In Vitro and In Silico Investigation of Polyacetylenes from Launaea capitata (Spreng.) Dandy as Potential COX-2, 5-LOX, and BchE Inhibitors
Abstract
1. Introduction
2. Results and Discussion
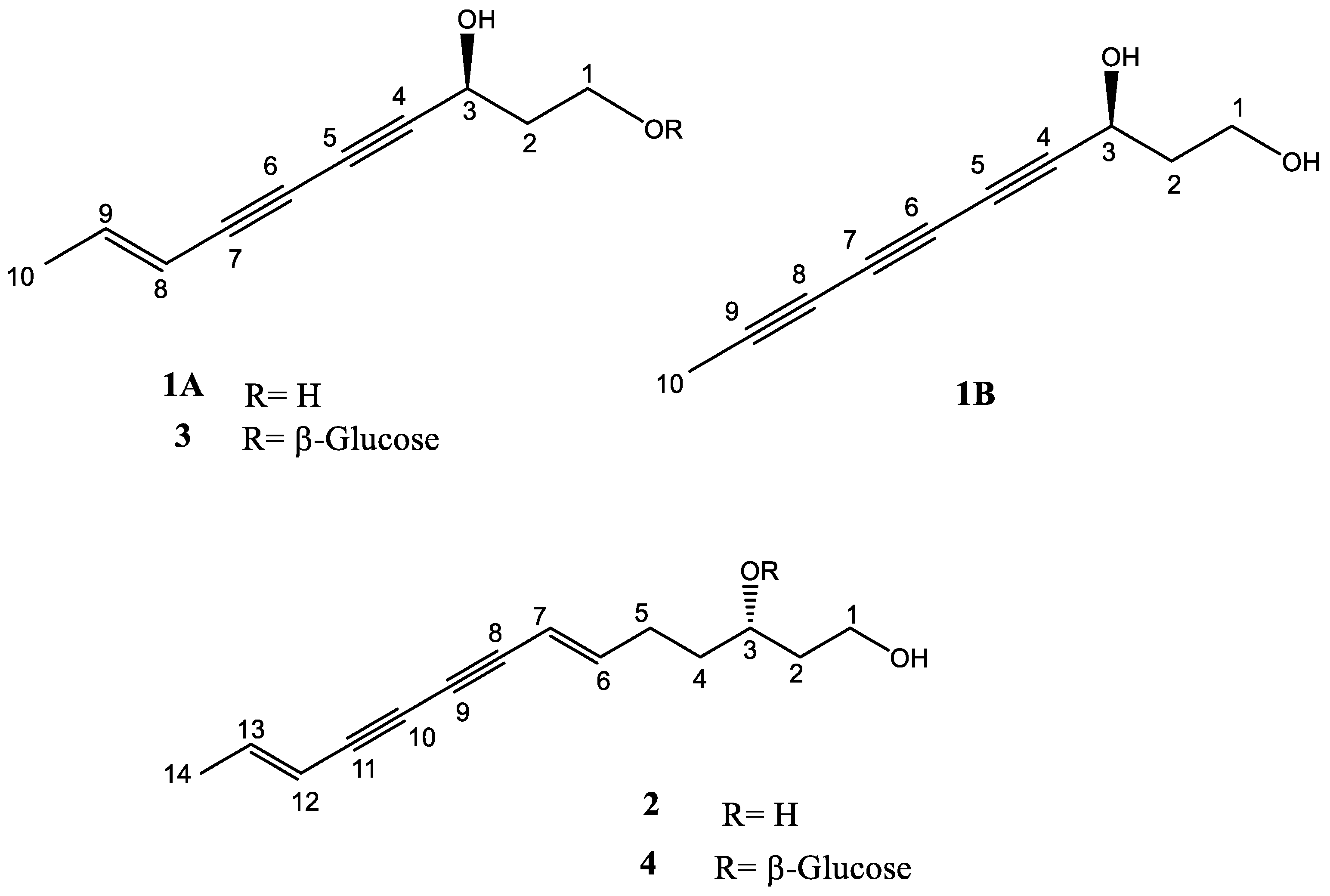
2.1. Identification of the Isolated Compounds
2.1.1. Identification of Compound 1A and 1B
2.1.2. Identification of Compound 2
2.1.3. Identification of Compound 3
2.1.4. Identification of Compound 4
2.2. In Vitro Enzyme Inhibition Assays
2.3. Docking Study
3. Materials and Methods
3.1. Plant Material
3.2. Chemicals and Instruments
3.3. Extraction and Purification
3.4. Enzyme Inhibition Assays
3.4.1. BchE Inhibition Assay
3.4.2. COX-2 Inhibition Assay
3.4.3. 5-LOX Inhibition Assay
3.5. Statistical Analysis
3.6. Docking Study
4. Limitation of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Orhan, I.E.; Banach, M.; Rollinger, J.M.; Barreca, D.; Weckwerth, W.; Bauer, R.; Bayer, E.A.; et al. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Perry, E.K.; Pickering, A.T.; Wang, W.W.; Houghton, P.J.; Perry, N.S. Medicinal plants and Alzheimer’s disease: From ethnobotany to phytotherapy. J. Pharm. Pharmacol. 1999, 51, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Morgan, A.R.; Touchard, S.; Leckey, C.; O’Hagan, C.; Nevado-Holgado, A.J.; Barkhof, F.; Bertram, L.; Blin, O.; Bos, I.; Dobricic, V.; et al. Inflammatory biomarkers in Alzheimer’s disease plasma. Alzheimer’s Dement. 2019, 15, 776–787. [Google Scholar] [CrossRef]
- Roy, A. Role of medicinal plants against Alzheimer’s disease. Int. J. Complement. Altern. Med. 2018, 11, 205–208. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Gan, R.Y.; Li, S.; Zhou, Y.; Li, A.N.; Xu, D.P.; Li, H.B. Antioxidant Phytochemicals for the Prevention and Treatment of Chronic Diseases. Molecules 2015, 20, 21138–21156. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Rapposelli, S.; Sestito, S.; Herrera-Bravo, J.; Arancibia-Diaz, A.; Salazar, L.A.; Yeskaliyeva, B.; Beyatli, A.; Leyva-Gómez, G.; González-Contreras, C.; et al. Multi-Target Mechanisms of Phytochemicals in Alzheimer’s Disease: Effects on Oxidative Stress, Neuroinflammation and Protein Aggregation. J. Pers. Med. 2022, 12, 1515. [Google Scholar] [CrossRef]
- Rubio-Perez, J.M.; Morillas-Ruiz, J.M. A review: Inflammatory process in Alzheimer’s disease, role of cytokines. Sci. World J. 2012, 2012, 756357. [Google Scholar] [CrossRef] [PubMed]
- McGeer, P.L.; McGeer, E.G. The inflammatory response system of brain: Implications for therapy of Alzheimer and other neurodegenerative diseases. Brain Res. Rev. 1995, 21, 195–218. [Google Scholar] [CrossRef]
