Metallocene-Naphthalimide Derivatives: The Effect of Geometry, DFT Methodology, and Transition Metals on Absorption Spectra
Abstract
1. Introduction
2. Results and Discussion
2.1. Geometry
2.2. Energetics
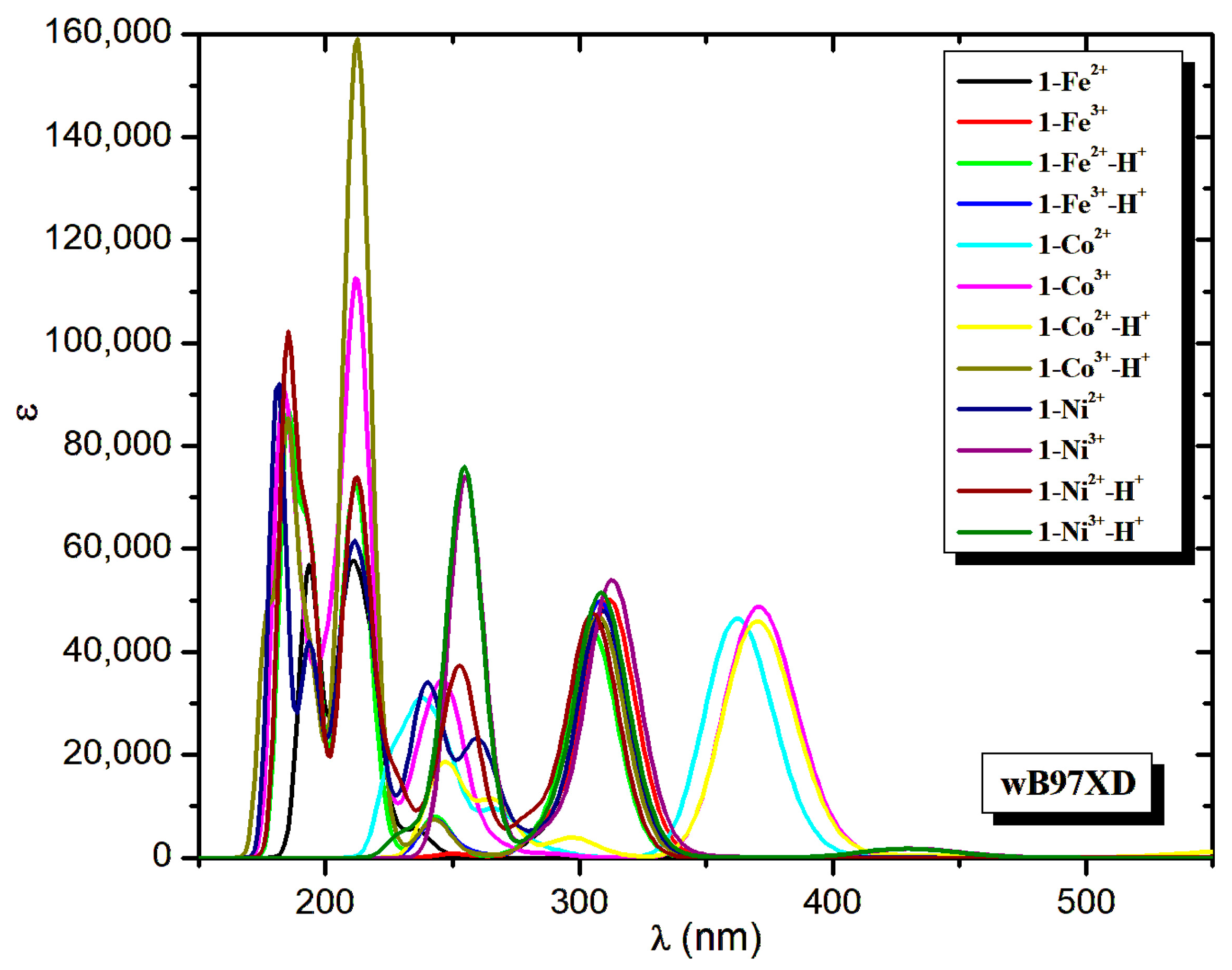
2.3. Absorption Spectra
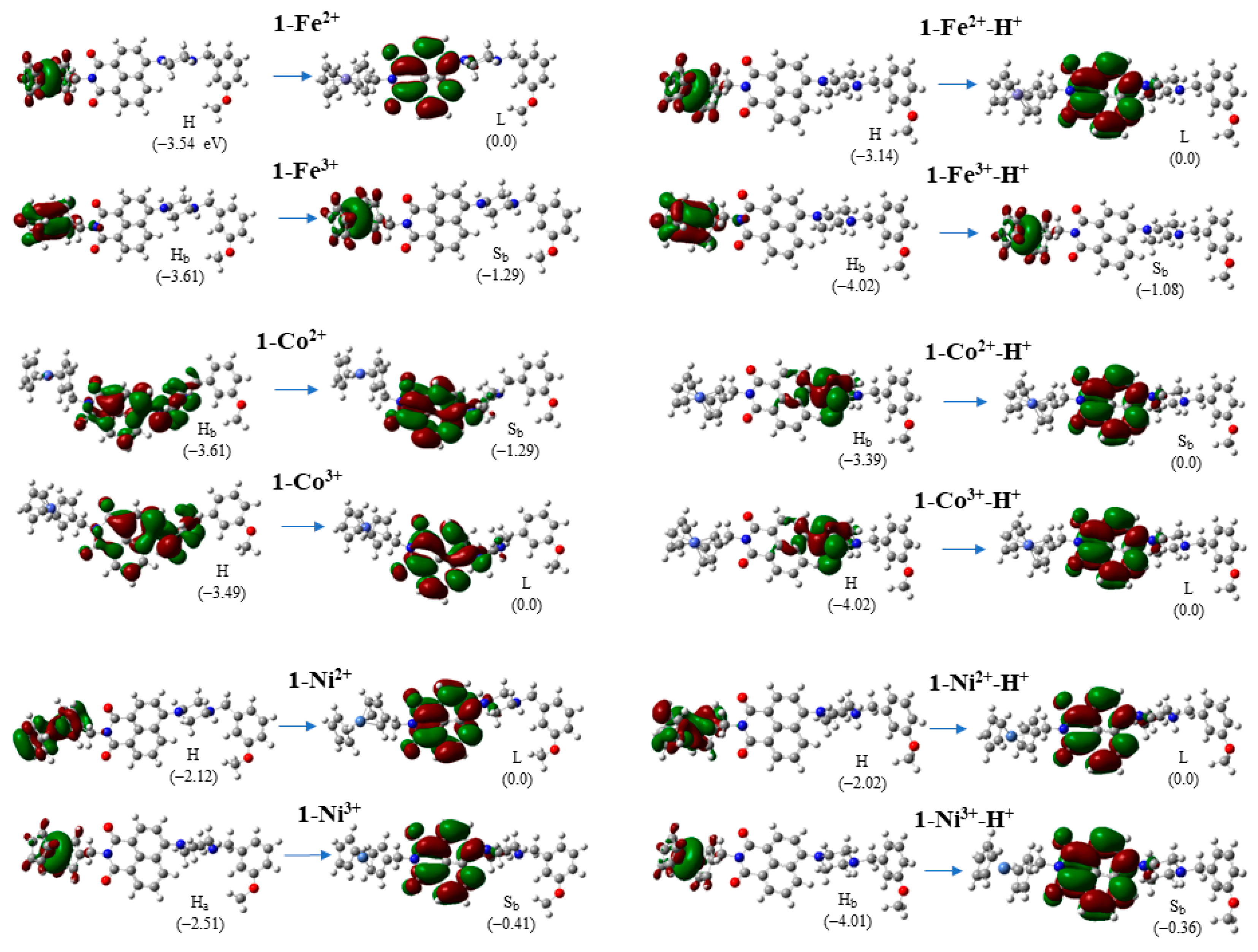
2.4. Molecular Orbitals
3. Computational Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Ling, J.; Daly, B.; Silverson, V.A.D.; de Silva, A.P. Taking baby steps in molecular logic-based computation. Chem. Commun. 2015, 51, 8403–8409. [Google Scholar] [CrossRef] [PubMed]
- Erbas-Cakmak, S.; Kolemen, S.; Sedgwick, A.C.; Gunnlaugsson, T.; James, T.D.; Yoon, J.; Akkaya, E.U. Molecular logic gates: The past, present and future. Chem. Soc. Rev. 2018, 47, 2228–2248. [Google Scholar] [CrossRef] [PubMed]
- Andréasson, J.; Pischel, U. Molecules for security measures: From keypad locks to advanced communication protocols. Chem. Soc. Rev. 2018, 47, 2266–2279. [Google Scholar] [CrossRef] [PubMed]
- Magri, D.C. Logical sensing with fluorescent molecular logic gates based on photoinduced electron transfer. Coord. Chem. Rev. 2021, 426, 213598. [Google Scholar] [CrossRef]
- Konry, T.; Walt, D.R. Intelligent Medical Diagnostics via Molecular Logic. J. Am. Chem. Soc. 2009, 131, 13232–13333. [Google Scholar] [CrossRef]
- Tzeli, D.; Petsalakis, I.D.; Theodorakopoulos, G. Molecular Logic Gates based on benzo-18-crown-6 ether of styrylquinoline. A theoretical study. Phys. Chem. Chem. Phys. 2016, 18, 32132–32145. [Google Scholar] [CrossRef]
- Magri, D.C.; de Silva, A.P. From PASS 1 to YES to AND logic: Building parallel processing into molecular logic gates by sequential addition of receptors. New J. Chem. 2010, 34, 476–481. [Google Scholar] [CrossRef]
- Andréasson, J.; Pischel, U. Smart molecules at work—Mimicking advanced logic operations. Chem. Soc. Rev. 2010, 39, 174–188. [Google Scholar] [CrossRef]
- Tzeli, D.; Petsalakis, I.; Theodorakopoulos, G. Theoretical study of the photophysical processes of a styryl-bodipy derivative eliciting an AND molecular logic gate response. Int. J. Quantum Chem. 2019, 119, e25958. [Google Scholar] [CrossRef]
- Tzeli, D.; Petsalakis, I.D.; Theodorakopoulos, G. The solvent effect on a styryl-bodipy derivative functioning as an AND molecular logic gate. Int. J. Quantum Chem. 2020, 120, e26181. [Google Scholar] [CrossRef]
