Constructing a Low–Cost Si–NSs@C/NG Composite by a Ball Milling–Catalytic Pyrolysis Method for Lithium Storage
Abstract
1. Introduction
2. Results and Discussion
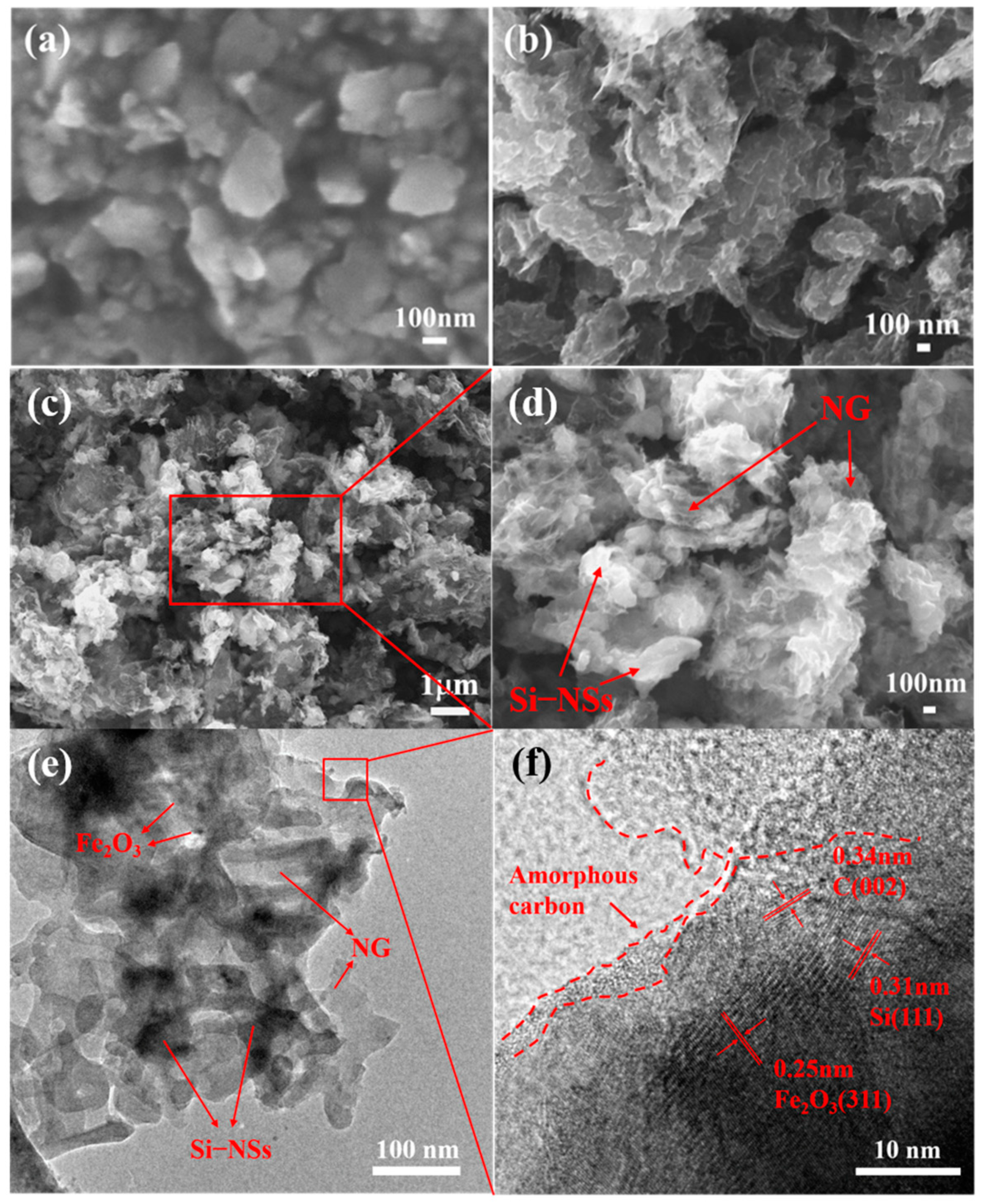
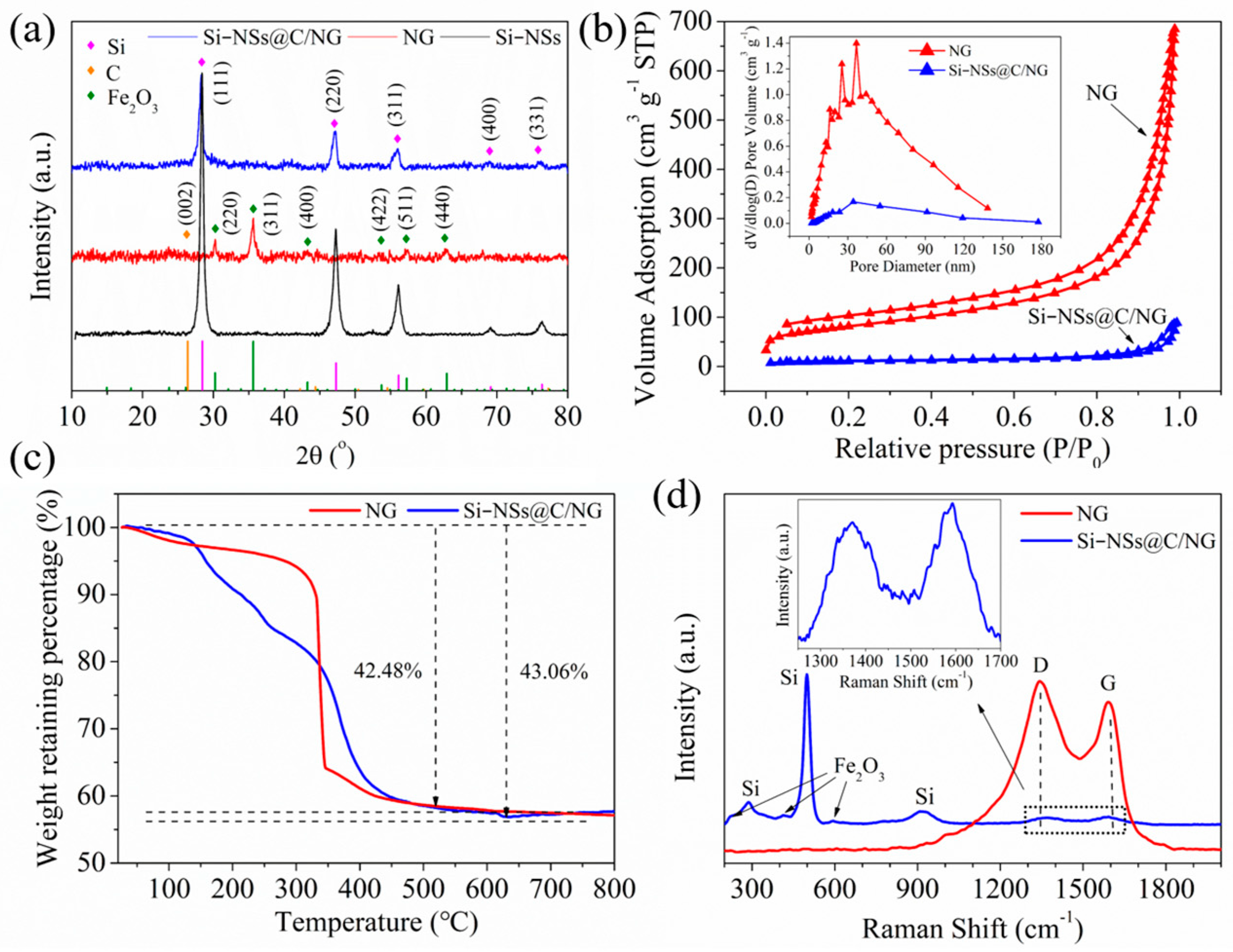
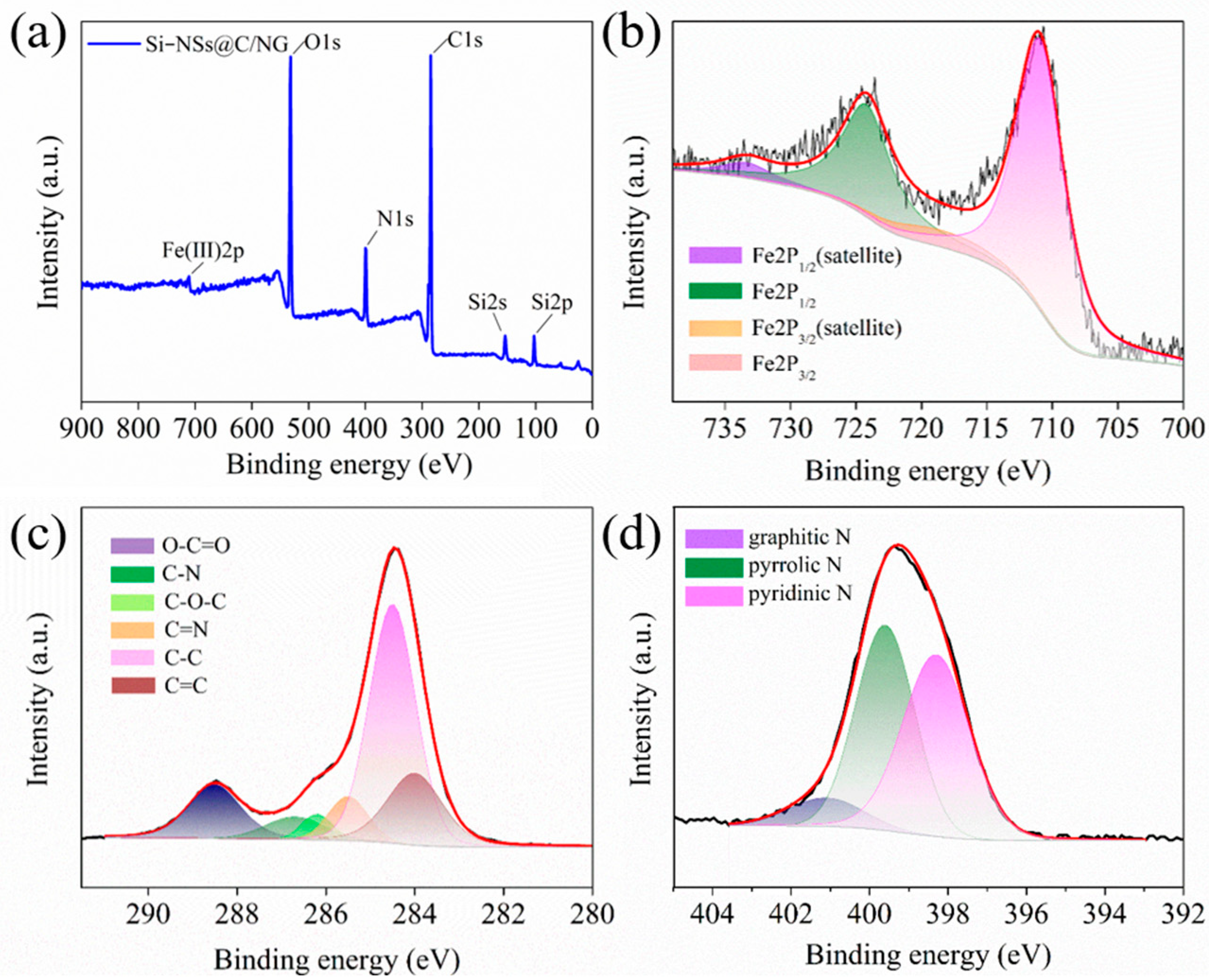
2.1. Synthesis and Microstructure Characterization
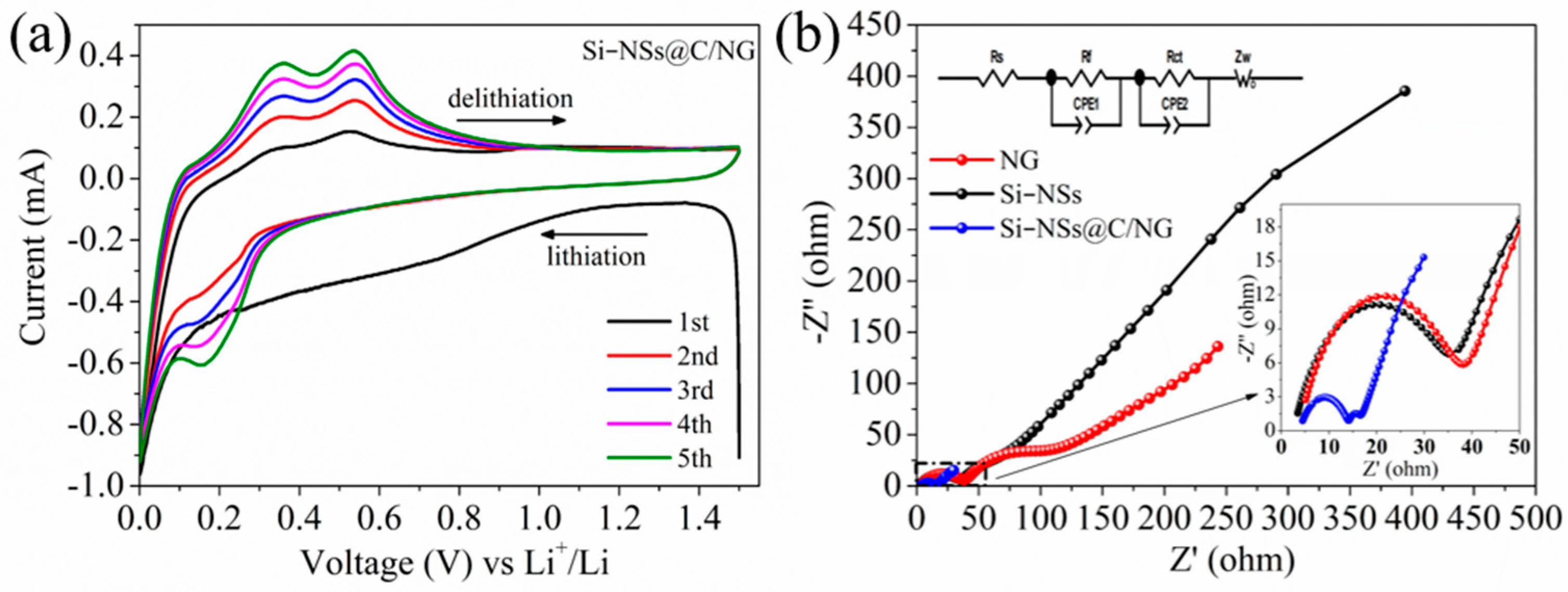
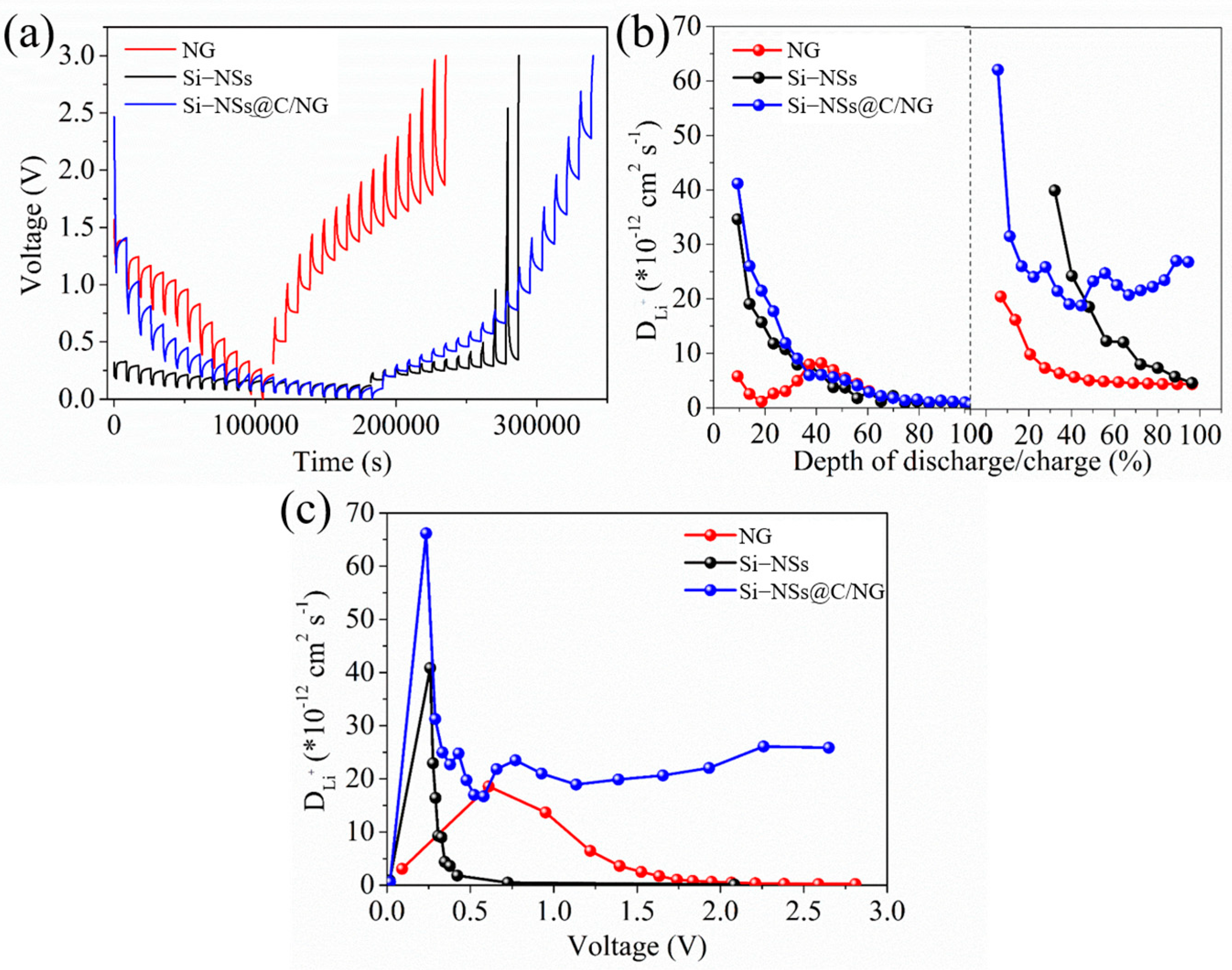
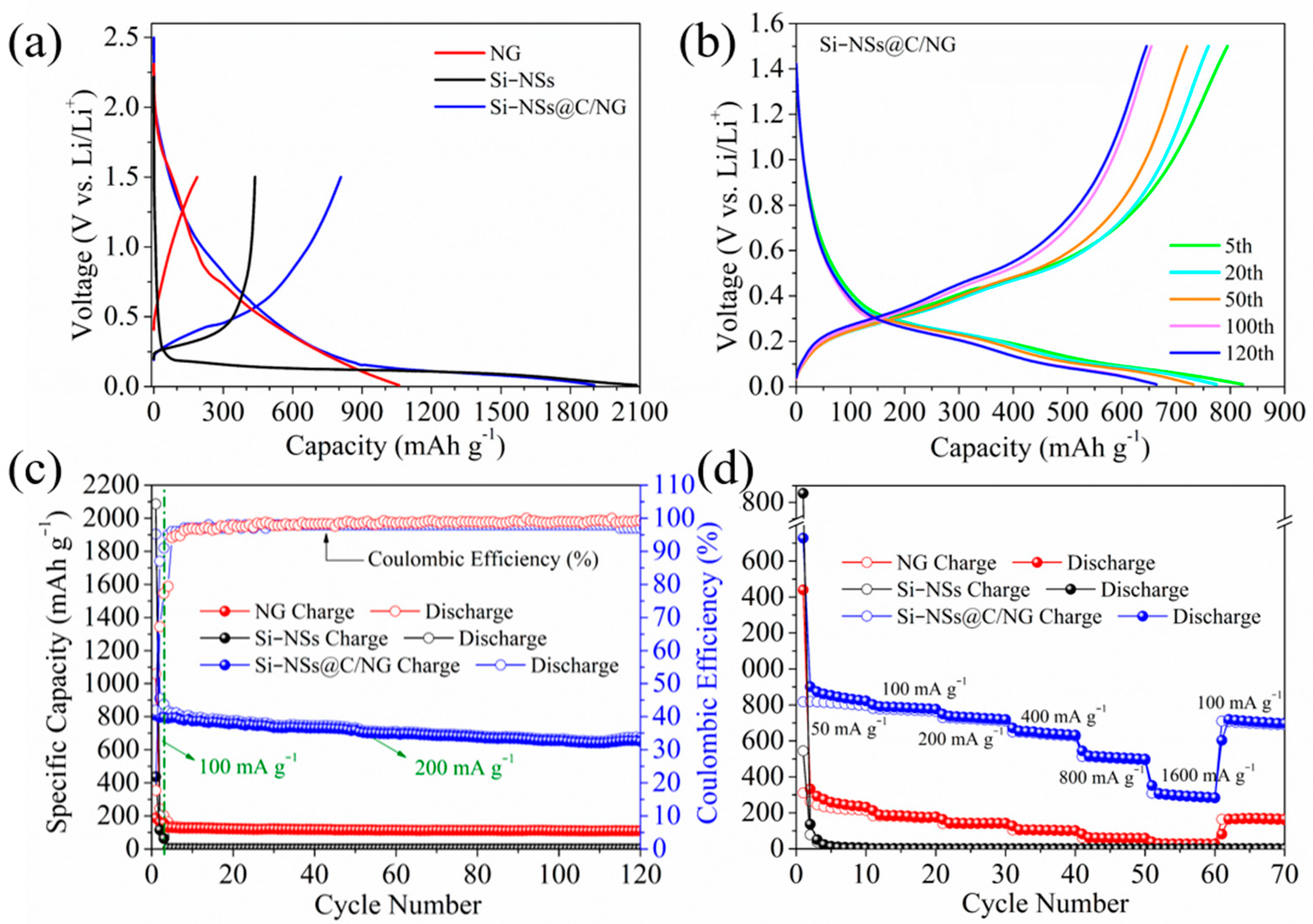
2.2. Electrochemical Performances
3. Conclusions
4. Materials and Methods
4.1. Materials Preparation
4.1.1. Preparation of g–C3N4 and the Si–NS Slurry
4.1.2. Preparation of Si–NSs@C/NG
4.2. Material Characterization
4.3. Electrochemical Measurement
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roy, K.; Banerjee, A.; Ogale, S. Search for New Anode Materials for High Performance Li-Ion Batteries. ACS Appl. Mater. Interfaces 2022, 14, 20326–20348. [Google Scholar] [CrossRef] [PubMed]
- Ai, W.; Kirkaldy, N.; Jiang, Y.; Offer, G.; Wang, H.; Wu, B. A composite electrode model for lithium-ion batteries with silicon/graphite negative electrodes. J. Power Source 2022, 527, 231142. [Google Scholar] [CrossRef]
- Chen, X.; Zheng, J.; Li, L.; Chu, W. Strategy for enhanced performance of silicon nanoparticle anodes for lithium-ion batteries. R. Soc. Chem. 2022, 12, 17889–17897. [Google Scholar] [CrossRef]
- An, H.; Jiang, L.; Li, F.; Wu, P.; Zhu, X.; Wei, S.; Zhou, Y. Hydrogel-Derived Three-Dimensional Porous Si-CNT@G Nanocomposite with High-Performance Lithium Storage. Acta Phys.-Chim. Sin. 2020, 36, 1905034. [Google Scholar] [CrossRef]
- Yang, N.; Sun, J.; Shao, R.; Cao, Z.; Zhang, Z.; Dou, M.; Niu, J.; Wang, F. Stable and conductive carbon networks enabling high-performance silicon anodes for lithium-ion batteries. Cell Rep. Phys. Sci. 2022, 3, 100862. [Google Scholar] [CrossRef]
