Effect of Poly(Vinyl Alcohol) Concentration and Chain Length on Polymer Nanogel Formation in Aqueous Dispersion Polymerization
Abstract
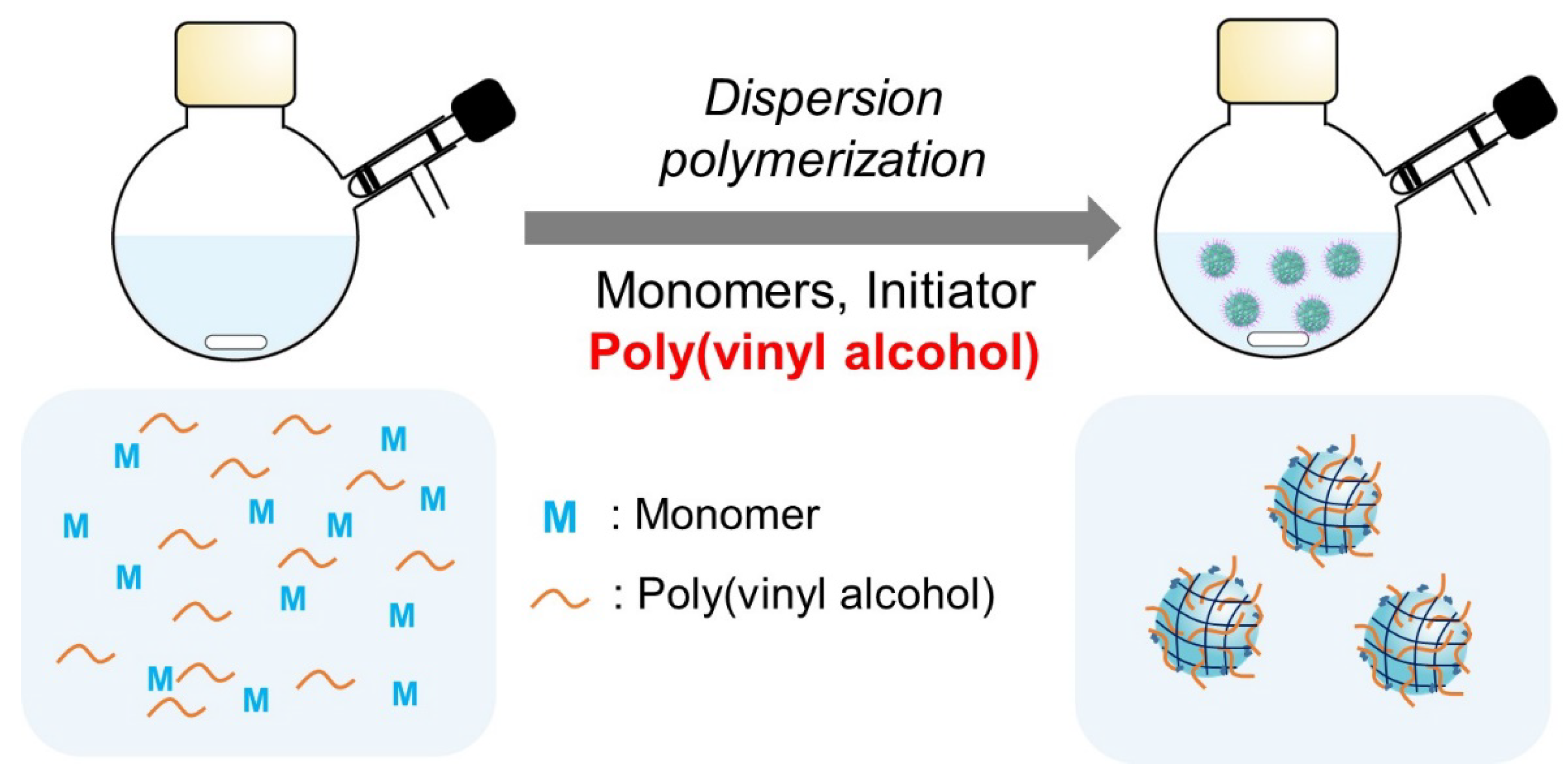
1. Introduction
2. Results and Discussion
2.1. Effect of PVA Addition on Nanogel Formation
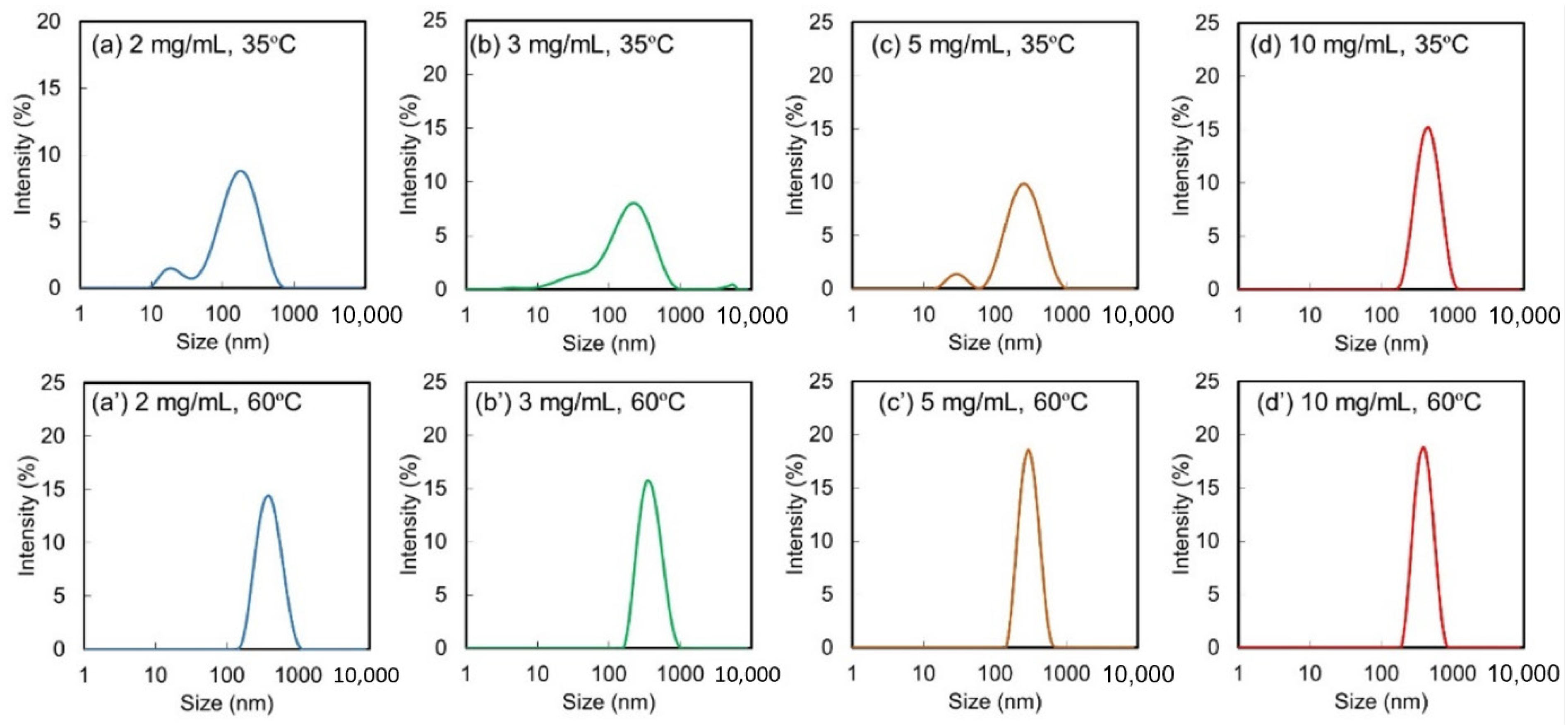
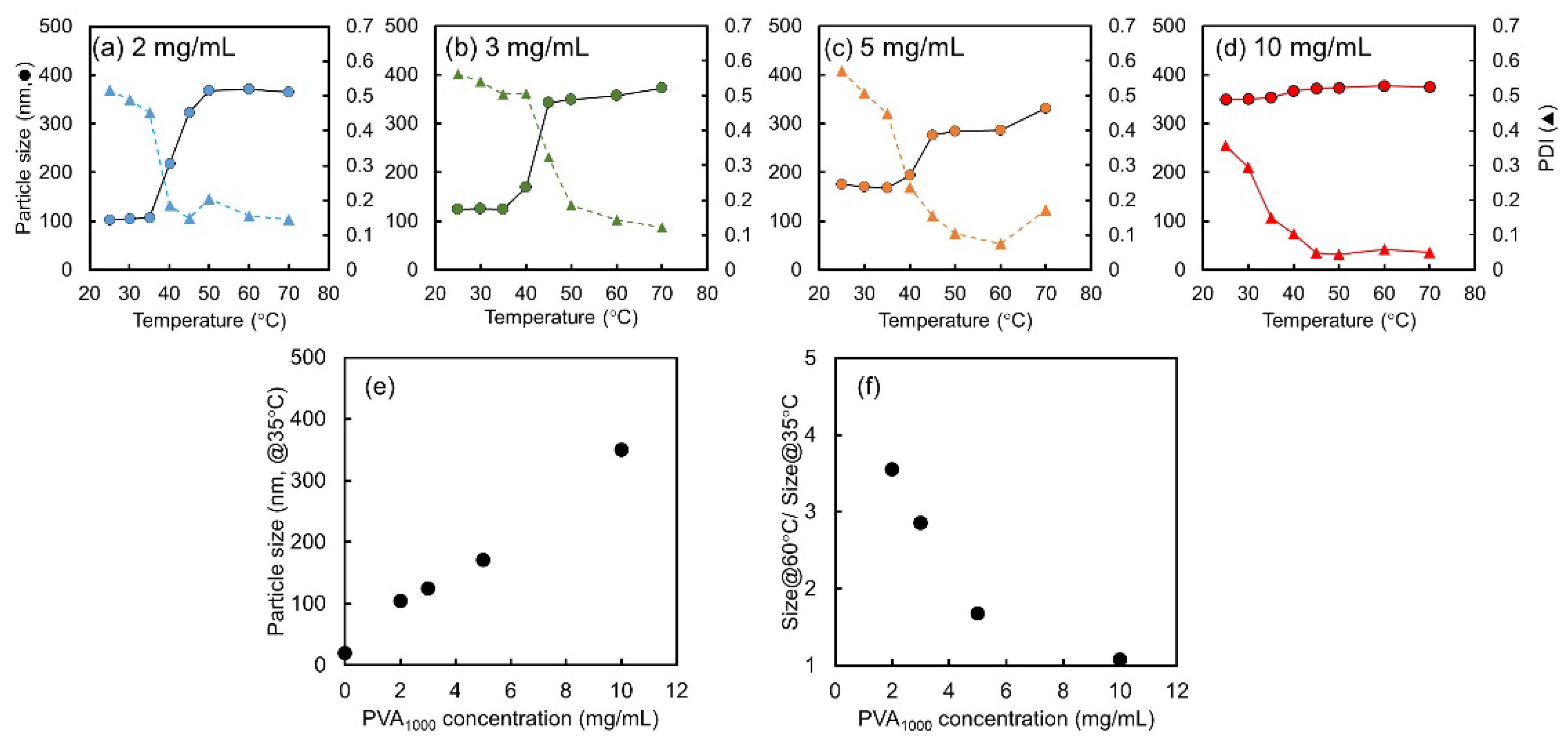
2.2. Effect of PVA1000 Concentration
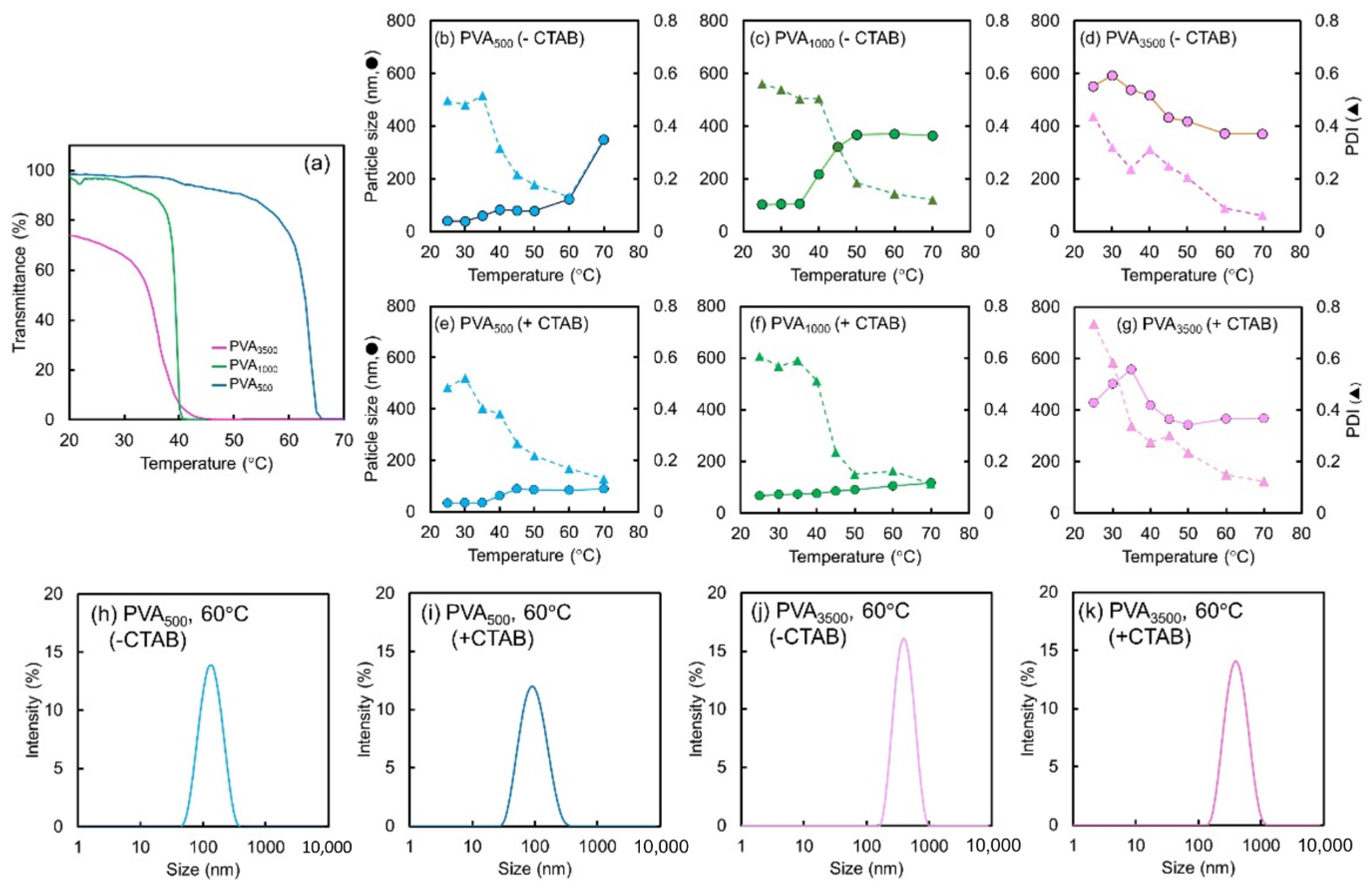
2.3. Effect of PVA Chain Length
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Apparatus
4.3. Precipitation/Dispersion Polymerizations
4.4. Transmittance Measurements
4.5. Particle Size Distributions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability Statement
Abbreviations
References
- Agnihotri, S.A.; Mallikarjuna, N.N.; Aminabhavi, T.M. Recent Advances on Chitosan-Based Micro- and Nanoparticles in Drug Delivery. J. Control. Release 2004, 100, 5–28. [Google Scholar] [CrossRef] [PubMed]
- Kono, K. Thermosensitive Polymer-Modified Liposomes. Adv. Drug. Deliver. Rev. 2001, 53, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Delcea, M.; Möhwald, H.; Skirtach, A.G. Stimuli-Responsive LbL Capsules and Nanoshells for Drug Delivery. Adv. Drug. Deliver. Rev. 2011, 63, 730–747. [Google Scholar] [CrossRef]
- Uskokovic, V.; Lee, K.; Lee, P.P.; Fischer, K.E.; Desai, T.A. Shape Effect in the Design of Nanowire-Coated Microparticles as Transepithelial Drug Delivery Devices. ACS Nano 2012, 6, 7832–7841. [Google Scholar] [CrossRef]
- Farokhzad, O.C.; Langer, R. Impact of Nanotechnology on Drug Delivery. ACS Nano 2009, 3, 16–20. [Google Scholar] [CrossRef]
