Targeting Chondroitin Sulphate Synthase 1 (Chsy1) Promotes Axon Growth Following Neurorrhaphy by Suppressing Versican Accumulation
Abstract
1. Introduction
2. Results
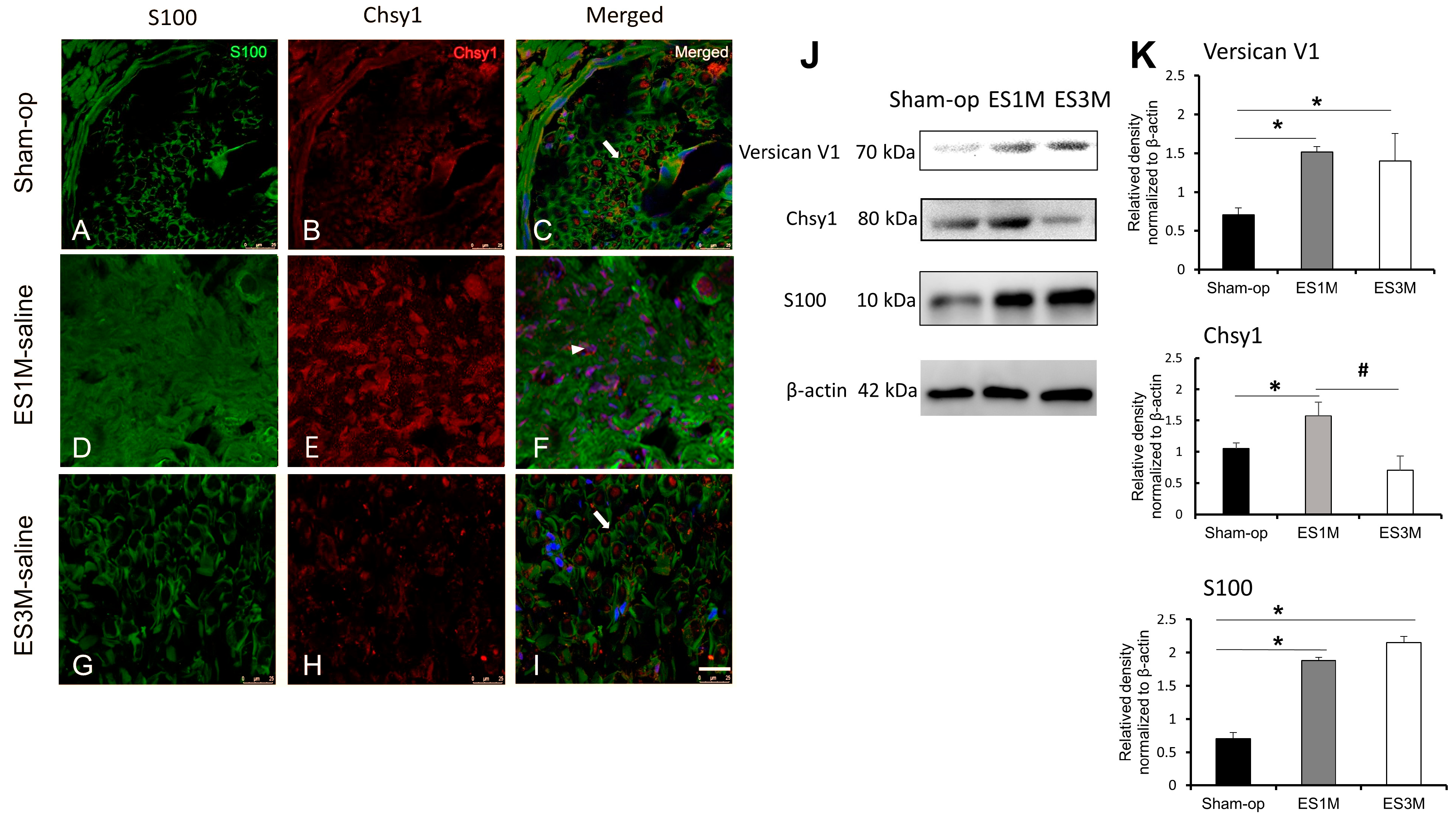
2.1. Expression of Chsy1 in the Regenerating Axon after End-to-Side Neurorrhaphy
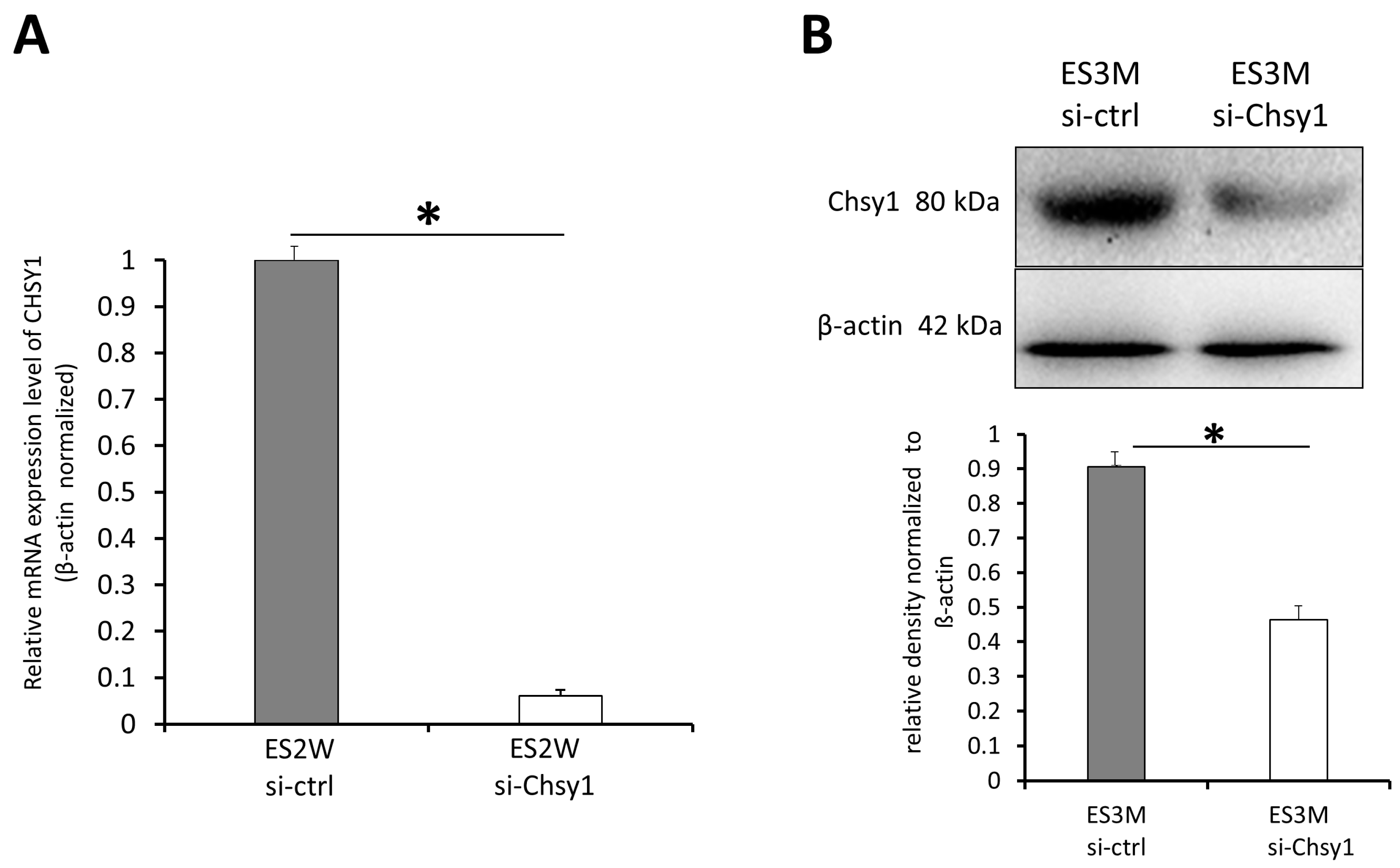
2.2. In Vivo Knockdown of Chsy1 after End-to-Side Neurorrhaphy
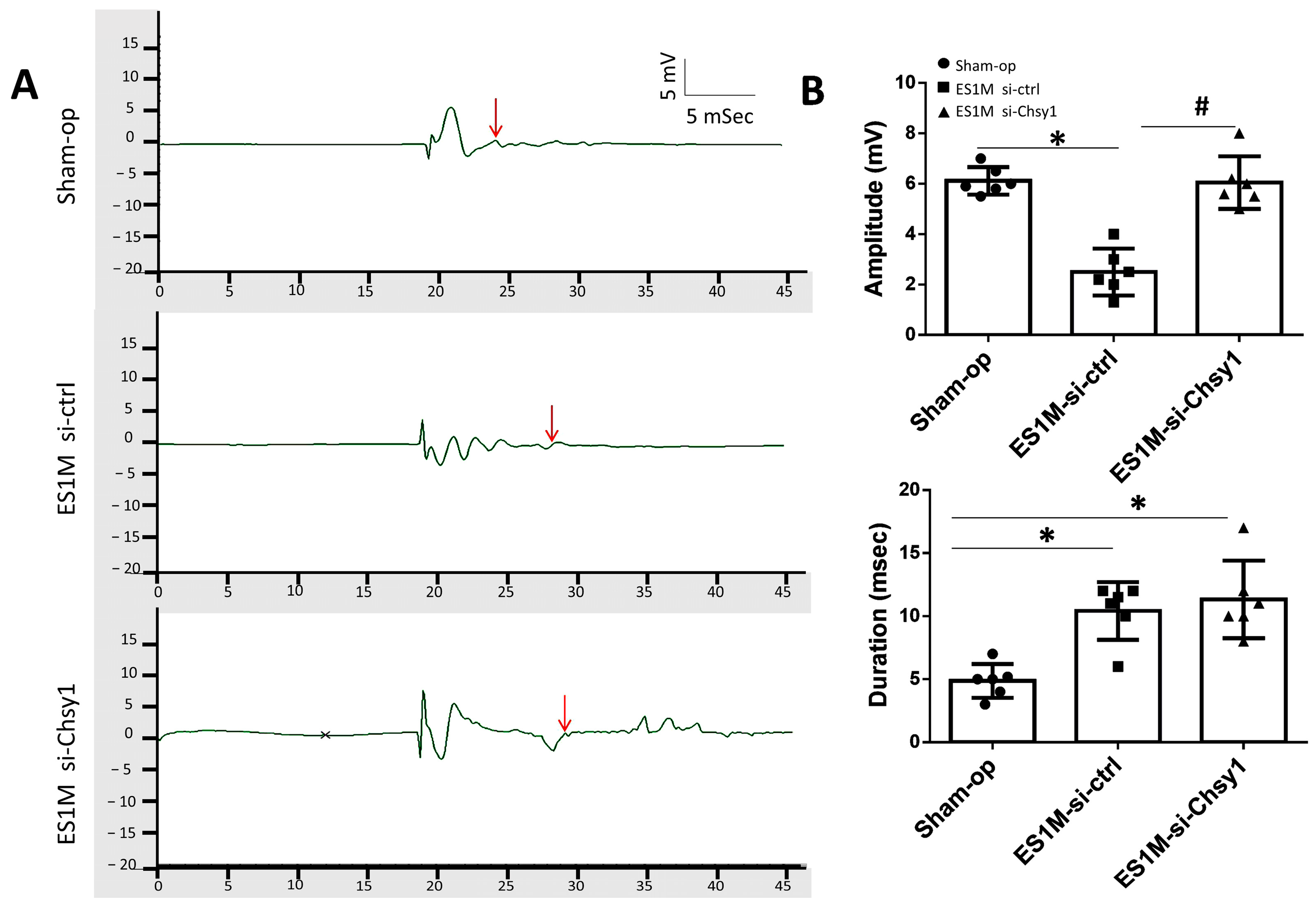
2.3. Effects of Chsy1-Silencing on Functional Recovery
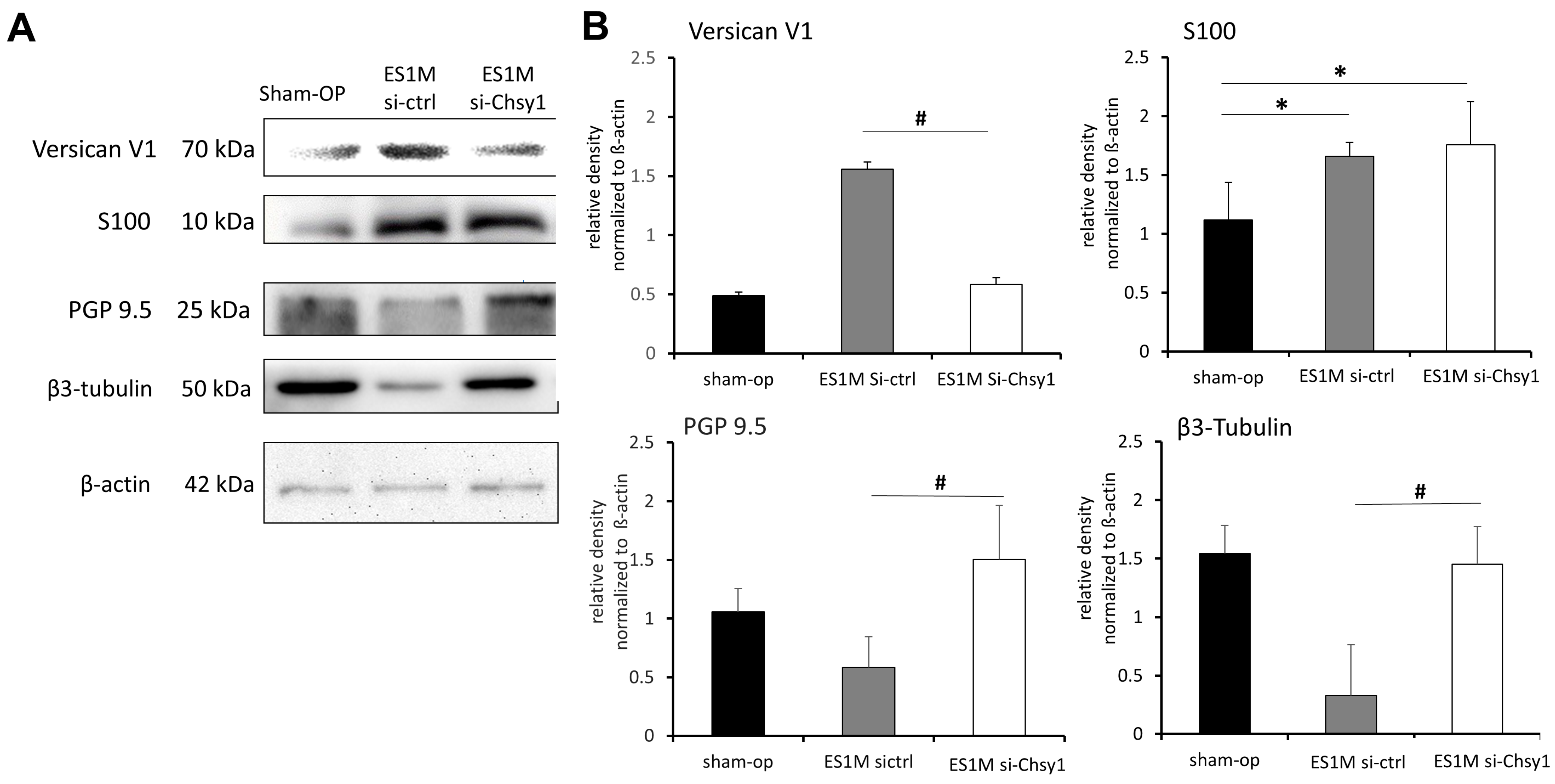
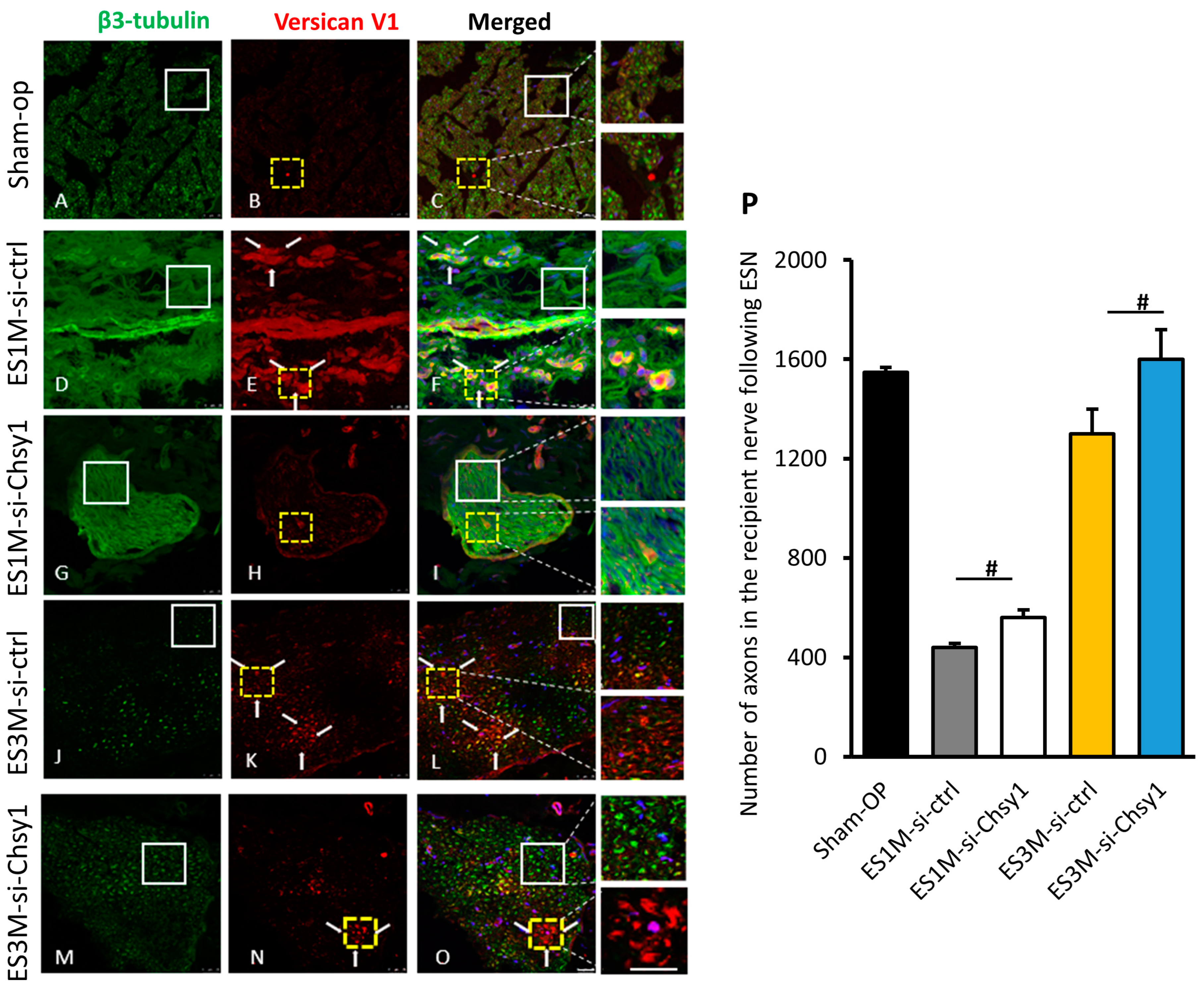
2.4. Effects of Chsy1-Silencing on Axonal Markers and Versican Expression
2.5. Morphological Studies of Nerve Regeneration after siRNA Treatment
3. Discussion
4. Materials and Methods
4.1. Experimental Animals
4.2. Surgical Procedures
4.3. siRNA Delivery in ESN Rats
4.4. RNA Isolation and Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
4.5. Compound Muscle Action Potential Recording
4.6. Immunofluorescence Stain
4.7. Western Blotting
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Liao, W.-C.; Chen, J.-R.; Wang, Y.-J.; Tseng, G.-F. The efficacy of end-to-end and end-to-side nerve repair (neurorrhaphy) in the rat brachial plexus. J. Anat. 2009, 215, 506–521. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.-C.; Wang, Y.-J.; Huang, M.-C.; Tseng, G.-F. Methylcobalamin Facilitates Collateral Sprouting of Donor Axons and Innervation of Recipient Muscle in End-to-Side Neurorrhaphy in Rats. PLoS ONE 2013, 8, e76302. [Google Scholar] [CrossRef] [PubMed]
