A Comprehensive Review on Pharmacological Activities of Pachypodol: A Bioactive Compound of an Aromatic Medicinal Plant Pogostemon Cablin Benth
Abstract
1. Introduction
2. Botanical Description
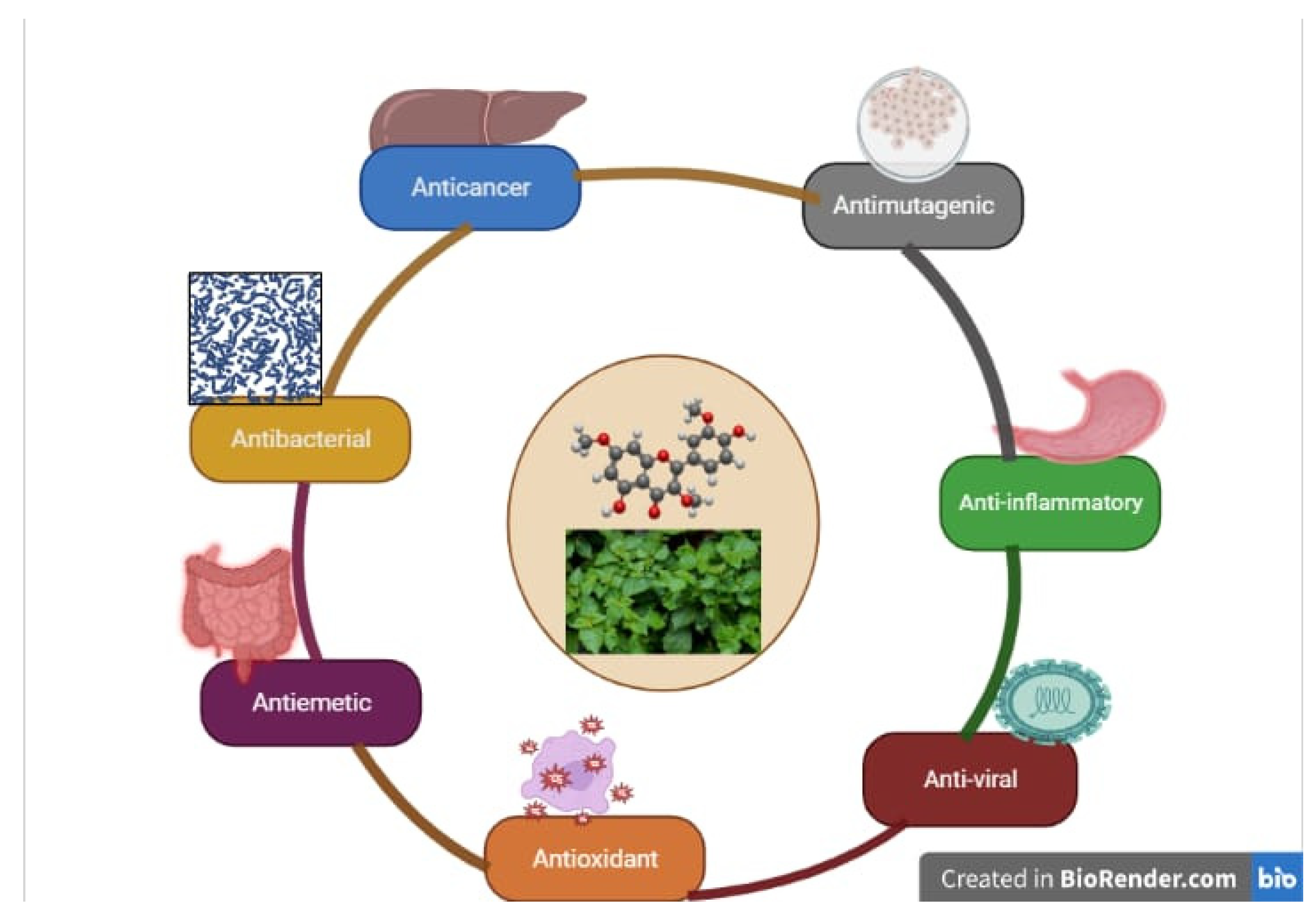
3. Pharmacological Activities
3.1. Antimicrobial Activity
3.2. Anti-Mutagenic Activity
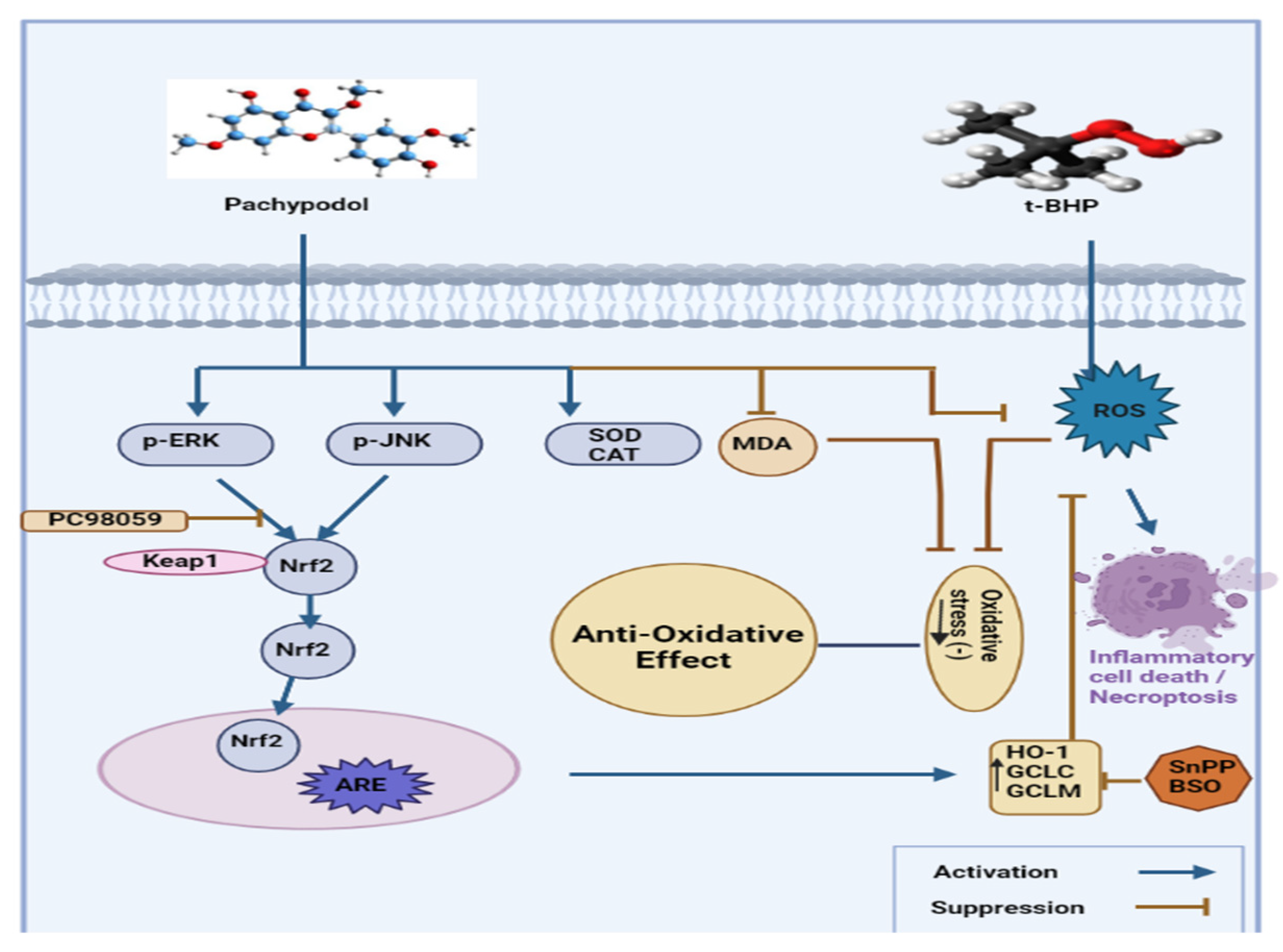
3.3. Anti-Oxidant Activity
3.4. Anti-Viral Activity
3.5. Anticancer Activity
3.6. Apoptosis Induction
3.7. Anti-Inflammatory Activity
3.8. Antiemetic Activity
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Harvey, A.L. Natural products in drug discovery. Drug Discov. 2008, 13, 894–901. [Google Scholar] [CrossRef]
- Meena, A.K.; Bansal, P.; Kumar, S. Plants-herbal wealth as a potential source of ayurvedic drugs. Asian J. Tradit. Med. 2009, 4, 152–170. [Google Scholar]
- Sucher, N.J.; Carles, M.C. Genome-based approaches to the authentication of medicinal plants. Planta Medica 2008, 74, 603–623. [Google Scholar] [CrossRef]
- Kumaraswamy, M.; Anuradha, M. Micropropagation of Pogostemon cablin Benth. through direct regeneration for production of true to type plants. Plant Tissue Cult. Biotechnol. 2010, 20, 81–89. [Google Scholar] [CrossRef]
- Saini, A.; Gahlawat, D.K.; Chauhan, C.; Gulia, S.K.; Ganie, S.A.; Yadav, S.S. Ethnomedicinal uses and phytochemistry of Abutilon indicum (Linn.) Sweet: An overview. J. Pharmacogn. Phytochem. 2015, 3, 66–72. [Google Scholar]
- Pharmacopoeia, C. Pharmacopoeia of the PR China; Press Chem Ind.: Beijing, China, 2010. [Google Scholar]
- Chen, M.; Zhang, J.; Lai, Y.; Wang, S.; Li, P.; Xiao, J.; Fu, C.; Hu, H.; Wang, Y. Analysis of Pogostemon cablin from pharmaceutical research to market performances. Expert Opin. Investig. Drugs 2013, 22, 245–257. [Google Scholar] [CrossRef]
- Puripattanavong, J.; Tewtrakul, S. Anti-allergic and anti-inflammatory compounds from Aglaia andamanica leaves. Songklanakarin J. Sci. Technol. 2015, 37, 37–41. [Google Scholar]
- Zhang, D.; Xiao, L.Y.; Cheng, Y.W.; Li, H.L.; Feng, Z.M.; Lin, P.Y.; Huang, K.R. Pharmacological action of Baoji Pill. Tradit. Chin. Drug Res. Clin. Pharmacol. 1998, 9, 212–214. [Google Scholar]
- Xian, Y.F.; Suo, J.; Huang, X.D.; Hou, S.Z.; Chen, J.N.; Ye, M.R.; Su, Z.R. A pharmacological study on anti-inflammatory effects of refined Huodan recipe. Chin. J. Exp. Tradit. Med. Formul. 2007, 13, 54–56. [Google Scholar]
- Verma, R.S.; Padalia, R.C.; Chauhan, A.; Singh, V.R. Chemical composition of leaves, inflorescence, whole aerial-parts and root essential oils of patchouli {Pogostemon cablin (Blanco) Benth.}. J. Essent. Oil Res. 2019, 31, 319–325. [Google Scholar] [CrossRef]
- Mohammadhosseini, M. A Comprehensive Review on New Methods for Processing, Separation and Identification of the Essential Oils; Islamic Azad University of Shahrood Press: Shahrood, Iran, 2016; pp. 61–73. [Google Scholar]
- Murugan, R.; Livingstone, C. Origin of the name ‘patchouli’and its history. Curr. Sci. 2010, 99, 1274–1276. [Google Scholar]
- Deguerry, F.; Pastore, L.; Wu, S.; Clark, A.; Chappell, J.; Schalk, M. The diverse sesquiterpene profile of patchouli, Pogostemon cablin, is correlated with a limited number of sesquiterpene synthases. Arch. Biochem. Biophys. 2006, 454, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Swamy, M.K.; Sinniah, U.R. A comprehensive review on the phytochemical constituents and pharmacological activities of Pogostemon cablin Benth.: An aromatic medicinal plant of industrial importance. Molecules 2015, 20, 8521–8547. [Google Scholar] [CrossRef]
