Effective Removal of Fe (III) from Strongly Acidic Wastewater by Pyridine-Modified Chitosan: Synthesis, Efficiency, and Mechanism
Abstract
1. Introduction
2. Results and Discussion
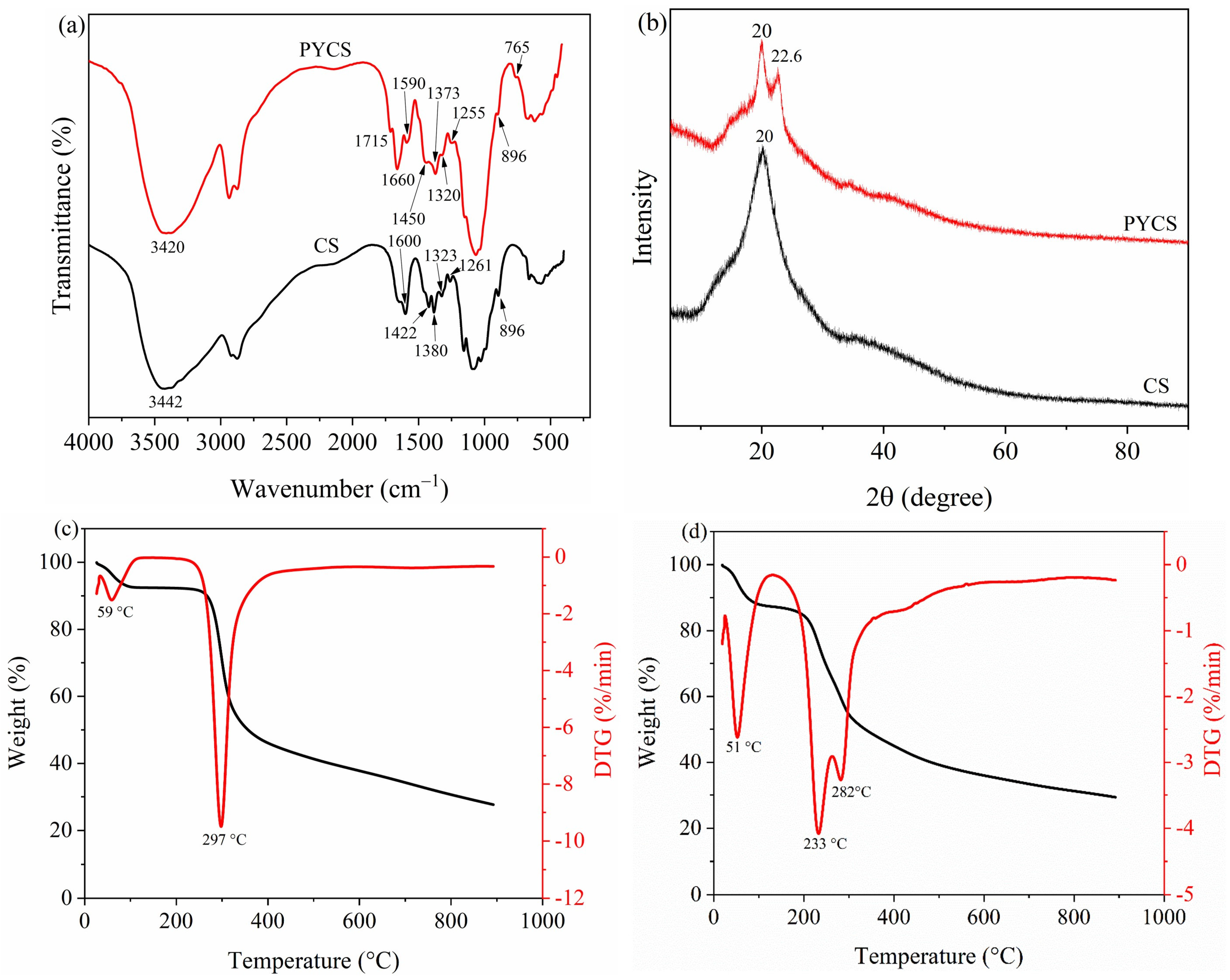
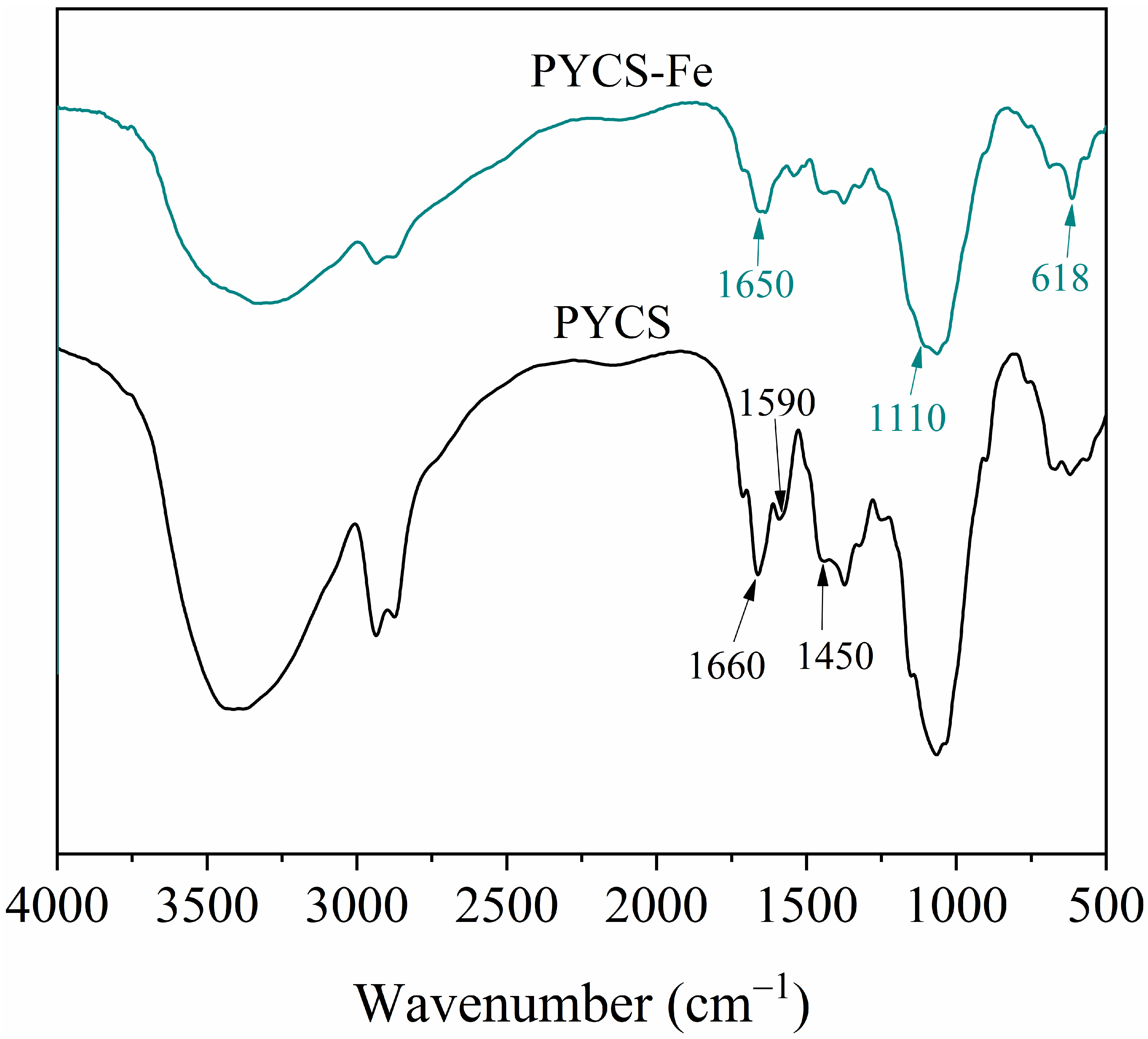
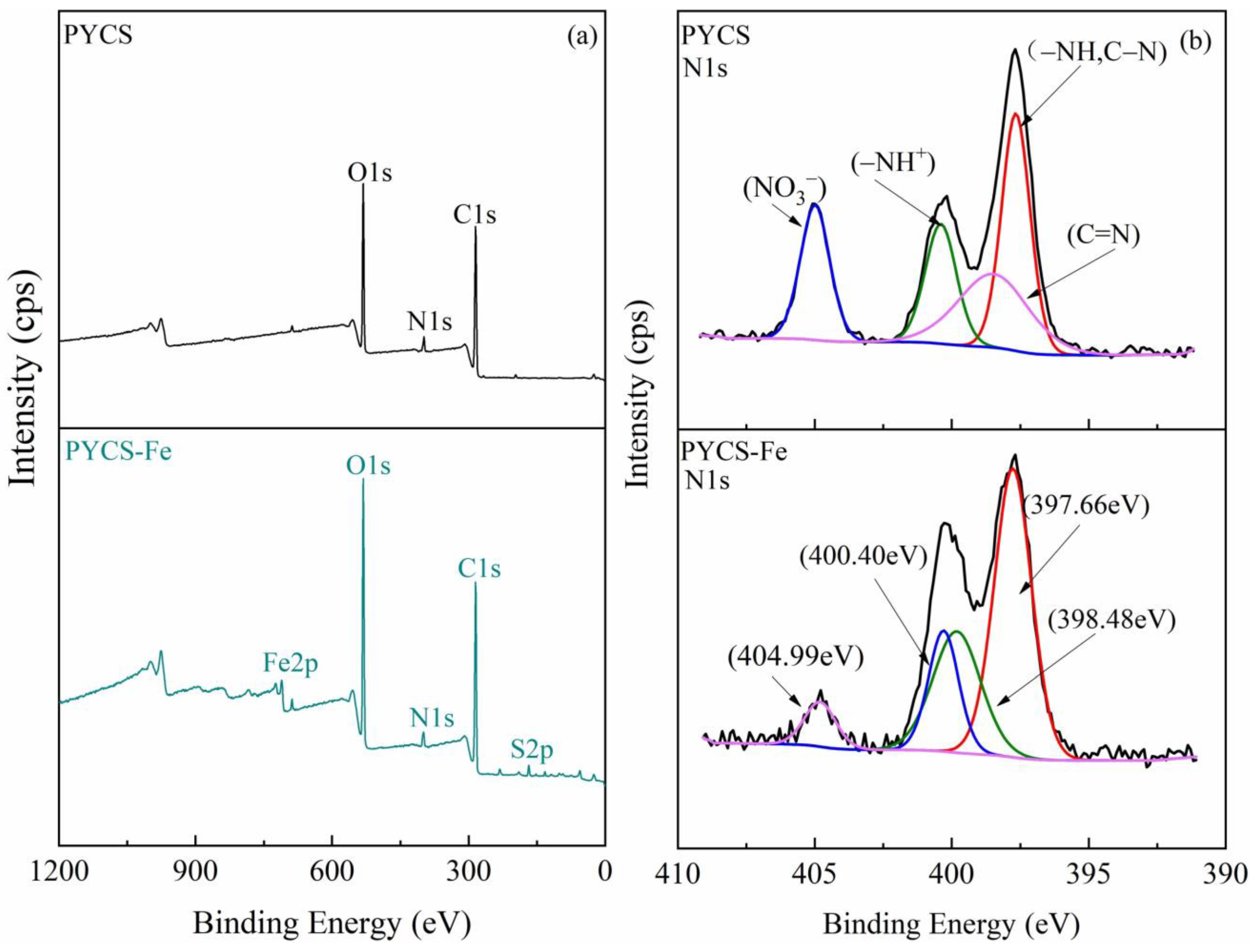
2.1. Characterization
2.2. Effect of pH on Adsorption
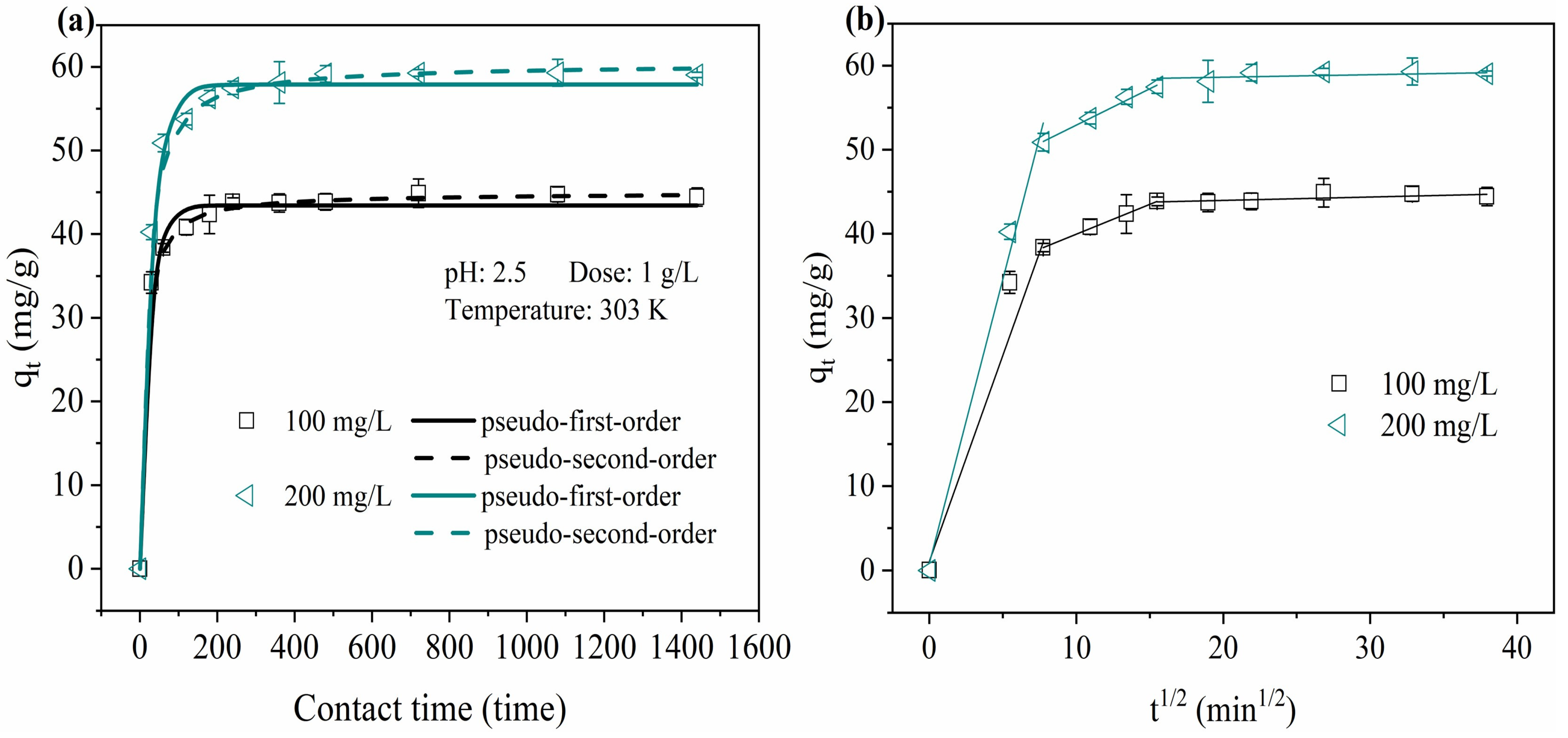
2.3. Adsorption Kinetics
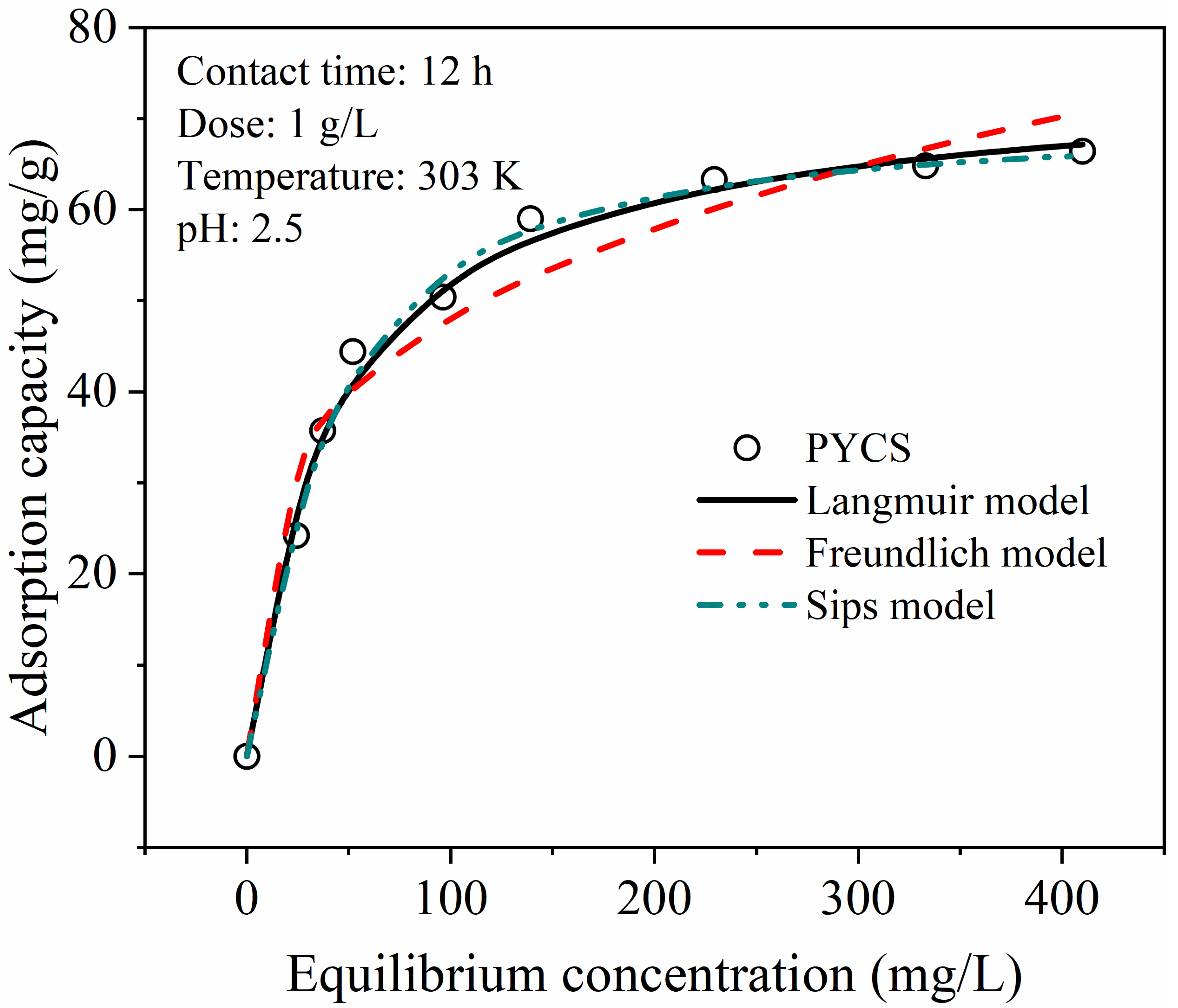
2.4. Adsorption Isotherm
2.5. Adsorption Thermodynamics
2.6. Regeneration and Reusability
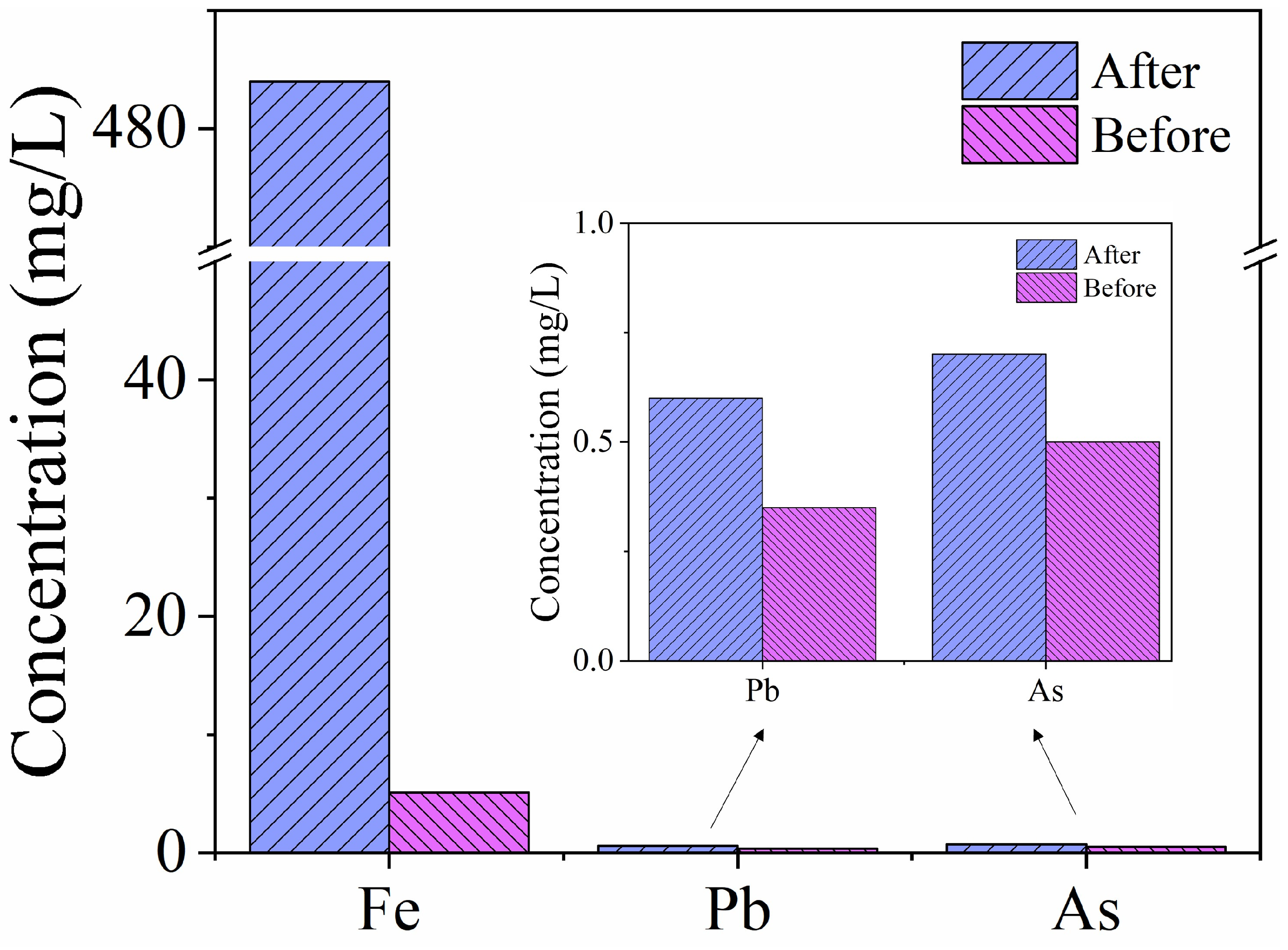
2.7. Application in Real Acidic Wastewater
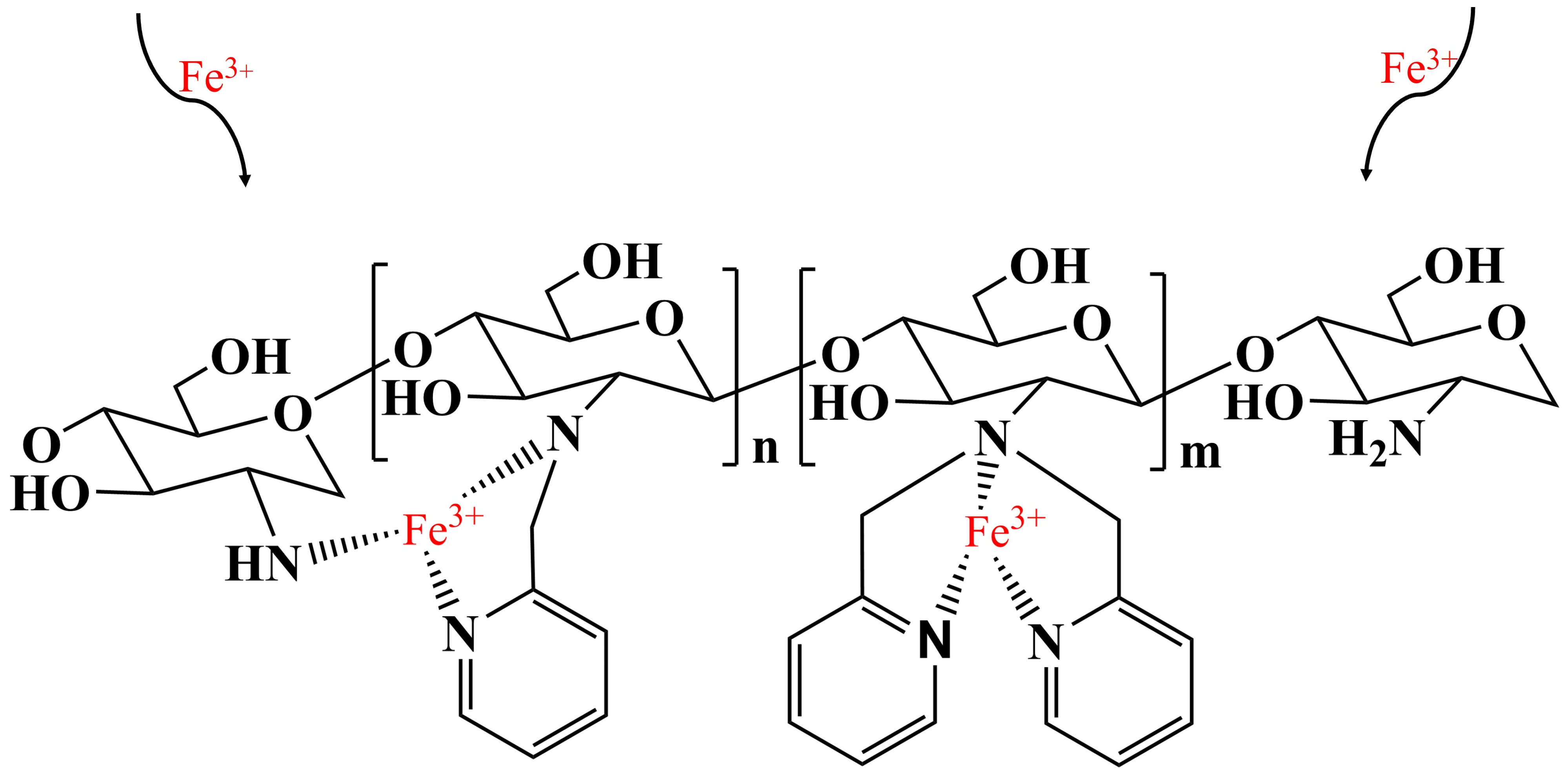
2.8. Adsorption Mechanism
3. Materials and Methods
3.1. Chemical and Materials
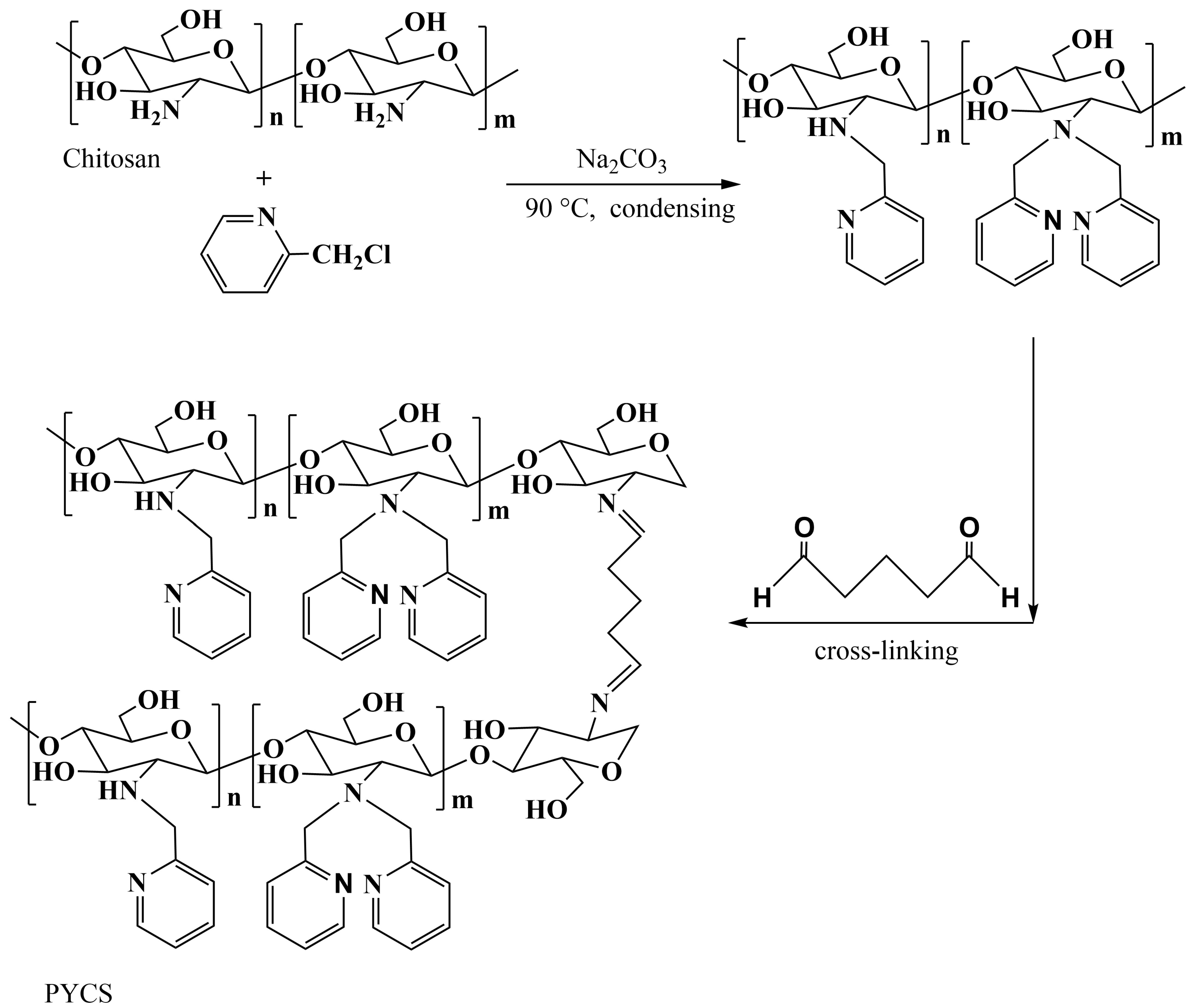
3.2. Preparation of Pyridine-Modified Chitosan
3.3. Characterization
3.4. Adsorption Experiments
3.5. Regeneration Studies
3.6. Application in Real Acidic Wastewater
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Zhang, W.; Oswal, H.; Renew, J.; Ellison, K.; Huang, C.H. Removal of heavy metals by aged zero-valent iron from flue-gas-desulfurization brine under high salt and temperature conditions. J. Hazard. Mater. 2019, 373, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Solinska, A.; Bajda, T. Modified zeolite as a sorbent for removal of contaminants from wet flue gas desulphurization wastewater. Chemosphere 2022, 286, 131772. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Zhu, F.; Kong, L.; Peng, X. Sulfate radical-based removal of chloride ion from strongly acidic wastewater: Kinetics and mechanism. J. Hazard. Mater. 2021, 410, 124540. [Google Scholar] [CrossRef] [PubMed]
- You, S.; Lu, J.; Tang, C.Y.; Wang, X. Rejection of heavy metals in acidic wastewater by a novel thin-film inorganic forward osmosis membrane. Chem. Eng. J. 2017, 320, 532–538. [Google Scholar] [CrossRef]
- Pi, X.; Sun, F.; Qu, Z.; Li, Y.; Gao, J. Hierarchical pore configuration in activated coke boosting direct desorption of desulfurization product H2SO4: A combined experimental and computational investigation. Fuel 2021, 298, 120697. [Google Scholar] [CrossRef]
- Guo, H.; Yuan, P.; Pavlovic, V.; Barber, J.; Kim, Y. Ammonium sulfate production from wastewater and low-grade sulfuric acid using bipolar- and cation-exchange membranes. J. Clean. Prod. 2021, 285, 124888. [Google Scholar] [CrossRef]
