Volatile Components, Antioxidant and Phytotoxic Activity of the Essential Oil of Piper acutifolium Ruiz & Pav. from Peru
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemical Characterization of the Essential Oil of P. acutifolium
| # | Compound | LRI Ref | LRI Exp | % | Molecular Formula/ Molecular Mass | Chemical Structure | Chemical Group |
|---|---|---|---|---|---|---|---|
| 1 | α-Pinene | 950 | 948 | 2.82 | C10H16 (136.23) | 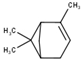 | Monoterpene hydrocarbon |
| 2 | β-Myrcene | 960 | 958 | 29.48 | C10H16 (136.23) | 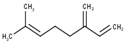 | Monoterpene hydrocarbon |
| 3 | α-Phellandrene | 972 | 969 | 38.18 | C10H16 (136.23) | 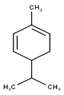 | Monoterpene hydrocarbon |
| 4 | o-Cymene | 1052 | 1042 | 1.55 | C10H14 (136.22) | 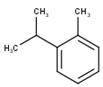 | Aromatic monoterpene hydrocarbon |
| 5 | β-Phellandrene | 1082 | 1075 | 21.88 | C10H16 (136.23) | 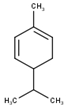 | Monoterpene hydrocarbon |
| 6 | α-Gurjunene | 1420 | 1419 | 0.38 | C15H24 (204.35) | 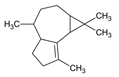 | Sesquiterpene hydrocarbon |
| 7 | β-Caryophyllene | 1585 | 1579 | 1.20 | C15H24 (204.35) | 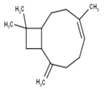 | Sesquiterpene Hydrocarbon |
| 8 | Humulene | 1452 | 1579 | 0.26 | C15H24 (204.35) | 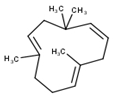 | Sesquiterpene hydrocarbon |
| 9 | Germacrene D | 1480 | 1515 | 0.85 | C15H24 (204.35) | 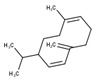 | Sesquiterpene hydrocarbon |
| 10 | Bicyclogermacrene | 1500 | 1492 | 0.63 | C15H24 (204.35) | 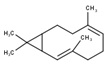 | Sesquiterpene hydrocarbon |
| 11 | δ-Cadinene | 1513 | 1510 | 0.94 | C15H24 (204.35) | 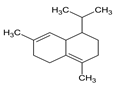 | Sesquiterpene hydrocarbon |
| - | Unknown I | - | 1600 | 0.78 | C15H26O (222.37) | n.d. | Oxygenated Sesquiterpene |
| - | Unknown II | - | 1623 | 0.27 | C15H26O (222.37) | n.d. | Oxygenated Sesquiterpene |
| 12 | Aromadendrene | 1656 | 1660 | 0.78 | C15H26O (222.37) | 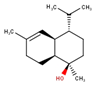 | Oxygenated Sesquiterpene |
| Aromatic monoterpene hydrocarbons | 1.55% | ||||||
| Monoterpene hydrocarbons | 92.36% | ||||||
| Sesquiterpenes hydrocarbons | 4.26% | ||||||
| Oxygenated sesquiterpenes | 1.83% | ||||||
| Total identified | 100.00% | ||||||
2.2. Antioxidant Activity of the Essential Oil of P. acutifolium
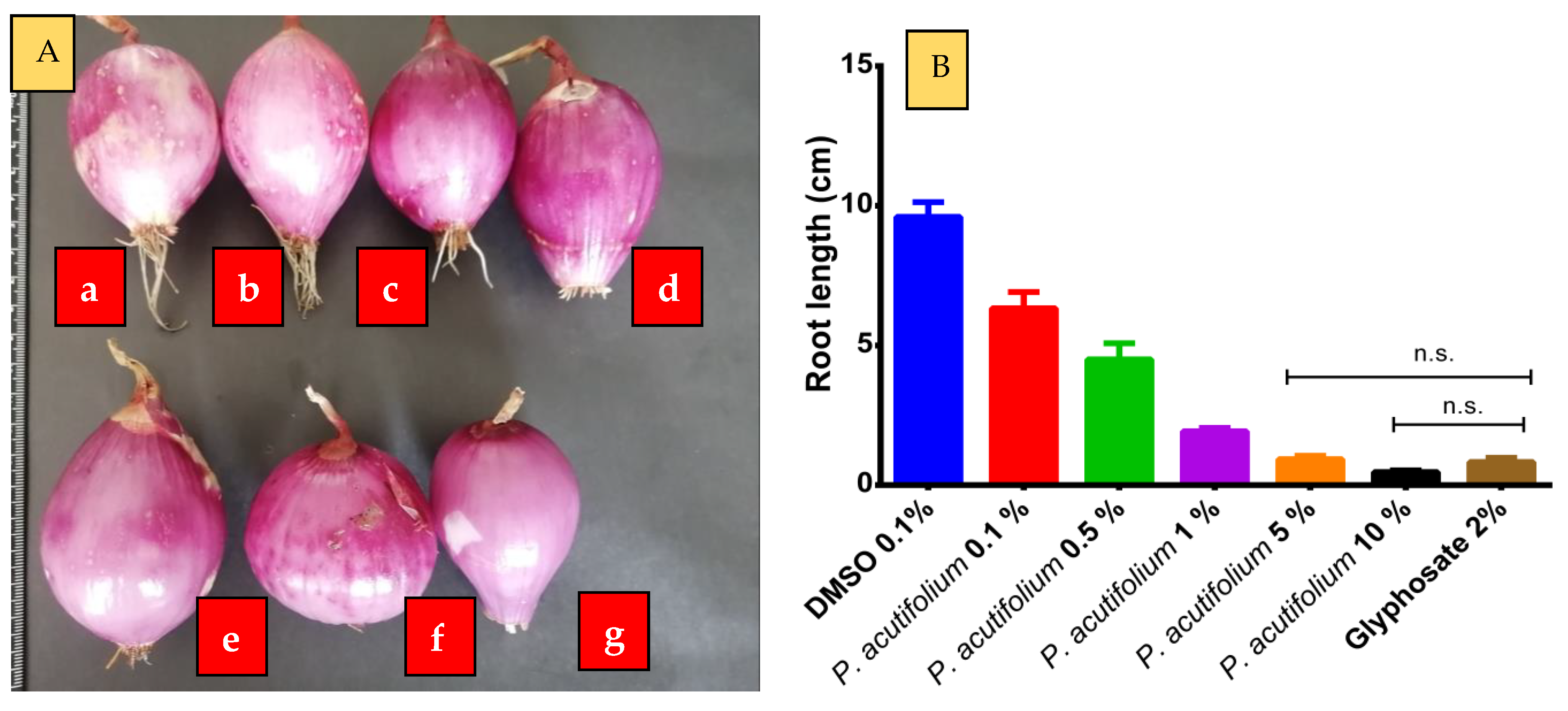
2.3. Phytotoxic Activity of the Essential Oil of P. acutifolium on L. sativa Seeds and A. cepa Bulbs
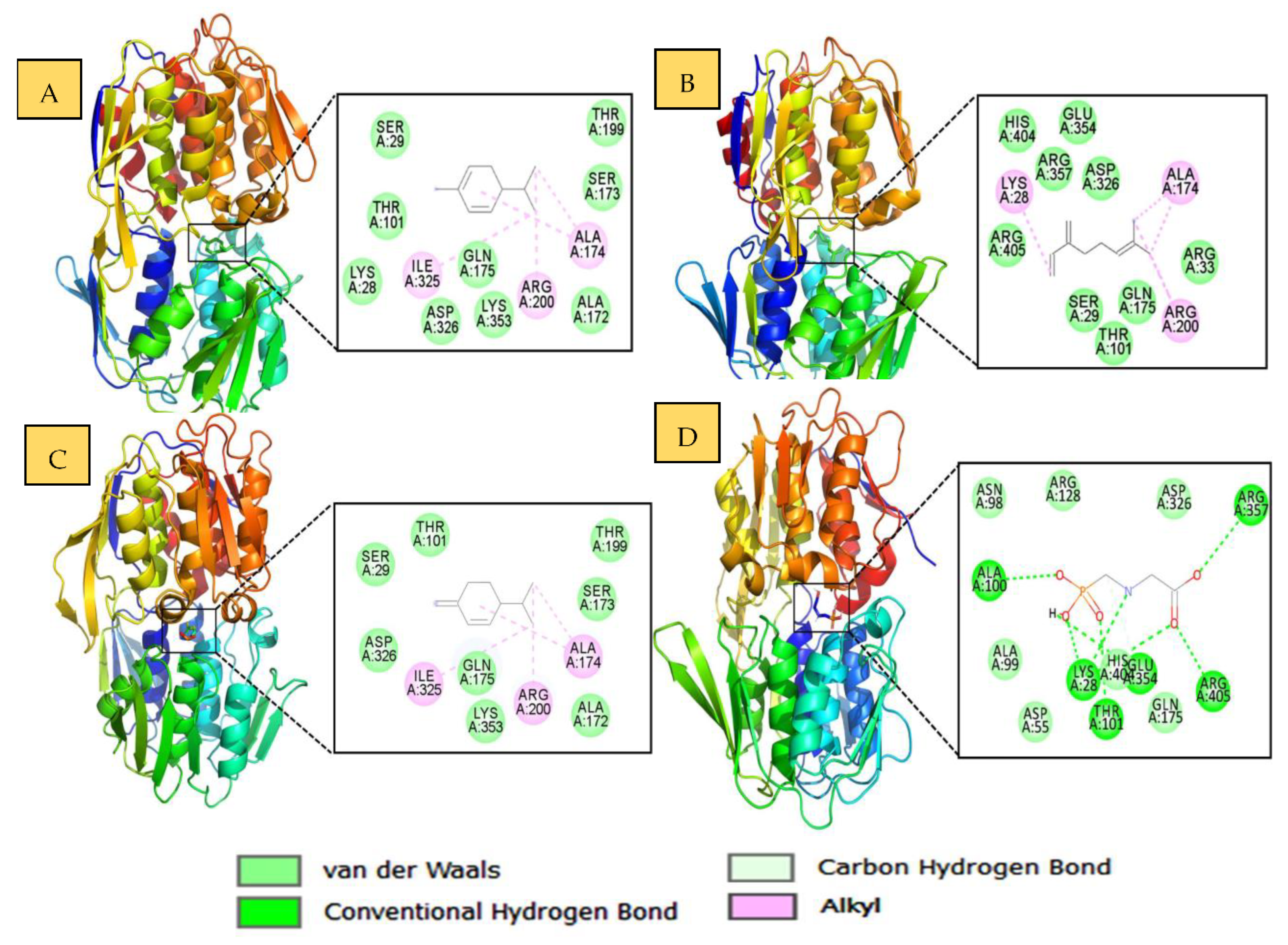
2.4. Molecular Docking Studies
