Potential Hypoglycemic and Antilipidemic Activity of Polyphenols from Passiflora ligularis (Granadilla)
Abstract
1. Introduction
2. Results
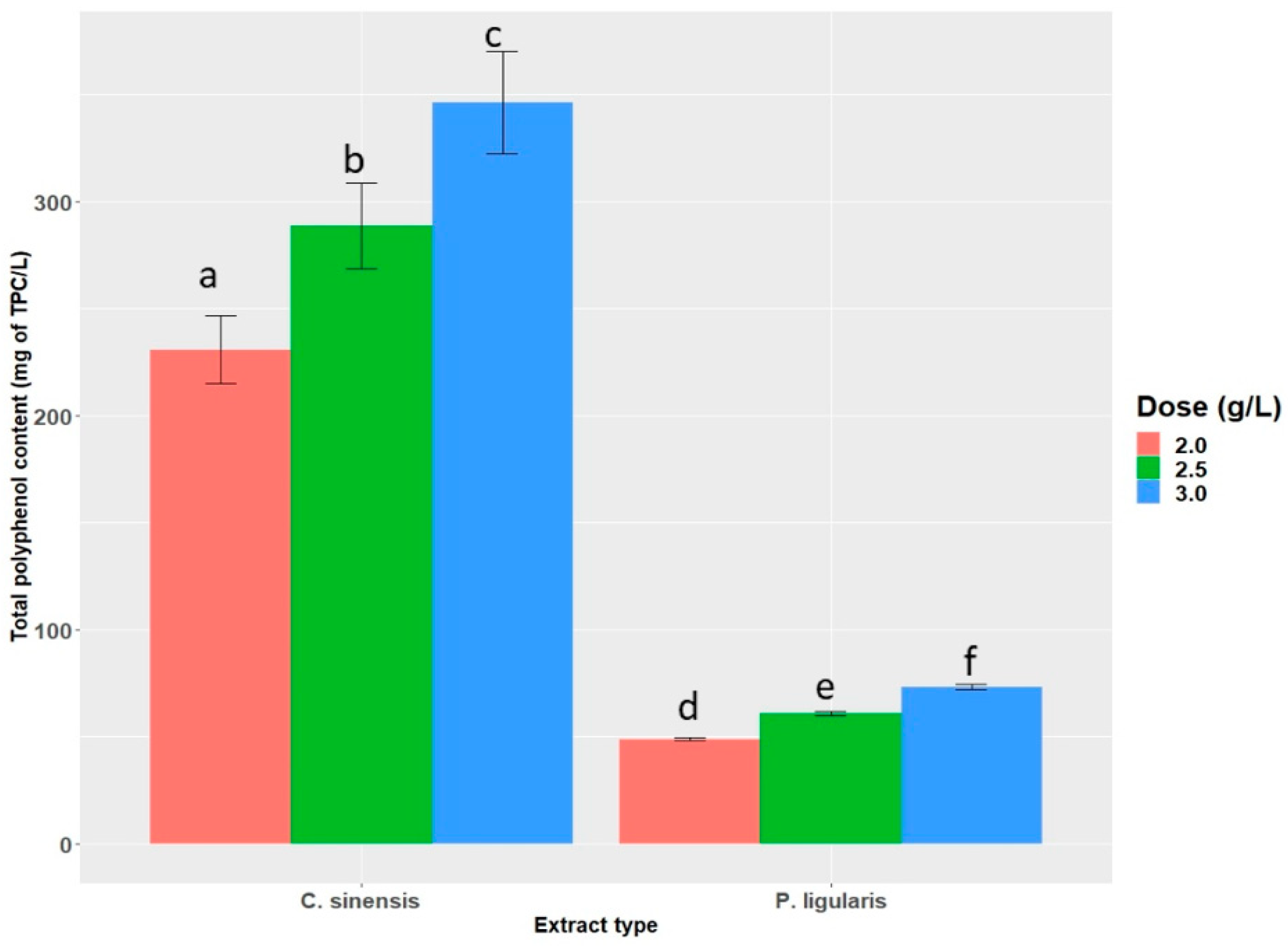
2.1. Total Polyphenol Content (TPC)
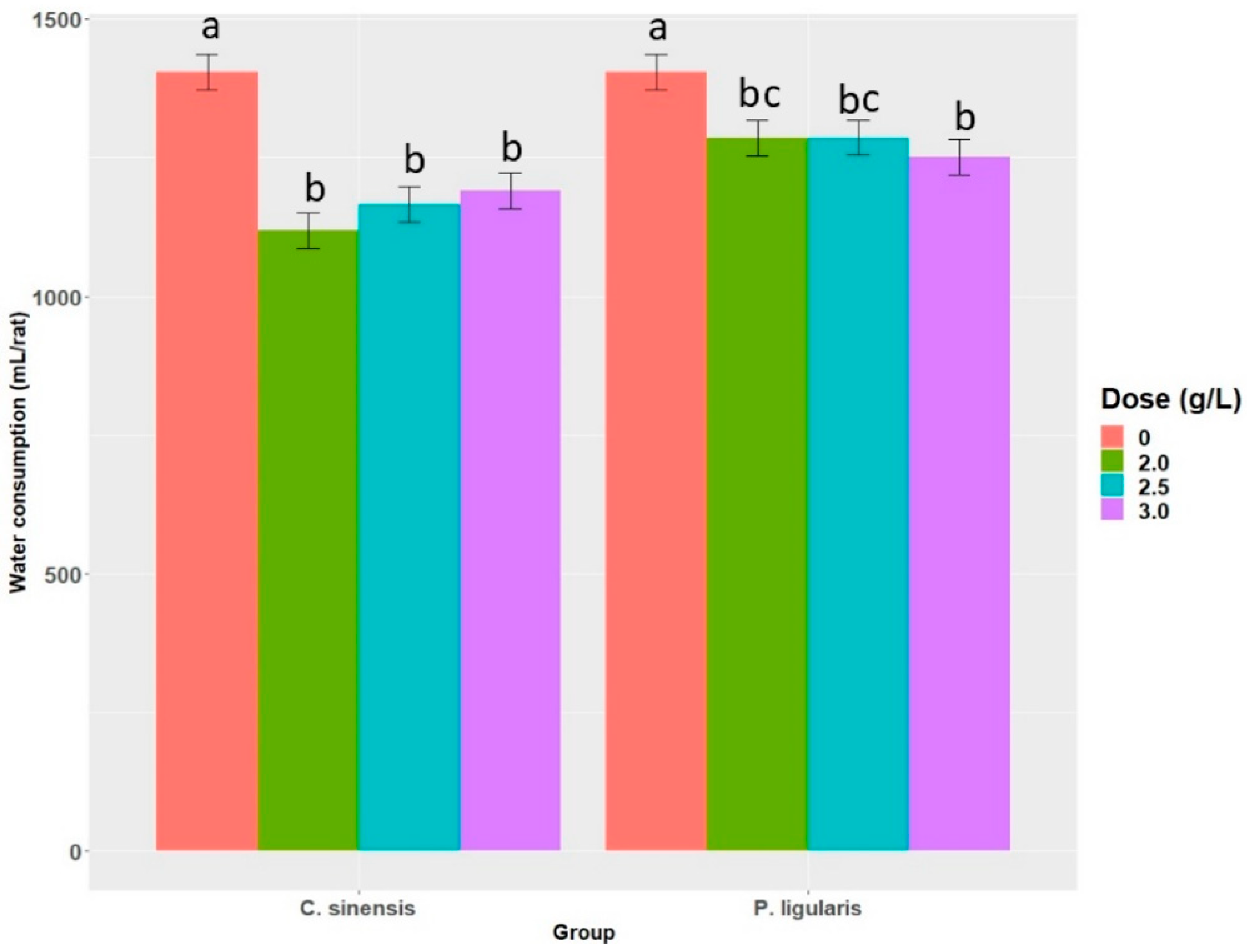
2.2. Water Consumption
2.3. Serum Glucose
2.4. Triglycerides
2.5. Cholesterol
2.6. Lipids in Feces
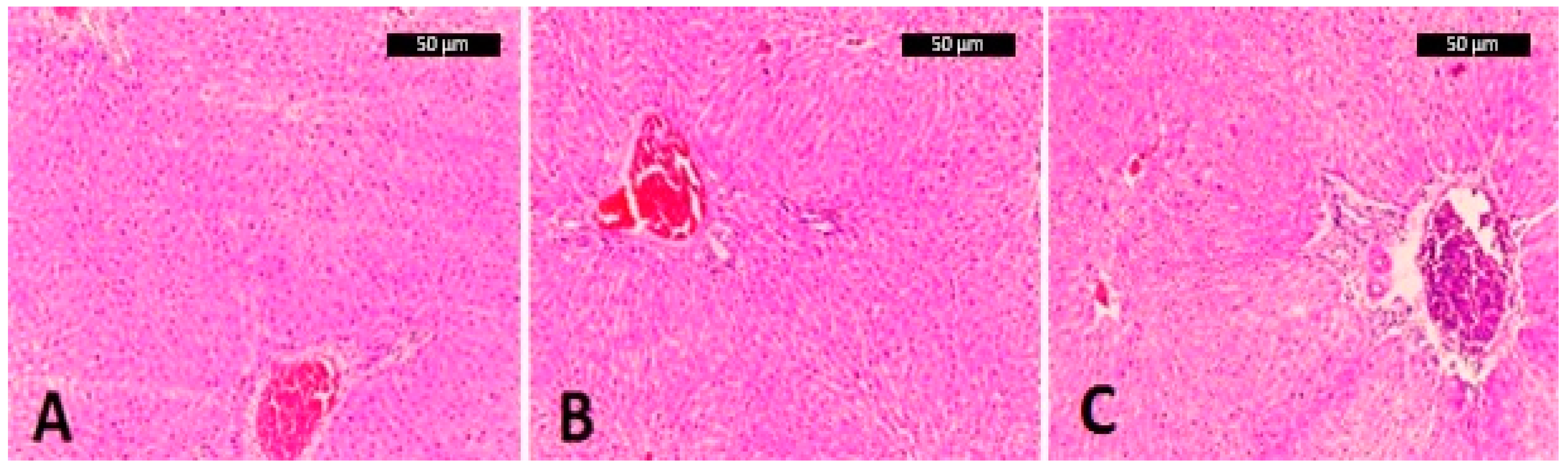
2.7. Liver Histology
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Polyphenol Extraction and Total Polyphenol Content (TPC)
4.3. Diets
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Kuriyama, S.; Shimazu, T.; Ohmori, K.; Kikuchi, N.; Nakaya, N.; Nishino, Y.; Tsubono, Y.; Tsuji, I. Green Tea Consumption and Mortality Due to Cardiovascular Disease, Cancer, and All Causes in Japan. JAMA 2006, 296, 1255–1265. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-S.; Quon, M.J.; Kim, J.-A. New insights into the mechanisms of polyphenols beyond antioxidant properties; lessons from the green tea polyphenol, epigallocatechin 3-gallate. Redox Biol. 2014, 2, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Angel-Isaza, J.; Carmona-Hernandez, J.C.; Narváez-Solarte, W.; Gonzalez-Correa, C.H. Polyphenols from Passiflora ligularis Regulate Inflammatory Markers and Weight Gain. Biomol. Concepts 2021, 12, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Carmona-Hernandez, J.C.; Taborda-Ocampo, G.; Valdez, J.C.; Bolling, B.W.; González-Correa, C.H. Polyphenol Extracts from Three Colombian Passifloras (Passion Fruits) Prevent Inflammation-Induced Barrier Dysfunction of Caco-2 Cells. Molecules 2019, 24, 4614. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Luo, M.; Wei, S. The Bioprotective Effects of Polyphenols on Metabolic Syndrome against Oxidative Stress: Evidences and Perspectives. Oxidative Med. Cell. Longev. 2019, 2019, 6713194. [Google Scholar] [CrossRef]
