Synergism of Photo-Induced Electron Transfer and Aggregation-Induced Quenching Mechanisms for Highly Sensitive Detection of Silver Ion and Captopril
Abstract
:1. Introduction
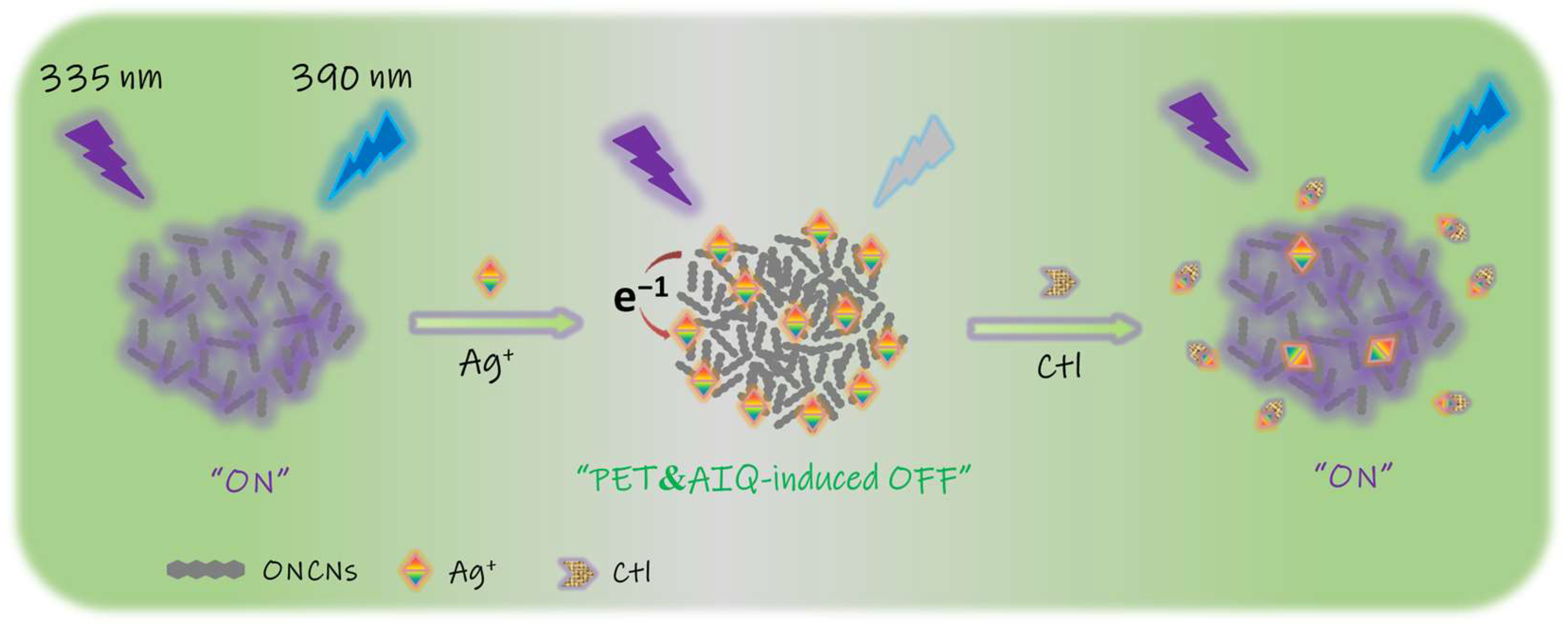
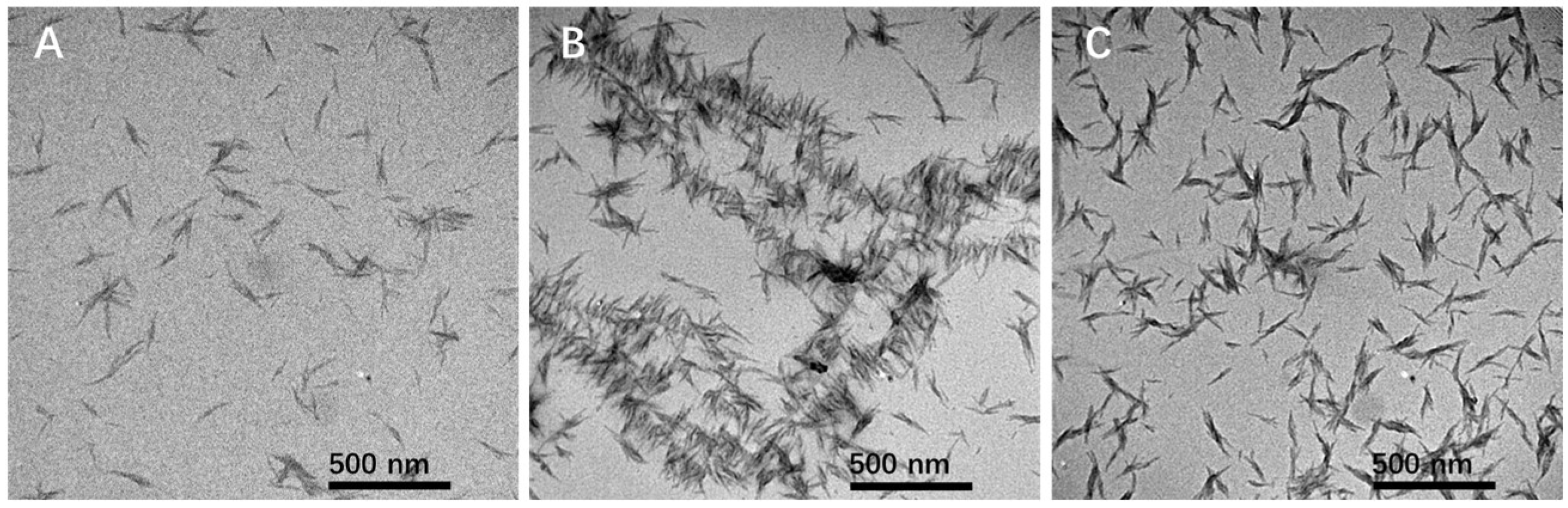
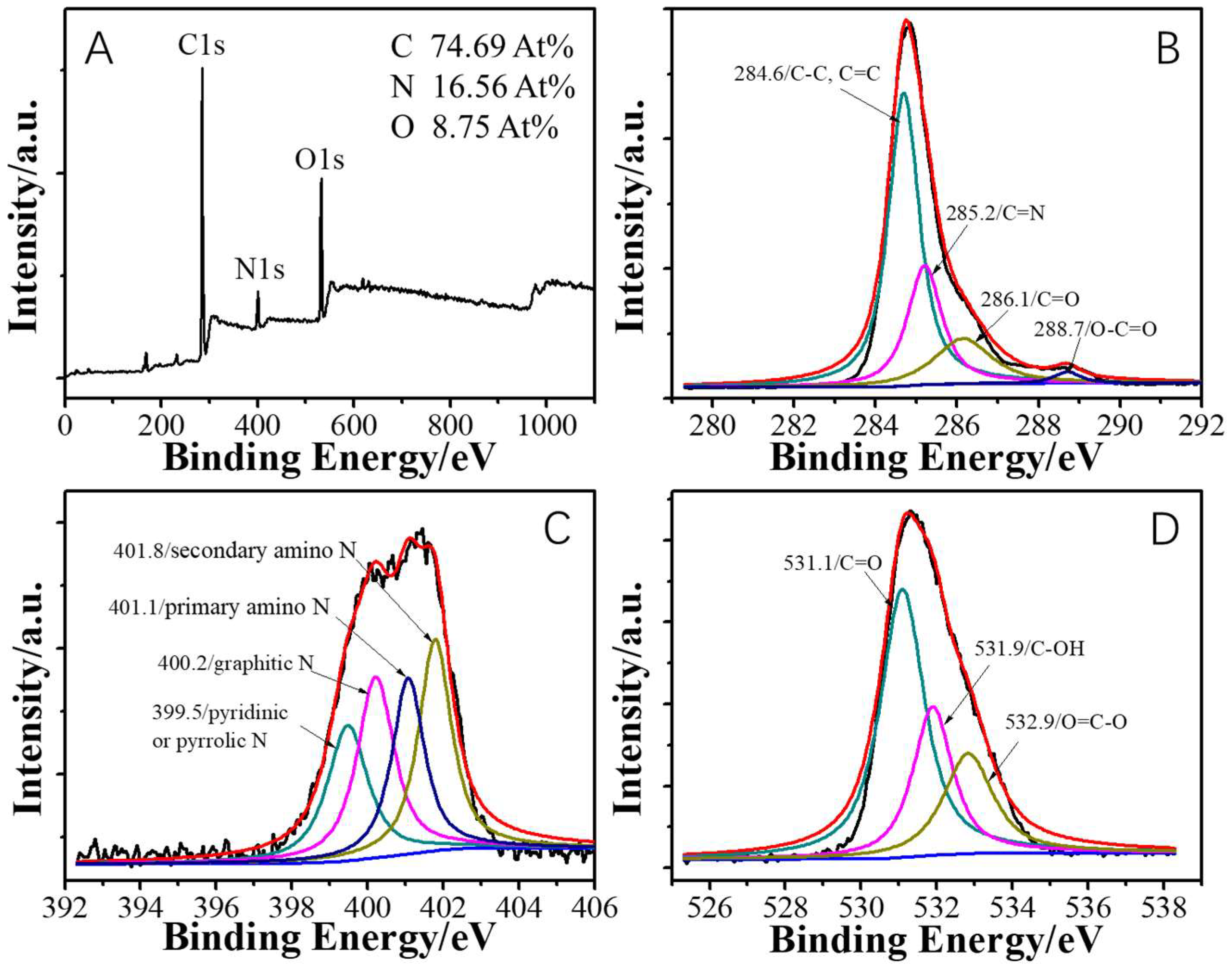
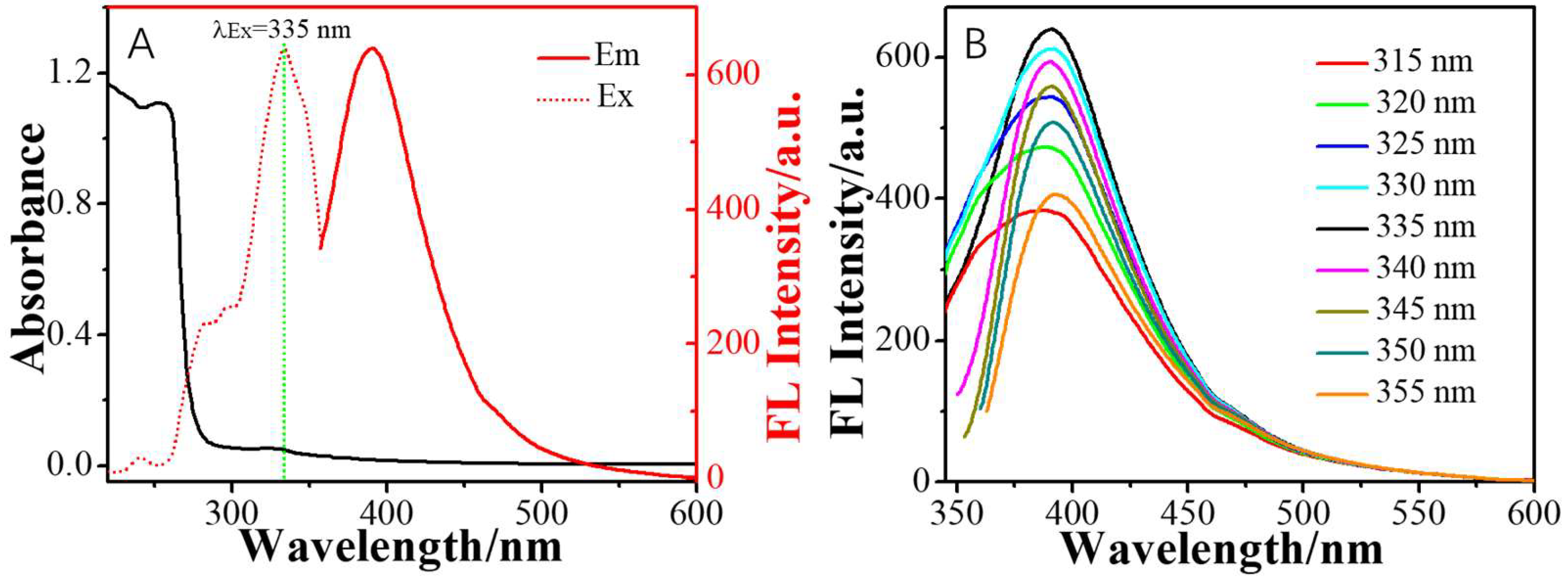
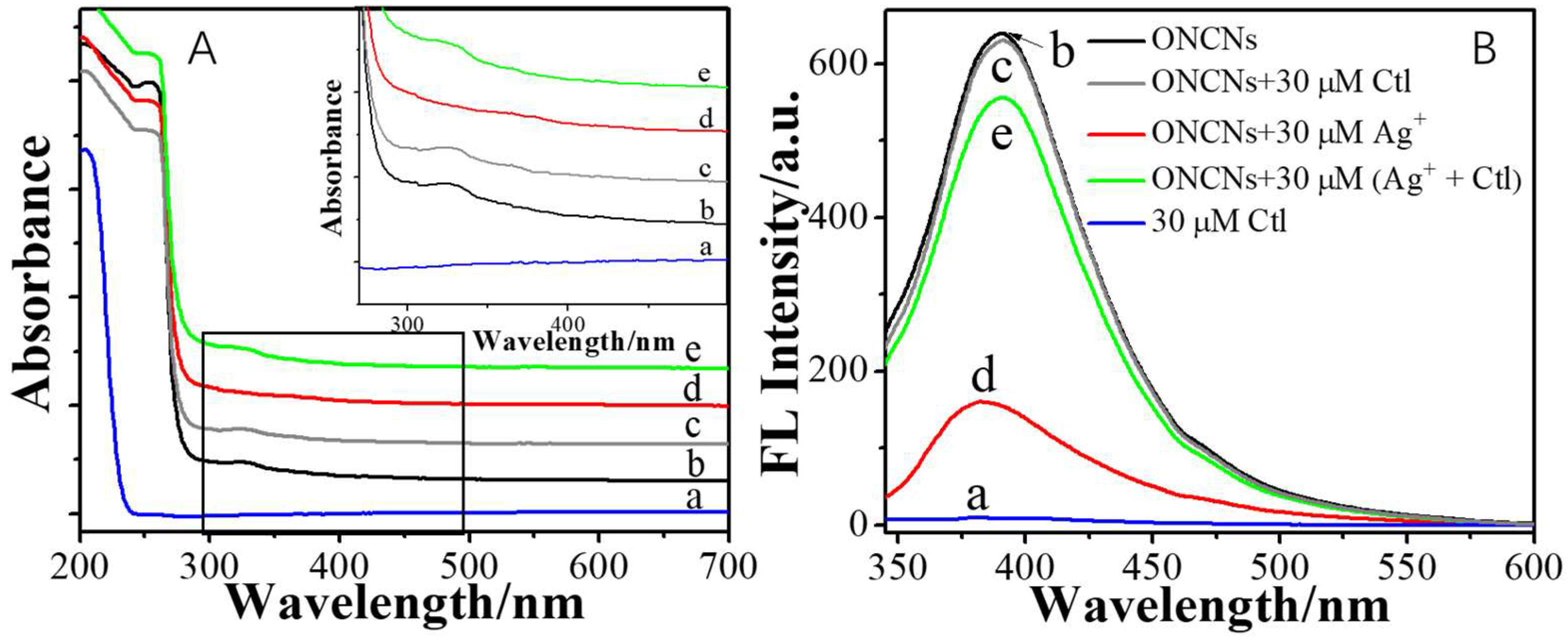
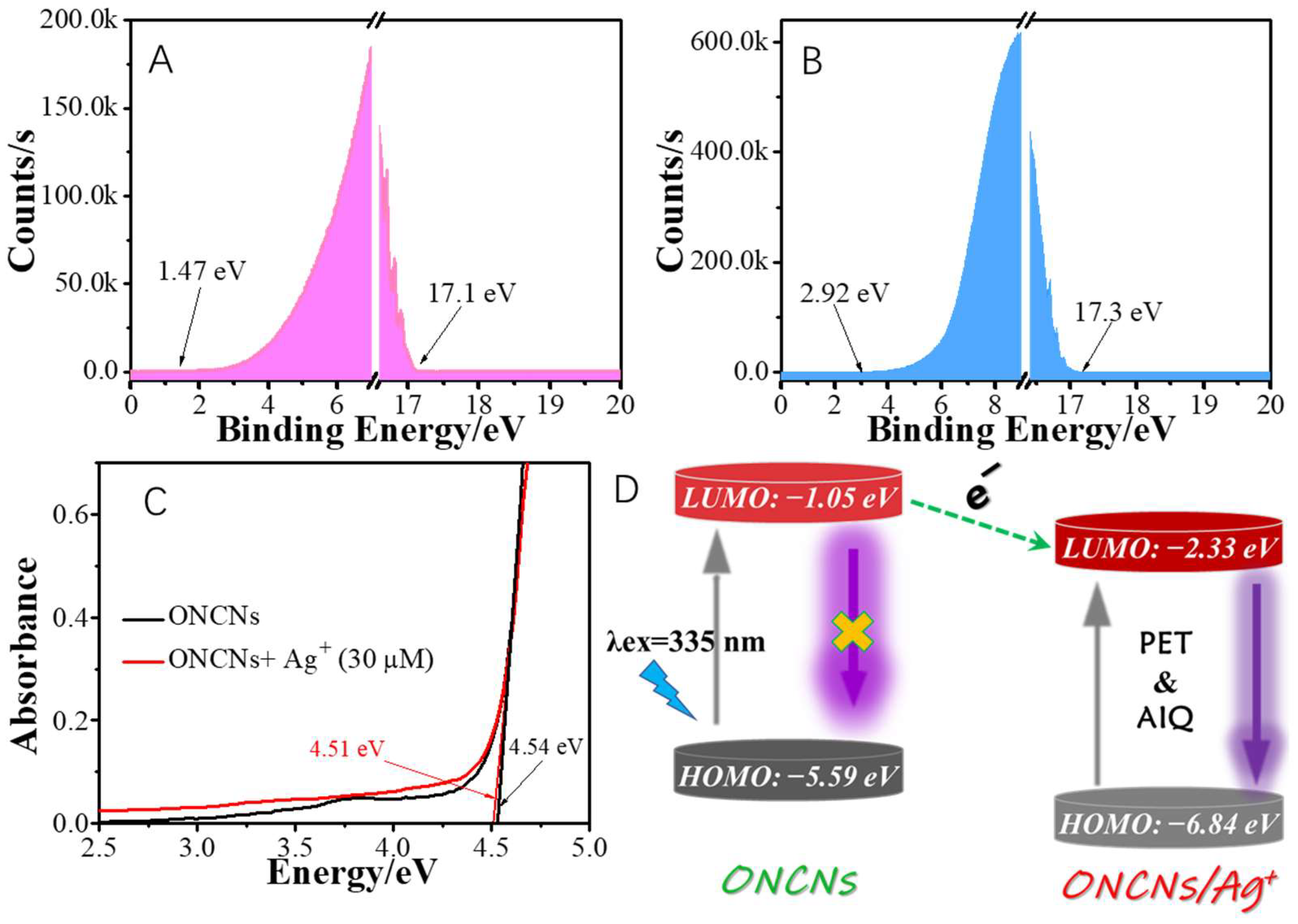
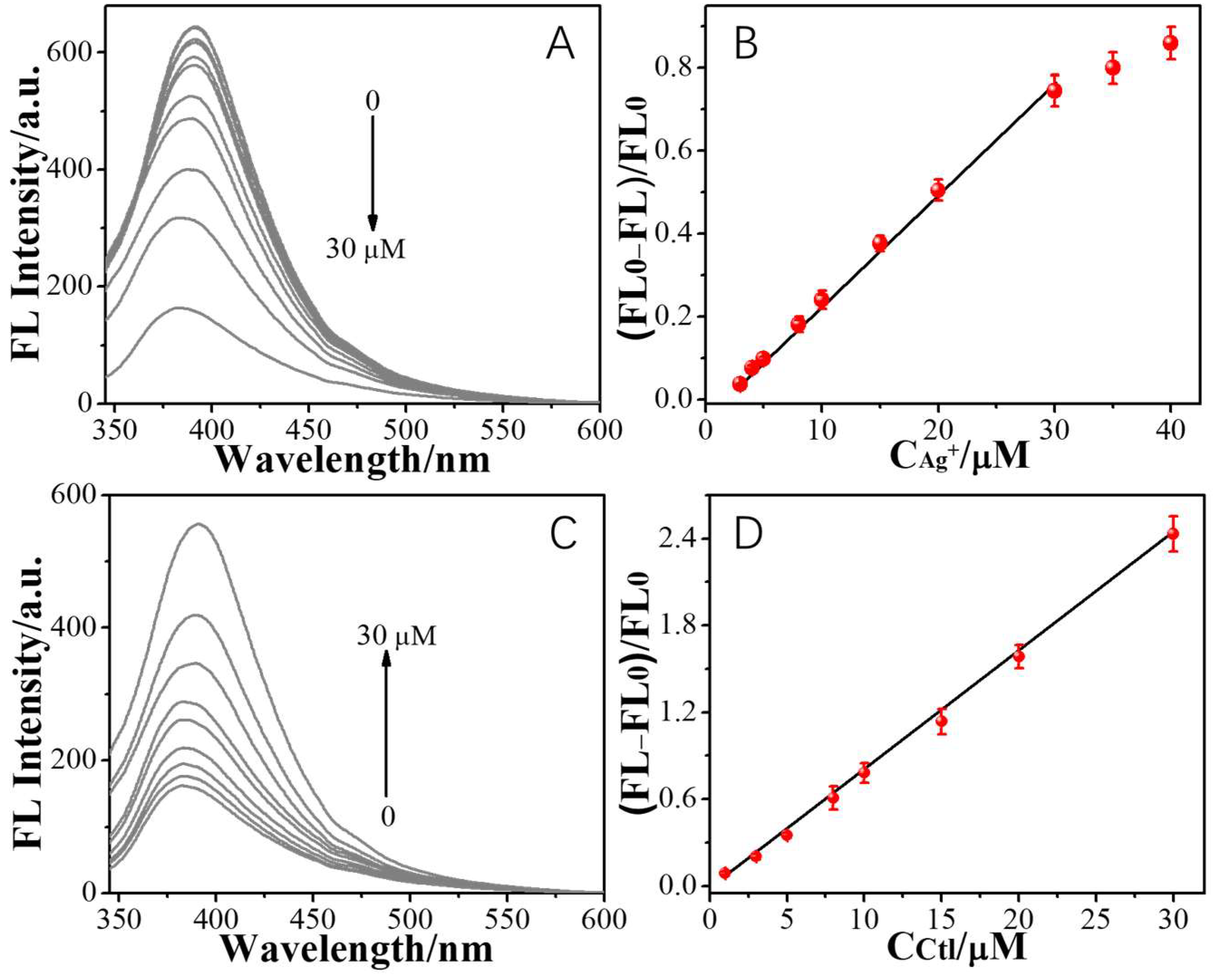
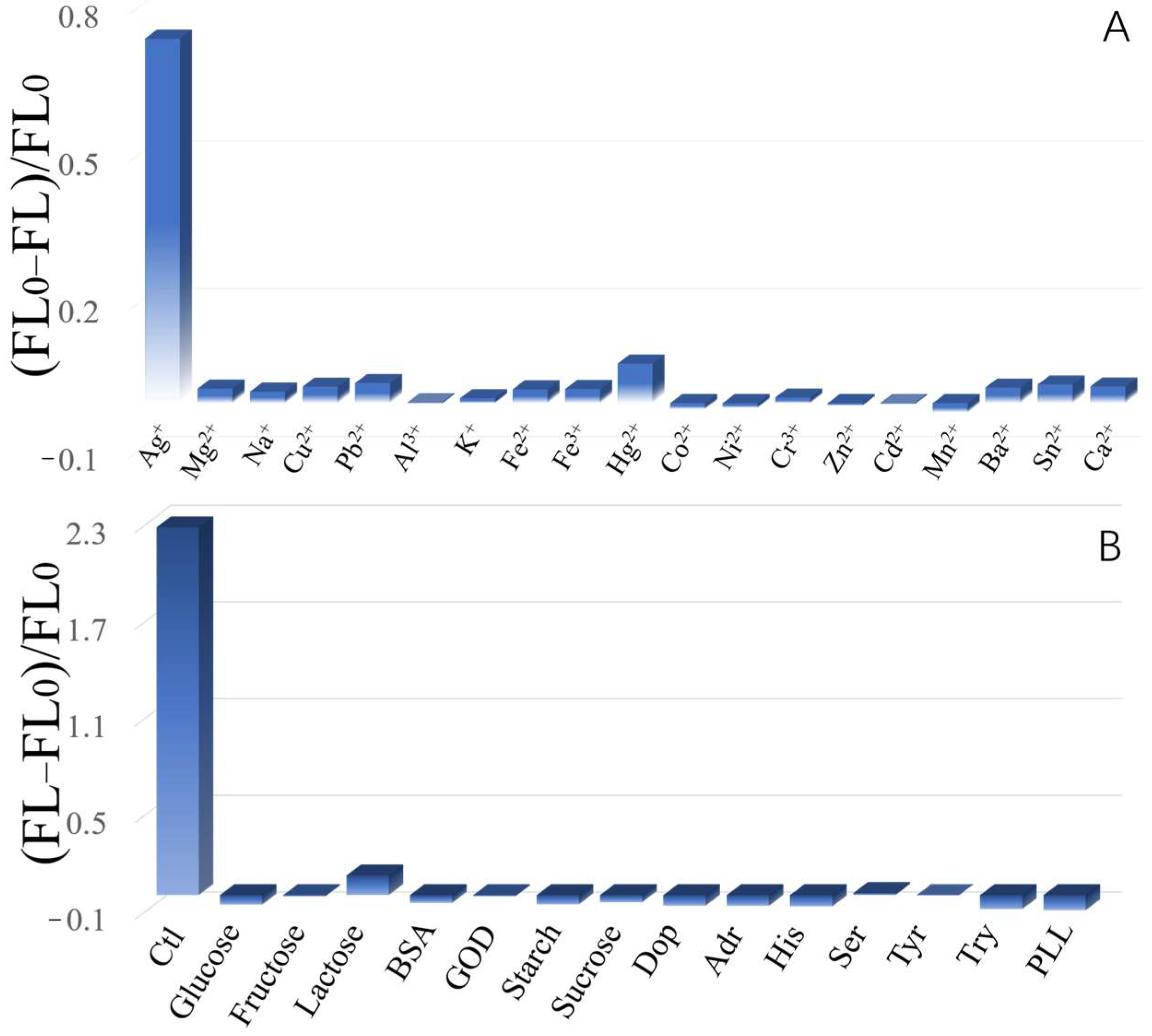
2. Results and Discussion
3. Materials and Methods
3.1. Reagents and Chemicals
3.2. Apparatus
3.3. Synthesis of Oxygen-Doped Nitrogen-Enrichment Carbon Nanoribbons (ONCNs)
3.4. Optimizing the Experimental Conditions
3.5. Fluorescent Detection Procedures for Ag+ and Ctl
3.6. Selectivity Investigation for Ctl
3.7. Procedure for Pharmaceutical Sample
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Ketki, B.; Dhiraj Devidas, B.; Udisha, S.; Pankaj, Y. Carbon-based, designer, and programmable fluorescent quantum dots for targeted biological and biomedical applications. Mater. Chem. Front. 2023, in press. [Google Scholar] [CrossRef]
- Xie, X.; Feng, S.; Lei, W.; Xia, M.; Wang, F.; Ni, Y. The facile detection and micromechanism of ATMP and DTPMP by fluorescence sensor based on nitrogen-doped carbon nanomaterials. Dye. Pigment. 2022, 207, 110659. [Google Scholar] [CrossRef]
- Othman, H.O.; Salehnia, F.; Hosseini, M.; Hassan, R.; Faizullah, A.; Ganjali, M.R. Fluorescence immunoassay based on nitrogen doped carbon dots for the detection of human nuclear matrix protein NMP22 as biomarker for early stage diagnosis of bladder cancer. Microchem. J. 2020, 157, 104966. [Google Scholar] [CrossRef]
