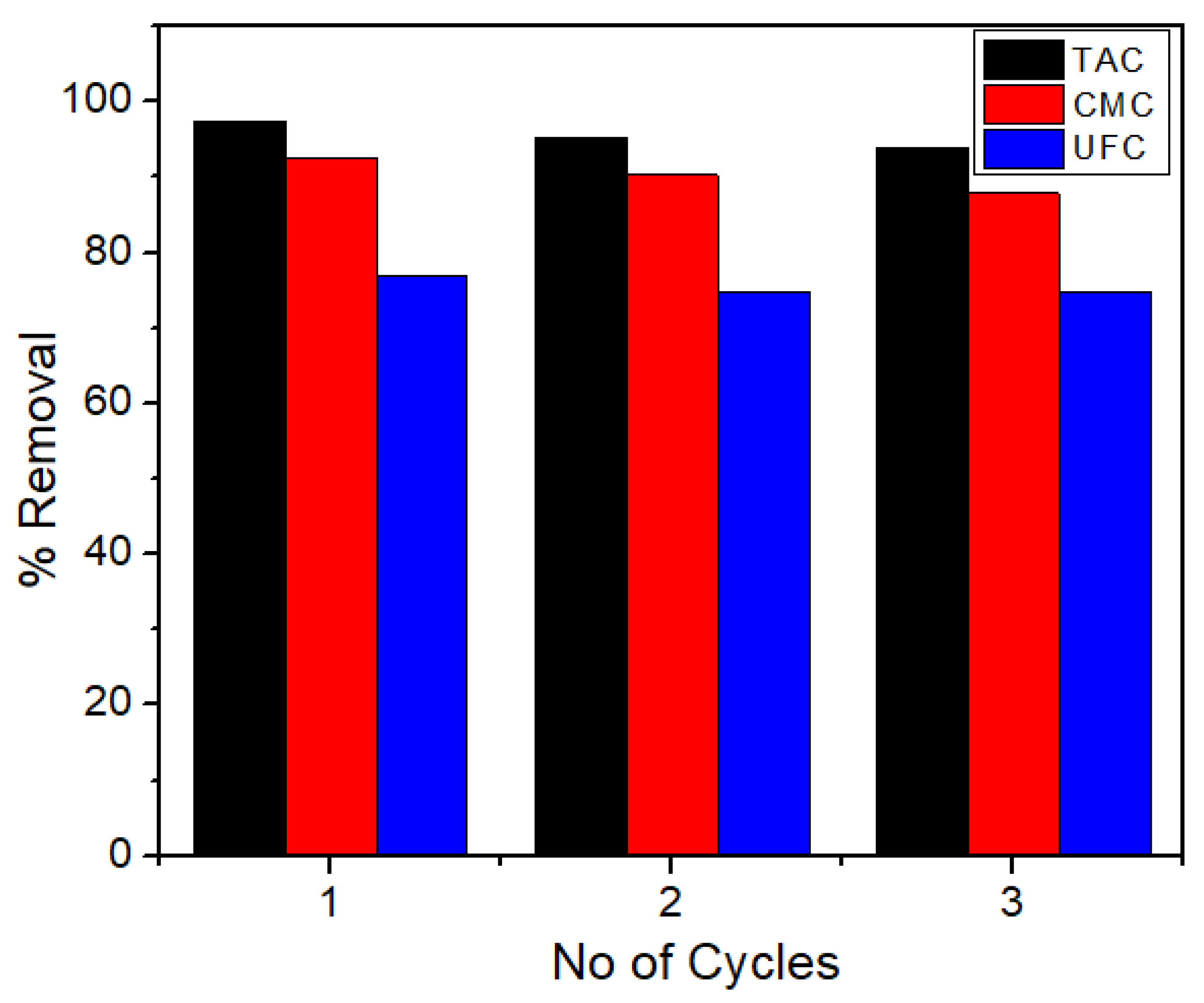
2.10. Recycling and Reusability of the Materials
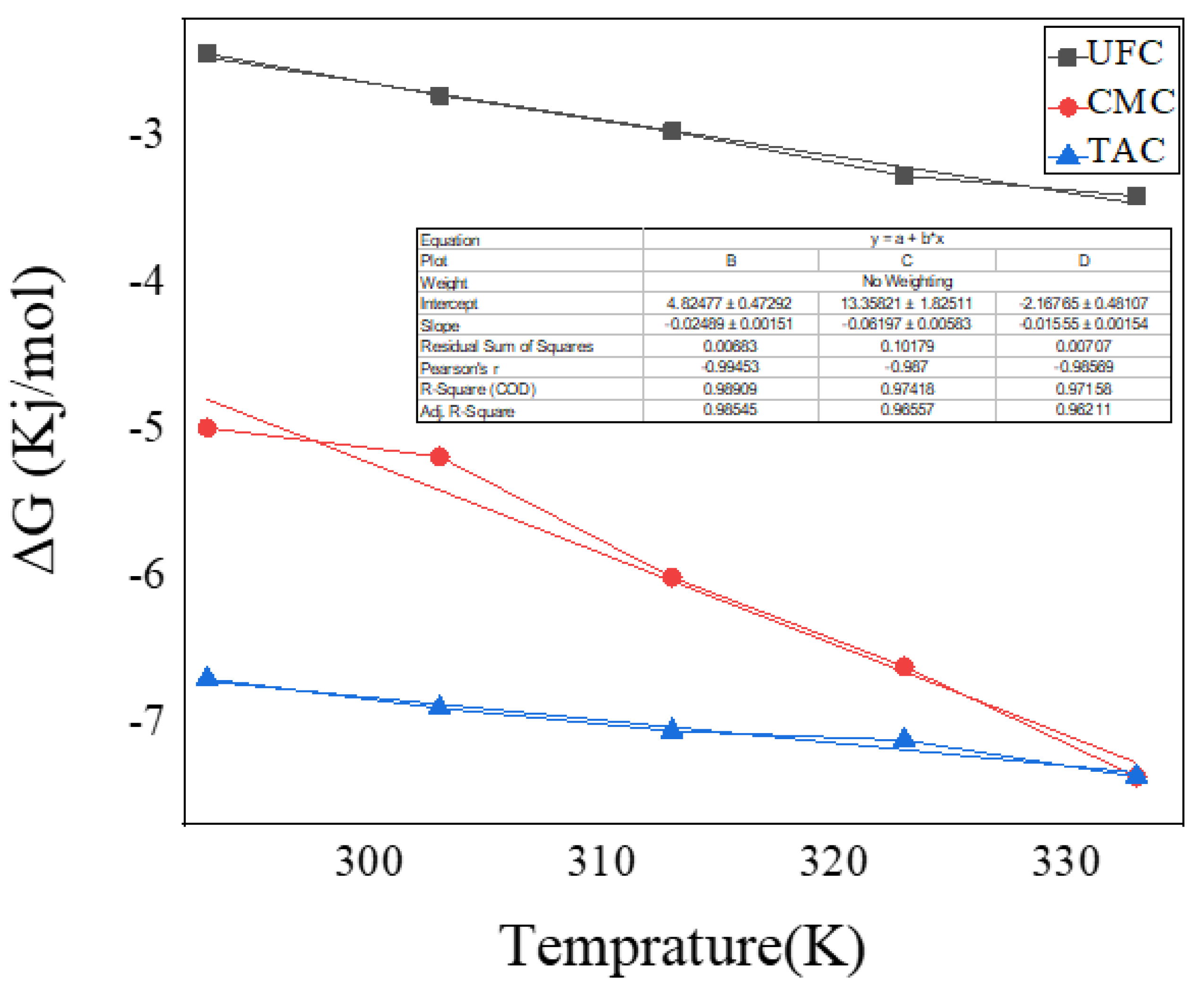
2.11. Adsorption Thermodynamics Studies
3. Experimental Methods
3.1. Materials Required
3.2. Preparation and Functionalization of the Adsorbents
3.3. Thermo-Chemical Modification of Fibers
3.4. Adsorption Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Kathirselvam, M.; Kumaravel, A.; Arthanarieswaran, V.P.; Saravanakumar, S.S. Isolation and characterization of cellulose fibers from Thespesia populnea barks: A study on physicochemical and structural properties. Int. J. Biol. Macromol. 2019, 129, 396–406. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, H.; Du, C.; Zhang, D.; Lin, H.; Chen, Y.; Xiong, J. Cellulose for Sustainable Triboelectric Nanogenerators. Adv. Energy Sustain. Res. 2022, 3, 2100161. [Google Scholar] [CrossRef]
- Kalia, S.; Dufresne, A.; Cherian, B.M.; Kaith, B.S.; Avérous, L.; Njuguna, J.; Nassiopoulos, E. Cellulose-Based Bio- and Nanocomposites: A Review. Int. J. Polym. Sci. 2011, 2011, 837875. [Google Scholar] [CrossRef]
- Miyamoto, T.; Takahashi, S.-I.; Ito, H.; Inagaki, H.; Noishiki, Y. Tissue biocompatibility of cellulose and its derivatives. J. Biomed. Mater. Res. 1989, 23, 125–133. [Google Scholar] [CrossRef]
- Khan, S.; Cao, Q.; Zheng, Y.M.; Huang, Y.Z.; Zhu, Y.G. Health risks of heavy metals in contaminated soils and food crops irrigated with wastewater in Beijing, China. Environ. Pollut. 2008, 152, 686–692. [Google Scholar] [CrossRef]
