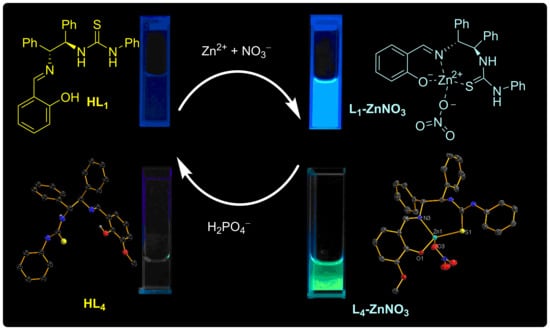
A Highly Selective and Sensitive Sequential Recognition Probe Zn2+ and H2PO4− Based on Chiral Thiourea Schiff Base
Abstract
1. Introduction
2. Results and Discussion
2.1. Fluorescence Spectroscopic Studies of HL1–HL6 and 3 in the Presence of Metal Ions
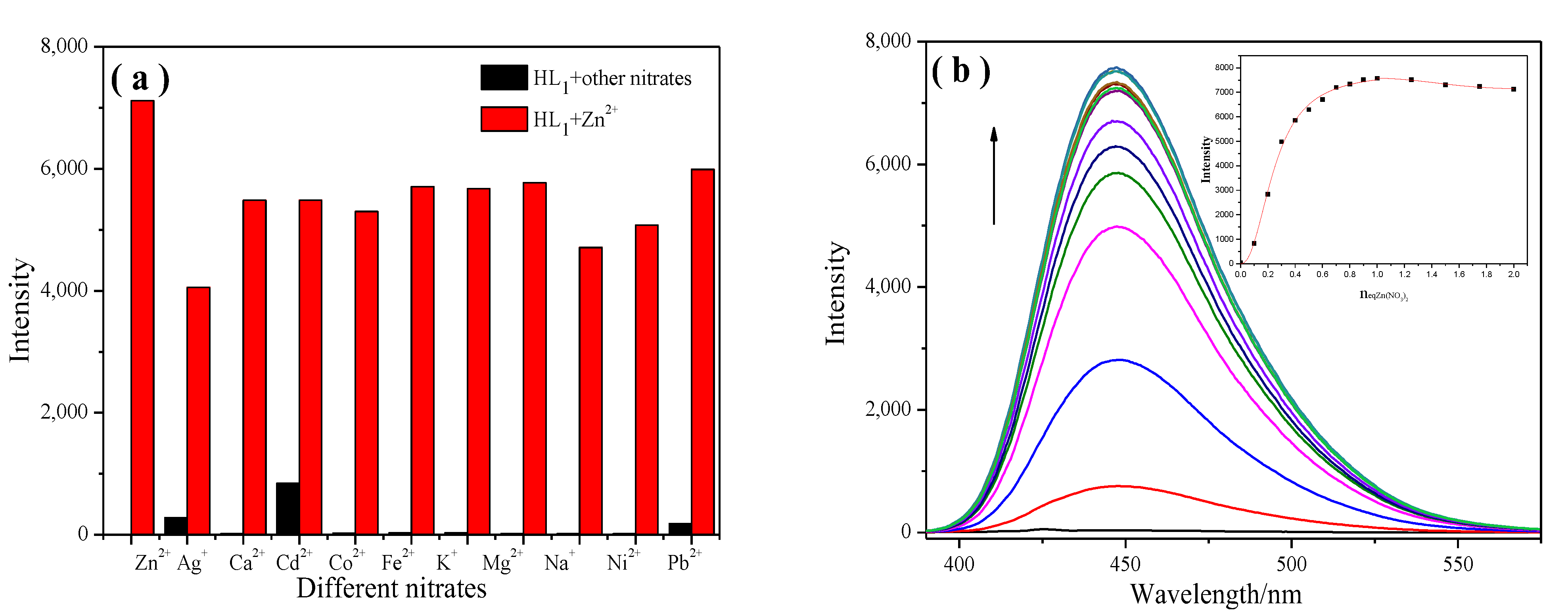
2.1.1. Cation Selectivity Experiments
2.1.2. Cation-Competitive Experiments
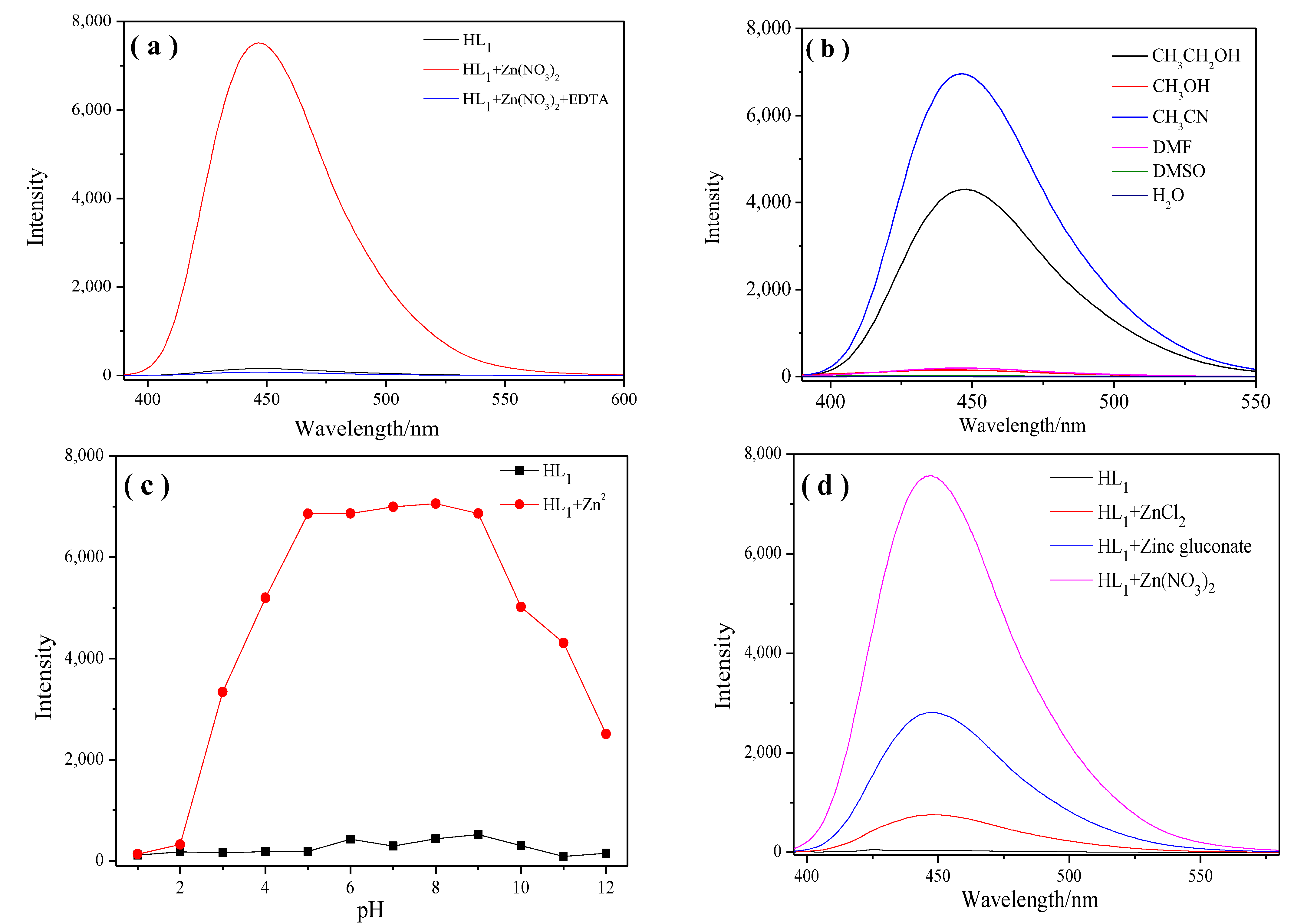
2.1.3. Study of the Reversibility and Dependence of Fluorescence on Solvent, pH, Different Zinc Salts
2.1.4. Zn2+ Titration Analysis
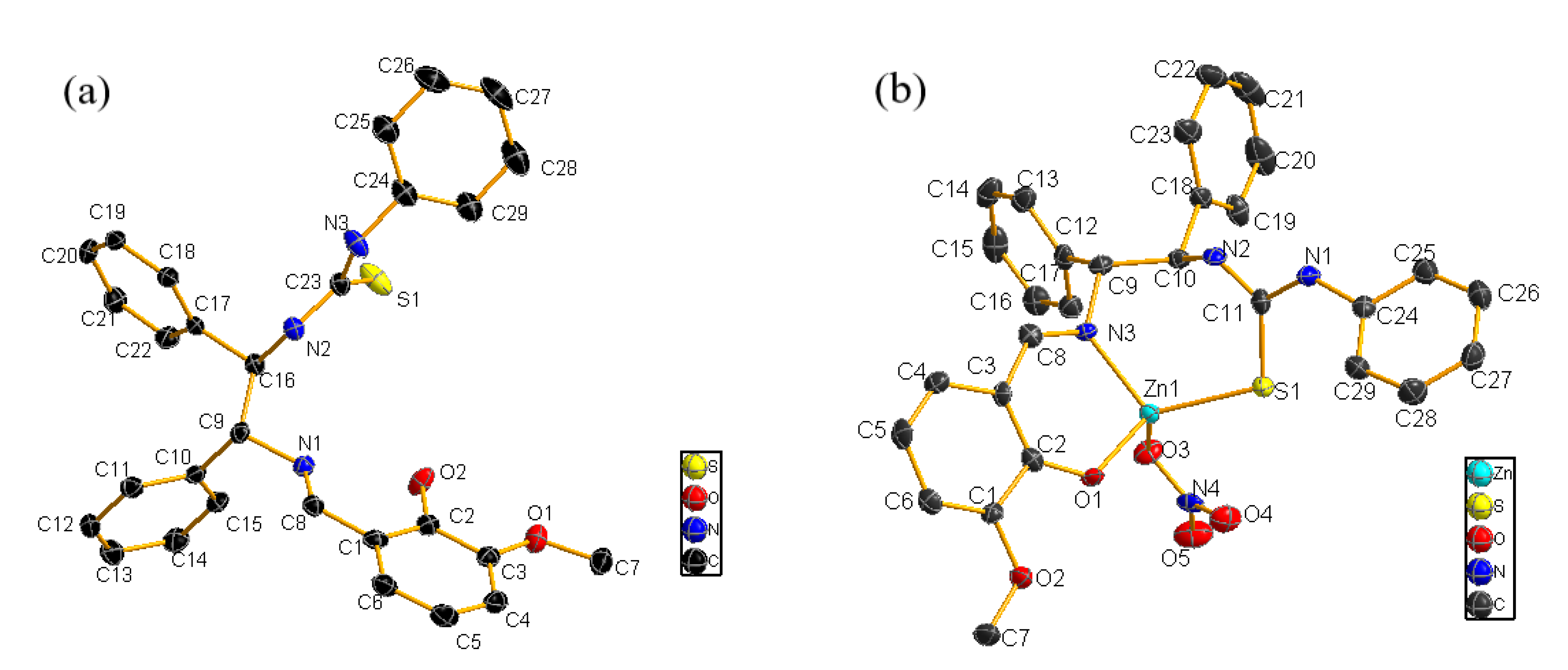
2.2. Binding Mechanism
2.3. Theoretical Study
2.4. Fluorescence Studies of L-ZnNO3 in the Presence of Anions
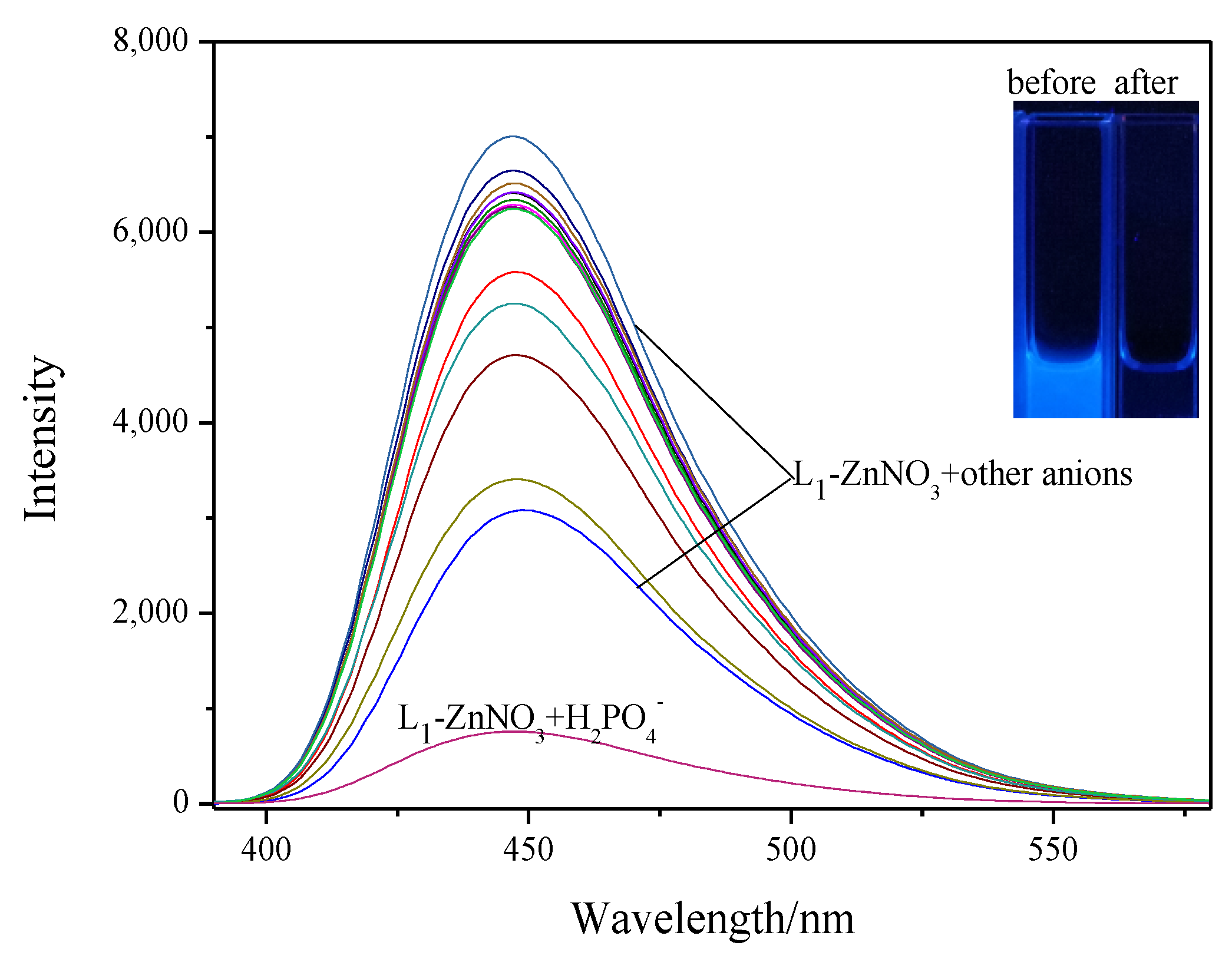
2.4.1. Anion Selectivity Experiments
2.4.2. Anion-Competitive Experiments
3. Materials and Methods
3.1. General Procedures
3.1.1. Reagents and Instruments
3.1.2. Synthesis of HL1–HL6 and Compound 3
3.2. Spectroscopic Measurements
3.3. Synthesis of the Sensor Zn(II) Complex and X-ray Crystallography
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Chowdhury, S.; Rooj, B.; Dutta, A.; Mandal, U. Review on recent advances in metal ions sensing using different fluorescent probes. J. Fluoresc. 2018, 28, 999–1021. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.J.; Feng, J.; Wang, Y.B.; Dong, C.; Shuang, S.M.; Wang, Y. Single fluorescein-based probe for selective colorimetric and fluorometric dual sensing of Al3+ and Cu2+. Sens. Actuators B Chem. 2017, 247, 451–460. [Google Scholar] [CrossRef]
- Zhan, Y.C.; Tsai, J.J.; Chen, Y.C. Zinc ion-based switch-on fluorescence-sensing probes for the detection of tetracycline. Molecules 2022, 27, 8403. [Google Scholar] [CrossRef] [PubMed]