- Khalesi, S.; Sun, J.; Buys, N.; Jamshidi, A.; Nikbakht-Nasrabadi, E.; Khosravi-Boroujeni, H. Green tea catechins and blood pressure: A systematic review and meta-analysis of randomised controlled trials. Eur. J. Nutr. 2014, 53, 1299–1311. [Google Scholar] [CrossRef]
- Amiot, M.J.; Riva, C.; Vinet, A. Effects of dietary polyphenols on metabolic syndrome features in humans: A systematic review. Obes. Rev. 2016, 17, 573–586. [Google Scholar] [CrossRef]
- He, X.; Luan, F.; Yang, Y.; Wang, Z.; Zhao, Z.; Fang, J.; Wang, M.; Zuo, M.; Li, Y. Passiflora edulis: An Insight into Current Researches on Phytochemistry and Pharmacology. Front. Pharmacol. 2020, 11, 617. [Google Scholar] [CrossRef]
- Saravanan, S.; Parimelazhagan, T. In vitro antioxidant, antimicrobial and anti-diabetic properties of polyphenols of Passiflora ligularis Juss. fruit pulp. Food Sci. Hum. Wellness 2014, 3, 56–64. [Google Scholar] [CrossRef]
- Sabogal-Palma, A.C.; Chávez-Marin, J.; Oliveros-Gomez, D.; MurilloPerea, E.; Méndez-Arteaga, J.J. Biological functionalities of passiflora maliformis from the south colombian range of mountains. Bioagro 2016, 28, 3–12. [Google Scholar]
- DANE. National Agropecuarian Survey (Encuesta Nacional Agropecuaria ENA), Boletin Tecnico. Comunicación Informative; Departamento Administrativo Nacional de Estadística—DANE: Bogota, Colombia, 2016.
- Chaparro, D.C.; Maldonado, M.E.; Franco, M.C.; Urango, L.A. Nutritional and antioxidant characteristics of the curuba larga fruit (Passiflora mollisima Bailey). Perspect. Nutr. Humana 2015, 13, 120–128. [Google Scholar] [CrossRef]
- Carmo, M.C.L.D.; Martins, I.M.; Magalhães, A.E.R.; Maróstica Júnior, M.R.; Macedo, J.A. Passion fruit (Passiflora edulis) leaf aqueous extract ameliorates intestinal epithelial barrier dysfunction and reverts inflammatory parameters in Caco-2 cells monolayer. Food Res. Int. 2020, 133, 109162. [Google Scholar] [CrossRef] [PubMed]
- Ożarowski, M.; Karpiński, T.M. Extracts and Flavonoids of Passiflora Species as Promising Anti-inflammatory and Antioxidant Substances. Curr. Pharm. Des. 2021, 27, 2582–2604. [Google Scholar] [CrossRef] [PubMed]