- You, X.; Chen, J.; Pan, S.; Lu, G.; Teng, L.; Lin, X.; Zhao, S.; Lin, J. Piperazine-functionalized porous anion exchange membranes for efficient acid recovery by diffusion dialysis. J. Membr. Sci. 2022, 654, 120560. [Google Scholar] [CrossRef]
- Akoto, J.D.; Chai, F.; Repo, E.; Yang, Z.; Wang, D.; Zhao, F.; Liao, Q.; Chai, L. Polyethyleneimine stabilized nanoscale zero-valent iron-magnetite (Fe3O4@nZVI-PEI) for the enhanced removal of arsenic from acidic aqueous solution: Performance and mechanisms. J. Environ. Chem. Eng. 2022, 10, 108589. [Google Scholar] [CrossRef]
- Silva, J.E.; Paiva, A.P.; Soares, D.; Labrincha, A.; Castro, F. Solvent extraction applied to the recovery of heavy metals from galvanic sludge. J. Hazard. Mater. 2005, 120, 113–118. [Google Scholar] [CrossRef]
- Nenov, V.; Dimitrova, N.; Dobrevsky, I. Recovery of sulphuric acid from waste aqueous solutions containing arsenic by ion exchange. Hydrometallurgy 1997, 44, 43–52. [Google Scholar] [CrossRef]
- Wang, P.; Tang, Y.; Liu, Y.; Wang, T.; Wu, P.; Lu, X.Y. Halloysite nanotube@carbon with rich carboxyl groups as a multifunctional adsorbent for the efficient removal of cationic Pb(II), anionic Cr(VI) and methylene blue (MB). Environ. Sci. Nano 2018, 5, 2257–2268. [Google Scholar] [CrossRef]
- Huang, Y.; Zheng, H.; Hu, X.; Wu, Y.; Tang, X.; He, Q.; Peng, S. Enhanced selective adsorption of lead(II) from complex wastewater by DTPA functionalized chitosan-coated magnetic silica nanoparticles based on anion-synergism. J. Hazard. Mater. 2022, 422, 126856. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhuang, S. Chitosan-based materials: Preparation, modification and application. J. Clean. Prod. 2022, 355, 131825. [Google Scholar] [CrossRef]
- Saleh, A.S.; Ibrahim, A.G.; Elsharma, E.M.; Metwally, E.; Siyam, T. Radiation grafting of acrylamide and maleic acid on chitosan and effective application for removal of Co(II) from aqueous solutions. Radiat. Phys. Chem. 2018, 144, 116–124. [Google Scholar] [CrossRef]
- Zhang, C.; Wen, H.; Huang, Y.; Shi, W. Adsorption of anionic surfactants from aqueous solution by high content of primary amino crosslinked chitosan microspheres. Int. J. Biol. Macromol. 2017, 97, 635–641. [Google Scholar] [CrossRef]