- Kalra, A.; Rao, E.P.; Khanuja, S. Cultivation and processing technologies of patchouli (Pogostemon cablin). J. Med. Arom. Plants Sci. 2006, 28, 414–419. [Google Scholar]
- Sumi, H. Fibrinolysis-accelerating activity of the essential oils and Shochu aroma. Aroma Res. 2003, 4, 60–63. [Google Scholar]
- Miyazawa, M.; Okuno, Y.; Nakamura, S.I.; Kosaka, H. Antimutagenic activity of flavonoids from Pogostemon cablin. J. Agric. Food Chem. 2000, 48, 642–647. [Google Scholar] [CrossRef]
- Wei, A.; Shibamoto, T. Antioxidant activities and volatile constituents of various essential oils. J. Agric. Food Chem. 2007, 55, 1737–1742. [Google Scholar] [CrossRef]
- Tripathi, Y.; Kaushik, P.; Pandey, B. Patchouli (Pogostemon cablin): A promising medicinal and aromatic crop for Northastern India. Recent Prog. Med. Plants 2005, 9, 289–310. [Google Scholar]
- Swamy, M.K.; Balasubramanya, S.; Anuradha, M. In vitro multiplication of Pogostemon cablin Benth. through direct regeneration. Afr. J. Biotechnol. 2010, 9, 2069–2075. [Google Scholar]
- Lal, M.; Dutta, S.; Munda, S.; Pandey, S.K. Identification and registration of a high essential oil yielding variety (Jor Lab L-14) of Lemongrass (Cymbopogon flexuosus L.) through mutation breeding technique. J. Essent. Oil-Bear. Plants 2018, 21, 1604–1611. [Google Scholar] [CrossRef]
- Kharkwal, M.; Shu, Q. The role of induced mutations in world food security. In Induced Plant Mutations in the Genomics Era; Food and Agriculture Organization of the United Nations: Rome, Italy, 2009; pp. 33–38. [Google Scholar]
- Lal, M. Jor Lab P-1 (Patchouli)(IC0625986; INGR18041), a Patchouli (Pogostemon cablin) Germplasm with High Essential Oil Yield and High Herbage Yield 3220 kg/year. IJPGR 2019, 32, 450–451. [Google Scholar]
- Kumar, K. Patchouli and India-A great leap forward. In Proceedings of the International Seminar of Prospectus and Potentials of Medicinal and Aromatic Crops, Bangalore, India, 18–19 June 2004. [Google Scholar]
- Sharmeen, J.B.; Mahomoodally, F.M.; Zengin, G.; Maggi, F. Essential Oils as Natural Sources of Fragrance Compounds for Cosmetics and Cosmeceuticals. Molecules 2021, 26, 666. [Google Scholar] [CrossRef] [PubMed]
- Chakrapani, P.; Venkatesh, K.; Singh, B.C.S.; Jyothi, B.A.; Kumar, P.; Amareshwari, P.; Roja, A.R. Phytochemical, pharmacological importance of Patchouli (Pogostemon cablin (Blanco) Benth) an aromatic medicinal plant. Int. J. Pharm. Sci. Rev. Res. 2013, 21, 7–15. [Google Scholar]
- Maheshwari, M.L.; Vasantha Kumar, T.; Sharma, N.; Chandel, K.P.S. Patchouli-an Indian perspective. Indian Perfum. 1993, 37, 9. [Google Scholar]
- Bhaskar, S.; Kumar, T.V. Agronomic bottlenecks, genetic barriers and marketing impediments in patchouli Pogostemon patchouli production. Open Access J. Med. Aromat. 2000, 22, 396–403. [Google Scholar]
- Angadi, S.; Vasanthakumar, T.P. Advances in Horticulture: Medicinal and Aromatic Plants; Chadha, K.L., Gupta, R., Eds.; Malhotra Publishing House: New Delhi, India, 1995. [Google Scholar]
- Guo, J.; Yuan, Y.; Liu, Z.; Zhu, J. Development and structure of internal glands and external glandular trichomes in Pogostemon cablin. PLoS ONE 2013, 8, e77862. [Google Scholar] [CrossRef]
- Xie, J.; Chai, T.; Xu, R.; Liu, D.; Yang, Y.-X.; Deng, Z.; Jin, H.; He, H. Induction of defense-related enzymes in patchouli inoculated with virulent Ralstonia solanacearum. Electron. J. Biotechnol. 2017, 27, 63–69. [Google Scholar] [CrossRef]