- Li, B.; Zhao, D.; Wang, F.; Zhang, X.; Li, W.; Fan, L. Recent advances in molecular logic gate chemosensors based on luminescent metal organic frameworks. Dalton Trans. 2021, 50, 14967–14977. [Google Scholar] [CrossRef]
- Katz, E.; Minko, S. Enzyme-based logic systems interfaced with signal-responsive materials and electrodes. Chem. Commun. 2015, 51, 3493–3500. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Wan, S.; Hou, W.; Zhang, L.; Xu, J.; Cui, C.; Wang, Y.; Hu, J.; Tan, W. A survey of advancements in nucleic acid-based logic gates and computing for applications in biotechnology and biomedicine. Chem. Commun. 2015, 51, 3723–3734. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.D.; Buhagiar, J.A.; Magri, D.C. 4-Amino-1,8-naphthalimide–ferrocene conjugates as potential multi-targeted anticancer and fluorescent cellular imaging agents. RSC Med. Chem. 2021, 12, 2060–2064. [Google Scholar] [CrossRef] [PubMed]
- Tzeli, D.; Petsalakis, I.D. Physical insights into molecular sensors, molecular logic gates and on photosensitizers in photodynamic therapy. J. Chem. 2019, 2019, 6793490. [Google Scholar] [CrossRef]
- Grech, J.; Spiteri, J.C.; Scerri, G.J.; Magri, D.C. Molecular logic with ferrocene-rylene conjugates: A comparison of naphthalenediimide, naphthalimide and perylenediimide Pourbaix sensor designs. Inorg. Chim. Acta 2023, 544, 121176. [Google Scholar] [CrossRef]
- Scerri, G.J.; Spiteri, J.C.; Mallia, C.J.; Magri, D.C. A lab-on-a-molecule with an enhanced fluorescent readout on detection of three chemical species. Chem. Comm. 2019, 55, 4961–4964. [Google Scholar] [CrossRef]
- Tzeliou, C.E.; Tzeli, D. 3-input AND molecular logic gate with enhanced fluorescence output: The key atom for the accurate prediction of the spectra. J. Chem. Inf. Model. 2022, 62, 6436–6448. [Google Scholar] [CrossRef]
- Zerafa, N.; Cini, M.; Magri, D.C. Molecular engineering of 1,3,5-triaryl-2-pyrazoline fluorescent logic systems responsive to acidity and oxidisability and attachment to polymer beads. Mol. Syst. Des. Eng. 2021, 6, 93–99. [Google Scholar] [CrossRef]
- Scerri, G.J.; Cini, M.; Schembri, J.S.; da Costa, P.F.; Johnson, A.D.; Magri, D.C. Redox-Enabled, pH-Disabled Pyrazoline–Ferrocene INHIBIT Logic Gates. ChemPhysChem 2017, 18, 1742–1745. [Google Scholar] [CrossRef]
