Flowers Like α-MoO3/CNTs/PANI Nanocomposites as Anode Materials for High-Performance Lithium Storage
Abstract
1. Introduction
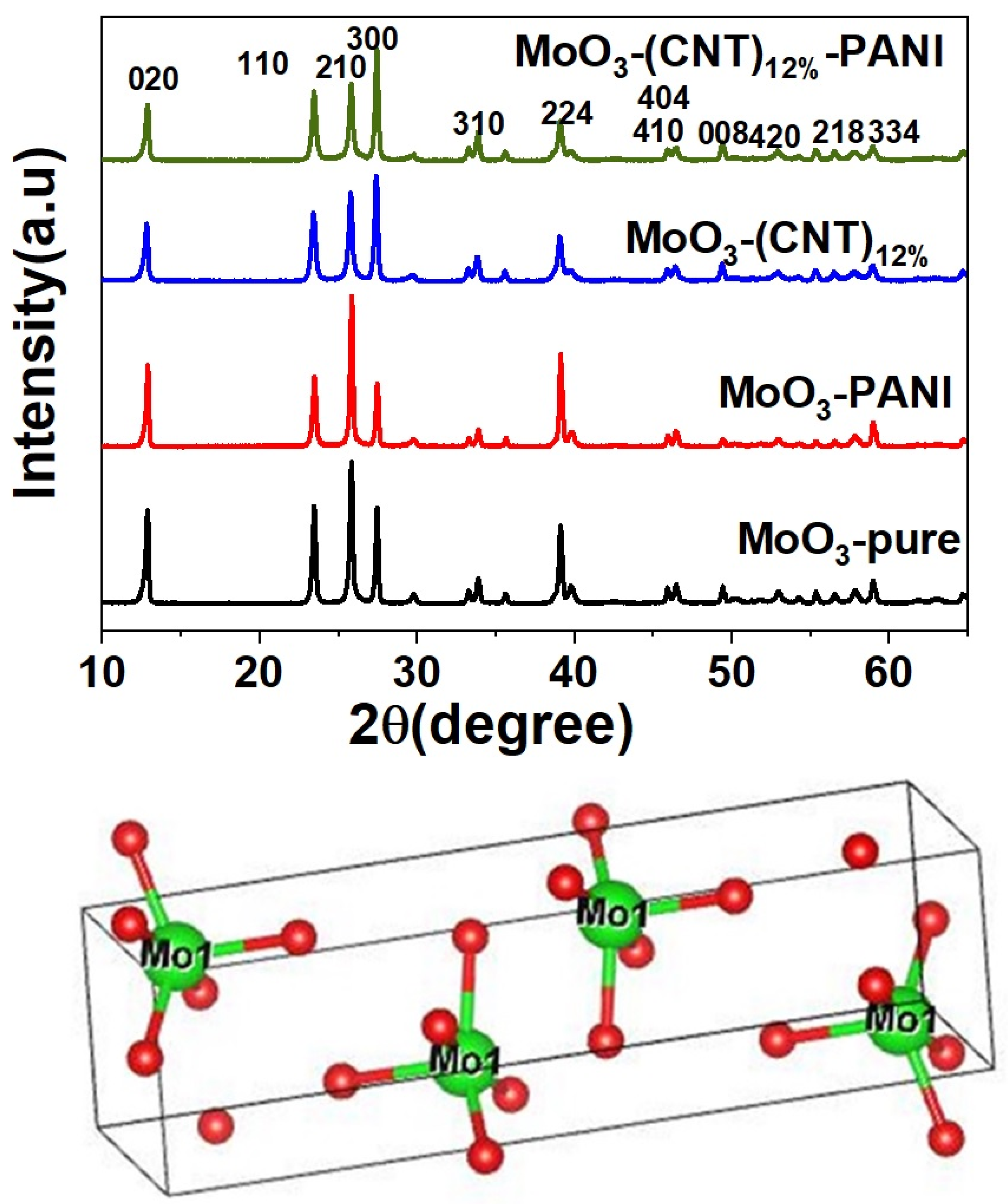
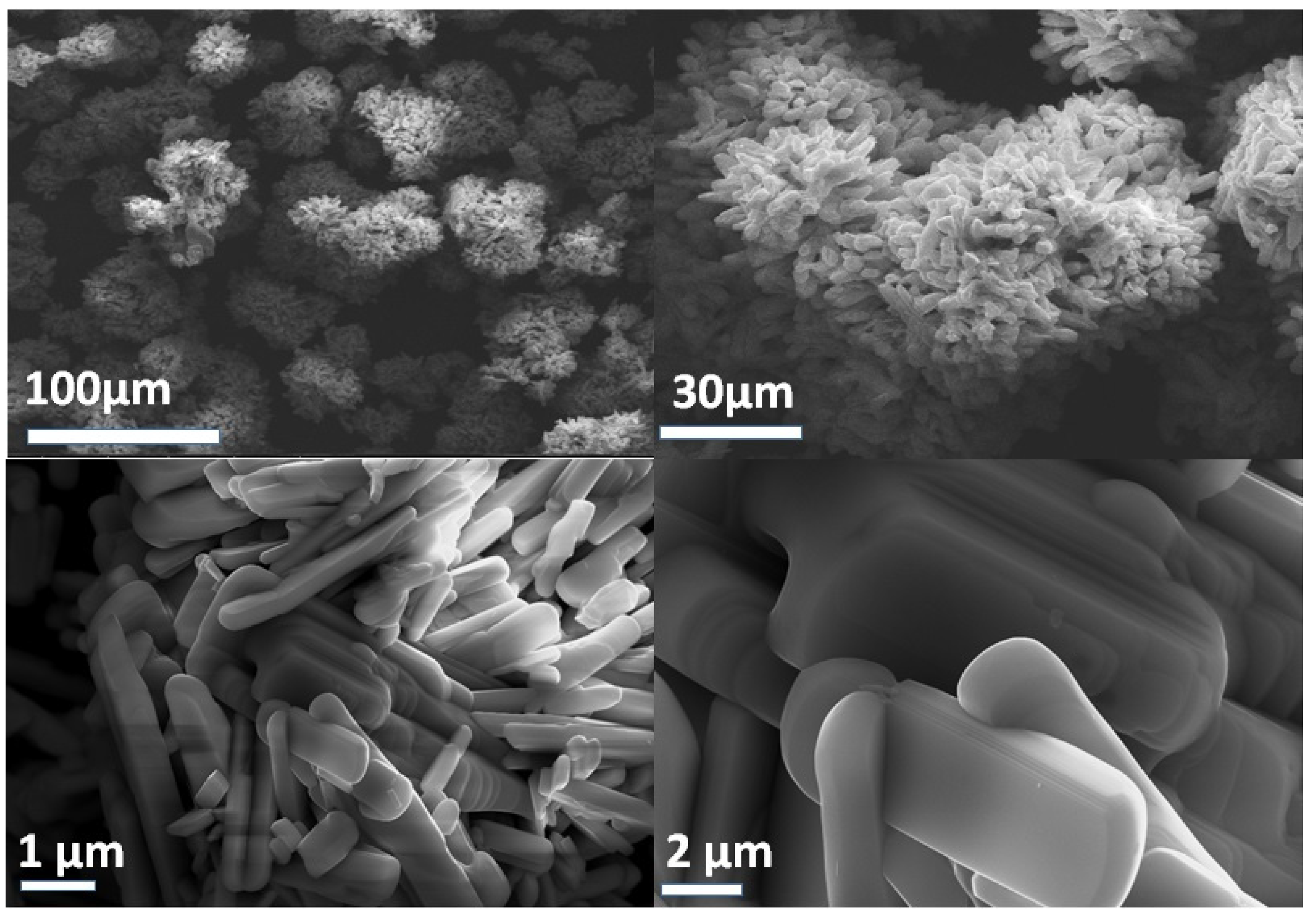