- Iuchi, K.; Aozasa, K.; Yamamoto, S.; Mori, T.; Tajima, K.; Minato, K.; Mukai, K.; Komatsu, H.; Tagaki, T.; Kobashi, Y.; et al. Non-Hodgkin’s lymphoma of the pleural cavity developing from long-standing pyothorax. Summary of clinical and pathological findings in thirty-seven cases. Jpn. J. Clin. Oncol. 1989, 19, 249–257. [Google Scholar] [PubMed]
- Tang, Y.; Shen, X.; Zhang, J.; Guo, D.; Kong, F.; Zhang, N. Extraction of cellulose nano-crystals from old corrugated container fiber using phosphoric acid and enzymatic hydrolysis followed by sonication. Carbohydr. Polym. 2015, 125, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Tumuluru, J.S.; Hess, J.R.; Boardman, R.D.; Wright, C.; Westover, T. Formulation, Pretreatment, and Densification Options to Improve Biomass Specifications for Co-Firing High Percentages with Coal. Ind. Biotechnol. 2012, 8, 113–132. [Google Scholar] [CrossRef]
- Abdelhamid, H.N.; Mathew, A.P. Cellulose-Based Materials for Water Remediation: Adsorption, Catalysis, and Antifouling. Front. Chem. Eng. 2021, 3, 5405–5441. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Hassaan, M.A.; El Nemr, A.; Hassaan, A. Health and environmental impacts of dyes: Mini review. Am. J. Environ. Sci. Eng. 2017, 1, 64–67. [Google Scholar]
- Malik, A.; McBain, D.; Wiedmann, T.O.; Lenzen, M.; Murray, J. Advancements in Input-Output Models and Indicators for Consumption-Based Accounting. J. Ind. Ecol. 2018, 23, 300–312. [Google Scholar] [CrossRef]
- Khan, A.; Noor, S.; Khan, M.S.; Khattak, R.; Malik, A.; Rahman, U.U.; Zekker, I.; Rahman, N.U.; Shah, L.A. Removal of crystal violet from wastewater using synthesized graphene quantum dots as adsorbents: Kinetic approach. Int. J. Environ. Sci. Technol. 2023, 1–14. [Google Scholar] [CrossRef]
