7-Geranyloxycinnamic Acid Isolated from Melicope lunu-ankenda Leaves Perturbs Colon Cancer and Breast Cancer Cell Lines’ Growth via Induction of Apoptotic Pathway
Abstract
1. Introduction
2. Results
2.1. Extraction using Different Solvents
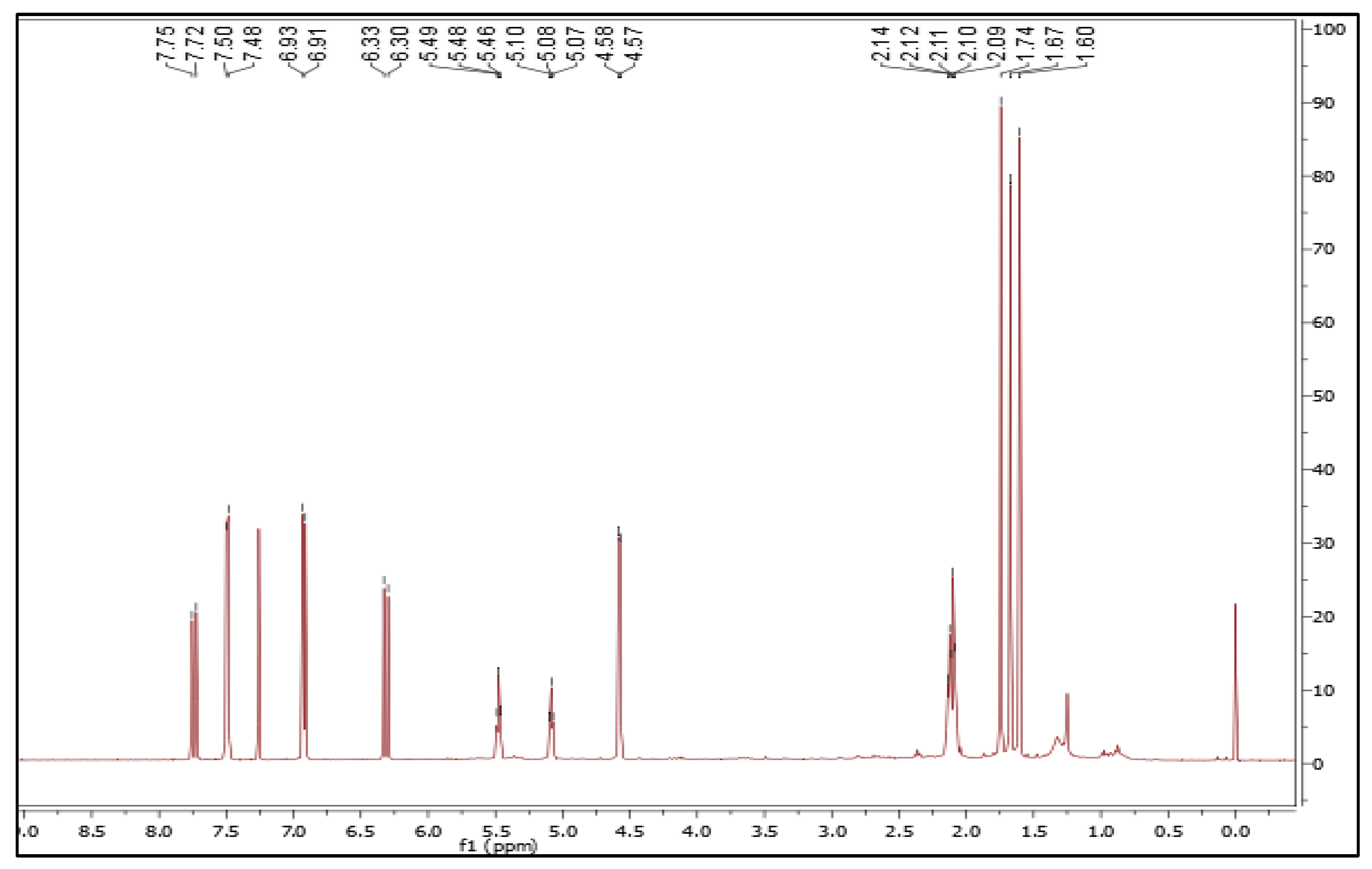
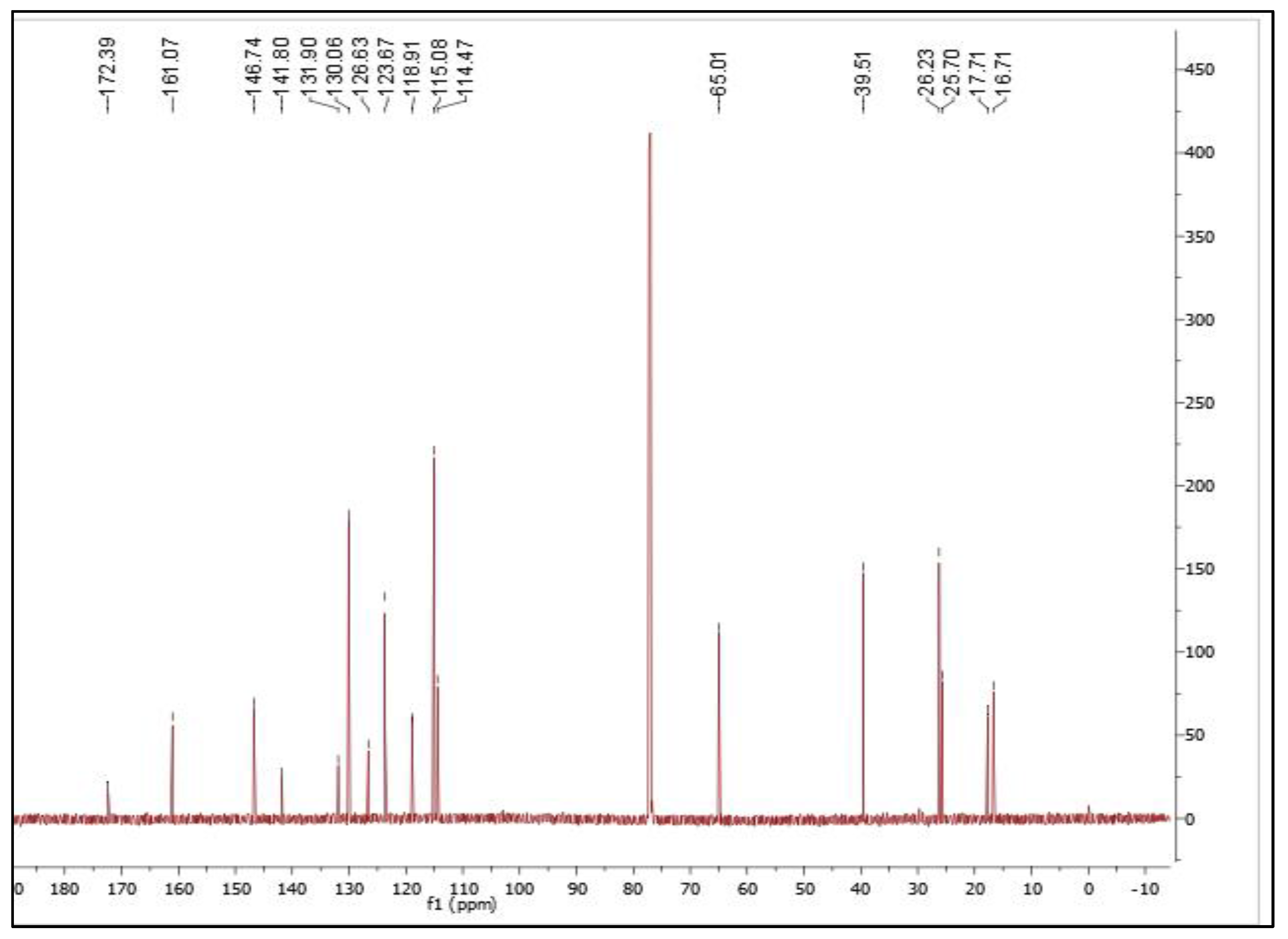
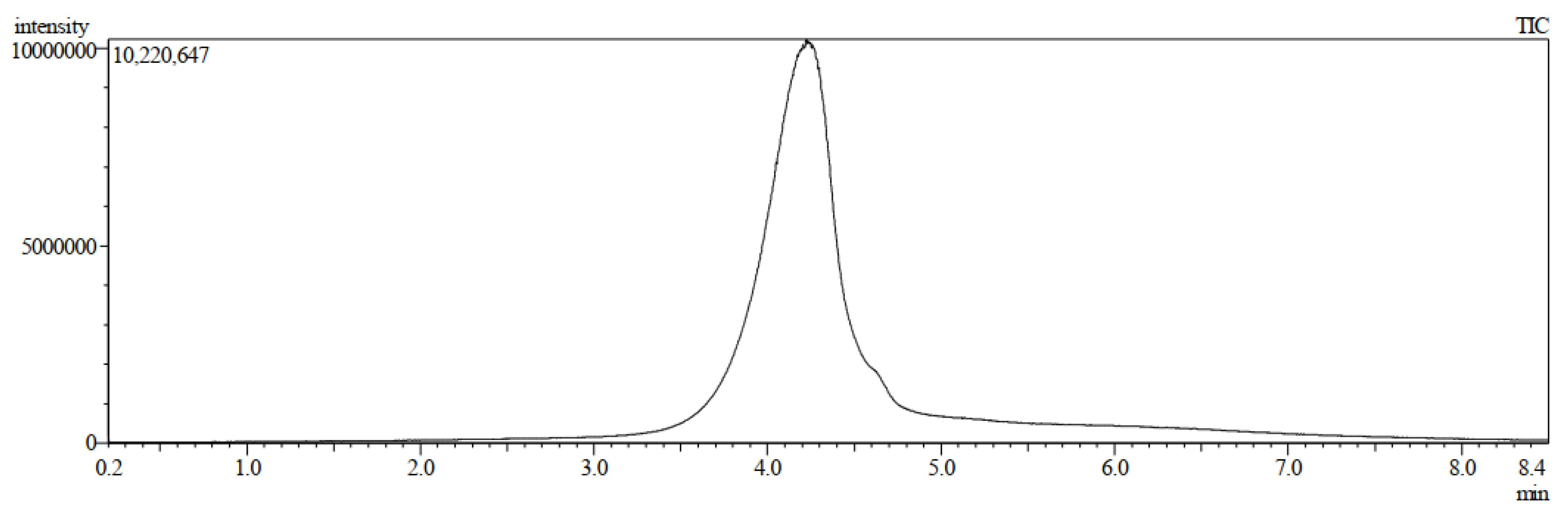
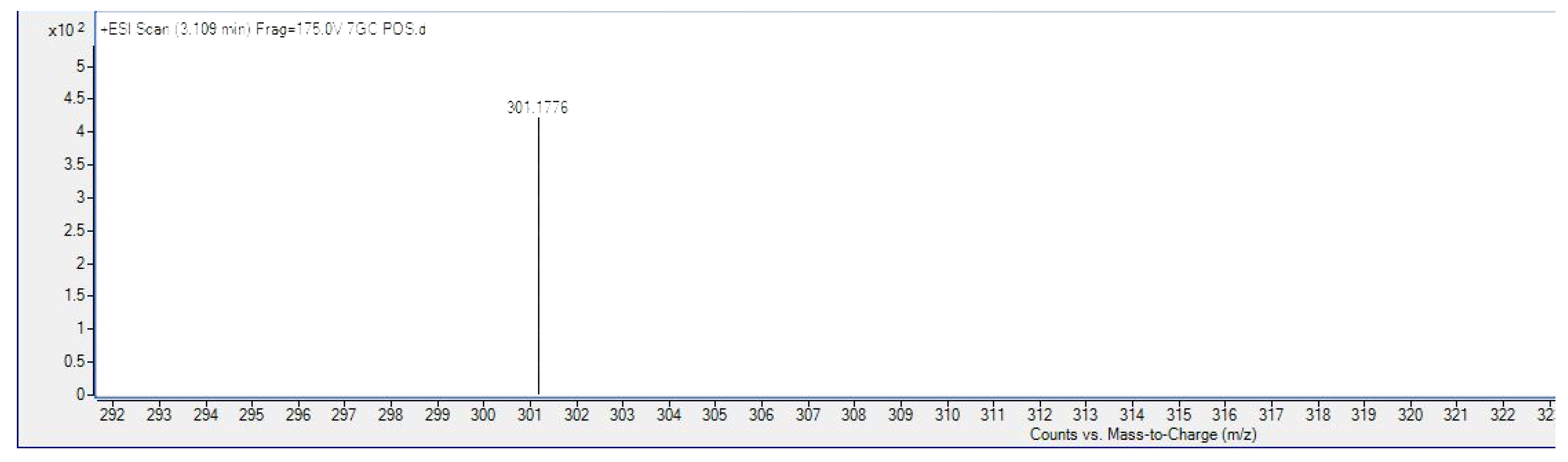
Isolation and Characterization of 7-Geranyloxycinnamic Acid from the Petroleum Ether Extract of M. lunu-Ankenda
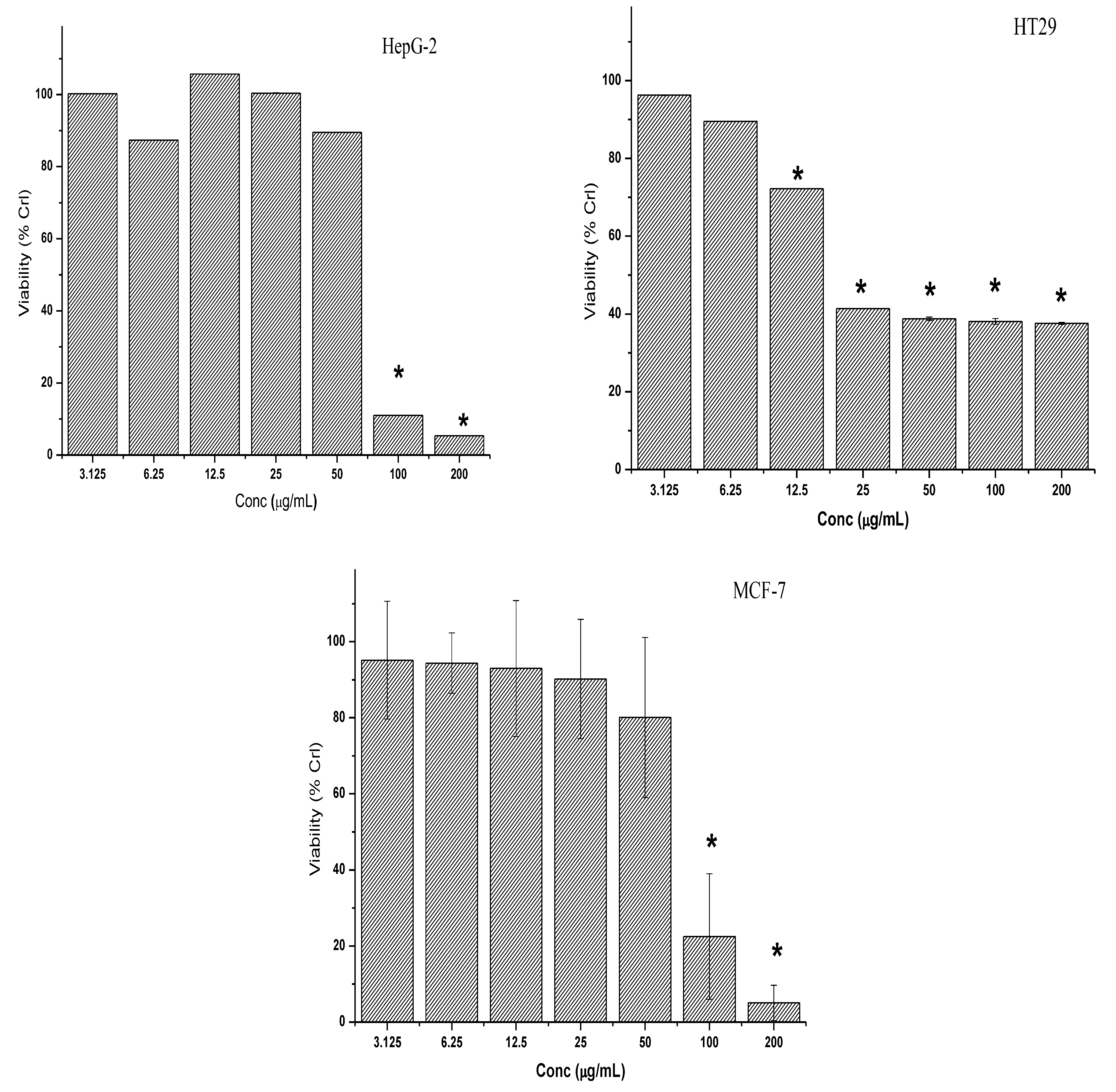
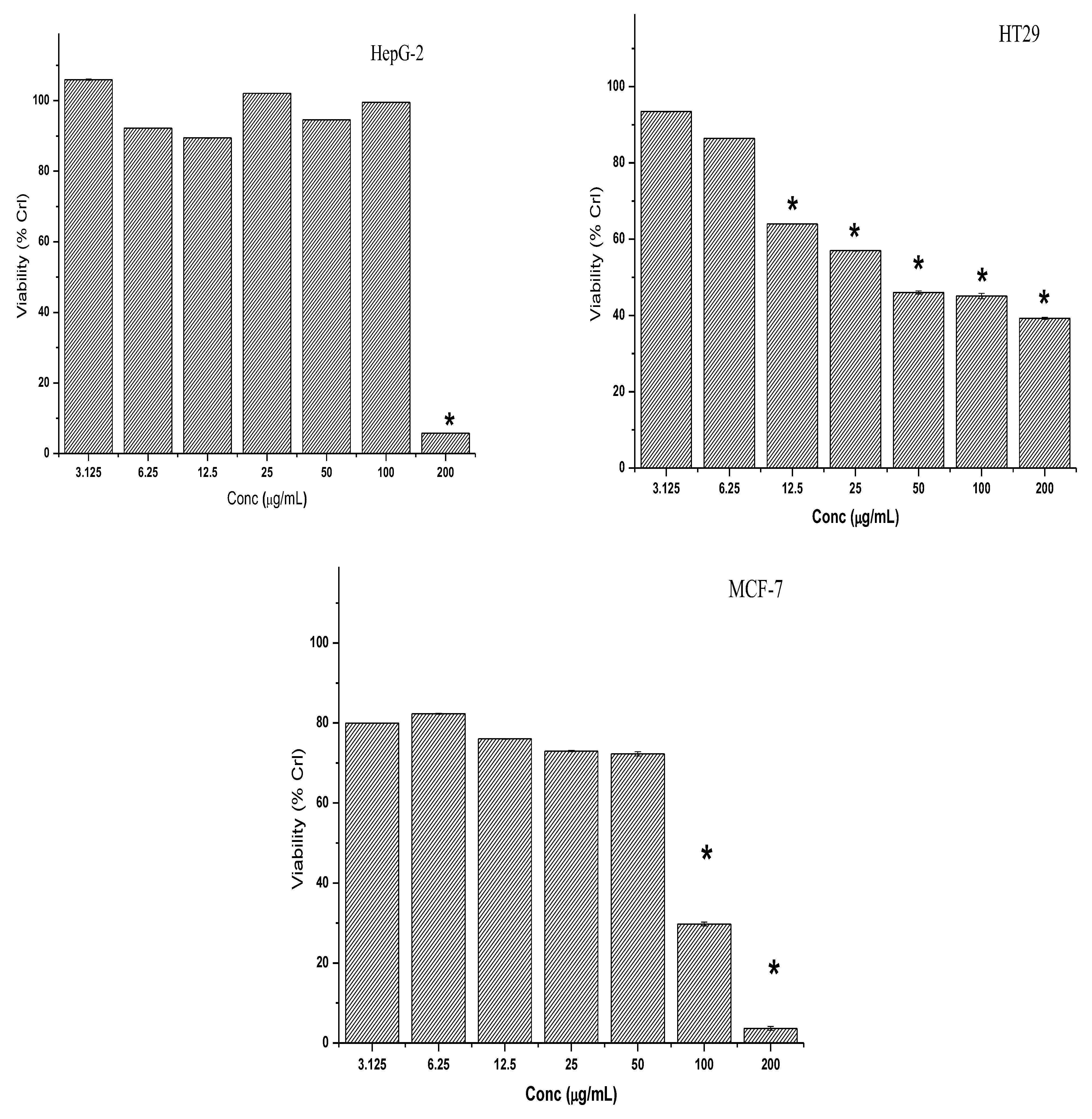
2.2. Cytotoxicity of Crude Extracts and 7-Geranyloxycinnamic Acid on Cancer Cell Lines
2.2.1. Cytotoxicity of Crude Extracts
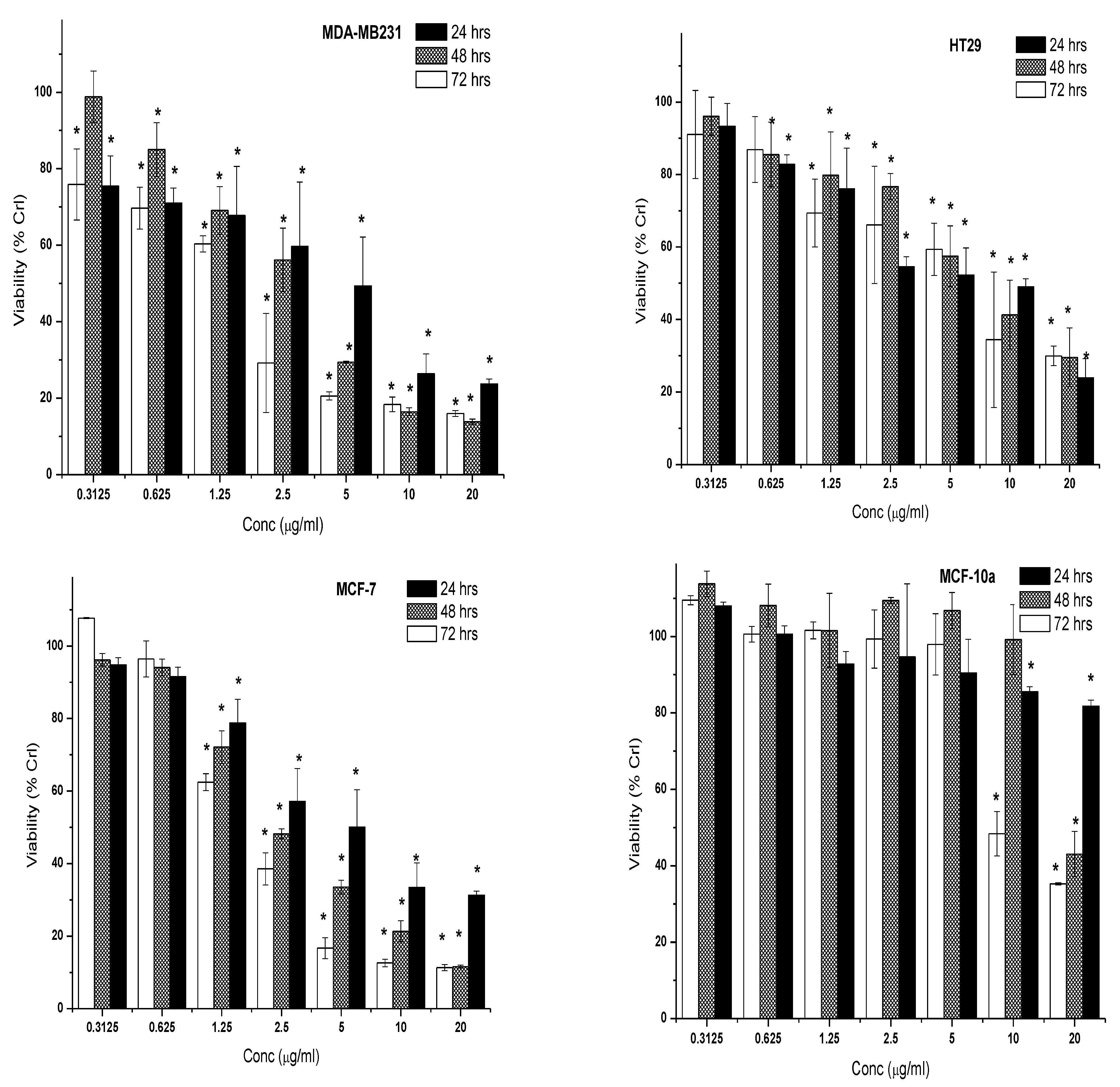
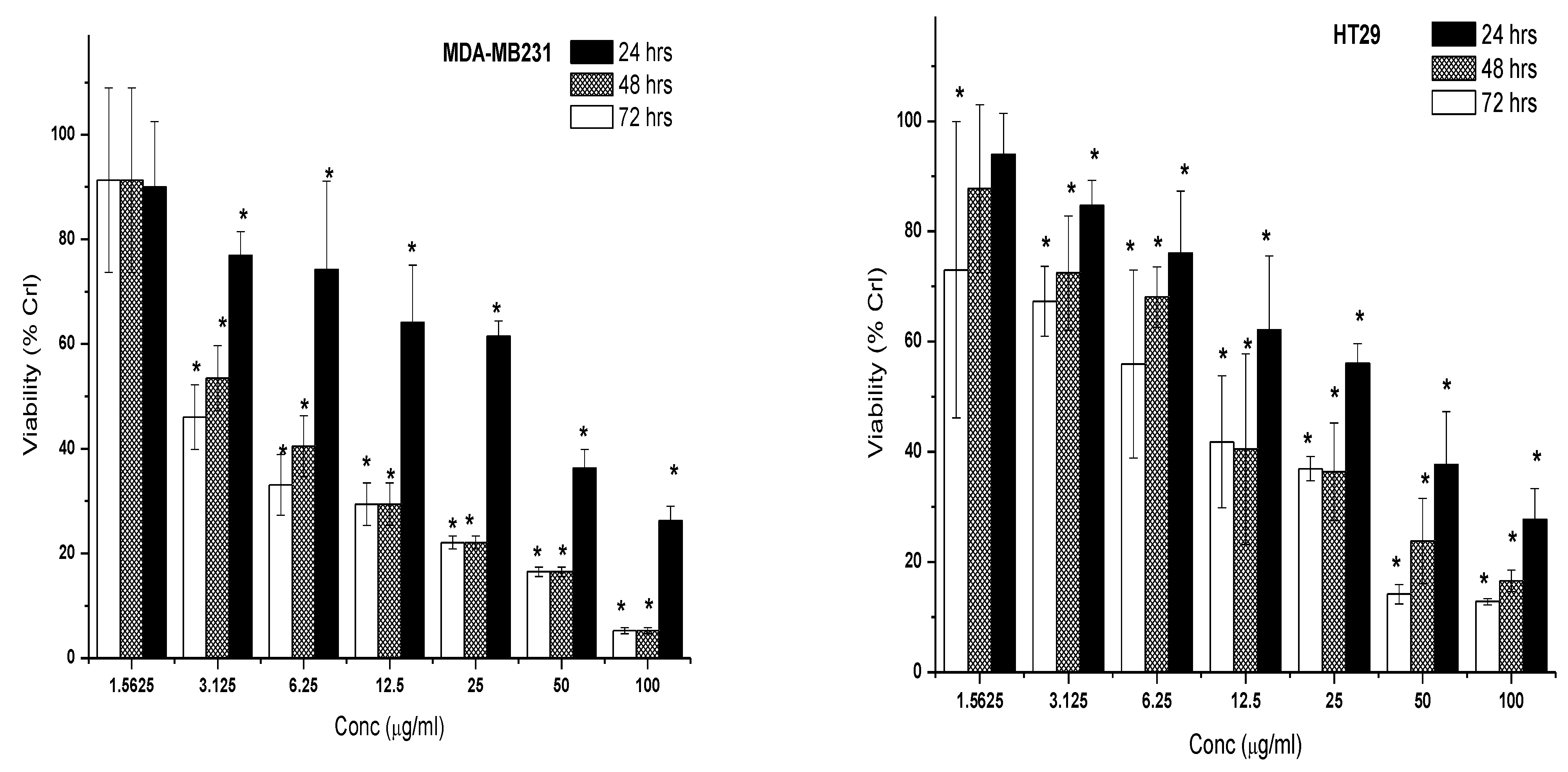
2.2.2. Cytotoxic Effect of 7-Geranyloxycinnamic Acid and 5-Fluorouracil
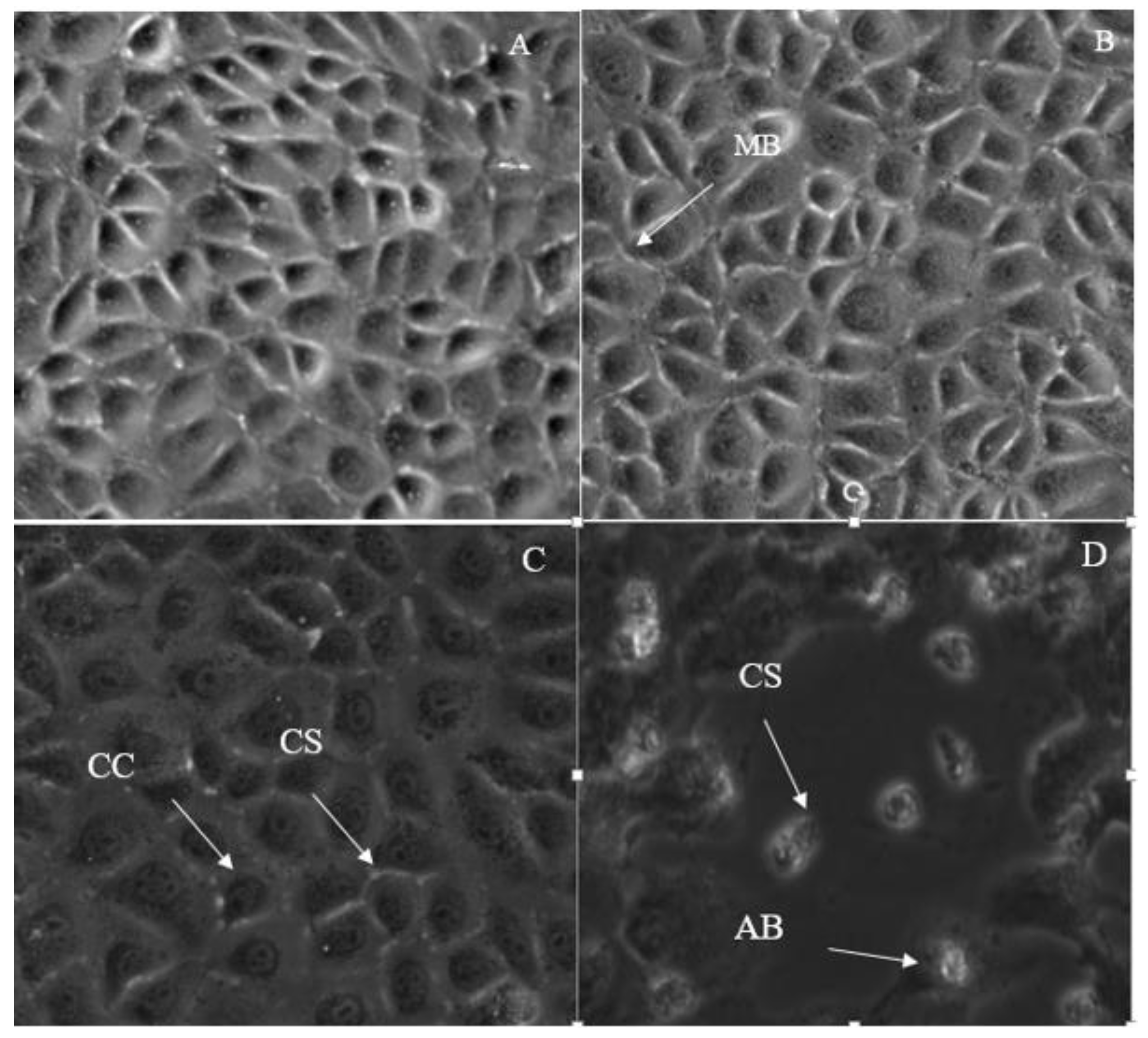
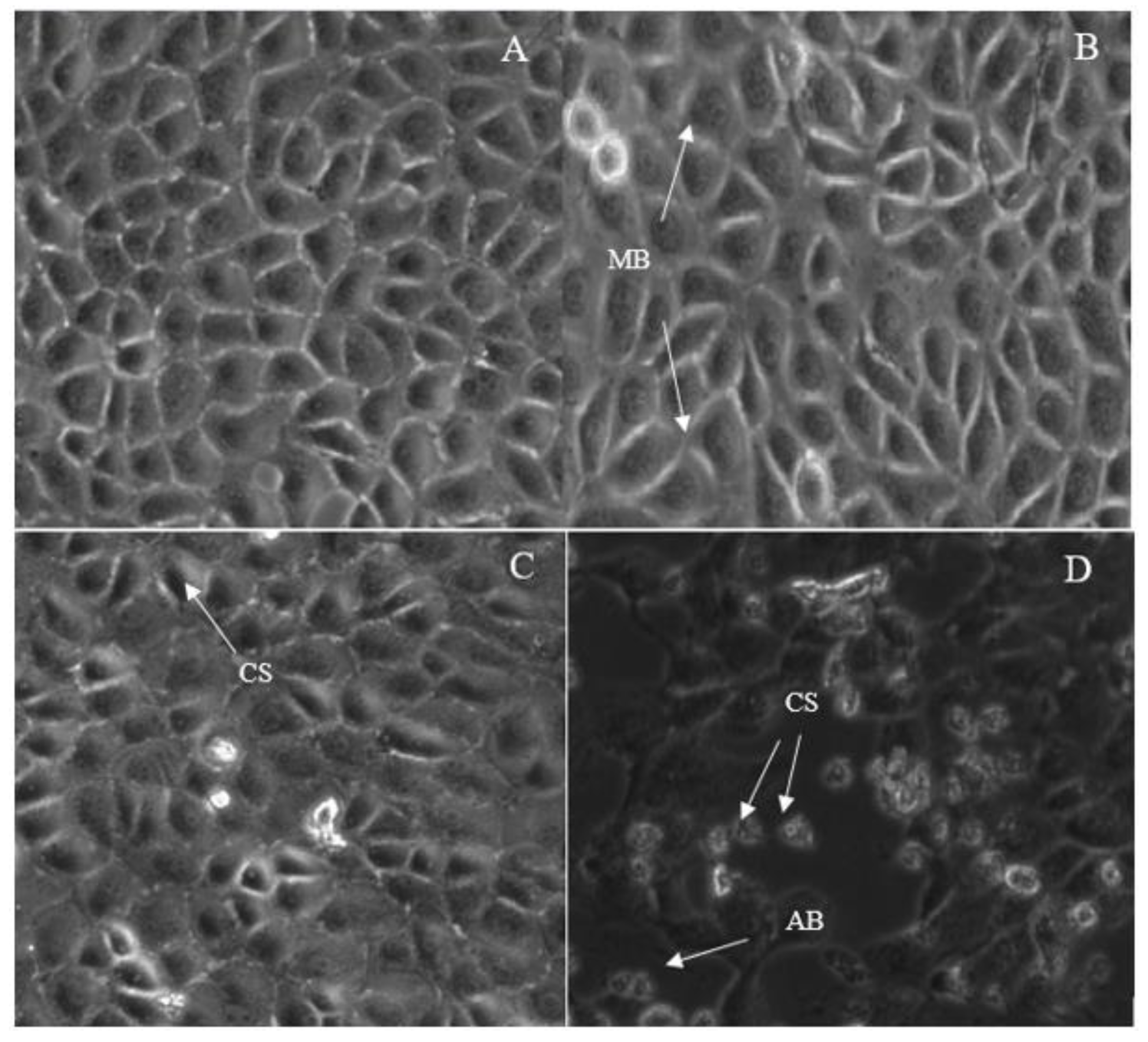
2.3. Morphological Changes in Breast Cancer Cell Lines after Treatment with IC50 Concentration of 7-Geranyloxycinnamic Acid
2.3.1. Morphological Changes in MCF-7 Cell Line
2.3.2. Morphological Changes in MDA-MB231 Cell Line
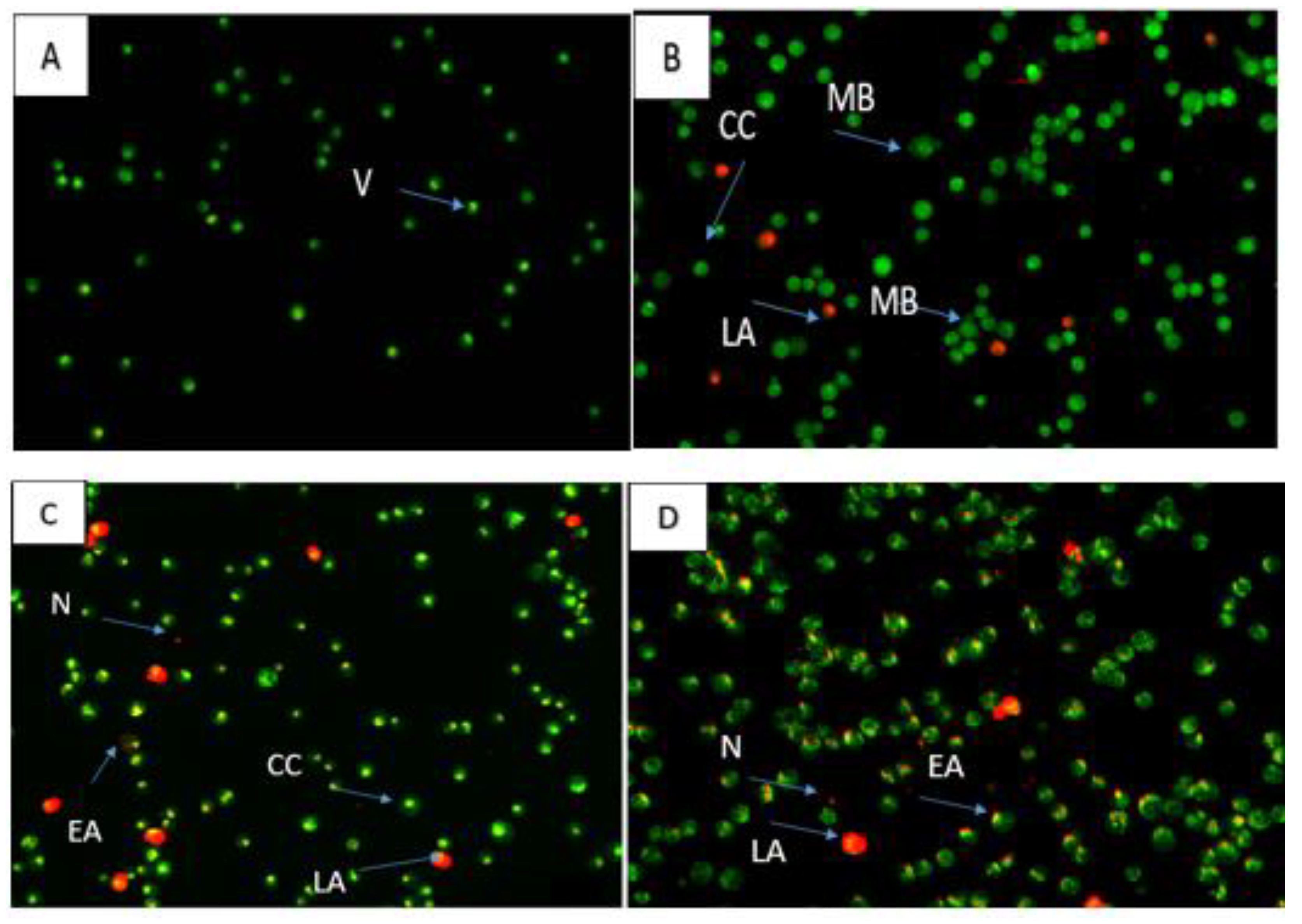
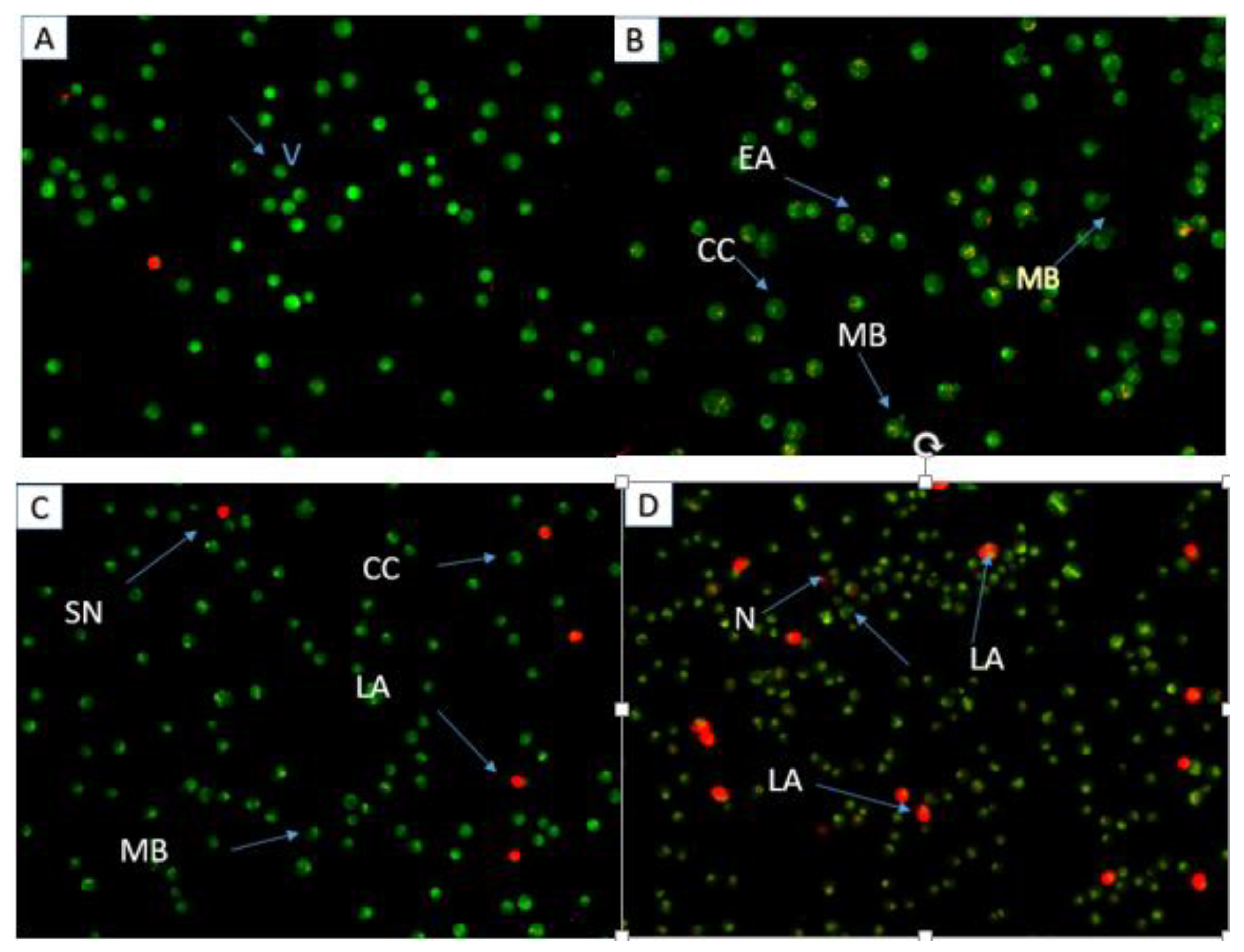
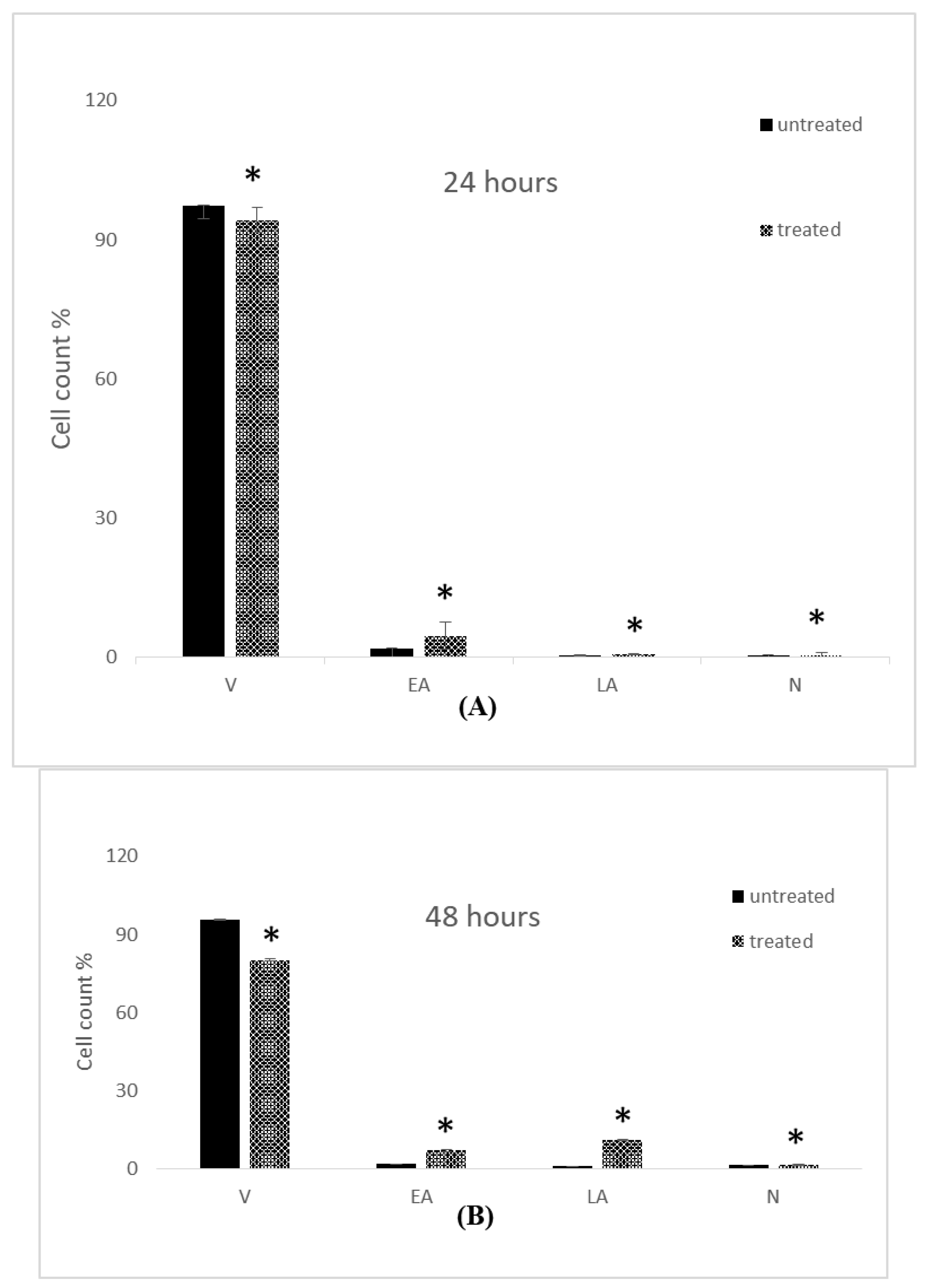
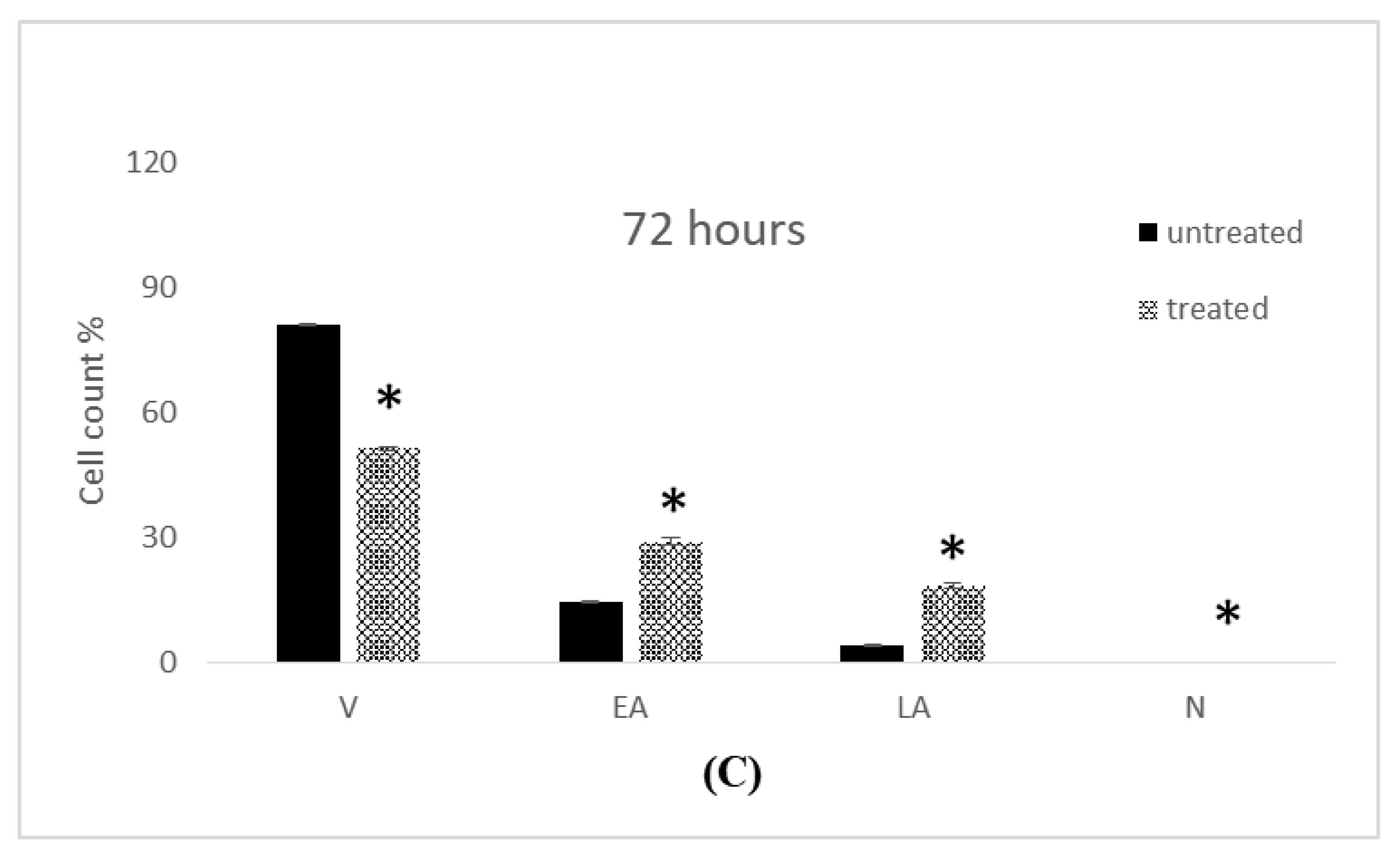
2.4. Apoptosis Assessment
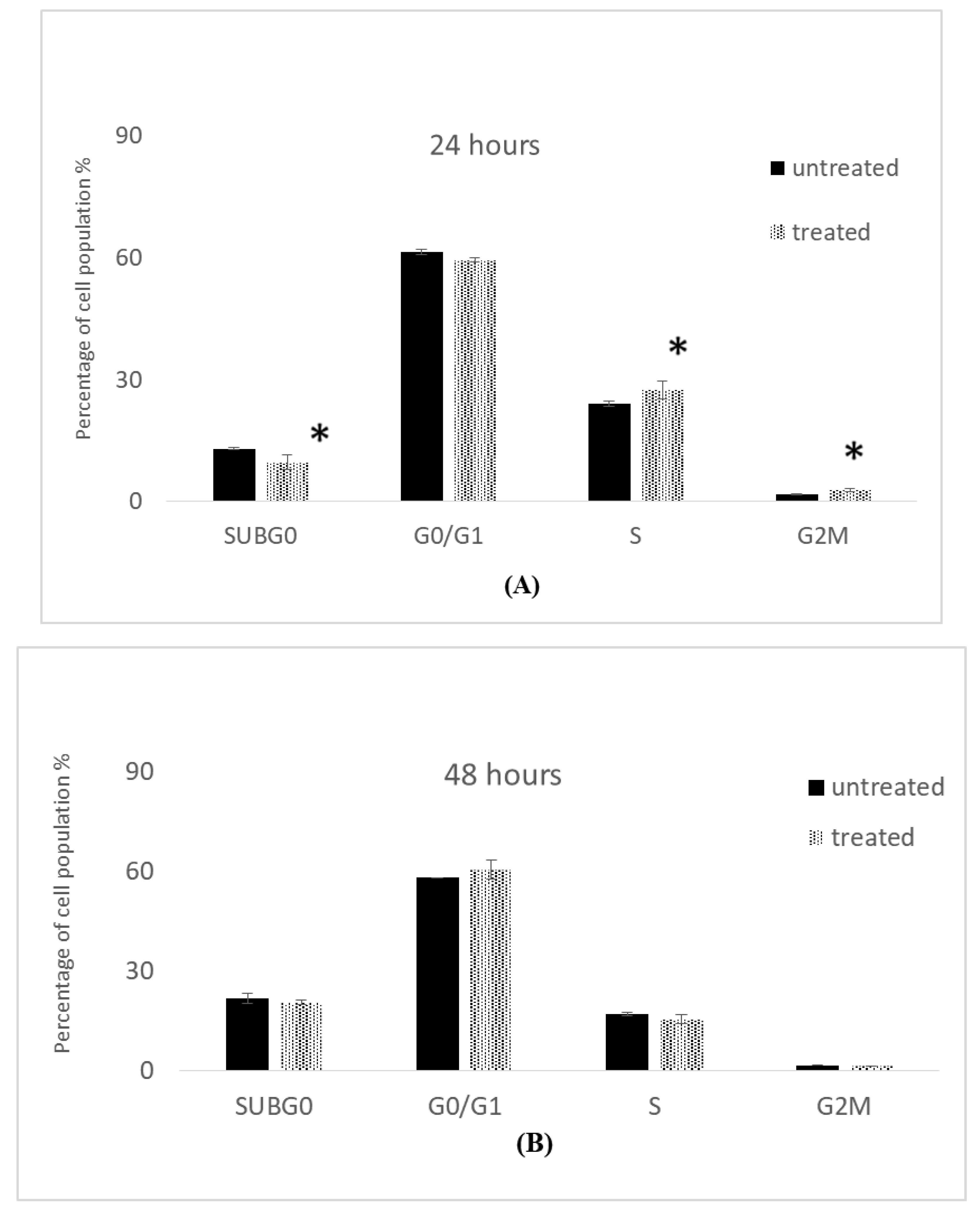
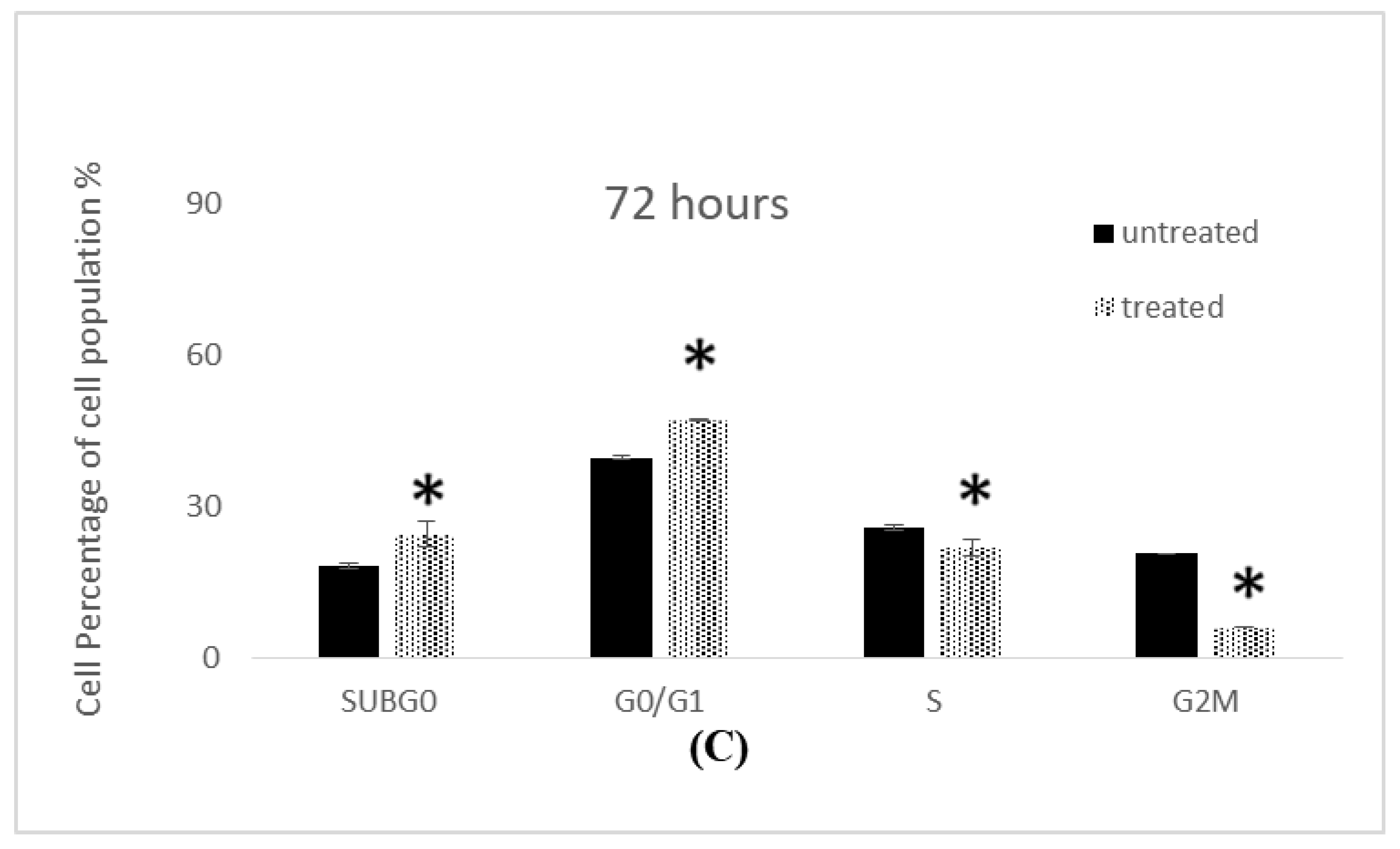
2.5. Cell Cycle Analysis
2.5.1. Cell Cycle Analysis of MCF-7 Cells
2.5.2. Cell Cycle Analysis of MDA-MB-231 Cells
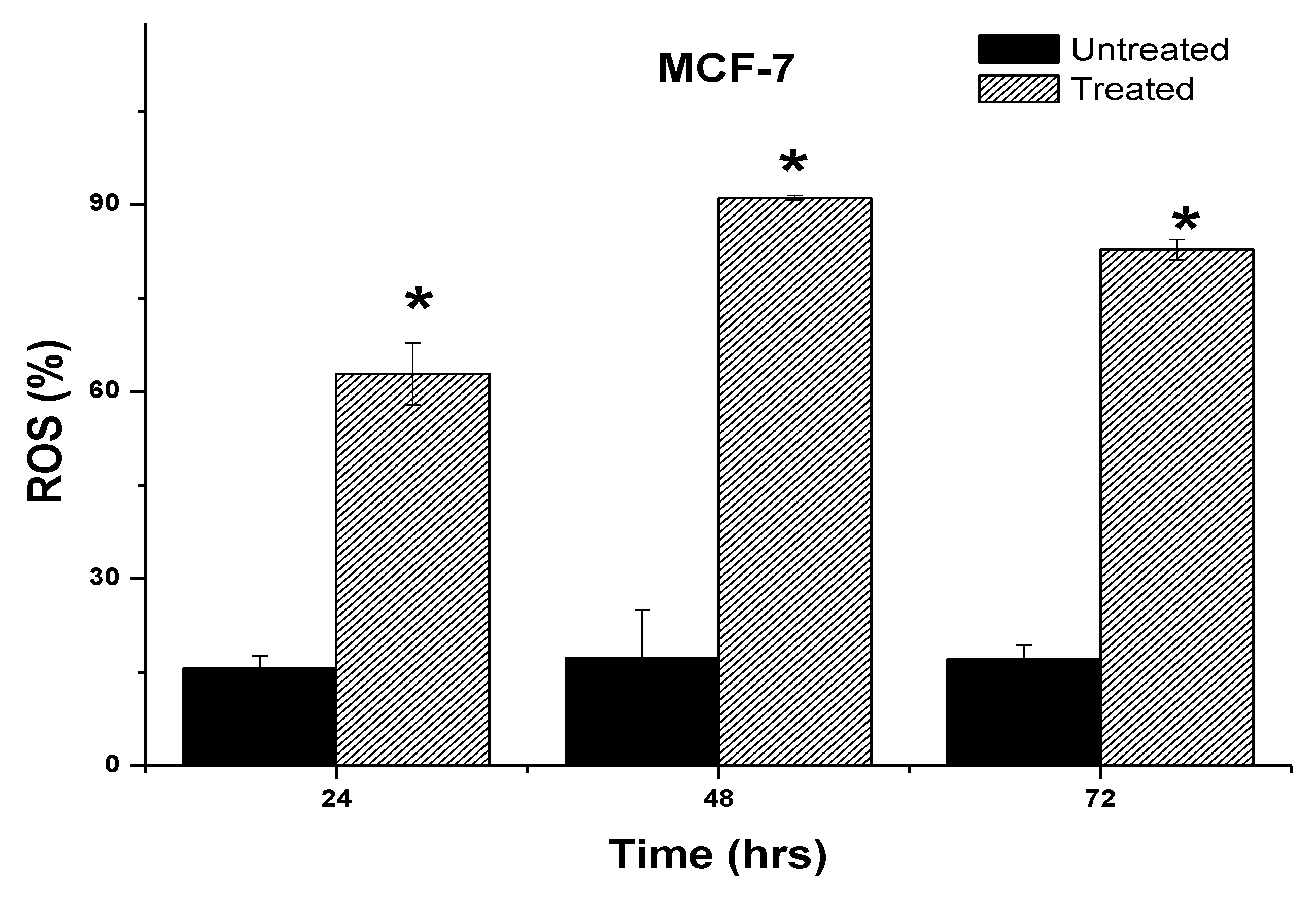
2.6. Induction of Intracellular Reactive Oxygen Species (ROS) by 7-Geranyloxycinnamic Acid
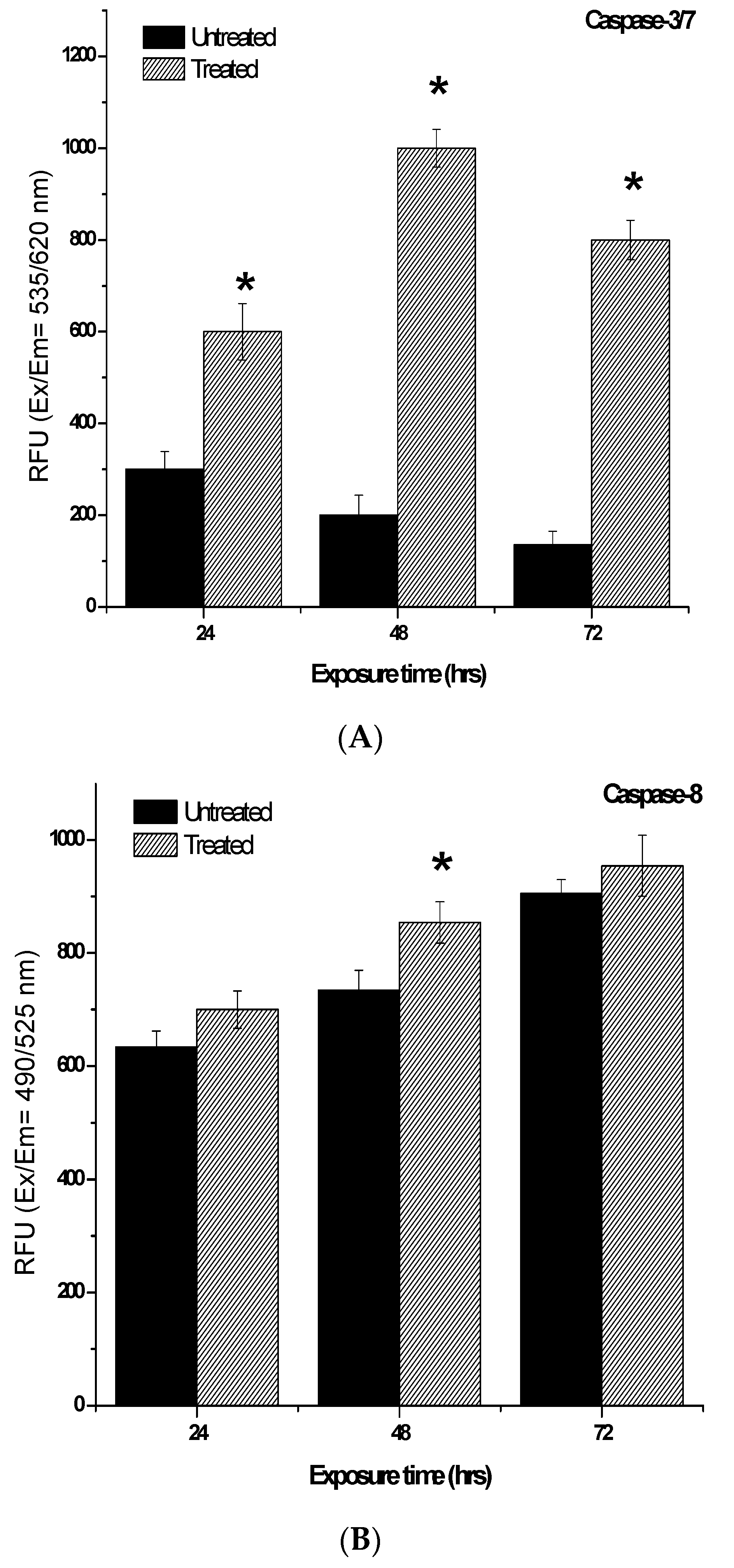
2.7. Determination of Caspases-3/7, -8 and -9 Activity Induced by 7-Geranyloxycinnamic Acid
3. Discussion
4. Materials and Methods
4.1. Plant Collection, Extraction, and Fractionation
4.2. Structure Characterization and Identification of 7-Geranyloxycinnamic Acid
4.2.1. Nuclear Magnetic Resonance Spectroscopy
4.2.2. Liquid Chromatography–Mass Spectrometry
4.3. Cell Culture
4.3.1. Cell Viability Assay
4.3.2. Qualitative Assessment of Cell Morphology Using Phase Contrast Microscopy
4.3.3. Acridine Orange and Propidium Iodide (AO/PI) Staining
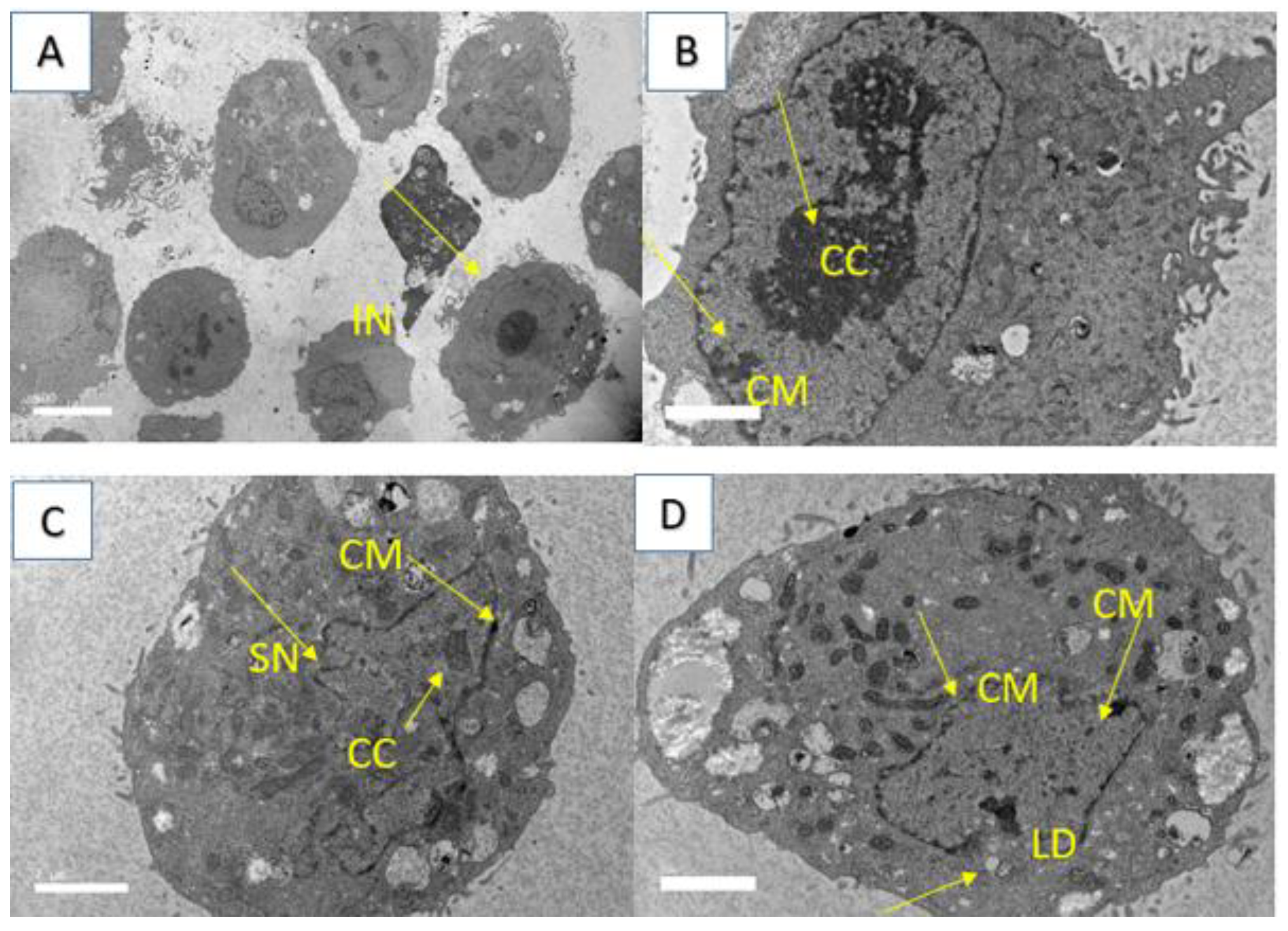
4.3.4. Transmission Electron Microscopy (TEM)
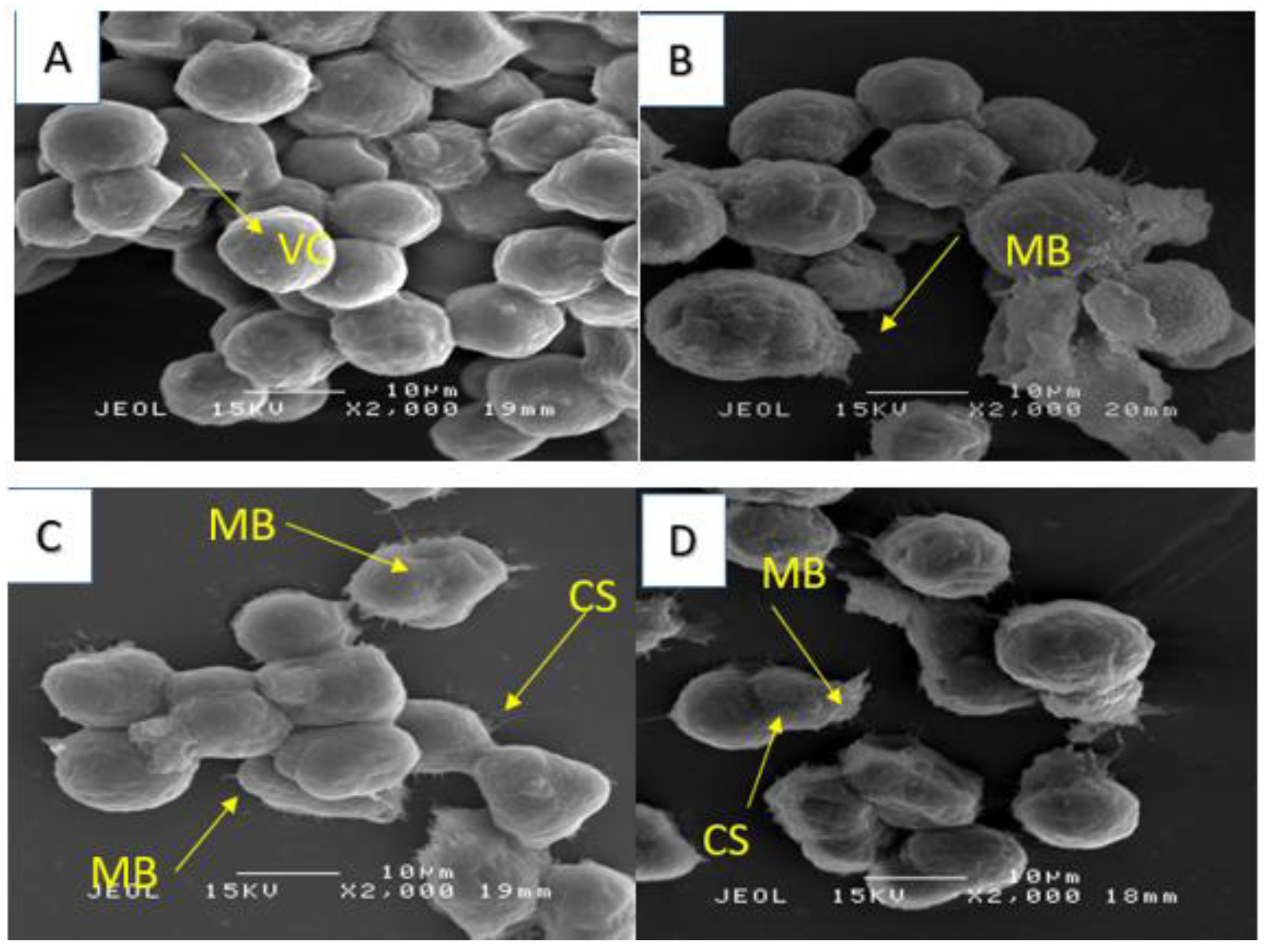
4.3.5. Scanning Electron Microscopy (SEM)
4.3.6. Annexin V-FITC Apoptosis Detection Assay
4.3.7. Intracellular Reactive Oxygen Species (ROS) assay
4.3.8. Cell Cycle Assay
4.3.9. Measurement of Caspase-3/7, -8, and -9 Activity
