The Differences of Nutrient Components in Edible and Feeding Coix Seed at Different Developmental Stages Based on a Combined Analysis of Metabolomics
Abstract
1. Introduction
2. Results and Discussion
2.1. Untargeted Metabolome Analysis of Fruits of Coix in Different Cultivars
2.2. Annotated Analysis of All Compounds
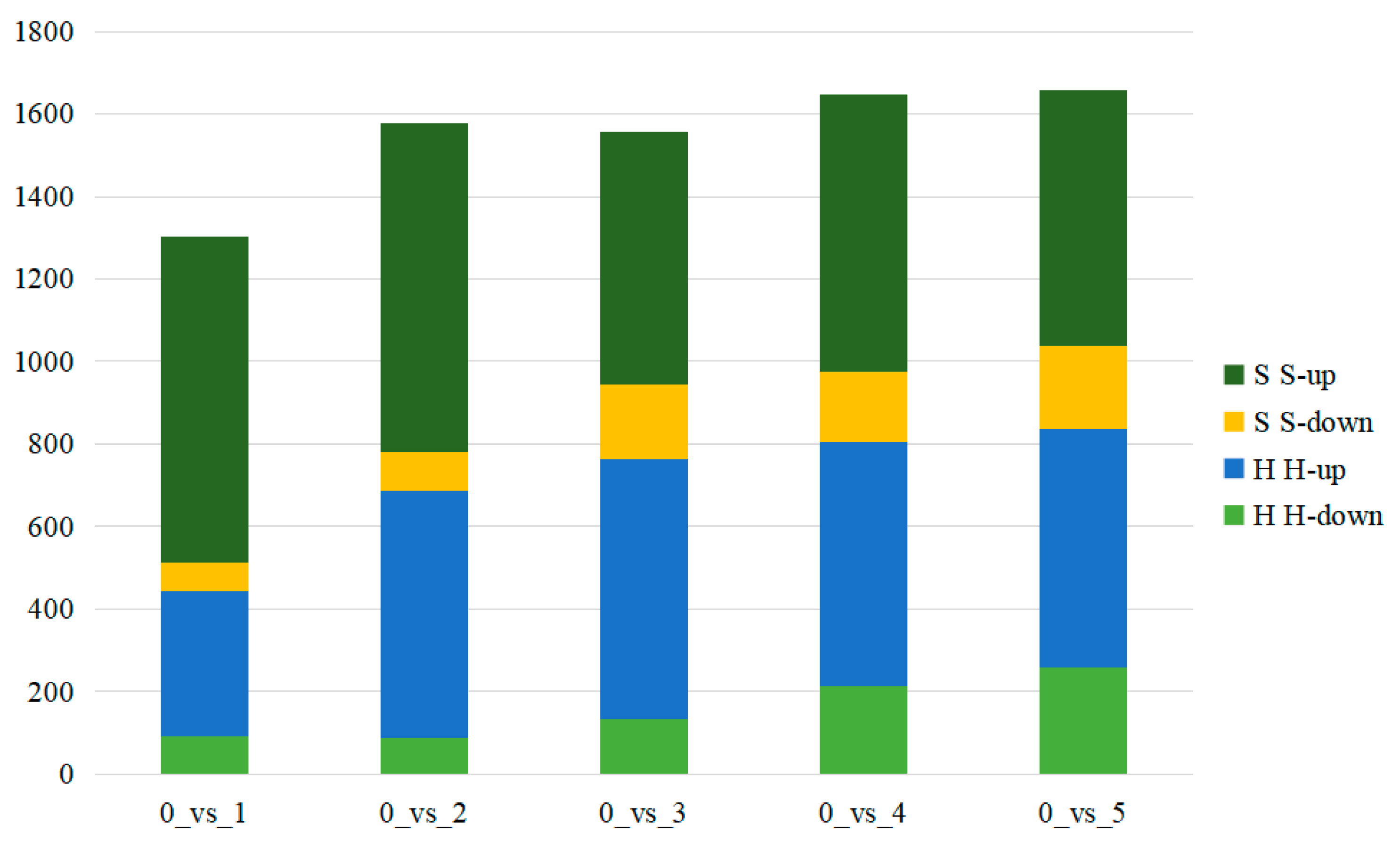
2.3. Screening and Analysis of Differential Compounds between the Two Cultivars
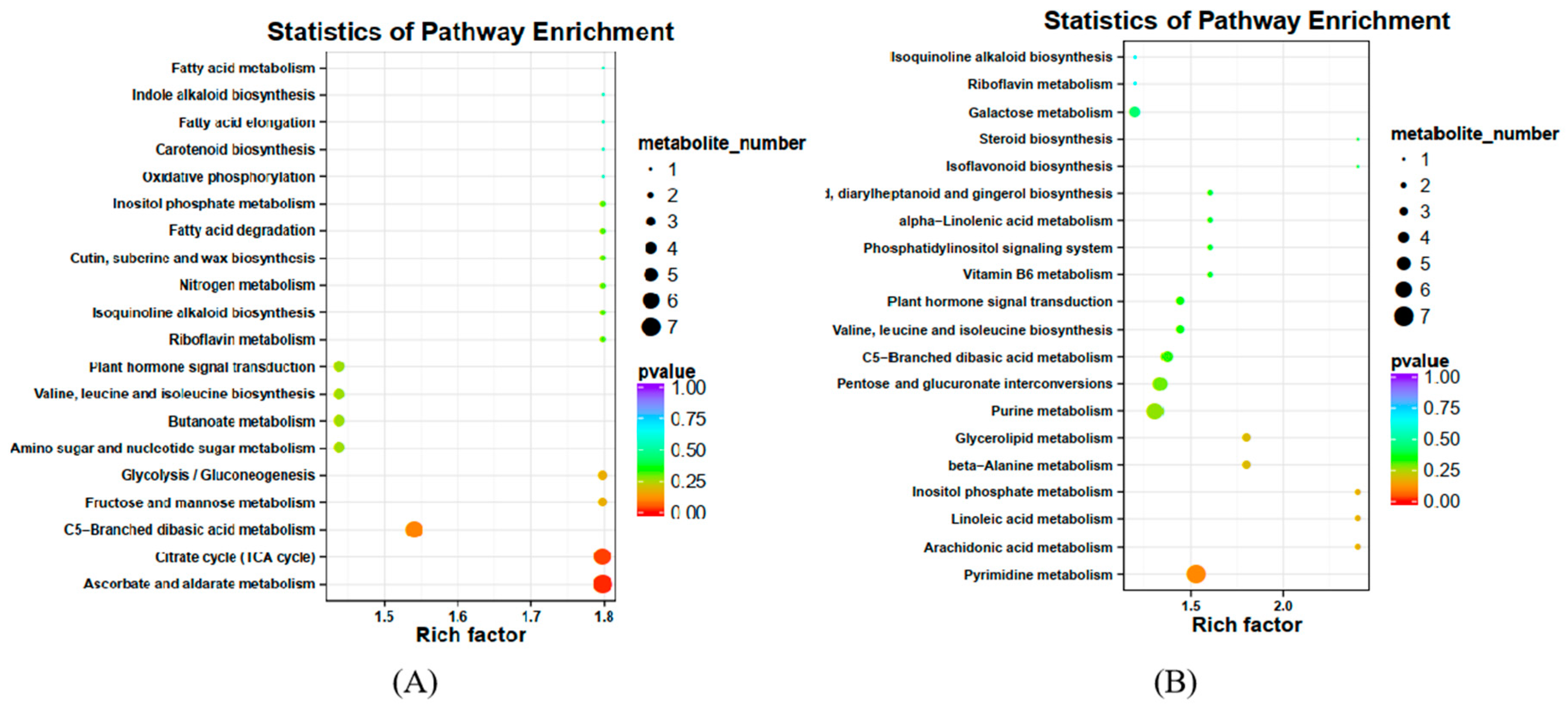
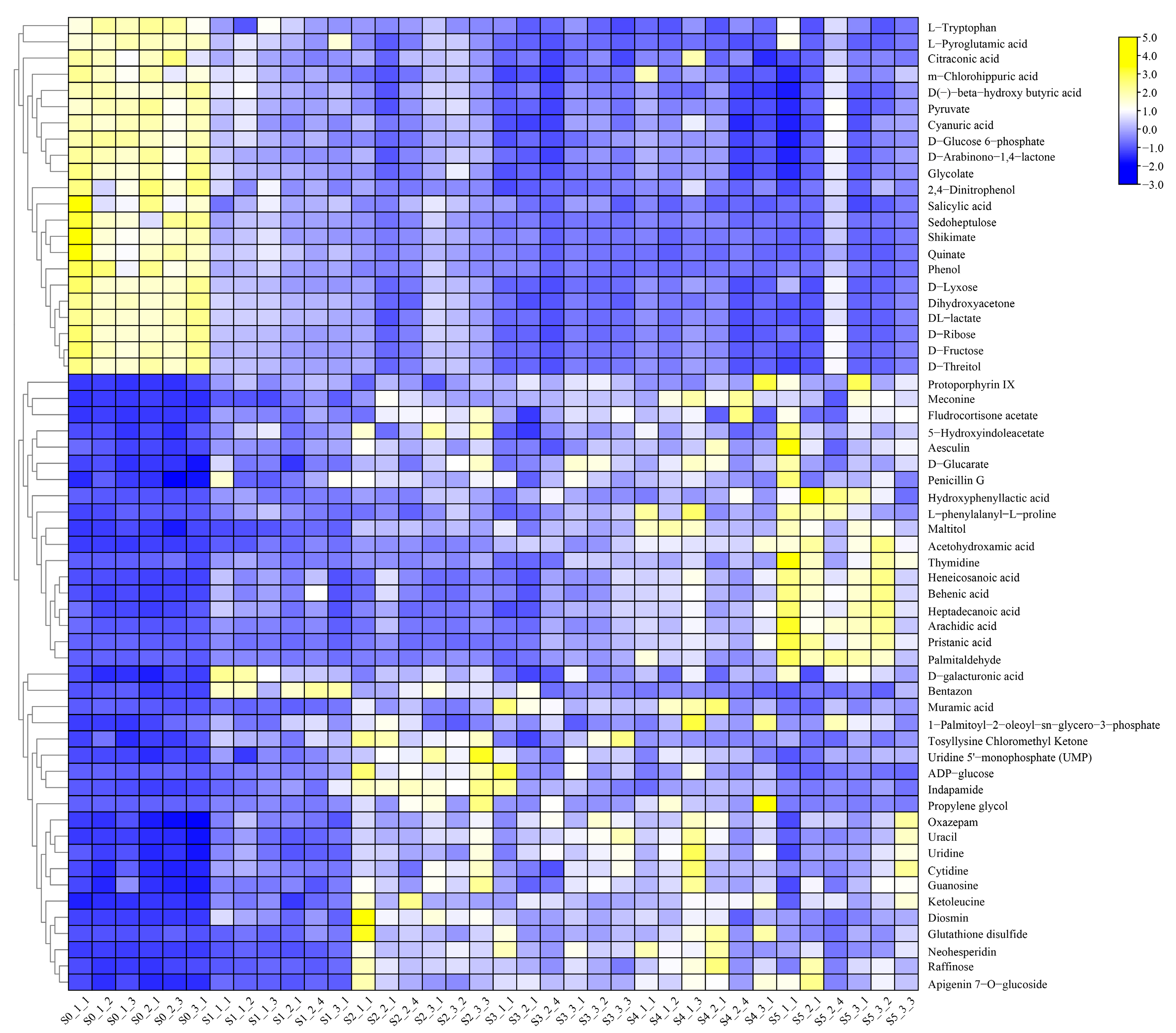
2.3.1. Analysis of Differential Metabolites in XBK
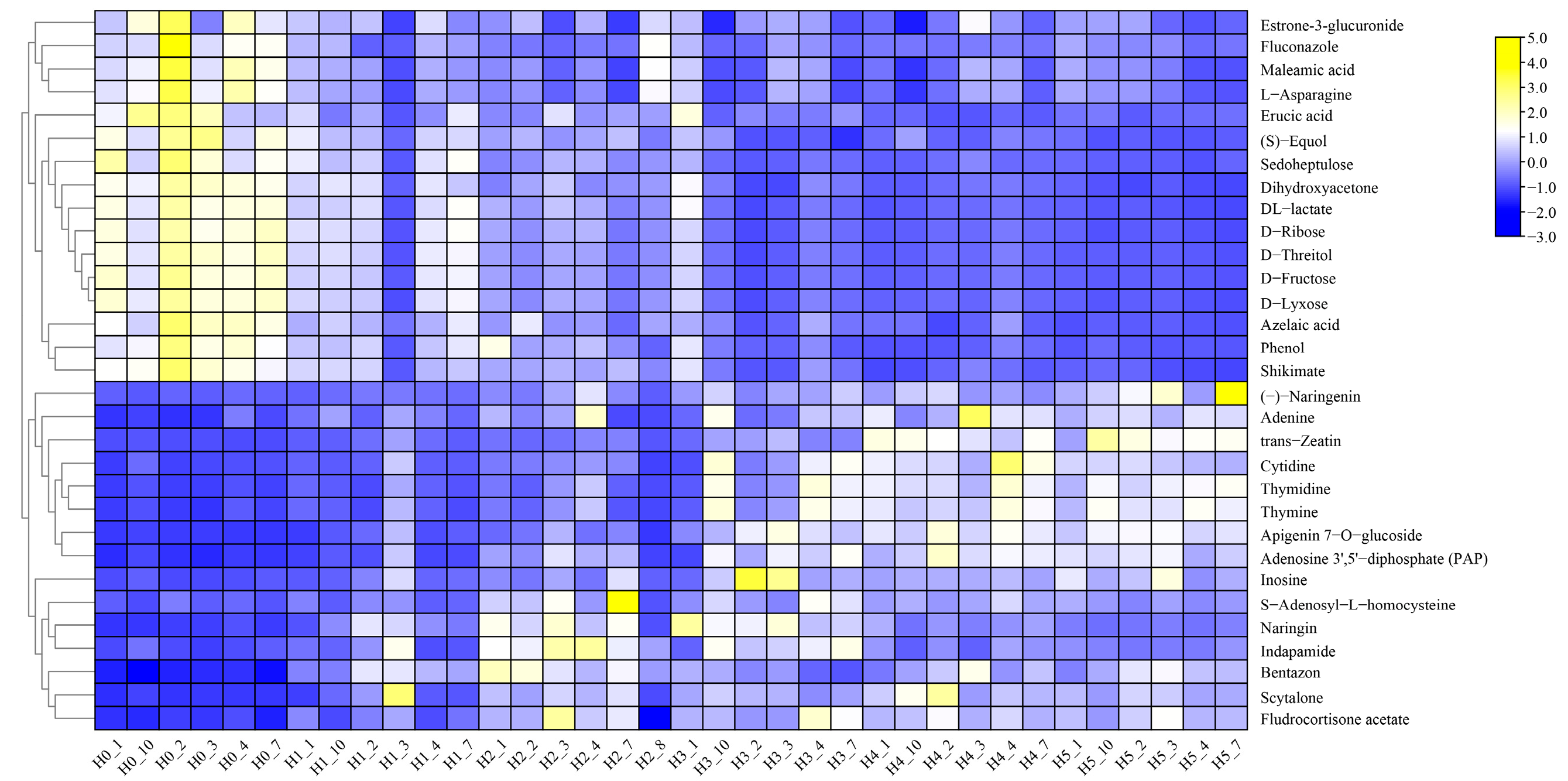
2.3.2. Analysis of Differential Metabolites in DHS
2.4. Differenence of Lipid Contents between the Two Cultivars
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Extraction of Metabolites
4.3. UPLC-MS/MS Analysis
4.4. Data Analysis of Metabolome
4.5. Determination of Total Lipid in Coix Seeds
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Kang, S.H.; Kim, B.; Choi, B.S.; Lee, H.O.; Kim, N.H.; Lee, S.J.; Kim, H.S.; Shin, M.J.; Kim, H.W.; Nam, K.; et al. Genome assembly and annotation of soft-shelled adlay (Coix lacryma-jobi Variety ma-yuen), a cereal and medicinal crop in the Poaceae family. Front. Plant. Sci. 2020, 11, 630. [Google Scholar] [CrossRef]
- Cai, Z.X.; Liu, H.J.; He, Q.Y.; Pu, M.W.; Chen, J.; Lai, J.; Li, X.; Jin, W.W. Differential genome evolution and speciation of Coix lacryma-jobi L. and Coix aquatica Roxb. hybrid guangxi revealed by repetitive sequence analysis and fine karyotyping. BMC Genom. 2014, 15, 1025. [Google Scholar] [CrossRef]
