Sol-Gel Derived Tungsten Doped VO2 Thin Films on Si Substrate with Tunable Phase Transition Properties
Abstract
1. Introduction
2. Experimental Section
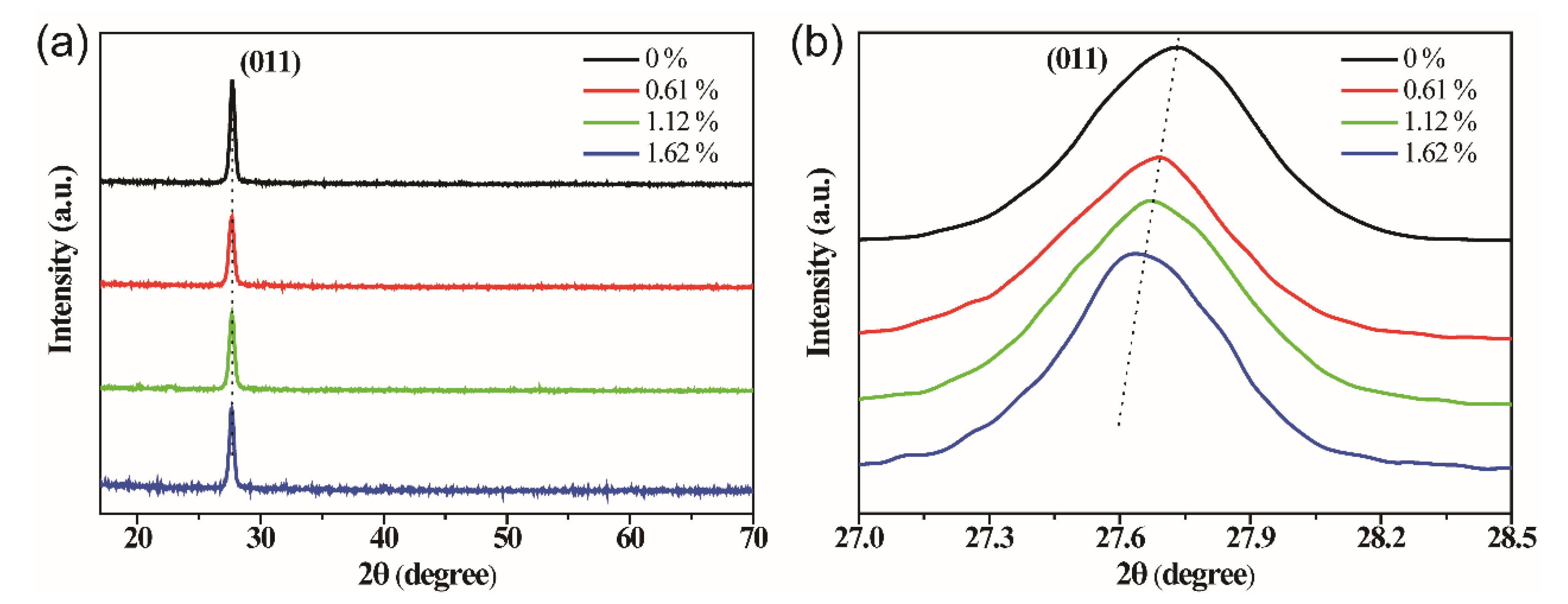
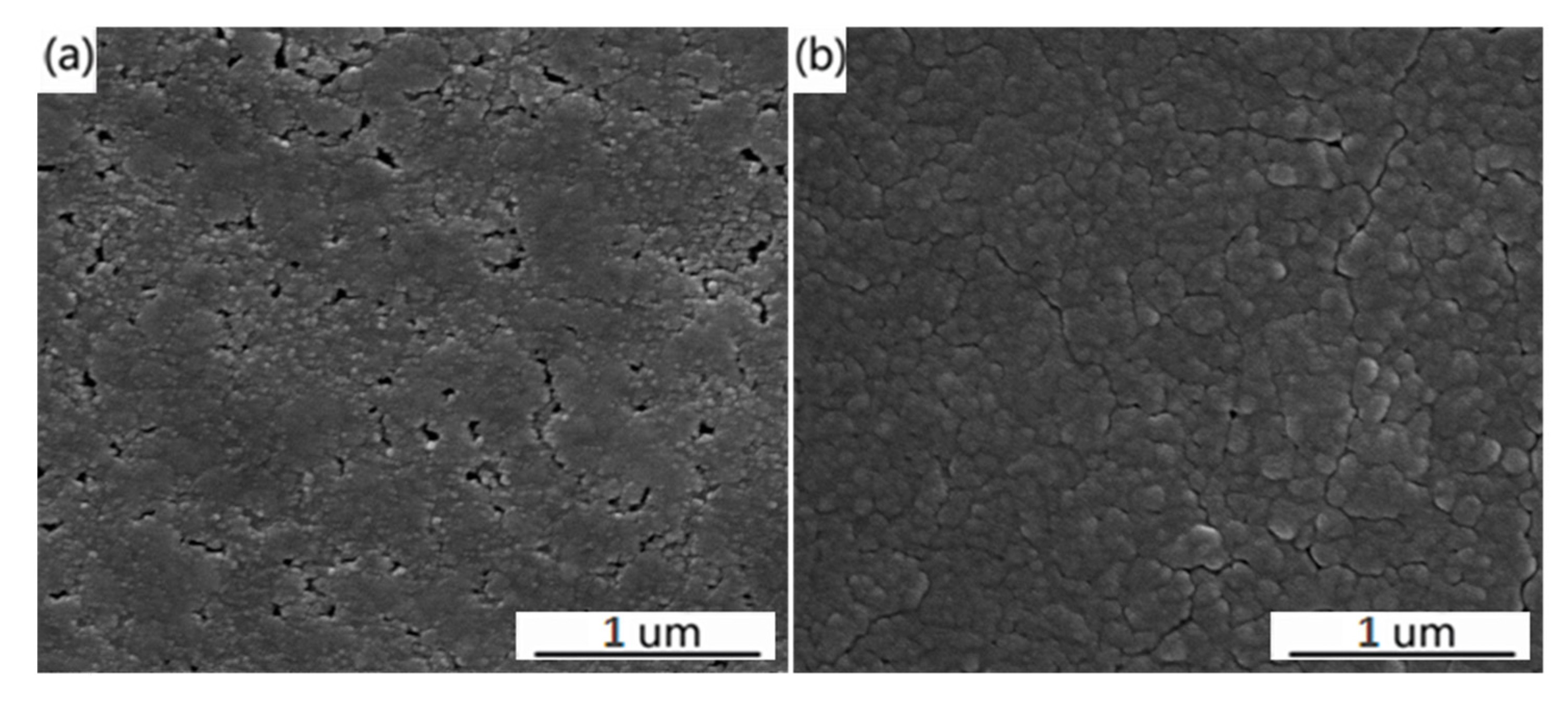
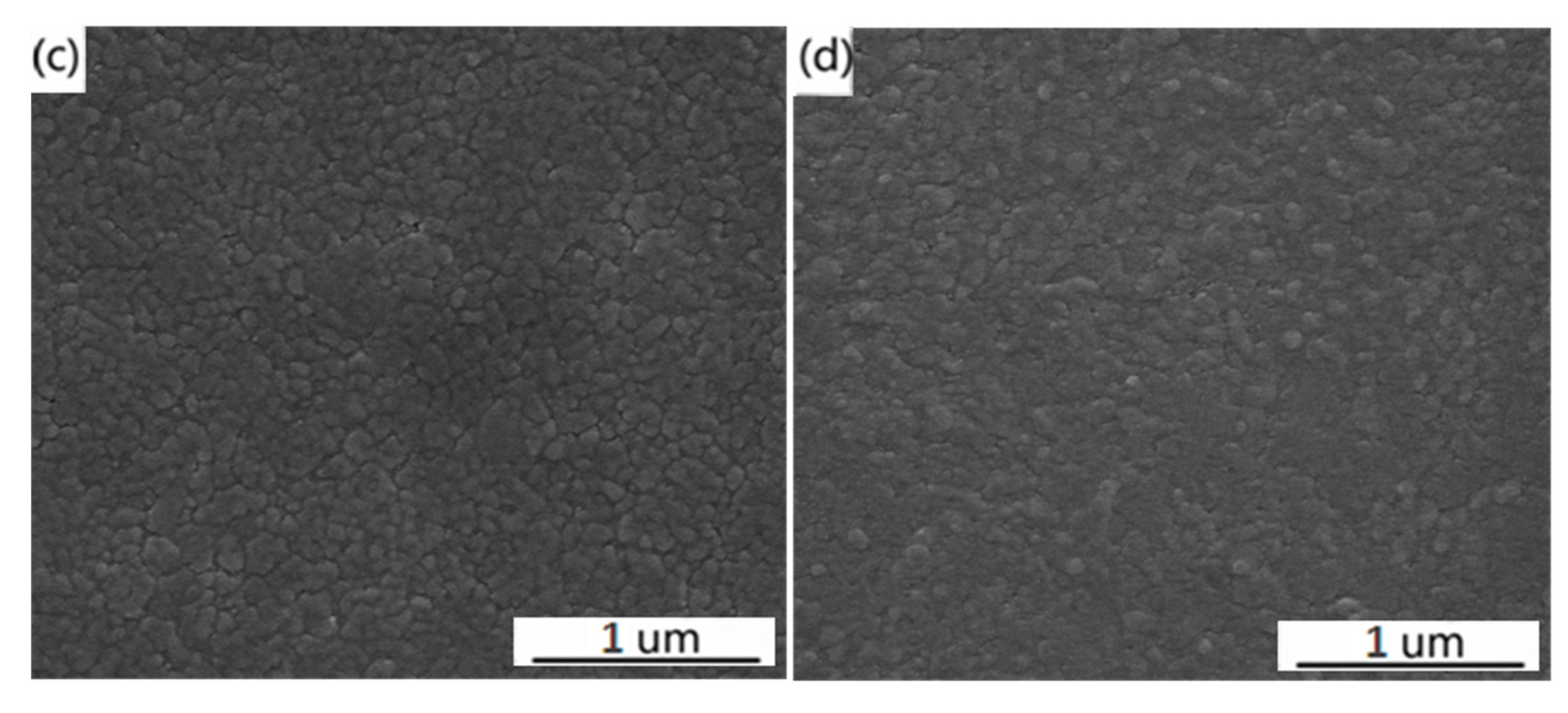
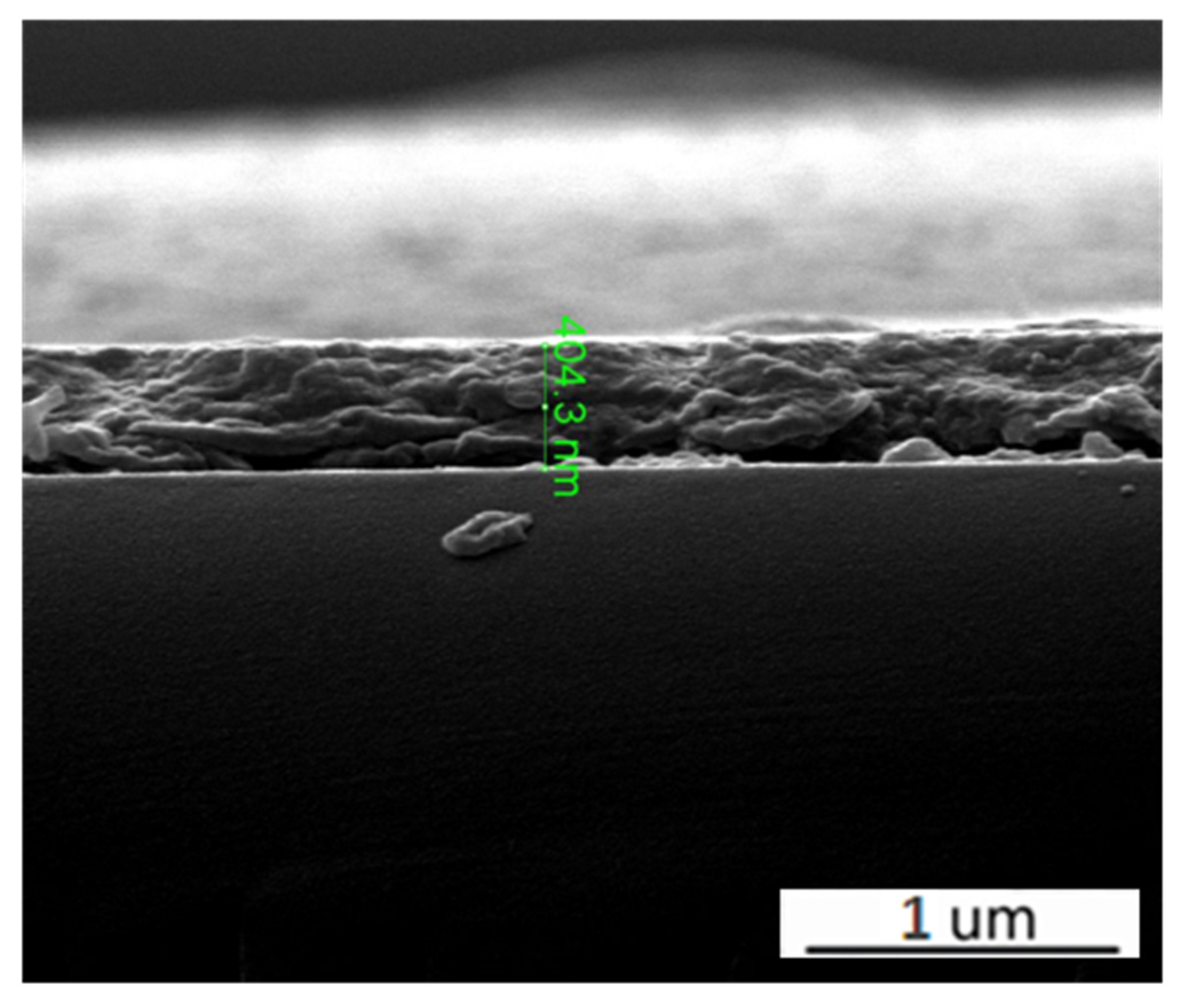
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Yi, D.; Wang, Y.; vant Erve, O.M.; Xu, L.; Yuan, H.; Veit, M.J.; Balakrishnan, P.P.; Choi, Y.; N’Diaye, A.T.; Shafer, P.; et al. Emergent electric field control of phase transformation in oxide superlattices. Nat. Commun. 2020, 11, 902. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Hu, G.; Starchenko, V.; Wan, G.; Dufresne, E.M.; Dong, Y.; Liu, H.; Zhou, H.; Jeen, H.; Saritas, K.; et al. Phase transition dynamics in a complex oxide heterostructure. Phys. Rev. Lett. 2022, 129, 235701. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Hippalgaonkar, K.; Yang, F.; Hong, J.; Ko, C.; Suh, J.; Liu, K.; Wang, K.; Urban, J.J.; Zhang, X.; et al. Anomalously low electronic thermal conductivity in metallic vanadium dioxide. Science 2017, 355, 371–374. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Lee, S.; Yang, S.; Delaire, O.; Wu, J.Q. Recent progress on physics and applications of vanadium dioxide. Mater. Today 2018, 21, 875–896. [Google Scholar] [CrossRef]
