NH2-Modified UiO-66: Structural Characteristics and Functional Properties
Abstract
1. Introduction
2. Results and Discussion
2.1. Structural Characteristics
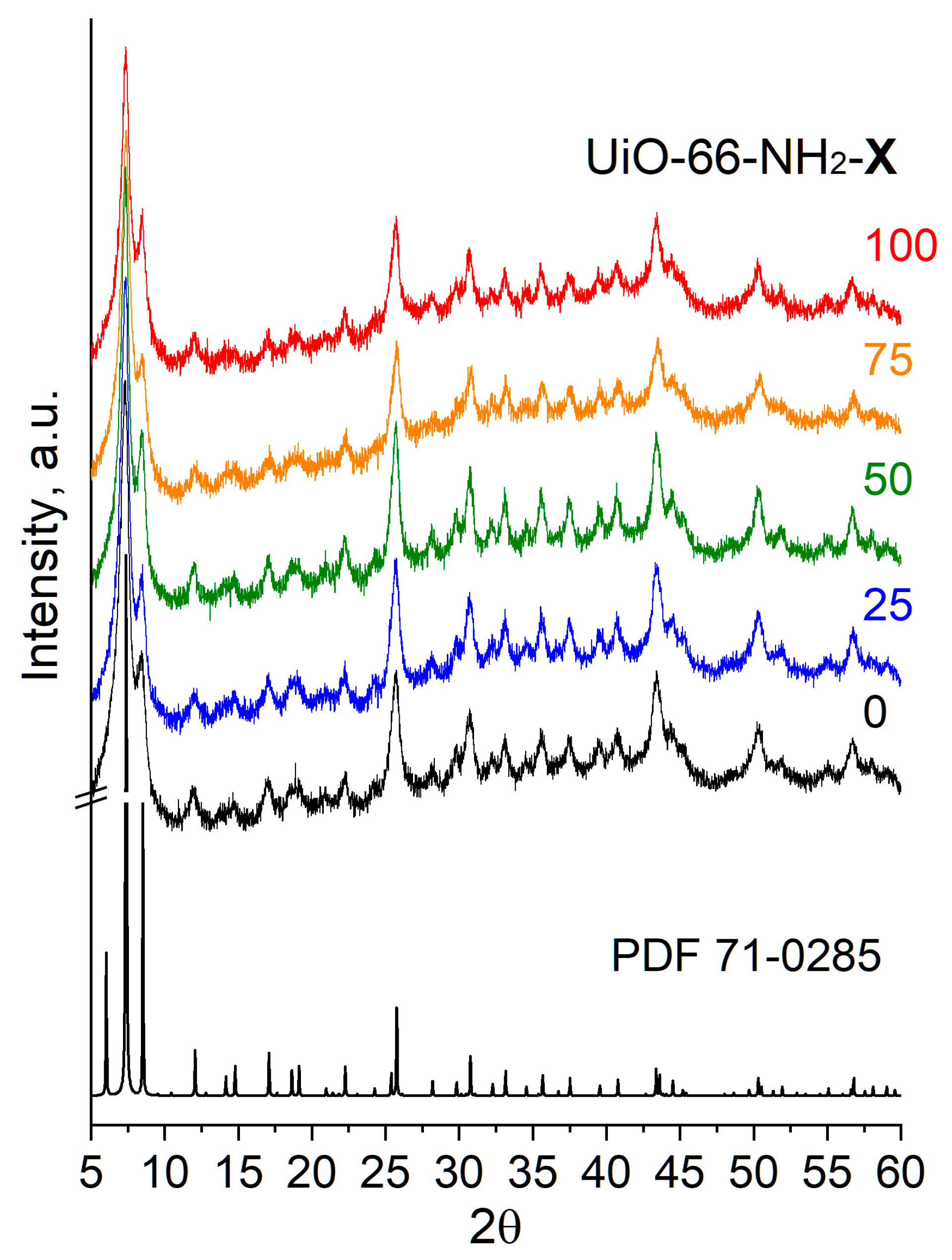
2.1.1. SEM and X-ray Diffraction
2.1.2. IR Spectroscopy
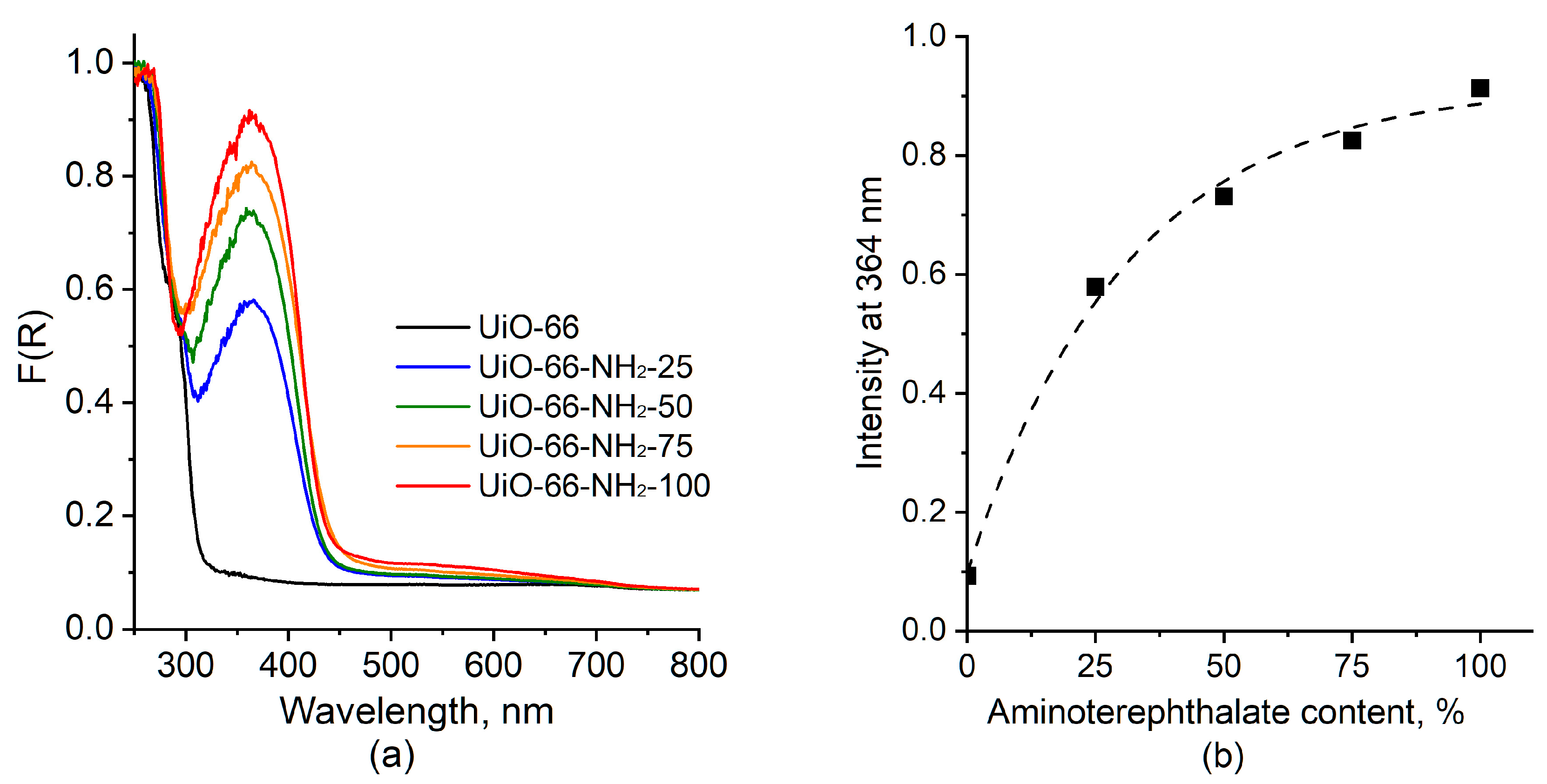
2.1.3. UV–Vis Spectroscopy
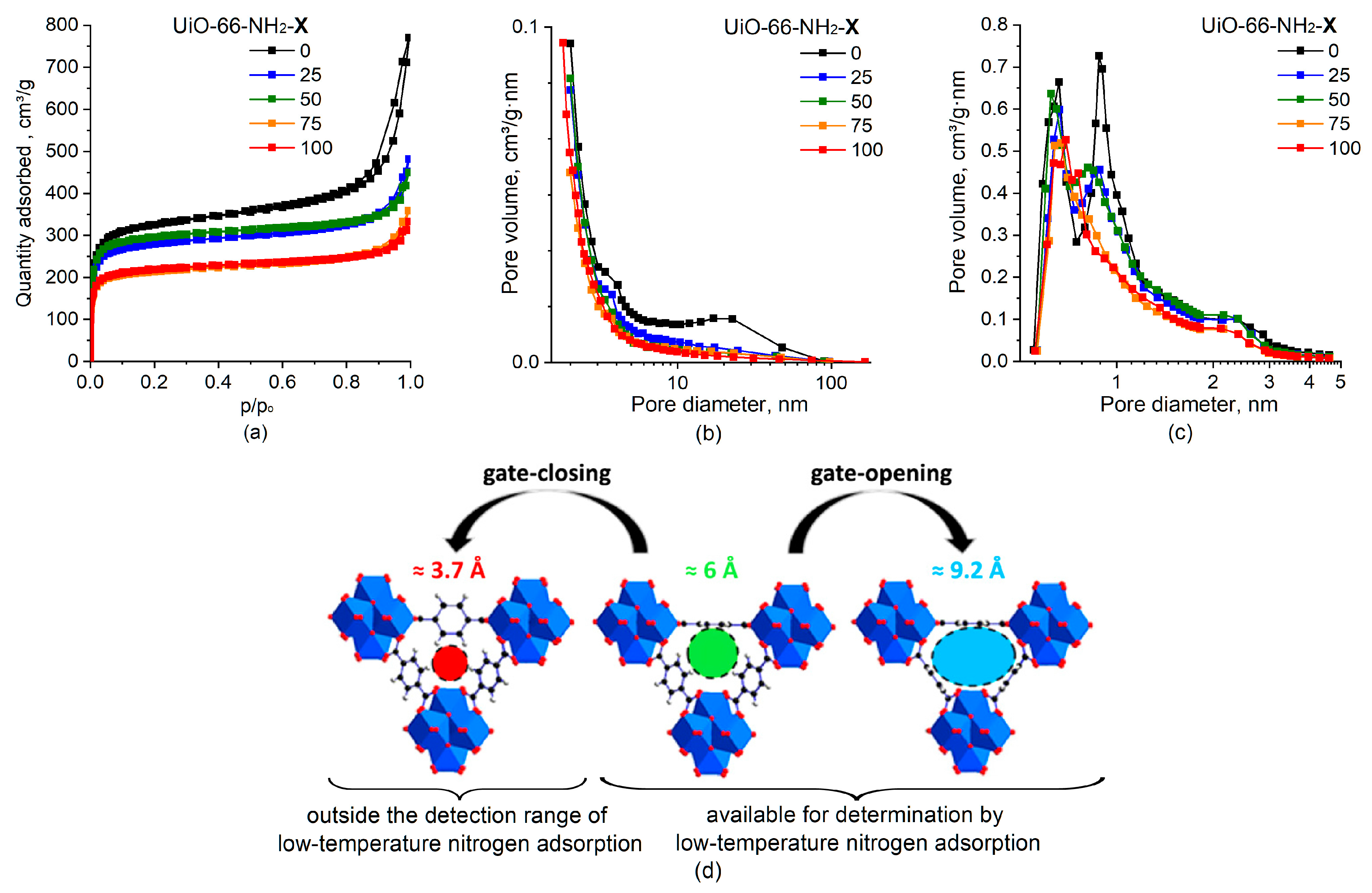
2.1.4. Textural Characteristics
2.2. Functional Proreties
2.2.1. Electrokinetic Properties
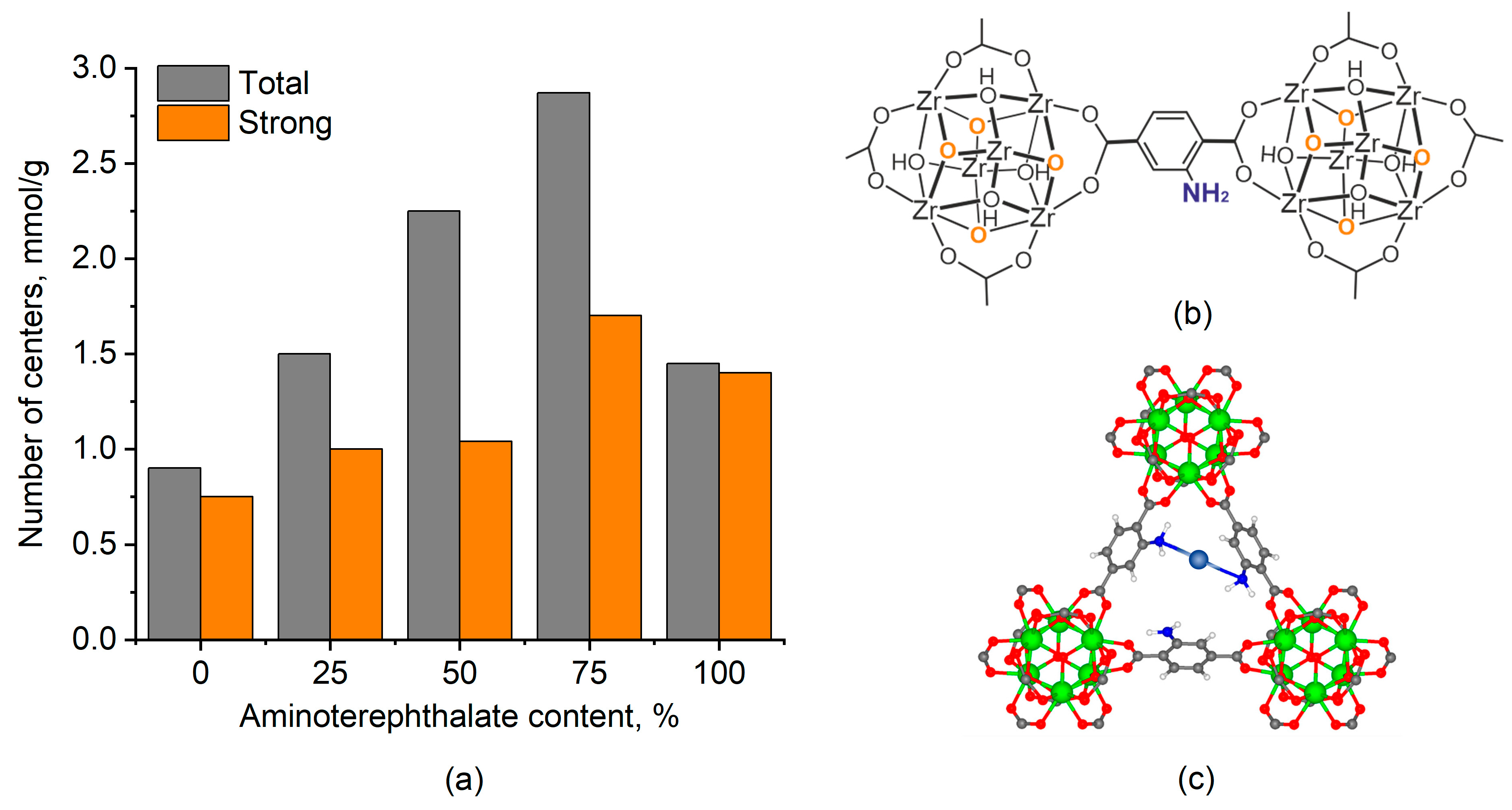
2.2.2. Basic Properties
2.3. UiO-66-NH2 as a Support
3. Materials and Methods
3.1. Materials Preparation
3.2. Low-Temperature Nitrogen Adsorption
3.3. X-ray Diffraction
3.4. IR Spetroscopy
3.5. UV–Vis Spectroscopy
3.6. Electrophoretic Light Scattering
3.7. Acid Adsorption
3.8. Scanning Electron Microscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chandel, A.K.; Garlapati, V.K.; Jeevan Kumar, S.P.; Hans, M.; Singh, A.K.; Kumar, S. The role of renewable chemicals and biofuels in building a bioeconomy. Biofuels Bioprod. Biorefining 2020, 14, 830–844. [Google Scholar] [CrossRef]
- Velvizhi, G.; Balakumar, K.; Shetti, N.P.; Ahmad, E.; Pant, K.K.; Aminabhavi, T.M. Integrated biorefinery processes for conversion of lignocellulosic biomass to value added materials: Paving a path towards circular economy. Bioresour. Technol. 2022, 343, 126151. [Google Scholar] [CrossRef] [PubMed]
- Timofeev, K.L.; Vodyankina, O. Selective oxidation of bio-based platform molecules and their conversion products over metal nanoparticle catalysts: A review. React. Chem. Eng. 2021, 6, 418–440. [Google Scholar] [CrossRef]
- Hu, L.; Lin, L.; Wu, Z.; Zhou, S.; Liu, S. Recent advances in catalytic transformation of biomass-derived 5-hydroxymethylfurfural into the innovative fuels and chemicals. Renew. Sustain. Energy Rev. 2017, 74, 230–257. [Google Scholar] [CrossRef]