- Veiseh, O.; Gunn, J.W.; Zhang, M.Q. Design and Fabrication of Magnetic Nanoparticles for Targeted Drug Delivery and Imaging. Adv. Drug. Deliver. Rev. 2010, 62, 284–304. [Google Scholar] [CrossRef]
- Kataoka, K.; Harada, A.; Nagasaki, Y. Block Copolymer Micelles for Drug Delivery: Design, Characterization and Biological Significance. Adv. Drug. Deliver. Rev. 2012, 64, 37–48. [Google Scholar] [CrossRef]
- Sharma, B.; Striegler, S. Nanogel Catalysts for the Hydrolysis of Underivatized Disaccharides Identified by a Fast Screening Assay. ACS Catalysis 2023, 13, 1614–1620. [Google Scholar] [CrossRef]
- Akl, M.A.; Sarhan, A.A.; Shoueir, K.R.; Atta, A.M. Application of Crosslinked Ionic Poly(Vinyl Alcohol) Nanogel as Adsorbents for Water Treatment. J. Dispers. Sci. Technol. 2013, 34, 1399–1408. [Google Scholar] [CrossRef]
- Richa; Roy Choudhury, A. Synthesis of a Novel Gellan-Pullulan Nanogel and Its Application in Adsorption of Cationic Dye from Aqueous Medium. Carbohydr. Polym. 2020, 227, 115291. [Google Scholar] [CrossRef] [PubMed]
- Asua, J.M. Miniemulsion Polymerization. Prog. Polym. Sci. 2002, 27, 1283–1346. [Google Scholar] [CrossRef]
- Landfester, K. Miniemulsion Polymerization and the Structure of Polymer and Hybrid Nanoparticles. Angew. Chem. Int. Ed. 2009, 48, 4488–4507. [Google Scholar] [CrossRef] [PubMed]
- Pan, G.; Sudol, E.D.; Dimonie, V.L.; El-Aasser, M.S. Nitroxide-Mediated Living Free Radical Miniemulsion Polymerization of Styrène. Macromolecules 2001, 34, 481–488. [Google Scholar] [CrossRef]
- Kitayama, Y.; Yorizane, M.; Minami, H.; Okubo, M. Preparation of Block Copolymer Particles by Two-Step, Reversible Chain Transfer Catalyzed Polymerization (RTCP) with Nitrogen Catalyst in Miniemulsion Systems. Polym. Chem. 2012, 3, 1394–1398. [Google Scholar] [CrossRef]