- Atchudan, R.; Edison, T.N.J.I.; Aseer, K.R.; Perumal, S.; Karthik, N.; Lee, Y.R. Highly fluorescent nitrogen-doped carbon dots derived from Phyllanthus acidus utilized as a fluorescent probe for label-free selective detection of Fe3+ ions, live cell imaging and fluorescent ink. Biosens. Bioelectron. 2017, 99, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Chen, M.; Guo, Y.; Zhou, J.; Shi, Q.; Sun, R. Oxidized nanocellulose facilitates preparing photoluminescent nitrogen-doped fluorescent carbon dots for Fe3+ ions detection and bioimaging. Chem. Eng. J. 2019, 384, 123260. [Google Scholar] [CrossRef]
- Li, J.; Zuo, G.; Qi, X.; Wei, W.; Pan, X.; Su, T.; Zhang, J.; Dong, W. Selective determination of Ag+ using Salecan derived nitrogen doped carbon dots as a fluorescent probe. Mater. Sci. Eng. C 2017, 77, 508–512. [Google Scholar] [CrossRef] [PubMed]
- Gu, T.; Zou, W.; Gong, F.; Xia, J.; Chen, C.; Chen, X. A specific nanoprobe for cysteine based on nitrogen-rich fluorescent quantum dots combined with Cu2+. Biosens. Bioelectron. 2017, 100, 79–84. [Google Scholar] [CrossRef]
- Zhang, Y.; Qin, H.; Huang, Y.; Zhang, F.; Liu, H.; Liu, H.; Wang, Z.J.; Li, R. Highly fluorescent nitrogen and boron doped carbon quantum dots for selective and sensitive detection of Fe3+. J. Mater. Chem. B 2021, 9, 4654–4662. [Google Scholar] [CrossRef]
- Yang, Z.; Li, H.; Xu, T.T.; She, M.Y.; Chen, J.; Jia, X.D.; Liu, P.; Liu, X.Y.; Li, J.L. Red emissive carbon dots as a fluorescent sensor for fast specific monitoring and imaging of polarity in living cells. J. Mater. Chem. A 2023, 11, 2679–2689. [Google Scholar] [CrossRef]