- Xu, C.; Paone, E.; Rodrıguez-Padron, D.; Luque, R.; Mauriello, F. Recent catalytic routes for the preparation and the upgrading of biomass derived furfural and 5-hydroxymethylfurfural. Chem. Soc. Rev. 2020, 49, 4273–4306. [Google Scholar] [CrossRef]
- Timofeev, K.L.; Kharlamova, T.S.; Ezhov, D.M.; Salaev, M.A.; Svetlichnyi, V.A.; Vodyankina, O.V. Hydroxymethylfurfural oxidation over unsupported Pd-Au alloy catalysts prepared by pulsed laser ablation: Synergistic and compositional effects. Appl. Catal. A Gen. 2023, 656, 119121. [Google Scholar] [CrossRef]
- Bai, Y.; Dou, Y.; Xie, L.-H.; Rutledge, W.; Li, J.-R.; Zhou, H.-C. Zr-based metal–organic frameworks: Design, synthesis, structure, and applications. Chem. Soc. Rev. 2016, 45, 2327–2367. [Google Scholar] [CrossRef]
- Hall, J.N.; Bollini, P. Structure, characterization, and catalytic properties of open-metal sites in metal organic frameworks. React. Chem. Eng. 2019, 4, 207–222. [Google Scholar] [CrossRef]
- Ding, Q.; Liu, Y.; Shi, C.; Xiao, J.; Dai, W.; Liu, D.; Chen, H.; Li, B.; Liu, J. Applications of ROS-Induced Zr-MOFs Platform in Multimodal Synergistic Therapy. Mini Rev. Med. Chem. 2021, 21, 1718–1733. [Google Scholar] [CrossRef]
- Chen, J.; Liu, R.; Guo, Y.; Chen, L.; Gao, H. Selective Hydrogenation of Biomass-Based 5-Hydroxymethylfurfural over Catalyst of Palladium Immobilized on Amine-Functionalized Metal–Organic Frameworks. ACS Catal. 2014, 5, 722–733. [Google Scholar] [CrossRef]
- Zhang, F.; Zheng, S.; Xiao, Q.; Zhong, Y.; Zhu, W.; Lin, A.; El-Shall, M.S. Synergetic catalysis of palladium nanoparticles encaged within amine-functionalized UiO-66 in the hydrodeoxygenation of vanillin in water. Green Chem. 2016, 18, 2900–2908. [Google Scholar] [CrossRef]
