A Novel Peptide Reagent for Investigating Disulfide-Coupled Folding Intermediates of Mid-Size Proteins
Abstract
1. Introduction
2. Results
2.1. Preparation of the Peptide Labeling Reagent, Maleimidohexanoyl-Arg5-Tyr-NH2 (Male-Arg5-Tyr-NH2)
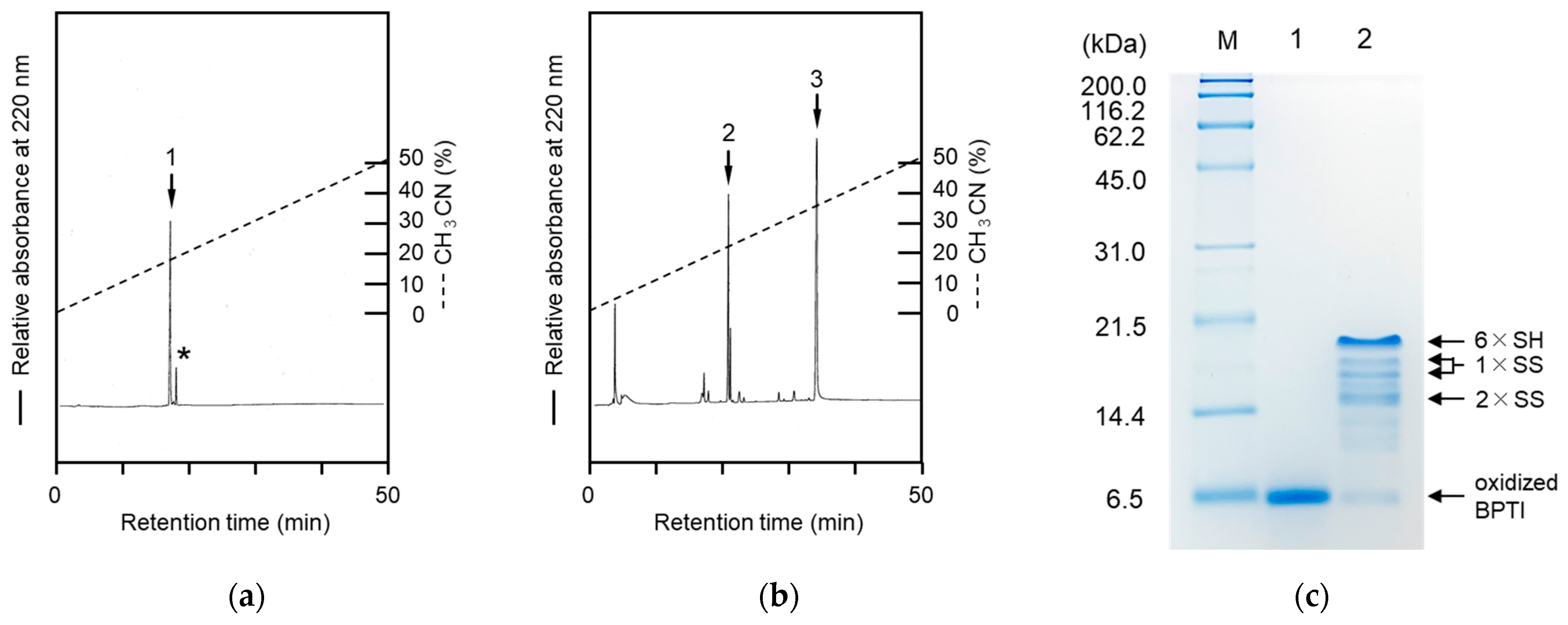
2.2. Tricine SDS-PAGE Analyses of the Labeled Folding Intermediates of BPTI Using the Male-Arg5-Tyr-NH2 Reagent
2.3. Labeling Reaction of the Folding Intermediates of BPTI Using the Male-Arg5-Tyr-NH2 Reagent
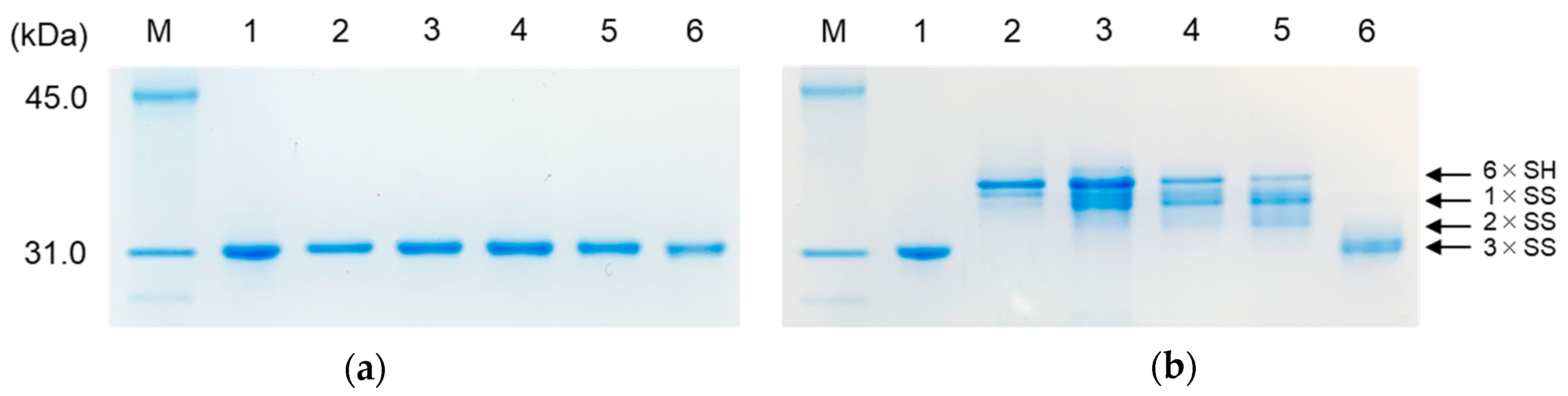
2.4. SDS-PAGE Analyses of the Labeled Folding Intermediates of Prococoonase ([K8D]-proCCN′) Using the Male-Arg5-Tyr-NH2 Reagent
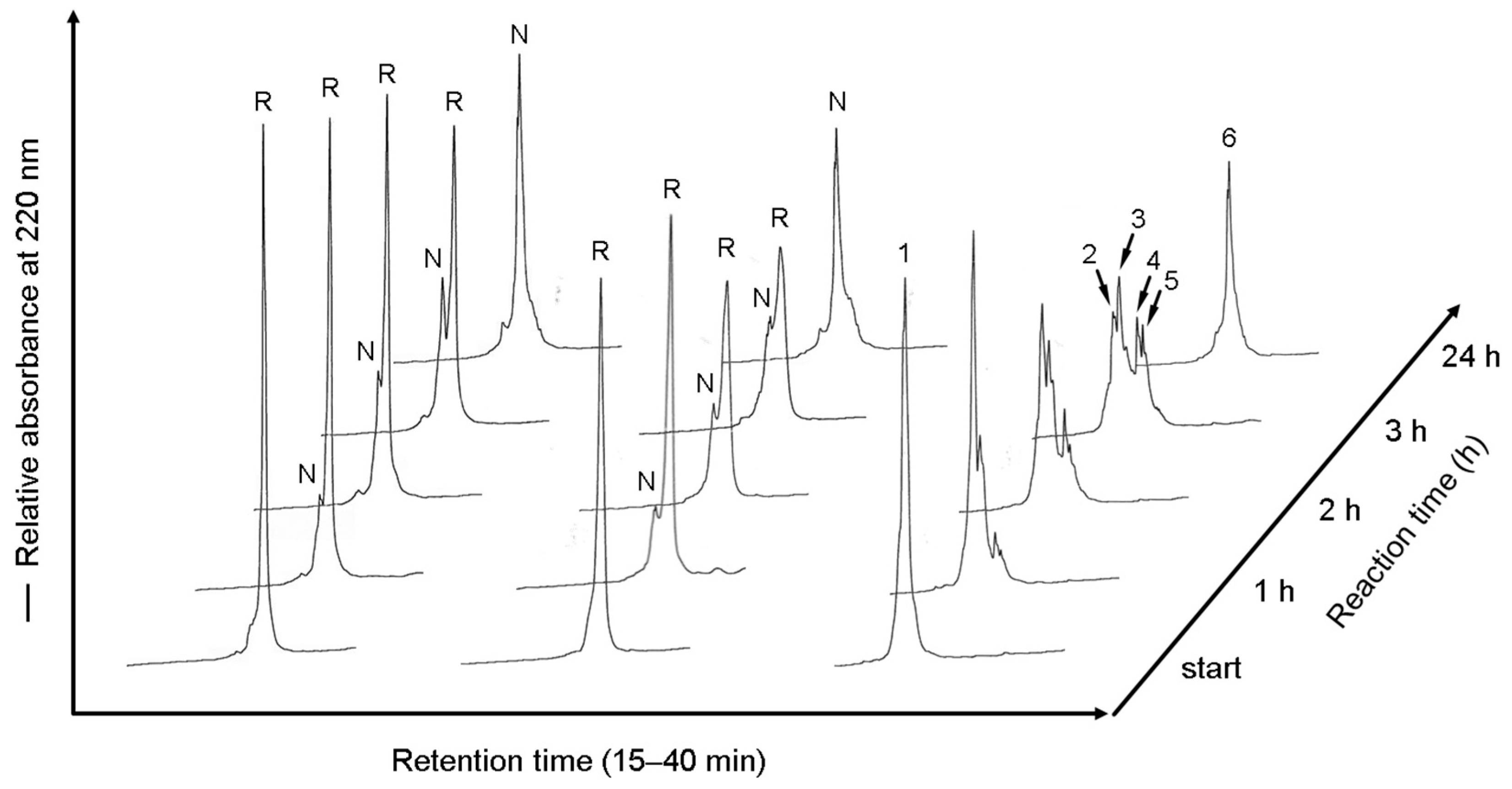
2.5. RP-HPLC Analyses of the Labeled Folding Intermediates of Prococoonase ([K8D]-proCCN′) Using the Male-Arg5-Tyr-NH2 Reagent
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of Maleimidohexanoyl-Arg5-Tyr-NH2 (Male-Arg5-Tyr-NH2)
4.3. Reduction of the Proteins
4.4. Labeling Reactions of the Folding Intermediate Des[5–55] of BPTI by the Peptide Reagent (Male-Arg5-Tyr-NH2)
4.5. Direct Labeling Reactions of the Refolding Intermediates of Proteins with the Peptide Reagent (Male-Arg5-Tyr-NH2)
4.6. Reversed-Phase High Performance Liquid Chromatography (RP-HPLC)
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Hidaka, Y.; Shimamoto, S. Folding of peptides and proteins: Role of disulfide bonds, recent developments. Biomol. Concepts 2013, 4, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Creighton, T.E. The disulfide folding pathway of BPTI. Science 1992, 256, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Weissman, J.S.; Kim, P.S. Reexamination of the folding of BPTI: Predominance of native intermediates. Science 1991, 253, 1386–1393. [Google Scholar] [CrossRef] [PubMed]