- Galtrey, C.M.; Fawcett, J. The role of chondroitin sulfate proteoglycans in regeneration and plasticity in the central nervous system. Brain Res. Rev. 2007, 54, 1–18. [Google Scholar] [CrossRef]
- Corvetti, L.; Rossi, F. Degradation of Chondroitin Sulfate Proteoglycans Induces Sprouting of Intact Purkinje Axons in the Cerebellum of the Adult Rat. J. Neurosci. 2005, 25, 7150–7158. [Google Scholar] [CrossRef]
- Iozzo, R.V.; Schaefer, L. Proteoglycan form and function: A comprehensive nomenclature of proteoglycans. Matrix Biol. 2015, 42, 11–55. [Google Scholar] [CrossRef]
- Asher, R.A.; Morgenstern, D.A.; Shearer, M.C.; Adcock, K.H.; Pesheva, P.; Fawcett, J.W. Versican Is Upregulated in CNS Injury and Is a Product of Oligodendrocyte Lineage Cells. J. Neurosci. 2002, 22, 2225–2236. [Google Scholar] [CrossRef] [PubMed]
- Bekku, Y.; Oohashi, T. Neurocan contributes to the molecular heterogeneity of the perinodal ECM. Arch. Histol. Cytol. 2010, 73, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Dutt, S.; Cassoly, E.; Dours-Zimmermann, M.T.; Matasci, M.; Stoeckli, E.T.; Zimmermann, D.R. Versican V0 and V1 direct the growth of peripheral axons in the developing chick hindlimb. J. Neurosci. 2011, 31, 5262–5270. [Google Scholar] [CrossRef]
- Yi, J.H.; Katagiri, Y.; Susarla, B.; Figge, D.; Symes, A.J.; Geller, H.M. Alterations in sulfated chondroitin glycosaminoglycans following controlled cortical impact injury in mice. J. Comp. Neurol. 2012, 520, 3295–3313. [Google Scholar] [CrossRef] [PubMed]
