Natamycin Has an Inhibitory Effect on Neofusicoccum parvum, the Pathogen of Chestnuts
Abstract
1. Introduction
2. Results and Analysis
2.1. Growth Curve of N. parvum
2.2. Inhibition Mechanism of Natamycin against N. parvum
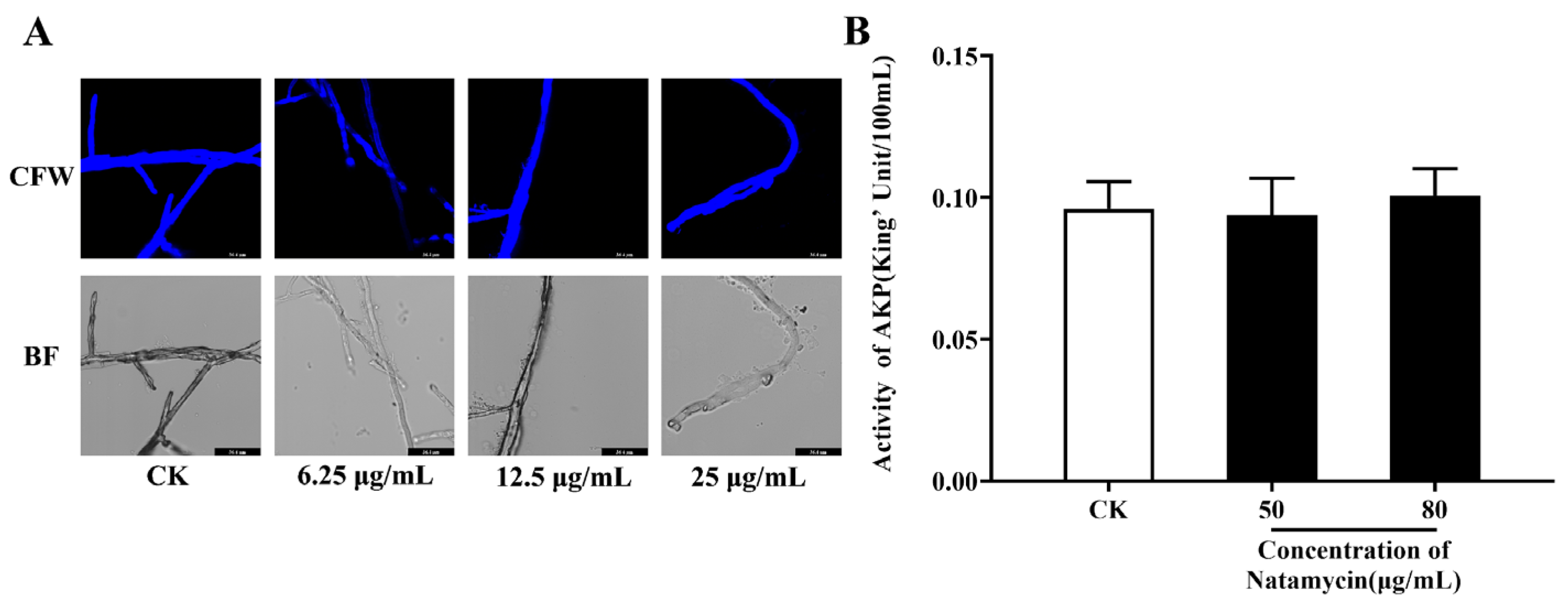
2.2.1. Effect of Natamycin on the Cell Wall of N. parvum
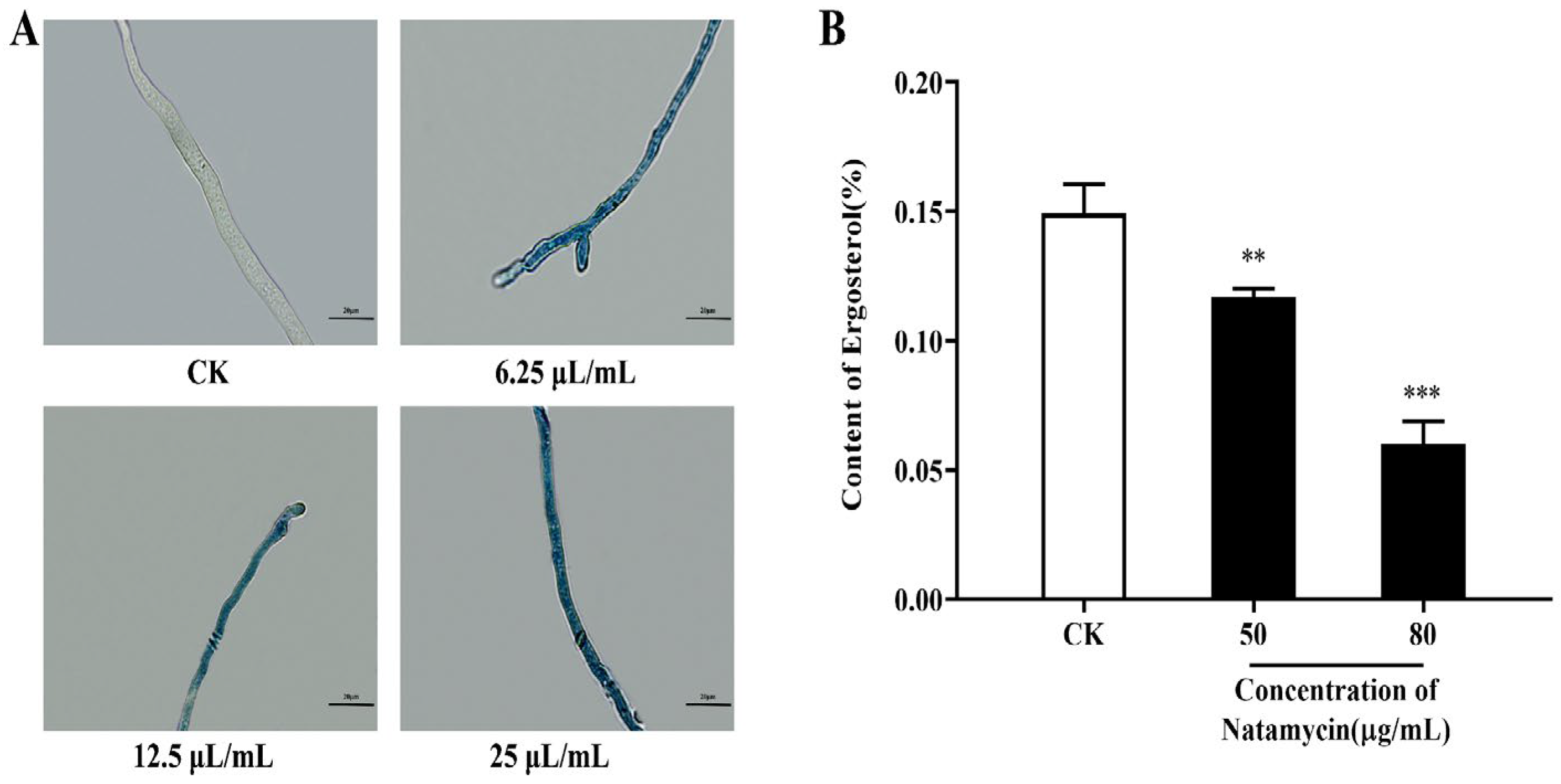
2.2.2. Effect of Natamycin on the Cell Membrane of N. parvum
2.2.3. Effect of Natamycin on the Leakage of the Cellular Contents of N. parvum
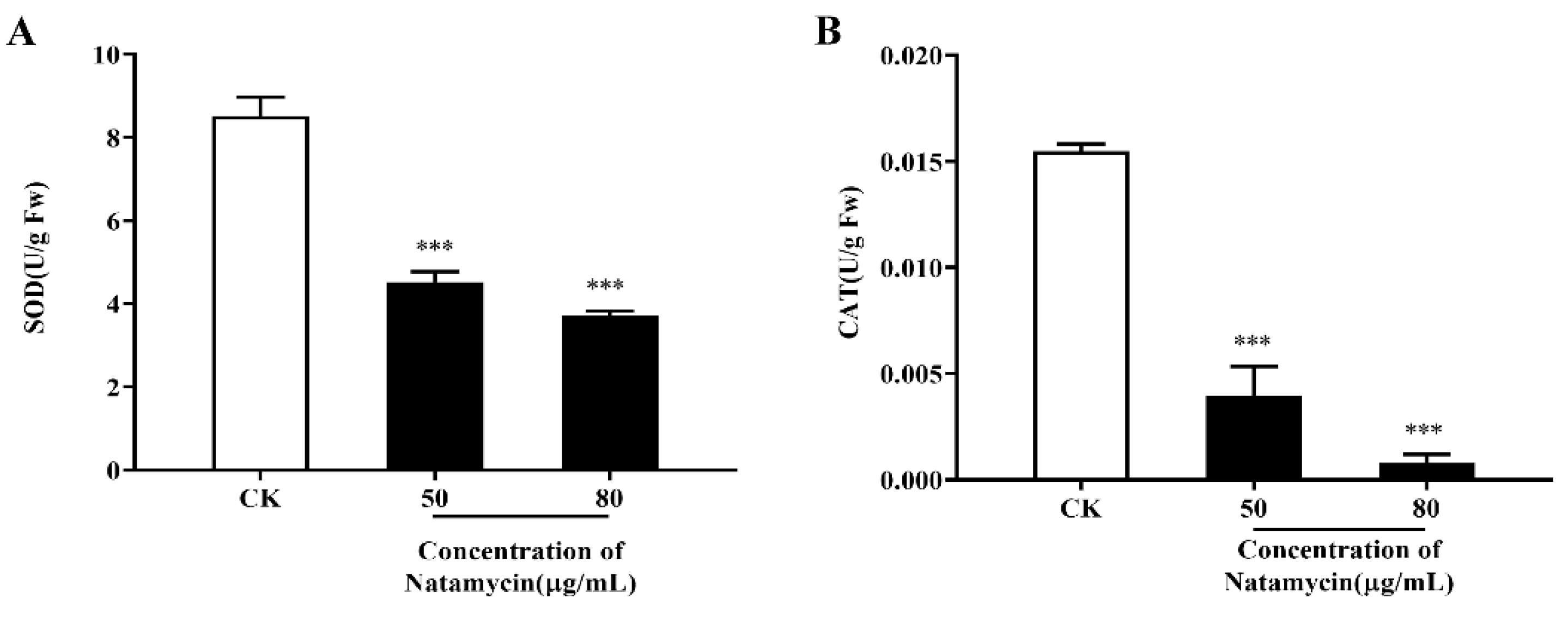
2.2.4. Effects of Natamycin on the Oxidative Stress Response of N. parvum
2.3. In Vivo Experiments
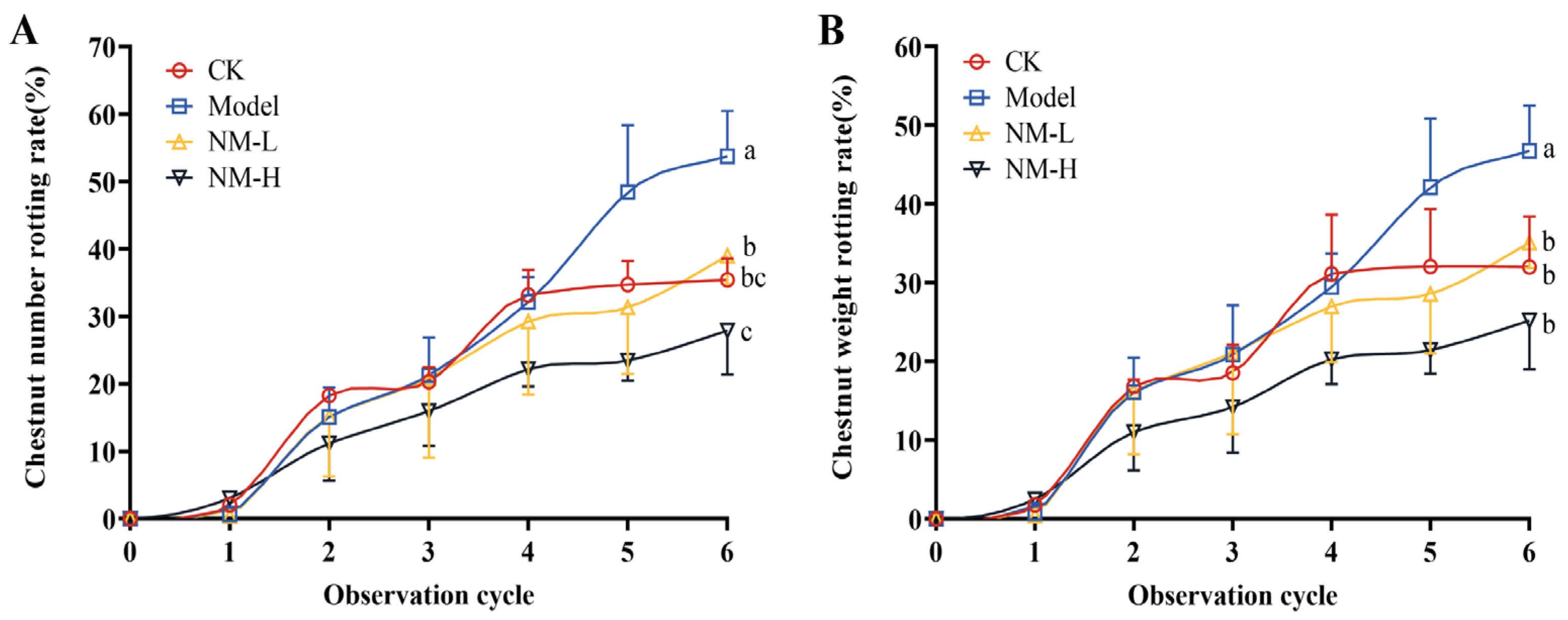
2.3.1. In Vivo Antifungal Efficacy of Natamycin
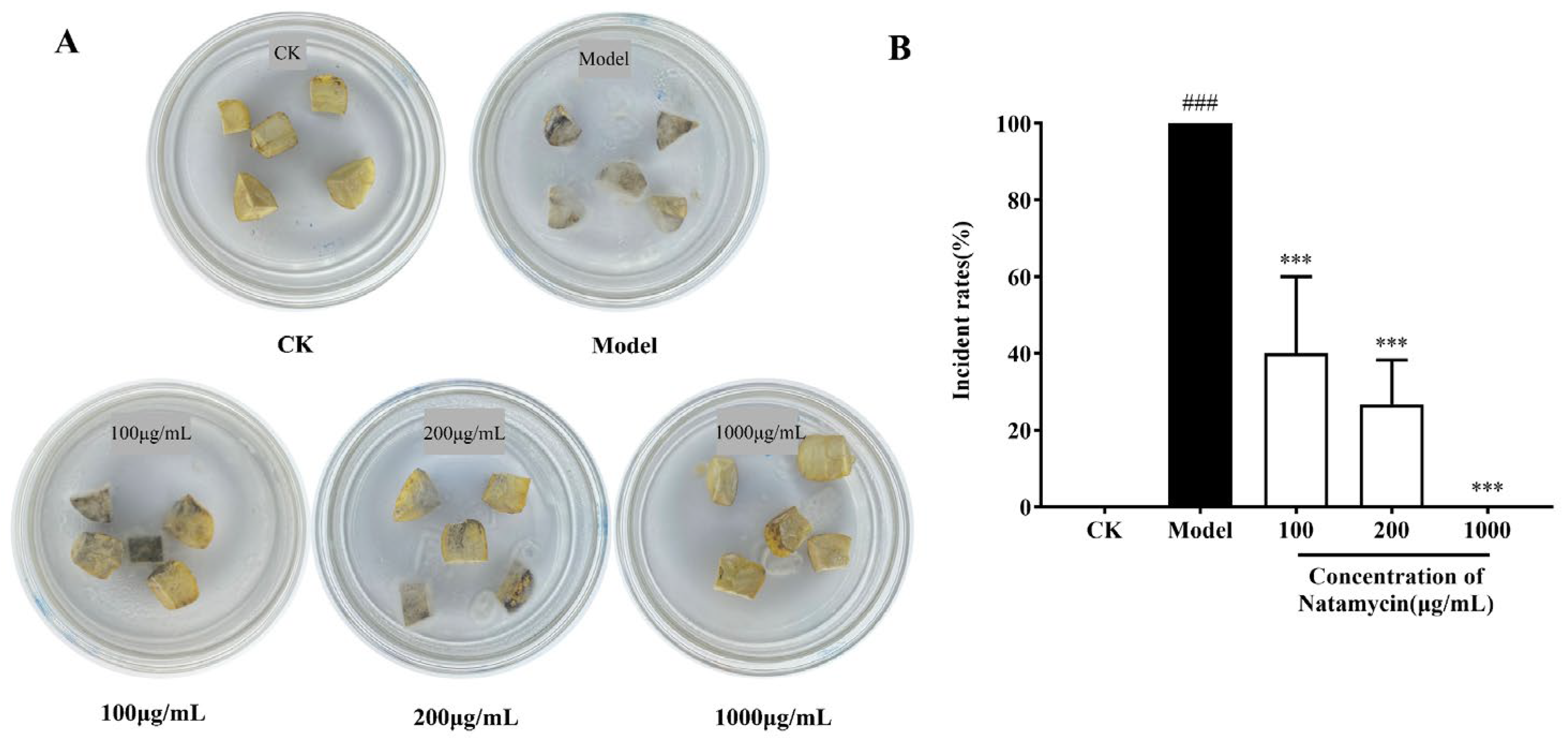
2.3.2. Effect of Natamycin Emulsion on Postharvest Ripening Infection of the Chestnut Shell Bucket
3. Discussion
4. Materials and Methods
4.1. Experimental Materials
4.2. Effect of Natamycin on the Growth Diameter of N. parvum
4.3. Inhibition Mechanism of Natamycin against N. parvum
4.3.1. Effect of Natamycin on the Cell Wall of N. parvum
4.3.2. Effect of Natamycin on the Cell Membrane of N. parvum
4.3.3. Effect of Natamycin on Ergosterol Synthesis in N. parvum
4.3.4. Effect of Natamycin on the Leakage of the Cellular Contents of N. parvum
4.3.5. Effect of Natamycin on Oxidative Stress in N. parvum
4.4. In Vivo Assays
4.4.1. In Vivo Antifungal Efficacy of Natamycin
