Study on the Enhancement Effect of Synergy between Multi-Size Functionalized Boron Nitride and Graphene Oxide on the Thermal Properties of Phase Change Composites
Abstract
:1. Introduction
2. Results and Discussion
2.1. Thermal Storage Properties of CPCM
2.2. Micromorphological Analysis of CPCM
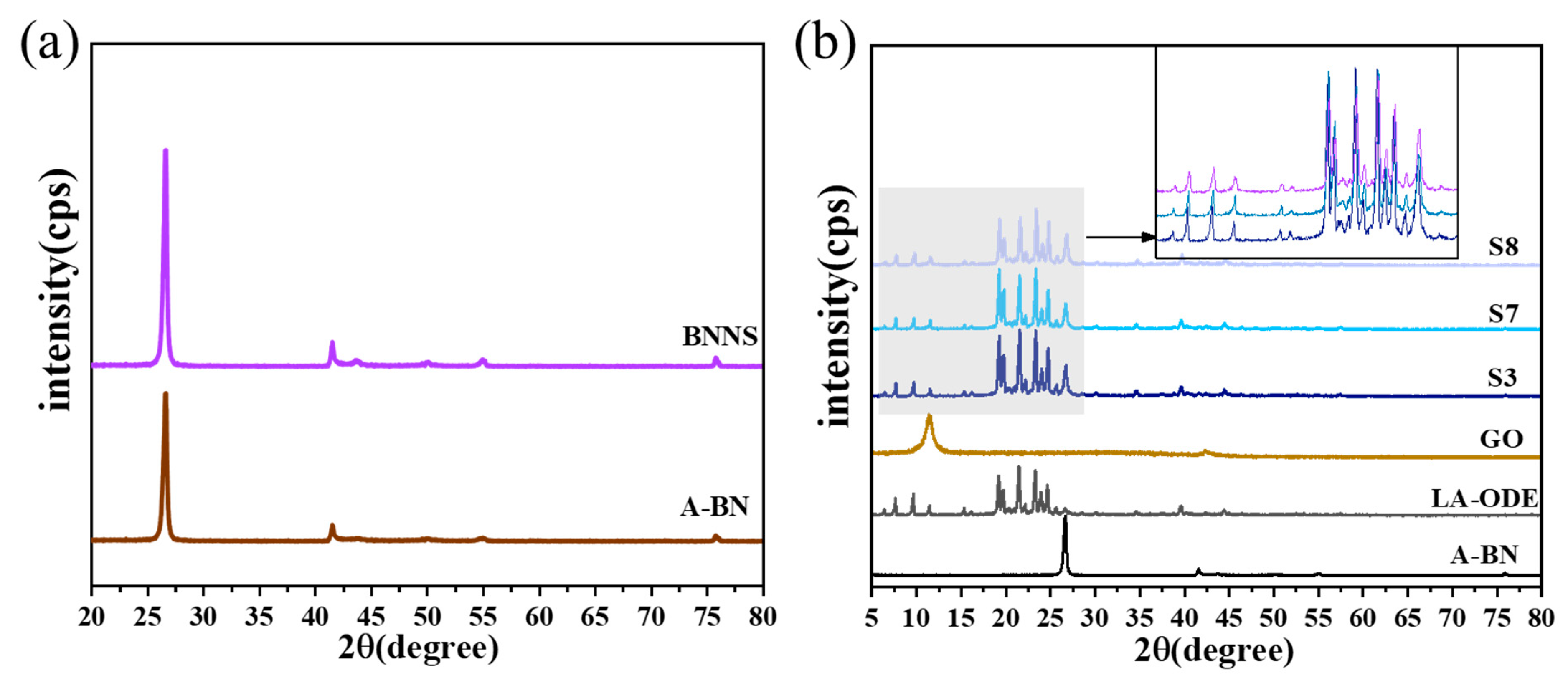
2.3. Crystal Structure of CPCM
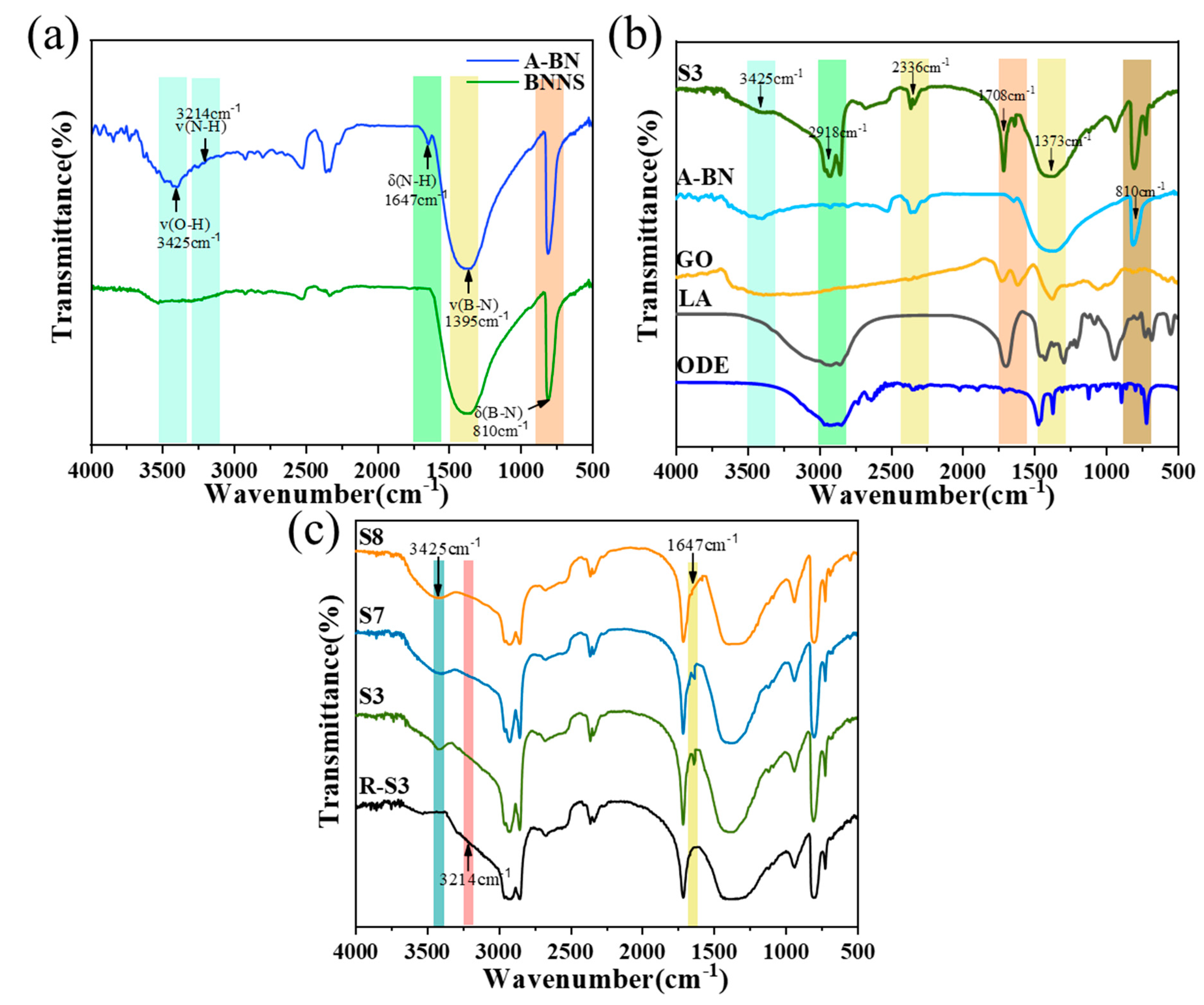
2.4. Chemical Structure of CPCM
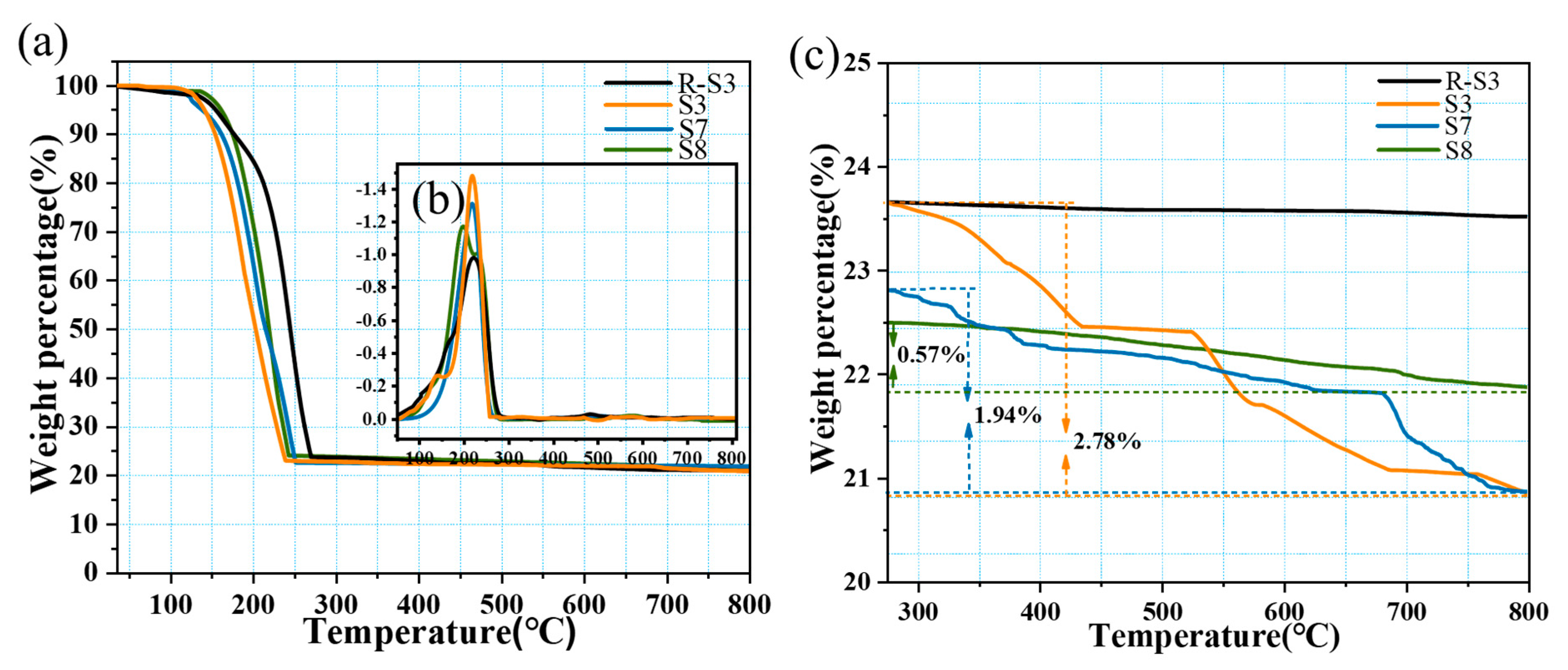
2.5. Thermal Stability of CPCM
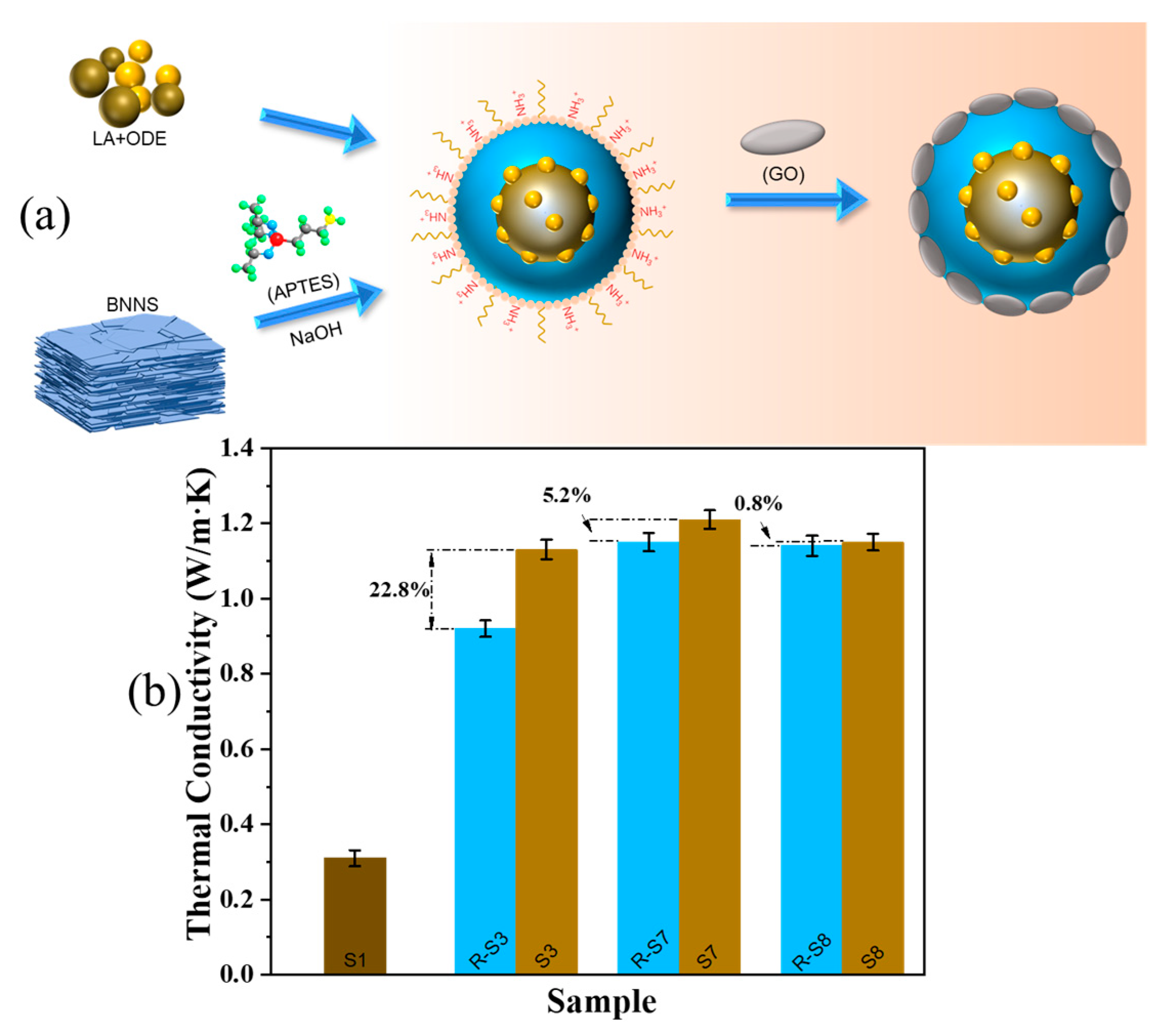
2.6. Thermal Conductivity of CPCM
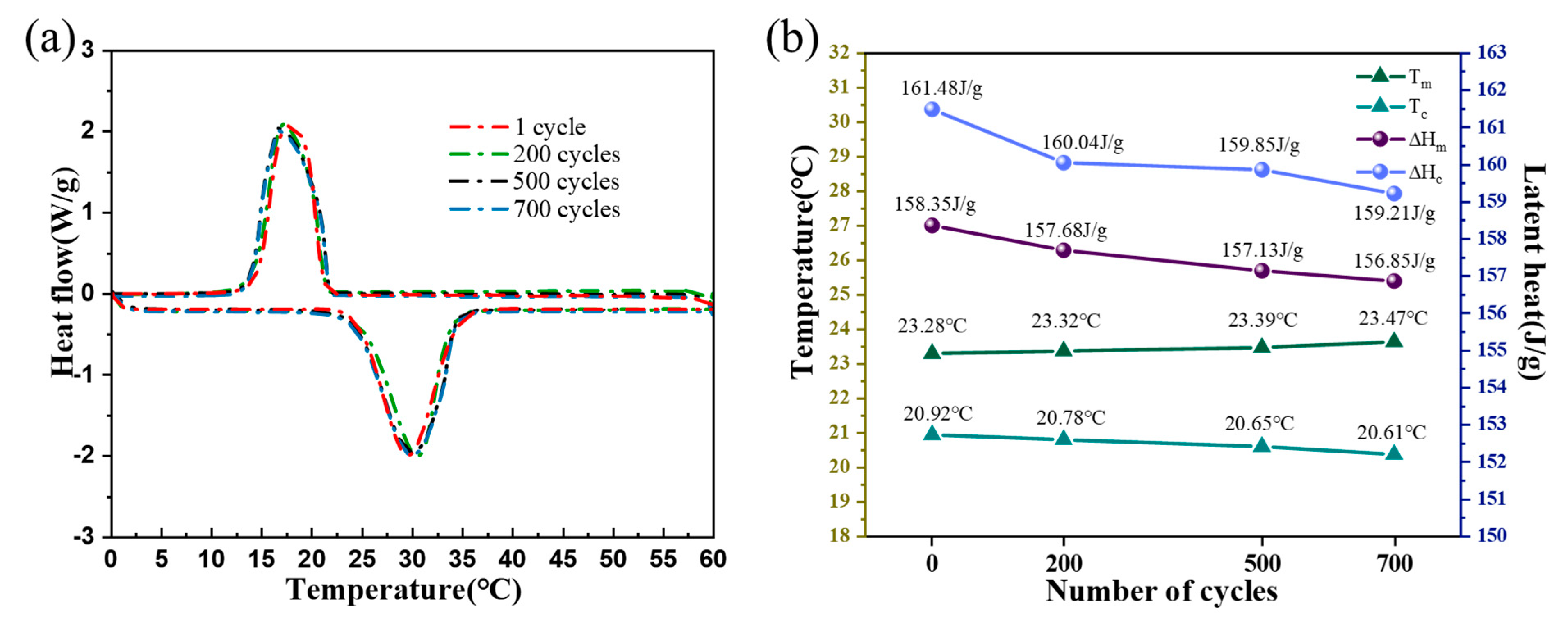
2.7. Cyclic Stability of CPCM
3. Materials and Methods
3.1. Materials
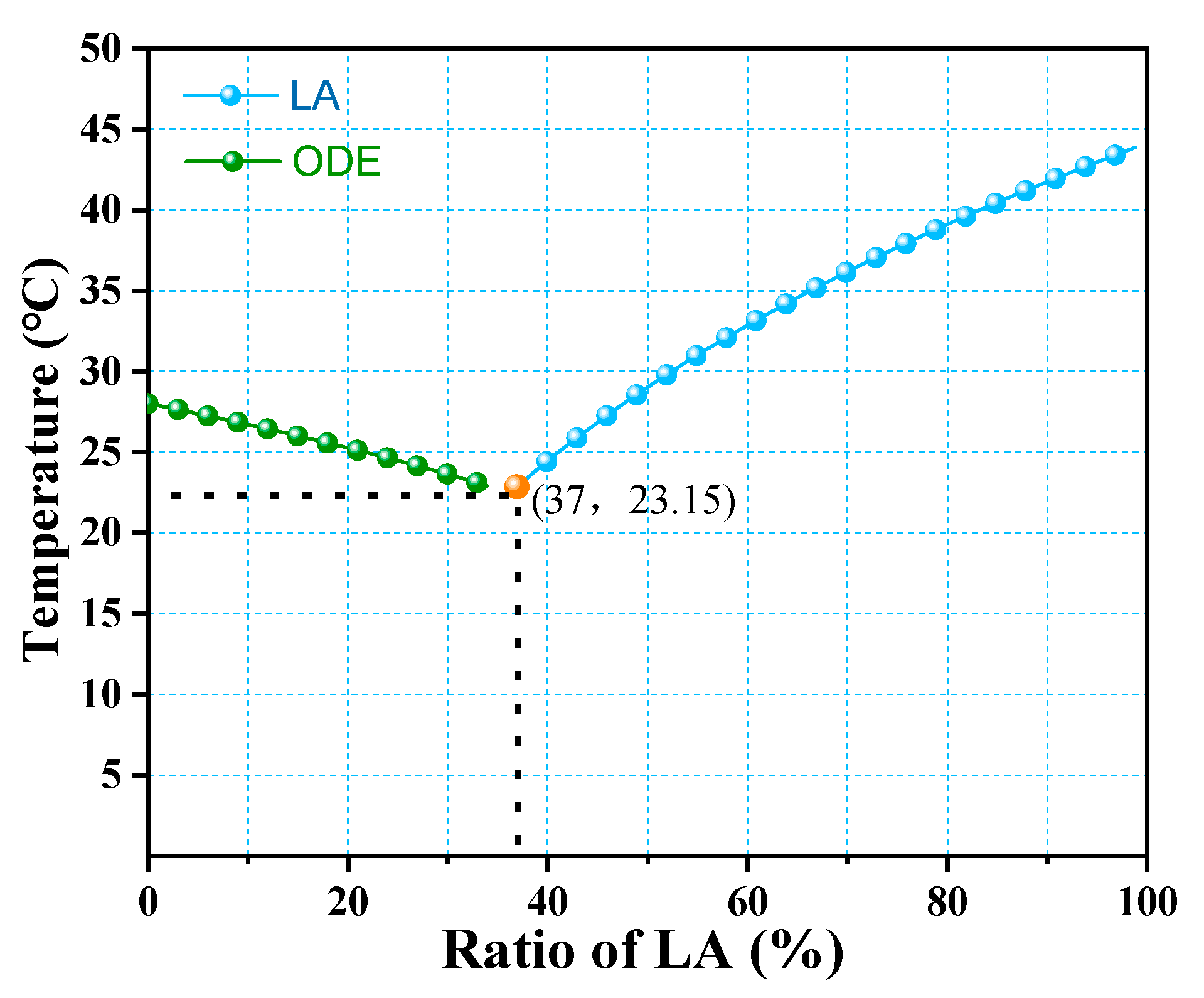
3.2. Determination of the Ratio of the LA-ODE Binary Eutectic Mixture
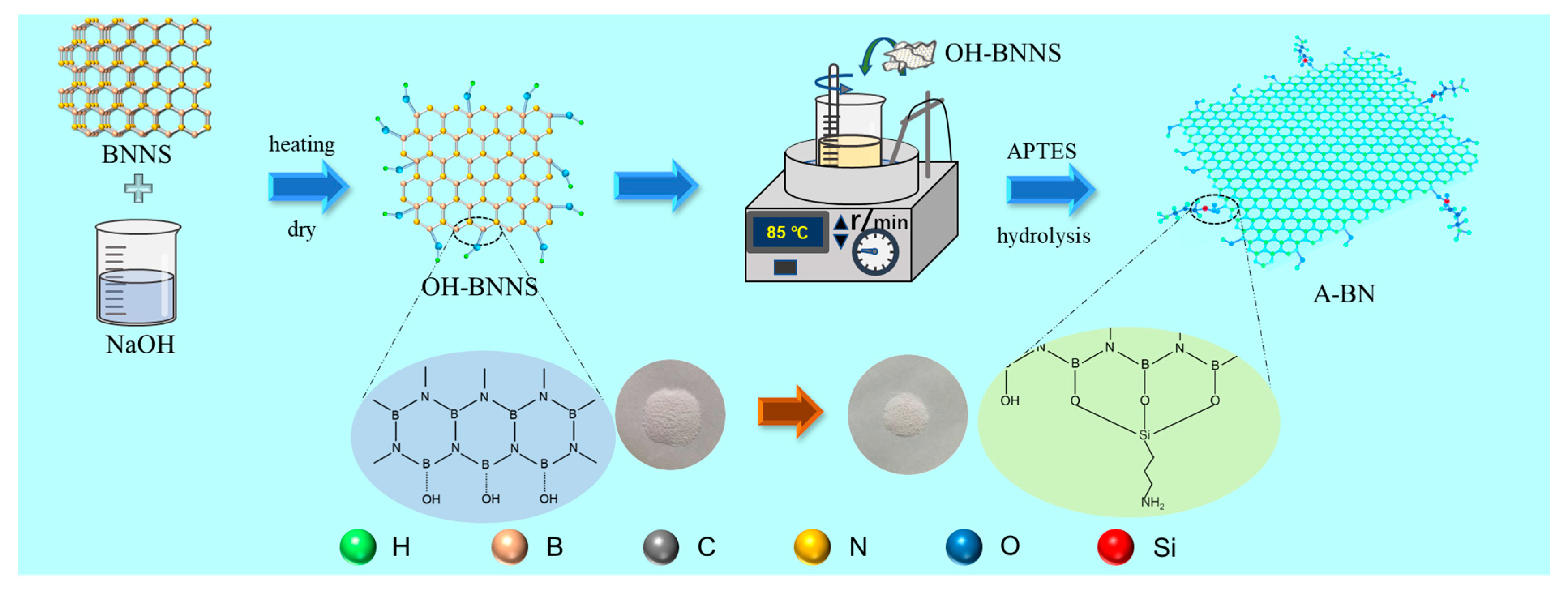
3.3. BNNS Surface Functionalization
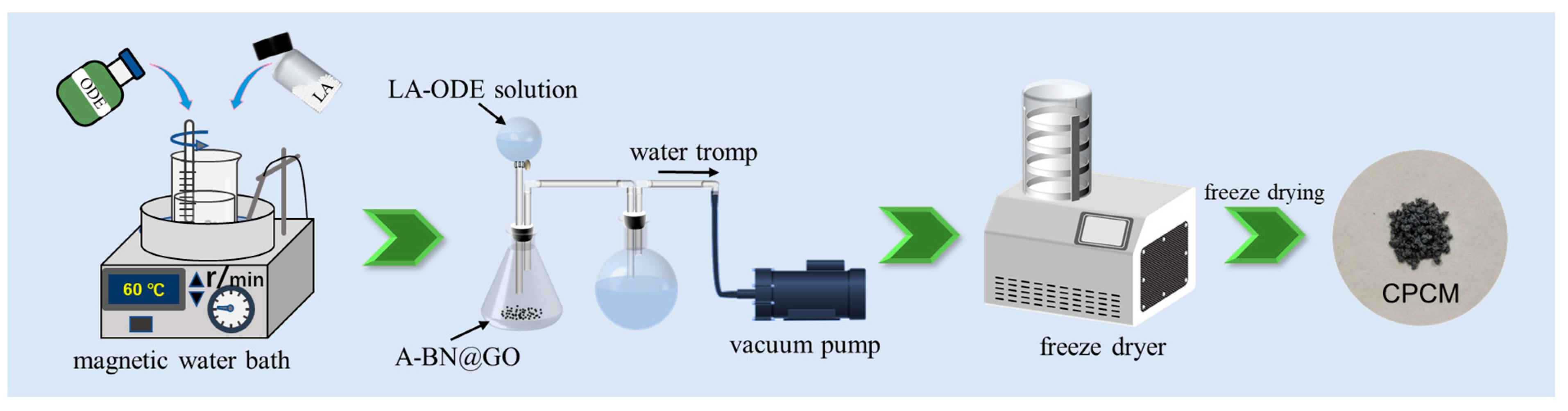
3.4. Preparation of CPCM
3.5. Characterization
4. Conclusions
- (1)
- The addition of A-BN and GO improved the thermal responsiveness of CPCM. With the addition of A-BN (50 nm) and GO at 12 wt% and 2 wt%, respectively, the latent heat of melting and the latent heat of solidification of CPCM could reach 158.35 J/g and 161.48 J/g, and the thermal conductivity reached 1.13 W/(m∙K), which was 265% higher than that of LA-ODE. CPCM had an ideal thermal stability, and the thermal properties of CPCM could still be maintained well after 700 thermal cycles.
- (2)
- The microstructure shows that the small size of A-BN means that it is easier to form a dense thermal conductivity network, and the good compatibility and interfacial connectivity between PCMs, A-BN, and GO ensure that the PCMs can be stored in the network without leakage. The XPS, FTIR, and XRD characterization results showed that BNNS successfully grafted the hydroxyl group and APTES, and the addition of A-BN with GO did not affect the crystal structure of the PCMs.
