Heavy Metals, Their Phytotoxicity, and the Role of Phenolic Antioxidants in Plant Stress Responses with Focus on Cadmium: Review
Abstract
1. Introduction
2. Main Plant Groups in Terms of HM Tolerance
3. Inactivation of HMs in Plants
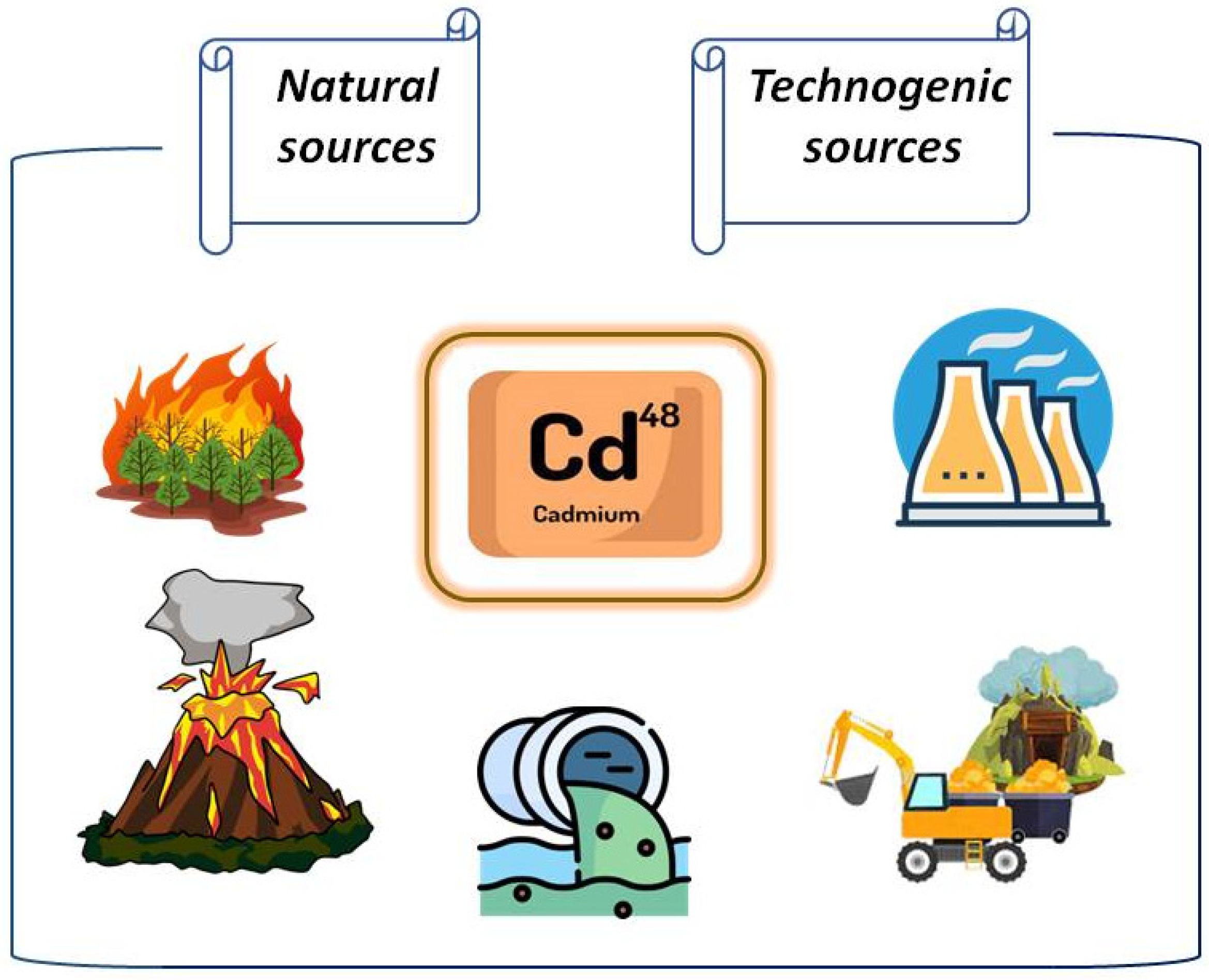
4. Cd, Its Uptake by Plants and Interaction with Other Metals
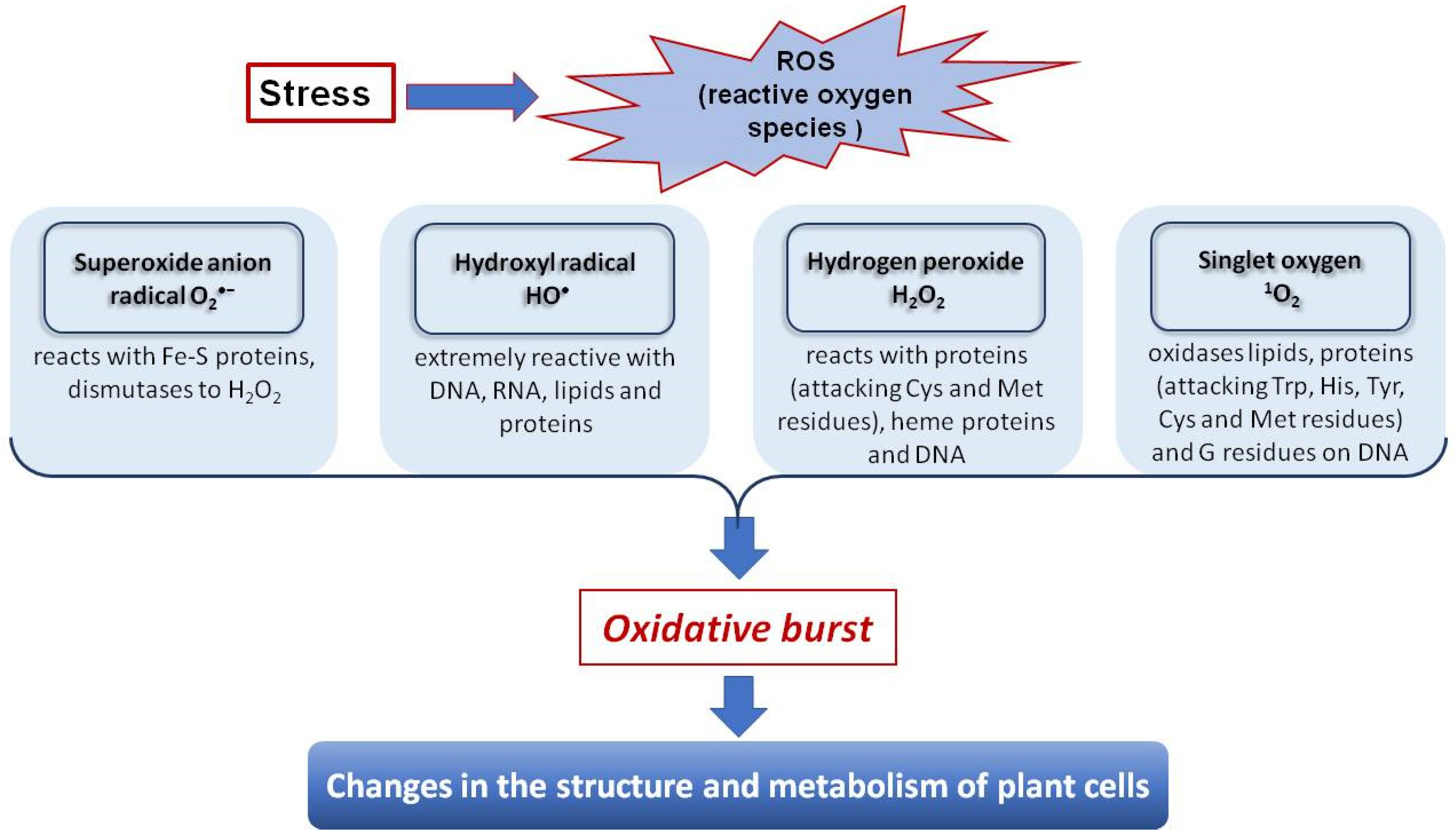
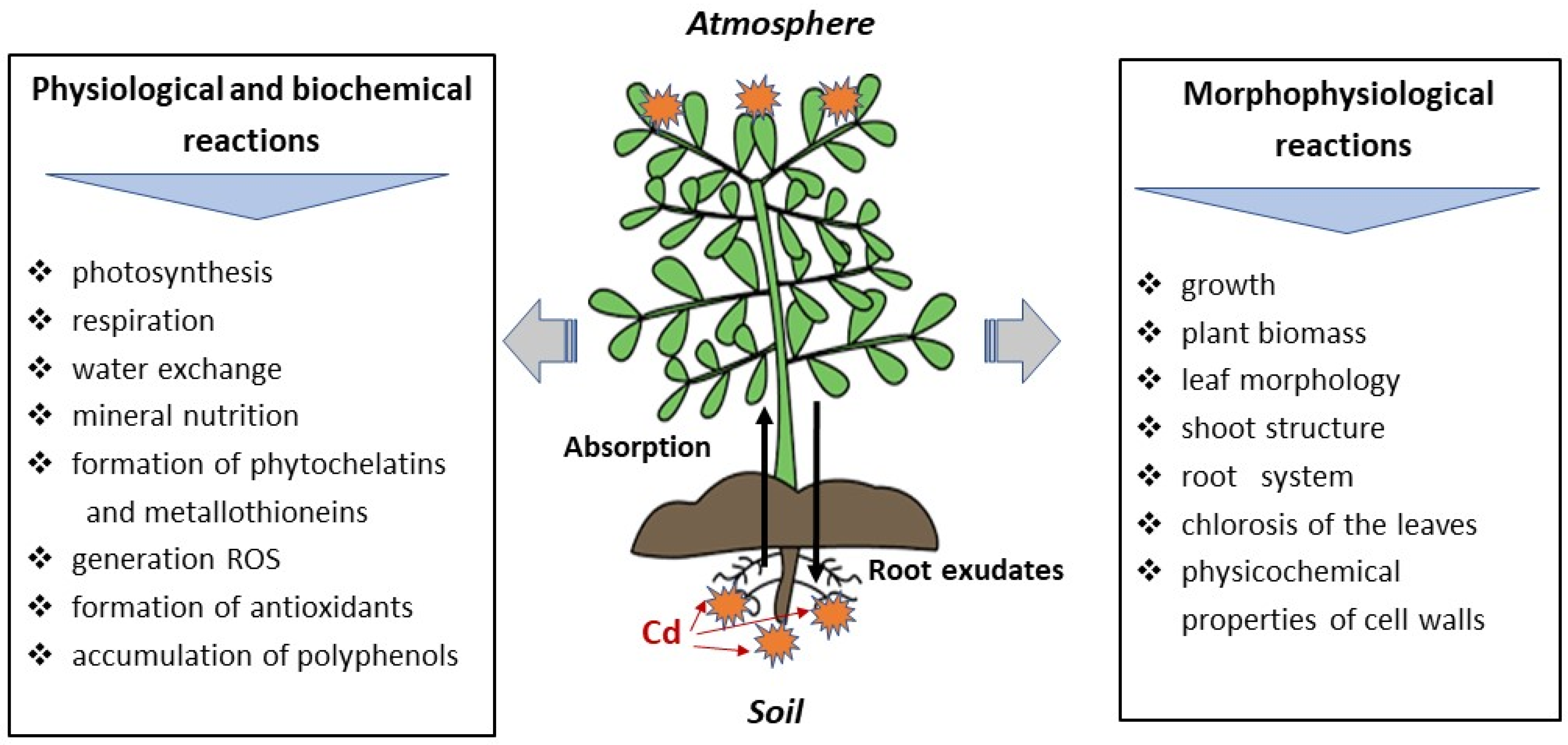
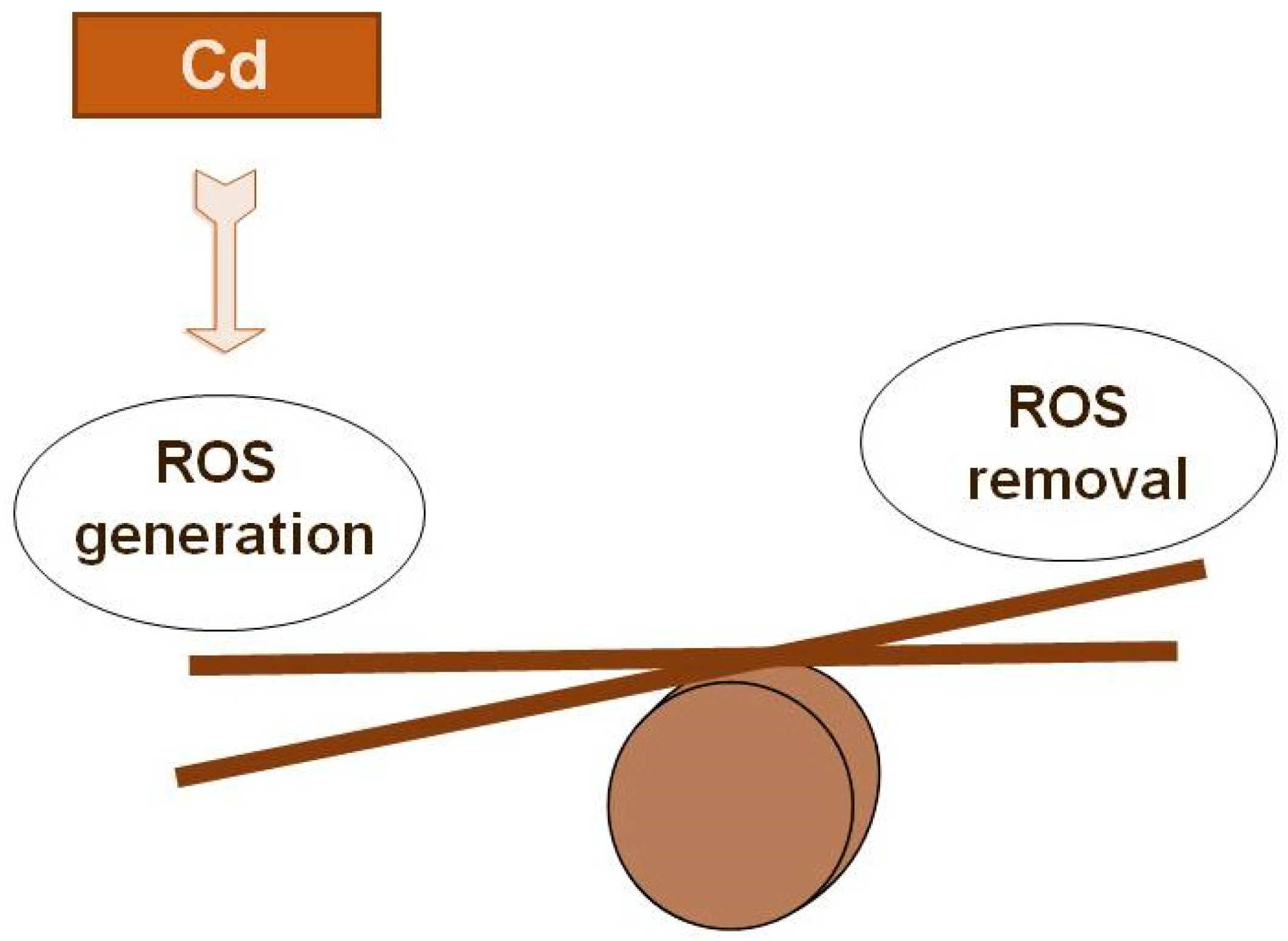
5. The Effect of Cd on Physiological and Biochemical Processes in Plants
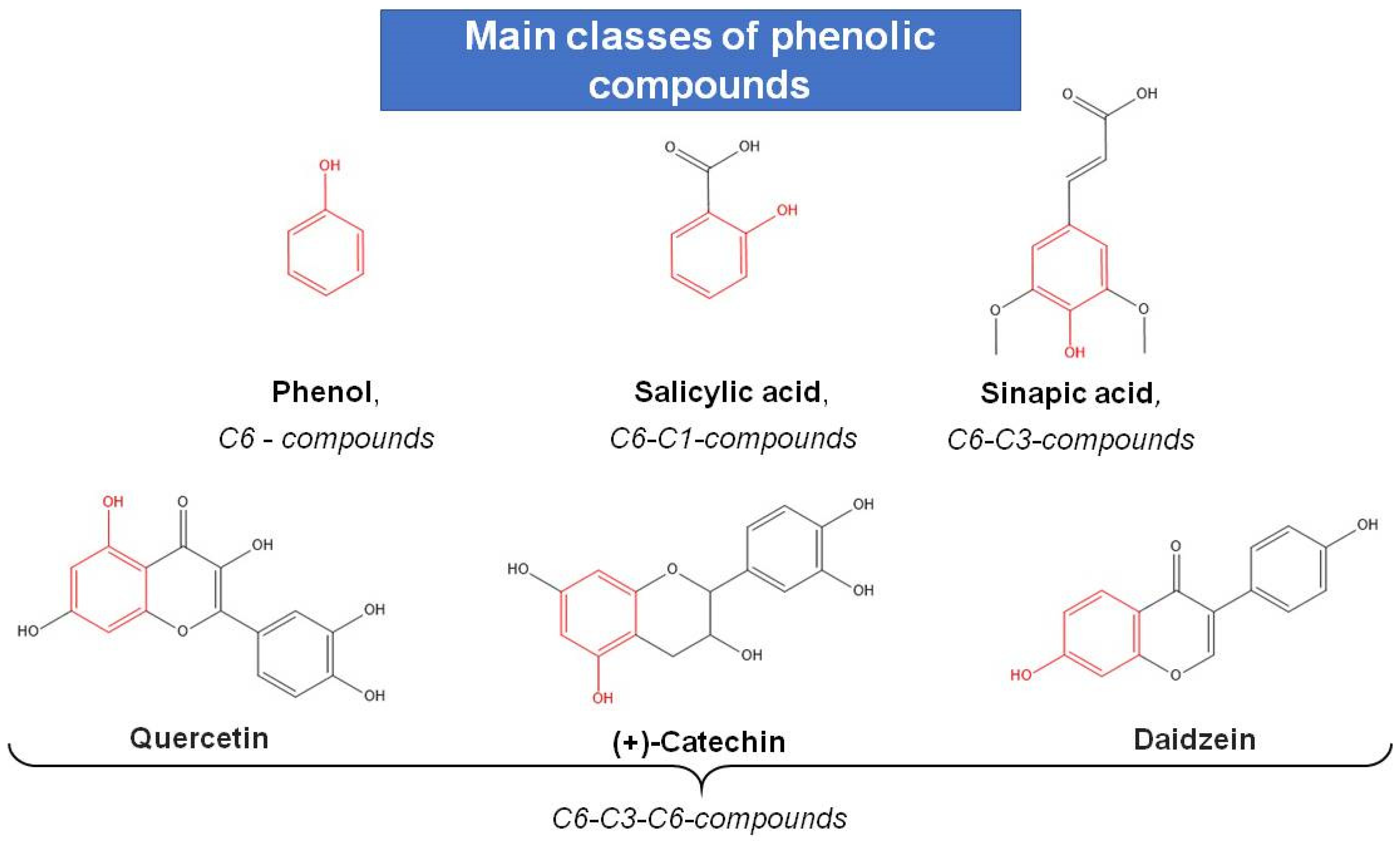
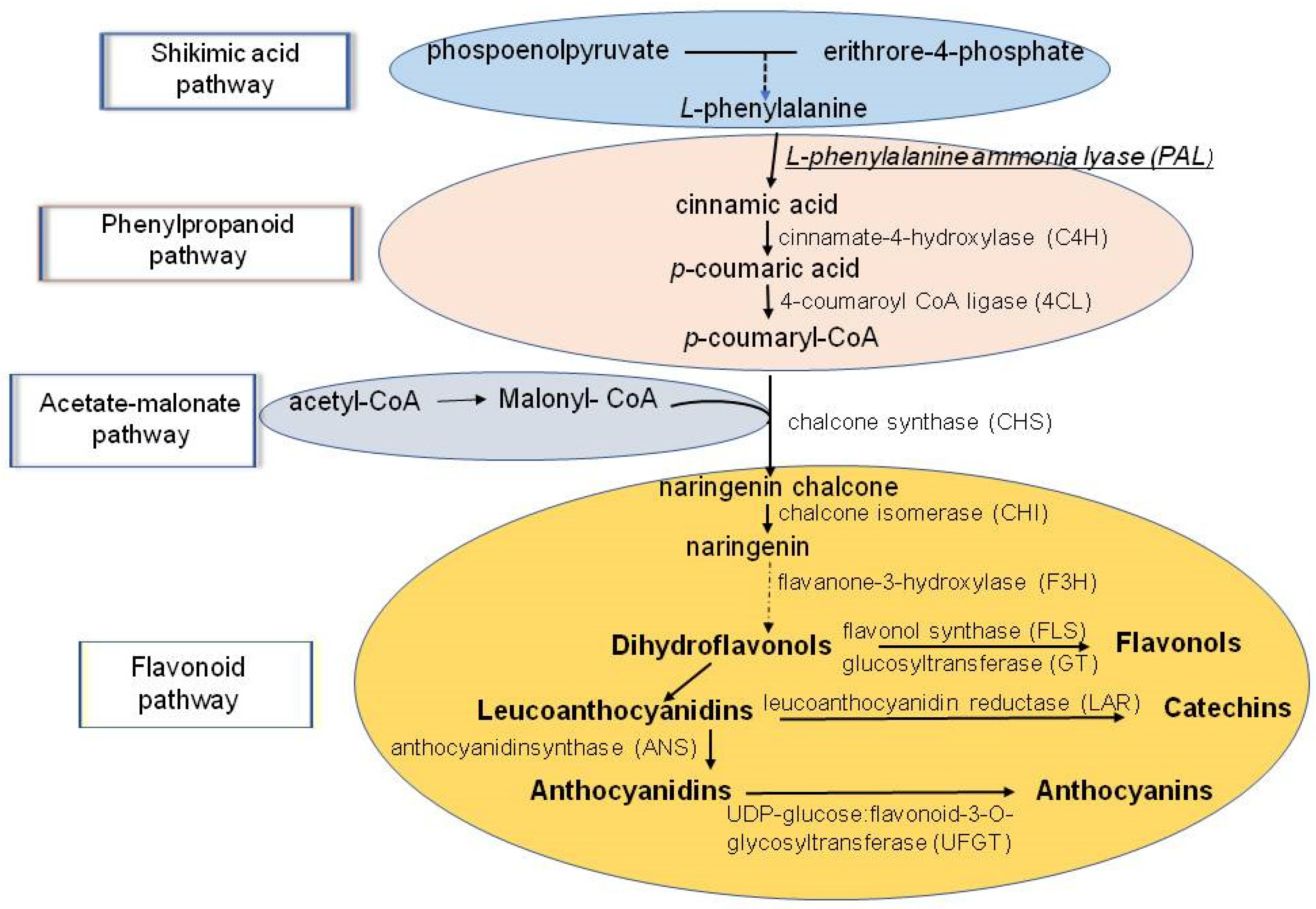
6. Phenolic Antioxidants (AO) and Their Role in Stress-Coping Strategies
| Plant Species | Plants’ Organs | Concentration Cd | PC | PC Level | Reference |
|---|---|---|---|---|---|
| Matricaria chamomill | Roots, shoots | 4.5 and 16.5 mg Cd/kg soil | Total PC | Increase | [177] |
| Malva parviflora | Roots, shoots | 40 μM Cd | Total PC, flavonoids | Increase | [179] |
| Vaccinium corymbosum | In vitro plantlets | 50 and 100 μM Cd | Total PC, chlorogenic acid | Increase | [176] |
| Linum usitatissimum | Callus culture | 15 мг/л Cd | Total PC | Increase | [57] |
| Camellia sinensis | Callus culture | 25 мг/л Cd | Total PC, flavans | Increase | [180] |
| Prosopis glandulosa | Leaf | 0.001 M Cd | Total PC | Decrease | [183] |
| Gallic, vanillic, and caffeic acids, rutin, and kaempferol-3-O-glucosides | Increase | ||||
| Withania somnifera | Aboveground organs of seedlings | 100 and 300 µM Cd | Total PC, flavonoids | Increase | [178] |
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Koller, M.; Saleh, H.M. Introductory chapter: Introducing heavy metals. In Heavy Metals, 2nd ed.; Saleh, H.M., Aglan, R., Eds.; IntechOpen Limited: London, UK, 2018; Volume 1, pp. 3–11. [Google Scholar] [CrossRef]
- Zhang, H.; Reynolds, M. Cadmium exposure in living organisms: A short review. Sci. Total Environ. 2019, 678, 761–767. [Google Scholar] [CrossRef] [PubMed]
- Maqsood, Q.; Hussain, N.; Mumtaz, M.; Bilal, M.; Iqbal, H.M.N. Novel strategies and advancement in reducing heavy metals from the contaminated environment. Arch. Microbiol. 2022, 204, 478. [Google Scholar] [CrossRef]
- Muszyńska, E.; Labudda, M. Dual role of metallic trace elements in stress biology—From negative to beneficial impact on plants. Int. J. Mol. Sci. 2019, 20, 3117. [Google Scholar] [CrossRef]
- Cimboláková, I.; Uher, I.; Lakticova, K.; Vargová, M.; Kimáková, T.; Papajová, I. Heavy metals and the environment. Environ. Factors Affect. Hum. Health 2020, 10, 29–58. [Google Scholar] [CrossRef]
- Jaiswal, A.; Verma, A.; Jaiswal, P. Detrimental effects of heavy metals in soil, plants, and aquatic ecosystems and in humans. J. Environ. Pathol. Toxicol. Oncol. 2018, 37, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Ghori, N.H.; Ghori, T.; Hayat, M.Q.; Imadi, S.R.; Gul, A.; Altay, V.; Ozturk, M. Heavy metal stress and responses in plants. Int. J. Environ. Sci. Technol. 2019, 16, 1807–1828. [Google Scholar] [CrossRef]
- Wang, W.; Chen, D.; Zhang, X.; Liu, D.; Cheng, Y.; Shen, F. Role of plant respiratory burst oxidase homologs in stress responses. Free Radic. Res. 2018, 52, 826–839. [Google Scholar] [CrossRef] [PubMed]
- Vodianitskii Iu, N. On hazardous heavy metals/metalloids in soils. Bull. Soil Inst. VV Dokuchaev. 2011, 68, 56–81. [Google Scholar] [CrossRef]
- McLaughlin, M.J.; Smolders, E.; Zhao, F.J.; Grant, C.; Montalvo, D. Chapter One Managing cadmium in agricultural systems. In Advances in Agronomy; Sparks, D., Ed.; Elesevier: Newark, DE, USA, 2021; Volume 166, pp. 1–129. [Google Scholar] [CrossRef]
- Fosu-Mensah, B.Y.; Addae, E.; Yirenya-Tawiah, D.; Nyame, F. Heavy metals concentration and distribution in soils and vegetation at Korle Lagoon area in Accra, Ghana. Cogent Environ. Sci. 2017, 3, 1405887. [Google Scholar] [CrossRef]
- Latif, A.; Bilal, M.; Asghar, W.; Azeem, M.; Ahmad, M.I.; Abbas, A.; Ahmad, M.Z.; Shahzad, T. Heavy metal accumulation in vegetables and assessment of their potential health risk. Int. J. Environ. Anal. Chem. 2018, 5, 2380–2391. [Google Scholar] [CrossRef]
- Lata, S.; Kaur, H.P.; Mishra, T. Cadmium bioremediation: A review. Int. J. Pharm. Sci. Res. 2019, 10, 4120–4128. [Google Scholar] [CrossRef]
- Robards, K.; Worsfold, P. Cadmium: Toxicology and analysis. A review. Analyst 1991, 116, 549–568. [Google Scholar] [CrossRef]
- Yunus, K.; Zuraidah, M.A.; John, A. A review on the accumulation of heavy metals in coastal sediment of Peninsular Malaysia. Ecofem. Clim. Chang. 2020, 1, 21–35. [Google Scholar] [CrossRef]
- Ulrich, A.E. Cadmium governance in Europe’s phosphate fertilizers: Not so fast? Sci. Total Environ. 2019, 650, 541–545. [Google Scholar] [CrossRef] [PubMed]
- Samrane, K.; Bouhaouss, A. Cadmium in phosphorous fertilizers: Balance and trends. Rasayan J. Chem. 2022, 15, 2103–2117. [Google Scholar] [CrossRef]
- Li, B.; Chen, Z.; Li, Y.; Yang, W.; Wang, W. Visualization analysis of graphene and its composites for heavy metal wastewater applications. Environ. Sci. Pollut. Res. 2019, 26, 27752–27760. [Google Scholar] [CrossRef]
- Nunes, N.; Ragonezi, C.; Gouveia, C.S.; Pinheiro de Carvalho, M.Â. Review of sewage sludge as a soil amendment in relation to current international guidelines: A heavy metal perspective. Sustainability 2021, 13, 2317. [Google Scholar] [CrossRef]
- Alamrani, N.A.; Almutairi, F.M.; Alatawi, N.M.; Mogharbel, A.T.; Al-Aoh, H.A.; Hajri, A.K.; Keshk, A.A.; Elsayed, N.H. Assessment and management of heavy metals pollution in Tabuk region Saudi Arabia, improvement for future development: A review. Wulfenia 2022, 29, 32–51. [Google Scholar]
- Li, H.; Watson, J.; Zhang, Y.; Lu, H.; Liu, Z. Environment-enhancing process for algal wastewater treatment, heavy metal control and hydrothermal biofuel production: A critical review. Bioresour. Technol. 2020, 298, 122421. [Google Scholar] [CrossRef]
- Qin, S.; Liu, H.; Nie, Z.; Rengel, Z.; Gao, W.; Li, C.; Zhao, P. Toxicity of cadmium and its competition with mineral nutrients for uptake by plants: A review. Pedosphere 2020, 30, 168–180. [Google Scholar] [CrossRef]
