Studies Relevant to the Functional Model of Mo-Cu CODH: In Situ Reactions of Cu(I)-L Complexes with Mo(VI) and Synthesis of Stable Structurally Characterized Heterotetranuclear MoVI2CuI2 Complex
Abstract
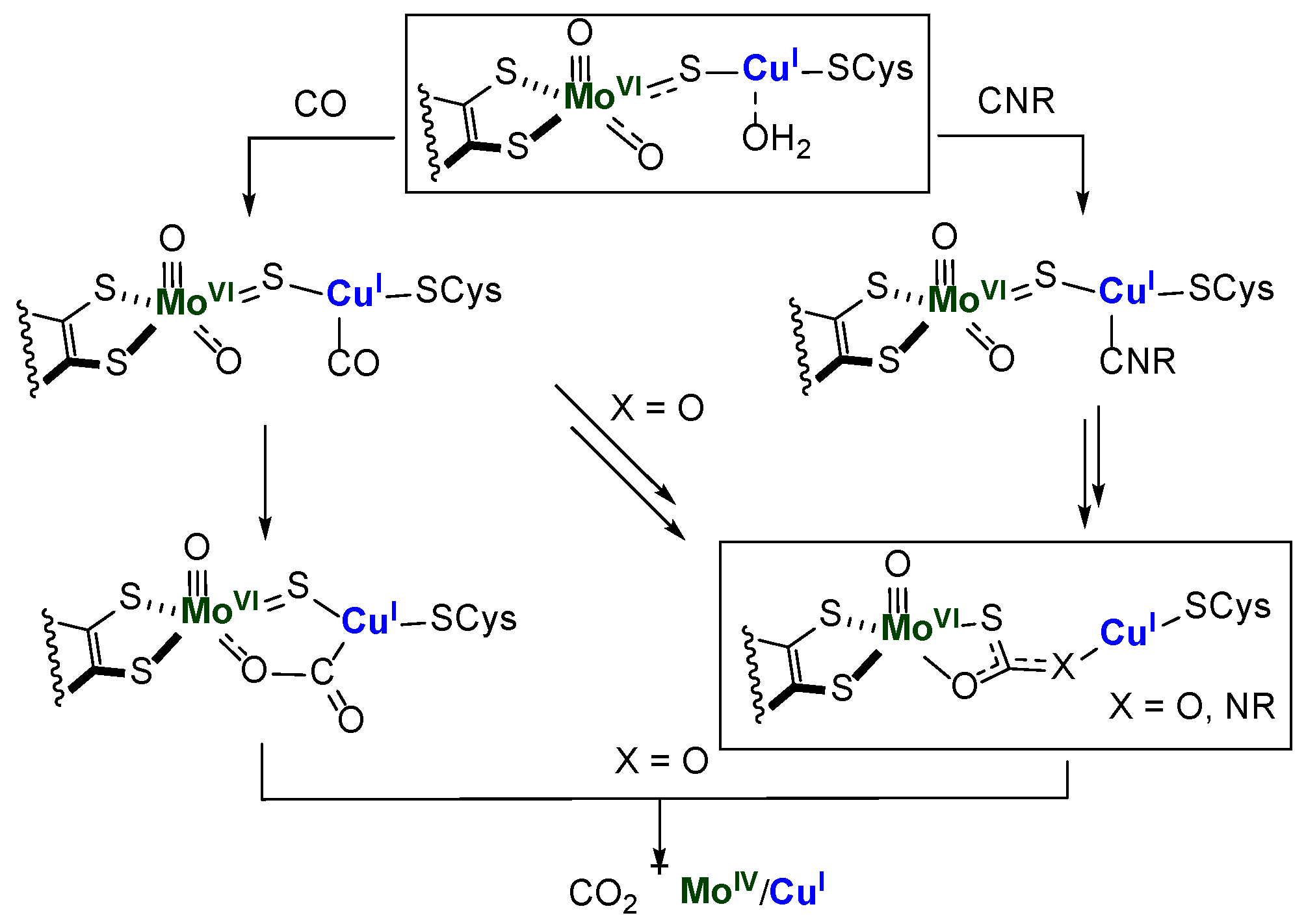
1. Introduction
2. Results and Discussion
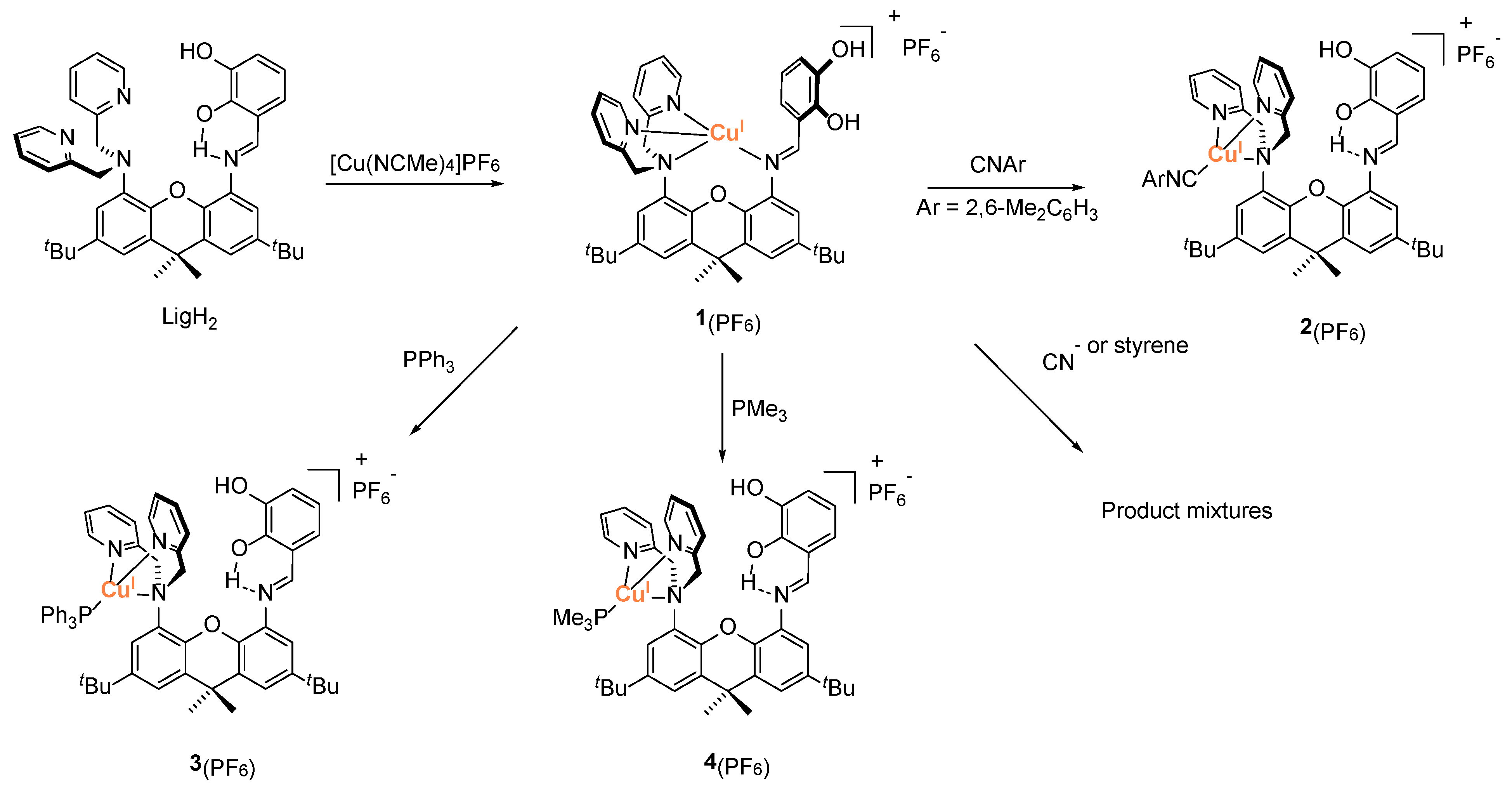
2.1. Synthesis and Characterization of Cu(I)-LigH2 Complexes Featuring an Additional L Ligand
2.2. Reactions of Cu-L Complexes (L = CNR, PR3, Styrene) with [MoO4]2−
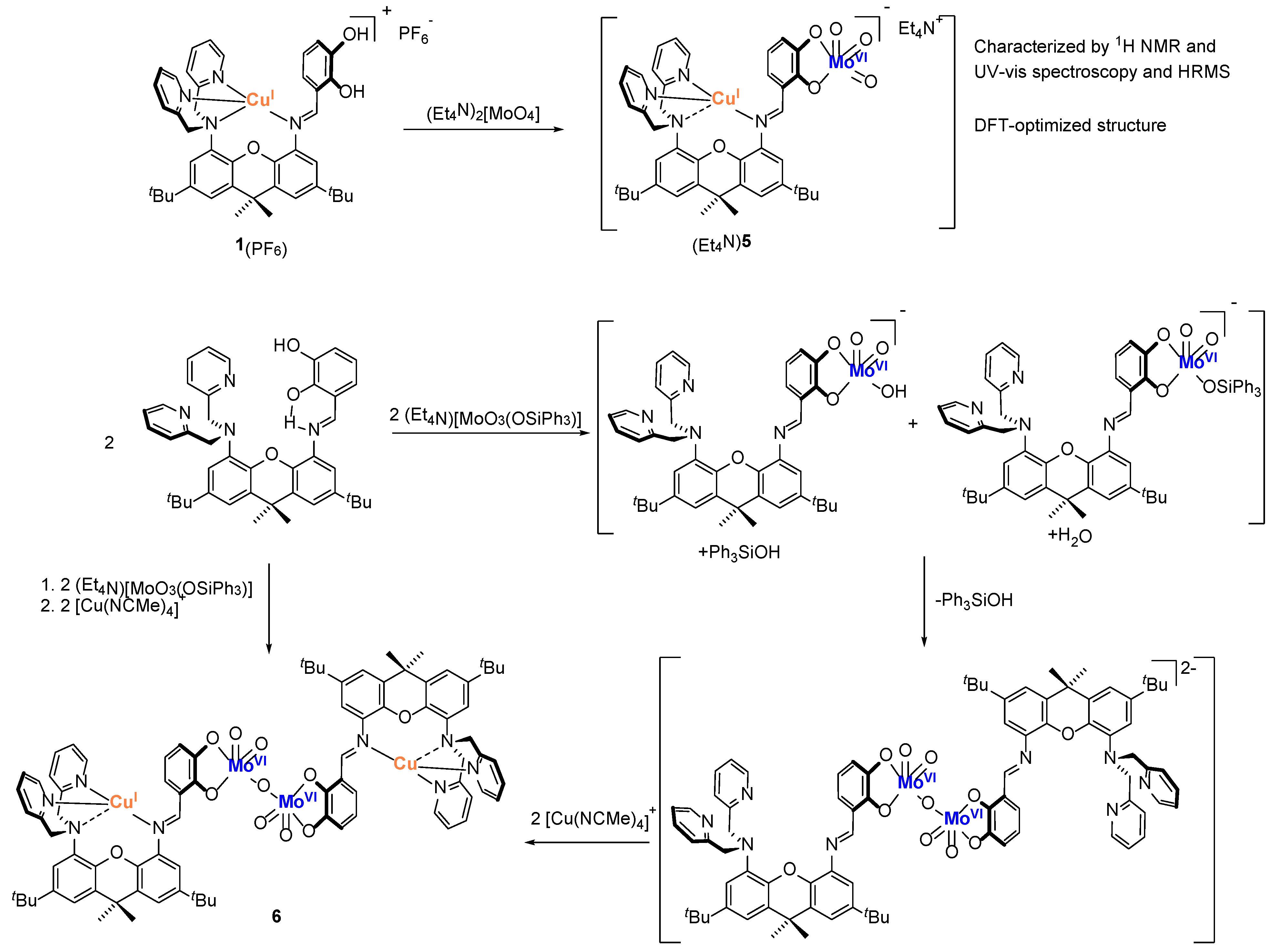
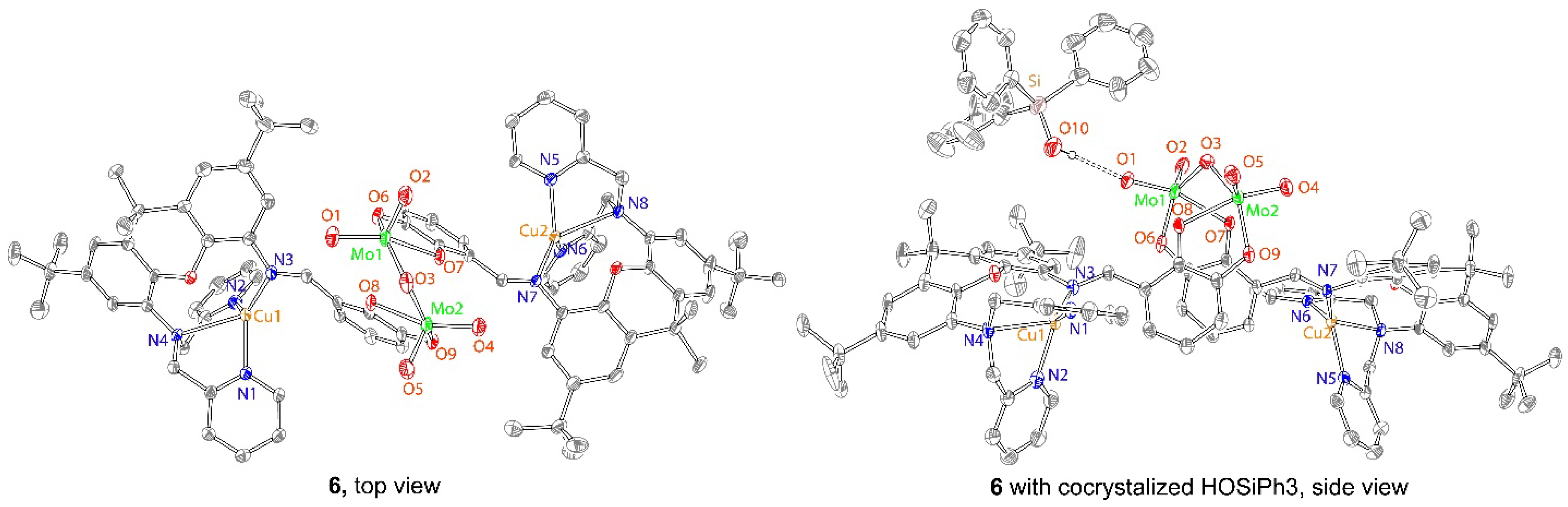
2.3. Synthesis and Characterization of Mo2Cu2 Heterodinuclear Complex
3. Summary and Conclusions
4. Materials and Methods
4.1. General
4.2. Synthetic Procedures and Compounds Characterization
4.3. X-ray Crystallographic Details
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hille, R.; Dingwall, S.; Wilcoxen, J. The aerobic CO dehydrogenase from Oligotropha carboxidovorans. J. Biol. Inorg. Chem. 2015, 20, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Mörsdorf, G.; Frunzke, K.; Gadkari, D.; Meyer, O. Microbial growth on carbon monoxide. Biodegradation 1992, 3, 61–82. [Google Scholar] [CrossRef]
- Burgmayer, S.J.N.; Kirk, M.L. The Role of the Pyranopterin Dithiolene Component of Moco in Molybdoenzyme Catalysis. In Structure and Bonding: Metallocofactors that Activate Small Molecules; Ribbe, M., Ed.; Springer Nature: Cham, Switzerland, 2019; Volume 179, pp. 101–151. [Google Scholar]