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Veronesi, U.; Boyle, P.; Goldhirsch, A.; Orecchia, R.; Viale, G. Breast cancer. Lancet 2005, 365, 1727–1741. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Q.; Wang, X.L.; He, D.H.; Cheng, Y.X. Protection against chemotherapy-and radiotherapy-induced side effects: A review based on the mechanisms and therapeutic opportunities of phytochemicals. Phytomedicine 2021, 80, 153402. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, H.; Chen, X. Drug resistance and combating drug resistance in cancer. Cancer Drug Resist. 2019, 2, 141. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wu, L.; Yan, G.; Chen, Y.; Zhou, M.; Wu, Y.; Li, Y. Inflammation and tumor progression: Signaling pathways and targeted intervention. Signal Transduct. Target. Ther. 2021, 6, 263. [Google Scholar] [CrossRef]
- Guan, X. Cancer metastases: Challenges and opportunities. Acta Pharm. Sin. B 2015, 5, 402–418. [Google Scholar] [CrossRef]
- Alugoju, P.; Chaitanya, N.S.; Swamy, V.K.; Kancharla, P.K. Phytotherapy for breast cancer. In A Theranostic and Precision Medicine Approach for Female-Specific Cancers; Academic Press: Cambridge, MA, USA, 2021; pp. 129–163. [Google Scholar]
- Yoo, S.; Kim, K.; Nam, H.; Lee, D. Discovering health benefits of phytochemicals with integrated analysis of the molecular network, chemical properties and ethnopharmacological evidence. Nutrients 2018, 10, 1042. [Google Scholar] [CrossRef] [PubMed]
- Matowa, P.R.; Gundidza, M.; Gwanzura, L.; Nhachi, C.F. A survey of ethnomedicinal plants used to treat cancer by traditional medicine practitioners in Zimbabwe. BMC Complement. Med. Ther. 2020, 20, 278. [Google Scholar] [CrossRef]
- Mohamed Eliaser, E.; Hui Ho, J.; Hashim, N.M.; Rukayadi, Y.; Lian Ee, G.C.; Abdull Razis, A.F. Phytochemical constituents and biological activities of melicope lunu-ankenda. Molecules 2018, 23, 2708. [Google Scholar] [CrossRef]
- Abdulwanis Mohamed, Z.; Mohamed Eliaser, E.; Mazzon, E.; Rollin, P.; Cheng Lian Ee, G.; Abdull Razis, A.F. Neuroprotective potential of secondary metabolites from Melicope lunu-ankenda (Rutaceae). Molecules 2019, 24, 3109. [Google Scholar] [CrossRef]
- AL-Zuaidy, M.H.; Hamid, A.A.; Ismail, A.; Mohamed, S.; Abdul Razis, A.F.; Mumtaz, M.W.; Salleh, S.Z. Potent antidiabetic activity and metabolite profiling of Melicope Lunu-ankenda leaves. J. Food Sci. 2016, 81, C1080–C1090. [Google Scholar] [CrossRef]
- Hashim, N.M.; Rahmani, M.; Ismail, H.B.M.; Sukari, M.A.; Lian, G.E.C. Biological activities of four Melicope species. Sains Malaysiana 2009, 38, 767–771. [Google Scholar]
- Johnson, A.J.; Kumar, A.; Rasheed, S.A.; Chandrika, S.P.; Chandrasekhar, A.; Baby, S.; Subramoniam, A. Antipyretic, analgesic, anti-inflammatory and antioxidant activities of two major chromenes from Melicope lunu-ankenda. J. Ethnopharmacol. 2010, 130, 267–271. [Google Scholar] [CrossRef] [PubMed]