- Asiri, A.M.; Al-Ghamdi, N.S.M.; Dzudzevic-Cancar, H.; Kumar, P.; Khan, S.A. Physicochemical and Photophysical investigation of newly synthesized carbazole containing pyrazoline-benzothiazole as fluorescent chemosensor for the detection of Cu2+, Fe3+ & Fe2+ metal ion. J. Mol. Struct. 2019, 1195, 670–680. [Google Scholar] [CrossRef]
- Shi, W.; Chen, Y.B.; Chen, X.; Xie, Z.F.; Hui, Y.H. Simple-structured, hydrazinecarbothioamide derivatived dual-channel optical probe for Hg2+ and Ag+. J. Lumin. 2016, 174, 56–62. [Google Scholar] [CrossRef]
- Gale, P.A.; Caltagirone, C. Fluorescent and colorimetric sensors for anionic species. Coord. Chem. Rev. 2018, 354, 2–27. [Google Scholar] [CrossRef]
- Banerjee, M.; Ta, S.; Ghosh, M.; Ghosh, A.; Das, D. Sequential fluorescence recognition of molybdenum(VI), arsenite, and phosphate ions in a ratiometric manner: A facile approach for discrimination of AsO2− and H2PO4−. ACS Omega 2019, 4, 10877–10890. [Google Scholar] [CrossRef]
- Wagh, Y.B.; Tayade, K.C.; Kuwar, A.; Sahoo, S.K.; Mayank; Singh, N.; Dalal, D.S. Exploration of highly selective fluorogenic “on–off” chemosensor for H2PO4- ions: ICT-based sensing and ATPase activity profiling. Luminescence 2020, 35, 379–384. [Google Scholar] [CrossRef]
- Lu, W.; Zhang, M.Y.; Liu, K.Y.; Fan, B.; Xia, Z.; Jiang, L.M. A fluoride-selective colorimetric and fluorescent chemosensor and its use for the design of molecular-scale logic devices. Sens. Actuators B Chem. 2011, 160, 1005–1010. [Google Scholar] [CrossRef]
- Hagimori, M.; Hara, F.; Mizuyama, N.; Fujino, T.; Saji, H.; Mukai, T. High-affinity ratiometric fluorescence probe based on 6-amino-2,2′-bipyridine scaffold for endogenous Zn2+ and its application to living cells. Molecules 2022, 27, 1287. [Google Scholar] [CrossRef]