- Wu, Z.N.; Wu, W.G.; Zhang, T.; Wang, B. Research progress of chemical constituents and pharmacological effects of Pogostemonis herba. From different habitats. ModernizationTrad. Chin. Med. Materia. Medica.World Sci.Technol. 2019, 21, 1227–1231. [Google Scholar]
- Croteau, R.; Munck, S.L.; Akoh, C.C.; Fisk, H.J.; Satterwhite, D.M. Biosynthesis of the sesquiterpene patchoulol from farnesyl pyrophosphate in leaf extracts of Pogostemon cablin (patchouli): Mechanistic considerations. Arch. Biochem. Biophys. 1987, 256, 56–68. [Google Scholar] [CrossRef]
- Akhila, A.; Nigam, M. GC-MS analysis of the essential oil of Pogostemon cablin (Patchouly oil). Fitoterapia 1984, 1, 363–365. [Google Scholar]
- Li, Y.C.; Xian, Y.F.; Su, Z.R.; Ip, S.P.; Xie, J.H.; Liao, J.B.; Wu, D.-W.; Li, C.; Chen, J.; Lin, Z.-X.; et al. Pogostone suppresses proinflammatory mediator production and protects against endotoxic shock in mice. J. Ethnopharmacol. 2014, 157, 212–221. [Google Scholar] [CrossRef]
- Silva, M.A.S.; Ehlert, P.A.D.; Ming, L.C.; Marques, M.O.M.; Vista, B. Composition and chemical variation during daytime of constituents of the essential oil of Pogostemon pachouli pellet leaves. Acta Hortic. 2004, 2, 145–148. [Google Scholar] [CrossRef]
- Wang, D.; Yin, Z.; Zhang, Q.; Ye, W.; Zhang, X.; Zhang, J. Nonvolatile chemical constituents from Pogostemon cablin. China J. Chin. Mater. Med. 2010, 35, 2704–2707. [Google Scholar]
- Kongkathip, N.; Sam-ang, P.; Kongkathip, B.; Pankaew, Y.; Tanasombat, M.; Udomkusonsri, P. Development of patchouli extraction with quality control and isolation of active compounds with antibacterial activity. Agric. Nat. Resour. 2009, 43, 519–525. [Google Scholar]
- Ali, H.A.; Chowdhury, A.A.; Rahman, A.K.; Borkowski, T.; Nahar, L.; Sarker, S.D. Pachypodol, a flavonol from the leaves of Calycopteris floribunda, inhibits the growth of CaCo 2 colon cancer cell line in vitro. Phytother Res. 2008, 22, 1684–1687. [Google Scholar] [CrossRef] [PubMed]
- Jeevitha, D.; Sadasivam, K.; Praveena, R. DFT investigation of pachypodol for exploring anti-oxidant action–Performance of B3LYP and M06-2X. Indian J. Chem. 2020, 59, 952–961. [Google Scholar]
- Yang, Y.; Kinoshita, K.; Koyama, K.; Takahashi, K.; Tai, T.; Nunoura, Y.; Watanabe, K. Anti-emetic principles of Pogostemon cablin (Blanco) Benth. Int. J. Phytomed. 1999, 6, 89–93. [Google Scholar] [CrossRef]
- González-Vázquez, R.; King Díaz, B.; Aguilar, M.I.; Diego, N.; Lotina-Hennsen, B. Pachypodol from Croton ciliatoglanduliferus Ort. as water-splitting enzyme inhibitor on thylakoids. J. Agric. Food Chem. 2006, 54, 1217–1221. [Google Scholar] [CrossRef]
- Huong, D.T.; Luong, D.V.; Thao, T.T.P.; Sung, T.V. A new flavone and cytotoxic activity of flavonoid constituents isolated from Miliusa balansae (Annonaceae). Die Pharmazie. Int. J. Pharm. Sci. 2005, 60, 627–629. [Google Scholar]
- Çitoğlu, G.S.; Sever, B.; Antus, S.; Baitz-Gacs, E.; Altanlar, N. Antifungal flavonoids from Ballota glandulosissima. Pharm. Biol. 2003, 41, 483–486. [Google Scholar] [CrossRef]
- Kim, E.K.; Kim, J.H.; Jeong, S.; Choi, Y.W.; Choi, H.J.; Kim, C.Y.; Kim, Y.M. Pachypodol, a methoxyflavonoid isolated from Pogostemon cablin bentham exerts antioxidant and cytoprotective effects in HepG2 cells: Possible role of ERK-dependent Nrf2 activation. Int. J. Mol. Sci. 2019, 20, 4082. [Google Scholar] [CrossRef] [PubMed]