- Huang, J.; Zhu, Y.; Feng, Y.; Han, Y.; Gu, Z.; Liu, R.; Yang, D.; Chen, K.; Zhang, X.; Sun, W.; et al. Research Progress on Key Materials and Technologies for Secondary Batteries. Acta Phys. Chim. Sin. 2022, 38, 2208008. [Google Scholar] [CrossRef]
- Lin, L.; Xu, X.; Chu, C.; Majeed, M.K.; Yang, J. Mesoporous Amorphous Silicon: A Simple Synthesis of a High-Rate and Long-Life Anode Material for Lithium-Ion Batteries. Angew. Chem. Int. Ed. 2016, 55, 14063–14066. [Google Scholar] [CrossRef]
- Orthner, H.; Wiggers, H.; Loewenich, M.; Kilian, S.; Bade, S.; Lyubina, J. Direct gas phase synthesis of amorphous Si/C nanoparticles as anode material for lithium ion battery. J. Alloy Compd. 2021, 870, 159315. [Google Scholar] [CrossRef]
- Kumar, P.; Berhaut, C.L.; Zapata Dominguez, D.; De Vito, E.; Tardif, S.; Pouget, S.; Lyonnard, S.; Jouneau, P.H. Nano-Architectured Composite Anode Enabling Long-Term Cycling Stability for High-Capacity Lithium-Ion Batteries. Small 2020, 16, e1906812. [Google Scholar] [CrossRef]
- Li, M.; Gu, J.; Feng, X.; He, H.; Zeng, C. Amorphous-silicon@silicon oxide/chromium/carbon as an anode for lithium–ion batteries with excellent cyclic stability. Electrochim. Acta 2015, 164, 163–170. [Google Scholar] [CrossRef]
- Shi, Q.; Zhou, J.; Ullah, S.; Yang, X.; Tokarska, K.; Trzebicka, B.; Ta, H.Q.; Rümmeli, M.H. A review of recent developments in Si/C composite materials for Li-ion batteries. Energy Storage Mater. 2021, 34, 735–754. [Google Scholar] [CrossRef]
- Zhang, W.; Fang, S.; Wang, N.; Zhang, J.; Shi, B.; Yu, Z.; Yang, J. A compact silicon-carbon composite with an embedded structure for high cycling coulombic efficiency anode materials in lithium-ion batteries. Inorg. Chem. Front. 2020, 7, 2487–2496. [Google Scholar] [CrossRef]