- Siebert, J.R.; Steencken, A.C.; Osterhout, D.J. Chondroitin Sulfate Proteoglycans in the Nervous System: Inhibitors to Repair. BioMed Res. Int. 2014, 2014, 845323. [Google Scholar] [CrossRef] [PubMed]
- Day, P.; Alves, N.; Daniell, E.; Dasgupta, D.; Ogborne, R.; Steeper, A.; Raza, M.; Ellis, C.; Fawcett, J.; Keynes, R.; et al. Targeting chondroitinase ABC to axons enhances the ability of chondroitinase to promote neurite outgrowth and sprouting. PLoS ONE 2020, 15, e0221851. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.L.; Yang, B.B.; Erwin, M.; Ang, L.C.; Finkelstein, J.; Yee, A.A. Versican G3 domain enhances cellular adhesion and proliferation of bovine intervertebral disc cells cultured in vitro. Life Sci. 2003, 73, 3399–3413. [Google Scholar] [CrossRef]
- Melendez-Vasquez, C.; Carey, D.J.; Zanazzi, G.; Reizes, O.; Maurel, P.; Salzer, J.L. Differential expression of proteoglycans at central and peripheral nodes of Ranvier. Glia 2005, 52, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Sheng, W.; Dong, H.; Lapierre, D.; Wan, Y.; Lu, W.-Y.; Yang, B.B. Versican isoforms modulate expression and function of nicotinic acetylcholine receptors. Int. J. Physiol. Pathophysiol. Pharmacol. 2009, 1, 64–75. [Google Scholar] [PubMed]
- Wight, T.N.; Kinsella, M.G.; Evanko, S.P.; Potter-Perigo, S.; Merrilees, M.J. Versican and the regulation of cell phenotype in disease. Biochim. Biophys. Acta BBA Bioenerg. 2014, 1840, 2441–2451. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, H.; Izumikawa, T.; Uyama, T.; Sugahara, K. Molecular Cloning of a Chondroitin Polymerizing Factor That Cooperates with Chondroitin Synthase for Chondroitin Polymerization. J. Biol. Chem. 2003, 278, 23666–23671. [Google Scholar] [CrossRef]