- Rajesh, K.S.; Bharath, B.R.; Rao, C.V.; Bhat, K.I.; Bhat, K.; Bhat, P. Neutralization of Naja naja venom induced lethality, edema and myonecrosis by ethanolic root extract of Coix lacryma-jobi. Toxicol. Rep. 2017, 4, 637–645. [Google Scholar] [CrossRef]
- Mustarichie, R.; Udin, Z.; Levita, J.; Musfiroh, I. Activity of leaf extracts of coix lachryma linn. and asparagus cochinchinensislinn. as breast anticancer drugs. Med. Health Sci. J. 2021, 9, 47–57. [Google Scholar] [CrossRef]
- Zhou, Q.; Yu, R.; Liu, T.; Li, Y.; Zhong, J.; Zhang, T.; Liu, Z.; Hu, Y. Coix seed diet ameliorates immune function disorders in experimental colitis mice. Nutrients 2021, 14, 123. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Qu, D.; Fu, R.P.; Guo, M.F.; Qin, Y.; Guo, J.; Chen, Y. A Tf-modified tripterine-loaded coix seed oil microemulsion enhances anti-cervical cancer treatment. Int. J. Nanomed. 2018, 13, 7275–7287. [Google Scholar] [CrossRef]
- Huang, C.C.; Lin, T.C.; Liu, C.H.; Hu, H.C.; Yu, S.Y.; Wu, S.J.; Yen, M.H.; Tsai, Y.H.; Chang, F.R. Lipid metabolism and its mechanism triggered by supercritical CO2 extract of adlay (Coix lacryma-jobi var. ma-yuen (Rom. Caill.) Stapf) bran in high-fat diet induced hyperlipidemic hamsters. Front. Pharmacol. 2021, 12, 785944. [Google Scholar] [CrossRef]
- Lin, L.Y.; Liao, Y.L.; Chen, M.H.; Chang, S.F.; Chen, K.C.; Peng, R.Y. Molecular action mechanism of Coixol from soft-shelled adlay on tyrosinase: The Future of Cosmetics. Molecules 2022, 27, 4626. [Google Scholar] [CrossRef]
- Zhu, F. Coix: Chemical composition and health effects. Trends Food Sci. Technol. 2017, 61, 160–175. [Google Scholar] [CrossRef]
- Devaraj, R.D.; Jeepipalli, S.P.; Xu, B. Phytochemistry and health promoting effects of Job’s tears (Coix lacryma-jobi)—A critical review. Food Biosci. 2020, 34, 100537. [Google Scholar] [CrossRef]