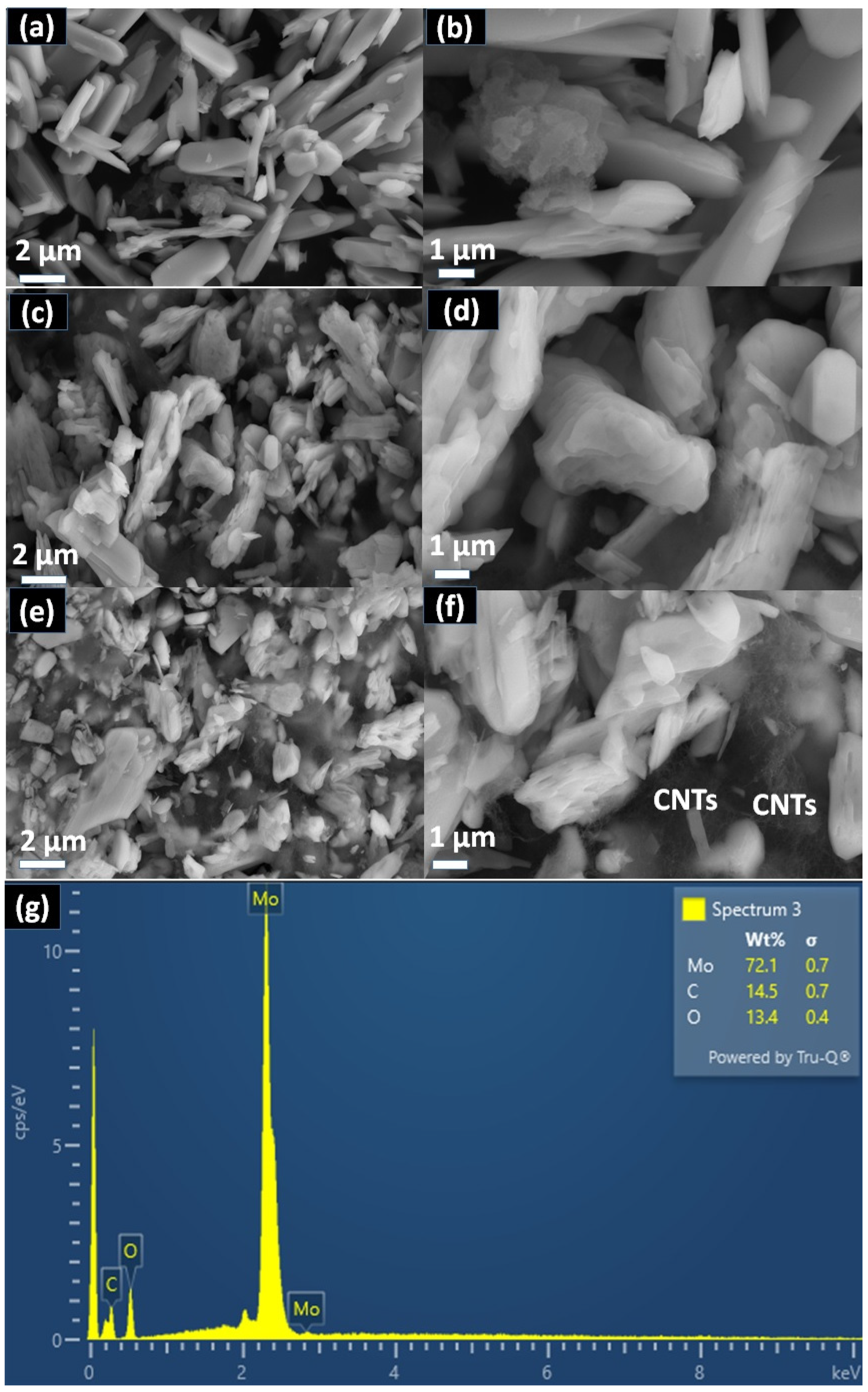
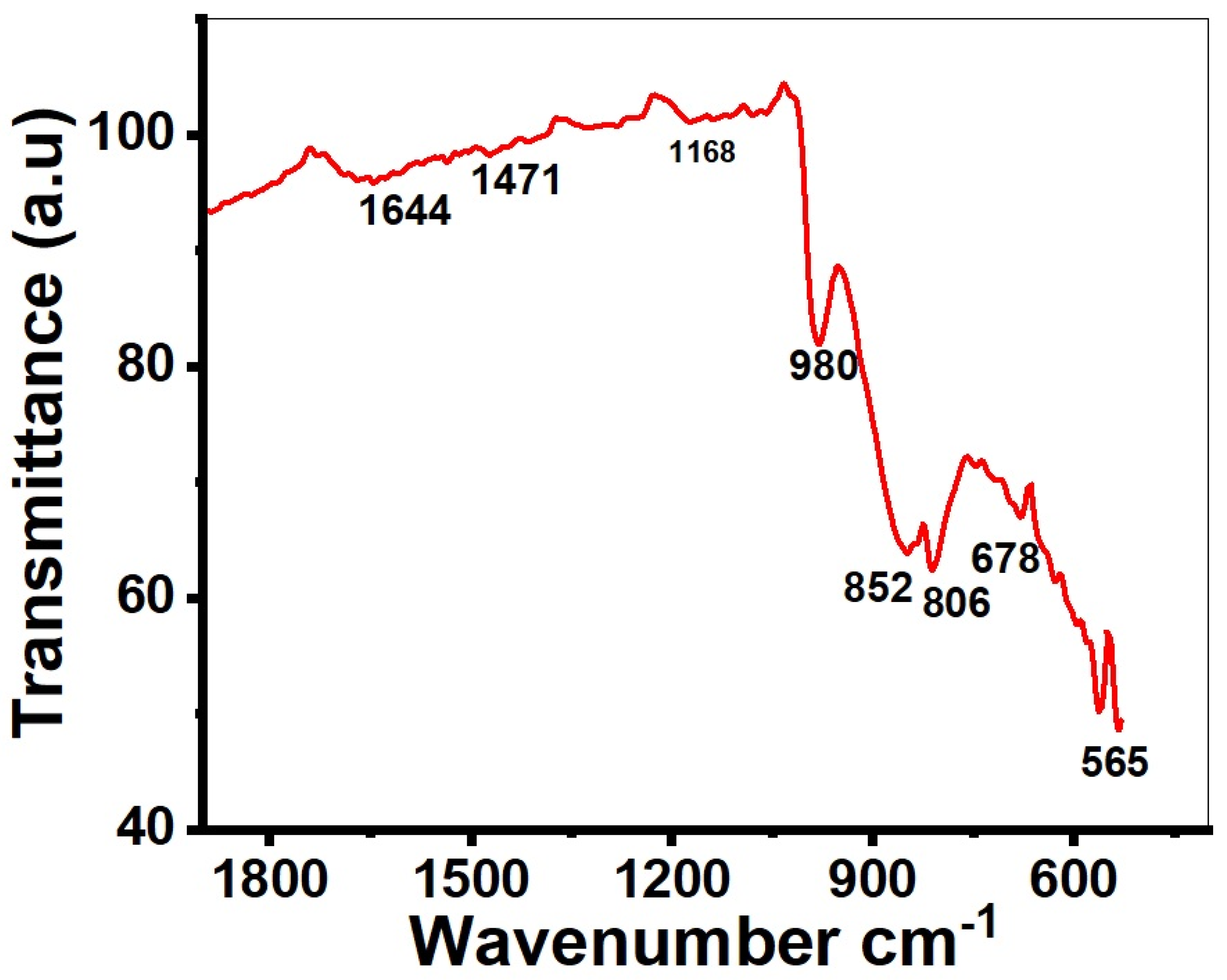