- (3)
- The thermal conductivity of CPCM with the addition of A-BN (50 nm) improved by 22.8% compared to R-S3, but the increase in thermal conductivity gradually decreased with the increase in size. The advantage of thermal conductivity gained by A-BN was no longer obvious when the size of A-BN was larger than 200 nm, indicating that the enhancement of thermal conductivity by the modification behavior is limited and there is a threshold value. This study can provide some help for the development of PCMs from other 2D nanosheet materials.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Imghoure, O.; Belouaggadia, N.; Ezzine, M.; Lbibb, R.; Younsi, Z. Performance evaluation of phase change materials for thermal comfort in a hot climate region. Appl. Therm. Eng. 2021, 186, 116509. [Google Scholar] [CrossRef]
- Charalambos, N.; Vassilis, N. A comprehensive review of recent advances in materials aspects of phase change materials in thermal energy storage. Energy Procedia 2019, 161, 385–394. [Google Scholar]
- Fan, L.; Khodadadi, J.M. Thermal conductivity enhancement of phase change materials for thermal energy storage: A review. Renew. Sustain. Energy Rev. 2011, 15, 24–46. [Google Scholar] [CrossRef]
- Kong, W.; Liu, Z.; Yang, Y.; Zhou, C.; Lei, Z. Preparation and characterizations of asphalt/lauric acid blends phase change materials for potential building materials. Constr. Build. Mater. 2017, 152, 568–575. [Google Scholar] [CrossRef]
- Dai, J.; Ma, F.; Fu, Z.; Li, C.; Jia, M.; Shi, K.; Wen, Y.; Wang, W. Applicability assessment of stearic acid/palmitic acid binary eutectic phase change material in cooling pavement. Renew. Energy 2021, 175, 748–759. [Google Scholar] [CrossRef]
- Sheng, N.; Rao, Z.; Zhu, C.; Zhu, C.; Habazaki, H. Enhanced thermal performance of phase change material stabilized with textile-structured carbon scaffolds. Sol. Energy Mater. Sol. Cells 2020, 205, 110241. [Google Scholar] [CrossRef]