- Kollmannsberger, K.L.; Kronthaler, L.; Jinschek, J.R.; Fischer, R.A. Defined metal atom aggregates precisely incorporated into metal–organic frameworks. Chem. Soc. Rev. 2022, 51, 9933–9959. [Google Scholar] [CrossRef]
- Xu, Z.; Wu, Z.; Huang, S.; Ye, K.; Jiang, Y.; Liu, J.; Liu, J.; Lu, X.; Li, B. A metal-organic framework-based immunomodulatory nanoplatform for anti-atherosclerosis treatment. J. Control. Release 2023, 354, 615–625. [Google Scholar] [CrossRef]
- Ding, Q.; Xu, Z.; Zhou, L.; Rao, C.; Li, W.; Muddassir, M.; Sakiyama, H.; Li, B.; Ouyang, Q.; Liu, J. A multimodal Metal-Organic framework based on unsaturated metal site for enhancing antitumor cytotoxicity through Chemo-Photodynamic therapy. J. Colloid Interface Sci. 2022, 621, 180–194. [Google Scholar] [CrossRef]
- Wang, G.; He, C.-T.; Huang, R.; Mao, J.; Wang, D.; Li, Y. Photoinduction of Cu Single Atoms Decorated on UiO-66-NH2 for Enhanced Photocatalytic Reduction of CO2 to Liquid Fuels. J. Am. Chem. Soc. 2020, 142, 19339–19345. [Google Scholar] [CrossRef]
- Wang, J.; Hu, Y.; Chen, G.; Dong, Y. Cu(II)/Cu(0)@UiO-66-NH2: Base metal@MOFs as heterogeneous catalysts for olefin oxidation and reduction. Chem. Commun. 2016, 52, 13116–13119. [Google Scholar] [CrossRef]
- Luan, Y.; Qi, Y.; Gao, H.; Zheng, N.; Wang, G. Synthesis of an amino-functionalized metal–organic framework at a nanoscale level for gold nanoparticle deposition and catalysis. J. Mater. Chem. A 2014, 2, 20588–20596. [Google Scholar] [CrossRef]