- Kuo, C.C.; Chen, H.H.; Chiang, W. Adlay (yì yĭ; “soft-shelled job’s tears”; the seeds of Coix lachryma-jobi L. var. ma-yuen Stapf) is a potential cancer chemopreventive agent toward multistage carcinogenesis processes. J. Tradit. Complement. Med. 2012, 2, 267–275. [Google Scholar] [CrossRef]
- Lu, Y.; Li, C.S.; Dong, Q. Chinese herb related molecules of cancer-cell-apoptosis: A minireview of progress between Kanglaite injection and related genes. J. Exp. Clin. Cancer Res. 2008, 27, 31. [Google Scholar] [CrossRef]
- Yang, M.; Zhu, S.J.; Shen, C.; Zhai, R.; Li, D.D.; Fang, M.; Xu, J.N.; Gan, Y.N.; Yang, L.; Ren, Z.Y.; et al. Clinical application of Chinese herbal injection for cancer care: Evidence-mapping of the systematic reviews, meta-analyses, and randomized controlled trials. Front. Pharmacol. 2021, 12, 666368. [Google Scholar] [CrossRef]
- Wang, Z.; Qi, F.; Cui, Y.; Zhao, L.; Sun, X.; Tang, W.; Cai, P. An update on Chinese herbal medicines as adjuvant treatment of anticancer therapeutics. Biosci. Trends 2018, 12, 220–239. [Google Scholar] [CrossRef]
- Bao, Y.; Yuan, Y.Z.; Xia, L.; Jiang, H.; Wu, W.R.; Zhang, X.J. Neutral lipid isolated from endosperm of Job’s tears inhibits the growth of pancreatic cancer cells via apoptosis, G2/M arrest, and regulation of gene expression. J. Gastroenterol. Hepatol. 2005, 20, 1046–1053. [Google Scholar] [CrossRef]
- Zeng, Y.W.; Yang, J.Z.; Chen, J.; Pu, X.Y.; Li, X.; Yang, X.M.; Yang, L.; Ding, Y.M.; Nong, M.Y.; Zhang, S.B.; et al. Actional mechanisms of active ingredients in functional food adlay for human health. Molecules 2022, 27, 4808. [Google Scholar] [CrossRef]