- Hu, X.J.; Wang, J.S.; Liu, Y.G.; Li, X.; Zeng, G.M.; Bao, Z.L.; Zeng, X.X.; Chen, A.W.; Long, F. Adsorption of chromium(VI) by ethylenediamine-modified cross-linked magnetic chitosan resin: Isotherms, kinetics and thermodynamics. J. Hazard. Mater. 2011, 185, 306–314. [Google Scholar] [CrossRef]
- Karthik, R.; Meenakshi, S. Removal of Pb(II) and Cd(II) ions from aqueous solution using polyaniline grafted chitosan. Chem. Eng. J. 2015, 263, 168–177. [Google Scholar] [CrossRef]
- Zhao, Z.; Huang, Y.; Wu, Y.; Li, S.; Yin, H.; Wang, J. α-ketoglutaric acid modified chitosan/polyacrylamide semi-interpenetrating polymer network hydrogel for removal of heavy metal ions. Colloids Surf. A Physicochem. Eng. Asp. 2021, 628, 127262. [Google Scholar] [CrossRef]
- Rosli, N.; Yahya, W.Z.N.; Wirzal, M.D.H. Crosslinked chitosan/poly(vinyl alcohol) nanofibers functionalized by ionic liquid for heavy metal ions removal. Int. J. Biol. Macromol. 2022, 195, 132–141. [Google Scholar] [CrossRef]
- Zong, L.; Liu, F.; Chen, D.; Zhang, X.; Ling, C.; Li, A. A novel pyridine based polymer for highly efficient separation of nickel from high-acidity and high-concentration cobalt solutions. Chem. Eng. J. 2018, 334, 995–1005. [Google Scholar] [CrossRef]
- Zou, B.; Zhang, S.; Sun, P.; Zhao, Q.; Zhang, W.; Zhang, X.; Ran, L.; Zhou, L.; Ye, Z. Synthesis of a novel Poly-chloromethyl styrene chelating resin containing Tri-pyridine aniline groups and its efficient adsorption of heavy metal ions and catalytic degradation of bisphenol A. Sep. Purif. Technol. 2021, 275, 119234. [Google Scholar] [CrossRef]
- Zahedifar, M.; Es-haghi, A.; Zhiani, R.; Sadeghzadeh, S.M. Synthesis of benzimidazolones by immobilized gold nanoparticles on chitosan extracted from shrimp shells supported on fibrous phosphosilicate. RSC Adv. 2019, 9, 6494–6501. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.G.; Fouda, A.; Elgammal, W.E.; Eid, A.M.; Elsenety, M.M.; Mohamed, A.E.; Hassan, S.M. New thiadiazole modified chitosan derivative to control the growth of human pathogenic microbes and cancer cell lines. Sci. Rep. 2022, 12, 21423. [Google Scholar] [CrossRef] [PubMed]
- Samuels, R.J. Solid state characterization of the structure of chitosan films. J. Polym. Sci. Polym. Phys. Ed. 1981, 19, 1081–1105. [Google Scholar] [CrossRef]
- Thien, D.; An, N.; Hoa, N. Preparation of Fully Deacetylated Chitosan for Adsorption of Hg(II) Ion from Aqueous Solution. Chem. Sci. 2015, 6, 1. [Google Scholar]