- Bhatta, S.R.; Bheemireddy, V.; Thakur, A. A Redox-Driven Fluorescence “Off–On” Molecular Switch Based on a 1,1′-Unsymmetrically Substituted Ferrocenyl Coumarin System: Mimicking Combinational Logic Operation. Organometallics 2017, 36, 829–838. [Google Scholar] [CrossRef]
- Karmakar, M.; Bhatta, S.R.; Giri, S.; Thakur, A. Oxidation-Induced Differentially Selective Turn-On Fluorescence via Photoinduced Electron Transfer Based on a Ferrocene-Appended Coumarin–Quinoline Platform: Application in Cascaded Molecular Logic. Inorg. Chem. 2020, 59, 4493–4507. [Google Scholar] [CrossRef] [PubMed]
- Magri, D.C.; Fava, M.C.; Mallia, C.J. A sodium-enabled ‘Pourbaix sensor’: A three-input AND logic gate as a ‘lab-on-a-molecule’ for monitoring Na+, pH and pE. Chem. Commun. 2014, 50, 1009–1011. [Google Scholar] [CrossRef] [PubMed]
- Magri, D.C.; Spiteri, J.C. Proof of principle of a three-input AND–INHIBIT–OR combinatorial logic gate array. Org. Biomol. Chem. 2017, 15, 6706–6709. [Google Scholar] [CrossRef]
- Magri, D.C.; Brown, G.J.; McClean, G.D.; de Silva, A.P. Communicating Chemical Congregation: A Molecular and Logic Gate with Three Chemical Inputs as a “Lab-on-a-Molecule” Prototype. J. Am. Chem. Soc. 2006, 128, 4950–4951. [Google Scholar] [CrossRef]
- Poddar, M.; Sivakumar, G.; Misra, R. Donor–acceptor substituted 1,8-naphthalimides: Design, synthesis, and structure–property relationship. J. Mater. Chem. C 2019, 7, 14798–14815. [Google Scholar] [CrossRef]
- Torres-Moya, I.; Carrillo, J.R.; Gómez, M.V.; Velders, A.H.; Donoso, B.; Rodríguez, A.M.; Díaz-Ortiz, A.; Navarrete, J.T.L.; Ortiz, R.P.; Prieto, P. Synthesis of D-π-A high-emissive 6-arylalkynyl-1,8-naphthalimides for application in Organic Field-Effect Transistors and optical waveguides. Dye. Pigment. 2021, 191, 109358. [Google Scholar] [CrossRef]
- Georgiev, N.I.; Dimitrova, M.D.; Krasteva, P.V.; Bojinov, V.B. A novel water-soluble 1,8-naphthalimide as a fluorescent pH-probe and a molecular logic circuit. J. Lumin. 2017, 187, 383–391. [Google Scholar] [CrossRef]
- Peng, H.; Shen, K.; Mao, S.; Shi, X.; Xu, Y.; Aderinto, S.O.; Wu, H. A Highly Selective and Sensitive Fluorescent Turn-on Probe for Al3+ Based on Naphthalimide Schiff Base. J. Fluoresc. 2017, 27, 1191–1200. [Google Scholar] [CrossRef]
- Yang, S.; Meng, F.; Tian, H.; Chen, K. Photostability of novel copolymers functionalized with laser dyes based on modified rhodamine 6G and 1,8-naphthalimide. Eur. Polym. J. 2002, 38, 911–919. [Google Scholar] [CrossRef]
