A Computational Evaluation of the Steric and Electronic Contributions in Stereoselective Olefin Polymerization with Pyridylamido-Type Catalysts
Abstract
1. Introduction
2. Results and Discussion
3. Methodology
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Sample Availability
References
- Resconi, L.; Cavallo, L.; Fait, A.; Piemontesi, F. Selectivity in Propene Polymerization with Metallocene Catalysts. Chem. Rev. 2000, 100, 1253–1346. [Google Scholar] [CrossRef] [PubMed]
- Correa, A.; Talarico, G.; Cavallo, L. Regiochemistry of propene insertion with group 4 polymerization catalysts from a theoretical perspective. J. Organomet. Chem. 2007, 692, 4519–4527. [Google Scholar] [CrossRef]
- Busico, V.; Cipullo, R.; Talarico, G.; Segre, A.L.; Caporaso, L. High-Field 13C NMR Characterization of Ethene-1-13C/Propene Copolymers Prepared with Cs-Symmetric ansa-Metallocene Catalysts: A Deeper Insight into the Regio- and Stereoselectivity of Syndiotactic Propene Polymerization. Macromolecules 1998, 31, 8720–8724. [Google Scholar] [CrossRef]
- Talarico, G.; Budzelaar, P.H.M. Variability of Chain Transfer to Monomer Step in Olefin Polymerization. Organometallics 2008, 27, 4098–4107. [Google Scholar] [CrossRef]
- Talarico, G.; Budzelaar, P.H.M. A Second Transition State for Chain Transfer to Monomer in Olefin Polymerization Promoted by Group 4 Metal Catalysts. J. Am. Chem. Soc. 2006, 128, 4524–4525. [Google Scholar] [CrossRef]
- Cavallo, L.; Guerra, G. A Density Functional and Molecular Mechanics Study of β-Hydrogen Transfer in Homogeneous Ziegler–Natta Catalysis. Macromolecules 1996, 29, 2729–2737. [Google Scholar] [CrossRef]
- De Rosa, C.; Auriemma, F. Single site metallorganic polymerization catalysis as a method to probe the properties of polyolefins. Polym. Chem. 2011, 2, 2155. [Google Scholar] [CrossRef]
- Baier, M.C.; Zuideveld, M.A.; Mecking, S. Post-Metallocenes in the Industrial Production of Polyolefins. Angew. Chem. Int. Ed. 2014, 53, 9722–9744. [Google Scholar] [CrossRef]
- Domski, G.J.; Edson, J.B.; Keresztes, I.; Lobkovsky, E.B.; Coates, G.W. Synthesis of a new olefin polymerization catalyst supported by an sp3-C donor via insertion of a ligand-appended alkene into the Hf–C bond of a neutral pyridylamidohafnium trimethyl complex. Chem. Commun. 2008, 46, 6137–6139. [Google Scholar] [CrossRef]