4.4.2. Effect of Natamycin Emulsion on the Postharvest Ripening Infection of the Chestnut Shell Bucket
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Zhang, J.H.; He, Y.J. Advances and development trends of Chinese chestnut research at home and abroad. World For. Res. 1999, 12, 7–12. [Google Scholar]
- Zhang, S.; Wang, L.T.; Fu, Y.J.; Jiang, J.C. Bioactive constituents, nutritional benefits and woody food applications of Castanea mollissima: A comprehensive review. Food Chem. 2022, 393, 133380. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Shi, X.H.; Zhao, Q.J.; Cui, Y.H.; OuYang, J.; Xu, F. Effect of cooking methods on nutritional quality and volatile compounds of Chinese chestnut (Castanea mollissima Blume). Food Chem. 2016, 201, 80–86. [Google Scholar] [CrossRef]
- Rodrigues, P.; Ferreira, T.; Nascimento-Gonçalves, E.; Seixas, F.; Gil da Costa, R.M.; Martins, T.; Neuparth, M.J.; Pires, M.J.; Lanzarin, G.; Félix, L.; et al. Dietary supplementation with chestnut (Castanea sativa) reduces abdominal adiposity in FVB/n mice: A preliminary study. Biomedicines 2020, 8, 75. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Li, Y.S.; Wang, Q.L.; Li, J.X.; Ou, Y.J.; Wang, J.Z.; Wang, W.Q. Production, purification and analysis of the isomalto-oligosaccharides from Chinese chestnut (Castanea mollissima Blume) and the prebiotics effects of them on proliferation of Lactobacillus. Food Bioprod. Process. 2017, 106, 75–81. [Google Scholar] [CrossRef]