- George, S.; Nair, S.A.; Johnson, A.J.; Venkataraman, R.; Baby, S. O-prenylated flavonoid, an antidiabetes constituent in Melicope lunu-ankenda. J. Ethnopharmacol. 2015, 168, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Ramli, I.; Kamarulzaman, N.H.; Shaari, K.; Ee, G.C.L. p-O-geranylcoumaric acid from Melicope lunu-ankenda. Nat. Prod. Res. 2004, 18, 289–294. [Google Scholar] [CrossRef]
- Abdulwanis Mohamed, Z.; Mohamed Eliaser, E.; Jaafaru, M.S.; Nordin, N.; Ioannides, C.; Abdull Razis, A.F. Neuroprotective Effects of 7-Geranyloxycinnamic Acid from Melicope lunu ankenda Leaves. Molecules 2020, 25, 3724. [Google Scholar] [CrossRef]
- Niero, E.L.D.O.; Machado-Santelli, G.M. Cinnamic acid induces apoptotic cell death and cytoskeleton disruption in human melanoma cells. J. Exp. Clin. Cancer Res. 2013, 32, 31. [Google Scholar] [CrossRef]
- Al-Abboodi, A.S.; Eid, E.E.; Azam, F.; Al-Qubaisi, M.S. Inclusion complex of clausenidin with hydroxypropyl-β-cyclodextrin: Improved physicochemical properties and anti-colon cancer activity. Saudi Pharm. J. 2021, 29, 223–235. [Google Scholar] [CrossRef]
- Kumar, R.; Saneja, A.; Panda, A.K. An Annexin V-FITC—Propidium Iodide-Based Method for Detecting Apoptosis in a Non-Small Cell Lung Cancer Cell Line. In Lung Cancer; Humana: New York, NY, USA, 2021; pp. 213–223. [Google Scholar]
- Aubry, J.P.; Blaecke, A.; Lecoanet-Henchoz, S.; Jeannin, P.; Herbault, N.; Caron, G.; Moine, V.; Bonnefoy, J.Y. Annexin V used for measuring apoptosis in the early events of cellular cytotoxicity. Cytom. J. Int. Soc. Anal. Cytol. 1999, 37, 197–204. [Google Scholar] [CrossRef]
- Baskić, D.; Popović, S.; Ristić, P.; Arsenijević, N.N. Analysis of cycloheximide-induced apoptosis in human leukocytes: Fluorescence microscopy using annexin V/propidium iodide versus acridin orange/ethidium bromide. Cell Biol. Int. 2006, 30, 924–932. [Google Scholar] [CrossRef]
- Solowey, E.; Lichtenstein, M.; Sallon, S.; Paavilainen, H.; Solowey, E.; Lorberboum-Galski, H. Evaluating medicinal plants for anticancer activity. Sci. World J. 2014, 2014, 721402. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Radwan, E.M. Treatment of MCF-7 and MDA-MB-231 Human Breast Cancer Cell Lines with Erythropoietin, Doxorubicin and Their Combination. Doctor’s Thesis, Universiti Putra Malaysia, UPM Serdang, Selangor, Malaysia, 2015. [Google Scholar]
- Alam, F.; Najum us Saqib, Q.; Waheed, A. Cytotoxic activity of extracts and crude saponins from Zanthoxylum armatum DC. against human breast (MCF-7, MDA-MB-468) and colorectal (Caco-2) cancer cell lines. BMC Complement. Altern. Med. 2017, 17, 368. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zhu, Q.; Chen, M.; Huang, Q.; Wang, W.; Li, Q.; Huang, Y.; Di, W. The changing 50% inhibitory concentration (IC50) of cisplatin: A pilot study on the artifacts of the MTT assay and the precise measurement of density-dependent chemoresistance in ovarian cancer. Oncotarget 2016, 7, 70803. [Google Scholar] [CrossRef] [PubMed]