- Ivanov, A.A.; Kosobryukhov, A.A. Ecophysiology of plants under cadmium toxicity: Photosynthetic and physiological responses. In Plant Ecophysiology and Adaptation under Climate Change: Mechanisms and Perspectives I.; Springer: Singapore, 2020; pp. 429–484. [Google Scholar] [CrossRef]
- Zulfiqar, U.; Ayub, A.; Hussain, S.; Waraich, E.A.; El-Esawi, M.A.; Ishfaq, M.; Ahmad, M.; Ali, M.; Maqsood, M.F. Cadmium toxicity in plants: Recent progress on morpho-physiological effects and remediation strategies. J. Soil Sci. Plant Nutr. 2022, 22, 212–269. [Google Scholar] [CrossRef]
- Khaliq, M.A.; James, B.; Chen, Y.H.; Saqib, H.S.A.; Li, H.H.; Jayasuriya, P.; Guo, W. Uptake, translocation, and accumulation of Cd and its interaction with mineral nutrients (Fe, Zn, Ni, Ca, Mg) in upland rice. Chemosphere 2019, 215, 916–924. [Google Scholar] [CrossRef]
- Suhani, I.; Sahab, S.; Srivastava, V.; Singh, R.P. Impact of cadmium pollution on food safety and human health. Curr. Opin. Toxicol. 2021, 27, 1–7. [Google Scholar] [CrossRef]
- Baker, A.J.M.; McGrath, S.P.; Reeves, R.D.; Smith, J.A.C. Metal hyperaccumulator plants: A review of the ecology and physiology of a biological resource for phytoremediation of metal-polluted soils. In Phytoremediation of Contaminated Soil and Water; Terry, N., Banuelos, G., Eds.; Phytoremediation of contaminated soils; Lewis Publisher: London, UK, 2020; pp. 85–197. [Google Scholar] [CrossRef]
- Seregin, I.V.; Kozhevnikova, A.D. Low-molecular-weight ligands in plants: Role in metal homeostasis and hyperaccumulation. Photosyn. Res. 2021, 150, 51–96. [Google Scholar] [CrossRef] [PubMed]
- Hasnaoui, S.E.; Fahr, M.; Keller, C.; Levard, C.; Angeletti, B.; Chaurand, P.; Smouni, A. Screening of native plants growing on a Pb/Zn mining area in eastern Morocco: Perspectives for phytoremediation. Plants 2020, 9, 1458. [Google Scholar] [CrossRef] [PubMed]
- Yan, A.; Wang, Y.; Tan, S.N.; Mohd Yusof, M.L.; Ghosh, S.; Chen, Z. Phytoremediation: A promising approach for revegetation of heavy metal-polluted land. Front. Plant Sci. 2020, 11, 359. [Google Scholar] [CrossRef]
- Bech, J. Special Issue on “Metallophytes for soil remediation”—Preface. Environ. Geochem. Health 2021, 43, 1319–1325. [Google Scholar] [CrossRef]
- Devi, R.; Behera, B.; Raza, M.B.; Mangal, V.; Altaf, M.A.; Kumar, R.; Singh, B. An insight into microbes mediated heavy metal detoxification in plants: A review. J. Soil Sci. Plant Nutr. 2022, 22, 914–936. [Google Scholar] [CrossRef]
- Mathys, W. Enzymes of heavy-metal-resistant and non-resistant populations of Silene cucubalus and their interaction with some heavy metals in vitro and in vivo. Physiol. Plant 1975, 33, 161–165. [Google Scholar] [CrossRef]
- Arnetoli, M.; Vooijs, R.; Gonnelli, C.; Gabbrielli, R.; Verkleij, J.A.; Schat, H. High-level Zn and Cd tolerance in Silene paradoxa L. from a moderately Cd-and Zn-contaminated copper mine tailing. Environ. Pollut. 2008, 156, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Lolkema, P.C.; Vooijs, R. Copper tolerance in Silene cucubalus. Planta 1986, 167, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Koszelnik-Leszek, A.; Bielecki, K. Physiological responses of nonmetallicolous and serpentine Silene vulgaris ecotypes cultivated in different soils. Environ. Prot. Eng. 2021, 47, 5–15. [Google Scholar] [CrossRef]
- Feki, K.; Tounsi, S.; Mrabet, M.; Mhadhbi, H.; Brini, F. Recent advances in physiological and molecular mechanisms of heavy metal accumulation in plants. Environ. Sci. Pollut. Res. 2021, 28, 64967–64986. [Google Scholar] [CrossRef]
- Yaashikaa, P.R.; Kumar, P.S.; Jeevanantham, S.; Saravanan, R. A review on bioremediation approach for heavy metal detoxification and accumulation in plants. Environ. Pollut. 2022, 301, 119035. [Google Scholar] [CrossRef] [PubMed]
- Zalewska, T.; Danowska, B. Marine environment status assessment based on macrophytobenthic plants as bio-indicators of heavy metals pollution. Mar. Pollut. Bull. 2017, 118, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Phillips, D.P.; Human, L.R.D.; Adams, J.B. Wetland plants as indicators of heavy metal contamination. Mar. Pollut. Bull. 2015, 92, 227–232. [Google Scholar] [CrossRef]
- Miramand, P.; Bentley, D. Heavy metal concentrations in two biological indicators (Patella vulgata and Fucus serratus) collected near the French nuclear fuel reprocessing plant of La Hague. Sci. Total Environ. 1992, 111, 135–149. [Google Scholar] [CrossRef]
- Sheoran, V.; Sheoran, A.S.; Poonia, P. Factors affecting phytoextraction: A review. Pedosphere 2016, 26, 148–166. [Google Scholar] [CrossRef]
- McGrath, S.P.; Zhao, F.J. Phytoextraction of metals and metalloids from contaminated soils. Curr. Opin. Biotechnol. 2003, 14, 277–282. [Google Scholar] [CrossRef] [PubMed]
- García-Sánchez, M.; Košnář, Z.; Mercl, F.; Aranda, E.; Tlustoš, P.A. Comparative study to evaluate natural attenuation, mycoaugmentation, phytoremediation, and microbial-assisted phytoremediation strategies for the bioremediation of an aged PAH-polluted soil. Ecotoxicol. Environ. Saf. 2018, 147, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.; Hadi, F. Phytoremediation of cadmium improved with the high production of endogenous phenolics and free proline contents in Parthenium hysterophorus plant treated exogenously with plant growth regulator and chelating agent. Environ. Sci. Pollut. Res. 2015, 22, 13305–13318. [Google Scholar] [CrossRef] [PubMed]
- Bali, A.S.; Sidhu, G.P.S.; Kumar, V. Root exudates ameliorate cadmium tolerance in plants: A review. Environ. Chem. Lett. 2020, 18, 1243–1275. [Google Scholar] [CrossRef]
- Rodrigues, M.; Ganança, J.F.T.; da Silva, E.M.; dos Santos, T.M.; Slaski, J.J.; Zimny, J.; Pinheiro de Carvalho, M.Â. Evidences of organic acids exudation in aluminium stress responses of two Madeiran wheat (Triticum aestivum L.) landraces. Genet. Resour. Crop. Evol. 2019, 66, 857–869. [Google Scholar] [CrossRef]
- Shrivastav, P.; Prasad, M.; Singh, T.B.; Yadav, A.; Goyal, D.; Ali, A.; Dantu, P.K. Role of nutrients in plant growth and development. In Contaminants in Agriculture; Springer: Cham, Switzerland, 2020; pp. 43–59. ISBN 978-3-030-41551-8. [Google Scholar]
- Dar, M.I.; Naikoo, M.I.; Green, I.D.; Sayeed, N.; Ali, B.; Khan, F.A. Heavy metal hyperaccumulation and hypertolerance in Brassicaceae. In Plants under Metal and Metalloid Stress; Springer: Singapore, 2018; pp. 263–276. [Google Scholar]