- Reggi, S.; Giromini, C.; Dell’Anno, M.; Baldi, A.; Rebucci, R.; Rossi, L. In Vitro Digestion of Chestnut and Quebracho Tannin Extracts: Antimicrobial Effect, Antioxidant Capacity and Cytomodulatory Activity in Swine Intestinal IPEC-J2 Cells. Animals 2020, 10, 195. [Google Scholar] [CrossRef]
- Hao, J.J.; Liu, H.; Donis-Gonzalez, I.R.; Lu, X.H.; Jones, A.D.; Fulbright, D.W. Antimicrobial Activity of Chestnut Extracts for Potential Use in Managing Soilborne Plant Pathogens. Plant Dis. 2012, 96, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Li, H.Y.; Cao, W.X.; Xie, J.W.; Che, H.X.; Liu, L.; Dong, X.F.; Song, L.; Xie, W.C. α-D-1, 6-glucan from Castanea mollissima Blume alleviates dextran sulfate sodium-induced colitis in vivo. Carbohydr. Polym. 2022, 289, 119410. [Google Scholar] [CrossRef]
- Dong, X.D.; Feng, Y.Y.; Liu, Y.N.; Ji, H.Y.; Yu, S.S.; Liu, A.J.; Yu, J. A novel polysaccharide from Castanea mollissima Blume: Preparation, characteristics and antitumor activities in vitro and in vivo. Carbohydr. Polym. 2020, 240, 116323. [Google Scholar] [CrossRef]
- Nazzaro, M.; Barbarisi, C.; Cara, F.L.; Volpe, M.G. Chemical and biochemical characterisation of an IGP ecotype chestnut subjected to different treatments. Food Chem. 2011, 128, 930–936. [Google Scholar] [CrossRef]
- Waqas, M.; Guarnaccia, V.; Spadaro, D. First report of nut rot caused by Neofusicoccum parvum on hazelnut (Corylus avellana) in Italy. Plant Dis. 2022, 106, 1987. [Google Scholar] [CrossRef] [PubMed]