- Cao, M.; Chen, H.; Chen, D.; Xu, Z.; Liu, S.H.; Chen, X.; Yin, J. Naphthalimide-based fluorescent probe for selectively and specifically detecting glutathione in the lysosomes of living cells. Chem. Commun. 2016, 52, 721–724. [Google Scholar] [CrossRef] [PubMed]
- Chena, W.; Zhang, Q. Recent progress in non-fullerene small molecule acceptors in organic solar cells (OSCs). J. Mater. Chem. C 2017, 5, 1275–1302. [Google Scholar] [CrossRef]
- Bezvikonnyi, O.; Gudeika, D.; Volyniuk, D.; Grazulevicius, J.V.; Bagdziunas, G. Pyrenyl substituted 1,8-naphthalimide as a new material for weak efficiency-roll-off red OLEDs: A theoretical and experimental study. New J. Chem. 2018, 42, 12492–12502. [Google Scholar] [CrossRef]
- Wu, A.; Xu, Y.; Qian, X. Novel naphthalimide–amino acid conjugates with flexible leucine moiety as side chain: Design, synthesis and potential antitumor activity. Bioorg. Med. Chem. 2009, 17, 592–599. [Google Scholar] [CrossRef]
- Ponce, R.; Herrera, H.; Mancheño, M.J.; Seoane, C.; Segura, J.L.; Mayorga, P.; Casado, J.; Teodomiro, J.; Facchetti, A.; Marks, T.J. Molecular and electronic-structure basis of the ambipolar behavior of naphthalimide-terthiophene derivatives: Implementation in organic field-effect transistors. Chem. Eur. J. 2013, 19, 12458–12467. [Google Scholar] [CrossRef]
- Greiner, R.; Schlücker, T.; Zgela, D.; Langhals, H. Fluorescent aryl naphthalene dicarboximides with large Stokes shifts and strong solvatochromism controlled by dynamics and molecular geometry. J. Mater. Chem. C 2016, 4, 11244–11252. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, Y.; Chen, S.; Wang, N.; Qi, Y.; Zhang, X.; Yang, M.; Huang, Y.; Li, M.; Yu, J.; et al. Facile Access to Twisted Intramolecular Charge-Transfer Fluorogens Bearing Highly Pretwisted Donor–Acceptor Systems Together with Readily Fine-Tuned Charge-Transfer Characters. Small 2017, 13, 1604113. [Google Scholar] [CrossRef]
- Gopikrishna, P.; Adilb, L.R.; Iyer, P.K. Bridge-driven aggregation control in dibenzofulvene–naphthalimide based donor–bridge–acceptor systems: [thin space (1/6-em)] enabling fluorescence enhancement, blue to red emission and solvatochromism. Mater. Chem. Front. 2017, 1, 2590–2598. [Google Scholar] [CrossRef]