- Feldman, F.; Koudoufio, M.; Desjardins, Y.; Spahis, S.; Delvin, E.; Levy, E. Efficacy of Polyphenols in the Management of Dyslipidemia: A Focus on Clinical Studies. Nutrients 2021, 13, 672. [Google Scholar] [CrossRef] [PubMed]
- Martins, G.R.; Monteiro, A.F.; Amaral, F.R.L.D.; da Silva, A.S. A validated Folin-Ciocalteu method for total phenolics quantification of condensed tannin-rich açaí (Euterpe oleracea Mart.) seeds extract. J. Food Sci. Technol. 2021, 58, 4693–4702. [Google Scholar] [CrossRef]
- Carmona-Hernandez, J.C.; Taborda-Ocampo, G.; González-Correa, C.H. Folin-Ciocalteu Reaction Alternatives for Higher Polyphenol Quantitation in Colombian Passion Fruits. Int. J. Food Sci. 2021, 2021, 8871301. [Google Scholar] [CrossRef] [PubMed]
- García-Casas, V.E.; Seiquer, I.; Pardo, Z.; Haro, A.; Recio, I.; Olías, R. Antioxidant Potential of the Sweet Whey-Based Beverage Colada after the Digestive Process and Relationships with the Lipid and Protein Fractions. Antioxidants 2022, 11, 1827. [Google Scholar] [CrossRef]
- Nakamura, Y.; Tonogai, Y. Effects of Grape Seed Polyphenols on Serum and Hepatic Lipid Contents and Fecal Steroid Excretion in Normal and Hypercholesterolemic Rats. J. Health Sci. 2002, 48, 570–578. [Google Scholar] [CrossRef]
- Mineo, S.; Noguchi, A.; Nagakura, Y.; Kobori, K.; Ohta, T.; Sakaguchi, E.; Ichiyanagi, T. Boysenberry Polyphenols Suppressed Elevation of Plasma Triglyceride Levels in Rats. J. Nutr. Sci. Vitaminol. 2015, 61, 306–312. [Google Scholar] [CrossRef]
- Yokozawa, T.; Nakagawa, T.; Kitani, K. Antioxidative Activity of Green Tea Polyphenol in Cholesterol-Fed Rats. J. Agric. Food Chem. 2002, 50, 3549–3552. [Google Scholar] [CrossRef]