- Fu, Z.-X.I.; Jiao, B.-H.; Nie, B.; Zhang, G.; Gao, T. A comprehensive generic-level phylogeny of the sunflower family: Implications for the systematics of Chinese Asteraceae. J. Syst. Evol. 2016, 54, 416–437. [Google Scholar] [CrossRef]
- Soković, M.; Skaltsa, H.; Ferreira, I. Editorial: Bioactive Phytochemicals in Asteraceae: Structure, Function, and Biological Activity. Front. Plant Sci. 2019, 10, 1464. [Google Scholar] [CrossRef]
- Marzouk, R.I.; El-Darier, S.M.; Kamal, S.A.; Nour, I.H. Comparative Taxonomic Study of Launaea Cass. (Asteraceae, Cichorioideae) in Egypt. Taxonomy. Taxonomy 2021, 1, 192–209. [Google Scholar] [CrossRef]
- Cheriti, A.; Belboukhari, M.; Belboukhari, N.N.B.; Djeradi, H.H.D. Phytochemical and biological studies on Launaea Cass. Genus (Asteracea) from Algerian sahara. Curr. Top. Phytochem. 2012, 11, 67–80. [Google Scholar]
- Mansour, R.M.A.; Ahmed, A.A.; Saleh, N.A.M. Flavone glycosides of some Launaea species. Phytochemistry 1983, 22, 2630–2631. [Google Scholar] [CrossRef]
- Khalil, H.E.; Aldakheel, T.S.; AlAhmed, A.; Emeka, P.M.; Kandeel, M. Anti-proliferative activity of leaves of Launaea capitata Asteraceae: Phytochemical, cytotoxicity and in silico studies. Trop. J. Pharm. Res. 2020, 19, 2129–2136. [Google Scholar] [CrossRef]
- Elsharkawy, E.R. Isolation of phytoconstituents and evaluation of anticancer and Antioxidant potential of Launaea mucronata (Forssk.) Muschl. subsp. Pak. J. Pharm. Sci. 2017, 30, 399–405. [Google Scholar] [PubMed]
- Emad, F.; Khalafalah, A.K.; El Sayed, M.A.; Mohamed, A.H.; Stadler, M.; Helaly, S.E. Three new polyacetylene glycosides (PAGs) from the aerial part of Launaea capitata (Asteraceae) with anti-biofilm activity against Staphylococcus aureus. Fitoterapia 2020, 143, 104548. [Google Scholar] [CrossRef]
- Saleem, M.; Parveen, S.; Riaz, N.; Tahir, M.N.; Ashraf, M.; Afzal, I.; Ali, M.S.; Malik, A.; Jabbar, A. New bioactive natural products from Launaea nudicaulis. Phytochem. Lett. 2012, 5, 793–799. [Google Scholar] [CrossRef]
- Abdel Bar, F.M.; Sherif, A.E.; ElNaggar, M.H. Galactolipids from Launaea capitata (Spreng.) Dandy with In Vitro Anti-Inflammatory and Neuroprotective Activities. Separations 2023, 10, 83. [Google Scholar] [CrossRef]
- Jung, H.J.; Hung, T.M.; Na, M.K.; Min, B.S.; Kwon, B.M.; Bae, K.H. ACAT inhibition of polyacetylenes from Gymnaster koraiensis. Nat. Prod. Sci. 2009, 15, 110–113. [Google Scholar]
- Jung, H.J.; Min, B.S.; Park, J.Y.; Kim, Y.H.; Lee, H.K.; Bae, K.H. Gymnasterkoreaynes A-F, cytotoxic polyacetylenes from Gymnaster koraiensis. J. Nat. Prod. 2002, 65, 897–901. [Google Scholar] [CrossRef]
- Wang, N.; Yao, X.; Ishii, R.; Kitanaka, S. Antiallergic agents from natural sources. 3. Structures and inhibitory effects on nitric oxide production and histamine release of five novel polyacetylene glucosides from Bidens parviflora WILLD. Chem. Pharm. Bull. 2001, 49, 938–942. [Google Scholar] [CrossRef]