- Khan, A.; Malik, A.; Humayun, M.; Shah, N.; Ismail, M.; Yahia, M.; Mohamed, R.M. Improvement of the adsorption efficiency of rice husk ash for crystal violet dye removal from aqueous medium. Egypt. J. Chem. 2022, 65, 427–435. [Google Scholar] [CrossRef]
- Malik, A.; Khan, A.; Humayun, M. Preparation and Chemical Modification of Rice Husk Char for the Removal of a Toxic Dye (Orange G) from Aqueous Medium. Z. Phys. Chem. 2018, 233, 375–392. [Google Scholar] [CrossRef]
- Tang, C.; Shu, Y.; Zhang, R.; Li, X.; Song, J.; Li, B.; Zhang, Y.; Ou, D. Comparison of the removal and adsorption mechanisms of cadmium and lead from aqueous solution by activated carbons prepared from Typha angustifolia and Salix matsudana. RSC Adv. 2017, 7, 16092–16103. [Google Scholar] [CrossRef]
- Kataria, N.; Garg, V. Removal of Congo red and Brilliant green dyes from aqueous solution using flower shaped ZnO nanoparticles. J. Environ. Chem. Eng. 2017, 5, 5420–5428. [Google Scholar] [CrossRef]
- Saini, J.; Garg, V.K.; Gupta, R.K.; Kataria, N. Removal of Orange G and Rhodamine B dyes from aqueous system using hydrothermally synthesized zinc oxide loaded activated carbon (ZnO-AC). J. Environ. Chem. Eng. 2017, 5, 884–892. [Google Scholar] [CrossRef]
- Gautam, R.K.; Chattopadhyaya, M.C. Nanomaterials for Wastewater Remediation; Butterworth-Heinemann: Oxford, UK, 2016. [Google Scholar]
- Norzilah, A.H.; Fakhru’L-Razi, A.; Choong, T.S.Y.; Chuah, A.L. Surface Modification Effects on CNTs Adsorption of Methylene Blue and Phenol. J. Nanomater. 2011, 2011, 495676. [Google Scholar] [CrossRef]