- Shao, Z.W.; Luo, H.J.; Jin, P. Recent progress in the phase-transition mechanism and modulation of vanadium dioxide materials. NPG Asia Mater. 2018, 10, 581–605. [Google Scholar] [CrossRef]
- Kim, H.; Kim, Y.; Kim, K.S.; Jeong, H.Y.; Jang, A.R.; Han, S.H.; Yoon, D.H.; Suh, K.S.; Shin, H.S.; Kim, T.; et al. Flexible thermochromic window based on hybridized VO2/graphene. ACS Nano 2013, 7, 5769–5776. [Google Scholar] [CrossRef]
- Yi, W.; Tsang, K.K.; Lam, S.K.; Bai, X.; Crowell, J.A.; Flores, E.A. Biological plausibility and stochasticity in scalable VO2 active memristor neurons. Nat. Commun. 2018, 9, 4661. [Google Scholar] [CrossRef]
- Jiang, W.; Zheng, T.; Wu, B.; Jiao, H.; Wang, X.; Chen, Y.; Zhang, X.; Peng, M.; Wang, H.; Lin, T.; et al. A versatile photodetector assisted by photovoltaic and bolometric effects. Light Sci. Appl. 2020, 9, 160. [Google Scholar] [CrossRef]
- Zhu, H.F.; Du, L.H.; Li, J.; Shi, Q.W.; Peng, B.; Li, Z.R.; Huang, W.X.; Zhu, L.G. Near-perfect terahertz wave amplitude modulation enabled by impedance matching in VO2 thin films. Appl. Phys. Lett. 2018, 112, 081103. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, Y.; Shi, Q.; Liang, S.; Huang, W.; Kou, W.; Yang, Z. Dynamic photoinduced controlling of the large phase shift of terahertz waves via vanadium dioxide coupling nanostructures. ACS Photonics 2018, 5, 3040–3050. [Google Scholar] [CrossRef]
- Li, Z.; Cao, C.; Li, M.; Wang, L.; Zhu, D.; Xu, F.; Huang, A.; Jin, P.; Yu, L.; Cao, X. Gradient variation oxygen-content vanadium-oxygen composite films with enhanced crystallinity and excellent durability for smart windows. ACS Appl. Mater. Interfaces 2023, 15, 9401–9411. [Google Scholar] [CrossRef] [PubMed]
- Muller, A.; Khadar, R.A.; Abel, T.; Negm, N.; Rosca, T.; Krammer, A.; Cavalieri, M.; Schueler, A.; Qaderi, F.; Bolten, J.; et al. Radio-frequency characteristics of Ge-doped vanadium dioxide thin films with increased transition temperature. ACS Appl. Electron. Mater. 2020, 2, 1263–1272. [Google Scholar] [CrossRef]
- Mutilin, S.V.; Yakovkina, L.V.; Seleznev, V.A.; Prinz, V.Y. Kinetics of catalyst-free and position-controlled low-pressure chemical vapor deposition growth of VO2 nanowire arrays on nanoimprinted Si substrates. Materials 2022, 15, 7863. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Huang, W.; Zhang, Y.; Qiao, S.; Wu, J.; Zhao, D.; Yan, J. Enhanced hydrophilicity of the Si substrate for deposition of VO2 film by sol-gel method. J. Mater. Sci. Mater. Electron. 2012, 23, 1610–1615. [Google Scholar] [CrossRef]