- Sudol, E.D.; Daniels, E.S.; Elaasser, M.S. Overview of Emulsion Polymerization—Stepping toward Prediction. ACS Symp. Ser. 1992, 492, 1–11. [Google Scholar]
- Ferguson, C.J.; Hughes, R.J.; Nguyen, D.; Pham, B.T.T.; Gilbert, R.G.; Serelis, A.K.; Such, C.H.; Hawkett, B.S. Ab Initio Emulsion Polymerization by RAFT-Controlled Self-Assembly. Macromolecules 2005, 38, 2191–2204. [Google Scholar] [CrossRef]
- Thickett, S.C.; Gilbert, R.G. Emulsion Polymerization: State of the Art in Kinetics and Mechanisms. Polymer 2007, 48, 6965–6991. [Google Scholar] [CrossRef]
- Smith, W.V.; Ewart, R.H. Kinetics of Emulsion Polymerization. J. Chem. Phys. 1948, 16, 592–599. [Google Scholar] [CrossRef]
- Kitayama, Y.; Okubo, M. A synthetic Route to Ultra-High Molecular Weight Polystyrene (>10(6)) with Narrow Molecular Weight Distribution by Emulsifier-Free, Emulsion Organotellurium-Mediated Living Radical Polymerization (emulsion TERP). Polym. Chem. 2016, 7, 2573–2580. [Google Scholar] [CrossRef]
- Khan, M.; Guimarães, T.R.; Kuchel, R.P.; Moad, G.; Perrier, S.; Zetterlund, P.B. Synthesis of Multicompositional Onion-like Nanoparticles via RAFT Emulsion Polymerization. Angew. Chem. Int. Ed. 2021, 60, 23281–23288. [Google Scholar] [CrossRef]
- Schmid, A.; Fujii, S.; Armes, S.P.; Leite, C.A.P.; Galembeck, F.; Minami, H.; Saito, N.; Okubo, M. Polystyrene—Silica Colloidal Nanocomposite Particles Prepared by Alcoholic Dispersion Polymerization. Chem. Mater. 2007, 19, 2435–2445. [Google Scholar] [CrossRef]