- El-Baz, A.A.A.; Hendy, I.A.; Dohdoh, A.M.; Srour, M.I. Adsorption technique for pollutants removal; current new trends and future challenges–A Review. Egypt. Int. J. Eng. Sci. Technol. 2020, 32, 1–24. [Google Scholar] [CrossRef]
- Kajjumba, G.W.; Emik, S.; Öngen, A. Modelling of Adsorption Kinetic Processes—Errors, Theory and Application. In Advanced Sorption Process Applications; IntechOpen: London, UK, 2019; pp. 1–19. [Google Scholar]
- Al-Ghouti, M.A.; Da’Ana, D.A. Guidelines for the use and interpretation of adsorption isotherm models: A review. J. Hazard. Mater. 2020, 393, 122383. [Google Scholar] [CrossRef] [PubMed]
- Agarry, S.E.; Aworanti, O.A. Kinetics, isothermal and thermodynamic modelling studies of hexavalent chromium ions adsorption from simulated wastewater onto Parkia biglobosa-Sawdust derived acid-steam activated carbon. Appl. J. Environ. Eng. Sci. 2017, 3, 58–76. [Google Scholar]
- Amin, M.T.; Alazba, A.A.; Shafiq, M. Successful Application of Eucalyptus Camdulensis Biochar in the Batch Adsorption of Crystal Violet and Methylene Blue Dyes from Aqueous Solution. Sustainability 2021, 13, 3600. [Google Scholar] [CrossRef]
- Akram, M.; Bhatti, H.N.; Iqbal, M.; Noreen, S.; Sadaf, S. Biocomposite efficiency for Cr (VI) adsorption: Kinetic, equilibrium and thermodynamics studies. J. Environ. Chem. Eng. 2017, 5, 400–411. [Google Scholar] [CrossRef]
- Alam, S.; Khan, M.S.; Umar, A.; Khattak, R.; Rahman, N.U.; Zekker, I.; Burlakovs, J.; Rubin, S.S.D.; Ghangrekar, M.M.; Bhowmick, G.D.; et al. Preparation of Pd–Ni Nanoparticles Supported on Activated Carbon for Efficient Removal of Basic Blue 3 from Water. Water 2021, 13, 1211. [Google Scholar] [CrossRef]