- Chung, D.D.L.; Xi, X. Factors that govern the electric permittivity of carbon materials in the graphite allotrope family. Carbon 2021, 184, 245–252. [Google Scholar] [CrossRef]
- Kuziel, A.W.; Dzido, G.; Turczyn, R. Ultra-long carbon nanotube-paraffin composites of record thermal conductivity and high phase change enthalpy among paraffin-based heat storage materials. J. Energy Storage 2021, 36, 102396. [Google Scholar] [CrossRef]
- Dong, G.; Zhao, L.; Tian, X. Effects of hard segment regulation on mechanical property and thermal stability of PPG-MDI polyurethane. J. Shandong Univ. Sci. Technol. (Nat. Sci.) 2022, 41, 75–81. [Google Scholar]
- Deng, Y.; Li, J.; Qian, T.; Guan, W.; Li, Y.; Yin, X. Thermal conductivity enhancement of polyethylene glycol/expanded vermiculite shape-stabilized composite phase change materials with silver nanowire for thermal energy storage. Chem. Eng. J. 2016, 295, 427–435. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, X.; Zhu, Z.; Xin, S. A low-temperature phase change material based on capric-stearic acid/expanded graphite for thermal energy storage. ACS Omega 2021, 6, 17988–17998. [Google Scholar] [CrossRef]
- Qian, Z.; Shen, H.; Fang, X.; Fan, L.; Zhao, N.; Xu, J. Phase change materials of paraffin in h-BN porous scaffolds with enhanced thermal conductivity and form stability. Energy Build. 2018, 158, 1184–1188. [Google Scholar] [CrossRef]
- Lin, Y.; Jia, Y.; Alva, G.; Fang, G. Review on thermal conductivity enhancement, thermal properties and applications of phase change materials in thermal energy storage. Renew. Sustain. Energy Rev. 2018, 82, 2730–2742. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Munyalo, J.M.; Tian, Z.; Ji, J. Preparation and thermophysical properties of low temperature composite phase change material octanoic-lauric acid/expanded graphite. J. Mol. Liq. 2019, 277, 577–583. [Google Scholar] [CrossRef]