- Domski, G.J.; Eagan, J.M.; De Rosa, C.; Di Girolamo, R.; LaPointe, A.M.; Lobkovsky, E.B.; Talarico, G.; Coates, G.W. Combined Experimental and Theoretical Approach for Living and Isoselective Propylene Polymerization. ACS Catal. 2017, 7, 6930–6937. [Google Scholar] [CrossRef]
- Boussie, T.R.; Diamond, G.M.; Goh, C.; Hall, K.A.; LaPointe, A.M.; Leclerc, M.K.; Murphy, V.; Shoemaker, J.A.W.; Turner, H.; Rosen, R.K.; et al. Nonconventional Catalysts for Isotactic Propene Polymerization in Solution Developed by Using High-Throughput-Screening Technologies. Angew. Chem. Int. Ed. 2006, 45, 3278–3283. [Google Scholar] [CrossRef] [PubMed]
- Cueny, E.S.; Johnson, H.C.; Anding, B.J.; Landis, C.R. Mechanistic Studies of Hafnium-Pyridyl Amido-Catalyzed 1-Octene Polymerization and Chain Transfer Using Quench-Labeling Methods. J. Am. Chem. Soc. 2017, 139, 11903–11912. [Google Scholar] [CrossRef] [PubMed]
- Shang, R.; Gao, H.; Luo, F.; Li, Y.; Wang, B.; Ma, Z.; Pan, L.; Li, Y. Functional Isotactic Polypropylenes via Efficient Direct Copolymerizations of Propylene with Various Amino-Functionalized α-Olefins. Macromolecules 2019, 52, 9280–9290. [Google Scholar] [CrossRef]
- Arriola, D.J.; Carnahan, E.M.; Hustad, P.D.; Kuhlman, R.L.; Wenzel, T.T. Catalytic Production of Olefin Block Copolymers via Chain Shuttling Polymerization. Science 2006, 312, 714–719. [Google Scholar] [CrossRef]
- Rocchigiani, L.; Busico, V.; Pastore, A.; Talarico, G.; Macchioni, A. Unusual Hafnium–Pyridylamido/ERn Heterobimetallic Adducts (ERn=ZnR2 or AlR3). Angew. Chem. Int. Ed. 2014, 53, 2157–2161. [Google Scholar] [CrossRef] [PubMed]
- Auriemma, F.; De Rosa, C.; Scoti, M.; Di Girolamo, R.; Malafronte, A.; Talarico, G.; Carnahan, E. Unveiling the molecular structure of ethylene/1-octene multi-block copolymers from chain shuttling technology. Polymer 2018, 154, 298–304. [Google Scholar] [CrossRef]
- Urciuoli, G.; Vittoria, A.; Talarico, G.; Luise, D.; De Rosa, C.; Busico, V.; Cipullo, R.; Ruiz de Ballesteros, O.; Auriemma, F. In-Depth Analysis of the Nonuniform Chain Microstructure of Multiblock Copolymers from Chain-Shuttling Polymerization. Macromolecules 2021, 54, 10891–10902. [Google Scholar] [CrossRef]