- Roa, K.; Oyarce, E.; Boulett, A.; Alsamman, M.; Oyarzún, D.; Pizarro, G.D.C.; Sánchez, J. Lignocellulose-based materials and their application in the removal of dyes from water: A review. Sustain. Mater. Technol. 2021, 29, e00320. [Google Scholar] [CrossRef]
- Malik, A.; Khan, A.; Shah, N.; Khan, M.S. The Kinetics and Equilibrium Thermodynamics Study on the Removal of Direct Blue and Titan Yellow Dyes from Aqueous Media by Modified Rice Husk Char. Z. Phys. Chem. 2020, 234, 485–503. [Google Scholar] [CrossRef]
- El-Azazy, M.; Dimassi, S.; El-Shafie, A.S.; Issa, A. Bio-Waste Aloe vera Leaves as an Efficient Adsorbent for Titan Yellow from Wastewater: Structuring of a Novel Adsorbent Using Plackett-Burman Factorial Design. Appl. Sci. 2019, 9, 4856. [Google Scholar] [CrossRef]
- Mittal, J.; Ahmad, R.; Mittal, A. Kahwa tea (Camellia sinensis) carbon—A novel and green low-cost adsorbent for the sequestration of titan yellow dye from its aqueous solutions. Desalination Water Treat. 2021, 227, 404–411. [Google Scholar] [CrossRef]
- Rastgordani, M.; Zolgharnein, J. Simultaneous Determination and Optimization of Titan Yellow and Reactive Blue 4 Dyes Removal Using Chitosan@hydroxyapatite Nanocomposites. J. Polym. Environ. 2021, 29, 1789–1807. [Google Scholar] [CrossRef]
- Abubakar, A.R.; Haque, M. Preparation of medicinal plants: Basic extraction and fractionation procedures for experimental purposes. J. Pharm. Bioallied Sci. 2020, 12, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Jeong, D.; Park, K.H.; Yu, J.-H.; Jung, S. Efficient Adsorption on Benzoyl and Stearoyl Cellulose to Remove Phenanthrene and Pyrene from Aqueous Solution. Polymers 2018, 10, 1042. [Google Scholar] [CrossRef] [PubMed]