- Sun, X.; Wang, C.; Zhang, X.; Zhai, X.; Feng, J.; Li, H. Nanodiamond-modified separators and optimized graphene anodes for highly enhanced performance of lithium-ion batteries. J. Alloy Compd. 2022, 929, 167204. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, H.; Tao, X.; Yang, Y.; Wang, J.; Yang, Z. Lithium silicates nanodots-decorated SiOx-C/graphene anode material with enhanced rate performance for lithium ion batteries. J. Alloy Compd. 2022, 924, 166521. [Google Scholar] [CrossRef]
- Cen, Y.; Sisson, R.; Qin, Q.; Liang, J. Current Progress of Si/Graphene Nanocomposites for Lithium-Ion Batteries. J. Carbon Res. 2018, 4, 18. [Google Scholar] [CrossRef]
- Tang, X.; Wen, G.; Song, Y. Stable silicon/3D porous N-doped graphene composite for lithium-ion battery anodes with self-assembly. Appl. Surf. Sci. 2018, 436, 398–404. [Google Scholar] [CrossRef]
- Liu, X.; Chao, D.; Zhang, Q.; Liu, H.; Hu, H.; Zhao, J.; Li, Y.; Huang, Y.; Lin, J.; Shen, Z.X. The roles of lithium-philic giant nitrogen-doped graphene in protecting micron-sized silicon anode from fading. Sci. Rep. 2015, 5, 15665. [Google Scholar] [CrossRef]
- Na, R.; Liu, Y.; Wu, Z.-P.; Cheng, X.; Shan, Z.; Zhong, C.-J.; Tian, J. Nano-Silicon composite materials with N-doped graphene of controllable and optimal pyridinic-to-pyrrolic structural ratios for lithium ion battery. Electrochim. Acta 2019, 321, 134742. [Google Scholar] [CrossRef]
- Gu, X.; Zhao, Y.; Sun, K.; Vieira, C.L.Z.; Jia, Z.; Cui, C.; Wang, Z.; Walsh, A.; Huang, S. Method of ultrasound-assisted liquid-phase exfoliation to prepare graphene. Ultrason. Sonochem. 2019, 58, 104630. [Google Scholar] [CrossRef]
- Tarcan, R.; Todor-Boer, O.; Petrovai, I.; Leordean, C.; Astilean, S.; Botiz, I. Reduced graphene oxide today. J. Mater. Chem. C 2020, 8, 1198–1224. [Google Scholar] [CrossRef]
