Reactive Disperse Dyes Bearing Various Blocked Isocyanate Groups for Digital Textile Printing Ink
Abstract
1. Introduction
2. Results
3. Materials and Methods
3.1. Materials
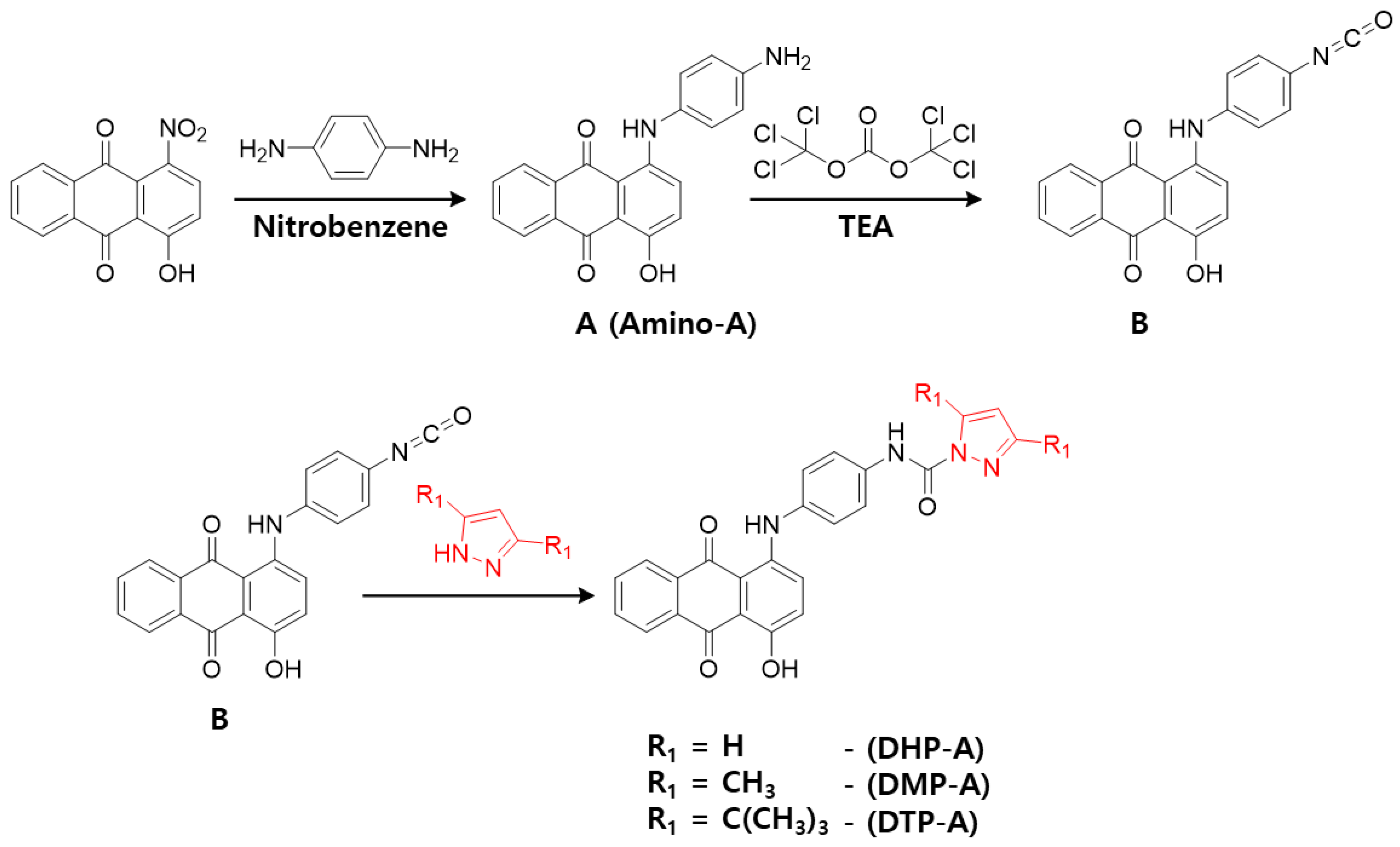
3.2. Synthesis and Characterization of Dyes
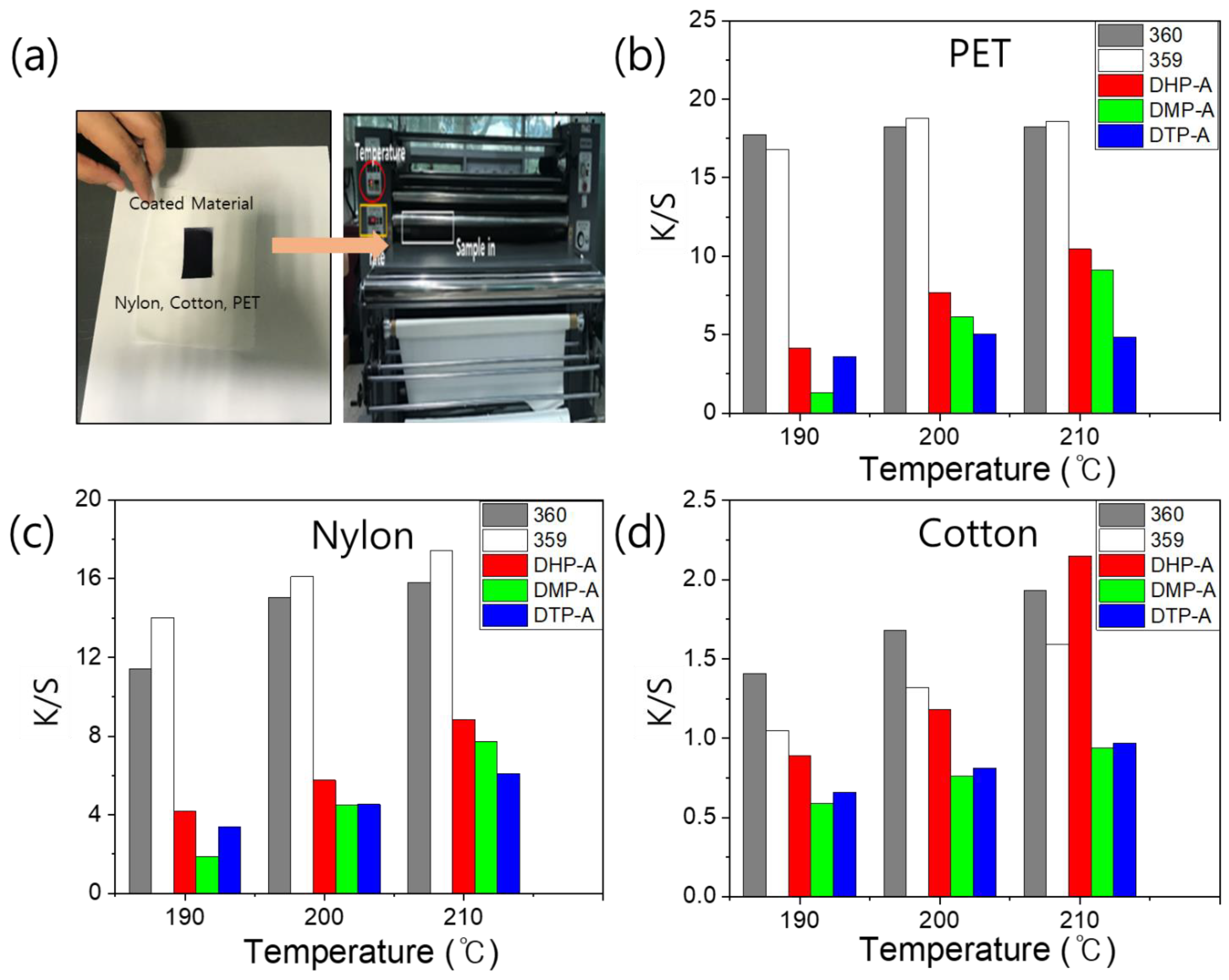
3.3. UV–Vis Spectrum and Color Strength
3.4. Thermal Transfer Printing
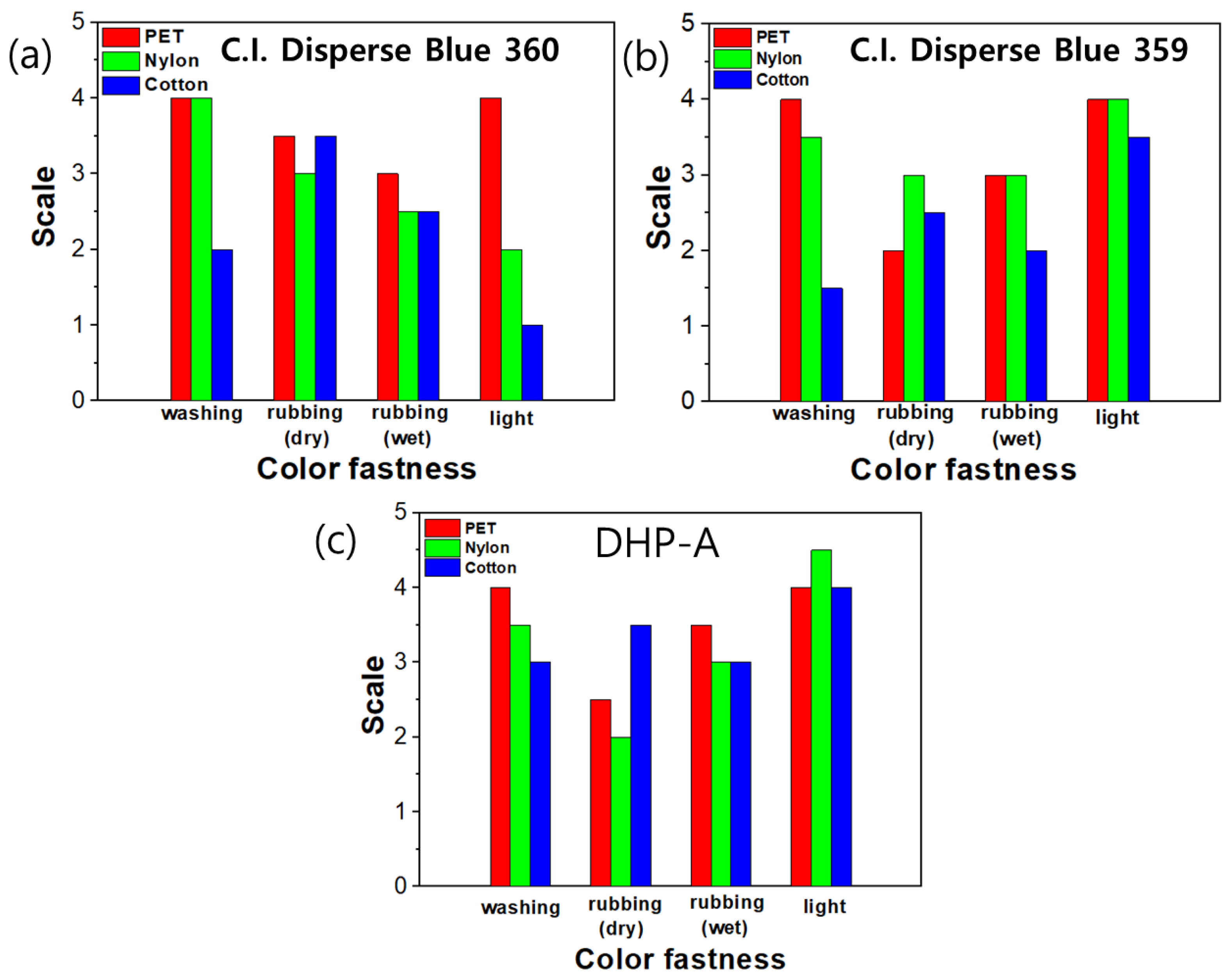
3.5. Fastness of Dyeing Fiber (Washing, Rubbing, and Light Fastness)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
Sample Availability
References
- Kumar, P.; Prasad, B.; Mishra, I.; Chand, S. Treatment of composite wastewater of a cotton textile mill by thermolysis and coagulation. J. Hazard. Mater. 2008, 151, 770–779. [Google Scholar] [CrossRef] [PubMed]
- Baeta, B.; Ramos, R.L.; Lima, D.R.S.; Aquino, S. Use of submerged anaerobic membrane bioreactor (SAMBR) containing powdered activated carbon (PAC) for the treatment of textile effluents. Water Sci. Technol. 2012, 65, 1540–1547. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Kwon, W.; Park, S.; Jeong, E. Optimization of self-crosslinking comonomer composition of polymer binder for DTP pigment ink. Text. Color. Finish. 2020, 32, 19–26. [Google Scholar] [CrossRef]
- Thompson, K.L. Digital Textile Printing: Colorfastness of Reactive Inks versus Pigment Inks. Master’s Thesis, Iowa State University, Ames, IA, USA, 2016. [Google Scholar] [CrossRef]