- Rodbumrer, P.; Arthan, D.; Uyen, U.; Yuvaniyama, J.; Svasti, J.; Wongsaengchantra, P.Y. Functional expression of a Bombyx mori cocoonase: Potential application for silk degumming. Acta Biochim. Biophys. Sin. 2012, 44, 974–983. [Google Scholar] [CrossRef] [PubMed]
- Berger, E.; Kafatos, F.C.; Felsted, R.L.; Law, J.H. Cocoonase. 3. Purification, preliminary characterization, and activation of the zymogen of an insect protease. J. Biol. Chem. 1971, 246, 4131–4137. [Google Scholar] [CrossRef] [PubMed]
- Felsted, R.L.; Kramer, K.J.; Law, J.H.; Berger, E.; Kafatos, F.C. Cocoonase. IV. Mechanism of activation of prococoonase from Antheraea polyphemus. J. Biol. Chem. 1973, 248, 3012–20. [Google Scholar] [CrossRef]
- Kramer, K.J.; Felsted, R.L.; Law, J.H. Cocoonase. V. Structural studies on an insect serine protease. J. Biol. Chem. 1973, 248, 3021–3028. [Google Scholar] [CrossRef]
- Sakata, N.; Ogata, A.; Takegawa, M.; Tajima, N.; Nishimura, M.; Hagiwara, T.; Miyazawa, M.; Shimamoto, S.; Hidaka, Y. The propeptide sequence assists the correct folding required for the enzymatic activity of cocoonase. Biochem. Biophys. Res. Commun. 2022, 624, 35–39. [Google Scholar] [CrossRef]
- Sakata, N.; Ogata, A.; Takegawa, M.; Murakami, Y.; Nishimura, M.; Miyazawa, M.; Hagiwara, T.; Shimamoto, S.; Hidaka, Y. Degradation-Suppressed Cocoonase for Investigating the Propeptide-Mediated Activation Mechanism. Molecules 2022, 27, 8063. [Google Scholar] [CrossRef]
- Rothwarf, D.M.; Scheraga, H.A. Regeneration of bovine pancreatic ribonuclease A. 1. Steady-state distribution. Biochemistry 1993, 32, 2671–2679. [Google Scholar] [CrossRef]
- Onda, M.; Tatsumi, E.; Takahashi, N.; Hirose, M. Refolding process of ovalbumin from urea-denatured state. Evidence for the involvement of nonproductive side chain interactions in an early intermediate. J. Biol. Chem. 1997, 272, 3973–3979. [Google Scholar] [CrossRef]