- Igbokwe, C.J.; Wei, M.; Feng, Y.Q.; Duan, Y.Q.; Ma, H.L.; Zhang, H.H. Coix seed: A review of its physicochemical composition, bioactivity, processing, application, functionality, and safety aspects. Food Rev. Int. 2021, 38 (Suppl. S1), 921–939. [Google Scholar] [CrossRef]
- Arora, R.K. Job’s-tears (Coix lacryma-jobi)—A minor food and fodder crop of northeastern India. Econ. Bot. 1977, 31, 358–366. [Google Scholar] [CrossRef]
- Yao, F.J.; Yin, G.H.; Mo, G.Y.; Luo, Y.Q.; Yuan, J.C. Coix lacryma-jobi L. growth and yield response to different sowing dates in Sichuan China. J. Sci. Appl. Biomed. 2013, 1, 8–14. [Google Scholar]
- Fu, Y.H.; Yang, C.L.; Meng, Q.Y.; Liu, F.Z.; Shen, G.; Zhou, M.Q.; Ao, M.H. Genetic diversity and structure of Coix lacryma-jobi L. from its world secondary diversity center, southwest China. Int. J. Genom. 2019, 2019, 9815697. [Google Scholar]
- Xi, X.J.; Zhu, Y.G.; Tong, Y.P.; Yang, X.L.; Tang, N.N.; Ma, S.M.; Li, S.; Cheng, Z. Assessment of the genetic diversity of different Job’s Tears (Coix lacryma-jobi L.) accessions and the active composition and anticancer effect of its seed oil. PLoS ONE 2016, 11, e0153269. [Google Scholar] [CrossRef]
- Guo, C.; Wang, Y.; Yang, A.; He, J.; Xiao, C.; Lv, S.; Han, F.; Yuan, Y.; Yuan, Y.; Dong, X.; et al. The Coix genome provides insights into Panicoideae evolution and papery hull domestication. Mol. Plant. 2020, 13, 309–320. [Google Scholar] [CrossRef]
- Luo, Y.; Zhou, S.; Li, L.X.; Sun, Z.; Liao, S.; Xiong, J.; Qiu, Y.; Liang, X. Nutritional quality analysis of “daheishan” coix with different mowing heights. China Feed. 2019, 35–38. [Google Scholar]