- Gause, T.M., 2nd; Sivak, W.N.; Marra, K.G. The role of chondroitinase as an adjuvant to peripheral nerve repair. Cells Tissues Organs 2014, 200, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Graham, J.B.; Muir, D. Chondroitinase C Selectively Degrades Chondroitin Sulfate Glycosaminoglycans that Inhibit Axonal Growth within the Endoneurium of Peripheral Nerve. PLoS ONE 2016, 11, e0167682. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.-C.; Liao, C.-K.; Tseng, T.-J.; Ho, Y.-J.; Chen, Y.-R.; Lin, K.-H.; Lai, T.-J.; Lan, C.-T.; Wei, K.-C.; Liu, C.-H. Chondroitin sulfate synthase 1 enhances proliferation of glioblastoma by modulating PDGFRA stability. Oncogenesis 2020, 9, 9. [Google Scholar] [CrossRef]
- Izumikawa, T.; Saigoh, K.; Shimizu, J.; Tsuji, S.; Kusunoki, S.; Kitagawa, H. A chondroitin synthase-1 (ChSy-1) missense mutation in a patient with neuropathy impairs the elongation of chondroitin sulfate chains initiated by chondroitin N-acetylgalactosaminyltransferase-1. Biochim. Biophys. Acta (BBA) Gen. Subj. 2013, 1830, 4806–4812. [Google Scholar] [CrossRef]
- Wight, T.N.; Kang, I.; Evanko, S.P.; Harten, I.A.; Chang, M.Y.; Pearce, O.M.T.; Allen, C.E.; Frevert, C.W. Versican—A Critical Extracellular Matrix Regulator of Immunity and Inflammation. Front. Immunol. 2020, 11, 512. [Google Scholar] [CrossRef]