- Zhang, Z.; Alva, G.; Gu, M.; Fang, G. Experimental investigation on n-octadecane/polystyrene/expanded graphite composites as form–stable thermal energy storage materials. Energy 2018, 157, 625–632. [Google Scholar] [CrossRef]
- Zhou, D.; Yuan, J.; Xiao, X.; Liu, Y. Preparation and Characterization of lauric–Myristic Acid/Expanded Graphite as Composite Phase Change Energy Storage Material. J. Nanomater. 2021, 2021, 1–11. [Google Scholar] [CrossRef]
- Cárdenas-Ramírez, C.; Gómez, M.A.; Jaramillo, F. Thermal reliability of organic-organic phase change materials and their shape-stabilized composites. J. Energy Storage 2021, 40, 102661. [Google Scholar] [CrossRef]
- Liu, B.; Gu, Y.; Ji, Y.; Zheng, G.; Ma, F.; Wang, J.; Wu, Y.; Long, F.; Zhou, B.; Chen, C. Thin-walled boron nitride micron square tube decorated by nanosheets: Preparation, characterization and adsorption property. Ceram. Int. 2021, 47, 14115–14123. [Google Scholar] [CrossRef]
- Matveev, A.; Permyakova, T.E.S.; Kovalskii, A.M.; Leibo, D.; Shchetinin, I.V.; Maslakov, K.I.; Golberg, D.V.; Shtansky, D.V.; Konopatsky, A.S. New insights into synthesis of nanocrystalline hexagonal BN. Ceram. Int. 2020, 46, 19866–19872. [Google Scholar] [CrossRef]
- Ren, J.; Stagl, L.; Innocenzi, P. Hydroxylated boron nitride materials: From structures to functional applications. J. Mater. Sci. 2020, 56, 4053–4079. [Google Scholar] [CrossRef]
- Wang, S.; Tao, B.; Yu, S.; Wei, C.; Zhou, T.; Chen, X.; Han, C.; Wang, C. Insight into the liquid-phase exfoliation to prepare BN nanosheets. Mater. Lett. 2020, 269, 127644. [Google Scholar] [CrossRef]
- Atinafu, D.G.; Dong, W.; Wang, J.; Huang, X.; Wang, J.; Gao, H.; Wang, G. Synthesis and Characterization of Paraffin/Metal Organic Gel Derived Porous Carbon/Boron Nitride Composite Phase Change Materials for Thermal Energy Storage. Eur. J. Inorg. Chem. 2018, 2018, 5167–5175. [Google Scholar] [CrossRef]