- Roy, A.; Shee, U.; Mukherjee, A.; Mandal, S.K.; Roy, P. Rhodamine-based dual chemosensor for Al3+ and Zn2+ ions with distinctly separated excitation and emission wavelengths. ACS Omega 2019, 4, 6864–6875. [Google Scholar] [CrossRef]
- Liu, D.D.; Zhang, M.Z.; Du, W.; Hu, L.; Li, F.; Tian, X.H.; Wang, A.D.; Zhang, Q.; Zhang, Z.P.; Wu, J.Y. A series of Zn(II) terpyridine-based nitrate complexes as two-photon fluorescent probe for identifying apoptotic and living cells via subcellular immigration. Inorg. Chem. 2018, 57, 7676–7683. [Google Scholar] [CrossRef] [PubMed]
- Winnett, M.R.; Mini, P.; Grace, M.R.; Tuck, K.L. Time-resolved terbium-based probe for the detection of zinc(II) ions: Investigation of the formation of a luminescent ternary complex. Inorg. Chem. 2020, 59, 118–127. [Google Scholar] [CrossRef]
- Bourassa, D.; Elitt, C.M.; McCallum, A.M.; Sumalekshmy, S.; McRae, R.L.; Morgan, M.T.; Sigel, N.; Perry, J.W.; Rosenberg, P.A.; Fahrni, C.J. Chromis-1, a ratiometric fluorescent probe optimized for two-photon microscopy reveals dynamic changes in labile Zn(II) in differentiating oligodendrocytes. ACS Sens. 2018, 3, 458–467. [Google Scholar] [CrossRef]
- Cho, J.; Verwilst, P.; Kang, M.J.; Pan, J.L.; Sharma, A.; Hong, C.S.; Kim, J.S.; Kim, S. Crown ether-appended calix[2]triazolium[2]arene as a macrocyclic receptor for the recognition of the H2PO4− anion. Chem. Commun. 2020, 56, 1038–1041. [Google Scholar] [CrossRef] [PubMed]
- Asthana, S.K.; Pandey, A.; Kumar, A.; Upadhyay, K.K. An incisive optical recognition of monohydrogen phosphate by a fluorescein-based chemodosimeter. New J. Chem. 2020, 44, 2201–2205. [Google Scholar] [CrossRef]