- Isaeva, V.I.; Chernyshev, V.V.; Vergun, V.V.; Arkhipov, D.A.; Deyko, G.S.; Glukhov, L.M.; Kapustin, G.I.; Tkachenko, O.P.; Kustov, L.M. The Impact of Functionality and Porous System of Nanostructured Carriers Based on Metal–Organic Frameworks of UiO-66-Type on Catalytic Performance of Embedded Au Nanoparticles in Hydroamination Reaction. Catalysts 2023, 13, 133. [Google Scholar] [CrossRef]
- Bajpai, V.K.; Haldorai, Y.; Khan, I.; Sonwal, S.; Singh, M.P.; Yadav, S.; Paray, B.A.; Jan, B.L.; Kang, S.-M.; Huh, Y.S.; et al. Au@Zr-based metal–organic framework composite as an immunosensing platform for determination of hepatitis B virus surface antigen. Microchim. Acta 2021, 188, 365. [Google Scholar] [CrossRef]
- Yin, D.; Li, C.; Ren, H.; Liu, J.; Liang, C. Gold-Palladium-Alloy-Catalyst Loaded UiO-66-NH2 for Reductive Amination with Nitroarenes Exhibiting High Selectivity. ChemistrySelect 2018, 3, 5092–5097. [Google Scholar] [CrossRef]
- Subudhi, S.; Mansingh, S.; Tripathy, S.P.; Mohanty, A.; Mohapatra, P.; Rath, D.; Parida, K. The fabrication of Au/Pd plasmonic alloys on UiO66-NH2: An efficient visible light-induced photocatalyst towards the Suzuki Miyaura coupling reaction under ambient conditions. Catal. Sci. Technol. 2019, 9, 6585–6597. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, S.; Zhang, Q. Doping copper ions in a metal-organic framework (UiO-66-NH2): Location effect examined by ultrafast spectroscopy. Chin. J. Chem. Phys. 2020, 33, 394–400. [Google Scholar] [CrossRef]
- Xiao, J.-D.; Shang, Q.; Xiong, Y.; Zhang, Q.; Luo, Y.; Yu, S.-H.; Jiang, H.-L. Boosting Photocatalytic Hydrogen Production of a Metal–Organic Framework Decorated with Platinum Nanoparticles: The Platinum Location Matters. Angew. Chem. Int. Ed. 2016, 55, 9389–9393. [Google Scholar] [CrossRef] [PubMed]