- Kawaguchi, S.; Ito, K. Dispersion Polymerization. In Polymer Particles; Okubo, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2005; Volume 175, pp. 299–328. [Google Scholar]
- Paine, A.J.; Luymes, W.; McNulty, J. Dispersion Polymerization of Styrene in Polar Solvents. 6. Influence of Reaction Parameters on Particle Size and Molecular Weight in Poly(N-vinylpyrrolidone)-Stabilized Reactions. Macromolecules 1990, 23, 3104–3109. [Google Scholar] [CrossRef]
- Tseng, C.M.; Lu, Y.Y.; El-Aasser, M.S.; Vanderhoff, J.W. Uniform Polymer Particles by Dispersion Polymerization in Alcohol. J. Polym. Sci. Part A Polym. Chem. 1986, 24, 2995–3007. [Google Scholar] [CrossRef]
- Wang, J.; Cormack, P.A.G.; Sherrington, D.C.; Khoshdel, E. Monodisperse, Molecularly Imprinted Polymer Microspheres Prepared by Precipitation Polymerization for Affinity Separation Applications. Angew. Chem. Int. Ed. 2003, 42, 5336–5338. [Google Scholar] [CrossRef]
- Li, W.H.; Stöver, H.D.H. Porous Monodisperse Poly(divinylbenzene) Microspheres by Precipitation Polymerization. J. Polym. Sci. A Polym. Chem. 1998, 36, 1543–1551. [Google Scholar] [CrossRef]
- Bai, F.; Yang, X.; Huang, W. Synthesis of Narrow or Monodisperse Poly(divinylbenzene) Microspheres by Distillation-Precipitation Polymerization. Macromolecules 2004, 37, 9746–9752. [Google Scholar] [CrossRef]
- Yoshimatsu, K.; Reimhult, K.; Krozer, A.; Mosbach, K.; Sode, K.; Ye, L. Uniform Molecularly Imprinted Microspheres and Nanoparticles Prepared by Precipitation Polymerization: The Control of Particle Size Suitable for Different Analytical Applications. Anal. Chim. Acta 2007, 584, 112–121. [Google Scholar] [CrossRef]