- Froese, R.D.J.; Hustad, P.D.; Kuhlman, R.L.; Wenzel, T.T. Mechanism of activation of a hafnium pyridyl–amide olefin polymerization catalyst: Ligand modification by monomer. J. Am. Chem. Soc. 2007, 129, 7831–7840. [Google Scholar] [CrossRef]
- Zuccaccia, C.; Busico, V.; Cipullo, R.; Talarico, G.; Froese, R.D.; Vosejpka, P.C.; Hustad, P.D.; Macchioni, A. On the first insertion of α-olefins in hafnium pyridyl-amido polymerization catalysts. Organometallics 2009, 28, 5445–5458. [Google Scholar] [CrossRef]
- Zuccaccia, C.; Macchioni, A.; Busico, V.; Cipullo, R.; Talarico, G.; Alfano, F.; Boone, H.W.; Frazier, K.A.; Hustad, P.D.; Stevens, J.C.; et al. Intra- and Intermolecular NMR Studies on the Activation of Arylcyclometallated Hafnium Pyridyl-Amido Olefin Polymerization Precatalysts. J. Am. Chem. Soc. 2008, 130, 10354–10368. [Google Scholar] [CrossRef]
- Busico, V.; Cipullo, R.; Pellecchia, R.; Rongo, L.; Talarico, G.; Macchioni, A.; Zuccaccia, C.; Froese, R.D.J.; Hustad, P.D. “Uni et Trini”: In Situ Diversification of (Pyridylamide)hafnium(IV) Catalysts. Macromolecules 2009, 42, 4369–4373. [Google Scholar] [CrossRef]
- De Rosa, C.; Di Girolamo, R.; Talarico, G. Expanding the Origin of Stereocontrol in Propene Polymerization Catalysis. ACS Catal. 2016, 6, 3767–3770. [Google Scholar] [CrossRef]
- Miller, S.A.; Bercaw, J.E. Mechanism of Isotactic Polypropylene Formation with C1-Symmetric Metallocene Catalysts. Organometallics 2006, 25, 3576–3592. [Google Scholar] [CrossRef]
- Cossee, P. Ziegler-Natta catalysis I. Mechanism of polymerization of α-olefins with Ziegler-Natta catalysts. J. Catal. 1964, 3, 80–88. [Google Scholar] [CrossRef]
- Cicolella, A.; Romano, E.; Barone, V.; De Rosa, C.; Talarico, G. Metallocenes and Beyond for Propene Polymerization: Energy Decomposition of Density Functional Computations Unravels the Different Interplay of Stereoelectronic Effects. Organometallics 2022, 41, 3872–3883. [Google Scholar] [CrossRef]
- Romano, E.; Budzelaar, P.H.M.; De Rosa, C.; Talarico, G. Unconventional Stereoerror Formation Mechanisms in Nonmetallocene Propene Polymerization Systems Revealed by DFT Calculations. J. Phys. Chem. A 2022, 126, 6203–6209. [Google Scholar] [CrossRef] [PubMed]