- Chavan, S.M.; Shearer, G.C.; Svelle, S.; Olsbye, U.; Bonino, F.; Ethiraj, J.; Lillerud, K.P.; Bordiga, S. Synthesis and Characterization of Amine-Functionalized Mixed-Ligand Metal–Organic Frameworks of UiO-66 Topology. Inorg. Chem. 2014, 53, 9509–9515. [Google Scholar] [CrossRef] [PubMed]
- Aghajanzadeh, M.; Zamani, M.; Molavi, H.; Manjili, H.K.; Danafar, H.; Shojaei, A. Preparation of Metal–Organic Frameworks UiO-66 for Adsorptive Removal of Methotrexate from Aqueous Solution. J. Inorg. Organomet. Polym. Mater. 2018, 28, 177–186. [Google Scholar] [CrossRef]
- Kandiah, M.; Nilsen, M.H.; Usseglio, S.; Jakobsen, S.; Olsbye, U.; Tilset, M.; Larabi, C.; Quadrelli, E.A.; Bonino, F.; Lillerud, K.P. Synthesis and Stability of Tagged UiO-66 Zr-MOFs. Chem. Mater. 2010, 22, 6632–6640. [Google Scholar] [CrossRef]
- Hadjiivanov, K.I.; Panayotov, D.A.; Mihaylov, M.Y.; Ivanova, E.Z.; Chakarova, K.K.; Andonova, S.M.; Drenchev, N.L. Power of Infrared and Raman Spectroscopies to Characterize Metal-Organic Frameworks and Investigate Their Interaction with Guest Molecules. Chem. Rev. 2021, 121, 1286–1424. [Google Scholar] [CrossRef]
- Lee, T.; Chang, Y.H.; Lee, H.L. Crystallization process development of metal–organic frameworks by linking secondary building units, lattice nucleation and luminescence: Insight into reproducibility. CrystEngComm 2017, 19, 426–441. [Google Scholar] [CrossRef]
- Shastri, A.; Das, A.K.; Krishnakumar, S.; Singh, P.J.; Sekhar, B.N.R. Spectroscopy of N,N-dimethylformamide in the VUV and IR regions: Experimental and computational studies. J. Chem. Phys. 2017, 147, 224305. [Google Scholar] [CrossRef]
- Chavan, S.; Vitillo, J.G.; Gianolio, D.; Zavorotynska, O.; Civalleri, B.; Jakobsen, S.; Nilsen, M.H.; Valenzano, L.; Lamberti, C.; Lillerud, K.P.; et al. H2 storage in isostructural UiO-67 and UiO-66 MOFs. Phys. Chem. Chem. Phys. 2012, 14, 1614–1626. [Google Scholar] [CrossRef]