- Shi, Q.; Huang, W.; Lu, T.; Zhang, Y.; Yue, F.; Qiao, S.; Xiao, Y. Nanostructured VO2 film with high transparency and enhanced switching ratio in THz range. Appl. Phys. Lett. 2014, 104, 071903. [Google Scholar] [CrossRef]
- Wu, J.; Huang, W.; Shi, Q.; Cai, J.; Zhao, D.; Zhang, Y.; Yan, J. Effect of annealing temperature on thermochromic properties of vanadium dioxide thin films deposited by organic sol-gel method. Appl. Surf. Sci. 2013, 268, 556–560. [Google Scholar] [CrossRef]
- Pattanayak, M.; Hoque, M.N.F.; Ho, Y.C.; Li, W.; Fan, Z.; Bernussi, A.A. Ultrahigh tunability of resistive switching in strongly correlated functional oxide. Appl. Mater. Today 2023, 30, 101642. [Google Scholar] [CrossRef]
- Outón, J.; Casas-Acuña, A.; Domínguez, M.; Blanco, E.; Delgado, J.J.; Ramírez-del-Solar, M. Novel laser texturing of W-doped VO2 thin film for the improvement of luminous transmittance in smart windows application. Appl. Surf. Sci. 2022, 608, 155180. [Google Scholar] [CrossRef]