- Falivene, L.; Cao, Z.; Petta, A.; Serra, L.; Poater, A.; Oliva, R.; Scarano, V.; Cavallo, L. Towards the online computer-aided design of catalytic pockets. Nat. Chem. 2019, 11, 872–879. [Google Scholar] [CrossRef]
- Falivene, L.; Cavallo, L.; Talarico, G. Buried Volume Analysis for Propene Polymerization Catalysis Promoted by Group 4 Metals: A Tool for Molecular Mass Prediction. ACS Catal. 2015, 5, 6815–6822. [Google Scholar] [CrossRef]
- Vermeeren, P.; van der Lubbe, S.C.C.; Fonseca Guerra, C.; Bickelhaupt, F.M.; Hamlin, T.A. Understanding chemical reactivity using the activation strain model. Nat. Protoc. 2020, 15, 649–667. [Google Scholar] [CrossRef]
- Bickelhaupt, F.M.; Houk, K.N. Analyzing Reaction Rates with the Distortion/Interaction-Activation Strain Model. Angew. Chem. Int. Ed. 2017, 56, 10070–10086. [Google Scholar] [CrossRef]
- Zhao, Y.; Xu, X.; Wang, Y.; Liu, T.; Li, H.; Zhang, Y.; Wang, L.; Wang, X.; Zhao, S.; Luo, Y. Ancillary ligand effects on α-olefin polymerization catalyzed by zirconium metallocene: A computational study. RSC Adv. 2022, 12, 21111–21121. [Google Scholar] [CrossRef] [PubMed]
- Falivene, L.; Kozlov, S.M.; Cavallo, L. Constructing Bridges between Computational Tools in Heterogeneous and Homogeneous Catalysis. ACS Catal. 2018, 8, 5637–5656. [Google Scholar] [CrossRef]
- Corradini, P.; Guerra, G.; Cavallo, L. Do New Century Catalysts Unravel the Mechanism of Stereocontrol of old Ziegler–Natta Catalysts? Acc. Chem. Res. 2004, 37, 231–241. [Google Scholar] [CrossRef]
- Bahri-Laleh, N.; Hanifpour, A.; Mirmohammadi, S.A.; Poater, A.; Nekoomanesh-Haghighi, M.; Talarico, G.; Cavallo, L. Computational modeling of heterogeneous Ziegler-Natta catalysts for olefins polymerization. Prog. Polym. Sci. 2018, 84, 89–114. [Google Scholar] [CrossRef]
- Kulyabin, P.S.; Uborsky, D.V.; Voskoboynikov, A.Z.; Canich, J.A.M.; Hagadorn, J.R. Pyridylamido hafnium complexes with a silylene bridge: Synthesis and olefin polymerization. Dalton Trans. 2020, 49, 6693–6702. [Google Scholar] [CrossRef] [PubMed]
- Luconi, L.; Rossin, A.; Tuci, G.; Tritto, I.; Boggioni, L.; Klosin, J.J.; Theriault, C.N.; Giambastiani, G. Facing unexpected reactivity paths with ZrIV–pyridylamido polymerization catalysts. Chem. Eur. J. 2012, 18, 671–687. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zuccaccia, C.; Tedesco, C.; D’Auria, I.; Macchioni, A.; Pellecchia, C. NMR Spectroscopy and X-Ray Characterisation of Cationic N-Heteroaryl-Pyridylamido Zr(IV) Complexes: A Further Level of Complexity for the Elusive Active Species of Pyridylamido Olefin Polymerisation Catalysts. Chem. Eur. J. 2014, 20, 232–244. [Google Scholar] [CrossRef]