- Wang, Z.X.; Gao, Y.F.; Yu, X.H.; Kong, F.Y.; Lv, W.X.; Wang, W. Photoluminescent coral-like carbon-branched polymers as nanoprobe for fluorometric determination of captopril. Microchim. Acta 2018, 185, 422. [Google Scholar] [CrossRef]
- Han, L.; Liu, T.; Cui, D.; Yi, J.; Jiang, W.; Li, X.; Niu, N.; Chen, L. Quantitative detection of captopril in urine by smartphone-assisted ratiometric fluorescence sensing platform. Spectrochim. Acta A 2022, 280, 121562. [Google Scholar] [CrossRef] [PubMed]
- Dewangan, L.; Chawre, Y.; Korram, J.; Karbhal, I.; Nagwanshi, R.; Jain, V.; Satnami, M.L. N-doped, silver, and cerium co-doped carbon quantum dots based sensor for detection of Hg2+ and captopril. Microchem. J. 2022, 182, 107867. [Google Scholar] [CrossRef]
- Jiang, X.; Qin, D.; Mo, G.; Feng, J.; Zheng, X.; Deng, B. Facile preparation of boron and nitrogen codoped green emission carbon quantum dots for detection of permanganate and captopril. Anal. Chem. 2019, 91, 11455–11460. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Wang, X.; Liu, H.; Wang, X.; Li, D.; Zhu, H.L.; Qian, Y. A high selective colorimetric fluorescent probe for detection of silver ions in vitro and in vivo and its application on test strips. Talanta 2022, 246, 123366. [Google Scholar] [CrossRef]
- Fan, X.Y.; Lv, J.P.; Li, R.; Chen, Y.F.; Zhang, S.; Liu, T.; Zhou, S.X.; Shao, X.D.; Wang, S.H.; Hu, G.Z.; et al. Paper test strip for silver ions detection in drinking water samples based on combined fluorometric and colorimetric methods. Arab. J. Chem. 2022, 16, 104492. [Google Scholar] [CrossRef]