- Bassyouni, M.; Hasan, S.W.U. The Use of Rice Straw and Husk Fibers as Reinforcements in Composites. In Biofiber Reinforcements in Composite Materials; Elsevier: Amsterdam, The Netherlands, 2015; pp. 385–422. [Google Scholar]
- Tsuji, W.; Nakao, T.; Ohigashi, K.; Maegawa, K.; Kobayashi, N.; Shukri, S.; Kasai, S.; Miyanaga, K. Chemical modification of cotton fiber by alkali-swelling and substitution reactions—Acetylation, cyanoethylation, benzoylation, and oleoylation. J. Appl. Polym. Sci. 1986, 32, 5175–5192. [Google Scholar] [CrossRef]

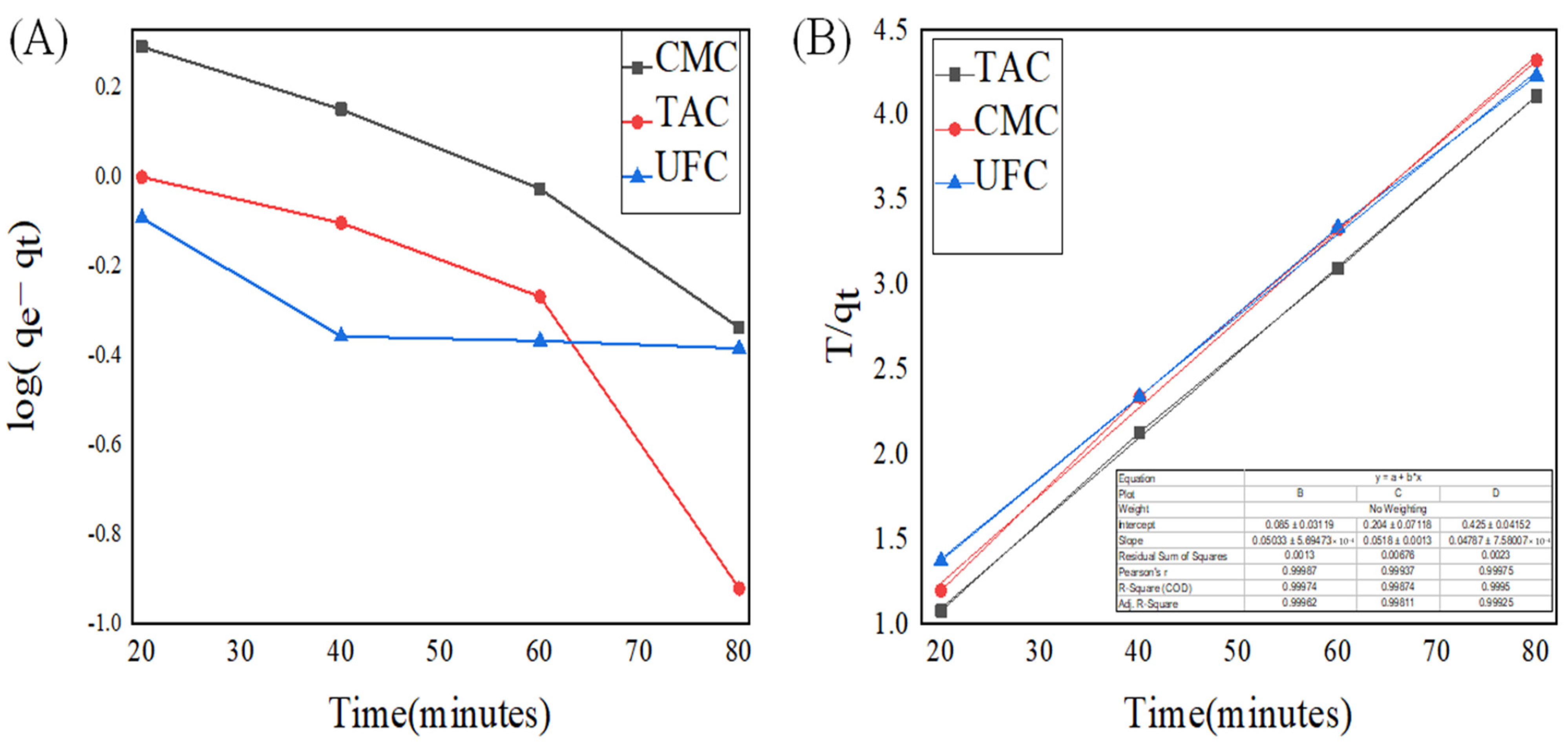

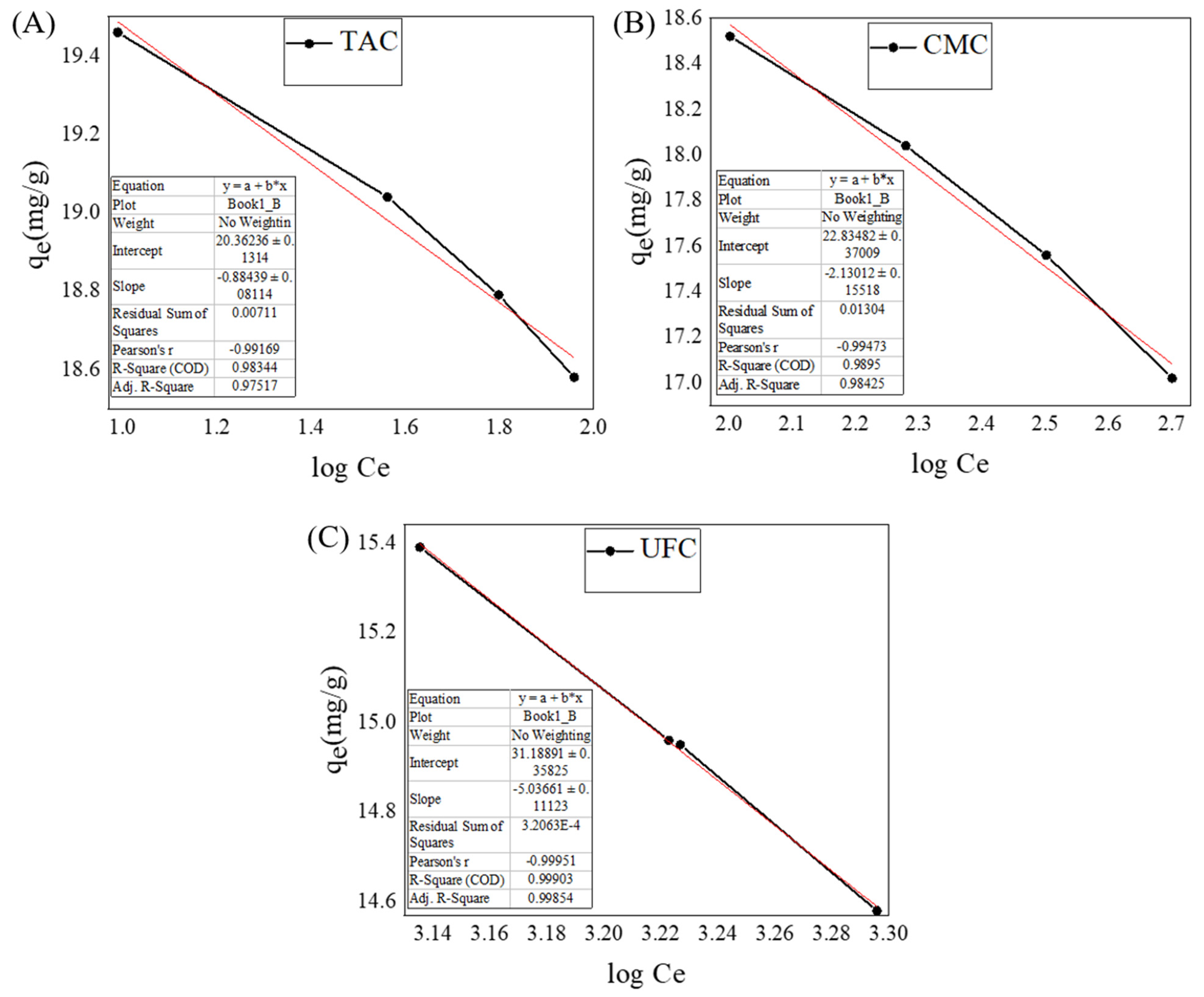
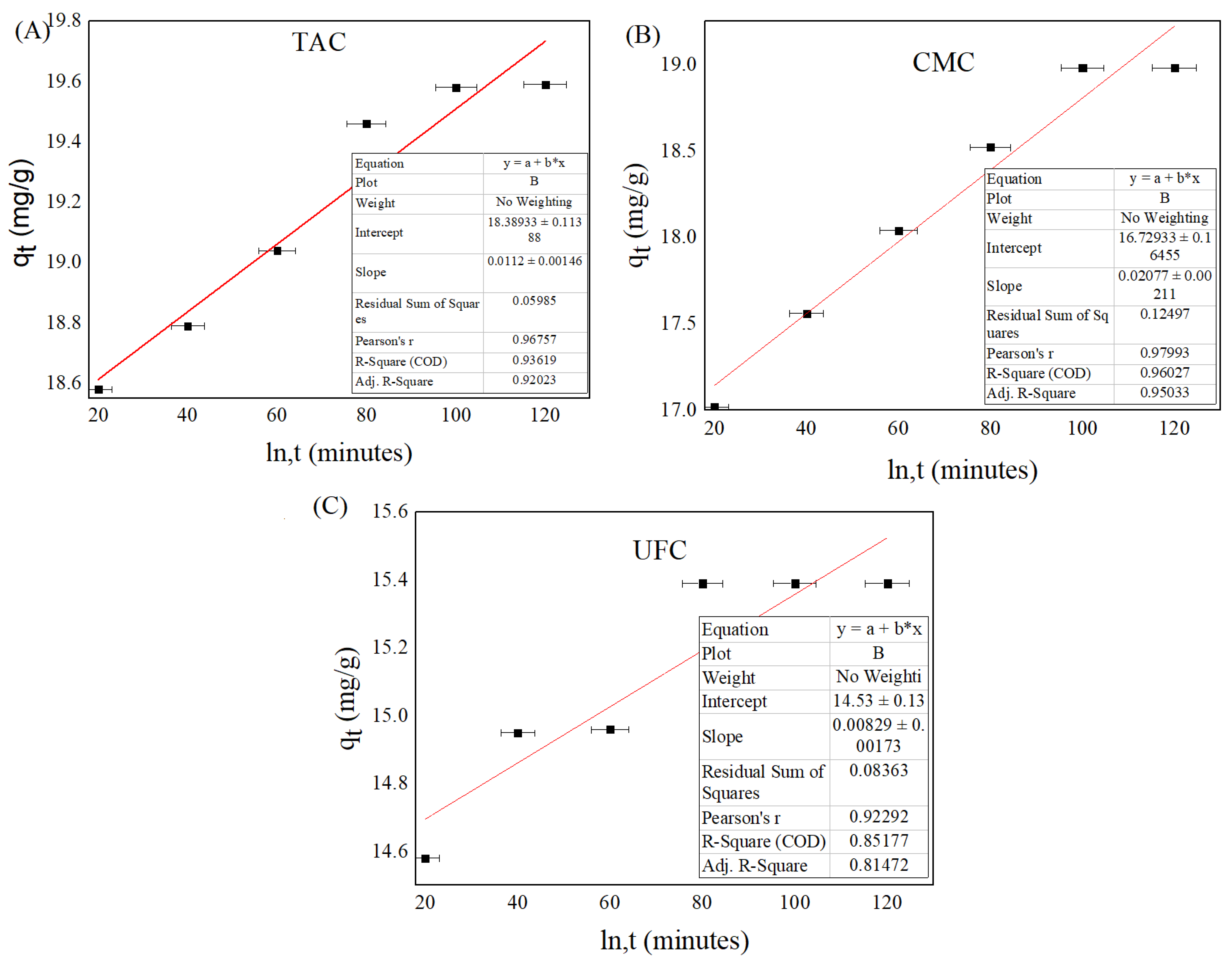
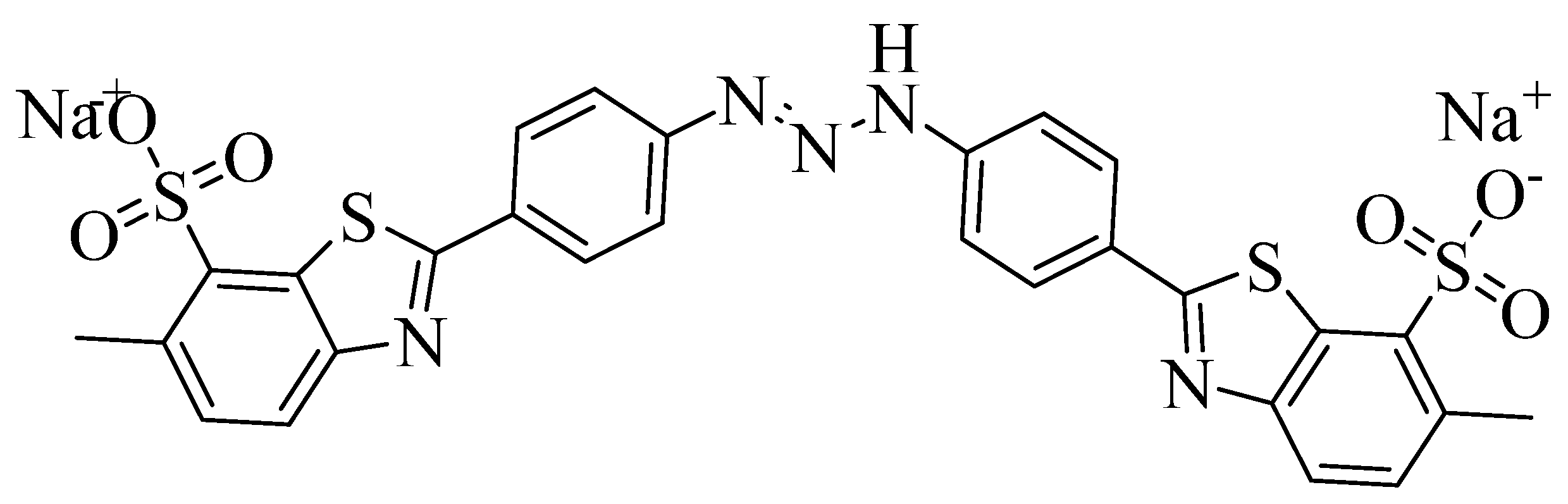
| Adsorbent | Pseudo-First Order | Pseudo-Second Order | Experimental qe | ||||
|---|---|---|---|---|---|---|---|
| k1 (min−1) | R2 | qe (mg/g) | k2 (g/mg/min) | R2 | qe (mg/g) | (mg/g) | |
| TAC | 0.0336 | 0.834 | 2.566 | 0.0297 | 0.999 | 19.88 | 19.59 |
| CMC | 0.0237 | 0.964 | 3.439 | 0.0157 | 0.998 | 19.15 | 18.98 |
| UFC | 0.0155 | 0.033 | 1.008 | 0.0024 | 0.906 | 18.00 | 15.39 |
| Adsorption Isotherm Models | Parameters | TAC | CMC | UFC |
|---|---|---|---|---|
| Langmuir | qm (mg/g) | 19.56 | 20.16 | 19.99 |
| KL (L mg−1) | 3.663 | 1.138 | 0.012 | |
| R2 | 0.990 | 0.999 | 0.999 | |
| Freundlich | n | 23.58 | 8.298 | 0.0003 |
| KF (L mg−1) | 751.1 | 390.9 | 44.30 | |