- He, C.J.; Li, Z.Y.; Liu, H.X.; Zhang, H.N.; Wang, L.Y.; Chen, H. Chemical compositions and antioxidant activity of adlay seed (Coix lachryma-jobi L.) oil extracted from four main producing areas in China. J. Food Sci. 2020, 85, 123–131. [Google Scholar] [CrossRef]
- Singh, R.; Low, E.-T.L.; Ooi, L.C.-L.; Ong-Abdullah, M.; Ting, N.-C.; Nagappan, J.; Nookiah, R.; Amiruddin, M.D.; Rosli, R.; Manaf, M.A.A. The oil palm SHELL gene controls oil yield and encodes a homologue of SEEDSTICK. Nature 2013, 500, 340–344. [Google Scholar] [CrossRef]
- Sun, L.; Miao, Z.; Cai, C.; Zhang, D.; Zhao, M.; Wu, Y.; Zhang, X.; Swarm, S.A.; Zhou, L.; Zhang, Z.J. GmHs1-1, encoding a calcineurin-like protein, controls hard-seededness in soybean. Nat. Genet. 2015, 47, 939–943. [Google Scholar] [CrossRef]
- Yang, Q.Q.; Wang, Z.R.; Chen, X.H.; Guo, Z.H.; Wen, L.Y.; Kan, J.Q. Evaluation of bitter compounds in Zanthoxylum schinifolium Sieb. et Zucc. by instrumental and sensory analyses. Food Chem. 2022, 390, 133180. [Google Scholar] [CrossRef]
- Shi, Q.Q.; Han, G.; Liu, Y.; Jiang, J.J.; Jia, Y.Y.; Li, X.G. Nutrient composition and quality traits of dried jujube fruits in seven producing areas based on metabolomics analysis. Food Chem. 2022, 385, 132627. [Google Scholar] [CrossRef]
- Hou, J.J.; Cao, C.M.; Xu, Y.W.; Yao, S.; Cai, L.Y.; Long, H.L.; Bi, Q.R.; Zhen, Y.Y.; Wu, W.Y.; Guo, D.A. Exploring lipid markers of the quality of coix seeds with different geographical origins using supercritical fluid chromatography mass spectrometry and chemometrics. Phytomedicine 2018, 45, 1–7. [Google Scholar] [CrossRef]
- Woo, J.H.; Li, D.P.; Wilsbach, K.; Orita, H.; Coulter, J.; Tully, E.; Kwon, T.K.; Xu, S.; Gabrielson, E. Coix seed extract, a commonly used treatment for cancer in China, inhibits NFkappaB and protein kinase C signaling. Cancer Biol. Ther. 2007, 6, 2005–2011. [Google Scholar] [CrossRef]