- Sun, D.; Liu, W.; Qiu, M.; Zhang, Y.; Li, Z. Introduction of a mediator for enhancing photocatalytic performance via post-synthetic metal exchange in metal–organic frameworks (MOFs). Chem. Commun. 2015, 51, 2056–2059. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.; Olivier, J.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Han, Y.; Liu, M.; Li, K.; Zuo, Y.; Wei, Y.; Xu, S.; Zhang, G.; Song, C.; Zhang, Z.; Guo, X. Facile synthesis of morphology and size-controlled zirconium metal–organic framework UiO-66: The role of hydrofluoric acid in crystallization. CrystEngComm 2015, 17, 6434–6440. [Google Scholar] [CrossRef]
- Ten, S.; Torbina, V.V.; Zaikovskii, V.I.; Kulinich, S.A.; Vodyankina, O.V. Bimetallic AgPd/UiO-66 Hybrid Catalysts for Propylene Glycol Oxidation into Lactic Acid. Materials 2020, 13, 5471. [Google Scholar] [CrossRef]
- Zhu, G.; Graver, R.; Emdadi, L.; Liu, B.; Choi, K.Y.; Liu, D. Synthesis of zeolite@metal–organic framework core–shell particles as bifunctional catalysts. RSC Adv. 2014, 4, 30673–30676. [Google Scholar] [CrossRef]
- Friebe, S.; Geppert, B.; Steinbach, F.; Caro, J. Metal–Organic Framework UiO-66 Layer: A Highly Oriented Membrane with Good Selectivity and Hydrogen Permeance. ACS Appl. Mater. Interfaces 2017, 9, 12878–12885. [Google Scholar] [CrossRef]
- Wu, Y.; Weckhuysen, B.M. Separation and Purification of Hydrocarbons with Porous Materials. Angew. Chem. Int. Ed. 2021, 60, 18930–18949. [Google Scholar] [CrossRef]
- Guan, T.; Li, X.; Fang, W.; Wu, D. Efficient removal of phosphate from acidified urine using UiO-66 metal-organic frameworks with varying functional groups. Appl. Surf. Sci. 2020, 501, 144074. [Google Scholar] [CrossRef]