- Krithika, S.; Sadiq, M.; Ramanujam, G.M.; Maruthapillai, A. Trimethoxy flavone, pachypodol containing pogostemon cablin leaf extract shows broad spectrum antimicrobial activity. Mater. Today Proc. 2022, 50, 297–300. [Google Scholar] [CrossRef]
- Dlamini, B.S.; Chen, C.-R.; Shyu, D.; Chang, C.-I. Flavonoids from Tithonia diversifolia and their antioxidant and antibacterial activity. Chem. Nat. Compds. 2020, 56, 906–908. [Google Scholar] [CrossRef]
- Arita, M.; Philipov, S.; Galabov, A.S. Phosphatidylinositol 4-kinase III beta is the target of oxoglaucine and pachypodol (Ro 09-0179) for their anti-poliovirus activities, and is located at upstream of the target step of brefeldin A. Microbiol. Immunol. 2015, 59, 338–347. [Google Scholar] [CrossRef]
- Zhang, Q.; Song, X.; Chen, X.; Jiang, R.; Peng, K.; Tang, X.; Liu, Z. Antiosteoporotic effect of hesperidin against ovariectomy-induced osteoporosis in rats via reduction of oxidative stress and inflammation. J. Biochem. Mol. Toxicol. 2021, 35, e22832. [Google Scholar] [CrossRef]
- Azhagumeena, C.; Bharathi, P.R. A review on phytochemistry and pharmacology of calycopteris floribunda roxb. LAM. Int. J. Org. Chem. 2020, 1, 1–8. [Google Scholar] [CrossRef]
- Bryan, H.K.; Olayanju, A.; Goldring, C.E.; Park, B.K. (The Nrf2 cell defence pathway: Keap1-dependent and-independent mechanisms of regulation. Biochem. Pharmacol. 2013, 85, 705–717. [Google Scholar] [CrossRef]
- Ryu, S.; McDonnell, K.; Choi, H.; Gao, D.; Hahn, M.; Joshi, N.; Park, S.-M.; Catena, R.; Do, Y.; Brazin, J.; et al. Suppression of miRNA-708 by polycomb group promotes metastases by calcium-induced cell migration. Cancer cell. 2013, 23, 63–76. [Google Scholar] [CrossRef]
- Marvibaigi, M.; Supriyanto, E.; Amini, N.; Abdul Majid, F.A.; Jaganathan, S.K. Preclinical and clinical effects of mistletoe against breast cancer. Biomed Res. Int. 2014, 2014, 785479. [Google Scholar] [CrossRef]
- Raju, R.; Cullen, J.K.; Bruce, Z.C.; Reddell, P.; Münch, G. Eupomatenes A–E: Neolignans isolated from the leaves of Australian rainforest plant Eupomatia laurina. Fitoterapia 2021, 153, 104972. [Google Scholar] [CrossRef]
- Tasleem, F.; Ahmed, S.; Azhar, I. Antiemetic activity of bergenin from Peltophorum roxburghii L. Indo Am. J. Pharm. Sci. 2015, 5, 760–764. [Google Scholar]
- Dongare, P.; Dhande, S.; Kadam, V. A Review on Pogostemon patchouli. J. Pharmacogn. Phytochem. 2014, 6, 41–47. [Google Scholar]
- Li, C.W.; Wu, X.L.; Zhao, X.N.; Su, Z.Q.; Chen, H.M.; Wang, X.F.; Zhang, X.J.; Zeng, H.; Chen, J.; Li, Y.C.; et al. Anti-inflammatory property of the ethanol extract of the root and rhizome of Pogostemon cablin (Blanco) Benth. Sci. World J. 2013, 2013, 434151. [Google Scholar] [CrossRef] [PubMed]
- Punithavathy, P.; Nalina, K.; Menon, T. Antifungal susceptibility testing of Candida tropicalis biofilms against fluconazole using calorimetric indicator resazurin. Indian J. Pathol. Microbiol. 2012, 55, 72. [Google Scholar]
- Agarwal, P.; Pal, K.; Pal, A.; Sharma, O.P. Antimicrobial activity in Aegle mermalos and Terminalia bellirica in different extract. Int. J. Adv. Life Sci. 2017, 10, 31–35. [Google Scholar]
- Monteiro, M.C.; de la Cruz, M.; Cantizani, J.; Moreno, C.; Tormo, J.R.; Mellado, E.; De Lucas, J.D.; Asensio, F.; Valiante, V.; Brakhage, A.; et al. A new approach to drug discovery: High-throughput screening of microbial natural extracts against Aspergillus fumigatus using resazurin. J. Biomol. Screen 2012, 17, 542–549. [Google Scholar] [CrossRef]