- Lei, C.; Wu, K.; Wu, L.; Liu, W.; Du, R.; Chen, F.; Fu, Q. Phase change material with anisotropically high thermal conductivity and excellent shape stability due to its robust cellulose/BNNS skeleton. J. Mater. Chem. A 2019, 7, 19364–19373. [Google Scholar] [CrossRef]
- Wang, Z.; Qu, M.; Yao, G.; Wang, Q.; Zhao, W.; Xia, C.; Liu, Q.; Sun, S.; Jia, Z. Influence factors of pore structure and crystal phase in preparation of porous ceramics with alkali residue and gold tailings. J. Shandong Univ. Sci. Technol. (Nat. Sci.) 2022, 41, 65–74. [Google Scholar]
- Mohd, M.N.; Badarudin, A.; Kazi, S.N.; Ming, N.; Misran, M.; Sadeghinezhad, E.; Mehrali, M.; Yusoff, N. Highly dispersed reduced graphene oxide and its hybrid complexes as effective additives for improving thermophysical property of heat transfer fluid. Int. J. Heat Mass Transfer. 2015, 87, 284–294. [Google Scholar] [CrossRef]
- Warzoha, R.J.; Fleischer, A.S. Heat flow at nanoparticle interfaces. Nano Energy 2014, 6, 137–158. [Google Scholar] [CrossRef]
- Liu, S.; Xin, S.; Jiang, S. Study of Capric–Palmitic Acid/Clay Minerals as Form-Stable Composite Phase-Change Materials for Thermal Energy Storage. ACS Omega 2021, 6, 24650–24662. [Google Scholar] [CrossRef]
- Qi, M.; Liu, L.; Chen, Q.; Lu, C.; Chen, W. Research on acoustic performance of composite micro-perforated plate structure based on porous materials. J. Shandong Univ. Sci. Technol. (Nat. Sci.) 2022, 41, 83–90. [Google Scholar]
- Du, J.; Bian, S.; Lin, L.; Wang, Y.; Sun, P.; Hu, X.; Wang, Z.; Liu, J. Influence of changes insoluble organic matter in coal on its surface hydrophobicity. J. Shandong Univ. Sci. Technol. (Nat. Sci.) 2022, 41, 50–60. [Google Scholar]
- Yang, J.; Tang, L.; Bao, R.; Bai, L.; Liu, Z.; Yang, W.; Xie, B.; Yang, M. largely enhanced thermal conductivity of poly (ethylene glycol)/boron nitride composite phase change materials for solar-thermal-electric energy conversion and storage with very low content of graphene nanoplatelets. Chem. Eng. J. 2017, 315, 481–490. [Google Scholar] [CrossRef]