- Ibrahim, A.H.; El-Mehalmey, W.A.; Haikal, R.R.; Safy, M.E.A.; Amin, M.; Shatla, H.R.; Karakalos, S.G.; Alkordi, M.H. Tuning the Chemical Environment within the UiO-66-NH2 Nanocages for Charge-Dependent Contaminant Uptake and Selectivity. Inorg. Chem. 2019, 58, 15078–15087. [Google Scholar] [CrossRef]
- Kosmulski, M. Surface Charging and Points of Zero Charge (Surfactant Science); CRC Press: Boca Raton, FL, USA, 2009; p. 1065. [Google Scholar]
- Kuwahara, Y.; Kango, H.; Yamashita, H. Catalytic Transfer Hydrogenation of Biomass-Derived Levulinic Acid and Its Esters to γ-Valerolactone over Sulfonic Acid-Functionalized UiO-66. ACS Sustain. Chem. Eng. 2016, 5, 1141–1152. [Google Scholar] [CrossRef]
- Xu, H.; Chen, M.; Ji, M. Solid Lewis acid-base pair catalysts constructed by regulations on defects of UiO-66 for the catalytic hydrogenation of cinnamaldehyde. Catal. Today 2022, 402, 52–59. [Google Scholar] [CrossRef]
- Caratelli, C.; Hajek, J.; Cirujano, F.G.; Waroquier, M.; Llabrés i Xamena, F.X.; Van Speybroeck, V. Nature of active sites on UiO-66 and beneficial influence of water in the catalysis of Fischer esterification. J. Catal. 2017, 352, 401–414. [Google Scholar] [CrossRef]
- Ouellette, R.J.; Rawn, J.D. 23—Amines and Amides. In Organic Chemistry Study Guide; Elsevier: Amsterdam, The Netherlands, 2015; pp. 465–494. [Google Scholar] [CrossRef]
- Giambiagi, M.; de Giambiagi, M.S.; Mundim, K.C. Definition of a multicenter bond index. Struct. Chem. 1990, 1, 423–427. [Google Scholar] [CrossRef]
- Wang, K.; Gu, J.; Yin, N. Efficient Removal of Pb(II) and Cd(II) Using NH2-Functionalized Zr-MOFs via Rapid Microwave-Promoted Synthesis. Ind. Eng. Chem. Res. 2017, 56, 1880–1887. [Google Scholar] [CrossRef]