- Kaznina, N.M.; Titov, A.F. The influence of cadmium on physiological processes and productivity of Poaceae plants. Biol. Bull. Russ. Acad. Sci. 2014, 4, 335–348. [Google Scholar] [CrossRef]
- Sidhu, G.P.S.; Bali, A.S.; Bhardwaj, R. Role of organic acids in mitigating cadmium toxicity in plants. In Cadmium Tolerance in Plants; Academic Press: Cambridge, MA, USA, 2019; pp. 255–279. [Google Scholar] [CrossRef]
- Katarína, K.; Elena, M.; Josef, J. Plant responses to stress induced by toxic metals and their nanoforms. In Handbook of Plant and Crop Stress, 4th ed.; CRC Press: Boca Raton, FL, USA, 2019; pp. 479–522. [Google Scholar] [CrossRef]
- Dueck, T.A.; Wolting, H.G.; Moet, D.R.; Pasman, F.J.M. Growth and reproduction of Silene cucubalus Wib. intermittently exposed to low concentrations of air pollutants, zinc and copper. New Phytol. 1987, 105, 633–645. [Google Scholar] [CrossRef]
- Yadav, V.; Arif, N.; Kováč, J.; Singh, V.P.; Tripathi, D.K.; Chauhan, D.K.; Vaculík, M. Structural modifications of plant organs and tissues by metals and metalloids in the environment: A review. Plant Physiol. Biochem. 2021, 159, 100–112. [Google Scholar] [CrossRef]
- Steveninck, V.R.F.M.; Steveninck, V.M.E.; Fernando, D.R.; Horst, W.J.; Marschner, H. Deposition of zinc phytate in globular bodies in roots of Deschampsia caespitosa ecotypes; a detoxification mechanism? J. Plant Physiol. 1987, 131, 247–257. [Google Scholar] [CrossRef]
- Haiying, Y.; Guo, J.; Li, Q.; Zhang, X.; Huang, H.; Huang, F.; Li, T. Characteristics of cadmium immobilization in the cell wall of root in a cadmium-safe rice line (Oryza sativa L.). Chemosphere 2020, 241, 125095. [Google Scholar] [CrossRef]
- Goncharuk, E.A.; Zagoskina, N.V. Reaction of cells of long-lived flax varieties with contrasting resistance to the action of cadmium ions. Bulletin of Kharkiv National Agrarian University. Ser. Biol. 2016, 3, 27–38. [Google Scholar]
- Šamec, D.; Karalija, E.; Šola, I.; Bok, V.V.; Salopek-Sondi, B. The role of polyphenols in abiotic stress response: The influence of molecular structure. Plants 2021, 10, 118. [Google Scholar] [CrossRef]
- Sterckeman, T.; Thomine, S. Mechanisms of cadmium accumulation in plants. Crit. Rev. Plant Sci. 2020, 39, 322–359. [Google Scholar] [CrossRef]
- Kosakivska, I.V.; Babenko, L.M.; Romanenko, K.O.; Korotka, I.Y.; Potters, G. Molecular mechanisms of plant adaptive responses to heavy metals stress. Int. J. Cell Biol. 2021, 45, 258–272. [Google Scholar] [CrossRef]
- Hasan, M.K.; Cheng, Y.; Kanwar, M.K.; Chu, X.Y.; Ahammed, G.J.; Qi, Z.Y. Responses of plant proteins to heavy metal stress—A review. Front. Plant Sci. 2017, 8, 1492. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.J.; Tang, Z.; Song, J.J.; Huang, X.Y.; Wang, P. Toxic metals and metalloids: Uptake, transport, detoxification, phytoremediation, and crop improvement for safer food. Mol. Plant 2022, 15, 27–44. [Google Scholar] [CrossRef]
- Martí-Guillén, J.M.; Pardo-Hernández, M.; Martínez-Lorente, S.E.; Almagro, L.; Rivero, R.M. Redox post-translational modifications and their interplay in plant abiotic stress tolerance. Front. Plant Sci. 2022, 13, 1027730. [Google Scholar] [CrossRef]
- Ahammed, G.J.; Li, C.X.; Li, X.; Liu, A.; Chen, S.; Zhou, J. Overexpression of tomato RING E3 ubiquitin ligase gene SlRING1 confers cadmium tolerance by attenuating cadmium accumulation and oxidative stress. Physiol. Plant. 2020, 173, 449–459. [Google Scholar] [CrossRef]
- Dou, X.; Dai, H.; Twardowska, I.; Wei, S. Hyperaccumulation of Cd by Rorippa globosa (Turcz.) Thell. from soil enriched with different Cd compounds, and impact of soil amendment with glutathione (GSH) on the hyperaccumulation efficiency. Environ. Pollut. 2019, 255, 113270. [Google Scholar] [CrossRef] [PubMed]
- Gieroń, Ż.; Sitko, K.; Zieleźnik-Rusinowska, P.; Szopiński, M.; Rojek-Jelonek, M.; Rostański, A.; Rudnicka, M.; Małkowski, E. Ecophysiology of Arabidopsis arenosa, a new hyperaccumulator of Cd and Zn. J. Hazard. Mater. 2021, 412, 125052. [Google Scholar] [CrossRef] [PubMed]
- Pradedova, E.V.; Nimaeva, O.D.; Salyaev, R.K. Redox processes in biological systems. Russ. J. Plant Physiol. 2017, 64, 822–832. [Google Scholar] [CrossRef]
- Hendrix, S.; Jozefczak, M.; Wójcik, M.; Deckers, J.; Vangronsveld, J.; Cuypers, A. Glutathione: A key player in metal chelation, nutrient homeostasis, cell cycle regulation and the DNA damage response in cadmium-exposed Arabidopsis thaliana. Plant Physiol. Biochem. 2020, 154, 498–507. [Google Scholar] [CrossRef]
- Kaznina, N.; Batova, Y.; Repkina, N.; Laidinen, G. Cadmium treatment effects on the growth and antioxidant system in barley plants under optimal and low temperatures. Acta Agric. Slov. 2018, 111, 169–176. [Google Scholar] [CrossRef]
- Li, S.; Han, X.; Lu, Z.; Qiu, W.; Yu, M.; Li, H.; He, Z.; Zhuo, R. MAPK Cascades and Transcriptional Factors: Regulation of Heavy Metal Tolerance in Plants. Int. J. Mol. Sci. 2022, 23, 4463. [Google Scholar] [CrossRef] [PubMed]
- He, S.Y.; He, Z.L.; Yang, X.E.; Stoella, P.J.; Baligar, V.C. Soil biogeochemistry, plant physiology, and phytoremediation of cadmium-contaminated soils. Adv. Agron. 2015, 134, 135–225. [Google Scholar] [CrossRef]
- Tanhan, P.; Kruatrachue, M.; Pokethitiyook, P.; Chaiyarat, R. Uptake and accumulation of cadmium, lead and zinc by Siam weed [Chromolaena odorata (L.) King & Robinson]. Chemosphere 2007, 68, 323–329. [Google Scholar] [CrossRef]
- Sooksawat, N.; Meetam, M.; Kruatrachue, M.; Pokethitiyook, P.; Nathalang, K. Phytoremediation potential of charophytes: Bioaccumulation and toxicity studies of cadmium, lead and zinc. J. Environ. Sci. 2013, 25, 596–604. [Google Scholar] [CrossRef] [PubMed]
- Tauqeer, H.M.; Ali, S.; Rizwan, M.; Ali, Q.; Saeed, R.; Iftikhar, U.; Ahmad, R.; Farid, M.; Abbasi, G.H. Phytoremediation of heavy metals by Alternanthera bettzickiana: Growth and physiological response. Ecotoxicol. Environ. Saf. 2016, 126, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Seth, C.S.; Misra, V.; Chauhan, L.K.S.; Singh, R.R. Genotoxicity of cadmium on root meristem cells of Allium cepa: Cytogenetic and Comet assay approach. Ecotoxicol. Environ. Saf. 2008, 71, 711–716. [Google Scholar] [CrossRef]