- Behlke, M.A. Progress towards in Vivo Use of siRNAs. Mol. Ther. 2006, 13, 644–670. [Google Scholar] [CrossRef] [PubMed]
- Pei, Y.; Hancock, P.J.; Zhang, H.; Bartz, R.; Cherrin, C.; Innocent, N.; Pomerantz, C.J.; Seitzer, J.; Koser, M.L.; Abrams, M.T.; et al. Quantitative evaluation of siRNA delivery in vivo. RNA 2010, 16, 2553–2563. [Google Scholar] [CrossRef]
- Xie, W.; Strong, J.A.; Zhang, J.-M. Active Nerve Regeneration with Failed Target Reinnervation Drives Persistent Neuropathic Pain. Eneuro 2017, 4, e0008-17. [Google Scholar] [CrossRef]
- Lin, J.; Bin Jo, S.; Kim, T.-H.; Kim, H.-W.; Chew, S.Y. RNA interference in glial cells for nerve injury treatment. J. Tissue Eng. 2020, 11, 2041731420939224. [Google Scholar] [CrossRef]
- Chu, Y.H.; Liao, W.C.; Ho, Y.J.; Huang, C.H.; Tseng, T.J.; Liu, C.H. Targeting Chondroitin Sulfate Reduces Invasiveness of Glioma Cells by Suppressing CD44 and Integrin beta1 Expression. Cells 2021, 10, 3594. [Google Scholar] [CrossRef] [PubMed]
- Kenagy, R.D.; Plaas, A.H.; Wight, T.N. Versican Degradation and Vascular Disease. Trends Cardiovasc. Med. 2006, 16, 209–215. [Google Scholar] [CrossRef]
- Loers, G.; Liao, Y.; Hu, C.; Xue, W.; Shen, H.; Zhao, W.; Schachner, M. Identification and characterization of synthetic chondroitin-4-sulfate binding peptides in neuronal functions. Sci. Rep. 2019, 9, 1064. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jia, H.; Li, W.-Y.; Guan, L.-X.; Deng, L.; Liu, Y.-C.; Liu, G.-B. Molecular examination of bone marrow stromal cells and chondroitinase ABC-assisted acellular nerve allograft for peripheral nerve regeneration. Exp. Ther. Med. 2016, 12, 1980–1992. [Google Scholar] [CrossRef]