- Annunziata, L.; Pappalardo, D.; Tedesco, C.; Pellecchia, C. Isotactic-specific polymerization of propene by a Cs-symmetric zirconium (IV) complex bearing a dianionic tridentate [−NNN−] amidomethylpyrrolidepyridine ligand. Macromolecules 2009, 42, 5572–5578. [Google Scholar] [CrossRef]
- De Rosa, C.; Di Girolamo, R.; Muñoz-García, A.B.; Pavone, M.; Talarico, G. Breaking Symmetry Rules Enhance the Options for Stereoselective Propene Polymerization Catalysis. Macromolecules 2020, 53, 2959–2964. [Google Scholar] [CrossRef]
- Talarico, G.; Budzelaar, P.H.M. Analysis of Stereochemistry Control in Homogeneous Olefin Polymerization Catalysis. Organometallics 2014, 33, 5974–5982. [Google Scholar] [CrossRef]
- Falivene, L.; Barone, V.; Talarico, G. Unraveling the role of entropy in tuning unimolecular vs. bimolecular reaction rates: The case of olefin polymerization catalyzed by transition metals. Mol. Catal. 2018, 452, 138–144. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision A.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Becke, A. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P. Density-functional approximation for the correlation energy of the inhomogeneous electron gas. Phys. Rev. B 1986, 33, 8822–8824, Erratum in Phys. Rev. B 1986, 34, 7406–7406. [Google Scholar]
- Schäfer, A.; Horn, H.; Ahlrichs, R. Fully optimized contracted Gaussian basis sets for atoms lithium to krypton. J. Chem. Phys. 1992, 97, 2571–2577. [Google Scholar] [CrossRef]
- Hay, P.J.; Wadt, W.R. Ab initio effective core potentials for molecular calculations. Potentials for K to Au including the outermost core orbitals. J. Chem. Phys. 1985, 82, 299–310. [Google Scholar] [CrossRef]
- Weigend, F.; Furche, F.; Ahlrichs, R. Gaussian basis sets of quadruple zeta valence quality for atoms H–Kr. J. Chem. Phys. 2003, 119, 12753–12762. [Google Scholar] [CrossRef]