- Hue Ngan, D.; Hoai, H.T.C.; Mai Huong, L.; Hansen, P.E.; Vang, O. Bio activities and chemical constituents of a Vietnamese medicinal plant Che Vang, Jasminum subtriplinerve Blume (Oleaceae). Nat. Prod. Res. 2008, 22, 942–949. [Google Scholar] [CrossRef]
- Adebayo, A.H.; Tan, N.H.; Akindahunsi, A.A.; Zeng, G.Z.; Zhang, Y.M. Anticancer and antiradical scavenging activity of Ageratum conyzoides L. (Asteraceae). Pharmacogn. Mag. 2010, 6, 62. [Google Scholar]
- Shen, Y.; Sun, Z.; Shi, P.; Wang, G.; Wu, Y.; Li, S.; Zheng, Y.; Huang, L.; Lin, L.; Lin, X.; et al. Anticancer effect of petroleum ether extract from Bidens pilosa L and its constituent’s analysis by GC-MS. J. Ethnopharmacol. 2018, 217, 126–133. [Google Scholar] [CrossRef]
- Ghazi-Khansaria, M.; Mojarrab, M.; Ahmadi, F.; Hosseinzadeh, L. The antiproliferative effects of petroleum ether extract of Artemisia aucheri on human cancerous cell lines. J. Rep. Pharm. Sci. 2013, 2, 61–66. [Google Scholar]
- Singh, N.P.; Ferreira, J.F.; Park, J.S.; Lai, H.C. Cytotoxicity of ethanolic extracts of Artemisia annua to Molt-4 human leukemia cells. Planta Med. 2011, 77, 1788–1793. [Google Scholar] [CrossRef]
- Njeru, S.N.; Muema, J.M. Antimicrobial activity, phytochemical characterization and gas chromatography-mass spectrometry analysis of Aspilia pluriseta Schweinf. extracts. Heliyon 2020, 6, e05195. [Google Scholar] [CrossRef]
- Hashim, N.M. Chemical Constituents and Biological Activity of Four Melicope Species (rutaceace). Doctor’s Thesis, Universiti Putra Malaysia, UPM Serdang, Selangor, Malaysia, 2005. [Google Scholar]
- Alomrani, A.; Badran, M.; Harisa, G.I.; ALshehry, M.; Alhariri, M.; Alshamsan, A.; Alkholief, M. The use of chitosan-coated flexible liposomes as a remarkable carrier to enhance the antitumor efficacy of 5-fluorouracil against colorectal cancer. Saudi Pharm. J. 2019, 27, 603–611. [Google Scholar] [CrossRef] [PubMed]
- AlQahtani, S.A.; Harisa, G.I.; Alomrani, A.H.; Alanazi, F.K.; Badran, M.M. Improved pharmacokinetic and biodistribution of 5-fluorouracil loaded biomimetic nanoerythrocytes decorated nanocarriers for liver cancer treatment. Colloids Surf. B Biointerfaces 2021, 197, 111380. [Google Scholar] [CrossRef] [PubMed]
- Murad, A.M.; Santiago, F.F.; Petroianu, A.; Rocha, P.R.; Rodrigues, M.A.; Rausch, M. Modified therapy with 5-fluorouracil, doxorubicin, and methotrexate in advanced gastric cancer. Cancer 1993, 72, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Oguri, T.; Achiwa, H.; Bessho, Y.; Muramatsu, H.; Maeda, H.; Niimi, T.; Sato, S.; Ueda, R. The role of thymidylate synthase and dihydropyrimidine dehydrogenase in resistance to 5-fluorouracil in human lung cancer cells. Lung Cancer 2005, 49, 345–351. [Google Scholar] [CrossRef]