- Seddaiu, S.; Mello, A.; Sechi, C.; Cerboneschi, A.; Linaldeddu, B.T. First Report of Neofusicoccum parvum associated with chestnut nut rot in Italy. Plant Dis. 2021, 105, 3743. [Google Scholar] [CrossRef]
- Phillips, A.J.; Alves, A.; Abdollahzadeh, J.; Slippers, B.; Wingfield, M.J.; Groenewald, J.Z.; Crous, P.W. The Botryosphaeriaceae: Genera and species known from culture. Stud. Mycol. 2013, 76, 51–167. [Google Scholar] [CrossRef] [PubMed]
- Crous, P.W.; Slippers, B.; Wingfield, M.J.; Rheeder, J.; Marasas, W.F.; Philips, A.J.; Alves, A.; Burgess, T.; Barber, P.; Groenewald, J.Z. Phylogenetic lineages in the Botryosphaeriaceae. Stud. Mycol. 2006, 55, 235–253. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Yang, Z.X. First Report of Neofusicoccum parvum Causing Brown Spots on Gallnuts of Rhus potaninii in China. Plant Dis. 2022, 106, 1071. [Google Scholar] [CrossRef] [PubMed]
- Haenzi, M.; Cochard, B.; Chablais, R.; Crovadore, J.; Lefort, F. Neofusicoccum parvum, A New Agent of Sequoia Canker and Dieback Identified in Geneva, Switzerland. Forests 2021, 12, 434. [Google Scholar] [CrossRef]
- Li, M.; Wang, Y.; Gong, D.Q.; Zhao, C.; Hu, M.J. First report of Neofusicoccum parvum causing leaf spot of Scaevola taccada in China. J. Plant Pathol. 2020, 102, 1341. [Google Scholar] [CrossRef]
- Guarnaccia, V.; Polizzi, G.; Papadantonakis, N.; Gullino, M.L. Neofusicoccum species causing branch cankers on avocado in Crete (Greece). J. Plant Pathol. 2020, 102, 1251–1255. [Google Scholar] [CrossRef]