- Wadt, W.R.; Hay, P.J. Ab initio effective core potentials for molecular calculations. Potentials for main group elements Na to Bi. J. Chem. Phys. 1985, 82, 284–298. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Barone, V.; Cossi, M. Quantum Calculation of Molecular Energies and Energy Gradients in Solution by a Conductor Solvent Model. J. Phys. Chem. A 1998, 102, 1995–2001. [Google Scholar] [CrossRef]
- Glendening, E.D. Natural Energy Decomposition Analysis: Explicit Evaluation of Electrostatic and Polarization Effects with Application to Aqueous Clusters of Alkali Metal Cations and Neutrals. J. Am. Chem. Soc. 1996, 118, 2473–2482. [Google Scholar] [CrossRef]
- Talarico, G.; Budzelaar, P.H.M. α-Agostic Interactions and Growing Chain Orientation for Olefin Polymerization Catalysts. Organometallics 2016, 35, 47–54. [Google Scholar] [CrossRef]
- Eagan, J.M.; Xu, J.; Di Girolamo, R.; Thurber, C.M.; Macosko, C.W.; LaPointe, A.M.; Bates, F.S.; Coates, G.W. Combining polyethylene and polypropylene: Enhanced performance with PE/iPP multiblock polymers. Science 2017, 355, 814–816. [Google Scholar] [CrossRef] [PubMed]
- De Rosa, C.; Di Girolamo, R.; Auriemma, F.; D’Avino, M.; Talarico, G.; Cioce, C.; Scoti, M.; Coates, G.W.; Lotz, B. Oriented Microstructures of Crystalline-Crystalline Block Copolymers Induced by Epitaxy and Competitive and Confined Crystallization. Macromolecules 2016, 49, 5576–5586. [Google Scholar] [CrossRef]
- De Rosa, C.; Di Girolamo, R.; Malafronte, A.; Scoti, M.; Talarico, G.; Auriemma, F.; Ruiz de Ballesteros, O. Polyolefins based crystalline block copolymers: Ordered nanostructures from control of crystallization. Polymer 2020, 196, 122423. [Google Scholar] [CrossRef]
- Di Girolamo, R.; Santillo, C.; Malafronte, A.; Scoti, M.; De Stefano, F.; Talarico, G.; Coates, G.W.; De Rosa, C. Structure and morphology of isotactic polypropylene–polyethylene block copolymers prepared with living and stereoselective catalyst. Polym. Chem. 2022, 13, 2950–2963. [Google Scholar] [CrossRef]
- De Rosa, C.; Di Girolamo, R.; Auriemma, F.; Talarico, G.; Malafronte, A.; Scarica, C.; Scoti, M. Controlling Size and Orientation of Lamellar Microdomains in Crystalline Block Copolymers. ACS Appl. Mater. Interfaces 2017, 9, 31252–31259. [Google Scholar] [CrossRef]