- Ladan, Z.; Amupitan, J.O.; Oyewale, O.A.; Ayo, R.G.; Temple, E.; Ladan, E. O Phytochemical screening of the leaf extracts of Hyptis spicigera plant. Afr. J. Pure Appl. Chem. 2014, 8, 83–88. [Google Scholar]
- Oda, Y.; Nakamura, S.I.; Oki, I.; Kato, T.; Shinagawa, H. Evaluation of the new system (umu-test) for the detection of environmental mutagens and carcinogens. Mutat. Res. Sect. Environ. Mutagen. 1985, 147, 219–229. [Google Scholar] [CrossRef]
- Yahagi, T.; Nagao, M.; Seino, Y.; Matsushima, T.; Sugimura, T.; Okada, M. (Mutagenicities of N-nitrosamines on Salmonella. Mutat. Res.Fundam. Mol. Mech. Mutagen. 1977, 48, 121–129. [Google Scholar] [CrossRef]
- Staveness, D.; Bosque, I.; Stephenson, C.R. Free radical chemistry enabled by visible light-induced electron transfer. Acc. Chem. Res. 2016, 49, 2295–2306. [Google Scholar] [CrossRef]
- Lü, J.M.; Lin, P.H.; Yao, Q.; Chen, C. Chemical and molecular mechanisms of antioxidants: Experimental approaches and model systems. J. Cell. Mol. Med. 2010, 14, 840–860. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, A.K. Nrf2 signaling in coordinated activation of antioxidant gene expression. Free Radic. Biol. Med. 2004, 36, 1199–1207. [Google Scholar] [CrossRef] [PubMed]
- Bromfield, J.J. A role for seminal plasma in modulating pregnancy outcomes in domestic species. Reproduction 2016, 152, R223–R232. [Google Scholar] [CrossRef]
- Ali, S.S.; Ahsan, H.; Zia, M.K.; Siddiqui, T.; Khan, F.H. Understanding oxidants and antioxidants: Classical team with new players. J. Food Biochem. 2020, 44, e13145. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Tang, S.; Zhu, H.; Chen, Z.; Zang, Z.; Zhang, Y.; He, C. Biomonitoring PFAAs in blood and semen samples: Investigation of a potential link between PFAAs exposure and semen mobility in China. Environ. Int. 2018, 113, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Ijaz, M.U.; Rauf, A.; Mustafa, S.; Ahmed, H.; Ashraf, A.; Al-Ghanim, K.; Mahboob, S. Pachypodol attenuates Perfluorooctane sulphonate-induced testicular damage by reducing oxidative stress. Saudi J. Biol. Sci. 2022, 29, 1380–1385. [Google Scholar] [CrossRef]
- Agarwal, A.; Leisegang, K.; Sengupta, P. Oxidative stress in pathologies of male reproductive disorders. Pathology 2020, 15–27. [Google Scholar]
- Ijaz, M.U.; Tahir, A.; Samad, A.; Anwar, H. Nobiletin ameliorates nonylphenol-induced testicular damage by improving biochemical, steroidogenic, hormonal, spermatogenic, apoptotic and histological profile. Hum. Exp. Toxicol. 2021, 40, 403–416. [Google Scholar] [CrossRef]
- Wan, H.T.; Lai, K.P.; Wong, C.K.C. Comparative analysis of PFOS and PFOA toxicity on Sertoli cells. Environ. Sci. Technol. 2020, 54, 3465–3475. [Google Scholar] [CrossRef]
- Frisch, M.; Trucks, G.; Schlegel, H. Gaussian 09; Revision a. 02 [CP]; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Zhang, H.Y.; Sun, Y.M.; Wang, X.L. Substituent Effects on O H Bond Dissociation Enthalpies and Ionization Potentials of Catechols: A DFT Study and Its Implications in the Rational Design of Phenolic Antioxidants and Elucidation of Structure–Activity Relationships for Flavonoid Antioxidants. Chem. Eur. J. 2003, 9, 502–508. [Google Scholar] [CrossRef]
- Mayer, J.M.; Rhile, I.J. Thermodynamics and kinetics of proton-coupled electron transfer: Stepwise vs. concerted pathways. Biochim. Biophys. Acta Bioenerg. (BBA) 2004, 1655, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Klein, E.; Lukeš, V.; Ilčin, M. DFT/B3LYP study of tocopherols and chromans antioxidant action energetics. Chem. Phys. 2007, 336, 51–57. [Google Scholar] [CrossRef]