- Ali, N.; Khan, A.; Bilal, M.; Malik, S.; Badshah, S.; Iqbal, H.M.N. Chitosan-Based Bio-Composite Modified with Thiocarbamate Moiety for Decontamination of Cations from the Aqueous Media. Molecules 2020, 25, 226. [Google Scholar] [CrossRef]
- Yu, C.; Liu, X.; Pei, J.; Wang, Y. Grafting of laccase-catalysed oxidation of butyl paraben and p-coumaric acid onto chitosan to improve its antioxidant and antibacterial activities. React. Funct. Polym. 2020, 149, 104511. [Google Scholar] [CrossRef]
- Zawadzki, J.; Kaczmarek, H. Thermal treatment of chitosan in various conditions. Carbohydr. Polym. 2010, 80, 394–400. [Google Scholar] [CrossRef]
- Chung, Y.C.; Tsai, C.F.; Li, C.F. Preparation and characterization of water-soluble chitosan produced by Maillard reaction. Fish Sci. 2006, 72, 1096–1103. [Google Scholar] [CrossRef]
- Chen, L.; Tang, J.; Wu, S.; Wang, S.; Ren, Z. Selective removal of Au(III) from wastewater by pyridine-modified chitosan. Carbohydr. Polym. 2022, 286, 119307. [Google Scholar] [CrossRef]
- Zhan, W.; Xu, C.; Qian, G.; Huang, G.; Tang, X.; Lin, B. Adsorption of Cu(II), Zn(II), and Pb(II) from aqueous single and binary metal solutions by regenerated cellulose and sodium alginate chemically modified with polyethyleneimine. RSC Adv. 2018, 8, 18723–18733. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Yu, Z.; Islam, S.M.; Shi, K.; Cheng, Y.; Yuan, M.; Zhao, J.; Sun, G.; Li, H.; Ma, S.; et al. Remarkable acid stability of polypyrrole-MoS4: A highly selective and efficient scavenger of heavy metals over a wide pH range. Adv. Funct. Mater. 2018, 28, 1800502. [Google Scholar] [CrossRef]
- Zou, B.; Zhang, S.; Sun, P.; Ye, Z.; Zhao, Q.; Zhang, W.; Zhou, L. Preparation of a novel Poly-chloromethyl styrene chelating resin containing heterofluorenone pendant groups for the removal of Cu(II), Pb(II), and Ni(II) from wastewaters. Colloids Interface Sci. Commun. 2021, 40, 100349. [Google Scholar] [CrossRef]
- Ma, J.; Shen, J.; Wang, C.; Wei, Y. Preparation of dual-function chelating resin with high capacity and adjustable adsorption selectivity to variety of heavy metal ions. J. Taiwan Inst. Chem. Eng. 2018, 91, 532–538. [Google Scholar] [CrossRef]
- Lagergren, S. About the theory of so-called adsorption of solution substances, Kung. Sven. Veten. Hand. 1898, 24, 1–39. [Google Scholar]