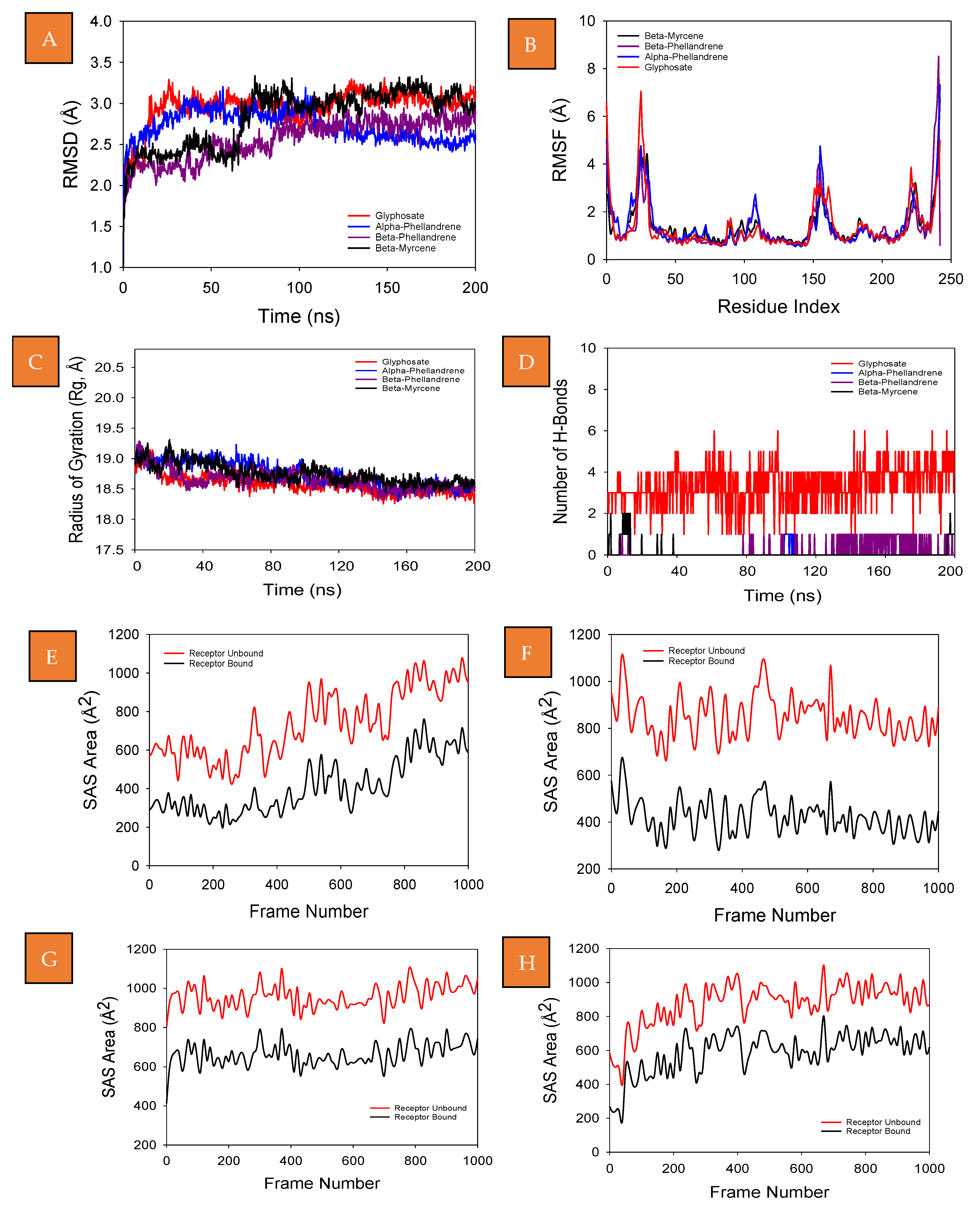
2.5. Molecular Dynamics Simulation
2.6. Molecular Mechanics Generalized Born Surface Area (MM-GBSA) Calculations
2.7. In-Silico Toxicological Study of P. acutifolium EO
3. Materials and Methods
3.1. Plant Material
3.2. Identification of the Volatile Compounds by Gas Chromatography–Mass Spectrometry (GC–MS)
3.3. Evaluation of the Antioxidant Activity of the EO of P. acutifolium against 2,2-Diphenyl-1-Picrylhydrazyl (DPPH) Radical
3.4. Evaluation of the Antioxidant Activity of the EO of P. acutifolium against 2,2′-Azinobis–(3-Ethylbenzothiazoline)-6- Sulfonic Acid (ABTS .+) Radical
3.5. Evaluation of the Antioxidant Activity of the EO of P. acutifolium against the Ferric Reducing/Antioxidant Power (FRAP)
3.6. Phytotoxicity Test on Lactuca sativa Seeds
3.7. Phytotoxicity Test on Allium cepa Bulbs
3.8. Molecular Docking
3.9. In-Silico Toxicity of the Volatile Components of P. acutifolium
3.10. Molecular Dynamics Simulation
3.11. Binding Free Energy Analysis
3.12. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Herrera-Calderon, O.; Chavez, H.; Enciso-Roca, E.C.; Común-Ventura, P.W.; Hañari-Quispe, R.D.; Figueroa-Salvador, L.; Loyola-Gonzales, E.L.; Pari-Olarte, J.B.; Aljarba, N.H.; Alkahtani, S.; et al. GC-MS Profile, Antioxidant Activity, and In Silico Study of the Essential Oil from Schinus molle L. Leaves in the Presence of Mosquito Juvenile Hormone-Binding Protein (MJHBP) from Aedes Aegypti. Biomed Res. Int. 2022, 2022, 5601531. [Google Scholar] [CrossRef]
- Raveau, R.; Fontaine, J.; Lounès-Hadj Sahraoui, A. Essential Oils as Potential Alternative Biocontrol Products against Plant Pathogens and Weeds: A Review. Foods 2020, 9, 365. [Google Scholar] [CrossRef] [PubMed]
- Popa, C.L.; Lupitu, A.; Mot, M.D.; Copolovici, L.; Moisa, C.; Copolovici, D.M. Chemical and Biochemical Characterization of Essential Oils and Their Corresponding Hydrolats from Six Species of the Lamiaceae Family. Plants 2021, 10, 2489. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Harmon, P.F.; Treadwell, D.D.; Carrillo, D.; Sarkhosh, A.; Brecht, J.K. Biocontrol Potential of Essential Oils in Organic Horticulture Systems: From Farm to Fork. Front. Nutr. 2022, 8, 1275. [Google Scholar] [CrossRef] [PubMed]
- Jackson-Davis, A.; White, S.; Kassama, L.S.; Coleman, S.; Shaw, A.; Mendonca, A.; Cooper, B.; Thomas-Popo, E.; Gordon, K.; London, L. A Review of Regulatory Standards and Advances in Essential Oils as Antimicrobials in Foods. J. Food Prot. 2023, 86, 100025. [Google Scholar] [CrossRef]
- Ebadollahi, A.; Ziaee, M.; Palla, F. Essential Oils Extracted from Different Species of the Lamiaceae Plant Family as Prospective Bioagents against Several Detrimental Pests. Molecules 2020, 25, 1556. [Google Scholar] [CrossRef]