- Basu, P.; Burgmayer, S.J.N. Recent Developments in the Study of Molybdoenzyme Models. J. Biol. Inorg. Chem. 2015, 20, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Dobbek, H.; Gremer, L.; Kiefersauer, R.; Huber, R.; Meyer, O. Catalysis at a dinuclear [CuSMo(AO)OH] cluster in a CO dehydrogenase resolved at 1.1-Å resolution. Proc. Natl. Acad. Sci USA 2002, 99, 15971–15976. [Google Scholar] [CrossRef]
- Wilcoxen, J.; Hille, R. The hydrogenase activity of the molybdenum/copper-containing carbon monoxide dehydrogenase of Oligotropha carboxidovorans. J. Biol. Chem. 2013, 288, 36052–36060. [Google Scholar] [CrossRef]
- Gnida, M.; Ferner, R.; Gremer, L.; Meyer, O.; Meyer-Klaucke, W. A novel binuclear [CuSMo] cluster at the active site of carbon monoxide dehydrogenase: Characterization by X-ray absorption spectroscopy. Biochemistry 2003, 42, 222–230. [Google Scholar] [CrossRef]
- Zhang, B.; Hemann, C.F.; Hille, R. Kinetic and spectroscopic studies of the molybdenum-copper CO dehydrogenase from Oligotropha carboxidovorans. J. Biol. Chem. 2010, 285, 12571–12578. [Google Scholar] [CrossRef]
- Shanmugam, M.; Wilcoxen, J.; Habel-Rodriguez, D.; Cutsail, G.E.; Kirk, M.L.; Hoffman, B.M.; Hille, R. 13C and 63,65Cu ENDOR studies of CO Dehydrogenase from Oligotropha carboxidovorans. Experimental Evidence in Support of a Copper–Carbonyl Intermediate. J. Am. Chem. Soc. 2013, 135, 17775–17782. [Google Scholar] [CrossRef]
- Siegbahn, P.E.M.; Shestakov, A.F. Quantum chemical modeling of CO oxidation by the active site of molybdenum CO dehydrogenase. J. Comput. Chem. 2005, 26, 888–898. [Google Scholar] [CrossRef]
- Hofmann, M.; Kassube, J.K.; Graf, T. The mechanism of Mo-/Cu-dependent CO dehydrogenase. J. Biol. Inorg. Chem. 2005, 10, 490–495. [Google Scholar] [CrossRef]