- Okaya, T.; Fujita, H.; Suzuki, A.; Kikuchi, K. Study on Chain Transfer Reaction of Poly(vinyl acetate) Radical with Poly(vinyl alcohol) in a Homogeneous System. Des. Monomers Polym. 2004, 7, 269–276. [Google Scholar] [CrossRef]
- Lee, A.; Tsai, H.Y.; Yates, M.Z. Steric Stabilization of Thermally Responsive N-Isopropylacrylamide Particles by Poly(vinyl alcohol). Langmuir 2010, 26, 18055–18060. [Google Scholar] [CrossRef]
- Tang, Z.; Weng, J.; Guan, Y.; Zhang, Y. Unexpected Large Depression of VPTT of a PNIPAM Microgel by Low Concentration of PVA. Macromol. Chem. Phys. 2017, 218, 1700364. [Google Scholar] [CrossRef]
- Matsumura, Y.; Maeda, H. A New Concept for Macromolecular Therapeutics in Cancer-Chemotherapy—Mechanism of Tumoritropic Accumulation of Proteins and the Antitumor Agent Smancs. Cancer Res. 1986, 46, 6387–6392. [Google Scholar] [PubMed]
- Blackburn, W.H.; Lyon, L.A. Size-Controlled Synthesis of Monodisperse Core/Shell Nanogels. Colloid Polym. Sci. 2008, 286, 563–569. [Google Scholar] [CrossRef]
- Takeuchi, T.; Kitayama, Y.; Sasao, R.; Yamada, T.; Toh, K.; Matsumoto, Y.; Kataoka, K. Molecularly Imprinted Nanogels Acquire Stealth in situ by Cloaking Themselves with Native Dysopsonic Proteins. Angew. Chem. Int. Ed. 2017, 56, 7088–7092. [Google Scholar] [CrossRef]
- Ichikawa, S.; Shimokawa, N.; Takagi, M.; Kitayama, Y.; Takeuchi, T. Size-Dependent Uptake of Electrically Neutral Amphipathic Polymeric Nanoparticles by Cell-Sized Liposomes and an Insight into Their Internalization Mechanism in Living Cells. Chem. Commun. 2018, 54, 4557–4560. [Google Scholar] [CrossRef]