- Hussain, A.; Ali, S.; Rizwan, M.; Zia-ur-Rehman, M. Morphological and physiological responses of plants to cadmium toxicity. In Cadmium Toxicity and Tolerance in Plants; Hasanuzzaman, M., Prasad, M.N.V., Fujita, M., Eds.; Elsever: London, UK, 2019; pp. 47–72. [Google Scholar]
- Madhu, P.M.; Sadagopan, R.S. Effect of heavy metals on growth and development of cultivated plants with reference to cadmium, chromium and lead–a review. J. Stress Physiol. Biochem. 2020, 16, 84–102. [Google Scholar]
- Lux, A.; Martinka, M.; Vaculík, M.; White, P.J. Root responses to cadmium in the rhizosphere: A review. J. Exp. Bot. 2011, 62, 21–37. [Google Scholar] [CrossRef]
- Yang, W.; Wu, F.; Ding, Z.; Zhang, X.; Zhao, F.; Wang, Y.; Yang, X. Cadmium accumulation and tolerance in seven ornamental willow genotypes. Bull. Environ. Contam. Toxicol. 2018, 101, 644–650. [Google Scholar] [CrossRef]
- Kumar, P.; Goud, E.L.; Devi, P.; Dey, S.R.; Dwivedi, P. Heavy Metals: Transport in Plants and Their Physiological and Toxicological Effects. In Plant Metal and Metalloid Transporters; Kumar, K., Srivastava, S., Eds.; Springer: Singapore, 2022. [Google Scholar] [CrossRef]
- Bano CAmist, N.; Singh, N.B. Morphological and Anatomical Modifications of Plants for Environmental Stresses. In Molecular Plant Abiotic Stress; Roychoudhury, A., Tripathi, D., Eds.; Wiley Online Library: Hoboken, NJ, USA, 2019; pp. 29–44. [Google Scholar] [CrossRef]
- Amirahmadi, E.; Ghorbani, M.; Moudrý, J. Effects of Zeolite on Aggregation, Nutrient Availability, and Growth Characteristics of Corn (Zea mays L.) in Cadmium-Contaminated Soils. Water Air Soil Pollut. 2022, 233, 436. [Google Scholar] [CrossRef]
- Weijie, X.; Shuzhen, H.; Khan, M.A.; Yu, C.; Linlin, X.; Zebin, R.; Liu, H.; Zhenhua, C.; Shengwei, C.; Ye, Z.; et al. Effect of water and fertilization management on Cd immobilization and bioavailability in Cd-polluted paddy soil. Chemosphere 2021, 276, 130168. [Google Scholar] [CrossRef]
- Zhang, Z.; Rengel, Z.; Meney, K. Cadmium accumulation and translocation in four emergent wetland species. Water Air Soil Pollut. 2010, 212, 239–249. [Google Scholar] [CrossRef]
- Su, Y.Y.; Cheng, Y.Q.; Qin, C.; Ahmed, N.; Mu, Y.H.; Mustafad, N.S.; Ashraf, M.; Zhang, L.X. Exogenous acetylcholine alleviates cadmium-induced phytotoxicity by modulating photosynthetic metabolism and antioxidant potential in tobacco (Nicotiana benthamiana). Photosynthetica 2020, 58, 984–994. [Google Scholar] [CrossRef]
- Aqeel, M.; Khalid, N.; Tufail, A.; Ahmad, R.Z.; Akhter, M.S.; Luqman, M.; Javed, M.T.; Irshad, M.K.; Alamri, S.; Hashem, M.; et al. Elucidating the distinct interactive impact of cadmium and nickel on growth, photosynthesis, metal-homeostasis, and yield responses of mung bean (Vigna radiata L.) varieties. Environ. Sci. Pollut. Res. 2021, 28, 27376–27390. [Google Scholar] [CrossRef]
- Adil, M.F.; Sehar, S.; Han, Z.; Lwalaba, J.L.W.; Jilani, G.; Zeng, F.; Chen, Z.-H.; Shamsi, I.H. Zinc alleviates cadmium toxicity by modulating photosynthesis, ROS homeostasis, and cation flux kinetics in rice. Environ. Pollut. 2020, 265, 114979. [Google Scholar] [CrossRef]
- Du, J.; Zeng, J.; Ming, X.; He, Q.; Tao, Q.; Jiang, M.; Gao, S.; Li, X.; Lei, T.; Pan, Y.; et al. The presence of zinc reduced cadmium uptake and translocation in Cosmos bipinnatus seedlings under cadmium/zinc combined stress. Plant Physiol. Biochem. 2020, 151, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Lasat, M.M.; Kochian, L.V. Physiology of Zn hyperaccumulation in Thlaspi caerulescens. In Phytoremediation of Contaminated Soil and Water; Terry, N., Bañuelos, G., Eds.; Lewis Publishers: Boca Raton, FL, USA, 2000; pp. 159–169. [Google Scholar]
- Sytar, O.; Ghosh, S.; Malinska, H.; Zivcak, M.; Brestic, M. Physiological and molecular mechanisms of metal accumulation in hyperaccumulator plants. Physiol. Plant Copy 2021, 173, 148–166. [Google Scholar] [CrossRef] [PubMed]
- Guidi Nissim, W.; Palm, E.; Mancuso, S.; Azzarello, E. Trace element phytoextraction from contaminated soil: A case study under Mediterranean climate. Environ. Sci. Pollut. Res. 2018, 25, 9114–9131. [Google Scholar] [CrossRef] [PubMed]
- García-Gómez, C.; Fernández, M.D. Impacts of metal oxide nanoparticles on seed germination, plant growth and development. Compr. Anal. Chem. 2019, 84, 75–124. [Google Scholar] [CrossRef]
- Zou, R.; Wang, L.; Li, Y.C.; Tong, Z.; Huo, W.; Chi, K.; Fan, H. Cadmium absorption and translocation of amaranth (Amaranthus mangostanus L.) affected by iron deficiency. Environ. Pollut. 2020, 256, 113410. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Chen, W.; He, X. Evaluation of hyperaccumulation potentials to cadmium (Cd) in six ornamental species (compositae). Int. J. Phytoremediat. 2018, 20, 1464–1469. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.; Liu, Y.; Liu, G.; Guo, Y.; Yang, Q.; Shi, J.; Hu, L.; Liang, Y.; Yin, Y.; Cai, Y.; et al. Aging and phytoavailability of newly introduced and legacy cadmium in paddy soil and their bioaccessibility in rice grain distinguished by enriched isotope tracing. J. Hazard. Mater. 2021, 417, 125998. [Google Scholar] [CrossRef]
- Kaur, B.; Singh, B.P.; Devashree, Y. Heavy Metal Sequestration in Plants. In Heavy Metals in Plants Physiological to Molecular Approach; CRC Press: Boca Raton, FL, USA, 2022; pp. 215–245. [Google Scholar] [CrossRef]
- Naeem, A.; Zafar, M.; Khalid, H.; Zia-ur-Rehman, M.; Ahmad, Z.; Ayub, M.A.; Qayyum, M.F. Cadmium-Induced Imbalance in Nutrient and Water Uptake by Plants. In Cadmium Toxicity and Tolerance in Plants; Elsevier: London, UK, 2019; pp. 299–326. [Google Scholar] [CrossRef]
- Urazgildin, R.V.; Kulagin, A.Y. Damage, Adaptations, and Strategies of Tree Species in Technogenesis Conditions: Structural and Functional Levels of Realization of Adaptive Potential. Biol. Bull. Russ. Acad. Sci. 2022, 12, 441–457. [Google Scholar] [CrossRef]
- Wani, K.I.; Zehra, A.; Choudhary, S.; Naeem, M.; Aftab, T. Cadmium, a Nonessential Heavy Metal: Uptake, Translocation, Signaling, Detoxification, and Impact on Amino Acid Metabolism. In Plant Metal and Metalloid Transporters; Springer: Singapore, 2022; pp. 73–89. [Google Scholar] [CrossRef]