- Stein, B.W.; Kirk, M.L. Orbital contributions to CO oxidation in Mo–Cu carbon monoxide dehydrogenase. Chem. Commun. 2014, 50, 1104–1106. [Google Scholar] [CrossRef]
- Rokhsana, D.; Large, T.A.; Dienst, M.C.; Retegan, M.; Neese, F. A realistic in silico model for structure/function studies of molybdenum–copper CO dehydrogenase. J. Biol. Inorg. Chem. 2016, 21, 491–499. [Google Scholar] [CrossRef]
- Xu, K.; Hirao, H. Revisiting the catalytic mechanism of Mo–Cu carbon monoxide dehydrogenase using QM/MM and DFT calculations. Phys. Chem. Chem. Phys. 2018, 20, 18938–18948. [Google Scholar] [CrossRef]
- Rovaletti, A.; Bruschi, M.; Moro, G.; Cosentino, U.; Greco, C. The challenging in silico description of carbon monoxide oxidation as catalyzed by molybdenum-copper CO dehydrogenase. Front. Chem. 2019, 6, 630. [Google Scholar] [CrossRef]
- Rovaletti, A.; Bruschi, M.; Moro, G.; Cosentino, U.; Ryde, U.; Greco, C. A thiocarbonate sink on the enzymatic energy landscape of aerobic CO oxidation? Answers from DFT and QM/MM models of MoCu CO-dehydrogenases. J. Catal. 2019, 372, 201–205. [Google Scholar] [CrossRef]
- Ritacca, A.G.; Rovaletti, A.; Moro, G.; Cosentino, U.; Ryde, U.; Sicilia, E.; Greco, C. Unraveling the reaction mechanism of Mo/Cu CO dehydrogenase using QM/MM calculations. ACS Catal. 2022, 12, 7336–7343. [Google Scholar] [CrossRef]
- Majumdar, A. Bioinorganic Modeling Chemistry of Carbon Monoxide Dehydrogenases: Description of Model Complexes, Current Status and Possible Future Scopes. Dalton Trans. 2014, 43, 12135–12145. [Google Scholar] [CrossRef]