- Ravasco, J.; Faustino, H.; Trindade, A.; Gois, P.M.P. Bioconjugation with Maleimides: A Useful Tool for Chemical Biology. Chemistry 2019, 25, 43–59. [Google Scholar] [CrossRef]
- Barron, E.S. Thiol groups of biological importance. Adv. Enzymol. Relat. Subj. Biochem. 1951, 11, 201–66. [Google Scholar] [PubMed]
- Narayan, M. Disulfide bonds: Protein folding and subcellular protein trafficking. FEBS J. 2012, 279, 2272–2282. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.R.; Lilie, H.; Rudolph, R.; Lange, C. L-Arginine increases the solubility of unfolded species of hen egg white lysozyme. Protein Sci. 2005, 14, 929–935. [Google Scholar] [CrossRef]
- Okumura, M.; Saiki, M.; Yamaguchi, H.; Hidaka, Y. Acceleration of disulfide-coupled protein folding using glutathione derivatives. FEBS J. 2011, 278, 1137–1144. [Google Scholar] [CrossRef] [PubMed]
- Okumura, M.; Kadokura, H.; Hashimoto, S.; Yutani, K.; Kanemura, S.; Hikima, T.; Hidaka, Y.; Ito, L.; Shiba, K.; Masui, S.; et al. Inhibition of the functional interplay between endoplasmic reticulum (ER) oxidoreduclin-1alpha (Ero1alpha) and protein-disulfide isomerase (PDI) by the endocrine disruptor bisphenol A. J. Biol. Chem. 2014, 289, 27004–27018. [Google Scholar] [CrossRef]
- Hames, B.D.; Rickwood, D. Gel Electrophoresis of Proteins: A Practical Approach, 3rd ed.; Oxford University Press: Oxford, NY, USA, 1998; pp. 1–92. [Google Scholar]
- Wu, H.H.; Thomas, J.A.; Momand, J. p53 protein oxidation in cultured cells in response to pyrrolidine dithiocarbamate: A novel method for relating the amount of p53 oxidation in vivo to the regulation of p53-responsive genes. Biochem. J. 2000, 351, 87–93. [Google Scholar] [CrossRef]
- Yamauchi, K.; Ebihara, Y.; Kawakami, Y. Redox status of serum apolipoprotein E and its impact on HDL cholesterol levels. Clin. Biochem. 2017, 50, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Okumura, M.; Shimamoto, S.; Hidaka, Y. A chemical method for investigating disulfide-coupled peptide and protein folding. FEBS J. 2012, 279, 2283–2295. [Google Scholar] [CrossRef]