- Leelajariyakul, S.; Noguchi, H.; Kiatkamjornwong, S. Surface-modified and micro-encapsulated pigmented inks for ink jet printing on textile fabrics. Prog. Org. Coat. 2008, 62, 145–161. [Google Scholar] [CrossRef]
- Ren, J.; Chen, G.; Li, X. A fine grained digital textile printing system based on image registration. Comput. Ind. 2017, 92, 152–160. [Google Scholar] [CrossRef]
- Elgammal, M.; Schneider, R.; Gradzielski, M. Development of self-curable hybrid pigment inks by miniemulsion polymerization for inkjet printing of cotton fabrics. Dyes Pigment. 2016, 133, 467–478. [Google Scholar] [CrossRef]
- Choi, S.; Cho, K.H.; Namgoong, J.W.; Kim, J.Y.; Yoo, E.S.; Lee, W.; Jung, J.W.; Choi, J. The synthesis and characterization of the perylene acid dye inks for digital textile printing. Dyes Pigm. 2019, 163, 381–391. [Google Scholar] [CrossRef]
- Javoršek, D.; Javoršek, A. Colour management in digital textile printing. Color. Technol. 2011, 127, 235–239. [Google Scholar] [CrossRef]
- Al-Etaibi, A.M.; El-Apasery, M.A. Dyeing performance of disperse dye on polyester fabrics using eco-friendly car-rier and their antioxidant and anticancer activities. Int. J. Environ. Res. Public Health 2019, 16, 4603. [Google Scholar] [CrossRef]
- Sadeghi-Kiakhani, M.; Safapour, S. Improvement of dyeing and antimicrobial properties of nylon fabrics modified using chitosan-poly(propylene imine) dendreimer hybrid. J. Ind. Eng. Chem. 2016, 33, 170–177. [Google Scholar] [CrossRef]