- Ho, Y.S.; McKay, G. Sorption of dye from aqueous solution by peat. Chem. Eng. J. 1998, 70, 115–124. [Google Scholar] [CrossRef]
- Weber Walter, J.; Morris, J.C. Kinetics of Adsorption on Carbon from Solution. J. Sanit. Eng. Div. Am. Soc. Civ. Eng. 1963, 89, 31–59. [Google Scholar] [CrossRef]
- Liu, L.; Hu, S.; Shen, G.; Farooq, U.; Zhang, W.; Lin, S.; Lin, K. Adsorption dynamics and mechanism of aqueous sulfachloropyridazine and analogues using the root powder of recyclable long-root Eichhornia crassipes. Chemosphere 2018, 196, 409–417. [Google Scholar] [CrossRef]
- Huang, Y.; Keller, A.A. EDTA functionalized magnetic nanoparticle sorbents for cadmium and lead contaminated water treatment. Water Res. 2015, 80, 159–168. [Google Scholar] [CrossRef]
- Repo, E.; Warchol, J.K.; Kurniawan, T.A.; Sillanpää, M.E.T. Adsorption of Co(II) and Ni(II) by EDTA- and/or DTPA-modified chitosan: Kinetic and equilibrium modeling. Chem. Eng. J. 2010, 161, 73–82. [Google Scholar] [CrossRef]
- Repo, E.; Warchoł, J.K.; Bhatnagar, A.; Sillanpää, M. Heavy metals adsorption by novel EDTA-modified chitosan–silica hybrid materials. J. Colloid Interface Sci. 2011, 358, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Repo, E.; Yin, D.; Sillanpää, M.E.T. Adsorption of Cd(II) and Pb(II) by a novel EGTA-modified chitosan material: Kinetics and isotherms. J. Colloid Interface Sci. 2013, 409, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Langmuir, I. The constitution and fundamental properties of solids and liquids. J. Am. Chem. Soc. 1917, 183, 102–105. [Google Scholar] [CrossRef]
- Freundlich, H. Über die Adsorption in Lösungen. Z. Phys. Chem. 1907, 57U, 385–470. [Google Scholar] [CrossRef]
- Zhao, F.; Repo, E.; Yin, D.; Meng, Y.; Jafari, S.; Sillanpää, M. EDTA-Cross-Linked β-Cyclodextrin: An Environmentally Friendly Bifunctional Adsorbent for Simultaneous Adsorption of Metals and Cationic Dyes. Environ. Sci. Technol. 2015, 49, 10570–10580. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Pan, J.; Ou, H.; Wang, X.; Guan, W.; Li, C.; Yan, Y.; Duan, Y. Adsorptive removal of Cr(III) and Fe(III) from aqueous solution by chitosan/attapulgite composites: Equilibrium, thermodynamics and kinetics. Chem. Eng. J. 2011, 167, 112–121. [Google Scholar] [CrossRef]