- Pour, F.N.; Pedrosa, B.; Oliveira, M.; Fidalgo, C.; Decreese, B.; Van Driessche, G.; Felix, C.; Rosa, N.; Alves, A.; Duarte, A.S.; et al. Unveiling the secretome of the fungal plant pathogen Neofusicoccum parvum induced by in vitro host mimicry. J. Fungi 2022, 8, 971. [Google Scholar] [CrossRef]
- Yan, J.Y.; Xie, Y.; Zhang, W.; Wang, Y.; Liu, J.K.; Hyde, K.D.; Seem, R.C.; Zhang, G.Z.; Wang, Z.Y.; Yao, S.W.; et al. Species of Botryosphaeriaceae involved in grapevine dieback in China. Fungal. Divers. 2013, 61, 221–236. [Google Scholar] [CrossRef]
- Tiwari, B.K.; Valdramidis, V.P.; O’Donnell, C.P.; Muthukumarappan, K.; Bourke, P.; Cullen, P.J. Application of natural antimicrobials for food preservation. J. Agric. Food Chem. 2018, 57, 5987–6000. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.A.; Qian, C.; Wu, J.; Liu, Z.; Khan, S.; Tao, Z.; Zhang, X.; Khan, I.U.; Zhong, X. Effects of natamycin and Lactobacillus plantarum on the chemical composition, microbial community, and aerobic stability of Hybrid pennisetum at different temperatures. RSC Adv. 2020, 10, 8692–8702. [Google Scholar] [CrossRef] [PubMed]
- GB 2760-2014; National Standards for Food Safety—Standerds for Food Additive. National Health and Family Planning: Beijing, China, 2014.