- Do, T.T.; Pham, H.D.; Manzhos, S.; Bell, J.M.; Sonar, P. Molecular Engineering Strategy for High Efficiency Fullerene-Free Organic Solar Cells Using Conjugated 1,8-Naphthalimide and Fluorenone Building Blocks. ACS Appl. Mater. Interfaces 2017, 9, 16967–16976. [Google Scholar] [CrossRef]
- Yu, H.; Guo, Y.; Zhu, W.; Havener, K.; Zheng, X. Recent advances in 1,8-naphthalimide-based small-molecule fluorescent probes for organelles imaging and tracking in living cells. Coord. Chem. Rev. 2021, 444, 214019. [Google Scholar] [CrossRef]
- Sugar, J.; Corliss, C. Atomic Energy Levels of the Iron-Period Elements: Potassium through Nickel. J. Phys. Chem. Ref. Data 1985, 14 (Suppl. 2), 1–664. [Google Scholar]
- Garcia-Riquelme, O.; Rico, F.R. Extended Analysis and the Ionization Potential of the Ni III Spectrum. Phys. Scr. 1992, 45, 212–230. [Google Scholar] [CrossRef]
- Adamo, C.; Barone, V. Toward reliable density functional methods without adjustable parameters: The PBE0 model. J. Chem. Phys. 1999, 110, 6158–6170. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Tao, J.; Perdew, J.P.; Staroverov, V.N.; Scuseria, G.E. Climbing the Density Functional Ladder: Nonempirical Meta-Generalized Gradient Approximation Designed for Molecules and Solids. Phys. Rev. Lett. 2003, 91, 146401. [Google Scholar] [CrossRef]
- Chai, J.-D.; Head-Gordon, M. Long-range corrected hybrid density functionals with damped atom-atom dispersion corrections. Phys. Chem. Chem. Phys. 2008, 10, 6615–6620. [Google Scholar] [CrossRef] [PubMed]
- Curtiss, L.A.; McGrath, M.P.; Blaudeau, J.-P.; Davis, N.E.; Binning, R.C., Jr.; Radom, L. Extension of Gaussian-2 theory to molecules containing third-row atoms Ga–Kr. J. Chem. Phys. 1995, 103, 6104. [Google Scholar] [CrossRef]
- Miertuš, S.; Scrocco, E.; Tomasi, J. Electrostatic interaction of a solute with a continuum. A direct utilization of AB initio molecular potentials for the prevision of solvent effects. Chem. Phys. 1981, 55, 117–129. [Google Scholar] [CrossRef]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum Mechanical Continuum Solvation Models. Chem. Rev. 2005, 105, 2999–3094. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parameterization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016.
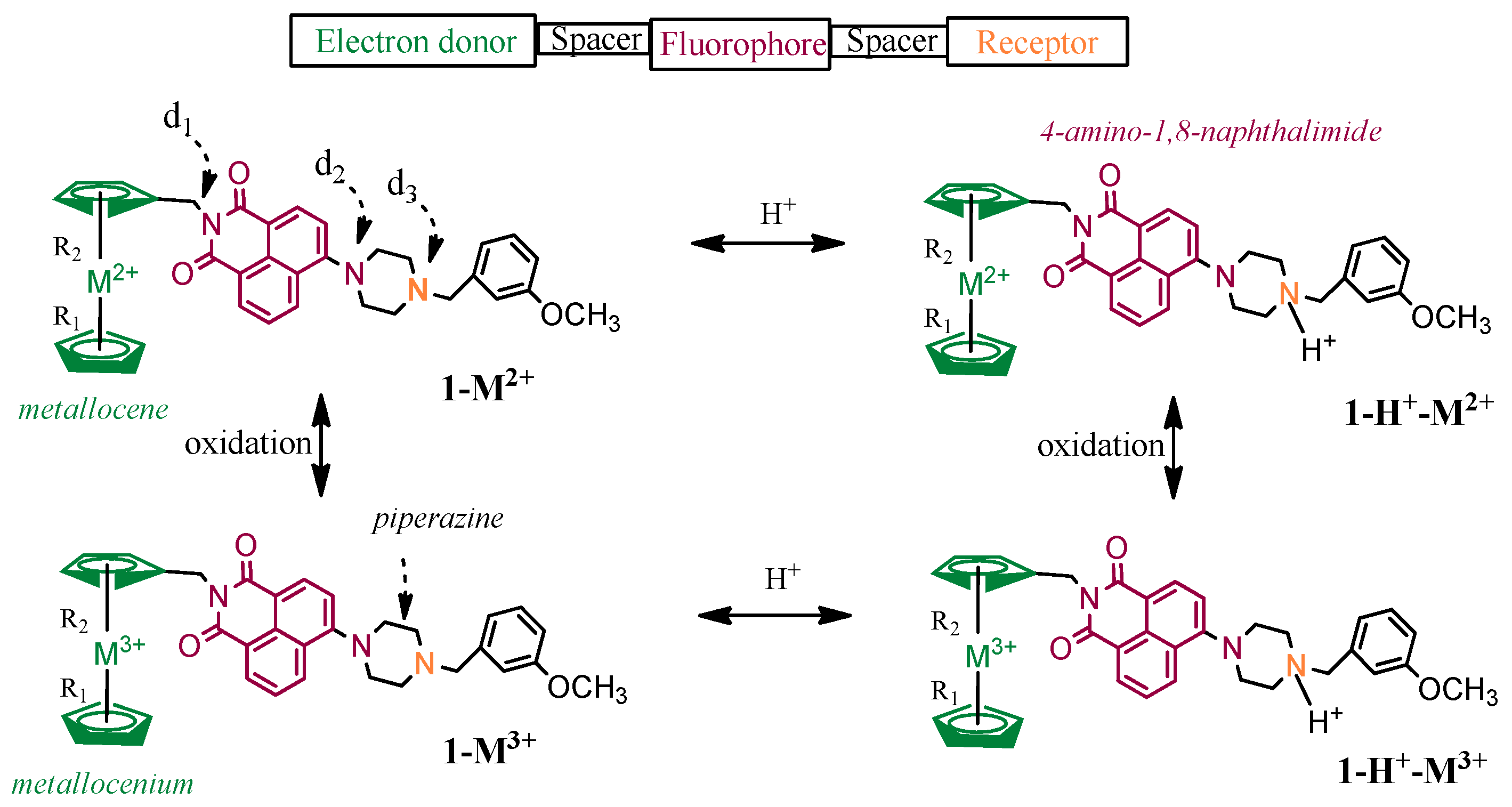
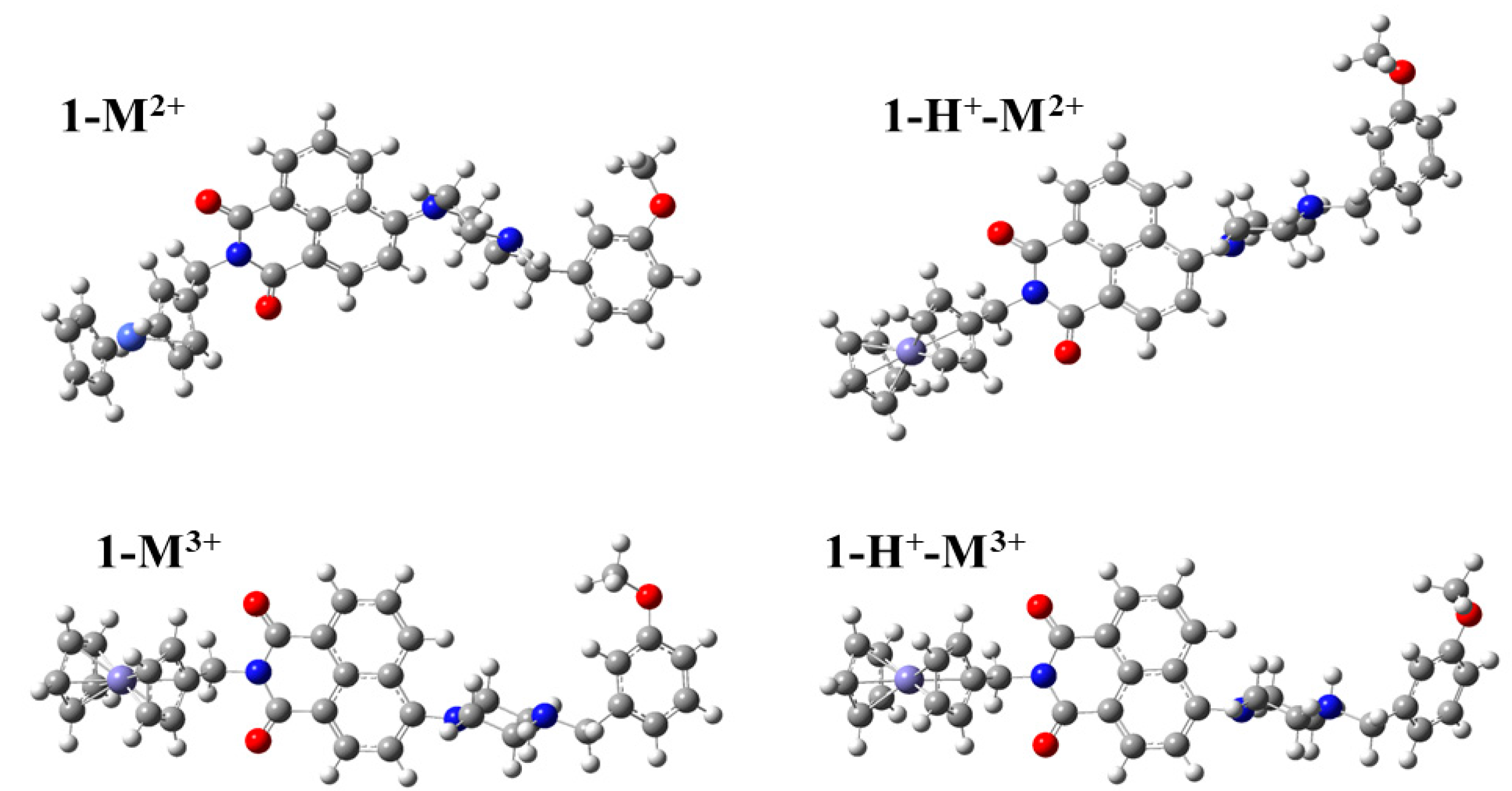