- Gao, Z.; Liu, G.G.; Ye, H.; Rauschendorfer, R.; Tang, D.; Xia, X. Facile colorimetric detection of silver ions with picomolar sensitivity. Anal. Chem. 2017, 89, 3622–3629. [Google Scholar] [CrossRef]
- Maruthupandi, M.; Mamat, M.H.; Stalin, T.; Vasimalai, N. On-off-on fluorescence sequential sensor for silver ions, thiamine and anti-counterfeiting application using mannitol derived carbon dots. Nano-Struct. Nano-Objects 2022, 30, 100868. [Google Scholar] [CrossRef]
- Kang, J.; Gao, P.; Zhang, G.; Shi, L.; Zhou, Y.; Wu, J.; Shuang, S.; Zhang, Y. Rapid sonochemical synthesis of copper nanoclusters with red fluorescence for highly sensitive detection of silver ions. Microchem. J. 2022, 178, 107370. [Google Scholar] [CrossRef]
- Jiang, L.; Ding, H.; Xu, M.; Hu, X.; Li, S.; Zhang, M.; Zhang, Q.; Wang, Q.; Lu, S.; Tian, Y.; et al. UV-Vis-NIR full-range responsive carbon dots with large multiphoton absorption cross sections and deep-red fluorescence at nucleoli and in vivo. Small 2020, 16, 2000680. [Google Scholar] [CrossRef]
- Ai, L.; Song, Z.; Nie, M.; Yu, J.; Liu, F.; Song, H.; Zhang, B.; Waterhouse, G.I.N.; Lu, S. Solid-state fluorescence from carbon dots widely tunable from blue to deep red through surface ligand modulation. Angew. Chem. Int. Edit. 2022, 62, e202217822. [Google Scholar] [CrossRef]
- Hu, L.; Li, X.Q.; Jia, Y.L.; Wei, M.J.; Li, H.Y.; Kong, F.Y.; Wang, W.; Wang, Z.X. Electropositive far-ultraviolet carbon nanoparticles-based photoluminescent photoinduced-electron transfer sensor for sodium dodecyl sulfate and sodium lauryl sulfonate detection. Dye. Pigment. 2022, 208, 110859. [Google Scholar] [CrossRef]