- Raederstorff, D.G.; Schlachter, M.F.; Elste, V.; Weber, P. Effect of EGCG on lipid absorption and plasma lipid levels in rats. J. Nutr. Biochem. 2003, 14, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Kim, J.; Cheng, J.; Ong, M.; Lao, W.-G.; Jin, X.-L.; Lin, Y.-G.; Xiao, L.; Zhu, X.-Q.; Qu, X.-Q. Green tea polyphenols ameliorate non-alcoholic fatty liver disease through upregulating AMPK activation in high fat fed Zucker fatty rats. World J. Gastroenterol. 2017, 23, 3805–3814. [Google Scholar] [CrossRef]
- Bose, M.; Lambert, J.D.; Ju, J.; Reuhl, K.R.; Shapses, S.; Yang, C.S. The major green tea polyphenol, (-)-epigallocatechin-3-gallate, inhibits obesity, metabolic syndrome, and fatty liver disease in high-fat-fed mice. J. Nutr. 2008, 138, 1677–1683. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-K.; Cheung, C.; Reuhl, K.R.; Liu, A.B.; Lee, M.-J.; Lu, Y.-P.; Yang, C.S. Effects of Green Tea Polyphenol (−)-Epigallocatechin-3-gallate on Newly Developed High-Fat/Western-Style Diet-Induced Obesity and Metabolic Syndrome in Mice. J. Agric. Food Chem. 2011, 59, 11862–11871. [Google Scholar] [CrossRef] [PubMed]
- Sae-Tan, S.; Grove, K.A.; Lambert, J.D. Weight control and prevention of metabolic syndrome by green tea. Pharmacol. Res. 2011, 64, 146–154. [Google Scholar] [CrossRef]
- Son, M.J.; Rico, C.W.; Nam, S.H.; Kang, M.Y. Effect of Oryzanol and Ferulic Acid on the Glucose Metabolism of Mice Fed with a High-Fat Diet. J. Food Sci. 2011, 76, H7–H10. [Google Scholar] [CrossRef] [PubMed]
- Ibitoye, O.B.; Ajiboye, T.O. Dietary phenolic acids reverse insulin resistance, hyperglycaemia, dyslipidaemia, inflammation and oxidative stress in high-fructose diet-induced metabolic syndrome rats. Arch. Physiol. Biochem. 2017, 124, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.S.; Butt, M.S.; Sultan, M.T.; Mushtaq, Z.; Ahmad, S.; Dewanjee, S.; De Feo, V.; Zia-Ul-Haq, M. Preventive role of green tea catechins from obesity and related disorders especially hypercholesterolemia and hyperglycemia. J. Transl. Med. 2015, 13, 79. [Google Scholar] [CrossRef] [PubMed]