- Maes, C.; Meersmans, J.; Lins, L.; Bouquillon, S.; Fauconnier, M.L. Essential Oil-Based Bioherbicides: Human Health Risks Analysis. Int. J. Mol. Sci. 2021, 22, 9396. [Google Scholar] [CrossRef]
- Werrie, P.Y.; Durenne, B.; Delaplace, P.; Fauconnier, M.L. Phytotoxicity of Essential Oils: Opportunities and Constraints for the Development of Biopesticides. A Review. Foods 2020, 9, 1291. [Google Scholar] [CrossRef]
- Caverzan, A.; Piasecki, C.; Chavarria, G.; Stewart, C.N.; Vargas, L. Defenses Against ROS in Crops and Weeds: The Effects of Interference and Herbicides. Int. J. Mol. Sci. 2019, 20, 1086. [Google Scholar] [CrossRef]
- Anwar, S.; Naseem, S.; Karimi, S.; Asi, M.R.; Akrem, A.; Ali, Z. Bioherbicidal Activity and Metabolic Profiling of Potent Allelopathic Plant Fractions Against Major Weeds of Wheat—Way Forward to Lower the Risk of Synthetic Herbicides. Front. Plant Sci. 2021, 12, 333. [Google Scholar] [CrossRef]
- Amorati, R.; Foti, M.C.; Valgimigli, L. Antioxidant Activity of Essential Oils. J. Agric. Food Chem. 2013, 61, 10835–10847. [Google Scholar] [CrossRef]
- Bagheri, H.; Manap, M.Y.B.A.; Solati, Z. Antioxidant Activity of Piper nigrum L. Essential Oil Extracted by Supercritical CO2 Extraction and Hydro-Distillation. Talanta 2014, 121, 220–228. [Google Scholar] [CrossRef]
- Mgbeahuruike, E.E.; Yrjönen, T.; Vuorela, H.; Holm, Y. Bioactive Compounds from Medicinal Plants: Focus on Piper Species. S. Afr. J. Bot. 2017, 112, 54–69. [Google Scholar] [CrossRef]
- de Feo, V. Ethnomedical Field Study in Northern Peruvian Andes with Particular Reference to Divination Practices. J. Ethnopharmacol. 2003, 85, 243–256. [Google Scholar] [CrossRef]
- Lognay, G.C.; Bouxin, P.; Marlier, M.; Haubruge, E.; Gaspar, C.; Rodriguez, A. Composition of the Essential Oil of Piper acutifolium Ruiz. and Pav. from Peru. J. Essent. Oil Res. 1996, 8, 689–691. [Google Scholar] [CrossRef]
- Flores, N.; Jiménez, I.A.; Giménez, A.; Ruiz, G.; Gutiérrez, D.; Bourdy, G.; Bazzocchi, I.L. Benzoic Acid Derivatives from Piper Species and Their Antiparasitic Activity. J. Nat. Prod. 2008, 71, 1538–1543. [Google Scholar] [CrossRef]
- do Carmo, D.F.M.; Amaral, A.C.F.; MacHado, G.M.C.; Leon, L.L.; de Andrade Silva, J.R. Chemical and Biological Analyses of the Essential Oils and Main Constituents of Piper Species. Molecules 2012, 17, 1819–1829. [Google Scholar] [CrossRef]
- da Silva, J.K.R.; Pinto, L.C.; Burbano, R.M.R.; Montenegro, R.C.; Guimarães, E.F.; Andrade, E.H.A.; Maia, J.G.S. Essential Oils of Amazon Piper Species and Their Cytotoxic, Antifungal, Antioxidant and Anti-Cholinesterase Activities. Ind. Crops Prod. 2014, 58, 55–60. [Google Scholar] [CrossRef]