- Minto, R.E.; Blacklock, B.J. Biosynthesis and function of polyacetylenes and allied natural products. Prog. Lipid Res. 2008, 47, 233–306. [Google Scholar] [CrossRef] [PubMed]
- Geng, C.A.; Yang, T.H.; Huang, X.Y.; Yang, J.; Ma, Y.B.; Li, T.Z.; Zhang, X.M.; Chen, J.J. Anti-hepatitis B virus effects of the traditional Chinese herb Artemisia capillaris and its active enynes. J. Ethnopharmacol. 2018, 224, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Bentley, R.K.; Jones, E.R.H.; Thaller, V. Natural acetylenes. Part XXX. Polyacetylenes from Lactuca (lettuce) species of the liguliflorae sub-family of the compositae. J. Chem. Soc. C Org. 1969, 7, 1096–1099. [Google Scholar] [CrossRef]
- He, J.; Shen, Y.; Jiang, J.-S.; Yang, Y.-N.; Feng, Z.-M.; Zhang, P.-C.; Yuan, S.-P.; Hou, Q. New polyacetylene glucosides from the florets of Carthamus tinctorius and their weak anti-inflammatory activities. Carbohydr. Res. 2011, 346, 1903–1908. [Google Scholar] [CrossRef]
- He, J.; Chen, Z.; Yang, Y.N.; Jiang, J.S.; Feng, Z.M.; Zhang, P.C. Chemical constituents from aqueous extract of Carthamus tinctorius. Chin. Pharm. J. 2014, 49, 455–458. [Google Scholar] [CrossRef]
- Gu, W.-J.D. Polyacetylenes from capitulum of Coreopsis tinctoria. Zhongcaoyao 2016, 47, 1834–1837. [Google Scholar]
- Li, L.B.; Xiao, G.D.; Xiang, W.; Yang, X.; Cao, K.X.; Huang, R.S. Novel substituted thiophenes and sulf-polyacetylene ester from Echinops ritro L. Molecules 2019, 24, 805. [Google Scholar] [CrossRef]
- Xu, K.; Feng, Z.M.; Yang, Y.N.; Jiang, J.S.; Zhang, P.C. Eight new eudesmane- and eremophilane-type sesquiterpenoids from Atractylodes lancea. Fitoterapia 2016, 114, 115–121. [Google Scholar] [CrossRef]
- Resch, M.; Heilmann, J.; Steigel, A.; Bauer, R. Further phenols and polyacetylenes from the rhizomes of Atractylodes lancea and their anti-inflammatory activity. Planta Med. 2001, 67, 437–442. [Google Scholar] [CrossRef]
- Murata, K.; Iida, D.; Ueno, Y.; Samukawa, K.; Ishizaka, T.; Kotake, T.; Matsuda, H. Novel polyacetylene derivatives and their inhibitory activities on acetylcholinesterase obtained from Panax ginseng roots. J. Nat. Med. 2017, 71, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Fu, A.; Zhang, L. Progress in molecular docking. Quant. Biol. 2019, 7, 83–89. [Google Scholar] [CrossRef]
- Pinzi, L.; Rastelli, G. Molecular docking: Shifting paradigms in drug discovery. Int. J. Mol. Sci. 2019, 20, 4331. [Google Scholar] [CrossRef]
- Rouzer, C.A.; Marnett, L.J. Structural and chemical biology of the interaction of cyclooxygenase with substrates and non-steroidal anti-inflammatory drugs. Chem. Rev. 2020, 120, 7592–7641. [Google Scholar] [CrossRef] [PubMed]