- Saeed, M.; Alshammari, Y.; Majeed, S.A.; Al-Nasrallah, E. Chemical Vapour Deposition of Graphene-Synthesis, Characterisation, and Applications: A Review. Molecules 2020, 25, 173856. [Google Scholar] [CrossRef] [PubMed]
- Hu, E.; Yu, X.-Y.; Chen, F.; Wu, Y.; Hu, Y.; Lou, X.W.D. Graphene Layers-Wrapped Fe/Fe5C2 Nanoparticles Supported on N-doped Graphene Nanosheets for Highly Efficient Oxygen Reduction. Adv. Energy Mater. 2018, 8, 1702476. [Google Scholar] [CrossRef]
- Tang, Y.; Wang, X.; Chen, J.; Wang, X.; Wang, D.; Mao, Z. PVP-assisted synthesis of g-C3N4-derived N-doped graphene with tunable interplanar spacing as high-performance lithium/sodium ions battery anodes. Carbon 2021, 174, 98–109. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Y.; Zhang, L.; Xie, F.; Vasileff, A.; Qiao, S.Z. Graphitic Carbon Nitride (g-C3N4)-Derived N-Rich Graphene with Tuneable Interlayer Distance as a High-Rate Anode for Sodium-Ion Batteries. Adv. Mater. 2019, 31, 1901261. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Zhou, X.; Xu, H.; Hu, T.; Cao, P.; Luo, C.; Wang, J.; Yao, H.; Tang, J.; Yang, J. Optimized design of 3D nitrogen-doped graphene-like carbon derived from g-C3N4 encapsulated nano-Si as high-performance anode for lithium-ion batteries. J. Electroanal. Chem. 2022, 907, 116048. [Google Scholar] [CrossRef]
- Jiang, X.; Liu, Y.; Wang, T.; Xia, B.; Qian, J.; Ran, J.; Zhang, Z.; Gao, D. High-temperature ferromagnetism in non-metal carbonitride: From nitrogen vacant g-C3N4 to N-doped graphene. J. Magn. Magn. Mater. 2021, 538, 168223. [Google Scholar] [CrossRef]
- Park, S.-W.; Ha, J.H.; Park, J.M.; Cho, B.W.; Choi, H.-J. 2D Silicon Nanosheets/Carbon Composites Based Foldable Anode Electrode for Lithium-Ion Batteries. J. Electrochem. Soc. 2020, 167, 020556. [Google Scholar] [CrossRef]