- Debonsi Navickiene, H.M.; Morandim, A.D.A.; Alécio, A.C.; Regasini, L.O.; Bergamo, D.C.B.; Telascrea, M.; Cavalheiro, A.J.; Lopes, M.N.; Bolzani, V.D.S.; Furlan, M.; et al. Composition and Antifungal Activity of Essential Oils from Piper Aduncum, Piper Arboreum and Piper Tuberculatum. Quim. Nova 2006, 29, 467–470. [Google Scholar] [CrossRef]
- da Silva, M.F.R.; Bezerra-Silva, P.C.; de Lira, C.S.; de Lima Albuquerque, B.N.; Agra Neto, A.C.; Pontual, E.V.; Maciel, J.R.; Paiva, P.M.G.; Navarro, D.M.d.A.F. Composition and Biological Activities of the Essential Oil of Piper Corcovadensis (Miq.) C. DC (Piperaceae). Exp. Parasitol. 2016, 165, 64–70. [Google Scholar] [CrossRef]
- da Silva, J.K.; da Trindade, R.; Alves, N.S.; Figueiredo, P.L.; Maia, J.G.S.; Setzer, W.N. Essential Oils from Neotropical Piper Species and Their Biological Activities. Int. J. Mol. Sci. 2017, 18, 2571. [Google Scholar] [CrossRef] [PubMed]
- Cicció, J.F. Essential Oil from the Leaves of Piper Augustum from “Alberto M. Brenes” Biological Preserve, Costa Rica. J. Essent. Oil Res. 2011, 17, 251–253. [Google Scholar] [CrossRef]
- Ruiz-Vásquez, L.; Mesia, L.R.; Ceferino, H.D.C.; Mesia, W.R.; Andrés, M.F.; Díaz, C.E.; Gonzalez-Coloma, A. Antifungal and Herbicidal Potential of Piper Essential Oils from the Peruvian Amazonia. Plants 2022, 11, 1793. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing: Carol Stream, IL, USA, 2017; p. 804. [Google Scholar]
- Sharopov, F.S.; Wink, M.; Setzer, W.N. Radical Scavenging and Antioxidant Activities of Essential Oil Components—An Experimental and Computational Investigation. Nat. Prod. Commun. 2015, 10, 153–156. [Google Scholar] [CrossRef]
- Rodriguez, E.J.; Saucedo-Hernández, Y.; Vander Heyden, Y.; Simó-Alfonso, E.F.; Ramis-Ramos, G.; Lerma-García, M.J.; Monteagudo, U.; Bravo, L.; Medinilla, M.; de Armas, Y.; et al. Chemical analysis and antioxidant activity of the essential oils of three Piperaceae species growing in the central region of Cuba. Nat. Prod. Commun. 2013, 8, 1325–1328. [Google Scholar] [CrossRef]
- Alminderej, F.; Bakari, S.; Almundarij, T.I.; Snoussi, M.; Aouadi, K.; Kadri, A. Antioxidant Activities of a New Chemotype of Piper cubeba L. Fruit Essential Oil (Methyleugenol/Eugenol): In Silico Molecular Docking and ADMET Studies. Plants 2020, 9, 1534. [Google Scholar] [CrossRef]
- Valarezo, E.; Flores-Maza, P.; Cartuche, L.; Ojeda-Riascos, S.; Ramírez, J. Phytochemical Profile, Antimicrobial and Antioxidant Activities of Essential Oil Extracted from Ecuadorian Species Piper Ecuadorense Sodiro. Nat. Prod. Res. 2020, 35, 6014–6019. [Google Scholar] [CrossRef]