| R2 | 0.818 | 0.998 | 0.985 | |
| Temkin | b (j/mol) | −0.384 | −0.924 | −2.187 |
| KKT (L/mg) | −23.00 | −10.72 | −6.191 | |
| R2 | 0.983 | 0.989 | 0.999 | |
| Elovich | α (mg/g min) | 18.389 | 16.729 | 14.530 |
| β (g/mg) | 0.0112 | 0.0207 | 0.0082 | |
| R2 | 0.936 | 0.960 | 0.851 |
| Temperature (°C) | ΔG (kJ/mol) | Distribution Coefficient (Keq or Kd) | ||||
|---|---|---|---|---|---|---|
| TAC | CMC | UFC | TAC | CMC | UFC | |
| 20 | −6.706 | −4.993 | −2.443 | 15.674 | 7.771 | 2.728 |
| 30 | −6.907 | −5.189 | −2.725 | 15.528 | 7.849 | 2.951 |
| 40 | −7.062 | −6.013 | −2.974 | 15.103 | 10.086 | 3.139 |
| 50 | −7.124 | −6.622 | −3.274 | 14.197 | 11.787 | 3.434 |
| 60 | −7.220 | −7.375 | −3.413 | 13.577 | 14.360 | 3.434 |
| ΔH kJ/mol | ΔS kJ/mol.K | |||||
| TAC | CMC | UFC | TAC | CMC | UFC | |
| 3.107 | 13.35 | 4.824 | 0.012 | 0.062 | 0.025 | |
| Adsorbent | Dye | Experimental Conditions | Adsorption Capacity (mg/g) | Reference |
|---|---|---|---|---|
| Modified cellulose lignin | TY | Adsorbent dose: 0.1 g, t: 80 min, Conc: 100 ppm, Sol: 50 mL, T: 25 °C, pH: 9 | 17.72 | [28] |
| Rice husk modified (KMRHC) | TY | Adsorbent dose 0.1 g, t: 90 min, Conc: 100 ppm, Sol: 50 mL, T: 40 °C, pH: 9 | 28.02 | [29] |
| Aloe vera leaves wastes-based sulfuric acid modified activated carbon (AV-SAC) | TY | Adsorbent dose: 0.1 g, t: 100 min, Conc: 100 ppm, Sol: 50 mL, T: 40 °C, pH: 8 | 11.92 | [30] |
| Kahwa tea (KTC) | TY | Adsorbent dose: 0.1 g, t: 180 min, Conc: 100 ppm, Sol: 50 mL, T: 40 °C, pH: 5 | 7.04 | [31] |
| Chitosan hydroxyapatite nanocomposites (CNNc) | TY | Adsorbent dose: 0.01 g, t: 60 min, Conc: 100 ppm, Sol: 50 mL, T: 25 °C, pH: 7 | 14.5 | [32] |