- Kallinteri, L.D.; Kostoula, O.K.; Savvaidis, I.N. Efficacy of nisin and/or natamycin to improve the shelf-life of Galotyri cheese. Food Microbiol. 2013, 36, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.Y.; Lu, X.X.; Li, J.K.; Liang, B.; Guo, X.Y.; Chen, S.H.; Zhang, P. Effect of natamycin on the freshness of green juglans. Food Ferment Ind. 2013, 39, 221–225. [Google Scholar]
- Zhou, H.L.; Liu, M.Y.; Ren, X.L.; Wu, Z.L.; Zhang, W. Antisepsis and Fresh-keeping Effects of Natamycin Coating Compounds Treatment on Red Global Grape. Agric. Sci. Technol. 2012, 13, 2012–2016. [Google Scholar]
- Guo, Y.L.; Zhang, Z.H.; Hong, W.J. Advances in the research of a new food Additive natamycin. China Food Addit. 2005, 4, 68–80. [Google Scholar]
- OuYang, Q.L.; Okwong, R.O.; Chen, Y.P.; Tao, N.G. Synergistic activity of cinnamaldehyde and citronellal against green mold in citrus fruit. Postharvest Biol. Technol. 2020, 162, 111095. [Google Scholar] [CrossRef]
- Arora, R.; Gupta, D.; Goyal, J.; Kaur, R. Voriconazole versus natamycin as primary treatment in fungal corneal ulcers. Clin. Experiment. Ophthalmol. 2011, 39, 434–440. [Google Scholar] [CrossRef]
- Meena, M.; Prajapati, P.; Ravichandran, C.; Sehrawat, R. Natamycin: A natural preservative for food applications—A review. Food Sci. Biotechnol. 2021, 30, 1481–1496. [Google Scholar] [CrossRef]
- Panagou, E.Z.; Vekiari, S.A.; Sourris, P.; Mallidis, C. Efficacy of hot water, hypochlorite, organic acids and natamycin in the control of post-harvest fungal infection of chestnuts. J. Hortic. Sci. Biotech. 2005, 80, 61–64. [Google Scholar] [CrossRef]