- Yao, H.; Zhou, Q.; Wang, J.; Chen, Y.Y.; Kan, X.T.; Wei, T.B.; Zhang, Y.M.; Lin, Q. Highly selective Fe3+ and F-/H2PO4- sensor based on a water-soluble cationic pillar[5]arene with aggregation-induced emission characteristic. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 221, 117215–117220. [Google Scholar] [CrossRef]
- Zhang, D.W.; Cochrane, J.R.; Martinez, A.; Gao, G.H. Recent advances in H2PO4− fluorescent sensors. RSC Adv. 2014, 4, 29735–29749. [Google Scholar] [CrossRef]
- Gray, R.W.; Wilz, D.R.; Caldas, A.E.; Jacob Lemann, J.R. The importance of phosphate in regulating plasma 1,25-(OH)2-vitamin D levels in humans: Studies in healthy subjects, in calcium-stone formers and in patients with primary hyperparathyroidism. J. Clin. Endocrinol. Metab. 1976, 45, 299–306. [Google Scholar] [CrossRef]
- Shi, B.B.; Zhang, Y.M.; Wei, T.B.; Lin, Q.; Yao, H.; Zhang, P.; You, X.M. A fluorescent and colorimetric chemosensor for dihydrogen phosphate ions based on 2-pyridine-1H-imidazo[4,5-b]phenazine–zinc ensemble. Sens. Actuators B Chem. 2014, 190, 555–561. [Google Scholar] [CrossRef]
- Liu, F.F.; Fan, C.B.; Pu, S.Z. A new “turn-on” fluorescent chemosensor for Zn2+ based on a diarylethene derivative and its practical applications. J. Photochem. Photobiol. A Chem. 2019, 371, 248–254. [Google Scholar] [CrossRef]
- Kumar, S.S.; Kumar, R.S.; Kumar, S.A. An “Off-On-Off” type fluorescent chemosensor for the relay detection of Zn2+ and H2PO4− in aqueous environment. Inorg. Chim. Acta 2020, 502, 119348. [Google Scholar] [CrossRef]