- Gourlay, C.; Nielsen, D.J.; White, J.M.; Knottenbelt, S.Z.; Kirk, M.L.; Young, C.G. Paramagnetic active site models for the molybdenum–copper carbon monoxide dehydrogenase. J. Am. Chem. Soc. 2006, 128, 2164–2165. [Google Scholar] [CrossRef] [PubMed]
- Gourlay, C.; Nielsen, D.J.; Evans, D.J.; White, J.M.; Young, C.G. Models for Aerobic Carbon Monoxide Dehydrogenase. Chem. Sci. 2018, 9, 876–888. [Google Scholar] [CrossRef] [PubMed]
- Takuma, M.; Ohki, Y.; Tatsumi, K. Sulfido-Bridged Dinuclear Molybdenum–Copper Complexes Related to the Active Site of CO Dehydrogenase: [(dithiolate)Mo(O)S2Cu(SAr)]2− (dithiolate = 1,2-S2C6H4, 1,2-S2C6H2-3,6-Cl2, 1,2-S2C2H4). Inorg. Chem. 2005, 44, 6034–6043. [Google Scholar] [CrossRef]
- Groysman, S.; Majumdar, A.; Zheng, S.-L.; Holm, R.H. Reactions of monodithiolene tungsten(VI) sulfido complexes with copper(I) in relation to the structure of the active site of carbon monoxide dehydrogenase. Inorg. Chem. 2010, 49, 1082–1089. [Google Scholar] [CrossRef]
- Mouchfiq, A.; Todorova, T.K.; Dey, S.; Fontecave, M.; Mougel, V. A Bioinspired Molybdenum–Copper Molecular Catalyst for CO2 Electroreduction. Chem. Sci. 2020, 11, 5503–5510. [Google Scholar] [CrossRef]
- Ghosh, D.; Sinhababu, S.; Santarsiero, B.D.; Mankad, N.P. A W/Cu Synthetic Model for the Mo/Cu Cofactor of Aerobic CODH indicates that Biochemical CO Oxidation Requires a Frustrated Lewis Acid/Base Pair. J. Am. Chem. Soc. 2020, 142, 12635–12642. [Google Scholar] [CrossRef]
- Hollingsworth, T.S.; Hollingsworth, R.L.; Lord, R.L.; Groysman, S. Cooperative Bimetallic Reactivity of a Heterodinuclear Molybdenum-Copper Model of Mo-Cu CODH. Dalton Trans. 2018, 47, 10017–10024. [Google Scholar] [CrossRef]