- Wang, O.; Liu, J.; Cheng, Q.; Guo, X.; Wang, Y.; Zhao, L.; Zhou, F.; Ji, B. Effects of Ferulic Acid and γ-Oryzanol on High-Fat and High-Fructose Diet-Induced Metabolic Syndrome in Rats. PLoS ONE 2015, 10, e0118135. [Google Scholar] [CrossRef] [PubMed]
- Koyama, Y.; Abe, K.; Sano, Y.; Ishizaki, Y.; Njelekela, M.; Shoji, Y.; Hara, Y.; Isemura, M. Effects of Green Tea on Gene Expression of Hepatic Gluconeogenic Enzymes in vivo. Planta Medica 2004, 70, 1100–1102. [Google Scholar] [CrossRef]
- Hurrle, S.; Hsu, W.H. The etiology of oxidative stress in insulin resistance. Biomed. J. 2017, 40, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Zhao, Y.; Suo, S.; Liu, Y.; Zhao, B. Green tea catechins ameliorate adipose insulin resistance by improving oxidative stress. Free. Radic. Biol. Med. 2012, 52, 1648–1657. [Google Scholar] [CrossRef] [PubMed]
- Brimson, J.M.; Prasanth, M.I.; Kumaree, K.K.; Thitilertdecha, P.; Malar, D.S.; Tencomnao, T.; Prasansuklab, A. Tea Plant (Camellia sinensis): A Current Update on Use in Diabetes, Obesity, and Cardiovascular Disease. Nutrients 2022, 15, 37. [Google Scholar] [CrossRef] [PubMed]
- Ansari, P.; Hannan, J.M.A.; Choudhury, S.T.; Islam, S.S.; Talukder, A.; Seidel, V.; Abdel-Wahab, Y.H.A. Antidiabetic Actions of Ethanol Extract of Camellia sinensis Leaf Ameliorates Insulin Secretion, Inhibits the DPP-IV Enzyme, Improves Glucose Tolerance, and Increases Active GLP-1 (7–36) Levels in High-Fat-Diet-Fed Rats. Medicines 2022, 9, 56. [Google Scholar] [CrossRef]
- Haidari, F.; Omidian, K.; Rafiei, H.; Zarei, M.; Shahi, M.M. Green Tea (Camellia sinensis) Supplementation to Diabetic Rats Improves Serum and Hepatic Oxidative Stress Markers. Iran J. Pharm. Res. 2013, 12, 109–114. [Google Scholar] [CrossRef]
- Paquette, M.; Larqué, A.S.M.; Weisnagel, S.J.; Desjardins, Y.; Marois, J.; Pilon, G.; Dudonné, S.; Marette, A.; Jacques, H. Strawberry and cranberry polyphenols improve insulin sensitivity in insulin-resistant, non-diabetic adults: A parallel, double-blind, controlled and randomised clinical trial. Br. J. Nutr. 2017, 117, 519–531. [Google Scholar] [CrossRef]