- Orlando, B.J.; Malkowski, M.G. Substrate-selective Inhibition of Cyclooxygeanse-2 by Fenamic Acid Derivatives Is Dependent on Peroxide Tone. J. Biol. Chem. 2016, 291, 15069–15081. [Google Scholar] [CrossRef]
- Hsu, K.-C.; HuangFu, W.-C.; Lin, T.E.; Chao, M.-W.; Sung, T.-Y.; Chen, Y.-Y.; Pan, S.-L.; Lee, J.-C.; Tzou, S.-C.; Sun, C.-M.; et al. A site-moiety map and virtual screening approach for discovery of novel 5-LOX inhibitors. Sci. Rep. 2020, 10, 10510. [Google Scholar] [CrossRef] [PubMed]
- Saura, P.; Maréchal, J.D.; Masgrau, L.; Lluch, J.M.; González-Lafont, À. Computational insight into the catalytic implication of head/tail-first orientation of arachidonic acid in human 5-lipoxygenase: Consequences for the positional specificity of oxygenation. Phys. Chem. Chem. Phys. 2016, 18, 23017–23035. [Google Scholar] [CrossRef]
- Rosenberry, T.L.; Brazzolotto, X.; Macdonald, I.R.; Wandhammer, M.; Trovaslet-Leroy, M.; Darvesh, S.; Nachon, F. Comparison of the Binding of Reversible Inhibitors to Human Butyrylcholinesterase and Acetylcholinesterase: A Crystallographic, Kinetic and Calorimetric Study. Molecules 2017, 22, 2098. [Google Scholar] [CrossRef]
- Kara, J.; Suwanhom, P.; Wattanapiromsakul, C.; Nualnoi, T.; Puripattanavong, J.; Khongkow, P.; Lee, V.S.; Gaurav, A.; Lomlim, L. Synthesis of 2-(2-oxo-2H-chromen-4-yl)acetamides as potent acetylcholinesterase inhibitors and molecular insights into binding interactions. Arch. Pharm. 2019, 352, 1800310. [Google Scholar] [CrossRef]
- Rahim, F.; Ullah, H.; Taha, M.; Hussain, R.; Sarfraz, M.; Iqbal, R.; Iqbal, N.; Khan, S.; Ali Shah, S.A.; Albalawi, M.A.; et al. Synthesis of New Triazole-Based Thiosemicarbazone Derivatives as Anti-Alzheimer’s Disease Candidates: Evidence-Based In Vitro Study. Molecules 2022, 28, 21. [Google Scholar] [CrossRef]
- Obregon, A.D.; Schetinger, M.R.; Correa, M.M.; Morsch, V.M.; da Silva, J.E.; Martins, M.A.; Bonacorso, H.G.; Zanatta, N. Effects per se of organic solvents in the cerebral acetylcholinesterase of rats. Neurochem. Res. 2005, 30, 379–384. [Google Scholar] [CrossRef]
- Atatreh, N.; Al Rawashdah, S.; Al Neyadi, S.S.; Abuhamdah, S.M.; Ghattas, M.A. Discovery of new butyrylcholinesterase inhibitors via structure-based virtual screening. J. Enzyme Inhib. Med. Chem. 2019, 34, 1373–1379. [Google Scholar] [CrossRef]
- Lee, C.; Liao, J.; Chen, S.; Yen, C.; Lee, Y.; Huang, S.; Huang, S.; Lin, C.; Chang, V.H. Fluorine-Modified Rutaecarpine Exerts Cyclooxygenase-2 Inhibition and Anti-inflammatory Effects in Lungs. Front. Pharmacol. 2019, 10, 91. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.H.; Cho, D.Y.; Choi, S.R.; Lee, J.Y.; Choi, D.K.; Kim, E.; Park, J.Y. Synthesis and biological evaluation of salicylic acid analogues of celecoxib as a new class of selective cyclooxygenase-1 inhibitor. Biol. Pharm. Bull. 2021, 44, 1230–1238. [Google Scholar] [CrossRef] [PubMed]