- Miao, G.D.; Qin, Y.; Guo, J.H.; Zhang, Q.; Bao, Y. Transcriptome characterization and expression profile of Coix lacryma-jobi L. in response to drought. PLoS ONE 2021, 16, e0256875. [Google Scholar] [CrossRef]
- Tang, W.; Wang, J.; Li, W.; Zhang, C.; Li, P.; Chen, J. Changes in Triacylglycerols Content and Quality Control Implications of Coix Seeds during Processing and Storage. Foods 2022, 11, 2462. [Google Scholar] [CrossRef]
- Kim, D.W.; Lee, W.J.; Asmelash Gebru, Y.; Choi, H.S.; Yeo, S.H.; Jeong, Y.J.; Kim, S.; Kim, Y.H.; Kim, M.K. Comparison of bioactive compounds and antioxidant activities of maclura tricuspidata fruit extracts at different maturity stages. Molecules 2019, 24, 567. [Google Scholar] [CrossRef]
- Erb, M.; Kliebenstein, D.J. Plant secondary metabolites as defenses, regulators, and primary metabolites: The blurred functional trichotomy. Plant Physiol. 2020, 184, 39–52. [Google Scholar] [CrossRef]
- Tvrzicka, E.; Kremmyda, L.S.; Stankova, B.; Zak, A. Fatty acids as biocompounds: Their role in human metabolism, health and disease—A review. Part 1: Classification, dietary sources and biological functions. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc. Czech Repub. 2011, 155, 117–130. [Google Scholar] [CrossRef]
- Sun, J.Y.; Feng, X.N.; Lyu, C.M.; Zhou, S.; Liu, Z.X. Effects of different processing methods on the lipid composition of hazelnut oil: A lipidomics analysis. Food Sci. Nutr. 2022, 11, 427–435. [Google Scholar] [CrossRef]
- Nagy, K.; Tiuca, I. Importance of fatty acids in physiopathology of human body. In Fatty Acids; IntechOpen: London, UK, 2017. [Google Scholar]