- Longley, D.B.; Harkin, D.P.; Johnston, P.G. 5-fluorouracil: Mechanisms of action and clinical strategies. Nat. Rev. Cancer 2003, 3, 330–338. [Google Scholar] [CrossRef]
- Hashemi-Moghaddam, H.; Kazemi-Bagsangani, S.; Jamili, M.; Zavareh, S. Evaluation of magnetic nanoparticles coated by 5-fluorouracil imprinted polymer for controlled drug delivery in mouse breast cancer model. Int. J. Pharm. 2016, 497, 228–238. [Google Scholar] [CrossRef]
- Lakkadwala, S.; Singh, J. Dual functionalized 5-fluorouracil liposomes as highly efficient nanomedicine for glioblastoma treatment as assessed in an in vitro brain tumor model. J. Pharm. Sci. 2018, 107, 2902–2913. [Google Scholar] [CrossRef]
- Ferreira, T.M.; Leonel, A.J.; Melo, M.A.; Santos, R.R.; Cara, D.C.; Cardoso, V.N.; Correia, M.I.; Alvarez-Leite, J.I. Oral supplementation of butyrate reduces mucositis and intestinal permeability associated with 5-fluorouracil administration. Lipids 2012, 47, 669–678. [Google Scholar] [CrossRef]
- de Barros, P.A.V.; Andrade, M.E.R.; de Vasconcelos Generoso, S.; Miranda, S.E.M.; Dos Reis, D.C.; Leocádio, P.C.L.; de Sales e Souza, É.L.; dos Santos Martins, F.; da Gama, M.A.S.; Cassali, G.D.; et al. Conjugated linoleic acid prevents damage caused by intestinal mucositis induced by 5-fluorouracil in an experimental model. Biomed. Pharmacother. 2018, 103, 1567–1576. [Google Scholar] [CrossRef]
- Ashkenazi, A. Directing cancer cells to self-destruct with pro-apoptotic receptor agonists. Nat. Rev. Drug Discov. 2008, 7, 1001–1012. [Google Scholar] [CrossRef]
- Taatjes, D.J.; Roth, J. Cell Imaging Techniques: Methods and Protocols; Humana Press: Totowa, NJ, USA, 2016. [Google Scholar]
- Hawkes, P.W.; Spence, J.C.H. Springer Handbook of Microscopy; Springer International Publishing: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Brady, H.J.M. Apoptosis Methods and Protocols; Humana Press: Totowa, NJ, USA, 2004. [Google Scholar]
- Scrochi, M.R.; Zanuzzi, C.N.; Fuentealba, N.; Nishida, F.; Bravi, M.E.; Pacheco, M.E.; Sguazza, G.H.; Gimeno, E.J.; Portiansky, E.L.; Muglia, C.I.; et al. Investigation of apoptosis in cultured cells infected with equine herpesvirus 1. Biotech. Histochem. 2017, 92, 560–568. [Google Scholar] [CrossRef]
- Tian, M.; Ma, Y.; Lin, W. Fluorescent probes for the visualization of cell viability. Acc. Chem. Res. 2019, 52, 2147–2157. [Google Scholar] [CrossRef]
- Kocabey, S.; Ekim Kocabey, A.; Schneiter, R.; Rüegg, C. Membrane-interacting DNA nanotubes induce cancer cell death. Nanomaterials 2021, 11, 2003. [Google Scholar] [CrossRef] [PubMed]
- Hawley, T.S.; Hawley, R.G. Flow Cytometry Protocols; Humana Press: Totowa, NJ, USA, 2004. [Google Scholar]
- Oyenihi, O.R.; Delgoda, R.; Matsabisa, M.G. Tagetes minuta leaf extracts triggered apoptosis in MCF-7 human breast cancer cell line. South Afr. J. Bot. 2021, 137, 359–364. [Google Scholar] [CrossRef]