- Han, X.; Zhao, D.; Meng, W.; Yang, H.; Zhao, M.; Duan, Y.; Tian, X. Graphene caging silicon nanoparticles anchored on graphene sheets for high performance Li-ion batteries. Appl. Surf. Sci. 2019, 484, 11–20. [Google Scholar] [CrossRef]
- Guo, H.; Chen, K.; Li, W.; Yuan, Z.; Yue, M.; Guo, Y.; Jiang, Y.; Zhao, L.; Wang, Y. Graphitic carbon nanochambers interweaved porous yolk-shell skeleton for long-lifespan lithium-ion batteries. J. Alloy Compd. 2022, 898, 162831. [Google Scholar] [CrossRef]
- Tang, X.; Zeng, D.; Chen, D.; Guo, W.; Fu, J.; Zou, L. Porous silicon particles embedded in N-doped graphene and carbon nanotube framework for high-performance lithium-ion batteries. J. Alloy Compd. 2022, 927, 167055. [Google Scholar] [CrossRef]
- Lv, K.; Wang, P.; Wang, C.; Shen, Z.; Lu, Z.; Zhang, H.; Zheng, M.; He, P.; Zhou, H. Oxygen-Deficient Ferric Oxide as an Electrochemical Cathode Catalyst for High-Energy Lithium-Sulfur Batteries. Small 2020, 16, 2000870. [Google Scholar] [CrossRef]
- Zaidi, S.D.A.; Wang, C.; Gyorgy, B.; Sun, C.; Yuan, H.; Tian, L.; Chen, J. Iron and silicon oxide doped/PAN-based carbon nanofibers as free-standing anode material for Li-ion batteries. J. Colloid Interface Sci. 2020, 569, 164–176. [Google Scholar] [CrossRef] [PubMed]
- Ting, P.-M.; Huang, J.-Y.; Muruganantham, R.; Liu, W.-R. Nitrogen-doping effects on few-layer graphene as an anode material for lithium-ion batteries. Mater. Today Commun. 2022, 31, 103498. [Google Scholar] [CrossRef]
- Liu, S.; Wei, W.; Wang, Y.; Ren, J. Novel sponge-like N-doped graphene film as high-efficiency electrode for Li-ion battery. Appl. Surf. Sci. 2019, 485, 529–535. [Google Scholar] [CrossRef]
- Ding, B.; Ahsan, Z.; Huang, X.; Cai, Z.; Ma, Y.; Song, G.; Yang, W.; Wen, C. Preparation and electrochemical properties of high capacity silicon-based composites for lithium-ion batteries. Synth. Met. 2020, 261, 116324. [Google Scholar] [CrossRef]