- Hatamian, M.; Nejad, A.R.; Kafi, M.; Souri, M.K.; Shahbazi, K. Growth characteristics of ornamental Judas tree (Cercis siliquastrum L.) seedling under different concentrations of lead and cadmium in irrigation water. Acta Sci. Pol. Hort Cultus 2019, 18, 87–96. [Google Scholar] [CrossRef]
- Kozhevnikova, A.D.; Seregin, I.V.; Aarts, M.G.M.; Schat, H. Intra-specific variation in zinc, cadmium and nickel hypertolerance and hyperaccumulation capacities in Noccaea caerulescens. Plant Soil 2020, 452, 479–498. [Google Scholar] [CrossRef]
- Popa, C.; Petrus, M.; Bratu, A.M. Alfalfa (Medicago sativa) Sprouts Respiratory Responses to Cadmium Stress Using IR LPAS. Molecules 2022, 27, 1891. [Google Scholar] [CrossRef]
- Yan, L.; Zhou, N.; Guo, X.; Dong, Q.; Gu, W.; Wang, K.; Yang, Y. Nutrition and Safety Evaluation of Hydroponic-cultured Pea SproutunderLead and Cadmium Stress. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2020; Volume 474, p. 22031. [Google Scholar] [CrossRef]
- Mostofa, M.G.; Rahman, M.M.; Ansary, M.M.U.; Fujita, M.; Phan, L.-S. Tran Interactive effects of salicylic acid and nitric oxide in enhancing rice tolerance to cadmium stress. Int. J. Mol. Sci. 2019, 20, 5798. [Google Scholar] [CrossRef]
- Fattahi, B.; Arzani, K.; Souri, M.K.; Barzegar, M. Effects of cadmium and lead on seed germination, morphological traits, and essential oil composition of sweet basil (Ocimum basilicum L.). Ind. Crops Prod. 2019, 138, 111584. [Google Scholar] [CrossRef]
- Szopiński, M.; Szopiński, M.; Sitko, K.; Gieroń, Ż.; Rusinowski, S.; Corso, M.; Hermans, C.; Verbruggen, N.; Małkowski, E. Toxic effects of Cd and Zn on the photosynthetic apparatus of the Arabidopsis halleri and Arabidopsis arenosa pseudo-metallophytes. Front. Plant Sci. 2019, 10, 748. [Google Scholar] [CrossRef]
- An, M.J.; Wang, H.; Fan, H.; Ippolito, J.A.; Meng, C.; Yulian, E.; Li, Y.; Wang, K.; Wei, C. Effects of modifiers on the growth, photosynthesis, and antioxidant enzymes of cotton under cadmium toxicity. J. Plant Growth Regul. 2019, 38, 1196–1205. [Google Scholar] [CrossRef]
- Grajek, H.; Rydzyński, D.; Piotrowicz-Cieślak, A.; Herman, A.; Maciejczyk, M.; Wieczorek, Z. Cadmium ion-chlorophyll interaction–Examination of spectral properties and structure of the cadmium-chlorophyll complex and their relevance to photosynthesis inhibition. Chemosphere 2020, 261, 127434. [Google Scholar] [CrossRef] [PubMed]
- Bansal, P.; Sharma, P. Effect of Pb2+ and Cd2+ on respiration and mitochondrial electron transport chain in germinating pea seeds (Pisum sativum L.). Indian J. Environ. Ecoplann. 2000, 3, 249–254, ISSN 00063134. [Google Scholar]
- Janeeshma, E.; Kalaji, H.M.; Puthur, J.T. Differential responses in the photosynthetic efficiency of Oryza sativa and Zea mays on exposure to Cd and Zn toxicity. Acta Physiol. Plant. 2021, 43, 12. [Google Scholar] [CrossRef]
- Li, X.; Li, Y.; Zhu, X.; Gui, X.; Ma, C.; Peng, W.; Li, Y.; Zhang, Y.; Huang, W.; Hua, D.; et al. Evaluation of the cadmium phytoextraction potential of tobacco (Nicotiana tabacum) and rhizosphere micro-characteristics under different cadmium levels. Chemosphere 2022, 286, 131714. [Google Scholar] [CrossRef] [PubMed]
- Özyiğit, İ.İ.; Baktibekova, D.; Hocaoglu-Ozyigit, A.; Kurmanbekova, G.; Chekirov, K.; Yalcin, I.E. The effects of cadmium on growth, some anatomical and physiological parameters of wheat (Triticum aestivum L.). Int. J. Life Sci. Biotechnol. 2021, 4, 235–253. [Google Scholar] [CrossRef]
- Kalai, T.; Chaoui, A.; Khamassi, K.; Jaime, A.; Silva, T.; Naceur, M.B.B.; Gouia, H.; Ben-Kaab, L.B. Cadmium and copper stress affect seedling growth and enzymatic activities in germinating barley seeds. Arch. Agron. Soil Sci. 2014, 60, 765–783. [Google Scholar] [CrossRef]
- Banerjee, A.; Roychoudhury, A. Explicating the cross-talks between nanoparticles, signaling pathways and nutrient homeostasis during environmental stresses and xenobiotic toxicity for sustainable cultivation of cereals. Chemosphere 2021, 286, 131827. [Google Scholar] [CrossRef]
- Zaid, A.; Mohammad, F.; Fariduddin, Q. Plant growth regulators improve growth, photosynthesis, mineral nutrient and antioxidant system under cadmium stress in menthol mint (Mentha arvensis L.). Physiol. Mol. Biol. Plants 2020, 26, 25–39. [Google Scholar] [CrossRef]
- Hussain, S.; Khaliq, A.; Noor, M.A.; Tanvee, M.; Hussain, H.A.; Hussain, S.; Shah, T.; Mehmood, T. Metal toxicity and nitrogen metabolism in plants: An overview. In The Carbon and Nitrogen Cycling in Soil; Datta, R., Meena, R.S., Pathan, S.I., Ceccherini, M.T., Eds.; Springer: Berlin/Heidelberg, Germany; Nature Singapore Pte Ltd.: Singapore, 2020; pp. 221–248. [Google Scholar] [CrossRef]
- Lebrazi, S.; Fikri-Benbrahim, K. Rhizobium-Legume Symbioses: Heavy metal effects and principal approaches for bioremediation of contaminated soil. In The Legumes for Soil Health and Sustainable Management; Meena, R.S., Yadav, A.D.G.S., Eds.; Springer: Berlin/Heidelberg, Germany; Nature Singapore Pte Ltd.: Singapore, 2018; pp. 205–233. [Google Scholar] [CrossRef]
- Haider, F.U.; Liqun, C.; Coulter, J.A.; Cheema, S.A.; Wu, J.; Zhang, R.; Wenjun, M.; Farooq, M. Cadmium toxicity in plants: Impacts and remediation strategies. Ecotoxicol. Environ. Saf. 2021, 211, 111887. [Google Scholar] [CrossRef]
- Juan, C.A.; Pérez de la Lastra, J.M.; Plou, F.J.; Pérez-Lebeña, E. The chemistry of reactive oxygen species (ROS) revisited: Outlining their role in biological macromolecules (DNA, lipids and proteins) and induced pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef]
- Mittler, R.; Zandalinas, S.I.; Fichman, Y.; Van Breusegem, F. Reactive oxygen species signalling in plant stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 663–679. [Google Scholar] [CrossRef]
- Sachdev, S.; Ansari, S.A.; Ansari, M.I.; Fujita, M.; Hasanuzzaman, M. Abiotic stress and reactive oxygen species: Generation, signaling, and defense mechanisms. Antioxidants 2021, 10, 277. [Google Scholar] [CrossRef]
- Fu, Y.; Mason, A.S.; Zhang, Y.; Lin, B.; Xiao, M.; Fu, D.; Yu, H. MicroRNA-mRNA expression profiles and their potential role in cadmium stress response in Brassica napus. BMC Plant Biol. 2019, 19, 570. [Google Scholar] [CrossRef]