- Donsante, A.; Xue, J.; Poth, K.M.; Hardcastle, N.S.; Diniz, B.; O’Connor, D.M.; Xia, Y.; Boulis, N.M. Controlling the Release of Neurotrophin-3 and Chondroitinase ABC Enhances the Efficacy of Nerve Guidance Conduits. Adv. Healthc. Mater. 2020, 9, e2000200. [Google Scholar] [CrossRef]
- Yu, H.; Xiang, L.; Xu, W.; Zhao, B.; Wang, Y.; Peng, J.; Lu, S. Chondroitinase ABC improves recovery of long sciatic nerve defects☆. Neural Regen. Res. 2012, 7, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; McKeon, R.J.; Bellamkonda, R.V. Sustained delivery of thermostabilized chABC enhances axonal sprouting and functional recovery after spinal cord injury. Proc. Natl. Acad. Sci. USA 2009, 107, 3340–3345. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.-Y.; Dong, H.; Wan, Y.; Li, J.; Yee, A.; Yang, B.B.; Lu, W.-Y. Versican G3 Domain Regulates Neurite Growth and Synaptic Transmission of Hippocampal Neurons by Activation of Epidermal Growth Factor Receptor. J. Biol. Chem. 2006, 281, 19358–19368. [Google Scholar] [CrossRef] [PubMed]
- Burnside, E.R.; Bradbury, E.J. Review: Manipulating the extracellular matrix and its role in brain and spinal cord plasticity and repair. Neuropathol. Appl. Neurobiol. 2014, 40, 26–59. [Google Scholar] [CrossRef] [PubMed]