- Kaluarachchige Don, U.I.; Kurup, S.S.; Hollingsworth, T.S.; Ward, C.L.; Lord, R.L.; Groysman, S. Synthesis and Cu(I)/Mo(VI) reactivity of a bifunctional heterodinucleating ligand on a xanthene platform. Inorg. Chem. 2021, 60, 14655–14666. [Google Scholar] [CrossRef]
- Do, T.H.; Brown, S.N. Mono- and Bimetallic Pentacoordinate Silicon Complexes of a Chelating Bis(catecholimine) Ligand. Dalton Trans. 2019, 48, 11565–11574. [Google Scholar] [CrossRef]
- Barybin, M.V.; Young, V.G.; Ellis, J.E. Syntheses and Structural Characterizations of the First 16-, 17-, and 18-Electron Homoleptic Isocyanide Complexes of Vanadium: Hexakis(2,6-dimethyl- phenyl isocyanide)vanadium(I, 0, –I)1. J. Am. Chem. Soc. 1998, 120, 429–430. [Google Scholar] [CrossRef]
- Carpenter, A.E.; Rheingold, A.L.; Figueroa, J.S. A Well-Defined Isocyano Analogue of HCo(CO)4. 1: Synthesis, Decomposition, and Catalytic 1,1-Hydrogenation of Isocyanides. Organometallics 2016, 35, 2309–2318. [Google Scholar] [CrossRef]
- Stephany, R.W.; de Bie, M.J.A.; Drenth, W. A C-NMR and IR study of isocyanides and some of their complexes. Org. Magn. Reason. 1974, 6, 45–47. [Google Scholar] [CrossRef]
- Wang, J.-J.; Holm, R.H. Silylation, Sulfidation, and Benzene-1,2-dithiolate Complexation Reactions of Oxo- and Oxosulfidomolybdates(VI) and -Tungstates(VI). Inorg. Chem. 2007, 46, 11156–11164. [Google Scholar] [CrossRef]
- Wang, J.-J.; Groysman, S.; Lee, S.C.; Holm, R.H. Synthesis of Structural Analogues of the Oxidized Sites in the Xanthine Oxidoreductase Enzyme Family. J. Am. Chem. Soc. 2007, 129, 7512–7513. [Google Scholar] [CrossRef] [PubMed]