- Araujo, C.A.; da Camara, C.A.G.; de Moraes, M.M.; de Vasconcelos, G.J.N.; Pereira, M.R.; Zartman, C.E. Chemical Composition of Essential Oils from Four Piper Species, Differentiation Using Multivariate Analysis and Antioxidant Activity. Nat. Prod. Res. 2020, 36, 436–439. [Google Scholar] [CrossRef]
- Ilić, Z.; Stanojević, L.; Milenković, L.; Šunić, L.; Milenković, A.; Stanojević, J.; Cvetković, D. The Yield, Chemical Composition, and Antioxidant Activities of Essential Oils from Different Plant Parts of the Wild and Cultivated Oregano (Origanum Vulgare L.). Horticulturae 2022, 8, 1042. [Google Scholar] [CrossRef]
- Kanissery, R.; Gairhe, B.; Kadyampakeni, D.; Batuman, O.; Alferez, F. Glyphosate: Its Environmental Persistence and Impact on Crop Health and Nutrition. Plants 2019, 8, 499. [Google Scholar] [CrossRef]
- Zhou, L.; Li, J.; Kong, Q.; Luo, S.; Wang, J.; Feng, S.; Yuan, M.; Chen, T.; Yuan, S.; Ding, C. Chemical Composition, Antioxidant, Antimicrobial, and Phytotoxic Potential of Eucalyptus grandis × E. urophylla Leaves Essential Oils. Molecules 2021, 26, 1450. [Google Scholar] [CrossRef]
- Ulukanli, Z.; Karabörklü, S.; Bozok, F.; Ates, B.; Erdogan, S.; Cenet, M.; Karaaslan, M.G. Chemical Composition, Antimicrobial, Insecticidal, Phytotoxic and Antioxidant Activities of Mediterranean Pinus Brutia and Pinus Pinea Resin Essential Oils. Chin. J. Nat. Med. 2014, 12, 901–910. [Google Scholar] [CrossRef]
- de Almeida, L.F.R.; Frei, F.; Mancini, E.; de Martino, L.; de Feo, V. Phytotoxic Activities of Mediterranean Essential Oils. Molecules 2010, 15, 4309. [Google Scholar] [CrossRef]
- Abd-Elgawad, A.M.; el Gendy, A.E.N.G.; Assaeed, A.M.; Al-Rowaily, S.L.; Alharthi, A.S.; Mohamed, T.A.; Nassar, M.I.; Dewir, Y.H.; Elshamy, A.I. Phytotoxic Effects of Plant Essential Oils: A Systematic Review and Structure-Activity Relationship Based on Chemometric Analyses. Plants 2020, 10, 36. [Google Scholar] [CrossRef]
- Surendran, S.; Qassadi, F.; Surendran, G.; Lilley, D.; Heinrich, M. Myrcene—What Are the Potential Health Benefits of This Flavouring and Aroma Agent? Front. Nutr. 2021, 8, 400. [Google Scholar] [CrossRef]
- Babalola, S.; Igie, N.; Odeyemi, I.; Thieme, G.; Kg, V. Molecular Docking, Drug-Likeness Analysis, In Silico Pharmacokinetics, and Toxicity Studies of p-Nitrophenyl Hydrazones as Anti-Inflammatory Compounds against COX-2, 5-LOX, and H þ /K þ ATPase. Pharmaceut. Fronts 2022, 4, 250–266. [Google Scholar] [CrossRef]