- Morishita, T.; Yoshida, A.; Hayakawa, N.; Kiguchi, K.; Cheubong, C.; Sunayama, H.; Kitayama, Y.; Takeuchi, T. Molecularly Imprinted Nanogels Possessing Dansylamide Interaction Sites for Controlling Protein Corona in Situ by Cloaking Intrinsic Human Serum Albumin. Langmuir 2020, 36, 10674–10682. [Google Scholar] [CrossRef]
- Cheubong, C.; Yoshida, A.; Mizukawa, Y.; Hayakawa, N.; Takai, M.; Morishita, T.; Kitayama, Y.; Sunayama, H.; Takeuchi, T. Molecularly Imprinted Nanogels Capable of Porcine Serum Albumin Detection in Raw Meat Extract for Halal Food Control. Anal. Chem. 2020, 92, 6401–6407. [Google Scholar] [CrossRef]
- Hayakawa, N.; Yamada, T.; Kitayama, Y.; Takeuchi, T. Cellular Interaction Regulation by Protein Corona Control of Molecularly Imprinted Polymer Nanogels Using Intrinsic Proteins. ACS Appl. Polym. Mater. 2020, 2, 1465–1473. [Google Scholar] [CrossRef]
- Hayakawa, N.; Kitayama, Y.; Igarashi, K.; Matsumoto, Y.; Takano, E.; Sunayama, H.; Takeuchi, T. Fc Domain-Imprinted Stealth Nanogels Capable of Orientational Control of Immunoglobulin G Adsorbed in Vivo. ACS Appl. Mater. Interfaces 2022, 14, 16074–16081. [Google Scholar] [CrossRef] [PubMed]
- Kitayama, Y.; Yamada, T.; Kiguchi, K.; Yoshida, A.; Hayashi, S.; Akasaka, H.; Igarashi, K.; Nishimura, Y.; Matsumoto, Y.; Sasaki, R.; et al. In vivo Stealthified Molecularly Imprinted Polymer Nanogels Incorporated with Gold Nanoparticles for Radiation Therapy. J. Mater. Chem. B 2022, 10, 6784–6791. [Google Scholar] [CrossRef]
- Kayal, S.; Ramanujan, R.V. Doxorubicin Loaded PVA Coated Iron Oxide Nanoparticles for Targeted Drug Delivery. Mater. Sci. Eng. C 2010, 30, 484–490. [Google Scholar] [CrossRef]