- Shang, Y.; Li, X.; Sun, T.Y.; Zhou, J.; Zhou, H.; Chen, K. Comparative theoretical researches on the anti-oxidant activity of δ-viniferin and ε-viniferin. J. Mol. Struct. 2021, 1245, 131062. [Google Scholar] [CrossRef]
- Joo, M.S.; Kim, W.D.; Lee, K.Y.; Kim, J.H.; Koo, J.H.; Kim, S.G. AMPK facilitates nuclear accumulation of Nrf2 by phosphorylating at serine 550. Mol. Cell. Biol. 2016, 36, 1931–1942. [Google Scholar] [CrossRef]
- Zipper, L.M.; Mulcahy, R.T. Erk activation is required for Nrf2 nuclear localization during pyrrolidine dithiocarbamate induction of glutamate cysteine ligase modulatory gene expression in HepG2 cells. Toxicol. Sci. 2003, 73, 124–134. [Google Scholar] [CrossRef]
- Gu, X.; Manautou, J.E. Molecular mechanisms underlying chemical liver injury. Expert Rev. Mol. Med. 2012, 14, e4. [Google Scholar] [CrossRef]
- Sandoval, I.V.; Carrasco, L. Poliovirus infection and expression of the poliovirus protein 2B provoke the disassembly of the Golgi complex, the organelle target for the antipoliovirus drug Ro-090179. Virol. J. 1997, 71, 4679–4693. [Google Scholar] [CrossRef]
- Arita, M.; Kojima, H.; Nagano, T.; Okabe, T.; Wakita, T.; Shimizu, H. Oxysterol-binding protein family I is the target of minor enviroxime-like compounds. Virol. J. 2013, 87, 4252–4260. [Google Scholar] [CrossRef]
- Chikara, S.; Nagaprashantha, L.D.; Singhal, J.; Horne, D.; Awasthi, S.; Singhal, S.S. Oxidative stress and dietary phytochemicals: Role in cancer chemoprevention and treatment. Cancer Lett. 2018, 413, 122–134. [Google Scholar] [CrossRef]
- Singh, S.; Sharma, B.; Kanwar, S.S.; Kumar, A. Lead phytochemicals for anticancer drug development. Front. Plant Sci. 2016, 7, 1667. [Google Scholar] [CrossRef]
- Alves-Silva, J.M.; Romane, A.; Efferth, T.; Salgueiro, L. North African medicinal plants traditionally used in cancer therapy. Front. Pharmacol. 2017, 8, 383. [Google Scholar] [CrossRef]
- Patel, S.; Panda, S. Emerging roles of mistletoes in malignancy management. Biotechnology 2014, 4, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Chien, J.-H.; Lee, S.-C.; Chang, K.-F.; Huang, X.-F.; Chen, Y.-T.; Tsai, N.-M. Extract of Pogostemon cablin possesses potent anticancer activity against colorectal cancer cells in vitro and in vivo. J. Evid. Based Complementary. Altern. Med. 2020, 2020, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Ye, Q.; Chen, N.Y.; Zhang, G.L. A new norditerpene from Miliusa balansae Finet et Gagnep. Chin. Chem. Lett. 2001, 12, 247–248. [Google Scholar]
- Kamperdick, C.; Van, N.H.; Van Sung, T. Constituents from Miliusa balansae (Annonaceae). Phytochemistry 2002, 61, 991–994. [Google Scholar] [CrossRef] [PubMed]
- Huong, D.T.; Van, N.T.H.; Kamperdick, C.; Kamperdick, C.; Anh, N.T.H.; Sung, T.V. Two new bis-styryl compounds from Miliusa balansae. Z. Naturforsch. 2008, 63, 335–338. [Google Scholar] [CrossRef]
- Itokawa, H.; Oshida, Y.; Ikuta, A.; Inatomi, H.; Ikegami, S. Flavonol glycosides from the flowers of Cucurbita pepo. Phytochemistry 1981, 20, 2421–2422. [Google Scholar] [CrossRef]
- Likhitayawuid, K.; Angerhofer, C. Cytotoxic and antimalarial bisbenzyl isoquinoline alkaloids from Sephania evecta. J. Nat. Prod. 1993, 56, 30–38. [Google Scholar] [CrossRef]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Boyd, M.R. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer. Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef]