- Du, W.W.; Yang, B.B.; Shatseva, T.A.; Yang, B.L.; Deng, Z.; Shan, S.W.; Lee, D.Y.; Seth, A.; Yee, A.J. Versican G3 Promotes Mouse Mammary Tumor Cell Growth, Migration, and Metastasis by Influencing EGF Receptor Signaling. PLoS ONE 2010, 5, e13828. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Chen, C.; Yi, S.; Wang, S.; Gong, L.; Liu, J.; Gu, X.; Zhao, Q.; Li, S. miR-sc8 Inhibits Schwann Cell Proliferation and Migration by Targeting Egfr. PLoS ONE 2015, 10, e0145185. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cao, L.; Yang, B.L.; Yang, B.B. The G3 domain of versican enhances cell proliferation via epidermial growth factor-like motifs. J. Biol. Chem. 1998, 273, 21342–21351. [Google Scholar] [CrossRef] [PubMed]
- Oberlin, C.; Béal, D.; Leechavengvongs, S.; Salon, A.; Dauge, M.; Sarcy, J. Nerve transfer to biceps muscle using a part of ulnar nerve for C5–C6 avulsion of the brachial plexus: Anatomical study and report of four cases. J. Hand Surg. 1994, 19, 232–237. [Google Scholar] [CrossRef]
- Liu, C.-H.; Chang, H.-M.; Yang, Y.-S.; Lin, Y.-T.; Ho, Y.-J.; Tseng, T.-J.; Lan, C.-T.; Li, S.-T.; Liao, W.-C. Melatonin Promotes Nerve Regeneration Following End-to-Side Neurorrhaphy by Accelerating Cytoskeletal Remodeling via the Melatonin Receptor-dependent Pathway. Neuroscience 2020, 429, 282–292. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-H.; Kuo, Y.-C.; Wang, C.-Y.; Hsu, C.-C.; Ho, Y.-J.; Chiang, Y.-C.; Mai, F.-D.; Lin, W.-J.; Liao, W.-C. Syndecan-3 contributes to the regulation of the microenvironment at the node of Ranvier following end-toside neurorrhaphy: Sodium image analysis. Histochem. Cell Biol. 2021, 155, 355–367. [Google Scholar] [CrossRef]