- Jing, R.; Zhang, H.; Huang, C.; Su, F.; Wu, B.; Sun, Z.; Xu, F.; Sun, L.; Xia, Y.; Peng, H.; et al. Construction of double cross-linking PEG/h-BN@GO polymeric energy-storage composites with high structural stability and excellent thermal performances. Colloids Surf. A 2022, 638, 128193. [Google Scholar] [CrossRef]
- Chi, B.; Yao, Y.; Cui, S.; Jin, X. Preparation of graphene oxide coated tetradecanol/expanded graphite composite phase change material for thermal energy storage. Mater. Lett. 2021, 282, 128666. [Google Scholar] [CrossRef]
- Lin, Y.; Kang, Q.; Wei, H.; Bao, H.; Jiang, P.; Mai, Y.; Huang, X. Spider Web-Inspired Graphene Skeleton-Based High Thermal Conductivity Phase Change Nanocomposites for Battery Thermal Management. Nano-Micro Lett. 2021, 13, 180. [Google Scholar] [CrossRef] [PubMed]
- Kausar, A. Shape memory polyurethane/graphene nanocomposites: Structures, properties, and applications. J. Plast. Film Sheeting 2019, 36, 151–166. [Google Scholar] [CrossRef]
- Cai, D.; Li, J.; Jiao, N. Preparation and thermophysical properties of graphene nanoplatelets-octadecane phase change composite materials. Acta Phys. Sin. 2019, 68, 10. [Google Scholar] [CrossRef]
- Liu, Z.; Zhao, S.; Yang, T.; Zhou, J. Improvement in mechanical properties in AlN-h-BN composites with high thermal conductivity. J. Adv. Ceram. 2021, 10, 1317–1325. [Google Scholar] [CrossRef]
- Liu, Z.; Li, J.; Liu, X. Novel Functionalized BN Nanosheets/Epoxy Composites with Advanced Thermal Conductivity and Mechanical Properties. ACS Appl. Mater. Interfaces 2020, 12, 6503–6515. [Google Scholar] [CrossRef]