- Tang, A.Y.L.; Lee, C.H.; Wang, Y.M.; Kan, C.W. Dye-ing-cotton-with-reactive-dyesa-comparison-between-conventional-water-based-and-solvent-assisted-PEG-basedreverse-micellar-dyeing-systems. Cellulose 2019, 26, 1399–1408. [Google Scholar] [CrossRef]
- Choi, G.-M.; Kim, K.-H. A study of the color reproducibility and color fastness of digital textile printing for nylon sublimation transfer. Res. J. Costume Cult. 2018, 26, 754–763. [Google Scholar] [CrossRef]
- Taylor, J.A. Recent developments in reactive dyes. Color. Technol. 2000, 30, 93–108. [Google Scholar] [CrossRef]
- Shan, B.; Tong, X.; Xiong, W.; Qiu, W.; Tang, B.; Lu, R.; Ma, W.; Luo, Y.; Zhang, S. A new kind of H-acid mono-azo-anthraquinone reactive dyes with surprising colour. Dyes Pigm. 2015, 123, 44–54. [Google Scholar] [CrossRef]
- Satake, A.; Ishizawa, Y.; Katagiri, H.; Kondo, S.I. Chloride Selective Macrocyclic Bisurea Derivatives with 2,2′-Binaphthalene Moieties as Spacers. J. Org. Chem. 2016, 81, 9848–9857. [Google Scholar] [CrossRef] [PubMed]
- Rolph, M.S.; Markowska, A.L.J.; Warriner, C.N.; O’Reilly, R.K. Blocked Isocyanates: From Analytical and Experimental Considerations to Non-polyurethane Applications. Polym. Chem. 2016, 7, 7351–7364. [Google Scholar] [CrossRef]
- Wicks, D.A.; Wicks, Z.W. Blocked isocyanates III: Part A. Mechanisms and chemistry. Prog. Org. Coat. 1999, 36, 148–172. [Google Scholar] [CrossRef]
- Mühlebach, A. Pyrazoles—A novel class of blocking agents for isocyanates. J. Polym. Sci. Part A Polym. Chem. 1994, 32, 753–765. [Google Scholar] [CrossRef]