- Zhang, Y.; Guan, W.; Song, H.; Wei, Y.; Jin, P.; Li, B.; Yan, C.; Pan, J.; Yan, Y. Coupled acid and base UiO-66-type MOFs supported on g-C3N4 as a bi-functional catalyst for one-pot production of 5-HMF from glucose. Microporous Mesoporous Mater. 2020, 305, 110328. [Google Scholar] [CrossRef]
- Tanabe, K. Solid Acids and Bases: Their Catalytic Properties, 1st ed.; Academic Press: Cambridge, MA, USA, 1971; 184p. [Google Scholar]

| Sample | SG | a, Å | V, Å3 | SSA, m2/g | VHK, cm3/g | VBJH, cm3/g |
|---|---|---|---|---|---|---|
| UiO-66 | Pm-3m | 20.86 | 9077 | 1269 | 0.50 | 0.83 |
| UiO-66-NH2-25 | Pm-3m | 20.83 | 9037 | 1091 | 0.43 | 0.40 |
| UiO-66-NH2-50 | Pm-3m | 20.83 | 9037 | 1158 | 0.45 | 0.31 |
| UiO-66-NH2-75 | Pm-3m | 20.77 | 8960 | 825 | 0.33 | 0.28 |
| UiO-66-NH2-100 | Pm-3m | 20.80 | 8999 | 854 | 0.34 | 0.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Timofeev, K.L.; Kulinich, S.A.; Kharlamova, T.S. NH2-Modified UiO-66: Structural Characteristics and Functional Properties. Molecules 2023, 28, 3916. https://doi.org/10.3390/molecules28093916
Timofeev KL, Kulinich SA, Kharlamova TS. NH2-Modified UiO-66: Structural Characteristics and Functional Properties. Molecules. 2023; 28(9):3916. https://doi.org/10.3390/molecules28093916
Chicago/Turabian StyleTimofeev, Konstantin L., Sergei A. Kulinich, and Tamara S. Kharlamova. 2023. "NH2-Modified UiO-66: Structural Characteristics and Functional Properties" Molecules 28, no. 9: 3916. https://doi.org/10.3390/molecules28093916
APA StyleTimofeev, K. L., Kulinich, S. A., & Kharlamova, T. S. (2023). NH2-Modified UiO-66: Structural Characteristics and Functional Properties. Molecules, 28(9), 3916. https://doi.org/10.3390/molecules28093916