| Molecule | R a | d1 b | d2 b | d3 b | R a | d1 b | d2 b | d3 b | R a | d1 b | d2 b | d3 b | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PBE0 | TPSSh | wB97XD | ||||||||||||
| 1-Fe2+ | 1.623 | 178.2 | 141.8 | 124.9 | 1.613 | 178.9 | 140.1 | 124.3 | 1.632 | 176.1 | 141.4 | 125.4 | ||
| c | 1.622 | 175.9 | 140.8 | 125.9 | 1.605 | 173.8 | 138.8 | 125.3 | ||||||
| 1-Fe3+ | 1.642 | 177.9 | 142.1 | 124.8 | 1.637 | 178.6 | 140.4 | 124.2 | 1.645 | 175.8 | 141.8 | 125.4 | ||
| 1-Fe2+-H+ | 1.621 | 177.9 | 143.2 | 126.7 | 1.613 | 178.5 | 141.6 | 126.6 | 1.631 | 175.7 | 142.9 | 126.7 | ||
| 1-Fe3+-H+ | 1.642 | 177.9 | 143.7 | 126.7 | 1.681 | 178.2 | 142.3 | 126.7 | 1.645 | 175.0 | 143.4 | 126.7 | ||
| 1-Co2+ | 1.695 | 178.7 | 150.0 | 125.0 | 1.664 | 179.2 | 150.2 | 124.4 | 1.717 | 177.3 | 145.6 | 124.9 | ||
| 1-Co3+ | 1.610 | 178.9 | 152.4 | 125.1 | 1.606 | 179.6 | 152.9 | 124.6 | 1.616 | 176.8 | 147.0 | 125.1 | ||
| c | 1.607 | 176.6 | 149.7 | 125.4 | 1.596 | 173.7 | 148.2 | 124.6 | ||||||
| 1-Co2+-H+ | 1.610 | 178.2 | 142.1 | 126.7 | 1.661 | 177.9 | 141.6 | 126.6 | 1.615 | 176.5 | 141.4 | 126.5 | ||
| 1-Co3+-H+ | 1.610 | 178.1 | 143.7 | 126.7 | 1.606 | 178.8 | 142.1 | 126.6 | 1.616 | 175.6 | 143.4 | 126.7 | ||
| 1-Ni2+ | 1.756 | 178.0 | 141.9 | 124.8 | 1.755 | 178.2 | 140.2 | 124.2 | 1.781 | 176.5 | 141.3 | 125.4 | ||
| 1-Ni3+ | 1.720 | 178.2 | 142.1 | 126.7 | 1.692 | 179.1 | 140.5 | 124.2 | 1.602 | 176.8 | 141.8 | 125.3 | ||
| 1-Ni2+-H+ | 1.658 | 179.2 | 143.1 | 126.5 | 1.809 | 180.0 | 141.6 | 126.4 | 1.796 | 177.1 | 142.7 | 126.5 | ||
| 1-Ni3+-H+ | 1.694 | 179.0 | 143.8 | 126.8 | 1.679 | 178.5 | 142.2 | 126.4 | 1.604 | 176.5 | 143.1 | 126.6 | ||
| Average/Fe d | 1.632 | 178.0 | 142.7 | 125.8 | 1.636 | 178.6 | 141.1 | 125.4 | 1.638 | 175.7 | 142.4 | 126.0 | ||
| Average/Co d | 1.631 | 178.5 | 147.0 | 125.9 | 1.634 | 178.9 | 146.7 | 125.6 | 1.641 | 176.5 | 144.3 | 125.8 | ||
| Average/Ni d | 1.707 | 178.6 | 142.7 | 126.2 | 1.734 | 178.9 | 141.1 | 125.3 | 1.696 | 176.7 | 142.2 | 125.9 | ||
| Average/M d | 1.656 | 178.3 | 144.1 | 125.9 | 1.668 | 178.8 | 143.0 | 125.4 | 1.658 | 176.3 | 143.0 | 125.9 | ||
| PBE0 | TPSSh | wB97XD | Expt | PBE0 | TPSSh | wB97XD | ||
|---|---|---|---|---|---|---|---|---|
| OE | BE | |||||||
| 1-Fe2+ → 1-Fe3+ | 5.30 | 5.38 | 5.45 | 1-Fe2+-H+ | 8.27 | 8.36 | 8.38 | |
| 1-Fe2+-H+ → 1-Fe3+-H+ | 5.45 | 5.62 | 5.66 | 1-Fe3+-H+ | 8.12 | 8.12 | 8.17 | |