- Wang, S.; Ma, L.L.; Liu, G.; Pu, S.Z. Diarylethene-based fluorescent and colorimetric chemosensor for the selective detection of Al3+ and CN−. Dyes Pigments 2019, 164, 257–266. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, X.; Feng, E.T.; Fan, C.B.; Pu, S.Z. A highly selective sequential recognition probe for Zn2+ and HSO4−/H2PO4− based on a diarylethene chemosensor. Spectrochim. Acta A 2021, 246, 119052. [Google Scholar] [CrossRef] [PubMed]
- Arabahmadi, R. Antipyrine-based Schiff base as fluorogenic chemosensor for recognition of Zn2+, Cu2+ and H2PO4− in aqueous media by comparator, half subtractor and integrated logic circuits. J. Photochem. Photobiol. A Chem. 2022, 426, 113762. [Google Scholar] [CrossRef]
- Liu, G.; Zhao, L. A simple colorimetric and on–off fluorescent chemosensor for biologically important anions based on thiourea groups. Spectrosc. Lett. 2012, 45, 424–429. [Google Scholar] [CrossRef]
- Chowdhury, B.; Sinha, S.; Ghosh, P. Substitution effect on near infrared absorbance based selective fluoride sensing of indole functionalized thiourea molecules. Eur. J. Org. Chem. 2019, 5, 1008–1015. [Google Scholar] [CrossRef]
- Sinha, S.; Dey, G.; Kumar, S.; Mathew, J.; Mukherjee, T.; Mukherjee, S.; Ghosh, S. Cysteamine-based cell-permeable Zn2+-specific molecular bioimaging materials: From animal to plant cells. ACS Appl. Mater. Interfaces 2013, 5, 11730–11740. [Google Scholar] [CrossRef]
- Powell, K.J.; Brown, P.L.; Byrne, R.H.; Gajda, T.; Hefter, G.; Leuz, A.-K.; Sjoberg, S.; Wanner, H. Chemical speciation of environmentally significant metals with inorganic ligands. Part 5: The Zn2+ + OH−, Cl−, CO32−, SO42−, and PO43− systems (IUPAC Technical Report). Pure Appl. Chem. 2013, 85, 2249–2311. [Google Scholar] [CrossRef]
- Hibberta, D.B.; Thordarson, P. The death of the Job plot, transparency, open science and online tools, uncertainty estimation methods and other developments in supramolecular chemistry data analysis. Chem. Commun. 2016, 52, 12792–12805. [Google Scholar] [CrossRef]