- Yang, R.; Xu, Y.; Xie, C.; Wang, H. Kubelka-Munk double constant theory of digital rotor spun color blended yarn. Dyes Pigment. 2019, 165, 151–156. [Google Scholar] [CrossRef]
- Xie, K.; Tang, J.; Fu, D.; Zhang, H.; Gao, A. Preparation of color polymeric nanomaterials containing dispersed dye by sunflower oil synergy and color transfer property by digital inkjet printing. Prog. Org. Coat. 2018, 125, 195–200. [Google Scholar] [CrossRef]
- Ghanavatkar, C.W.; Mishra, V.R.; Sekar, N. Benzothiazole-pyridone and benzothiazole-pyrazole clubbed emissive azo dyes and dyeing application on polyester fabric UPF, biological, photophysical and fastness properties with correlative computational assessments. Spectrochim. Acta A. Mol. Biomol. Spectrosc. 2020, 230, 118064. [Google Scholar] [CrossRef] [PubMed]
- Vodyanitskii, Y.N.; Savichev, A.T. The influence of organic matter on soil color using the regression equations of optical parameters in the system CIE- L*a*b*. Ann. Agrar. Sci. 2017, 15, 380–385. [Google Scholar] [CrossRef]
- Razvan, G.; Pérez, M.M.; Herrera, L.J.; Rivas, M.J.; Yebra, A.; Paravina, R.D. Color difference thresholds in dental ceramics. J. Dent. 2010, 38, 57–64. [Google Scholar] [CrossRef]
- Pawar, A.B.; More, S.P.; Adivarekar, R.V. Dyeing of Polyester and Nylon with Semi-synthetic Azo Dye by Chemical Modification of Natural Source Areca Nut. Nat. Prod. Bioprospec. 2017, 8, 23–29. [Google Scholar] [CrossRef] [PubMed]