| Thermal Activated Cellulose (TAC) | TY | Adsorbent dose: 0.1 g, t: 80 min, Conc: 100 ppm, Sol: 50 mL, T: 25 °C, pH: 7 | 19.46 | This study |
| Un-Functionalized Cellulose (UFC) | TY | Adsorbent dose: 0.1 g, t: 80 min, Conc: 100 ppm, Sol: 50 mL, T: 25 °C, pH: 7 | 15.39 | This study |
| Chemically Modified Cellulose (CMC) | TY | Adsorbent dose: 0.1 g, t: 80 min, Conc: 100 ppm, Sol: 50 mL, T: 25 °C, pH: 7 | 18.52 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahman, U.U.; Humayun, M.; Khan, A.; Farooq, S.; Sadiq, M.; Bououdina, M.; Shah, N. Thermo-Chemical Modification of Cellulose for the Adsorptive Removal of Titan Yellow from Wastewater. Molecules 2023, 28, 3955. https://doi.org/10.3390/molecules28093955
Rahman UU, Humayun M, Khan A, Farooq S, Sadiq M, Bououdina M, Shah N. Thermo-Chemical Modification of Cellulose for the Adsorptive Removal of Titan Yellow from Wastewater. Molecules. 2023; 28(9):3955. https://doi.org/10.3390/molecules28093955
Chicago/Turabian StyleRahman, Ubaid Ur, Muhammad Humayun, Abbas Khan, Saima Farooq, Muhammad Sadiq, Mohamed Bououdina, and Nasrullah Shah. 2023. "Thermo-Chemical Modification of Cellulose for the Adsorptive Removal of Titan Yellow from Wastewater" Molecules 28, no. 9: 3955. https://doi.org/10.3390/molecules28093955
APA StyleRahman, U. U., Humayun, M., Khan, A., Farooq, S., Sadiq, M., Bououdina, M., & Shah, N. (2023). Thermo-Chemical Modification of Cellulose for the Adsorptive Removal of Titan Yellow from Wastewater. Molecules, 28(9), 3955. https://doi.org/10.3390/molecules28093955