- Domski, G.J.; Rose, J.M.; Coates, G.W.; Bolig, A.D.; Brookhart, M. Living alkene polymerization: New methods for the precision synthesis of polyolefins. Prog. Polym. Sci. 2007, 32, 30–92. [Google Scholar] [CrossRef]
- De Rosa, C.; Ruiz de Ballesteros, O.; Auriemma, F.; Talarico, G.; Scoti, M.; Di Girolamo, R.; Malafronte, A.; Piemontesi, F.; Liguori, D.; Camurati, I.; et al. Crystallization Behavior of Copolymers of Isotactic Poly(1-butene) with Ethylene from Ziegler–Natta Catalyst: Evidence of the Blocky Molecular Structure. Macromolecules 2019, 52, 9114–9127. [Google Scholar] [CrossRef]
- De Rosa, C.; Ruiz de Ballesteros, O.; Di Girolamo, R.; Malafronte, A.; Auriemma, F.; Talarico, G.; Scoti, M. The blocky structure of Ziegler–Natta “random” copolymers: Myths and experimental evidence. Polym. Chem. 2020, 11, 34–38. [Google Scholar] [CrossRef]
- Cipullo, R.; Busico, V.; Fraldi, N.; Pellecchia, R.; Talarico, G. Improving the Behavior of Bis(phenoxyamine) Group 4 Metal Catalysts for Controlled Alkene Polymerization. Macromolecules 2009, 42, 3869–3872. [Google Scholar] [CrossRef]
- Han, J.W.; Hollmann, F.; Luque, R.; Song, I.K.; Talarico, G.; Tatsumi, T.; Yan, N. Molecular Catalysis for the Chemistry of the future: A perspective. Mol. Catal. 2022, 522, 112233. [Google Scholar] [CrossRef]
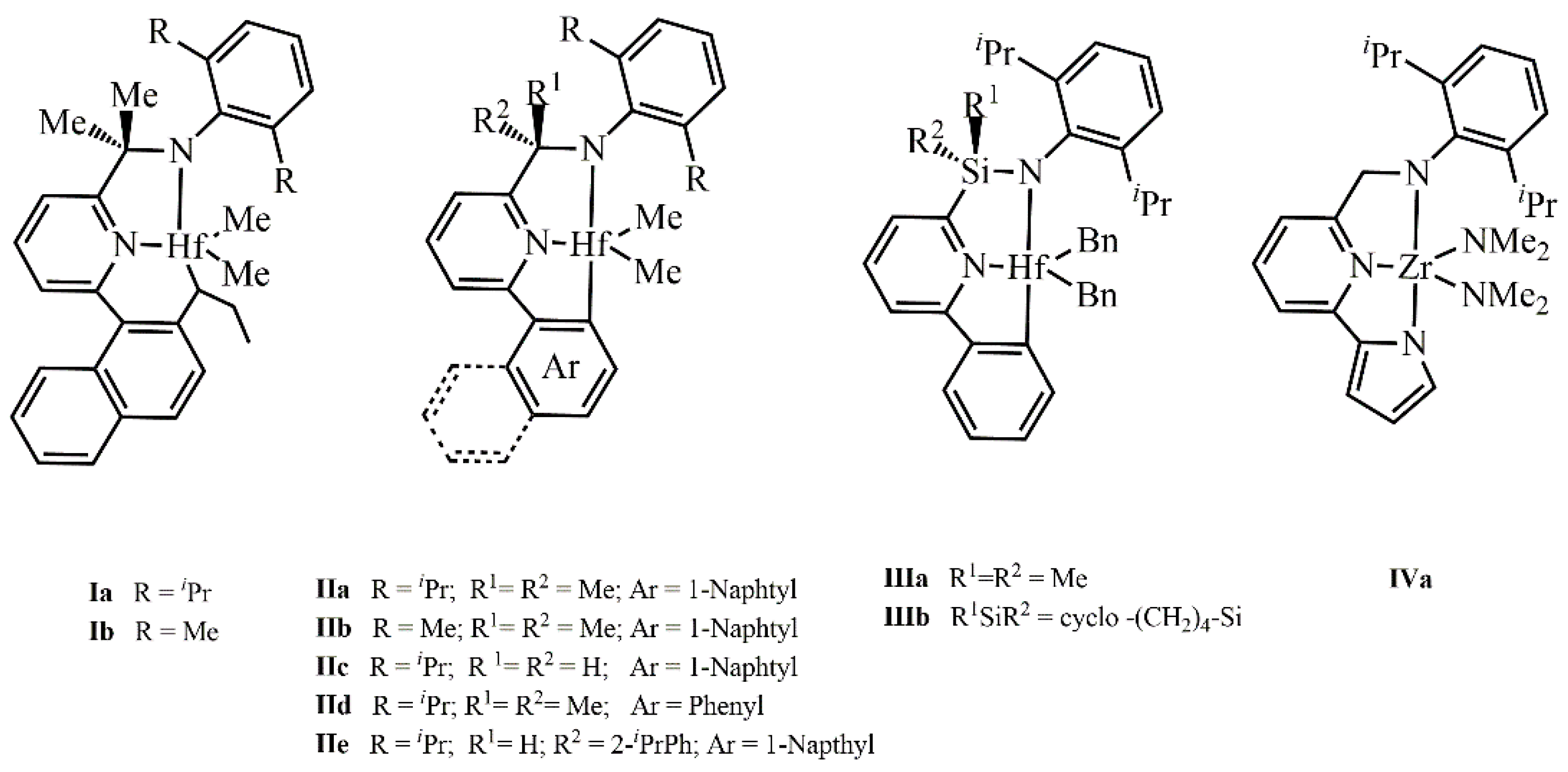
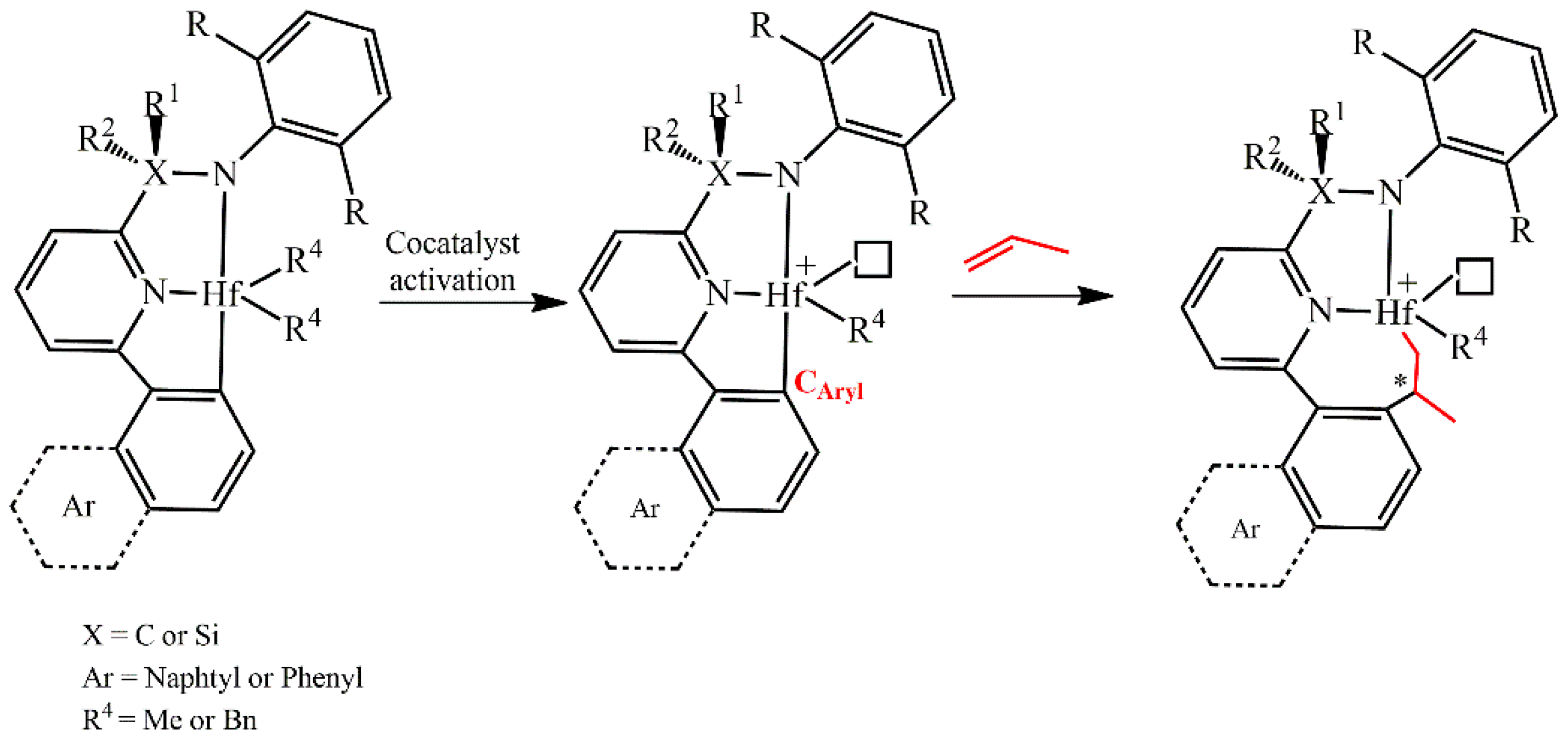
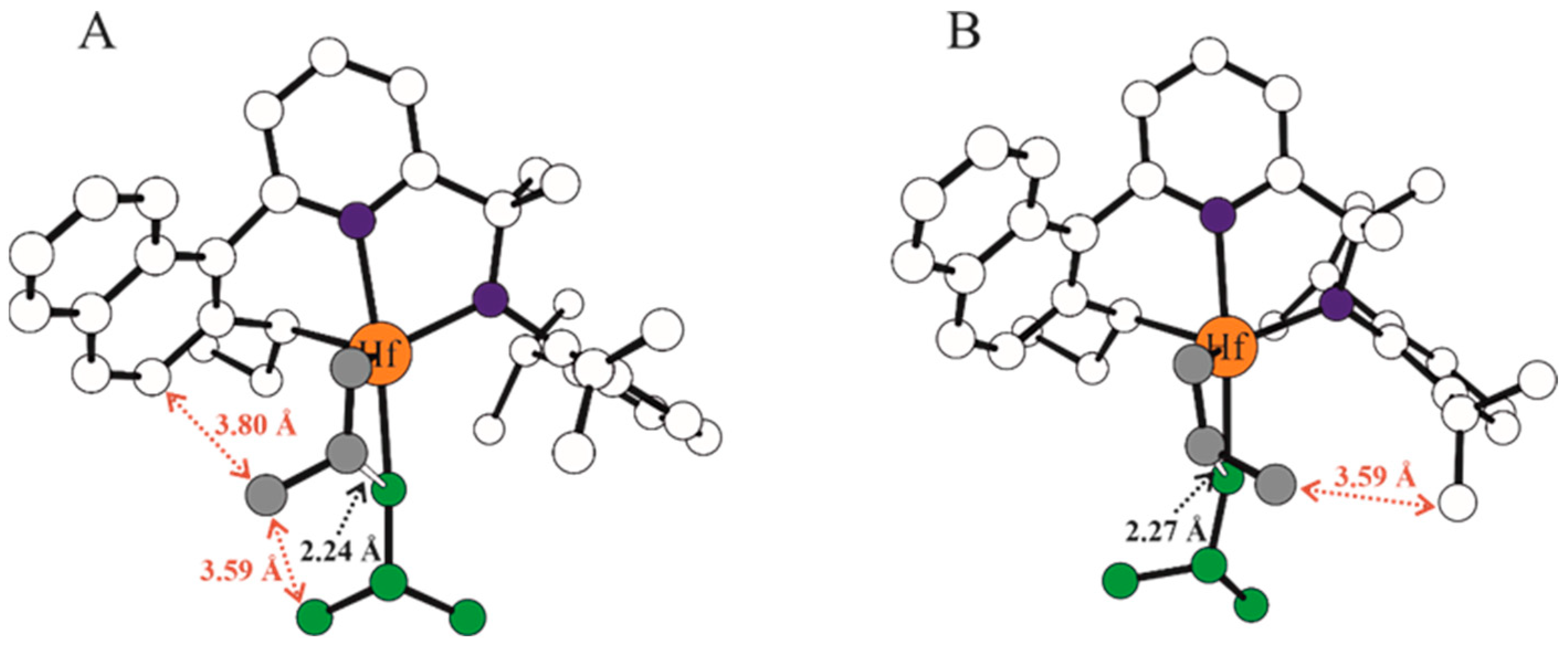
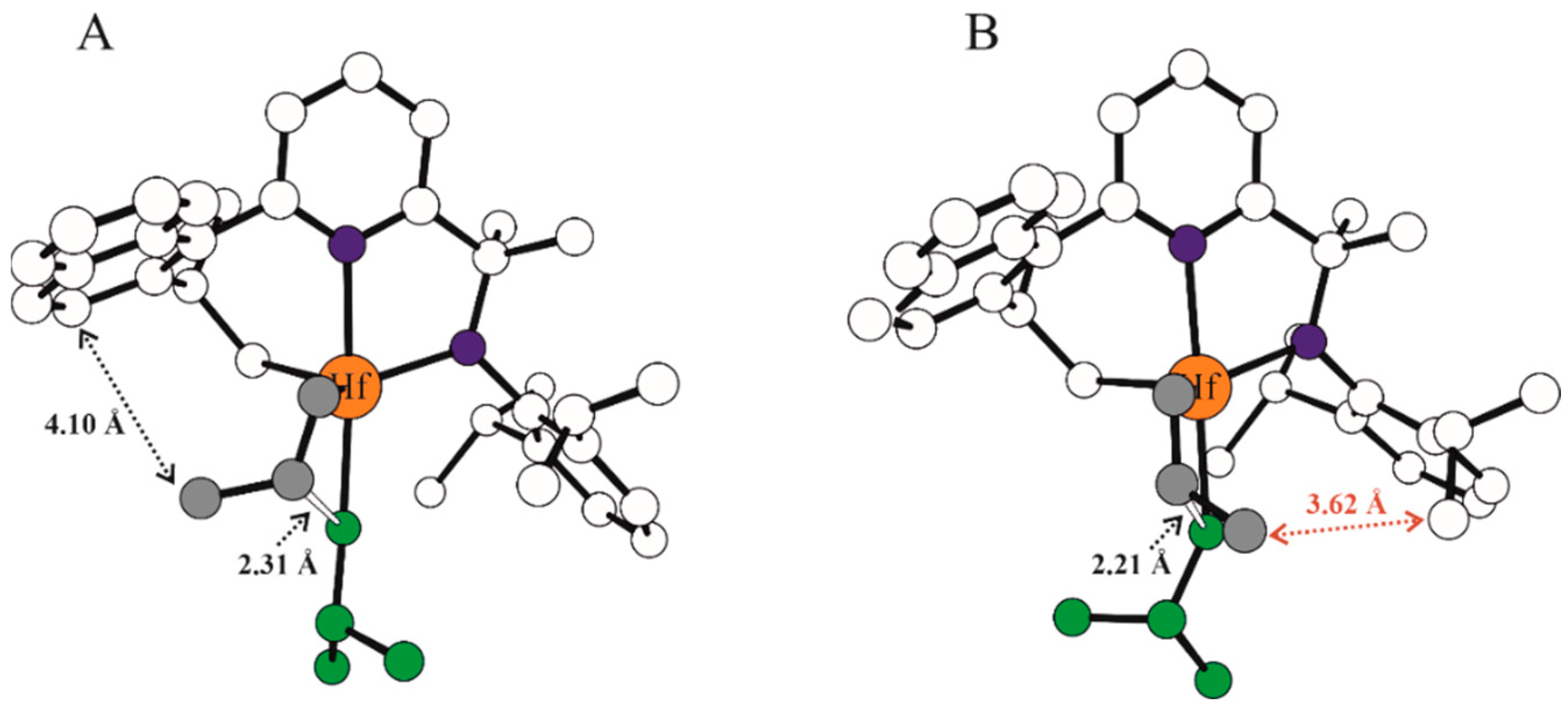
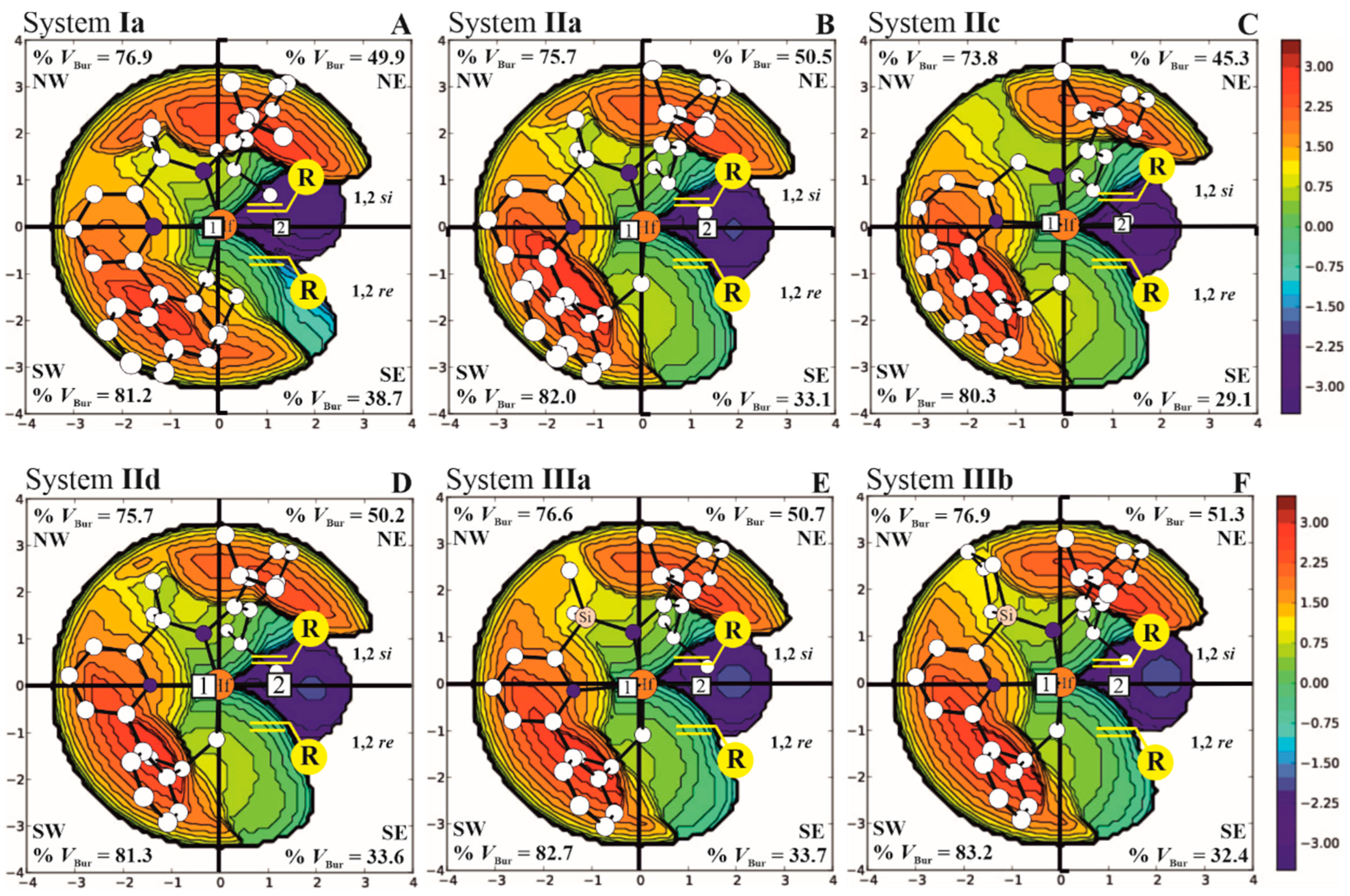
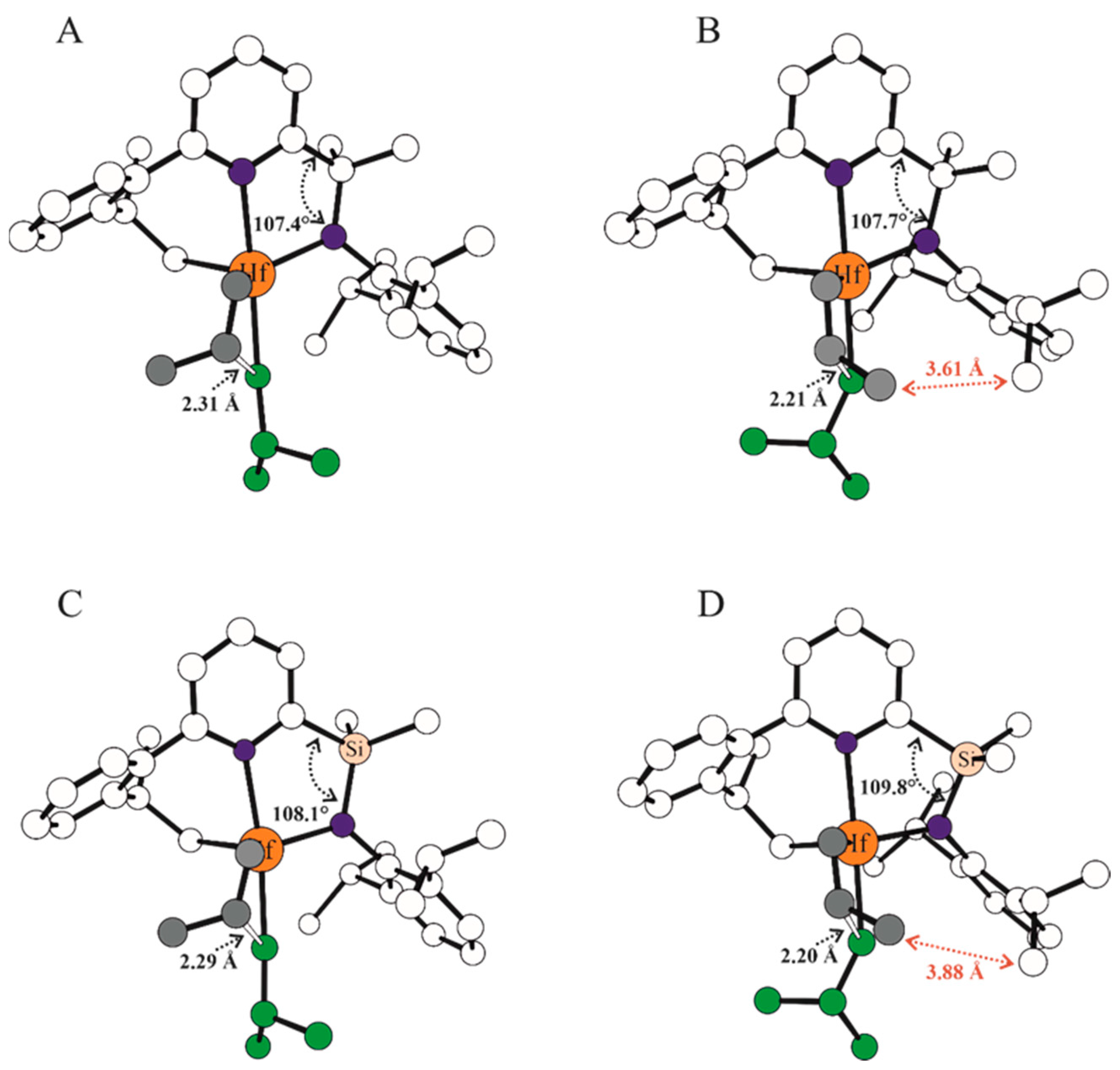
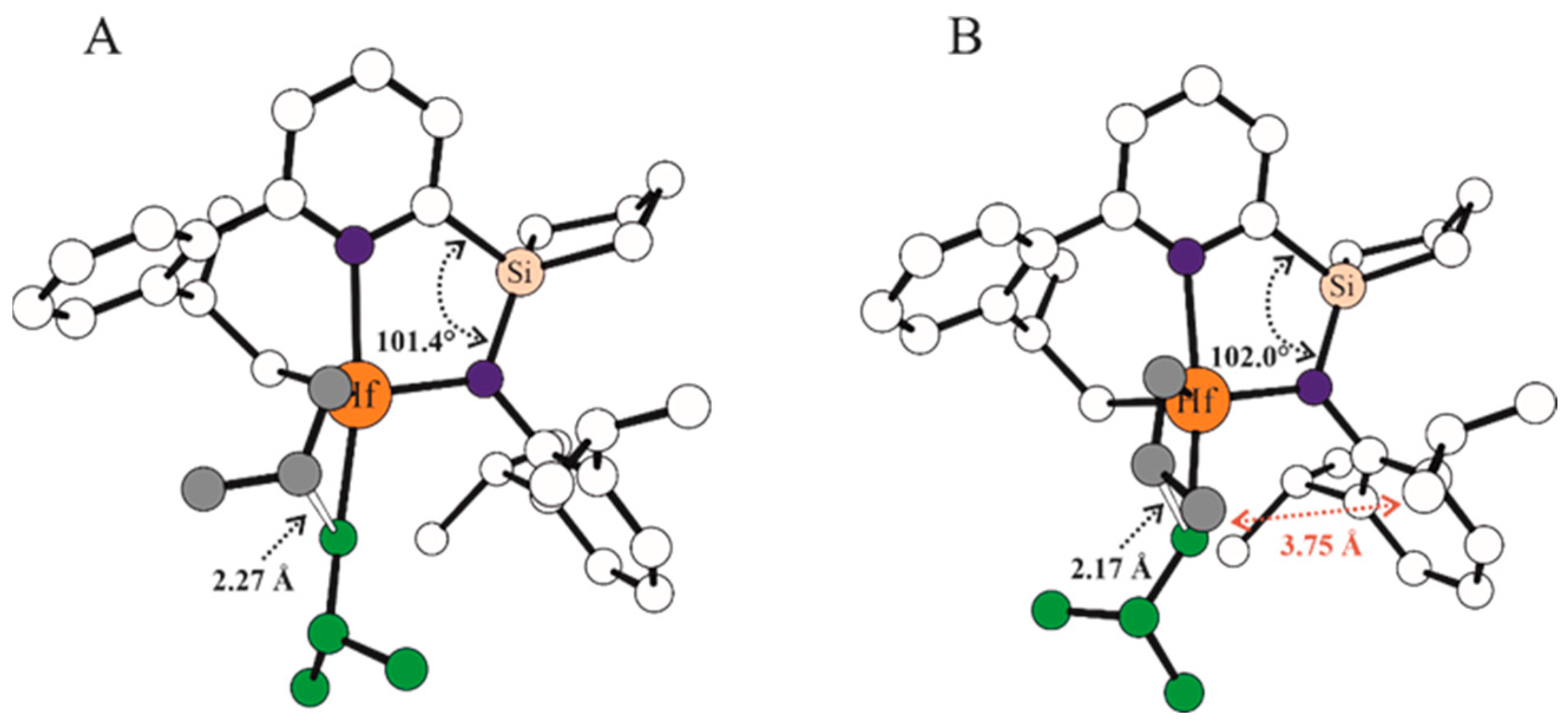
| Systems | ΔE(ΔG)Stereo a) | ΔE(ΔG)Stereo b) |
|---|---|---|
| Ia | 2.4 (1.4) | 2.8 (1.4) |
| Ib | −0.6 (−0.2) | −0.5 (−0.1) |
| IIa | 4.0 (3.1) | 3.6 (2.8) |
| IIb | 0.8 (0.5) | 0.5 (0.2) |
| IIc | 1.2 (1.4) | 1.3 (2.0) |
| IId | 3.8 (3.1) | 3.2 (2.5) |
| IIe | 3.8 (4.4) | 3.8 (4.1) |
| IIIa | 2.2 (2.5) | 2.2 (2.5) |
| IIIb | 4.6 (4.1) | 4.6 (4.1) |
| IVa (site 1) | 1.8 (2.4) | 2.2 (2.4) |
| IVa (site 2) | 3.1 (2.4) | 3.6 (2.9) |
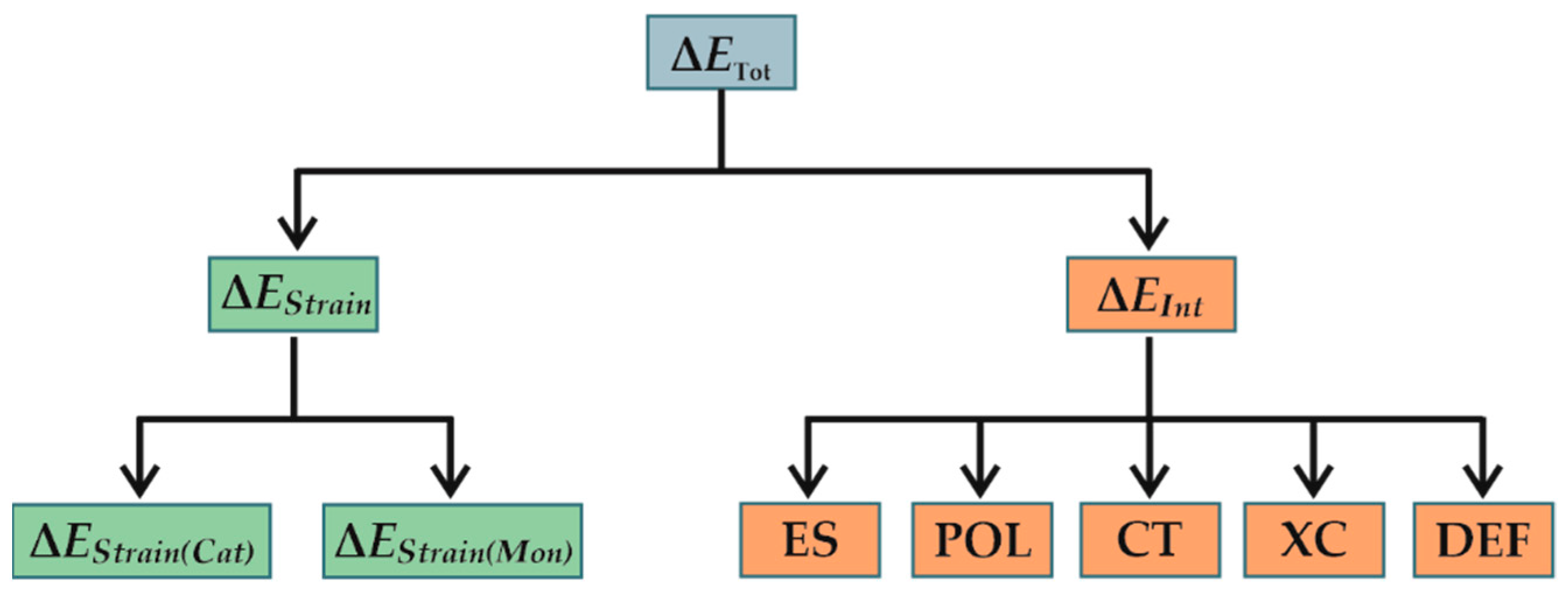
| 1,2 re Insertion | 1,2 si Insertion | 1,2 (si-re) Insertion a) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ΔETot | ΔEInt | ΔEStrain | ΔEStrain(Cat) | ΔEStrain(Mon) | ΔETot | ΔEInt | ΔEStrain | ΔEStrain(Cat) | ΔEStrain(Mon) | ΔΔEInt | ΔΔEStrain | ΔΔEStrain(Cat) | ΔΔEStrain(Mon) | |