- Okumura, M.; Shimamoto, S.; Nakanishi, T.; Yoshida, Y.; Konogami, T.; Maeda, S.; Hidaka, Y. Effects of positively charged redox molecules on disulfide-coupled protein folding. FEBS Lett. 2012, 586, 3926–3930. [Google Scholar] [CrossRef] [PubMed]
- Nishino, H.; Kitamura, M.; Okada, S.; Miyake, R.; Okumura, M.; Muraoka, T. Cysteine-based protein folding modulators for trapping intermediates and misfolded forms. RSC Adv. 2022, 12, 26658–26664. [Google Scholar] [CrossRef] [PubMed]
- Kafatos, F.C.; Law, J.H.; Tartakoff, A.M. Cocoonase. II. Substrate specificity, inhibitors, and classification of the enzyme. J. Biol. Chem. 1967, 242, 1488–1494. [Google Scholar] [CrossRef] [PubMed]
- Perona, J.J.; Craik, C.S. Structural basis of substrate specificity in the serine proteases. Protein Sci. 1995, 4, 337–360. [Google Scholar] [CrossRef]
- Stojanovski, B.M.; Chen, Z.; Koester, S.K.; Pelc, L.A.; Di Cera, E. Role of the I16-D194 ionic interaction in the trypsin fold. Sci. Rep. 2019, 9, 18035. [Google Scholar] [CrossRef]
- Merrifield, R.B. Solid Phase Peptide Synthesis. I. The Synthesis of a Tetrapeptide. J. Am. Chem. Soc. 1963, 85, 2149–2154. [Google Scholar] [CrossRef]
- Goto, M.; Yoshino, S.; Hiroshima, K.; Kawakami, T.; Murota, K.; Shimamoto, S.; Hidaka, Y. The Molecular Basis of Heat-Stable Enterotoxin for Vaccine Development and Cancer Cell Detection. Molecules 2023, 28, 1128. [Google Scholar] [CrossRef]
- Shimamoto, S.; Fukutsuji, M.; Osumi, T.; Goto, M.; Toyoda, H.; Hidaka, Y. Topological Regulation of the Bioactive Conformation of a Disulfide-Rich Peptide, Heat-Stable Enterotoxin. Molecules 2020, 25, 4798. [Google Scholar] [CrossRef]