| 1-Co2+ → 1-Co3+ | 3.49 | 3.17 | 3.48 | 1-Co2+-H+ | 7.67 | 8.12 | 7.71 | |
| 1-Co2+-H+ → 1-Co3+-H+ | 3.28 | 3.35 | 3.14 | 1-Co3+-H+ | 7.87 | 7.94 | 8.05 | |
| 1-Ni2+ → 1-Ni3+ | 3.55 | 3.38 | 3.57 | 1-Ni2+-H+ | 8.27 | 8.37 | 8.41 | |
| 1-Ni2+-H+ → 1-Ni3+-H+ | 3.69 | 3.53 | 3.74 | 1-Ni3+-H+ | 8.13 | 8.22 | 8.24 | |
| Fe2+ → Fe3+ | 30.97 | 31.13 | 31.04 | 30.651 a | ||||
| Co2+ → Co3+ | 33.35 | 33.40 | 33.82 | 33.5 a | ||||
| Ni2+ → Ni3+ | 35.20 | 35.04 | 33.68 | 35.187 b | ||||
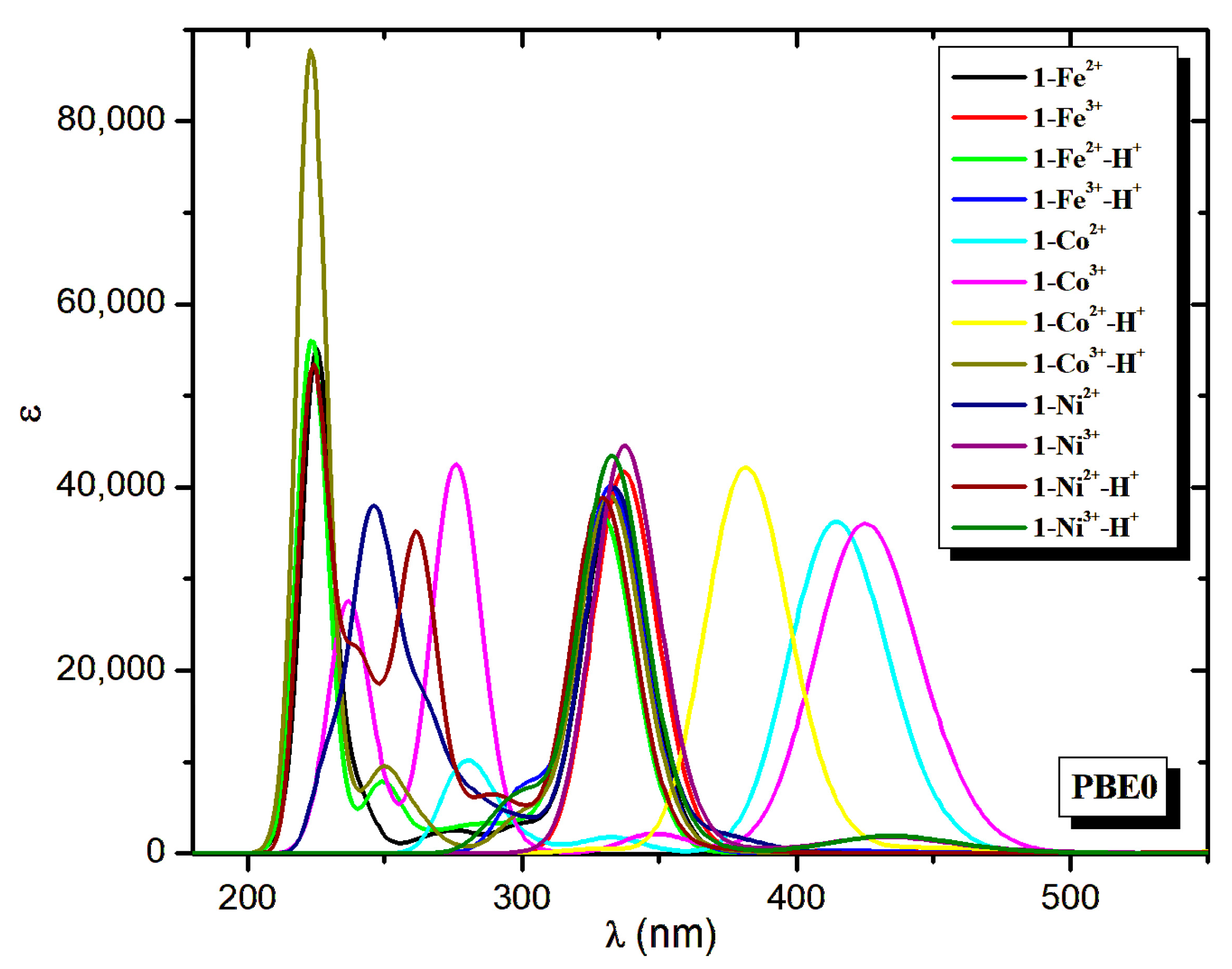
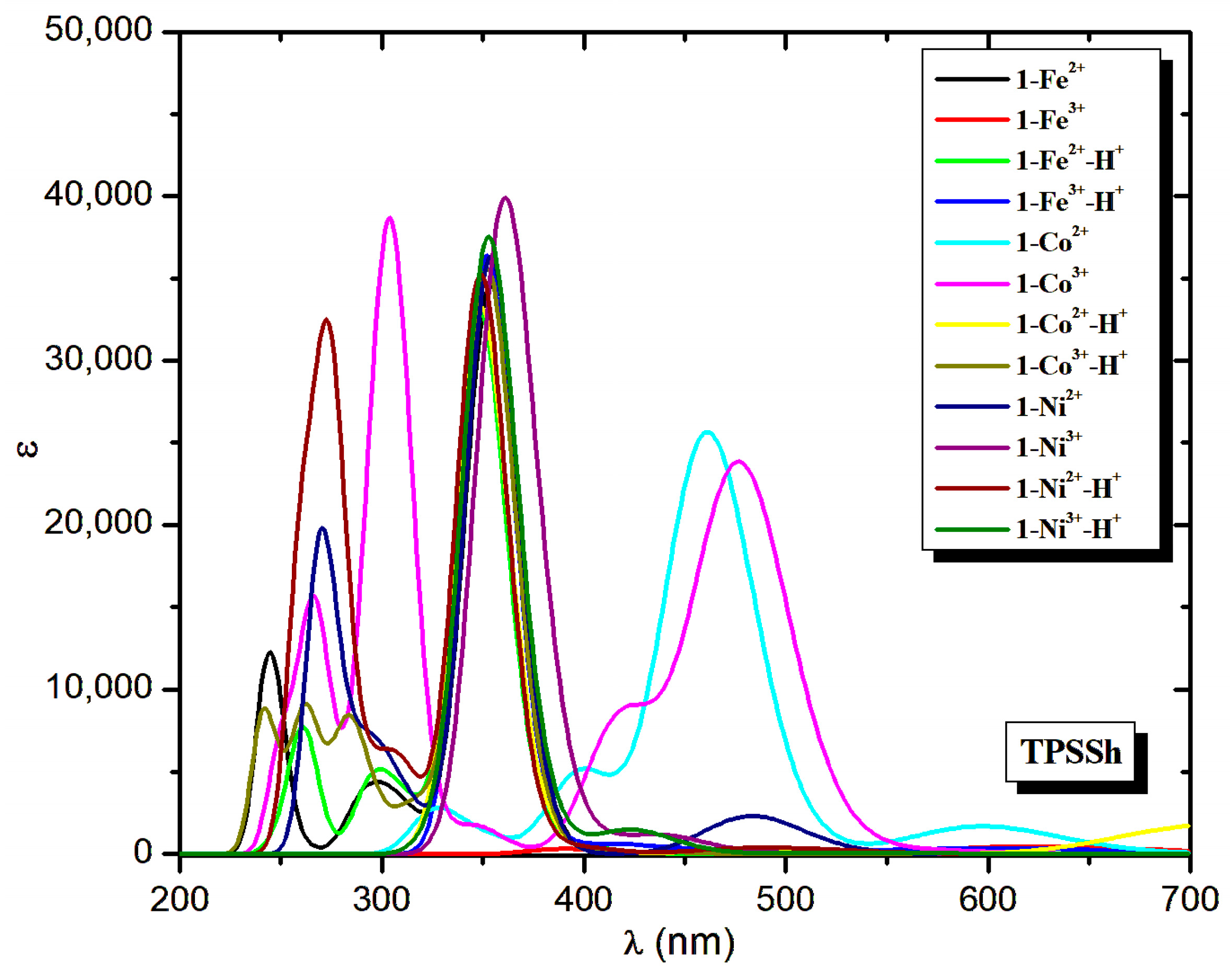
| Molecule | Peaks | λ | ΔΕ | f | Main Excitation |
|---|---|---|---|---|---|
| 1-Fe2+ | S→S | 443.7 | 2.795 | 0.0009 | H → L |
| S→T | 443.5 | 2.796 | 0.0000 | H → L | |
| S→S | 333.5 | 3.718 | 0.4260 | H-6 → L | |
| 1-Fe3+ | D→D | 597.4 | 2.076 | 0.0000 | Hβ → Sβ |
| D→D | 382.7 | 3.240 | 0.0000 | Hβ → Lβ | |
| D→D | 377.9 | 3.281 | 0.0001 | Hα → Lα | |
| D→D | 337.2 | 3.677 | 0.4444 | H-3β → Lβ | |
| 1-Fe2+-H+ | S→S | 475.7 | 2.607 | 0.0000 | H → L |
| S→T | 473.4 | 2.619 | 0.0000 | H → L | |
| S→S | 329.2 | 3.766 | 0.4069 | H-4 → L | |
| 1-Fe3+-H+ | D→D | 455.0 | 2.725 | 0.0000 | Hβ → Sβ |
| D→D | 339.2 | 3.656 | 0.0019 | Sα → Lα | |
| D→D | 339.1 | 3.657 | 0.0213 | Hβ → Lβ | |
| D→D | 331.8 | 3.736 | 0.3888 | Hα → Lα | |
| 1-Co2+ | D→D | 619.6 | 2.001 | 0.0000 | Hβ → Sβ |
| D→D | 414.9 | 2.988 | 0.3969 | Hα → Lα | |
| D→D | 334.2 | 3.710 | 0.0000 | Hβ → Lβ | |
| 1-Co3+ | S→T | 630.5 | 1.967 | 0.0000 | H → L |
| S→S | 425.8 | 2.912 | 0.3871 | H → L | |
| 1-Co2+-H+ | D→D | 436.7 | 2.839 | 0.0001 | Hβ → Sβ |
| D→D | 380.5 | 3.259 | 0.2684 | Sα → L+6α | |
| 1-Co3+-H+ | S→T | 605.7 | 2.047 | 0.0000 | H → L |
| S→S | 339.3 | 3.654 | 0.0171 | H → L | |
| S→S | 331.7 | 3.738 | 0.4204 | H-1 → L | |
| 1-Ni2+ | S→T | 783.6 | 1.582 | 0.0000 | H → L |
| S→S | 781.0 | 1.588 | 0.0006 | H → L | |
| S→S | 333.7 | 3.715 | 0.4337 | H-5 → L | |
| 1-Ni3+ | D→D | 565.5 | 2.193 | 0.0001 | Sα → Lα |
| D→D | 442.3 | 2.803 | 0.0000 | Hβ → Sβ | |
| D→D | 385.2 | 3.219 | 0.0004 | Hβ → Lβ | |
| D→D | 337.4 | 3.675 | 0.3463 | H-3α/β → L+1α/β | |
| 1-Ni2+-H+ | S→T | 834.0 | 1.487 | 0.0000 | H → L |
| S→S | 833.2 | 1.488 | 0.0001 | H → L | |
| S→S | 329.5 | 3.763 | 0.4245 | H-4 → L | |
| 1-Ni3+-H+ | D→D | 361.5 | 3.430 | 0.0001 | Hβ → Sβ |
| D→D | 339.9 | 3.648 | 0.0184 | Hβ → Lβ | |