- Sun, K.; Wheeler, C.; Hillier, J.A.; Ye, S.; Zeimpekis, I.; Urbani, A.; Kalfagiannis, N.; Muskens, O.L.; de Groot, C.H. Room temperature phase transition of W-doped VO2 by atomic layer deposition on 200 mm Si wafers and flexible substrates. Adv. Opt. Mater. 2023, 10, 2201326. [Google Scholar] [CrossRef]
- Shi, J.; Zhou, S.; You, B.; Wu, L. Preparation and thermochromic property of tungsten-doped vanadium dioxide particles. Sol. Energy Mater. Sol. Cells 2007, 91, 1856–1862. [Google Scholar] [CrossRef]
- Jin, P.; Nakao, S.; Tanemura, S. Tungsten doping into vanadium dioxide thermochromic films by high-energy ion implantation and thermal annealing. Thin Solid Film. 1998, 324, 151–158. [Google Scholar] [CrossRef]
- Shi, Q.; Huang, W.; Yan, J.; Zhang, Y.; Mao, M.; Zhang, Y.; Xu, Y.; Zhang, Y. Preparation and phase transition characterization of VO2 thin film on single crystal Si (100) substrate by sol-gel process. J. Sol-Gel Sci. Technol. 2011, 59, 591–597. [Google Scholar] [CrossRef]
- Jepsen, P.U.; Fischer, B.M.; Thoman, A.; Helm, H.; Suh, J.Y.; Lopez, R.; Haglund, R.F., Jr. Metal-insulator phase transition in a VO2 thin film observed with terahertz spectroscopy. Phys. Rev. B 2006, 74, 205103. [Google Scholar] [CrossRef]