- Available online: http://supramolecular.org (accessed on 9 May 2016).
- Thordarson, P. Determining association constants from titration experiments in supramolecular chemistry. Chem. Soc. Rev. 2011, 40, 1305–1323. [Google Scholar] [CrossRef] [PubMed]
- Datta, B.K.; Thiyagarajan, D.; Ramesh, A.; Das, G. A sole multi-analyte receptor responds with three distinct fluorescence signals: Traffic signal like sensing of Al3+, Zn2+ and F−. Dalton Trans. 2015, 44, 13093–13099. [Google Scholar] [CrossRef]
- Valencia, L.; Pérez-Lourido, P.; Bastida, R.; Macias, A. Dinuclear Zn(II) polymer consisting of channels formed by π,π-stacking interactions with a flow of nitrate anions through the channels. Cryst. Growth Des. 2008, 8, 2080–2082. [Google Scholar] [CrossRef]
- Dolai, M.; Mistri, T.; Panja, A.; Ali, M. Diversity in supramolecular self-assembly through hydrogen-bonding interactions of non-coordinated aliphatic –OH group in a series of heterodinuclear CuIIM (M=NaI, ZnII, HgII, SmIII, BiIII, PbII and CdII). Inorg. Chim. Acta 2013, 399, 95–104. [Google Scholar] [CrossRef]
- Zhu, Y.; Xia, C.K.; Meng, S.C.; Chen, J.; Chen, J.; Xie, J.M. Syntheses, structures, properties and DFT calculations of coordination polymers constructed by 2,6-bis(benzimidazolyl)pyridine. Polyhedron 2013, 61, 181–187. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Montgomery, J.A.; Vreven, T.; Kudin, K.N.; Burant, J.C.; et al. Revision A.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Miehlich, B.; Savin, A.; Stoll, H.; Preuss, H. Results obtained with the correlation energy density functionals of becke and Lee, Yang and Parr. Chem. Phys. Lett. 1989, 157, 200–206. [Google Scholar] [CrossRef]