- Liu, T.T.; Gou, L.J.; Zeng, H.; Zhou, G.; Dong, W.R.; Cui, Y.; Cai, Q.; Chen, Y.X. Inhibitory Effect and Mechanism of Dill Seed Essential Oil on Neofusicoccum parvum in Chinese Chestnut. Separations 2022, 9, 296. [Google Scholar] [CrossRef]
- Aparicio, J.F.; Barreales, E.G.; Payero, T.D.; Vicente, C.M.; de Pedro, A.; Santos-Aberturas, J. Biotechnological production and application of the antibiotic pimaricin: Biosynthesis and its regulation. Appl. Microbiol. Biotechnol. 2016, 100, 61–78. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, R.; Yu, Z.; Xue, L. Superoxide dismutase (SOD) and catalase (CAT) activity assay protocols for caenorhabditis elegans. Bio-Protocol 2017, 7, e2505. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; OuYang, Q.L.; Tao, N.G. Plasma membrane damage contributes to antifungal activity of citronellal against Penicillium digitatum. J. Food Sci. Technol. 2016, 53, 3853–3858. [Google Scholar] [CrossRef]
- Di Ciaccio, L.S.; Catalano, A.V.; López, P.G.; Rojas, D.; Cristos, D.; Fortunato, R.H.; Salvat, A.E. In vitro antifungal activity of Peltophorum dubium (Spreng.) taub. extracts against Aspergillus flavus. Plants 2020, 9, 438. [Google Scholar] [CrossRef]