- Yarla, N.S.; Pathuri, G.; Gali, H.; Terzyan, S.; Panneerselvam, J.; Chandrakesan, P.; Scotti, M.T.; Houchen, C.; Madka, V.; Rao, C.V. Discovery and Development of a Novel mPGES-1/5-LOX Dual Inhibitor LFA-9 for Prevention and Treatment of Chronic Inflammatory Diseases. J. Inflamm. Res. 2020, 13, 1261–1278. [Google Scholar] [CrossRef] [PubMed]
- Shaaban, M.; Kamal, A.; Faggal, S.; Farag, N.; Aborehab, N.; El-Sahar, A.; Mohamed, K. Design, synthesis, and biological evaluation of new pyrazoloquinazoline derivatives as dual COX-2/5-LOX inhibitors. Archiv. Pharm. 2020, 353, 2000027. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Eberhardt, J.; Santos-Martins, D.; Tillack, A.F.; Forli, S. AutoDock Vina 1.2.0: New Docking Methods, Expanded Force Field, and Python Bindings. J. Chem. Inf. Model. 2021, 61, 3891–3898. [Google Scholar] [CrossRef]
- DeLano, W.L. Pymol: An open-source molecular graphics tool. CCP4 Newsl. Protein Crystallogr. 2002, 40, 82–92. [Google Scholar]

| C/H Position | 1A | 1B | ||
|---|---|---|---|---|
| 13C | 1H | 13C | 1H | |
| 1 | 59.1, CH2 | 3.66, m a | 58.9, CH2 | 3.66, m a |
| 2 | 41.3, CH2 | 1.86, m b | 41.2, CH2 | 1.86, m b |
| 3 | 60.2, CH | 4.58, t (6.8) c | 60.1, CH | 4.55, t (6.8) c |
| 4 | 83.5, C | --- | 79.2, C | --- |
| 5 | 69.70, C | --- | 69.7, C | --- |
| 6 | 72.4, C | --- | 64.9, C | --- |
| 7 | 78.1, C | --- | 59.0, C | --- |
| 8 | 110.5, CH | 5.59, d (15.8) | 64.5, C | --- |
| 9 | 145.1, CH | 6.31, dq (6.9, 15.8) | 78.0, C | --- |
| 10 | 18.9, CH3 | 1.82, dd (6.9, 1.6) | 3.81, C | 1.99, s |
| C/H Position | 2 | 3 | 4 | |||
|---|---|---|---|---|---|---|
| 13C | 1H | 13C | 1H | |||
| 1 | 60.1, CH2 | 3.70, t (6.3) | 66.6, CH2 | Ha: 3.99, dd (10.0, 5.2) Hb: 3.72, m | 59.5, CH2 | Ha: 3.66, m Hb: 3.76, m |
| 2 | 40.8, CH2 | 1.61, m | 38.6, CH2 | 1.79, brd (5.2) | 38.2, CH2 | 1.75, m |
| 3 | 69.1, CH | 3.67, m | 59.9, CH | 4.66, t (6.2) | 77.9, CH | 3.89, m |
| 4 | 37.5, CH2 | 1.51, m | 83.5, C | --- | 35.4, CH2 | Ha: 1.71, m Hb: 1.65, m |
| 5 | 30.5, CH2 | 2.20, m 2.28, m | 69.9, C | --- | 30.1, CH2 | 2.30, dd (14.5, 7.2) |
| 6 | 149.2, CH | 6.30, m b | 72.3, C | --- | 149.7, CH | 6.32, m |
| 7 | 109.2, CH | 5.63, d (15.7) a | 78.1, C | --- | 109.8, CH | 5.64, d (16.7) |
| 8 | 80.4, C | --- | 110.2, CH | 5.5, d (15.8) | 80.6, C | --- |
| 9 | 73.1, C | --- | 145.2, CH | 6.32, dd (15.7, 7.0) | 73.2, C | --- |
| 10 | 73.4, C | --- | 19.0, CH3 | 1.82, d (6.3) | 73.3, C | --- |
| 11 | 80.3, C | --- | 80.3, C | --- | ||