- Wang, Z.X.; Kong, F.Y.; Wang, W. Near-ultraviolet fluorescent “ON-OFF-ON” switching sensors based on nitrogen-enriched dual-color single-functional polymer carbon nanosheets. Chem.-A Eur. J. 2016, 23, 665–675. [Google Scholar] [CrossRef] [PubMed]
- Geng, X.; Sun, Y.; Li, Z.; Yang, R.; Zhao, Y.; Guo, Y.; Xu, J.; Li, F.; Wang, Y.; Lu, S.; et al. Retrosynthesis of tunable fluorescent garbon dots for precise long-term mitochondrial tracking. Small 2019, 15, 1901517. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.X.; Hu, L.; Gao, Y.F.; Kong, F.Y.; Li, H.Y.; Zhu, J.; Fang, H.L.; Wang, W. Aggregation-induced emission behavior of dual-NIR-emissive zinc-doped carbon nanosheets for ratiometric anthrax biomarker detection. ACS Appl. Bio Mater. 2020, 3, 9031–9042. [Google Scholar] [CrossRef]
- Zheng, M.; Jia, H.; Zhao, B.; Zhang, C.; Dang, Q.; Ma, H.; Xu, K.; Tan, Z.A. Gram-scale room-temperature synthesis of solid-state fluorescent carbon nanodots for bright electroluminescent light emitting diodes. Small 2023, 19, e2206715. [Google Scholar] [CrossRef]
- Wang, Z.X.; Hu, L.; Li, X.Q.; Jia, Y.L.; Wang, T.; Wang, W. Boron-enriched rice-like homologous carbon nanoclusters with a 51.5% photoluminescent quantum yield for highly sensitive determination of endogenous hydroxyl radicals in living cells. J. Mater. Chem. B 2023, 11, 1523–1532. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, J.; Yang, Y.; Liu, X.; Qiu, J.; Tian, Y. Tunable full-color solid-state fluorescent carbon dots for light emitting diodes. Carbon 2022, 190, 22–31. [Google Scholar] [CrossRef]