- Ferraz, C.A.; Pastorinho, M.R.; Palmeira-de-Oliveira, A.; Sousa, A.C.A. Ecotoxicity of Plant Extracts and Essential Oils: A Review. Environ. Pollut. 2022, 292, 118319. [Google Scholar] [CrossRef]
- Rojas-Armas, J.P.; Arroyo-Acevedo, J.L.; Palomino-Pacheco, M.; Herrera-Calderón, O.; Ortiz-Sánchez, J.M.; Rojas-Armas, A.; Calva, J.; Castro-Luna, A.; Hilario-Vargas, J. The Essential Oil of Cymbopogon Citratus Stapt and Carvacrol: An Approach of the Antitumor Effect on 7,12-Dimethylbenz-[α]-Anthracene (DMBA)-Induced Breast Cancer in Female Rats. Molecules 2020, 25, 3284. [Google Scholar] [CrossRef]
- Herrera-Calderon, O.; Alvarado-Puray, C.; Arroyo-Acevedo, J.; Rojas-Armas, J.; Chumpitaz-Cerrate, V.; Hañari-Quispe, R.; Valenzuela-Herrera, R. Phytochemical Screening, Total Phenolic Content, Antioxidant, and Cytotoxic Activity of Five Peruvian Plants on Human Tumor Cell Lines. Pharmacognosy. Res. 2018, 10, 161–165. [Google Scholar] [CrossRef]
- Herrera-Calderon, O.; Chacaltana-Ramos, L.J.; Huayanca-Gutiérrez, I.C.; Algarni, M.A.; Alqarni, M.; Batiha, G.E.S. Chemical Constituents, In Vitro Antioxidant Activity and In Silico Study on NADPH Oxidase of Allium sativum L. (Garlic) Essential Oil. Antioxidants 2021, 10, 1844. [Google Scholar] [CrossRef]
- Enciso-Roca, E.C.; Aguilar-Felices, E.J.; Tinco-Jayo, J.A.; Arroyo-Acevedo, J.L.; Herrera-Calderon, O. Biomolecules with Antioxidant Capacity from the Seeds and Sprouts of 20 Varieties of Chenopodium Quinoa Willd. (Quinoa). Plants 2021, 10, 2417. [Google Scholar] [CrossRef] [PubMed]
- Vieira, C.; Marcon, C.; Droste, A. Phytotoxic and Cytogenotoxic Assessment of Glyphosate on Lactuca Sativa L. Braz. J. Biol. 2022, 84. [Google Scholar] [CrossRef] [PubMed]
- Haq, I.; Kumari, V.; Kumar, S.; Raj, A.; Lohani, M.; Bhargava, R.N. Evaluation of the Phytotoxic and Genotoxic Potential of Pulp and Paper Mill Effluent Using Vigna Radiata and Allium Cepa. Adv. Biol. 2016, 2016, 1–10. [Google Scholar] [CrossRef]
- de Oliveira, M.V.D.; Bittencourt Fernandes, G.M.; da Costa, K.S.; Vakal, S.; Lima, A.H. Virtual Screening of Natural Products against 5-Enolpyruvylshikimate-3-Phosphate Synthase Using the Anagreen Herbicide-like Natural Compound Library. RSC Adv. 2022, 12, 18834–18847. [Google Scholar] [CrossRef] [PubMed]
- Xiong, G.; Wu, Z.; Yi, J.; Fu, L.; Yang, Z.; Hsieh, C.; Yin, M.; Zeng, X.; Wu, C.; Lu, A.; et al. ADMETlab 2.0: An Integrated Online Platform for Accurate and Comprehensive Predictions of ADMET Properties. Nucleic. Acids. Res. 2021, 49, W5–W14. [Google Scholar] [CrossRef]
- Shaw, D.E.; Maragakis, P.; Lindorff-Larsen, K.; Piana, S.; Dror, R.O.; Eastwood, M.P.; Bank, J.A.; Jumper, J.M.; Salmon, J.K.; Shan, Y.; et al. Atomic-Level Characterization of the Structural Dynamics of Proteins. Science 2010, 330, 341–346. [Google Scholar] [CrossRef]