- Dwivedy, A.K.; Prakash, B.; Chanotiya, C.S.; Bisht, D.; Dubey, N.K. Chemically characterized Mentha cardiaca L. essential oil as plant based preservative in view of efficacy against biodeteriorating fungi of dry fruits, aflatoxin secretion, lipid peroxidation and safety profile assessment. Food Chem. Toxicol. 2017, 106, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Tao, N.G.; OuYang, Q.L.; Jia, L. Citral inhibits mycelial growth of Penicillium italicum by a membrane damage mechanism. Food Control 2014, 41, 116–121. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gou, L.-J.; Liu, T.-T.; Zeng, Q.; Dong, W.-R.; Wang, L.; Long, S.; Su, J.-T.; Chen, Y.-X.; Zhou, G. Natamycin Has an Inhibitory Effect on Neofusicoccum parvum, the Pathogen of Chestnuts. Molecules 2023, 28, 3707. https://doi.org/10.3390/molecules28093707
Gou L-J, Liu T-T, Zeng Q, Dong W-R, Wang L, Long S, Su J-T, Chen Y-X, Zhou G. Natamycin Has an Inhibitory Effect on Neofusicoccum parvum, the Pathogen of Chestnuts. Molecules. 2023; 28(9):3707. https://doi.org/10.3390/molecules28093707
Chicago/Turabian StyleGou, Lin-Jing, Tian-Tian Liu, Qi Zeng, Wan-Rong Dong, Lu Wang, Sha Long, Jiang-Tao Su, Yu-Xin Chen, and Gao Zhou. 2023. "Natamycin Has an Inhibitory Effect on Neofusicoccum parvum, the Pathogen of Chestnuts" Molecules 28, no. 9: 3707. https://doi.org/10.3390/molecules28093707
APA StyleGou, L.-J., Liu, T.-T., Zeng, Q., Dong, W.-R., Wang, L., Long, S., Su, J.-T., Chen, Y.-X., & Zhou, G. (2023). Natamycin Has an Inhibitory Effect on Neofusicoccum parvum, the Pathogen of Chestnuts. Molecules, 28(9), 3707. https://doi.org/10.3390/molecules28093707