- Zhu, L.; Shen, D.; Wang, Q.; Luo, K.H. Green synthesis of tunable fluorescent carbon quantum dots from lignin and their application in anti-counterfeit printing. ACS Appl. Mater. Interfaces 2021, 13, 56465–56475. [Google Scholar] [CrossRef]
- Lu, S.; Sui, L.; Liu, J.; Zhu, S.; Chen, A.; Jin, M.; Yang, B. Near-infrared photoluminescent polymer-carbon nanodots with two-photon fluorescence. Adv. Mater. 2017, 29, 1603443. [Google Scholar] [CrossRef]
- Meng, T.; Wang, Z.; Yuan, T.; Li, X.; Li, Y.; Zhang, Y.; Fan, L. Gram-scale synthesis of highly efficient rare-earth-element-free red/green/blue solid-state bandgap fluorescent carbon quantum rings for white light-emitting diodes. Angew. Chem. Int. Edit. 2021, 60, 16343–16348. [Google Scholar] [CrossRef]
- Wang, Z.X.; Hu, L.; Wang, W.J.; Kong, F.Y.; Wei, M.J.; Fang, H.L.; Li, Q.L.; Wang, W. One-pot green preparation of deep-ultraviolet and dual-emission carbon nanodots for dual-channel ratiometric determination of polyphenol in tea sample. Microchim. Acta 2022, 189, 241. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Li, Q.L.; Hu, L.; Zhu, H.Y.; Wang, W.J.; Kong, F.Y.; Li, H.Y.; Wang, Z.X.; Wang, W. Ratiometric detection of p-nitrophenol and its derivatives using a dual-emissive neuron cell-like carbonized probe based on a π⋯π stacking quenching mechanism. Analyst 2021, 146, 4566–4575. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.X.; Gao, Y.F.; Yu, X.H.; Balasubramanian, P.; Kong, F.Y.; Wang, W.; Chen, W.; Peng, H.P. Boron carbon oxyphosphide heterostructured nanodots with phosphate tunable emission for switchable dual detection channels of 6-mercaptopurine assay. Talanta 2021, 226, 122067. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.X.; Ding, S.N. One-pot green synthesis of high quantum yield oxygen-doped, nitrogen-rich, photoluminescent polymer carbon nanoribbons as an effective fluorescent sensing platform for sensitive and selective detection of silver(I) and mercury(II) ions. Anal. Chem. 2014, 86, 7436–7445. [Google Scholar] [CrossRef] [PubMed]
- Rybakiewicz, R.; Gawrys, P.; Tsikritzis, D.; Emmanouil, K.; Kennou, S.; Zagorska, M.; Pron, A. Electronic properties of semiconducting naphthalene bisimide derivatives-ultraviolet photoelectron spectroscopy versus electrochemistry. Electrochim. Acta 2013, 96, 13–17. [Google Scholar] [CrossRef]
- Liu, T.; Fu, L.-X.; Yin, C.-H.; Wu, M.; Chen, L.-G.; Niu, N. Design of smartphone platform by ratiometric fluorescent for visual detection of silver ions. Microchem. J. 2022, 174, 107016. [Google Scholar] [CrossRef]
- Pu, Z.-F.; Peng, J.; Wen, Q.-L.; Li, Y.; Ling, J.; Liu, P.; Cao, Q.-E. Photocatalytic synthesis of BSA-Au nanoclusters with tunable fluorescence for highly selective detection of silver ion. Dye. Pigment. 2021, 193, 109533. [Google Scholar] [CrossRef]
- Ma, Y.-X.; Lv, W.-J.; Chen, Y.-L.; Na, M.; Liu, J.-J.; Han, Y.-X.; Ma, S.-D.; Chen, X.-G. Facile preparation of orange-emissive carbon dots for the highly selective detection of silver ions. New J. Chem. 2019, 43, 5070–5076. [Google Scholar] [CrossRef]
- Xiao, S.-J.; Zhao, X.-J.; Chu, Z.-J.; Xu, H.; Liu, G.-Q.; Huang, C.-Z.; Zhang, L. New off-on sensor for captopril sensing based on photoluminescent MoOx quantum dots. ACS Omega 2017, 2, 1666–1671. [Google Scholar] [CrossRef]
| Sample | τ1/ns | A1/% | τ2/ns | A2/% | τave/ns | a QE/% | b kET/s−1 |