- Ruwizhi, N.; Aderibigbe, B.A. Cinnamic acid derivatives and their biological efficacy. Int. J. Mol. Sci. 2020, 21, 5712. [Google Scholar] [CrossRef] [PubMed]
- Qi, G.; Chen, J.; Shi, C.; Wang, Y.; Mi, S.; Shao, W.; Yu, X.; Ma, Y.; Ling, J.; Huang, J. Cinnamic acid (CINN) induces apoptosis and proliferation in human nasopharyngeal carcinoma cells. Cell. Physiol. Biochem. 2016, 40, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Macey, M.G. Flow Cytometry: Principles and Applications; Humana Press: Totowa, NJ, USA, 2007. [Google Scholar]
- Gray, J.W.; Darzynkiewicz, Z. Techniques in Cell Cycle Analysis; Humana Press: Totowa, NJ, USA, 2014. [Google Scholar]
- Mensah-Osman, E.J. Mechanism of DNA Damage, Cell Cycle Arrest and Apoptosis in Indolent B-cell Lymphomas; Wayne State University: Detroit, MI, USA, 2003. [Google Scholar]
- Hunke, M.; Martinez, W.; Kashyap, A.; Bokoskie, T.; Pattabiraman, M.; Chandra, S. Antineoplastic actions of cinnamic acids and their dimers in breast cancer cells: A comparative study. Anticancer. Res. 2018, 38, 4469–4474. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhao, M.; Zhang, M.H.; Gao, W.; Guo, Z.; Zhang, X.; Zhang, J.; Cao, J.; Pu, Y.; He, B. Cinnamaldehyde-based poly (ester-thioacetal) to generate reactive oxygen species for fabricating reactive oxygen species-responsive nanoparticles. Biomacromolecules 2018, 19, 4658–4667. [Google Scholar] [CrossRef]
- Park, J.; Baek, S.H. Combination therapy with cinnamaldehyde and hyperthermia induces apoptosis of a549 non-small cell lung carcinoma cells via regulation of reactive oxygen species and mitogen-activated protein kinase family. Int. J. Mol. Sci. 2020, 21, 6229. [Google Scholar] [CrossRef]
- Miao, T.; Deng, Q.; Gao, H.; Fu, X.; Li, S. Theoretical studies on DNA-cleavage mechanism of copper (II) complexes: Probing generation of reactive oxygen species. J. Chem. Inf. Model. 2018, 58, 859–866. [Google Scholar] [CrossRef]
- Meeran, S.M.; Katiyar, S.; Katiyar, S.K. Berberine-induced apoptosis in human prostate cancer cells is initiated by reactive oxygen species generation. Toxicol. Appl. Pharmacol. 2008, 229, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Yesuthangam, Y.; Pandian, S.; Venkatesan, K.; Gandhidasan, R.; Murugesan, R. Photogeneration of reactive oxygen species and photoinduced plasmid DNA cleavage by novel synthetic chalcones. J. Photochem. Photobiol. B Biol. 2011, 102, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Boice, A.; Bouchier-Hayes, L. Targeting apoptotic caspases in cancer. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2020, 1867, 118688. [Google Scholar] [CrossRef] [PubMed]
- McArthur, K.; Kile, B.T. Apoptotic caspases: Multiple or mistaken identities? Trends Cell Biol. 2018, 28, 475–493. [Google Scholar] [CrossRef]