- Manzano, M.; Giron, M.D.; Vilchez, J.D.; Sevillano, N.; El-Azem, N.; Rueda, R.; Salto, R.; Lopez-Pedrosa, J.M. Apple polyphenol extract improves insulin sensitivity in vitro and in vivo in animal models of insulin resistance. Nutr. Metab. 2016, 13, 32. [Google Scholar] [CrossRef]
- Charan, J.; Kantharia, N.D. How to calculate sample size in animal studies? J. Pharmacol. Pharmacother. 2013, 4, 303–306. [Google Scholar] [CrossRef]
- Dell, R.B.; Holleran, S.; Ramakrishnan, R. Sample Size Determination. ILAR J. 2002, 43, 207–213. [Google Scholar] [CrossRef]
- Fenwick, N.; Griffin, G.; Gauthier, C. The welfare of animals used in science: How the “Three Rs” ethic guides improvements. Can. Vet. J. 2009, 50, 523–530. [Google Scholar]
- Pinzón, I.M.P.; Fischer, G.; Corredor, G. Determination of the maturity stages of the fruit of the gulupa (Passiflora edulis Sims). Agron. Colomb. 2007, 25, 83–95. [Google Scholar]
- Benevenga, N.J.; Calvert, C.; Eckhert, C.D.; Fahey, G.D.; Greger, J.L.; Keen, L.C.; Knapka, J.J.; Magalhaes, H.; Bucknell, O.; Olav, T.; et al. Nutrient requirements of the laboratory rat. In Nutrient Requirements of Laboratory Animals, 4th ed.; National Academies Press: Washington, VA, USA, 1995. [Google Scholar]
- Bresan Malafaia, A.; Nunes Nassif, P.A.; Marcondes Ribas, C.A.P.; Luiz Ariede, B.; Negume Sue, K.; Aguiar Cruiz, M. Obesity induction with sucrose in rats. Arq. Bras. Cir. Dig. 2013, 26, 17–21. [Google Scholar] [CrossRef]
- Helrich, K. Official Methods of Analysis, 15th ed.; Association of Official Analytical Chemists (AOAC): Arlington, VA, USA, 1990. [Google Scholar]
- National Research Council. Guide for the Care and Use of Laboratory Animals; The National Academies Press: Washington, DC, USA, 2001. [Google Scholar]
- Brenes-Pino, F. Histopathology of the liver biopsy, clinical approach. AMC 2008, 50, 19–22. [Google Scholar]

| Group | Dose (mL/L) | Cholesterol | Triglycerides | Glucose |