- Shehap, A.M.; Nasr, R.A.; Mahfouz, M.A.; Ismail, A.M. Preparation and characterizations of high doping chitosan/MMT nanocomposites films for removing iron from ground water. J. Environ. Chem. Eng. 2021, 9, 104700. [Google Scholar] [CrossRef]
- Marques, J.L.; Lütke, S.F.; Frantz, T.S.; Espinelli, J.B.S.; Carapelli, R.; Pinto, L.A.A.; Cadaval, T.R.S. Removal of Al(III) and Fe(III) from binary system and industrial effluent using chitosan films. Int. J. Biol. Macromol. 2018, 120, 1667–1673. [Google Scholar] [CrossRef]
- Habiba, U.; Siddique, T.A.; Talebian, S.; Lee, J.J.L.; Salleh, A.; Ang, B.C.; Afifi, A.M. Effect of deacetylation on property of electrospun chitosan/PVA nanofibrous membrane and removal of methyl orange, Fe(III) and Cr(VI) ions. Carbohydr. Polym. 2017, 177, 32–39. [Google Scholar] [CrossRef]
- Wang, M.; Xu, L.; Zhai, M.; Peng, J.; Li, J.; Wei, G. γ-ray radiation-induced synthesis and Fe(III) ion adsorption of carboxymethylated chitosan hydrogels. Carbohydr. Polym. 2008, 74, 498–503. [Google Scholar] [CrossRef]
- Ngah, W.S.W.; Ab Ghani, S.; Kamari, A. Adsorption behaviour of Fe(II) and Fe(III) ions in aqueous solution on chitosan and cross-linked chitosan beads. Bioresour. Technol. 2005, 96, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Khalil, M.M.H.; Al-Wakeel, K.Z.; Rehim, S.S.A.E.; Monem, H.A.E. Efficient removal of ferric ions from aqueous medium by amine modified chitosan resins. J. Environ. Chem. Eng. 2013, 1, 566–573. [Google Scholar] [CrossRef]
- Li, W.; Wei, H.; Liu, Y.; Li, S.; Wang, G.; Han, H. Fabrication of novel starch-based composite hydrogel microspheres combining Diels-Alder reaction with spray drying for MB adsorption. J. Environ. Chem. Eng. 2021, 9, 105929. [Google Scholar] [CrossRef]
- Sultan, M.; Mansor, E.S.; Nagieb, Z.A.; Elsayed, H. Fabrication of highly efficient nano-composite films based on ZnO-g-C3N4 @ PAA-g-(HEC/PVA)-Fe3+ for removal of methylene blue dye from water. J. Water Process. Eng. 2021, 42, 102184. [Google Scholar] [CrossRef]
- Hu, H.; Gao, H.; Chen, D.; Li, G.; Tan, Y.; Liang, G.; Zhu, F.; Wu, Q. Ligand-Directed Regioselectivity in Amine–Imine Nickel-Catalyzed 1-Hexene Polymerization. ACS Catal. 2015, 5, 122–128. [Google Scholar] [CrossRef]
- Bertóti, I.; Mohai, M.; László, K. Surface modification of graphene and graphite by nitrogen plasma: Determination of chemical state alterations and assignments by quantitative X-ray photoelectron spectroscopy. Carbon 2015, 84, 185–196. [Google Scholar] [CrossRef]