- Rao, X.; Huang, X.; Zhou, Z.; Lin, X. An improvement of the 2ˆ(–delta delta CT) method for quantitative real-time polymerase chain reaction data analysis. Biostat. Bioinform. Biomath. 2013, 3, 71–85. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.-H.; Ho, Y.-J.; Wang, C.-Y.; Hsu, C.-C.; Chu, Y.-H.; Hsu, M.-Y.; Chen, S.-J.; Hsiao, W.-C.; Liao, W.-C. Targeting Chondroitin Sulphate Synthase 1 (Chsy1) Promotes Axon Growth Following Neurorrhaphy by Suppressing Versican Accumulation. Molecules 2023, 28, 3742. https://doi.org/10.3390/molecules28093742
Liu C-H, Ho Y-J, Wang C-Y, Hsu C-C, Chu Y-H, Hsu M-Y, Chen S-J, Hsiao W-C, Liao W-C. Targeting Chondroitin Sulphate Synthase 1 (Chsy1) Promotes Axon Growth Following Neurorrhaphy by Suppressing Versican Accumulation. Molecules. 2023; 28(9):3742. https://doi.org/10.3390/molecules28093742
Chicago/Turabian StyleLiu, Chiung-Hui, Ying-Jui Ho, Che-Yu Wang, Chao-Chun Hsu, Yin-Hung Chu, Min-Yen Hsu, Shiu-Jau Chen, Wen-Chuan Hsiao, and Wen-Chieh Liao. 2023. "Targeting Chondroitin Sulphate Synthase 1 (Chsy1) Promotes Axon Growth Following Neurorrhaphy by Suppressing Versican Accumulation" Molecules 28, no. 9: 3742. https://doi.org/10.3390/molecules28093742
APA StyleLiu, C.-H., Ho, Y.-J., Wang, C.-Y., Hsu, C.-C., Chu, Y.-H., Hsu, M.-Y., Chen, S.-J., Hsiao, W.-C., & Liao, W.-C. (2023). Targeting Chondroitin Sulphate Synthase 1 (Chsy1) Promotes Axon Growth Following Neurorrhaphy by Suppressing Versican Accumulation. Molecules, 28(9), 3742. https://doi.org/10.3390/molecules28093742