|---|---|---|---|---|
| (mg/dL) | (mg/dL) | (mg/dL) | ||
| Control | 0.0 | 106.5 ± 9.6 | 119.0 ± 3.7 b | 187.0 ± 42.8 b |
| Passiflora ligularis | 2.0 | 111.5 ± 6.4 | 112.5 ± 16.0 b | 144.7 ± 23.2 ab |
| 2.5 | 110.5 ± 14.7 | 111.5 ± 12.4 b | 123.2 ± 18.6 a | |
| 3.0 | 98.3 ± 10.8 | 98.3 ± 57 a | 122.2 ± 33.3 a | |
| Camellia sinensis | 2.0 | 108.5 ± 13.7 | 115.2 ± 13.8 b | 163.7 ± 21.7 b |
| 2.5 | 109.7 ± 14.1 | 109.7 ± 6.1 b | 165.5 ± 32.3 b | |
| 3.0 | 112.2 ± 11.6 | 98.5 ± 3.1 a | 156.7 ± 17.7 b |
| Group | Dose (mL/L) | Lipids (%) |
|---|---|---|
| Control | 0.0 | 2.45 ± 0.02 a |
| Passiflora ligularis | 2.0 | 2.56 ± 0.03 b |
| 2.5 | 2.57 ± 0.04 b | |
| 3.0 | 2.57 ± 0.03 b | |
| Camellia sinensis | 2.0 | 2.55 ± 0.01 b |
| 2.5 | 2.55 ± 0.01 b | |
| 3.0 | 2.56 ± 0.03 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Angel-Isaza, J.; Carmona-Hernandez, J.C.; González-Correa, C.H.; Narváez-Solarte, W.V. Potential Hypoglycemic and Antilipidemic Activity of Polyphenols from Passiflora ligularis (Granadilla). Molecules 2023, 28, 3551. https://doi.org/10.3390/molecules28083551
Angel-Isaza J, Carmona-Hernandez JC, González-Correa CH, Narváez-Solarte WV. Potential Hypoglycemic and Antilipidemic Activity of Polyphenols from Passiflora ligularis (Granadilla). Molecules. 2023; 28(8):3551. https://doi.org/10.3390/molecules28083551
Chicago/Turabian StyleAngel-Isaza, Jaime, Juan Carlos Carmona-Hernandez, Clara Helena González-Correa, and William Vicente Narváez-Solarte. 2023. "Potential Hypoglycemic and Antilipidemic Activity of Polyphenols from Passiflora ligularis (Granadilla)" Molecules 28, no. 8: 3551. https://doi.org/10.3390/molecules28083551
APA StyleAngel-Isaza, J., Carmona-Hernandez, J. C., González-Correa, C. H., & Narváez-Solarte, W. V. (2023). Potential Hypoglycemic and Antilipidemic Activity of Polyphenols from Passiflora ligularis (Granadilla). Molecules, 28(8), 3551. https://doi.org/10.3390/molecules28083551