- Deng, J.; Yuk, H.; Wu, J.; Varela, C.E.; Chen, X.; Roche, E.T.; Guo, C.F.; Zhao, X. Electrical Bioadhesive Interface for Bioelectronics. Nat. Mater. 2021, 20, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Hu, H.; Wu, Y.; Liu, S. Thermo- and Light-Regulated Fluorescence Resonance Energy Transfer Processes within Dually Responsive Microgels. Polym. Chem. 2011, 2, 363–371. [Google Scholar] [CrossRef]
- Perelman, L.A.; Moore, T.; Singelyn, J.; Sailor, M.J.; Segal, E. Preparation and Characterization of a pH- and Thermally Responsive Poly(N-isopropylacrylamide-coacrylic acid)/Porous SiO2 Hybrid. Adv. Funct. Mater. 2010, 20, 826–833. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kitayama, Y.; Takigawa, S.; Harada, A. Effect of Poly(Vinyl Alcohol) Concentration and Chain Length on Polymer Nanogel Formation in Aqueous Dispersion Polymerization. Molecules 2023, 28, 3493. https://doi.org/10.3390/molecules28083493
Kitayama Y, Takigawa S, Harada A. Effect of Poly(Vinyl Alcohol) Concentration and Chain Length on Polymer Nanogel Formation in Aqueous Dispersion Polymerization. Molecules. 2023; 28(8):3493. https://doi.org/10.3390/molecules28083493
Chicago/Turabian StyleKitayama, Yukiya, Shunsuke Takigawa, and Atsushi Harada. 2023. "Effect of Poly(Vinyl Alcohol) Concentration and Chain Length on Polymer Nanogel Formation in Aqueous Dispersion Polymerization" Molecules 28, no. 8: 3493. https://doi.org/10.3390/molecules28083493
APA StyleKitayama, Y., Takigawa, S., & Harada, A. (2023). Effect of Poly(Vinyl Alcohol) Concentration and Chain Length on Polymer Nanogel Formation in Aqueous Dispersion Polymerization. Molecules, 28(8), 3493. https://doi.org/10.3390/molecules28083493