- Lu, Z.; Li, B.; Yang, D.; Lv, H.; Xue, M.; Zhang, C. A self-assembled silicon/phenolic resin-based carbon core-shell nanocomposite as an anode material for lithium-ion batteries. R. Soc. Chem. 2018, 8, 3477–3482. [Google Scholar] [CrossRef]
- Zeng, H.; Han, F. Large-Scale Synthesis of Silicon-Based Nanocomposites in Air Atmosphere for Lithium-Ion Batteries by Ball-Milling Method. J. Electron. Mater. 2022, 51, 4329–4336. [Google Scholar] [CrossRef]
- Zhou, Y.-Y.; Zhang, Z.-Y.; Zhang, H.-Z.; Li, Y.; Weng, Y. Progress and perspective of vanadium-based cathode materials for lithium ion batteries. Tungsten 2021, 3, 279–288. [Google Scholar] [CrossRef]
- Chen, Z.-Z.; Hou, J.-G.; Zhou, J.; Huang, P.; Wang, H.-Q.; Xu, C.-X. Carbon shell coated hollow NiCoSex composite as high-performance anode for lithium storage. Rare Met. 2021, 40, 3185–3194. [Google Scholar] [CrossRef]
- Shi, Q.; Wang, H.; Zhou, J.; Ta, H.Q.; Wang, J.; Lian, X.; Kurtyka, K.; Trzebicka, B.; Gemming, T.; Rümmeli, M.H. Synergistic protection of Si anode based on multi-dimensional graphitic carbon skeletons. Nano Res. 2022, 15, 8146–8155. [Google Scholar] [CrossRef]
- Sun, Y.-Q.; Fu, W.; Hu, Y.-X.; Vaughan, J.; Wang, L.-Z. The role of tungsten-related elements for improving the electrochemical performances of cathode materials in lithium ion batteries. Tungsten 2021, 3, 245–259. [Google Scholar] [CrossRef]
- Hu, J.-Z.; Liu, W.-J.; Zheng, J.-H.; Li, G.-C.; Bu, Y.-F.; Qiao, F.; Lian, J.-B.; Zhao, Y. Coral-like cobalt selenide/carbon nanosheet arrays attached on carbon nanofibers for high-rate sodium-ion storage. Rare Met. 2022. [Google Scholar] [CrossRef]
- Lin, J.; He, J.; Chen, Y.; Li, Q.; Yu, B.; Xu, C.; Zhang, W. Pomegranate-Like Silicon/Nitrogen-doped Graphene Microspheres as Superior-Capacity Anode for Lithium-Ion Batteries. Electrochim. Acta 2016, 215, 667–673. [Google Scholar] [CrossRef]