- Zhou, Y.; Qiu, S.; Chu, F.; Yang, W.; Qiu, Y.; Qian, L.; Hu, W.; Song, L. High-performance flexible polyurethane foam based on hierarchical BN@MOF-LDH@APTES structure: Enhanced adsorption, mechanical and fire safety properties. J. Colloid Interface Sci. 2022, 609, 794–806. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, D.; Fei, H.; Xu, Y.; Zeng, Z.; Ye, W. Preparation and properties of lauric acid-octadecanol/expanded graphite shape-stabilized phase change energy storage material. Mater. Today Commun. 2022, 31, 103325. [Google Scholar] [CrossRef]
- Motahar, S.; Nikkam, N.; Alemrajabi, A.A.; Khodabandeh, R.; Toprak, M.S.; Muhammed, M.A. A novel phase change material containing mesoporous silica nanoparticles for thermal storage: A study on thermal conductivity and viscosity. Int. Commun. Heat Mass Transfer. 2014, 56, 114–120. [Google Scholar] [CrossRef]
- Li, C.; Yu, H.; Song, Y.; Wang, M.; Liu, Z. A n-Octadecane/hierarchically porous TiO2 form-stable PCM for thermal energy storage. Renew. Energy 2020, 145, 1465–1473. [Google Scholar] [CrossRef]
- Wen, R.; Zhu, X.; Yang, C.; Sun, Z.; Zhang, L.; Xu, Y.; Qiao, J.; Wu, X.; Min, X.; Huang, Z. A novel composite phase change material from lauric acid, nano-Cu and attapulgite: Preparation, characterization and thermal conductivity enhancement. J. Energy Storage 2022, 46, 103921. [Google Scholar] [CrossRef]
- Eshkalak, K.E.; Sadeghzadeh, S.; Jalaly, M. Thermal resistance analysis of hybrid graphene-boron nitride nanosheets: The effect of geometry, temperature, size, strain and structural defects. Comput. Mater. Sci. 2020, 174, 109484. [Google Scholar] [CrossRef]
- Elston, S.J. Optics and Nonlinear Optics of Liquid Crystals. J. Mod. Opt. 1994, 41, 1517–1518. [Google Scholar] [CrossRef]
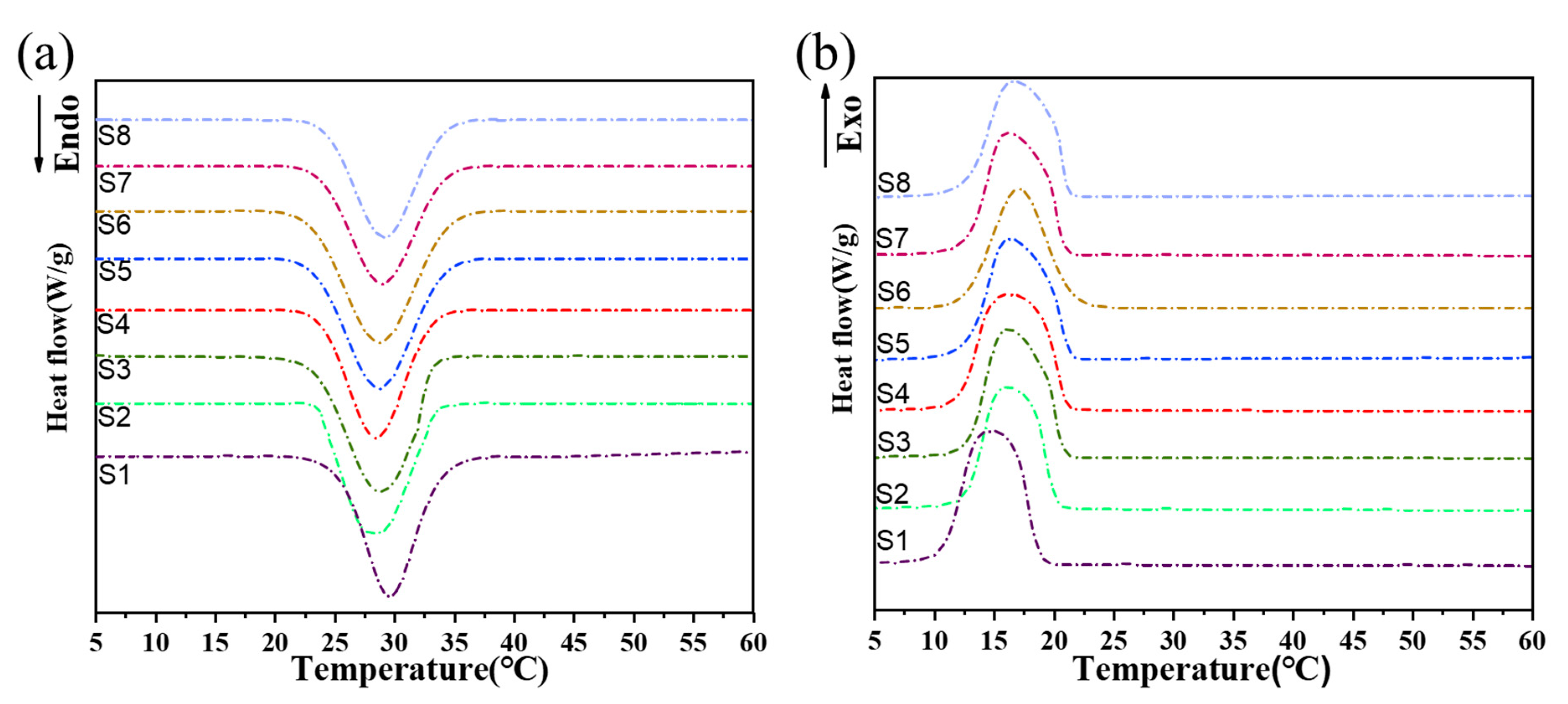



| Sample | Composite PCMs | Melting | Crystallization | |||
|---|---|---|---|---|---|---|
| S1 | 24.05 | |||||
| S2 | 23.64 | |||||
| S3 | 23.33 | |||||
| S4 | 23.28 | |||||
| S5 | 23.14 | |||||
| S6 | 23.10 | |||||
| S7 | 23.37 | |||||
| S8 | 23.45 | |||||
| Number of Cycles | Melting | Crystallization | ||||||
|---|---|---|---|---|---|---|---|---|