- Azimychetabi, Z.; Nodehi, M.S.; Moghadam, T.K.; Motesharezadeh, B. Cadmium stress alters the essential oil composition and the expression of genes involved in their synthesis in peppermint (Mentha piperita L.). Ind. Crops Prod. 2021, 168, 113602. [Google Scholar] [CrossRef]
- Bamagoos, A.A.; Alharby, H.F.; Abbas, G. Differential uptake and translocation of cadmium and lead by Quinoa: A multivariate comparison of physiological and oxidative stress responses. Toxics 2022, 10, 68. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Aziz, U.; Sahli, A.A.; Alyemeni, M.N.; Ahmad, P. Combined kinetin and spermidine treatments ameliorate growth and photosynthetic inhibition in Vigna angularis by up-regulating antioxidant and nitrogen metabolism under cadmium stress. Biomolecules 2020, 10, 147. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef] [PubMed]
- Eseberri, I.; Trepiana, J.; Léniz, A.; Gómez-García, I.; Carr-Ugarte, H.; González, M.; Portillo, M.P. Variability in the beneficial effects of phenolic compounds: A review. Nutrients 2022, 14, 1925. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Khan, A.; Ahmad, I.; Alghamdi, S.; Rajab, B.S.; Babalghith, A.O.; Alshahrani, M.Y.; Islam, S.; Islam, M.R. Flavonoids a bioactive compound from medicinal plants and its therapeutic applications. Biomed. Res. Int. 2022, 2022, 5445291. [Google Scholar] [CrossRef]
- Jakubczyk, K.; Dec, K.; Kałduńska, J.; Kawczuga, D.; Kochman, J.; Janda, K. Reactive oxygen species-sources, functions, oxidative damage. Pol. Merkur. Lek. Organ Pol. Tow. Lek. 2020, 48, 124–127. [Google Scholar]
- Sies, H.; Belousov, V.V.; Chandel, N.S.; Davies, M.J.; Jones, D.P.; Mann, G.E.; Murphy, M.P.; Yamamoto, M.; Winterbourn, C. Defining roles of specific reactive oxygen species (ROS) in cell biology and physiology. Nat. Rev. Mol. Cell Biol. 2022, 23, 499–515. [Google Scholar] [CrossRef] [PubMed]
- Locato, V.; De Gara, L. Programmed cell death in plants: An overview. In Plant Programmed Cell Death: Methods and Protocols; De Gara, L., Locato, V., Eds.; Humana Press: New York, NY, USA, 2018; pp. 1–8. ISBN 978-1-4939-7668-3. [Google Scholar] [CrossRef]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of phenylpropanoid pathway and the role of polyphenols in plants under abiotic stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef] [PubMed]
- Kolupaev, Y.E.; Karpets, Y.; Yastreb, T.O.; Shemet, S.A.; Bhardwaj, R. Antioxidant system and plant cross-adaptation against metal excess and other environmental stressors. In Metal Toxicity in Higher Plants; Landi, M., Shemet, S.A., Fedenko, V.S., Eds.; Nova Science Publishers: New York, NY, USA, 2020; pp. 21–67. ISBN 978-1-53616-790-0. [Google Scholar]
- Dumanović, J.; Nepovimova, E.; Natić, M.; Kuča, K.; Jaćević, V. The significance of reactive oxygen species and antioxidant defense system in plants: A concise overview. Front. Plant Sci. 2021, 11, 552969. [Google Scholar] [CrossRef] [PubMed]
- Mongkhonsin, B.; Nakbanpote, W.; Hokura, A.; Nuengchamnong, N.; Maneechai, S. Phenolic compounds responding to zinc and/or cadmium treatments in Gynura pseudochina (L.) DC. extracts and biomass. Plant Physiol. Biochem. 2016, 109, 549–560. [Google Scholar] [CrossRef] [PubMed]
- Belščak-Cvitanović, A.; Durgo, K.; Huđek, A.; Bačun-Družina, V.; Komes, D. Overview of polyphenols and their properties. In Polyphenols: Properties, Recovery, and Applications; Galanakis, C.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 3–44. ISBN 978-0-12-813573-0. [Google Scholar] [CrossRef]
- Rana, B.; Chahal, K. Phenolic Compounds Under Stress. In Plant Metabolites under Environmental Stress, 1st ed.; Hithamani, G., Naveen, J., Pushpalatha, H.G., Eds.; Apple Academic Press: New York, NY, USA, 2023; pp. 203–218. ISBN 9781003304869. [Google Scholar] [CrossRef]
- Olszowy, M. What is responsible for antioxidant properties of polyphenolic compounds from plants? Plant Physiol. Biochem. 2019, 144, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Soto-Hernandez, M.; Garcia-Mateos, R.; Palma-Tenango, M. Plant Physiological Aspects of Phenolic Compounds; BoD—Books on Demand: Nordstedt, Germany, 2019; 120p, ISBN 978-1-78985-640-8. [Google Scholar]
- Platzer, M.; Kiese, S.; Herfellner, T.; Schweiggert-Weisz, U.; Miesbauer, O.; Eisner, P. Common trends and differences in antioxidant activity analysis of phenolic substances using single electron transfer based assays. Molecules 2021, 26, 1244. [Google Scholar] [CrossRef]
- Al-Mamary, M.A.; Moussa, Z. Antioxidant activity: The presence and impact of hydroxyl groups in small molecules of natural and synthetic origin. In Antioxidants—Benefits, Sources, Mechanisms of Action; IntechOpen: London, UK, 2021; pp. 318–377. ISBN 978-1-83968-866-9. [Google Scholar]
- Dias, M.C.; Pinto, D.C.G.A.; Silva, A.M.S. Plant flavonoids: Chemical characteristics and biological activity. Molecules 2021, 26, 5377. [Google Scholar] [CrossRef]
- Santos, S.A.O.; Félix, R.; Pais, A.C.S.; Rocha, S.M.; Silvestre, A.J.D. The quest for phenolic compounds from macroalgae: A review of extraction and identification methodologies. Biomolecules 2019, 9, 847. [Google Scholar] [CrossRef]
- Wen, W.; Alseekh, S.; Fernie, A.R. Conservation and diversification of flavonoid metabolism in the plant kingdom. Curr. Opin. Plant Biol. 2020, 55, 100–108. [Google Scholar] [CrossRef]
- Chalaker-Scott, L.; Fuchigami, L.H. The role of phenolic compounds in plant stress responses. In Low Temperature Stress Physiology in Crops; CRC press: Boca Raton, FL, USA, 2018; pp. 67–80. [Google Scholar] [CrossRef]
- De la Rosa, L.A.; Moreno-Escamilla, J.O.; Rodrigo-García, J.; Alvarez-Parrilla, E. Phenolic compounds. In Postharvest Physiology and Biochemistry of Fruits and Vegetables; Woodhead publishing: Cambridge, UK, 2019; pp. 253–271. [Google Scholar] [CrossRef]
- Shoeva, O.Y.; Khlestkina, E.K. Anthocyanins participate in the protection of wheat seedlings against cadmium stress. Cereal Res. Commun. 2018, 46, 242–252. [Google Scholar] [CrossRef]
- Moazzen, A.; Öztinen, N.; Ak-Sakalli, E.; Koşar, M. Structure-antiradical activity relationships of 25 natural antioxidant phenolic compounds from different classes. Heliyon 2022, 8, e10467. [Google Scholar] [CrossRef]