- Ji, D.; Wan, Y.; Yang, Z.; Li, C.; Xiong, G.; Li, L.; Han, M.; Guo, R.; Luo, H. Nitrogen-doped graphene enwrapped silicon nanoparticles with nitrogen-doped carbon shell: A novel nanocomposite for lithium-ion batteries. Electrochim. Acta 2016, 192, 22–29. [Google Scholar] [CrossRef]
- Chen, Q.; Zhu, R.; He, Q.; Liu, S.; Wu, D.; Fu, H.; Du, J.; Zhu, J.; He, H. In situ synthesis of a silicon flake/nitrogen-doped graphene-like carbon composite from organoclay for high-performance lithium-ion battery anodes. R. Soc. Chem. 2019, 55, 2644–2647. [Google Scholar]
- Mi, H.; Li, Y.; Zhu, P.; Chai, X.; Sun, L.; Zhuo, H.; Zhang, Q.; He, C.; Liu, J. Insitu coating of nitrogen-doped graphene-ike nanosheets on silicon as a stable anode for high-performance lithium-ion batteries. J. Mater. Chem. A 2014, 2, 11254–11260. [Google Scholar] [CrossRef]
- Ji, D.; Yang, Z.; Xiong, L.; Luo, H.; Xiong, G.; Zhu, Y.; Wan, Y. Effect of Si content on structure and electrochemical performance of ternary nanohybrids integrating Si nanoparticles, N-doped carbon shell, and nitrogen-doped graphene. R. Soc. Chem. 2017, 7, 4209–4215. [Google Scholar] [CrossRef]
- Ou, J.; Jin, F.; Wang, H.; Wu, S.; Zhang, H. Carbon coated Si nanoparticles anchored to graphene sheets with excellent cycle performance and rate capability for Lithium-ion battery anodes. Surf. Coat. Technol. 2021, 418, 127262. [Google Scholar] [CrossRef]


| Electrochemical Properties | ||||
|---|---|---|---|---|
| Anodes Material /Ref. | Preparation Methods | Current Density (mAg−1) | Specific Capacity (mAhg−1) /Cycle Number | Capacity Retention Rate (%) |
| 3DNG–P@Si [16] | improved Hummers method and electrostatic self–assembly | 1000 | 1017/200 | 75 |
| PSNGM [43] | spray drying | 100 | 1142/150 | 96.1 |