| Ia | −12.0 | −46.9 | 34.9 | 15.8 | 19.1 | −9.2 | −45.7 | 36.5 | 18.8 | 17.7 | 1.2 | 1.6 | 3.0 | −1.4 |
| Ib | −8.5 | −45.8 | 37.3 | 18.7 | 18.6 | −9.0 | −45.5 | 36.5 | 17.8 | 18.7 | 0.3 | −0.8 | −0.9 | 0.1 |
| IIa | −2.8 | −41.9 | 39.1 | 24.7 | 14.4 | 0.8 | −45.8 | 46.6 | 27.4 | 19.2 | −3.9 | 7.5 | 2.7 | 4.8 |
| IIb | −1.4 | −42.2 | 40.9 | 25.3 | 15.6 | −0.9 | −45.1 | 44.2 | 25.1 | 19.2 | −2.9 | 3.4 | −0.2 | 3.5 |
| IIc | −3.7 | −43.0 | 39.3 | 23.3 | 16.0 | −2.5 | −46.4 | 43.9 | 23.7 | 20.2 | −3.4 | 4.6 | 0.4 | 4.2 |
| IId | −4.0 | −40.9 | 36.9 | 22.2 | 14.7 | −0.8 | −44.9 | 44.1 | 24.7 | 19.4 | −4.0 | 7.2 | 2.5 | 4.7 |
| IIe | −5.2 | −41.9 | 36.7 | 21.4 | 15.3 | −1.4 | −45.3 | 43.9 | 25.3 | 18.6 | −3.4 | 7.2 | 3.9 | 3.3 |
| IIIa | −0.6 | −40.5 | 39.9 | 24.1 | 15.8 | 1.5 | −45.9 | 47.4 | 28.1 | 19.3 | −5.4 | 7.5 | 4.0 | 3.5 |
| IIIb | −1.3 | −40.4 | 39.1 | 23.0 | 16.1 | 3.3 | −43.6 | 46.7 | 24.4 | 22.3 | −3.2 | 7.6 | 1.4 | 6.2 |
| IVa (site1) | −0.8 | −44.7 | 39.9 | 22.7 | 21.2 | 1.4 | −45.0 | 46.4 | 24.9 | 21.5 | −0.3 | 2.5 | 2.2 | 0.3 |
| IVa (site2) | −2.3 | −47.8 | 45.5 | 25.2 | 20.3 | 1.3 | −46.5 | 47.9 | 27.7 | 20.2 | 1.3 | 2.4 | 2.5 | −0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Anania, O.; De Rosa, C.; Talarico, G. A Computational Evaluation of the Steric and Electronic Contributions in Stereoselective Olefin Polymerization with Pyridylamido-Type Catalysts. Molecules 2023, 28, 3768. https://doi.org/10.3390/molecules28093768
D’Anania O, De Rosa C, Talarico G. A Computational Evaluation of the Steric and Electronic Contributions in Stereoselective Olefin Polymerization with Pyridylamido-Type Catalysts. Molecules. 2023; 28(9):3768. https://doi.org/10.3390/molecules28093768
Chicago/Turabian StyleD’Anania, Olga, Claudio De Rosa, and Giovanni Talarico. 2023. "A Computational Evaluation of the Steric and Electronic Contributions in Stereoselective Olefin Polymerization with Pyridylamido-Type Catalysts" Molecules 28, no. 9: 3768. https://doi.org/10.3390/molecules28093768
APA StyleD’Anania, O., De Rosa, C., & Talarico, G. (2023). A Computational Evaluation of the Steric and Electronic Contributions in Stereoselective Olefin Polymerization with Pyridylamido-Type Catalysts. Molecules, 28(9), 3768. https://doi.org/10.3390/molecules28093768