| Tm (°C) | Percentage Change (%) | ΔHm (J/g) | Percentage Change (%) | Tc (°C) | Percentage Change (%) | ΔHc (J/g) | Percentage Change (%) | |
| 1 | 23.28 | n.a | 158.35 | n.a | 20.92 | n.a | 161.48 | n.a |
| 200 | 23.32 | 0.17 | 157.68 | −0.42 | 20.78 | −0.66 | 160.04 | −0.89 |
| 500 | 23.39 | 0.47 | 157.13 | −0.77 | 20.65 | −1.29 | 159.85 | −1.01 |
| 700 | 23.47 | 0.81 | 156.85 | −0.94 | 20.61 | −1.48 | 159.21 | −1.41 |
| Sample | LA (g) | ODE (g) | A-BN (g) | GO (g) | Total (g) |
|---|---|---|---|---|---|
| S1 | 3.00 | 7.00 | 0 | 0 | 10 |
| S2, | 2.61 | 6.09 | 1.2 | 0.1 | 10 |
| S3, S7, S8 | 2.58 | 6.02 | 1.2 | 0.2 | 10 |
| S4 | 2.55 | 5.95 | 1.2 | 0.3 | 10 |
| S5 | 2.52 | 5.88 | 1.2 | 0.4 | 10 |
| S6 | 2.49 | 5.81 | 1.2 | 0.5 | 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xin, S.; Zhao, Z.; Liu, S.; Liu, J.; Li, M. Study on the Enhancement Effect of Synergy between Multi-Size Functionalized Boron Nitride and Graphene Oxide on the Thermal Properties of Phase Change Composites. Molecules 2023, 28, 3797. https://doi.org/10.3390/molecules28093797
Xin S, Zhao Z, Liu S, Liu J, Li M. Study on the Enhancement Effect of Synergy between Multi-Size Functionalized Boron Nitride and Graphene Oxide on the Thermal Properties of Phase Change Composites. Molecules. 2023; 28(9):3797. https://doi.org/10.3390/molecules28093797
Chicago/Turabian StyleXin, Song, Zhiwen Zhao, Shangxiao Liu, Jiedong Liu, and Mengya Li. 2023. "Study on the Enhancement Effect of Synergy between Multi-Size Functionalized Boron Nitride and Graphene Oxide on the Thermal Properties of Phase Change Composites" Molecules 28, no. 9: 3797. https://doi.org/10.3390/molecules28093797
APA StyleXin, S., Zhao, Z., Liu, S., Liu, J., & Li, M. (2023). Study on the Enhancement Effect of Synergy between Multi-Size Functionalized Boron Nitride and Graphene Oxide on the Thermal Properties of Phase Change Composites. Molecules, 28(9), 3797. https://doi.org/10.3390/molecules28093797