| 12 | 110.8, CH | 5.61, d (15.7) a | 110.9, CH | 5.61, d (16.7) | ||
| 13 | 144.5, CH | 6.28, m b | 144.4, CH | 6.28, m | ||
| 14 | 18.9, CH3 | 1.83, d (6.8) | 18.9, CH3 | 1.82, d (6.8) | ||
| 1` | 104.1, CH | 4.30, d (7.7) | 103.9, CH | 4.36, d (7.8) | ||
| 2` | 74.7, CH | 3.19, t (8.2) | 75.3, CH | 3.16, m | ||
| 3` | 77.6, CH | 3.40, m | 78.1, CH | 3.34, m | ||
| 4` | 71.1, CH | 3.34, m | 71.6, CH | 3.22, m | ||
| 5` | 77.4, CH | 3.30, m | 77.7, CH | 3.26, m | ||
| 6` | 62.3, CH2 | Ha: 3.87, d (11.5) Hb: 3.70, m | 62.7, CH2 | Ha: 3.87, m Hb: 3.71, d (5.2) | ||
| Sample Code | COX-2 | 5-LOX | BchE |
|---|---|---|---|
| 1A/1B | 170.48 ± 20.15 | >200 | 58.60 ± 5.21 |
| 2 | >200 | >200 | 179.02 ± 15.62 |
| 3 | >200 | 80.96 ± 5.79 | 48.81 ± 6.34 |
| 4 | 146.38 ± 7.70 | 34.59 ± 4.26 | 14.77 ± 1.55 |
| NDGA * | 4.70 ± 0.76 | 5.65 ± 0.89 | --- |
| Rivastigmine | --- | --- | 14.06 ± 1.48 |
| Donepezil | --- | --- | 5.77 ± 0.61 |
| Compound | Binding Energy (kcal/mol) | ||
|---|---|---|---|
| Cyclooxygenase-II (COX-2) | 5-Lipooxygenase (5-LOX) | Butyrylcholinesterase (BchE) | |
| 1A | −5.263 | −5.602 | −5.399 |
| 1B | −5.179 | −5.838 | −5.17 |
| 1A/1B (simultaneous docking) | −7.861 | −8.703 | −8.549 |
| 2 | −4.983 | −6.422 | −5.525 |
| 3 | −6.987 | −8.032 | −7.018 |
| 4 | −6.895 | −8.132 | −7.305 |
| Cocrystallized ligand | −8.004 | −6.218 | −8.049 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdel Bar, F.M.; Mira, A.; Foudah, A.I.; Alossaimi, M.A.; Alkanhal, S.F.; Aldaej, A.M.; ElNaggar, M.H. In Vitro and In Silico Investigation of Polyacetylenes from Launaea capitata (Spreng.) Dandy as Potential COX-2, 5-LOX, and BchE Inhibitors. Molecules 2023, 28, 3526. https://doi.org/10.3390/molecules28083526
Abdel Bar FM, Mira A, Foudah AI, Alossaimi MA, Alkanhal SF, Aldaej AM, ElNaggar MH. In Vitro and In Silico Investigation of Polyacetylenes from Launaea capitata (Spreng.) Dandy as Potential COX-2, 5-LOX, and BchE Inhibitors. Molecules. 2023; 28(8):3526. https://doi.org/10.3390/molecules28083526
Chicago/Turabian StyleAbdel Bar, Fatma M., Amira Mira, Ahmed I. Foudah, Manal A. Alossaimi, Shatha F. Alkanhal, Alanoud M. Aldaej, and Mai H. ElNaggar. 2023. "In Vitro and In Silico Investigation of Polyacetylenes from Launaea capitata (Spreng.) Dandy as Potential COX-2, 5-LOX, and BchE Inhibitors" Molecules 28, no. 8: 3526. https://doi.org/10.3390/molecules28083526
APA StyleAbdel Bar, F. M., Mira, A., Foudah, A. I., Alossaimi, M. A., Alkanhal, S. F., Aldaej, A. M., & ElNaggar, M. H. (2023). In Vitro and In Silico Investigation of Polyacetylenes from Launaea capitata (Spreng.) Dandy as Potential COX-2, 5-LOX, and BchE Inhibitors. Molecules, 28(8), 3526. https://doi.org/10.3390/molecules28083526