- Groysman, S.; Wang, J.-J.; Tagore, R.; Lee, S.C.; Holm, R.H. A Biomimetic Approach to Oxidized Sites in the Xanthine Oxidoreductase Family: Synthesis and Stereochemistry of Tungsten(VI) Analogue Complexes. J. Am. Chem. Soc. 2008, 130, 12794–12807. [Google Scholar] [CrossRef] [PubMed]
- Partyka, D.V.; Holm, R.H. Oxygen/Sulfur Substitution Reactions of Tetraoxometalates Effected by Electrophilic Carbon and Silicon Reagents. Inorg. Chem. 2004, 43, 8609–8616. [Google Scholar] [CrossRef] [PubMed]
- Bheemaraju, A.; Beattie, J.W.; Danylyuk, Y.; Rochford, J.; Groysman, S. Synthesis, Structures, and Reactivity of Copper(I) Complexes Supported by a Rigid Dinucleating Ligand. Eur. J. Inorg. Chem. 2014, 34, 5865–5873. [Google Scholar] [CrossRef]
- Hollingsworth, R.L.; Bheemaraju, A.; Lenca, N.; Lord, R.L.; Groysman, S. Divergent Reactivity of a New Dinuclear Xanthene-Bridged Bis(iminopyridine) di-Nickel Complex with Alkynes. Dalton Trans. 2017, 46, 5605–5616. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated Space-group and Crystal Structure Determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]


| 2(PF6) | 6 | |
|---|---|---|
| Formula | C51H55CuN5O3•PF6•C4H10O | C84H88Cu2Mo2N8O11•C18H16OSi•6(C2H3N) |
| Fw, g/mol | 1068.670 | 2227.30 |
| temperature | 100 K | 100 K |
| X-ray source | MoKα | MoKα |
| cryst syst | triclinic | monoclinic |
| space group | P-1 | P21/n |
| color | yellow | orange |
| Z | 2 | 4 |
| a, Å | 13.6310(5) | 20.546(5) |
| b, Å | 14.4630(6) | 21.591(7) |
| c, Å | 15.8288(6) | 25.679(7) |
| α, deg | 112.847(1) | 90.00 |
| β, deg | 92.606(1) | 109.253(6) |
| γ, deg | 109.383(1) | 90.00 |
| V, A3 | 2657.65(18) | 10754(5) |
| dcalcd, g/cm3 | 1.335 | 1.376 |
| μ, mm−1 | 0.512 | 0.696 |