- Zhang, Z.; Gao, Y.; Chen, Z.; Du, J.; Cao, C.; Kang, L.; Luo, H. Thermochromic VO2 Thin Films: Solution-Based Processing, Improved Optical Properties, and Lowered Phase Transformation Temperature. Langmuir 2010, 26, 10738–10744. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Jiang, W.; Liu, H. Synthesis and electrical properties of tungsten-doped vanadium dioxide nanopowders by thermolysis. J. Phys. Chem. C 2007, 111, 1119–1122. [Google Scholar] [CrossRef]
- Liang, S.; Shi, Q.; Zhu, H.; Peng, B.; Huang, W. One-Step Hydrothermal Synthesis of W-Doped VO2 (M) Nanorods with a Tunable Phase-Transition Temperature for Infrared Smart Windows. ACS Omega 2016, 1, 1139–1148. [Google Scholar] [CrossRef]
- Continenza, A.; Massidda, S.; Posternak, M. Self-energy corrections in VO2 within a model GW scheme. Phys. Rev. B 1999, 60, 15699–15704. [Google Scholar] [CrossRef]
- Goodenough, J.B. The two components of the crystallographic transition in VO2. J. Solid State Chem. 1971, 3, 490–500. [Google Scholar] [CrossRef]
- Lopez, R.; Haynes, T.E.; Boatner, L.A.; Feldman, L.C.; Haglund, R.F. Size effects in the structural phase transition of VO2 nanoparticles. Phys. Rev. B 2002, 65. [Google Scholar] [CrossRef]
- Du, J.; Gao, Y.; Luo, H.; Kang, L.; Zhang, Z.; Chen, Z.; Cao, C. Significant changes in phase-transition hysteresis for Ti-doped VO2 films prepared by polymer-assisted deposition. Sol. Energy Mater. Sol. Cells 2011, 95, 469–475. [Google Scholar] [CrossRef]