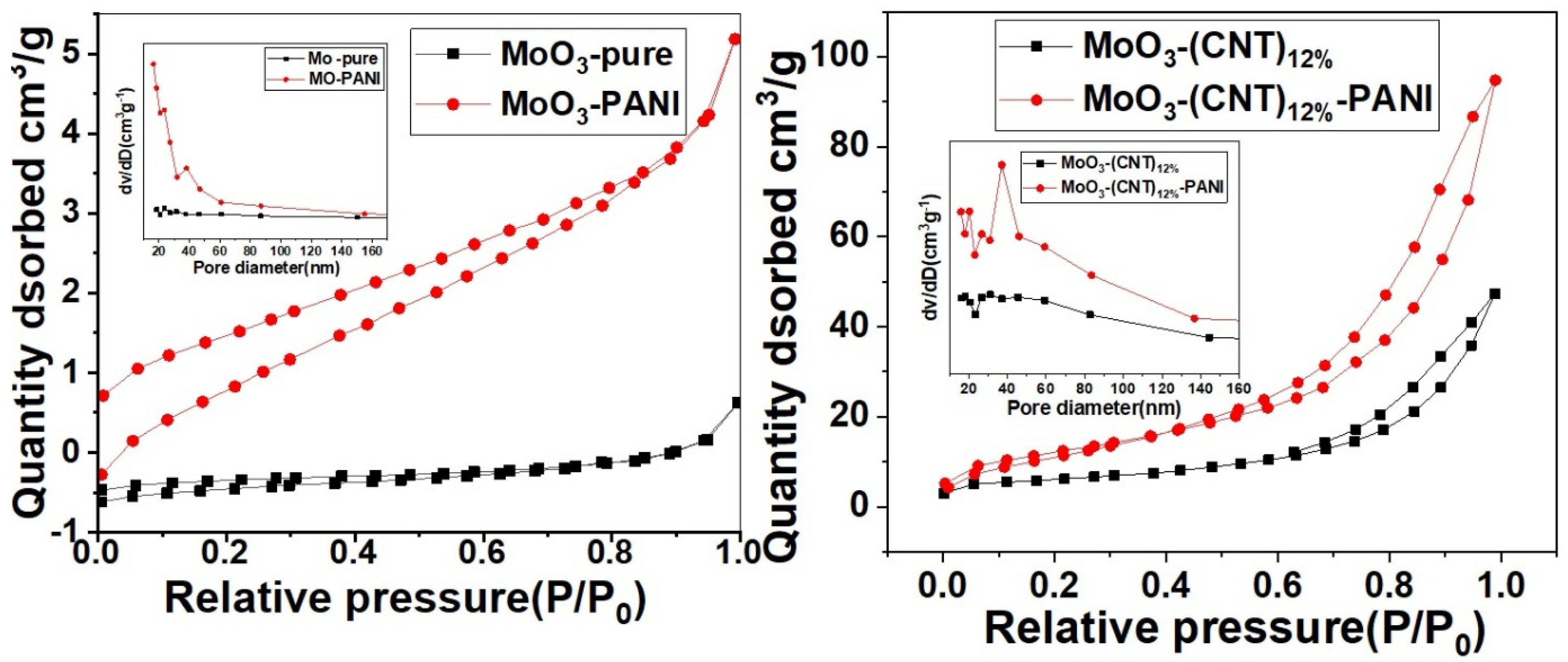