- McComb, S.; Chan, P.K.; Guinot, A.; Hartmannsdottir, H.; Jenni, S.; Dobay, M.P.; Bourquin, J.P.; Bornhauser, B.C. Efficient apoptosis requires feedback amplification of upstream apoptotic signals by effector caspase-3 or-7. Sci. Adv. 2019, 5, eaau9433. [Google Scholar] [CrossRef]
- Calori, I.R.; Piva, H.L.; Tedesco, A.C. Targeted cancer therapy using alpha-cyano-4-hydroxycinnamic acid as a novel vector molecule: A proof-of-concept study. J. Drug Deliv. Sci. Technol. 2020, 57, 101633. [Google Scholar] [CrossRef]
- Jan, H.; Shah, M.; Andleeb, A.; Faisal, S.; Khattak, A.; Rizwan, M.; Drouet, S.; Hano, C.; Abbasi, B.H. Plant-based synthesis of zinc oxide nanoparticles (ZnO-NPs) using aqueous leaf extract of aquilegia pubiflora: Their antiproliferative activity against HepG2 cells inducing reactive oxygen species and other in vitro properties. Oxidative Med. Cell. Longev. 2021, 4786227. [Google Scholar] [CrossRef]
- Fulda, S.; Debatin, K.M. Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene 2006, 25, 4798–4811. [Google Scholar] [CrossRef]
- Kianpour Rad, S.; Kanthimathi, M.S.; Abd Malek, S.N.; Lee, G.S.; Looi, C.Y.; Wong, W.F. Cinnamomum cassia suppresses caspase-9 through stimulation of AKT1 in MCF-7 cells but not in MDA-MB-231 cells. PLoS ONE 2015, 10, e0145216. [Google Scholar] [CrossRef]
- Machana, S.; Weerapreeyakul, N.; Barusrux, S.; Nonpunya, A.; Sripanidkulchai, B.; Thitimetharoch, T. Cytotoxic and apoptotic effects of six herbal plants against the human hepatocarcinoma (HepG2) cell line. Chin. Med. 2011, 6, 39. [Google Scholar] [CrossRef]









| Dried Material (g) | Weight and % Yield of Petroleum Ether Extract | Weight and %Yield of Chloroform Extract | Weight and %Yield of Methanol Extract |
|---|---|---|---|
| 960 g | 61.62 g and 6.48% | 41.93 g and 4.37% | 102.16 g and 10.64% |
| Carbon Position | Isolated Compound | Reference (Hashim et al., 2005) | ||
|---|---|---|---|---|
| δC (ppm) in CDCL3 | δH (J in Hz) | δC (ppm) in DCL3 | δH (J in Hz) | |
| 1 | 172.4 | ----- | 172.68 | ----- |
| 2 | 114.5 | 6.33 (1H, d, 15 Hz) | 114.38 | 6.26 (1H, d, 16) |
| 3 | 146.7 | 7.75 (1H, d, 15 Hz) | 146.8 | 7.72 (1H, d, 16) |
| 4 | 126.6 | ----- | 126.57 | ----- |
| 5,9 | 130.1 | 7.50 (2H, d, 10 Hz) | 130.07 | 7.48 (2H, d, 8.25) |
| 6,8 | 115.1 | 6.93 (2H, d, 10 Hz) | 115.07 | 6.91 (2H, d, 8.25) |
| 7 | 161.1 | ----- | 161.08 | ----- |
| 1′ | 65.0 | 4.58 (2H, d, 5 Hz) | 64.99 | 4.58 (2H, d, 6.4) |
| 2′ | 118.9 | 5.49 (1H, t, 5 Hz) | 118.88 | 5.47 (1H, t, 6.4) |
| 3′ | 141.8 | ----- | 141.77 | ----- |
| 4′ | 39.5 | 2.10 (2H, d, 5 Hz) | 39.5 | 2.10 (2H, m) |
| 5′ | 26.2 | 2.14 (2H, t, 10 Hz) | 26.23 | 2.10 (2H, m) |
| 6′ | 123.7 | 5.10 (1H, t, 10 Hz) | 123.66 | 5.07 (1H, t, 7.8) |
| 7′ | 131.9 | ----- | 131.88 | ----- |
| 8′ | 17.7 | 1.60 (3H, s) | 16.69 | 1.60 (3H, s) |
| 9′ | 16.7 | 1.74 (3H, s) | 17.69 | 1.74 (3H, s) |