- Lewandowska, M.; Keyl, A.; Feussner, I. Wax biosynthesis in response to danger: Its regulation upon abiotic and biotic stress. New Phytol. 2020, 227, 698–713. [Google Scholar] [CrossRef]
- Lee, S.B.; Suh, M.C. Regulatory mechanisms underlying cuticular wax biosynthesis. J. Exp. Bot. 2022, 73, 2799–2816. [Google Scholar] [CrossRef]
- Abdoul-Aziz, S.; Zhang, Y.D.; Wang, J.Q. Milk odd and branched chain fatty acids in dairy cows: A review on dietary factors and its consequences on human health. Animals 2021, 11, 3210. [Google Scholar] [CrossRef]
- Pfeuffer, M.; Jaudszus, A. Pentadecanoic and Heptadecanoic Acids: Multifaceted odd-chain fatty acids. Adv. Nutr. 2016, 7, 730–734. [Google Scholar] [CrossRef]
- Shen, J.Q.; Li, Z.; Zhou, L.B.; Wang, C.; Zhang, G.B.; Ren, Y.; Xu, J.X.; Shao, M.B. Progress on main components and functions of coix seed oil. China Oils Fats 2020, 45, 6. [Google Scholar]
- Dunn, W.B.; Broadhurst, D.; Begley, P.; Zelena, E.; Francis-McIntyre, S.; Anderson, N.; Brown, M.; Knowles, J.D.; Halsall, A.; Haselden, J.N.; et al. Procedures for large-scale metabolic profiling of serum and plasma using gas chromatography and liquid chromatography coupled to mass spectrometry. Nat. Protoc. 2011, 6, 1060–1083. [Google Scholar] [CrossRef]
- Want, E.J.; Wilson, I.D.; Gika, H.; Theodoridis, G.; Plumb, R.S.; Shockcor, J.; Holmes, E.; Nicholson, J.K. Global metabolic profiling procedures for urine using UPLC-MS. Nat. Protoc. 2010, 5, 1005–1018. [Google Scholar] [CrossRef]
- Wang, J.L.; Zhang, T.; Shen, X.T.; Liu, J.; Zhao, D.L.; Sun, Y.W.; Wang, L.; Liu, Y.J.; Gong, X.Y.; Liu, Y.X.; et al. Serum metabolomics for early diagnosis of esophageal squamous cell carcinoma by UHPLC-QTOF/MS. Metabolomics 2016, 12, 116. [Google Scholar] [CrossRef]