- Paulpandi, R.Q.; Ramasamy, S.; Paulraj, M.S.; Baños, F.G.D.; Villora, G.; Cerón-Carrasco, J.P.; Pérez-Sanchez, H.; Enoch, I.V.M.V. Enhanced Zn2+ ion-sensing behavior of a benzothiazole derivative on encapsulation by β- cyclodextrin. RSC Adv. 2016, 6, 15670–15677. [Google Scholar] [CrossRef]
- Dong, Z.; Le, X.; Zhou, P.; Dong, C.; Ma, J. An “off–on–off” fluorescent probe for the sequential detection of Zn2+ and hydrogen sulfide in aqueous solution. New J. Chem. 2014, 38, 1802–1808. [Google Scholar] [CrossRef]
- Dong, Z.; Le, X.; Zhou, P.; Dong, C.; Ma, J. Sequential recognition of zinc ion and hydrogen sulfide by a new quinoline derivative with logic gate behavior. RSC Adv. 2014, 4, 18270–18277. [Google Scholar] [CrossRef]
- Shyamal, M.; Mazumdar, P.; Maity, S.; Samanta, S.; Sahoo, G.P.; Misra, A. Highly selective turn-on fluorogenic chemosensor for robust quantification of Zn(II) based on aggregation induced emission enhancement feature. ACS Sens. 2016, 1, 739–747. [Google Scholar] [CrossRef]
- He, X.; Xie, Q.; Fan, J.; Xu, C.; Xu, W.; Li, Y.; Ding, F.; Deng, H.; Chen, H.; Shen, J. Dual-functional chemosensor with colorimetric/ratiometric response to Cu(II)/Zn(II) ions and its applications in bioimaging and molecular logic gates. Dyes Pigments 2020, 177, 108255. [Google Scholar] [CrossRef]
- Moradi, S.; Molavipordanjani, S.; Hosseinimehr, S.J.; Emami, S. Benzo[d]imidazo[2,1-b] thiazole-based fluorescent sensor for Zn2+ ion detection. J. Photochem. Photobiol. A 2020, 389, 112184. [Google Scholar] [CrossRef]