- Outirite, M.; Lagrenée, M.; Lebrini, M.; Traisnel, M.; Jama, C.; Vezin, H.; Bentiss, F. Ac impedance X-ray photoelectron spectroscopy and density functional theory studies of 3,5-bis(n-pyridyl)-1,2,4-oxadiazoles as efficient corrosion inhibitors for carbon steel surface in hydrochloric acid solution. Electrochim. Acta 2010, 55, 1670–1681. [Google Scholar] [CrossRef]
- Lebrini, M.; Lagrenée, M.; Traisnel, M.; Gengembre, L.; Vezin, H.; Bentiss, F. Enhanced corrosion resistance of mild steel in normal sulfuric acid medium by 2,5-bis(n-thienyl)-1,3,4-thiadiazoles: Electrochemical, X-ray photoelectron spectroscopy and theoretical studies. Appl. Surf. Sci. 2007, 253, 9267–9276. [Google Scholar] [CrossRef]
- Latha, G.; Rajendran, N.; Rajeswari, S. Influence of alloying elements on the corrosion performance of alloy 33 and alloy 24 in seawater. J. Mater. Eng. Perform. 1997, 6, 743–748. [Google Scholar] [CrossRef]
- Wang, L.L.; Ling, C.; Li, B.S.; Zhang, D.S.; Li, C.; Zhang, X.P.; Shi, Z.F. Highly efficient removal of Cu(II) by novel dendritic polyamine-pyridine-grafted chitosan beads from complicated salty and acidic wastewaters. RSC Adv. 2020, 10, 19943–19951. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.P.; Liu, F.Q.; Zhu, C.Q.; Xu, C.; Chen, D.; Wei, M.-M.; Liu, J.; Li, C.H.; Ling, C.; Li, A.M.; et al. A novel tetraethylenepentamine functionalized polymeric adsorbent for enhanced removal and selective recovery of heavy metal ions from saline solutions. RSC Adv. 2015, 5, 75985–75997. [Google Scholar] [CrossRef]
- Babić, B.M.; Milonjić, S.K.; Polovina, M.J.; Kaludierović, B.V. Point of zero charge and intrinsic equilibrium constants of activated carbon cloth. Carbon 1999, 37, 477–481. [Google Scholar] [CrossRef]



| Kinetic Models | Parameters | Concentration (mg/L) | |
|---|---|---|---|
| 100 | 200 | ||
| Pseudo-first-order | qe,exp (mg/g) | 44.88 | 59.29 |
| qe,cal (mg/g) | 43.43 | 57.87 | |
| k1 (min−1) | 0.047 | 0.037 | |
| RMSE | 1.36 | 1.47 | |
| Pseudo-second-order | qe,cal (mg/g) | 44.96 | 60.38 |
| k2 (g/mg·min) | 0.0022 | 0.0012 | |
| RMSE | 0.38 | 0.72 | |
| Intra-particle diffusion | ki,1 (mg/g·min1/2) | 4.90 | 6.75 |
| ki,2 (mg/g·min1/2) | 0.71 | 0.86 | |
| ki,3 (mg/g·min1/2) | 0.04 | 0.03 | |
| Ci,1 (mg/g) | 1.10 | 0.86 | |
| Ci,2 (mg/g) | 32.89 | 44.30 | |