| 2θ, deg | 55.00 | 55.96 |
| R1 a (all data) | 0.0428 | 0.0996 |
| wR2 b (all data) | 0.1011 | 0.1977 |
| R1 a [(I > 2σ)] | 0.0372 | 0.0682 |
| wR2 b [(I > 2σ)] | 0.0969 | 0.1760 |
| GOF c | 1.040 | 1.022 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaluarachchige Don, U.I.; Almaat, A.S.; Ward, C.L.; Groysman, S. Studies Relevant to the Functional Model of Mo-Cu CODH: In Situ Reactions of Cu(I)-L Complexes with Mo(VI) and Synthesis of Stable Structurally Characterized Heterotetranuclear MoVI2CuI2 Complex. Molecules 2023, 28, 3644. https://doi.org/10.3390/molecules28083644
Kaluarachchige Don UI, Almaat AS, Ward CL, Groysman S. Studies Relevant to the Functional Model of Mo-Cu CODH: In Situ Reactions of Cu(I)-L Complexes with Mo(VI) and Synthesis of Stable Structurally Characterized Heterotetranuclear MoVI2CuI2 Complex. Molecules. 2023; 28(8):3644. https://doi.org/10.3390/molecules28083644
Chicago/Turabian StyleKaluarachchige Don, Umesh I., Ahmad S. Almaat, Cassandra L. Ward, and Stanislav Groysman. 2023. "Studies Relevant to the Functional Model of Mo-Cu CODH: In Situ Reactions of Cu(I)-L Complexes with Mo(VI) and Synthesis of Stable Structurally Characterized Heterotetranuclear MoVI2CuI2 Complex" Molecules 28, no. 8: 3644. https://doi.org/10.3390/molecules28083644
APA StyleKaluarachchige Don, U. I., Almaat, A. S., Ward, C. L., & Groysman, S. (2023). Studies Relevant to the Functional Model of Mo-Cu CODH: In Situ Reactions of Cu(I)-L Complexes with Mo(VI) and Synthesis of Stable Structurally Characterized Heterotetranuclear MoVI2CuI2 Complex. Molecules, 28(8), 3644. https://doi.org/10.3390/molecules28083644