- Jovanovic, S.V.; Steenken, S.; Tosic, M.; Marjanovic, B.; Simic, M.G. Flavonoids as antioxidants. J. Am. Chem. Soc. 1994, 116, 4846–4851. [Google Scholar] [CrossRef]
- Brunetti, C.; Fini, A.; Sebastiani, F.; Gori, A.; Tattini, M. Modulation of Phytohormone Signaling: A Primary Function of Flavonoids in Plant–Environment Interactions. Front.Plant Sci. 2018, 9, 1042. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.I.; Kim, H.Y.; Kim, J.; Oh, M.-M.; Son, J.E. Quantitative Analysis of UV-B Radiation Interception in 3D Plant Structures and Intraindividual Distribution of Phenolic Contents. Int. J. Mol. Sci. 2021, 22, 2701. [Google Scholar] [CrossRef] [PubMed]
- Golovatskaya, I.F.; Laptev, N.I. Effect of UV-B radiation on plants growth, active constituents, and productivity. In Plants and Their Interaction to Environmental Pollution; Elsevier: Amsterdam, The Netherlands, 2023; pp. 25–60. [Google Scholar] [CrossRef]
- Horn, P.J. Where do the electrons go? How numerous redox processes drive phytochemical diversity: Redox processes in phytochemistry. Phytochem. Rev. 2021, 20, 367–407. [Google Scholar] [CrossRef]
- Igamberdiev, A.U.; Bykova, N.V. Mitochondria in photosynthetic cells: Coordinating redox control and energy balance. Plant Physiol. 2022, 191, 2104–2119. [Google Scholar] [CrossRef]
- Ferreyra, M.L.F.; Serra, P.; Casati, P. Recent advances on the roles of flavonoids as plant protective molecules after UV and high light exposure. Physiol. Plant. 2021, 173, 736–749. [Google Scholar] [CrossRef]
- Tarakhovsky, Y.S.; Kim, Y.A.; Abdrasilov, B.S.; Muzafarov, E.N. Flavonoids: Biochemistry. Biophysics, Medicine; Synchrobook: Pushchino, Russia, 2013. [Google Scholar]
- Juárez-Maldonado, A.; González-Morales, S.; Cabrera-De la Fuente, M.; Medrano-Macías, J.; Benavides-Mendoza, A. Nanometals as promoters of nutraceutical quality in crop plants. In Impact of Nanoscience in Food Industry; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press: London, UK, 2018; pp. 277–310. [Google Scholar] [CrossRef]
- Nobahar, A.; Carlier, J.D.; Miguel, M.G.; Costa, M.C. A review of plant metabolites with metal interaction capacity: A green approach for industrial applications. BioMetals 2021, 34, 761–793. [Google Scholar] [CrossRef]
- Gebre, S.H. Bio-inspired synthesis of metal and metal oxide nanoparticles: The key role of phytochemicals. J. Clust. Sci. 2022, 34, 665–704. [Google Scholar] [CrossRef]
- Anjitha, K.S.; Sameena, P.P.; Puthur, J.T. Functional aspects of plant secondary metabolites in metal stress tolerance and their importance in pharmacology. Plant Stress 2021, 2, 100038. [Google Scholar] [CrossRef]
- Agati, G.; Azzarello, E.; Pollastri, S.; Tattini, M. Flavonoids as antioxidants in plants: Location and functional significance. Plant Sci. 2012, 196, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Slabbert, N.E. Complexation of Condensed Tannins with Metal Ions. In Plant Polyphenols; Hemingway, R.W., Laks, P.E., Eds.; Basic Life Sciences; Springer: Boston, MA, USA, 1992; Volume 1, pp. 421–436. ISBN 978-1-4613-6540-2. [Google Scholar]
- Benzie, I.F.F.; Devaki, M. The ferric reducing/antioxidant power (FRAP) assay for non-enzymatic antioxidant capacity: Concepts, procedures, limitations and applications. In Measurement of Antioxidant Activity and Capacity: Recent Trends and Applications; Apak, R., Capanoglu, E., Shahidi, F., Eds.; Wiley: Oxford, UK, 2018; pp. 77–106. [Google Scholar] [CrossRef]
- Spiegel, M.; Cel, K.; Sroka, Z. The mechanistic insights into the role of pH and solvent on antiradical and prooxidant properties of polyphenols—Nine compounds case study. Food Chem. 2023, 407, 134677. [Google Scholar] [CrossRef]
- Mucha, P.; Skoczyńska, A.; Małecka, M.; Hikisz, P.; Budzisz, E. Overview of the Antioxidant and Anti-Inflammatory Activities of Selected Plant Compounds and Their Metal Ions Complexes. Molecules 2021, 26, 4886. [Google Scholar] [CrossRef] [PubMed]
- Karak, P. Biological Activities of Flavonoids: An Overview. Int. J. Pharm. Sci. Res. 2019, 10, 1567–1574. [Google Scholar] [CrossRef]
- Pulido, R.; Bravo, L.; Saura-Calixto, F. Antioxidant activity of dietary polyphenols as determined by a modified ferric reducing/antioxidant power assay. J. Agric. Food Chem. 2000, 48, 3396–3402. [Google Scholar] [CrossRef]
- Kao, T.H.; Chen, B.H. Functional components in soybean cake and their effects on antioxidant activity. J. Agric. Food Chem. 2006, 54, 7544–7555. [Google Scholar] [CrossRef]
- Eghbaliferiz, S.; Iranshahi, M. Prooxidant activity of polyphenols, flavonoids, anthocyanins and carotenoids: Updated review of mechanisms and catalyzing metals. Phytother. Res. 2016, 30, 1379–1391. [Google Scholar] [CrossRef] [PubMed]
- Corrente, G.A.; Malacaria, L.; Beneduci, A.; Furia, E.; Marino, T.; Mazzone, G. Experimental and theoretical study on the coordination properties of quercetin towards aluminum(III), iron(III) and copper(II) in aqueous solution. J. Mol. Liq. 2021, 325, 115171–115182. [Google Scholar] [CrossRef]
- Karonen, M. Insights into Polyphenol–Lipid Interactions: Chemical Methods, Molecular Aspects and Their Effects on Membrane Structures. Plants 2022, 11, 1809. [Google Scholar] [CrossRef] [PubMed]
- Milić, B.L.; Djilas, S.M.; Canadanovic-Brunet, J.M. Antioxidative activity of phenolic compounds on the metalion breakdown of lipid peroxidation system. Food Chem. 1998, 61, 443–447. [Google Scholar] [CrossRef]
- Pandeya, D.; Campbell, L.M.; Puckhaber, L.; Suh, C.; Rathore, K.S. Gossypol and related compounds are produced and accumulate in the aboveground parts of the cotton plant, independent of roots as the source. Planta 2023, 257, 21. [Google Scholar] [CrossRef] [PubMed]
- Zha, M.; Lian, L.; Wen, M.; Ercisli, S.; Ren, Y.; Jiang, Z.; Zhang, L. The Oxidation Mechanism of Flavan-3-ols by an Enzymatic Reaction Using Liquid Chromatography–Mass Spectrometry-Based Metabolomics Combined with Captured o-Quinone Intermediates of Flavan-3-ols by o-Phenylenediamine. J. Agric. Food Chem. 2022, 70, 5715–5727. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; de Bruijn, W.J.C.; van Zadelhoff, A.; Lin, Z.; Vincken, J.P. Browning of Epicatechin (EC) and Epigallocatechin (EGC) by Auto-Oxidation. J. Agric. Food Chem. 2020, 68, 13879–13887. [Google Scholar] [CrossRef] [PubMed]