| Synthetic Dyes | Solution in CHCl3 | Thermal Properties | |
|---|---|---|---|
| UVmax (nm) | (L/mol·cm) | Td at 5% Loss (oC) | |
| Amino-A | 588 | 1.63 103 | 228 |
| DHP-A | 582 | 1.32 103 | 219 |
| DMP-A | 585 | 1.46 103 | 156 |
| DTP-A | 570 | 1.56 103 | 132 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, S.; Kim, G.; Bae, H.; Kim, H.; Seo, E.; Choi, S.; Jeong, J.; Jung, H.; Lee, S.; Cheong, I.; et al. Reactive Disperse Dyes Bearing Various Blocked Isocyanate Groups for Digital Textile Printing Ink. Molecules 2023, 28, 3812. https://doi.org/10.3390/molecules28093812
Jeong S, Kim G, Bae H, Kim H, Seo E, Choi S, Jeong J, Jung H, Lee S, Cheong I, et al. Reactive Disperse Dyes Bearing Various Blocked Isocyanate Groups for Digital Textile Printing Ink. Molecules. 2023; 28(9):3812. https://doi.org/10.3390/molecules28093812
Chicago/Turabian StyleJeong, Subin, Giyoung Kim, Hyoungeun Bae, Hyeokjin Kim, Eunjeong Seo, Sujeong Choi, Jieun Jeong, Hyocheol Jung, Sangho Lee, Inwoo Cheong, and et al. 2023. "Reactive Disperse Dyes Bearing Various Blocked Isocyanate Groups for Digital Textile Printing Ink" Molecules 28, no. 9: 3812. https://doi.org/10.3390/molecules28093812
APA StyleJeong, S., Kim, G., Bae, H., Kim, H., Seo, E., Choi, S., Jeong, J., Jung, H., Lee, S., Cheong, I., Kim, J., & Park, Y. (2023). Reactive Disperse Dyes Bearing Various Blocked Isocyanate Groups for Digital Textile Printing Ink. Molecules, 28(9), 3812. https://doi.org/10.3390/molecules28093812