- Liu, D.; Cheng, H.; Xing, X.; Zhang, C.; Zheng, W. Thermochromic properties of W-doped VO2 thin films deposited by aqueous sol-gel method for adaptive infrared stealth application. Infrared Phys. Technol. 2016, 77, 339–343. [Google Scholar] [CrossRef]
- Wan, D.; Xiong, P.; Chen, L.; Shi, S.; Ishaq, A.; Luo, H.; Gao, Y. High-performance thermal sensitive W-doped VO2(B) thin film and its identification by first-principles calculations. Appl. Surf. Sci. 2017, 397, 30–39. [Google Scholar] [CrossRef]
- Takami, H.; Kanki, T.; Ueda, S.; Kobayashi, K.; Tanaka, H. Electronic Structure of W-Doped VO2 Thin Films with Giant Metal-Insulator Transition Investigated by Hard X-ray Core-Level Photoemission Spectroscopy. Appl. Phys. Express 2010, 3, 063201. [Google Scholar] [CrossRef]
- Reyes, J.M.; Sayer, M.; Mansingh, A.; Chen, R. Transport Properties of Impurity-Doped VO2. Can. J. Phys. 1976, 54, 413–423. [Google Scholar] [CrossRef]
- Mai, L.Q.; Hu, B.; Hu, T.; Chen, W.; Gu, E.D. Electrical property of Mo-doped VO2 nanowire array film by melting- quenching sol-gel method. J. Phys. Chem. B 2006, 110, 19083–19086. [Google Scholar] [CrossRef] [PubMed]