- Shimamoto, S.; Mitsuoka, N.; Takahashi, S.; Kawakami, T.; Hidaka, Y. Chemical Digestion of the-Asp-Cys- Sequence for Preparation of Post-translationally Modified Proteins. Protein J. 2020, 39, 711–716. [Google Scholar] [CrossRef]
- Schagger, H. Tricine-SDS-PAGE. Nat. Protoc. 2006, 1, 16–22. [Google Scholar] [CrossRef]

| des[5–55] | Peak † | [M + H]+calcd. | [M + H]+obs. | Number of Thiol and Disulfide Bond(s) | Number of the Peptide Reagent(s) |
| a | 8826.4 Da | 8821.8 Da | 2 × SH, 2 × SS | 2 | |
| [K8D]-proCCN′ | Peak ‡ | [M + H]+calcd. | [M + H]+obs. | Number of Thiol and Disulfide Bond(s) | Number of the Peptide Reagents |
| 1 | 31,927 Da | 31,933 Da | 6 × SH | 6 | |
| 2 | 29,616 Da | 29,612 Da | 4 × SH, 1 × SS | 4 | |
| 3 | 29,616 Da | 29,596 Da | 4 × SH, 1 × SS | 4 | |
| 4 | 27,304 Da | 27,299 Da | 2 × SH, 2 × SS | 2 | |
| 5 | 27,304 Da | 27,260 Da | 2 × SH, 2 × SS | 2 | |
| 6 | 24,992 Da | 24,969 Da | 3 × SS | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakata, N.; Murakami, Y.; Miyazawa, M.; Shimamoto, S.; Hidaka, Y. A Novel Peptide Reagent for Investigating Disulfide-Coupled Folding Intermediates of Mid-Size Proteins. Molecules 2023, 28, 3494. https://doi.org/10.3390/molecules28083494
Sakata N, Murakami Y, Miyazawa M, Shimamoto S, Hidaka Y. A Novel Peptide Reagent for Investigating Disulfide-Coupled Folding Intermediates of Mid-Size Proteins. Molecules. 2023; 28(8):3494. https://doi.org/10.3390/molecules28083494
Chicago/Turabian StyleSakata, Nana, Yuri Murakami, Mitsuhiro Miyazawa, Shigeru Shimamoto, and Yuji Hidaka. 2023. "A Novel Peptide Reagent for Investigating Disulfide-Coupled Folding Intermediates of Mid-Size Proteins" Molecules 28, no. 8: 3494. https://doi.org/10.3390/molecules28083494
APA StyleSakata, N., Murakami, Y., Miyazawa, M., Shimamoto, S., & Hidaka, Y. (2023). A Novel Peptide Reagent for Investigating Disulfide-Coupled Folding Intermediates of Mid-Size Proteins. Molecules, 28(8), 3494. https://doi.org/10.3390/molecules28083494