- Sreekanth, P.; Narayana, K.; Shridhar, N.B.; Bhat, A. Toxicity studies of Calycopteris floribunda Lam. in calf, rabbit and rat. J. Ethnopharmacol. 2006, 107, 229–233. [Google Scholar] [CrossRef]
- Datta, B.K.; Datta, S.K.; Rashid, M.A.; Nash, R.J.; Sarker, S.D. A sesquiterpene acid and flavonoids from Polygonum viscosum. Phytochemistry 2000, 54, 201–205. [Google Scholar] [CrossRef]
- Uddin, S.J.; Shilpi, J.A.; Delazar, A.; Nahar, L.; Sarker, S.D. Free radical scavenging activity of some Bangladeshi plant extracts. Adv.Tradit. Med. 2004, 4, 187–195. [Google Scholar]
- Uddin, S.J.; Nahar, L.; Shilpi, J.A.; Shoeb, M.; Borkowski, T.; Gibbons, S.; Middleton, M.; Byres, M.; Sarker, S.D. Gedunin, a limonoid from Xylocarpus granatum, inhibits the growth of CaCo-2 colon cancer cell line in vitro. Phytother. Res. 2007, 21, 757–761. [Google Scholar] [CrossRef]
- Liu, X.; Yang, D.L.; Liu, J.J.; Xu, K.; Wu, G.H. Modeling of supercritical fluid extraction of flavonoids from Calycopteris floribunda leaves. Chem. Pap. 2014, 68, 316–323. [Google Scholar] [CrossRef]
- Yoon, T.J.; Yoo, Y.C.; Kang, T.B.; Shimazaki, K.I.; Song, S.K.; Lee, K.H.; Kim, J.B. (Lectins isolated from Korean mistletoe (Viscum album coloratum) induce apoptosis in tumor cells. Cancer Lett. 1999, 136, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Friedel, W.E.; Matthes, H.; Bock, P.R.; Zänker, K.S. Systematic evaluation of the clinical effects of supportive mistletoe treatment within chemo-and/or radiotherapy protocols and long-term mistletoe application in nonmetastatic colorectal carcinoma: Multicenter, controlled, observational cohort study. J. Soc. Integr. Oncol. 2009, 7, 137–145. [Google Scholar] [PubMed]
- Burkhart, J.; Peter, M.; Heusser, M.D. In vitro investigation into the potential of a mistletoe extract to alleviate adverse effects of cyclophosphamide. Altern. Ther. Health. Med. 2020, 16, 40. [Google Scholar]
- Junren, C.; Xiaofang, X.; Mengting, L.; Qiuyun, X.; Gangmin, L.; Huiqiong, Z.; Guanru, C.; Xin, X.; Yanpeng, Y.; Fu, P.; et al. Pharmacological activities and mechanisms of action of Pogostemon cablin Benth: A review. Chin. Med. 2021, 16, 1–20. [Google Scholar] [CrossRef]
- Kuo, P.C.; Schroeder, R.A. The emerging multifaceted roles of nitric oxide. Ann. Surg. 1995, 221, 220. [Google Scholar] [CrossRef]
- Moncada, S.; Palmer, R.; Higgs, E. Nitric oxide: Physiology, pathophysiology, and pharmacology. Pharmacol. Rev. 1991, 43, 109–142. [Google Scholar]
- ICHIKAwA, K.; Kinoshita, T.; Sankawa, U. The Screening of Chinese Crude Drugs for Ca2+ Antagonist Activity: Identifiaction of Active Principles from the Aerial Part of Pogostemon cablin and the Fruits of Prunus mume. Chem. Pharm. Bull. 1989, 37, 345–348. [Google Scholar] [CrossRef] [PubMed]

| Activities | Tests | Target | Results | References |
|---|---|---|---|---|
| Antimicrobial activity | Resazurin assay | Staphylococcus aureus 6538 and Klebsiella pneumonia ATCC 27736 | Growth inhibited | [47] |
| Resazurin assay | Staphylococcus aureus (MTCC 737), Bacillus subtilis (NK-1), Salmonella enterica (ATCC 14028), and Escherichia coli (MTCC 1302). | Low bacteriostatic effect against bacteria strains, except for B. subtilis, against which it showed a moderate inhibition effect | [48] | |
| Antiviral activity | ADP-Glo Lipid Kinase Systems kit | Phosphatidylinositol 4-kinase III beta (PI4KB) | Interferes with brefeldin A (BFA)-Golgi disassembly, BFA targets viral RNA synthesis | [49] |