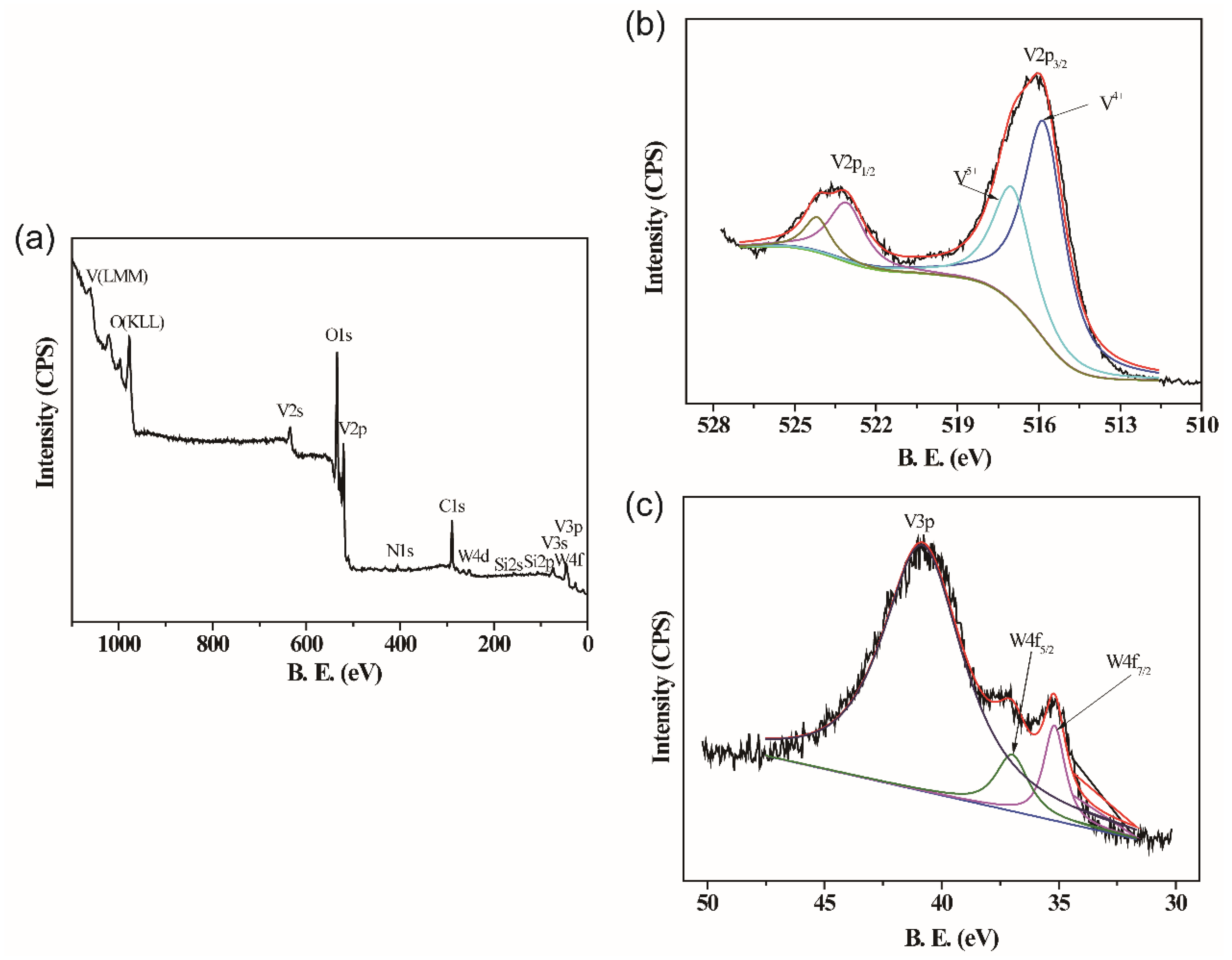


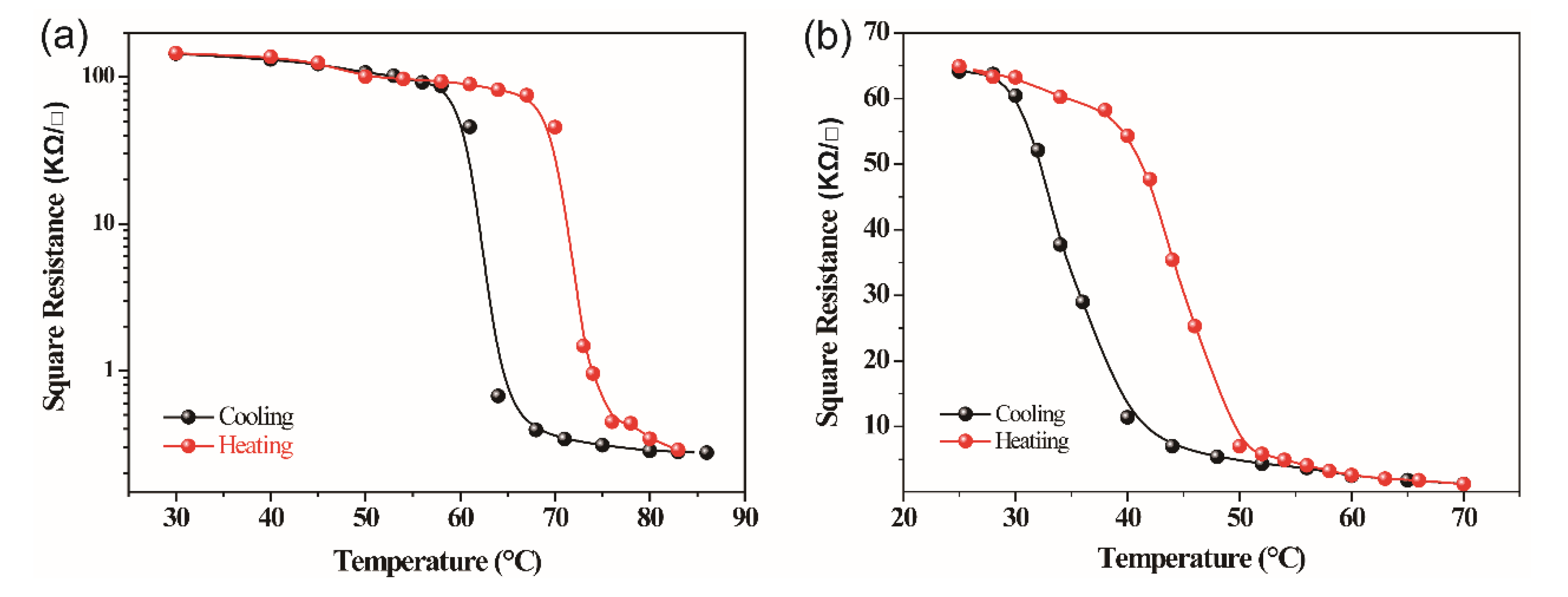
| Doping Level (at.%) | Theating (°C) | Tcooling (°C) | Tc (°C) | ΔT (°C) |
|---|---|---|---|---|
| Heating | Cooling | |||
| 0 | 72.3 | 62.4 | 67.35 | 9.9 |
| 0.61 | 45.3 | 50.5 | 47.9 | 5.2 |
| 1.12 | 37.4 | 43.4 | 40.4 | 6.0 |
| 1.62 | 28.7 | 38.0 | 33.35 | 9.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, X.; Li, Y.; Zhang, Y. Sol-Gel Derived Tungsten Doped VO2 Thin Films on Si Substrate with Tunable Phase Transition Properties. Molecules 2023, 28, 3778. https://doi.org/10.3390/molecules28093778
Ding X, Li Y, Zhang Y. Sol-Gel Derived Tungsten Doped VO2 Thin Films on Si Substrate with Tunable Phase Transition Properties. Molecules. 2023; 28(9):3778. https://doi.org/10.3390/molecules28093778
Chicago/Turabian StyleDing, Xiaoming, Yanli Li, and Yubo Zhang. 2023. "Sol-Gel Derived Tungsten Doped VO2 Thin Films on Si Substrate with Tunable Phase Transition Properties" Molecules 28, no. 9: 3778. https://doi.org/10.3390/molecules28093778
APA StyleDing, X., Li, Y., & Zhang, Y. (2023). Sol-Gel Derived Tungsten Doped VO2 Thin Films on Si Substrate with Tunable Phase Transition Properties. Molecules, 28(9), 3778. https://doi.org/10.3390/molecules28093778