- Manquián-Cerda, K.; Cruces, E.; Escudey, M.; Zúñiga, G.; Calderón, R. Interactive effects of aluminum and cadmium on phenolic compounds, antioxidant enzyme activity and oxidative stress in blueberry (Vaccinium corymbosum L.) plantlets cultivated in vitro. Ecotoxicol. Environ. Saf. 2018, 150, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Kováčik, J.; Dresler, S.; Sowa, I.; Babula, P.; Antunes, E. Calcium-enriched biochar modulates cadmium uptake depending on external cadmium dose. Environ. Pollut. 2022, 313, 120178. [Google Scholar] [CrossRef]
- Pandey, A.; Agrawal, M.; Agrawal, S.B. Ultraviolet-B and Heavy Metal-Induced Regulation of Secondary Metabolites in Medicinal Plants: A Review. Metabolites 2023, 13, 341. [Google Scholar] [CrossRef] [PubMed]
- Zoufan, P.; Azad, Z.; Rahnama Ghahfarokhie, A.; Kolahi, M. Modification of Oxidative Stress through Changes in Some Indicators Related to Phenolic Metabolism in Malva Parviflora Exposed to Cadmium. Ecotoxicol. Environ. Saf. 2020, 187, 109811. [Google Scholar] [CrossRef]
- Zubova, M.; Nechaeva, T.; Kartashov, A.; Zagoskina, N. Regulation of the phenolic compounds accumulation in the tea-plant callus culture with a separate and combined effect of light and cadmium ions. Biol. Bull. 2020, 47, 593–604. [Google Scholar] [CrossRef]
- Zagoskina, N.V.; Zubova, M.Y.; Nechaeva, T.L.; Zhivukhina, E.A. Action of cadmium ions on culture in vitro of tea (Camellia Sinensis L.) plant. Visn. Khar’kivs’kogo Natsional’nogo Agrar. Univ. 2015, 3, 29–37. [Google Scholar]
- Jamla, M.; Khare, T.; Joshi, S.; Patil, S.; Penna, S.; Kumar, V. Omics approaches for understanding heavy metal responses and tolerance in plants. Curr. Plant Biol. 2021, 27, 100213. [Google Scholar] [CrossRef]
- González-Mendoza, D.; Troncoso-Rojas, R.; Gonzalez-Soto, T.; Grimaldo-Juarez, O.; Ceceña-Duran, C.; Duran-Hernandez, D.; Gutiérrez-Miceli, F. Changes in the phenylalanine ammonia lyase activity, total phenolic compounds, and flavonoids in Prosopis glandulosa treated with cadmium and copper. An. Acad. Bras. Cienc. 2018, 90, 1465–1472. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Li, J.; Lin, X.; Wang, L.; Yang, X.; Xia, X.; Ke, Q. Changes in plant anthocyanin levels in response to abiotic stresses: A meta-analysis. Plant Biotechnol. Rep. 2022, 16, 497–508. [Google Scholar] [CrossRef]
- Xu, Z.; Rothstei, S.J. ROS-Induced anthocyanin production provides feedback protection by scavenging ROS and maintaining photosynthetic capacity in Arabidopsis. Plant Signal Behav. 2018, 13, e1451708. [Google Scholar] [CrossRef]
- Mukherjee, S.; Chatterjee, N.; Sircar, A.; Maikap, S.; Singh, A.; Acharyya, S.; Paul, S. A Comparative Analysis of Heavy Metal Effects on Medicinal Plants. Appl. Biochem. Biotechnol. 2022, 195, 2483–2518. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, D.; Singh, S.; Kumar, V.; Romero, R.; Prasad, R.; Singh, J. Antioxidant enzymes regulation in plants in reference to reactive oxygen species (ROS) and reactive nitrogen species (RNS). Plant Gene 2019, 19, 100182. [Google Scholar] [CrossRef]
- Bai, S.; Tao, R.; Tang, Y.; Yin, L.; Ma, Y.; Ni, J.; Teng, Y. BBX16, a B-box protein, positively regulates light-induced anthocyanin accumulation by activating MYB10 in red pear. Plant Biotechnol. J. 2019, 17, 1985–1997. [Google Scholar] [CrossRef]
- Naing, A.H.; Kim, C.K. Abiotic stress-induced anthocyanins in plants: Their role in tolerance to abiotic stresses. Physiol. Plant. 2021, 172, 1711–1723. [Google Scholar] [CrossRef] [PubMed]
- Altangerel, N.; Ariunbold, G.O.; Gorman, C.; Alkahtani, M.H.; Borrego, E.J.; Bohlmeyer, D.; Scully, M.O. In vivo diagnostics of early abiotic plant stress response via Raman spectroscopy. Proc. Natl. Acad. Sci. USA 2017, 114, 3393–3396. [Google Scholar] [CrossRef] [PubMed]
- Adzhieva, V.F.; Babak, O.G.; Shoeva, O.Y.; Kilchevsky, A.V.; Khlestkina, E.K. Molecular-genetic mechanisms underlying fruit and seed coloration in plants. Vavilov J. Genet. Breed. 2015, 19, 561–573. [Google Scholar] [CrossRef]
- Glagoleva, A.Y.; Shmakov, N.A.; Shoeva, O.Y.; Vasiliev, G.V.; Shatskaya, N.V.; Börner, A.; Khlestkina, E.K. Metabolic pathways and genes identified by RNA-seq analysis of barley near-isogenic lines differing by allelic state of the Black lemma and pericarp (Blp) gene. BMC Plant Biol. 2017, 17, 182. [Google Scholar] [CrossRef]
- Shoeva, O.Y.; Gordeeva, E.I.; Khlestkina, E.K. The Regulation of Anthocyanin Synthesis in the Wheat Pericarp. Molecules 2014, 19, 20266–20279. [Google Scholar] [CrossRef] [PubMed]
- Chaves-Silva, S.; Dos Santos, A.L.; Chalfun-Júnior, A.; Zhao, J.; Peres, L.E.; Benedito, V.A. Understanding the genetic regulation of anthocyanin biosynthesis in plants–tools for breeding purple varieties of fruits and vegetables. Phytochemistry 2018, 153, 11–27. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Y.; Lu, H.Q.; Jiang, K.X.; Wang, Y.R.; Wang, Y.P.; Jiang, J.J. The Flavonoid Biosynthesis and Regulation in Brassica napus: A Review. Int. J. Mol. Sci. 2023, 24, 357. [Google Scholar] [CrossRef] [PubMed]
- Fahad, S.; Saud, S.; Chen, Y.; Wu, C.; Wang, D. (Eds.) Abiotic Stress in Plants; BoD–Books on Demand: Nordstedt, Germany, 2021; p. 494. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goncharuk, E.A.; Zagoskina, N.V. Heavy Metals, Their Phytotoxicity, and the Role of Phenolic Antioxidants in Plant Stress Responses with Focus on Cadmium: Review. Molecules 2023, 28, 3921. https://doi.org/10.3390/molecules28093921
Goncharuk EA, Zagoskina NV. Heavy Metals, Their Phytotoxicity, and the Role of Phenolic Antioxidants in Plant Stress Responses with Focus on Cadmium: Review. Molecules. 2023; 28(9):3921. https://doi.org/10.3390/molecules28093921
Chicago/Turabian StyleGoncharuk, Evgenia A., and Natalia V. Zagoskina. 2023. "Heavy Metals, Their Phytotoxicity, and the Role of Phenolic Antioxidants in Plant Stress Responses with Focus on Cadmium: Review" Molecules 28, no. 9: 3921. https://doi.org/10.3390/molecules28093921
APA StyleGoncharuk, E. A., & Zagoskina, N. V. (2023). Heavy Metals, Their Phytotoxicity, and the Role of Phenolic Antioxidants in Plant Stress Responses with Focus on Cadmium: Review. Molecules, 28(9), 3921. https://doi.org/10.3390/molecules28093921