| D→D | 332.0 | 3.735 | 0.2374 | H-1α → L+1α |
| Molecule | λ | ΔΕ | f | λ | ΔΕ | f | λ | ΔΕ | f | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PBE0 | TPSSh | wB97XD | |||||||||
| 1-Fe2+ | 333.5 | 3.718 | 0.426 | 353.5 | 3.508 | 0.234 | 308.9 | 4.013 | 0.503 | ||
| a | 333.5 | 3.717 | 0.425 | 353.9 | 3.503 | 0.274 | |||||
| 1-Fe3+ | 337.2 | 3.677 | 0.444 | 357.4 | 3.469 | 0.250 | 311.9 | 3.975 | 0.551 | ||
| 1-Fe2+-H+ | 329.2 | 3.766 | 0.407 | 348.5 | 3.557 | 0.365 | 305.3 | 4.062 | 0.484 | ||
| 1-Fe3+-H+ | 331.8 | 3.736 | 0.389 | 352.1 | 3.521 | 0.278 | 308.4 | 4.020 | 0.356 | ||
| 1-Co2+ | 414.9 | 2.988 | 0.397 | 461.2 | 2.689 | 0.277 | 362.5 | 3.421 | 0.517 | ||
| 1-Co3+ | 425.8 | 2.912 | 0.387 | 477.2 | 2.598 | 0.262 | 370.8 | 3.344 | 0.541 | ||
| a | 426.0 | 2.910 | 0.309 | 480.7 | 2.579 | 0.240 | |||||
| 1-Co2+-H+ | 380.5 | 3.259 | 0.268 | 348.3 | 3.560 | 0.339 | 370.3 | 3.349 | 0.510 | ||
| 1-Co3+-H+ | 331.7 | 3.738 | 0.420 | 352.4 | 3.518 | 0.336 | 307.8 | 4.028 | 0.516 | ||
| 1-Ni2+ | 333.7 | 3.715 | 0.434 | 353.7 | 3.506 | 0.187 | 309.2 | 4.010 | 0.533 | ||
| 1-Ni3+ | 337.4 | 3.675 | 0.346 | 359.9 | 3.445 | 0.124 | 311.6 | 3.979 | 0.370 | ||
| 1-Ni2+-H+ | 329.5 | 3.763 | 0.425 | 348.6 | 3.556 | 0.329 | 305.4 | 4.059 | 0.523 | ||
| 1-Ni3+-H+ | 332.0 | 3.735 | 0.237 | 352.2 | 3.520 | 0.279 | 308.0 | 4.026 | 0.501 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tzeliou, C.E.; Tzeli, D. Metallocene-Naphthalimide Derivatives: The Effect of Geometry, DFT Methodology, and Transition Metals on Absorption Spectra. Molecules 2023, 28, 3565. https://doi.org/10.3390/molecules28083565
Tzeliou CE, Tzeli D. Metallocene-Naphthalimide Derivatives: The Effect of Geometry, DFT Methodology, and Transition Metals on Absorption Spectra. Molecules. 2023; 28(8):3565. https://doi.org/10.3390/molecules28083565
Chicago/Turabian StyleTzeliou, Christina Eleftheria, and Demeter Tzeli. 2023. "Metallocene-Naphthalimide Derivatives: The Effect of Geometry, DFT Methodology, and Transition Metals on Absorption Spectra" Molecules 28, no. 8: 3565. https://doi.org/10.3390/molecules28083565
APA StyleTzeliou, C. E., & Tzeli, D. (2023). Metallocene-Naphthalimide Derivatives: The Effect of Geometry, DFT Methodology, and Transition Metals on Absorption Spectra. Molecules, 28(8), 3565. https://doi.org/10.3390/molecules28083565