| Anti-mutagenic | Ames test | S. typhimurium TA100 | Suppress SOS-inducing activity | [18] |
| Antioxidant | Terminal deoxynucleotidyl transferase nick-end labelling (TUNEL) assay | Rats | Cytokine levels and Oxidative stress reduced | [50] |
| Cell-based assays | HepG2 cells | Pachypodol protected HepG2 cells from cell death caused by oxidative stress and reduced ROS production | [46] | |
| Superoxide anion radical-scavenging method | Leaves of C. floribunda extracts | High antioxidant activity | [51] | |
| HAT, SPLET, AND SET-PT | Rats | ERK activation, Nrf2-regulated enzyme activation, increase in GSH levels | [52] | |
| Anti-cancerous | MTT assays | Human ovarian cancer cells (A2780) | Inhibition of cell growth | [53] |
| Brine shrimp lethality assay and Promega’s Cell Titer 96 non-radioactive cell proliferation assay | Cancer Coli-2 (CaCo-2) colon cancer cell line | Showed high toxicity against Cancer Coli-2 (CaCo-2) | [51] | |
| MTT assay | Chinese mistletoe MCF7 cell line | Positive cytotoxic effect | [54] | |
| Likhiwitayawuid method, ELISA | Human cell lines; human epidemoid carcinoma (Kb), Hep-G2 (Hepatoma-G2), RD (Rhabdosarcoma) | Showed strong cytotoxicity against cancer cell lines | [44] | |
| Anti-inflammatory | MTT assays | RBL2H3 and RAW264.7 cells of Aglaia andamanica leaves | Pachypodol exhibited cytotoxicity (25–30%) at 100 mM | [8] |
| Nitric oxide (NO) production assay | Leaves of Eupomatia laurina tree | High levels of cell growth inhibition. | [55] | |
| Formalin test | TNF, IL-1, COX-2, PGE2, SOD and glutathione peroxidase | Inhibited expression of inflammatory mediators | [56] | |
| Anti-emetic | New assay method | Young 4-day-old male chicks | 5-HT3, 5-HT4, or NK1 receptor antagonism | [56] |
| Screening | Young chicks | Anti-emetic properties are demonstrated at doses of 10–50 mg/kg | [15] | |
| Antagonistic 5-HT3, 5-HT4, or NK-1 receptor action test | Digestive organ muscles | Inward Ca2+ efflux through cell membranes | [57] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fatima, S.; Farzeen, I.; Ashraf, A.; Aslam, B.; Ijaz, M.U.; Hayat, S.; Sarfraz, M.H.; Zafar, S.; Zafar, N.; Unuofin, J.O.; et al. A Comprehensive Review on Pharmacological Activities of Pachypodol: A Bioactive Compound of an Aromatic Medicinal Plant Pogostemon Cablin Benth. Molecules 2023, 28, 3469. https://doi.org/10.3390/molecules28083469
Fatima S, Farzeen I, Ashraf A, Aslam B, Ijaz MU, Hayat S, Sarfraz MH, Zafar S, Zafar N, Unuofin JO, et al. A Comprehensive Review on Pharmacological Activities of Pachypodol: A Bioactive Compound of an Aromatic Medicinal Plant Pogostemon Cablin Benth. Molecules. 2023; 28(8):3469. https://doi.org/10.3390/molecules28083469
Chicago/Turabian StyleFatima, Sehrish, Iqra Farzeen, Asma Ashraf, Bilal Aslam, Muhammad Umar Ijaz, Sumreen Hayat, Muhammad Hassan Sarfraz, Saima Zafar, Nimrah Zafar, Jeremiah Oshiomame Unuofin, and et al. 2023. "A Comprehensive Review on Pharmacological Activities of Pachypodol: A Bioactive Compound of an Aromatic Medicinal Plant Pogostemon Cablin Benth" Molecules 28, no. 8: 3469. https://doi.org/10.3390/molecules28083469
APA StyleFatima, S., Farzeen, I., Ashraf, A., Aslam, B., Ijaz, M. U., Hayat, S., Sarfraz, M. H., Zafar, S., Zafar, N., Unuofin, J. O., Lebelo, S. L., & Muzammil, S. (2023). A Comprehensive Review on Pharmacological Activities of Pachypodol: A Bioactive Compound of an Aromatic Medicinal Plant Pogostemon Cablin Benth. Molecules, 28(8), 3469. https://doi.org/10.3390/molecules28083469