- Chong, J.; Xia, J.G. MetaboAnalystR: An R package for flexible and reproducible analysis of metabolomics data. Bioinformatics 2018, 34, 4313–4314. [Google Scholar] [CrossRef]
- GB/T 5009.6-2003; Determination of Fat in Foods. The Standardization Administration of the People’s Republic of China: Beijing, China, 2003.





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, X.; Li, Y.; Zhou, S.; Guo, C.; Dong, X.; Li, Q.; Guo, J.; Wang, Y.; Huang, L. The Differences of Nutrient Components in Edible and Feeding Coix Seed at Different Developmental Stages Based on a Combined Analysis of Metabolomics. Molecules 2023, 28, 3759. https://doi.org/10.3390/molecules28093759
Wei X, Li Y, Zhou S, Guo C, Dong X, Li Q, Guo J, Wang Y, Huang L. The Differences of Nutrient Components in Edible and Feeding Coix Seed at Different Developmental Stages Based on a Combined Analysis of Metabolomics. Molecules. 2023; 28(9):3759. https://doi.org/10.3390/molecules28093759
Chicago/Turabian StyleWei, Xiaoyan, Yong Li, Shufeng Zhou, Chao Guo, Xiaolong Dong, Qishuang Li, Juan Guo, Yanan Wang, and Luqi Huang. 2023. "The Differences of Nutrient Components in Edible and Feeding Coix Seed at Different Developmental Stages Based on a Combined Analysis of Metabolomics" Molecules 28, no. 9: 3759. https://doi.org/10.3390/molecules28093759
APA StyleWei, X., Li, Y., Zhou, S., Guo, C., Dong, X., Li, Q., Guo, J., Wang, Y., & Huang, L. (2023). The Differences of Nutrient Components in Edible and Feeding Coix Seed at Different Developmental Stages Based on a Combined Analysis of Metabolomics. Molecules, 28(9), 3759. https://doi.org/10.3390/molecules28093759