| 10′ | 25.7 | 1.67 (3H, s) | 25.68 | 1.67 (3H, s) |
| Extract | HepG2 | MCF-7 | HT-29 |
|---|---|---|---|
| Petroleum ether | 75.181 ± 0.011 | 76.107 ± 0.011 | 20.645 ± 0.023 |
| Chloroform | 152.809 ± 0.150 | 76.199 ±0.038 | 19.662 ± 0.013 |
| Methanol | 77.012 ± 0.007 | 89.332 ± 0.031 | 181.020 ± 0.043 |
| Cell Lines | Cell Lines (IC50 in µg/mL) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MDA-MB231 | HT29 | MCF-7 | MCF-10a | |||||||||
| Incubation (h)/Compound | 24 | 48 | 72 | 24 | 48 | 72 | 24 | 48 | 72 | 24 | 48 | 72 |
| 7-geranyloxycinnamic acid | 5.368 ± 0.777 | 3.975 ± 0.857 | 1.732 ± 0.060 | 9.057 ± 0.596 | 6.797 ± 0.306 | 6.748 ± 0.522 | 4.936 ± 0.345 | 3.335 ± 0.728 | 1.847 ± 0.212 | ND | 94.201 ± 0.429 | 48.814 ± 0.386 |
| 5-fluorouracil | 34.464 ± 1.541 | 4.445 ± 0.613 | 2.505 ± 0.448 | 34.904 ± 1.316 | 10.336 ± 0.610 | 7.952 ± 0.905 | 30.252 ± 1.156 | 4.446 ± 0.595 | 2.163 ± 0.606 | NA | ||
| Test Compounds | IC50 (μg/mL) | Selectivity Index (SI) | |
| MCF-10a Cells | MDA-MB231 Cells | ||
| 7-geranyloxycinnamic acid (24 h) | ND | 5.368 ± 0.777 | 0 |
| 7-geranyloxycinnamic acid (48 h) | 94.201 ± 0.429 | 3.975 ± 0.857 | 24 |
| 7-geranyloxycinnamic acid (72 h) | 48.814 ± 0.386 | 1.732 ± 0.060 | 28 |
| Test Compounds | IC50 (μg/mL) | Selectivity Index (SI) | |
| MCF-10a Cells | MCF-7 Cells | ||
| 7-geranyloxycinnamic acid (24 h) | ND | 4.936 ± 0.345 | 0 |
| 7-geranyloxycinnamic acid (48 h) | 94.201 ± 0.429 | 3.335 ± 0.728 | 28 |
| 7-geranyloxycinnamic acid (72 h) | 48.814 ± 0.386 | 1.847 ± 0.212 | 26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eliaser, E.M.; Mohd. Hashim, N.; Rukayadi, Y.; Abdull Razis, A.F. 7-Geranyloxycinnamic Acid Isolated from Melicope lunu-ankenda Leaves Perturbs Colon Cancer and Breast Cancer Cell Lines’ Growth via Induction of Apoptotic Pathway. Molecules 2023, 28, 3612. https://doi.org/10.3390/molecules28083612
Eliaser EM, Mohd. Hashim N, Rukayadi Y, Abdull Razis AF. 7-Geranyloxycinnamic Acid Isolated from Melicope lunu-ankenda Leaves Perturbs Colon Cancer and Breast Cancer Cell Lines’ Growth via Induction of Apoptotic Pathway. Molecules. 2023; 28(8):3612. https://doi.org/10.3390/molecules28083612
Chicago/Turabian StyleEliaser, Enas Mohamed, Najihah Mohd. Hashim, Yaya Rukayadi, and Ahmad Faizal Abdull Razis. 2023. "7-Geranyloxycinnamic Acid Isolated from Melicope lunu-ankenda Leaves Perturbs Colon Cancer and Breast Cancer Cell Lines’ Growth via Induction of Apoptotic Pathway" Molecules 28, no. 8: 3612. https://doi.org/10.3390/molecules28083612
APA StyleEliaser, E. M., Mohd. Hashim, N., Rukayadi, Y., & Abdull Razis, A. F. (2023). 7-Geranyloxycinnamic Acid Isolated from Melicope lunu-ankenda Leaves Perturbs Colon Cancer and Breast Cancer Cell Lines’ Growth via Induction of Apoptotic Pathway. Molecules, 28(8), 3612. https://doi.org/10.3390/molecules28083612