| Ci,3 (mg/g) | 43.19 | 58.03 | |
| Isothermal Models | Parameters | |
|---|---|---|
| Langmuir | qm,exp (mg/g) | 66.20 |
| qm,cal (mg/g) | 73.83 | |
| KL (L/mg) | 0.024 | |
| RMSE | 1.76 | |
| Freundlich | nF | 3.70 |
| KF (mg(1−n) Ln/g) | 13.93 | |
| RMSE | 4.33 | |
| Sips | qe,cal (mg/g) | 68.93 |
| nS | 1.27 | |
| KS (L/mg) | 0.027 | |
| RMSE | 1.41 |
| Adsorbent | Conditions | Adsorption Capacity (mg/g) | Reference | |
|---|---|---|---|---|
| pH | T (K) | |||
| Chitosan/attapulgite | 3 | 308 | 47.17 | [47] |
| Chitosan/MMT | 5.5 | 298 | 7.03 | [48] |
| Chitosan films | 4.5 | 298 | 299.04 | [49] |
| Chitosan/PVA | 3 | 298 | 136 | [50] |
| Carboxymethylated chitosan | 4.7 | 298 | 18.5 | [51] |
| Chitosan-EGDE | 3 | 298 | 46.30 | [52] |
| Amine-modified chitosan resins | 2.5 | 298 | 109.61 | [53] |
| PYCS | 2.5 | 303 | 66.20 | This work |
| Sample | ΔS (J/mol·K) | ΔH (kJ/mol) | ΔG (kJ/mol) | |||
|---|---|---|---|---|---|---|
| 303 K | 313 K | 323 K | 333 K | |||
| PYCS | 36.49 | 8.14 | −2.91 | −3.27 | −3.64 | −4.00 |
| Fe (III) | As (III) | Pb (II) | SO42− | pH |
|---|---|---|---|---|
| 484 ± 10 | 0.7 ± 0.1 | 0.6 ± 0.1 | 1260 | 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Liu, H.; Zhu, J.; Liu, X.; Li, L.; Huang, Y.; Fu, B.; Fan, G.; Wang, Y. Effective Removal of Fe (III) from Strongly Acidic Wastewater by Pyridine-Modified Chitosan: Synthesis, Efficiency, and Mechanism. Molecules 2023, 28, 3445. https://doi.org/10.3390/molecules28083445
Zhang L, Liu H, Zhu J, Liu X, Li L, Huang Y, Fu B, Fan G, Wang Y. Effective Removal of Fe (III) from Strongly Acidic Wastewater by Pyridine-Modified Chitosan: Synthesis, Efficiency, and Mechanism. Molecules. 2023; 28(8):3445. https://doi.org/10.3390/molecules28083445
Chicago/Turabian StyleZhang, Lei, Heng Liu, Jiaqi Zhu, Xueling Liu, Likun Li, Yanjun Huang, Benquan Fu, Guozhi Fan, and Yi Wang. 2023. "Effective Removal of Fe (III) from Strongly Acidic Wastewater by Pyridine-Modified Chitosan: Synthesis, Efficiency, and Mechanism" Molecules 28, no. 8: 3445. https://doi.org/10.3390/molecules28083445
APA StyleZhang, L., Liu, H., Zhu, J., Liu, X., Li, L., Huang, Y., Fu, B., Fan, G., & Wang, Y. (2023). Effective Removal of Fe (III) from Strongly Acidic Wastewater by Pyridine-Modified Chitosan: Synthesis, Efficiency, and Mechanism. Molecules, 28(8), 3445. https://doi.org/10.3390/molecules28083445