- Bowers, K.J.; Chow, D.E.; Xu, H.; Dror, R.O.; Eastwood, M.P.; Gregersen, B.A.; Klepeis, J.L.; Kolossvary, I.; Moraes, M.A.; Sacerdoti, F.D.; et al. Scalable Algorithms for Molecular Dynamics Simulations on Commodity Clusters. In Proceedings of the 2006 ACM/IEEE Conference on Supercomputing, New York, NY, USA, 11–17 November 2007; p. 43. [Google Scholar] [CrossRef]
- Kulke, M.; Vermaas, J.V. Reversible Unwrapping Algorithm for Constant-Pressure Molecular Dynamics Simulations. J. Chem. Theory Comput. 2022, 18, 6161–6171. [Google Scholar] [CrossRef]

| Antioxidant | Trolox Equivalent Antioxidant Capacity (µmol TE/g) | IC50 (µg/mL) |
|---|---|---|
| DPPH | 8.10 ± 0.05 | 160.12 ± 0.30 |
| ABTS | 150.56 ± 8.27 | 138.10 ± 0.06 |
| FRAP | 64.16 ± 1.40 | 450.10 ± 0.05 |
| Groups | Seed Germination (%) | Root Length (cm) | Hypocotyl Length (cm) | Root Length/Stem Length |
|---|---|---|---|---|
| Negative control: DMSO 0.1% | 96.33 ± 5.51 * | 2.57 ± 0.06 * | 6.67 ± 0.58 * | 0.28 |
| P. acutifolium 0.1% | 36.67 ± 5.77 * | 2.10 ± 0.17 * | 4.67 ± 0.58 * | 0.31 |
| P. acutifolium 0.5% | 25.33 ± 5.03 * | 1.57 ± 0.06 * | 3.33 ± 0.58 * | 0.32 |
| P. acutifolium 1% | 12.33 ± 2.52 * | 1.10 ± 0.10 * | 1.83 ± 0.29 * | 0.38 |
| P. acutifolium 5% | 4.00 ± 1.73 | 0.93 ± 0.06 | 1.07 ± 0.12 | 0.47 |
| P. acutifolium 10% | 0.0 ± 0.00 * | 0.60 ± 0.17 * | 0.63 ± 0.15 * | 0.49 |
| Positive control: glyphosate 2% | 6.67 ± 2.89 | 1.03 ± 0.25 | 1.03 ± 0.25 | 0.08 |
| Ligand | Binding Energy (kcal/mol) | Interactions |
|---|---|---|
| α-Phellandrene | −5.8 | Alkyl bond: Ala174, Arg200, Ile325 |
| β-Myrcene | −4.7 | Alkyl bond: Lys28, Ala174, Arg200 |
| β-Phellandrene | −5.2 | Alkyl bond: Ala174, Arg200, Ile325 |
| Glyphosate (Control) | −6.3 | Hydrogen Bond: Lys28, Thr101, Ala100, Arrg405, Arg357 |
| Energies (kcal/mol) | 3FJZ-Beta-Phellandrene | 3FJZ-Beta-Myrcene | 3FJZ-Glyphosate | 3FJZ-Alpha-Phellandrene |
|---|---|---|---|---|
| ΔGbind | −36.59 ± 2.63 | −35.75 ± 2.99 | −48.53 ± 4.1 | −46.15 ± 1.13 |
| ΔGbindLipo | −13.96 ± 1.03 | −11.50 ± 3.1 | −19.83 ± 2.3 | −13.43 ± 1.6 |
| ΔGbindvdW | −11.10 ± 2.0 | −10.63 ± 2.63 | −12.68 ± 2.17 | −14.160 ± 3.0 |
| ΔGbindCoulomb | −8.12 ± 1.99 | −13.66 ± 2.88 | −2.14 ± 1.01 | −6.22 ± 0.99 |
| ΔGbindHbond | −0.41 ± 0.22 | −1.87 ± 0.5 | −0.06 ± 0.01 | −0.62 ± 0.16 |
| ΔGbindSolvGB | 16.5 ± 1.09 | 60.54 ± 2.8 | 13.65 ± 2.27 | 21.2 ± 1.7 |
| GbindCovalent | 1.56 ± 1.2 | 4.22 ± 1.07 | 0.85 ± 0.5 | 2.66 ± 1.12 |
| Toxicity | Environmental Toxicity | ||||||
|---|---|---|---|---|---|---|---|