- Sethupathi, M.; Jayamani, A.; Muthusankar, G.; Sakthivel, P.; Sekar, K.; Gandhid, S.; Sengottuvelan, N.; Gopu, G.; Selvaraju, C. Colorimetric and fluorescence sensing of Zn2+ ion and its bio-imaging applications based on macrocyclic “tet a” derivative. J. Photochem. Photobiol. B 2020, 207, 11854. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT-Integrated space-group and crystal-structure determination. Acta Crystallogr. A-Found. Adv. 2015, A71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C Struct. Chem. 2015, C71, 3–8. [Google Scholar] [CrossRef]




| Parameter (Bounds) | Optimized | Error | Initial |
|---|---|---|---|
| K (0 → ∞) | 8607.30 M−1 | ±9.1006% | 100.00 M−1 |
| K11 (0 → ∞) | 3376.76 M−1 | ±5.2092% | 1000.00 M−1 |
| K12 (0 → ∞) | 4790.08 M−1 | ±26.9494% | 100.00 M−1 |
| K11 (0 → ∞) | 3576.53 M−1 | ±9.1090% | 1000.00 M−1 |
| K21 (0 → ∞) | −277,004.73 M−1 | ±−4.6365% | 100.00 M−1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.; Huang, Y.; Lu, A.; Wang, Z.; Li, H. A Highly Selective and Sensitive Sequential Recognition Probe Zn2+ and H2PO4− Based on Chiral Thiourea Schiff Base. Molecules 2023, 28, 4166. https://doi.org/10.3390/molecules28104166
Yang S, Huang Y, Lu A, Wang Z, Li H. A Highly Selective and Sensitive Sequential Recognition Probe Zn2+ and H2PO4− Based on Chiral Thiourea Schiff Base. Molecules. 2023; 28(10):4166. https://doi.org/10.3390/molecules28104166
Chicago/Turabian StyleYang, Shan, Yichuan Huang, Aidang Lu, Ziwen Wang, and Hongyan Li. 2023. "A Highly Selective and Sensitive Sequential Recognition Probe Zn2+ and H2PO4− Based on Chiral Thiourea Schiff Base" Molecules 28, no. 10: 4166. https://doi.org/10.3390/molecules28104166
APA StyleYang, S., Huang, Y., Lu, A., Wang, Z., & Li, H. (2023). A Highly Selective and Sensitive Sequential Recognition Probe Zn2+ and H2PO4− Based on Chiral Thiourea Schiff Base. Molecules, 28(10), 4166. https://doi.org/10.3390/molecules28104166