|---|---|---|---|---|---|---|---|
| ONCNs | 4.2 | 100 | - | - | 4.2 | - | - |
| ONCNs + Ag+ | 3.2 | 89.2 | 7.5 | 10.8 | 3.7 | 12.1 | 3.0 × 107 |
| ONCNs-Ag++ Ctl | 4.2 | 100 | - | - | 4.2 | ~0 | ~0 |
| Sample | Measured (µM) a | Standard Value (µM) a | Ctl Founded (µM) a | Recovery (%, n = 3) | RSD (%, n = 3) |
|---|---|---|---|---|---|
| 1 | 17.94 | 10 | 30.04 | 107.5 | 2.47 |
| 2 | 18.44 | 20 | 36.48 | 94.9 | 3.96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, J.; Hu, L.; Meng, X.; Li, F.; Wang, W.; Shi, G.; Wang, Z. Synergism of Photo-Induced Electron Transfer and Aggregation-Induced Quenching Mechanisms for Highly Sensitive Detection of Silver Ion and Captopril. Molecules 2023, 28, 3650. https://doi.org/10.3390/molecules28093650
Zhu J, Hu L, Meng X, Li F, Wang W, Shi G, Wang Z. Synergism of Photo-Induced Electron Transfer and Aggregation-Induced Quenching Mechanisms for Highly Sensitive Detection of Silver Ion and Captopril. Molecules. 2023; 28(9):3650. https://doi.org/10.3390/molecules28093650
Chicago/Turabian StyleZhu, Jing, Lei Hu, Xiangying Meng, Feng Li, Wenjuan Wang, Guiyang Shi, and Zhongxia Wang. 2023. "Synergism of Photo-Induced Electron Transfer and Aggregation-Induced Quenching Mechanisms for Highly Sensitive Detection of Silver Ion and Captopril" Molecules 28, no. 9: 3650. https://doi.org/10.3390/molecules28093650
APA StyleZhu, J., Hu, L., Meng, X., Li, F., Wang, W., Shi, G., & Wang, Z. (2023). Synergism of Photo-Induced Electron Transfer and Aggregation-Induced Quenching Mechanisms for Highly Sensitive Detection of Silver Ion and Captopril. Molecules, 28(9), 3650. https://doi.org/10.3390/molecules28093650