| NG/Si@NC [44] | solution–mixing and carbonization | 500 | 938/100 | 82 |
| Nano–Si@NG [18] | modified Hummers method | 2000 | 552/200 | 53 |
| Si/NG [45] | carbonization and low–temperature chemical reduction | 1000 | 1138/240 | 42 |
| G–Si [46] | carbon–thermal method | 200 | 819/50 | 62 |
| Si/NC/NG [47] | solution–mixing and carbonization method | 500 | 1210/100 | 58 |
| mSi@GNG [17] | modified Hummers method and freeze–drying | 500 | 510/80 | 58 |
| Si@NC/G [48] | drying and high–temperature calcination | 100 | 1045/200 | 56 |
| Si–NSs@C/NG | ball milling–catalytic pyrolysis method | 200 | 654/120 | 81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Song, N.-J.; Ma, C.-L.; Zhao, Y.; Li, Y.; Li, J.; Li, X.-M.; Kong, Q.-Q.; Chen, C.-M. Constructing a Low–Cost Si–NSs@C/NG Composite by a Ball Milling–Catalytic Pyrolysis Method for Lithium Storage. Molecules 2023, 28, 3458. https://doi.org/10.3390/molecules28083458
Zhang Q, Song N-J, Ma C-L, Zhao Y, Li Y, Li J, Li X-M, Kong Q-Q, Chen C-M. Constructing a Low–Cost Si–NSs@C/NG Composite by a Ball Milling–Catalytic Pyrolysis Method for Lithium Storage. Molecules. 2023; 28(8):3458. https://doi.org/10.3390/molecules28083458
Chicago/Turabian StyleZhang, Qi, Ning-Jing Song, Can-Liang Ma, Yun Zhao, Yong Li, Juan Li, Xiao-Ming Li, Qing-Qiang Kong, and Cheng-Meng Chen. 2023. "Constructing a Low–Cost Si–NSs@C/NG Composite by a Ball Milling–Catalytic Pyrolysis Method for Lithium Storage" Molecules 28, no. 8: 3458. https://doi.org/10.3390/molecules28083458
APA StyleZhang, Q., Song, N.-J., Ma, C.-L., Zhao, Y., Li, Y., Li, J., Li, X.-M., Kong, Q.-Q., & Chen, C.-M. (2023). Constructing a Low–Cost Si–NSs@C/NG Composite by a Ball Milling–Catalytic Pyrolysis Method for Lithium Storage. Molecules, 28(8), 3458. https://doi.org/10.3390/molecules28083458