| # | HH | AMES Toxicity | Carcinogenicity | Bioconcentration Factors | IGC50 | LC50FM | LC50DM |
| 1 | 0.196 | 0.002 | 0.056 | 2.986 | 4.327 | 5.287 | 5.948 |
| 2 | 0.61 | 0.025 | 0.802 | 2.021 | 4.471 | 5.331 | 5.45 |
| 3 | 0.76 | 0.011 | 0.344 | 2.36 | 3.08 | 3.674 | 4.176 |
| 4 | 0.03 | 0.032 | 0.456 | 2.231 | 3.598 | 4.033 | 4.107 |
| 5 | 0.269 | 0.024 | 0.874 | 2.154 | 4.09 | 4.242 | 4.328 |
| 6 | 0.174 | 0.007 | 0.048 | 3.263 | 4.796 | 5.861 | 6.647 |
| 7 | 0.26 | 0.012 | 0.357 | 3.129 | 4.024 | 4.957 | 6.196 |
| 8 | 0.182 | 0.002 | 0.938 | 3.083 | 3.323 | 4.45 | 5.997 |
| 9 | 0.411 | 0.012 | 0.9 | 2.864 | 4.253 | 4.966 | 5.405 |
| 10 | 0.582 | 0.005 | 0.062 | 3.262 | 4.214 | 6.018 | 6.783 |
| 11 | 0.172 | 0.018 | 0.229 | 3.174 | 3.562 | 5.018 | 5.582 |
| 12 | 0.256 | 0.011 | 0.043 | 3.295 | 4.705 | 5.41 | 6.639 |
| Glyphosate | 0.184 | 0.02 | 0.041 | 0.151 | 2.351 | 3.794 | 3.503 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cuadros-Siguas, C.F.; Herrera-Calderon, O.; Batiha, G.E.-S.; Almohmadi, N.H.; Aljarba, N.H.; Apesteguia-Infantes, J.A.; Loyola-Gonzales, E.; Tataje-Napuri, F.E.; Kong-Chirinos, J.F.; Almeida-Galindo, J.S.; et al. Volatile Components, Antioxidant and Phytotoxic Activity of the Essential Oil of Piper acutifolium Ruiz & Pav. from Peru. Molecules 2023, 28, 3348. https://doi.org/10.3390/molecules28083348
Cuadros-Siguas CF, Herrera-Calderon O, Batiha GE-S, Almohmadi NH, Aljarba NH, Apesteguia-Infantes JA, Loyola-Gonzales E, Tataje-Napuri FE, Kong-Chirinos JF, Almeida-Galindo JS, et al. Volatile Components, Antioxidant and Phytotoxic Activity of the Essential Oil of Piper acutifolium Ruiz & Pav. from Peru. Molecules. 2023; 28(8):3348. https://doi.org/10.3390/molecules28083348
Chicago/Turabian StyleCuadros-Siguas, Carmela Fiorella, Oscar Herrera-Calderon, Gaber El-Saber Batiha, Najlaa Hamed Almohmadi, Nada H. Aljarba, José Alfonso Apesteguia-Infantes, Eddie Loyola-Gonzales, Freddy Emilio Tataje-Napuri, José Francisco Kong-Chirinos, José Santiago Almeida-Galindo, and et al. 2023. "Volatile Components, Antioxidant and Phytotoxic Activity of the Essential Oil of Piper acutifolium Ruiz & Pav. from Peru" Molecules 28, no. 8: 3348. https://doi.org/10.3390/molecules28083348
APA StyleCuadros-Siguas, C. F., Herrera-Calderon, O., Batiha, G. E.-S., Almohmadi, N. H., Aljarba, N. H., Apesteguia-Infantes, J. A., Loyola-Gonzales, E., Tataje-Napuri, F. E., Kong-Chirinos, J. F., Almeida-Galindo, J. S., Chávez, H., & Pari-Olarte, J. B. (2023). Volatile Components, Antioxidant and Phytotoxic Activity of the Essential Oil of Piper acutifolium Ruiz & Pav. from Peru. Molecules, 28(8), 3348. https://doi.org/10.3390/molecules28083348