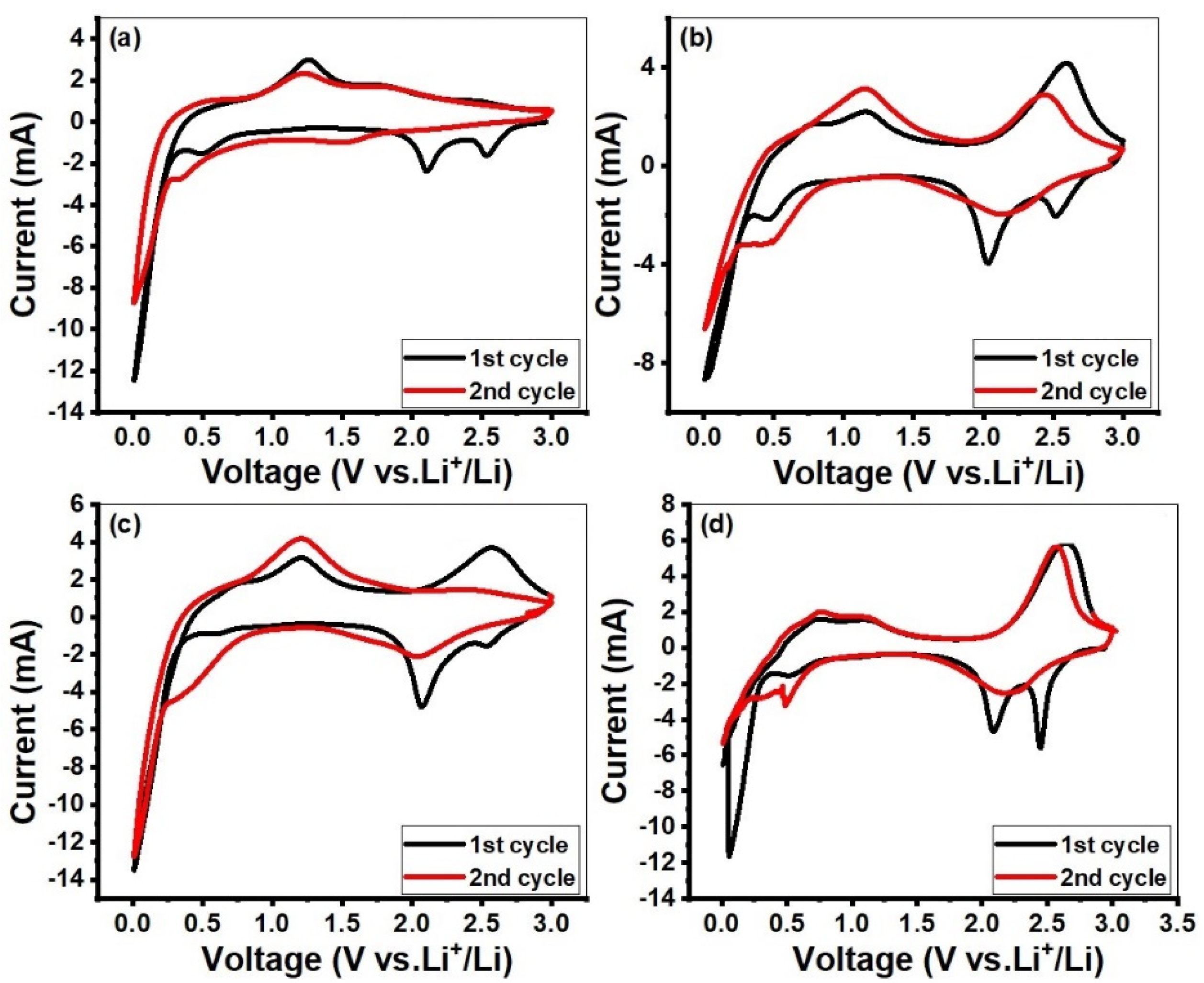
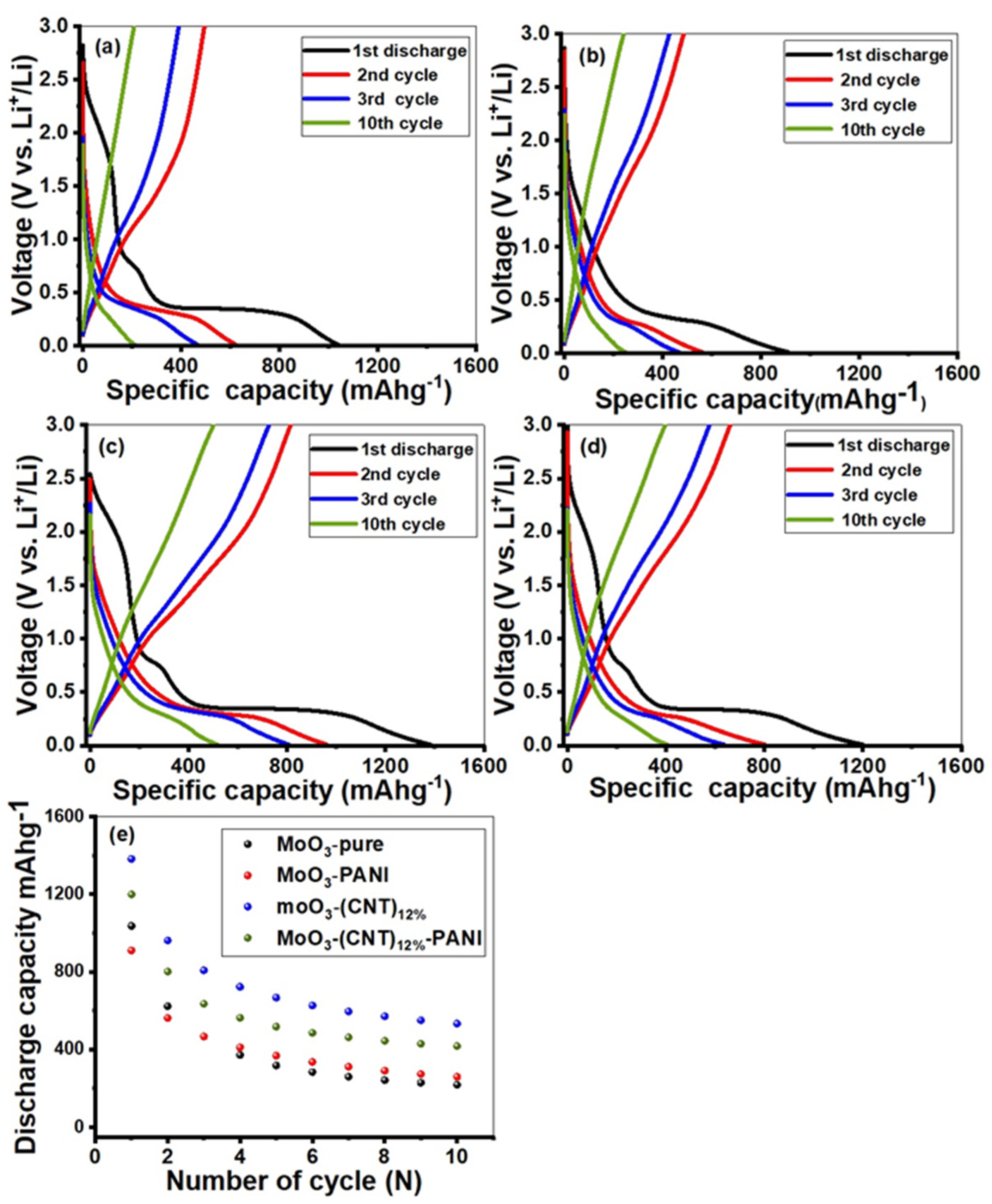
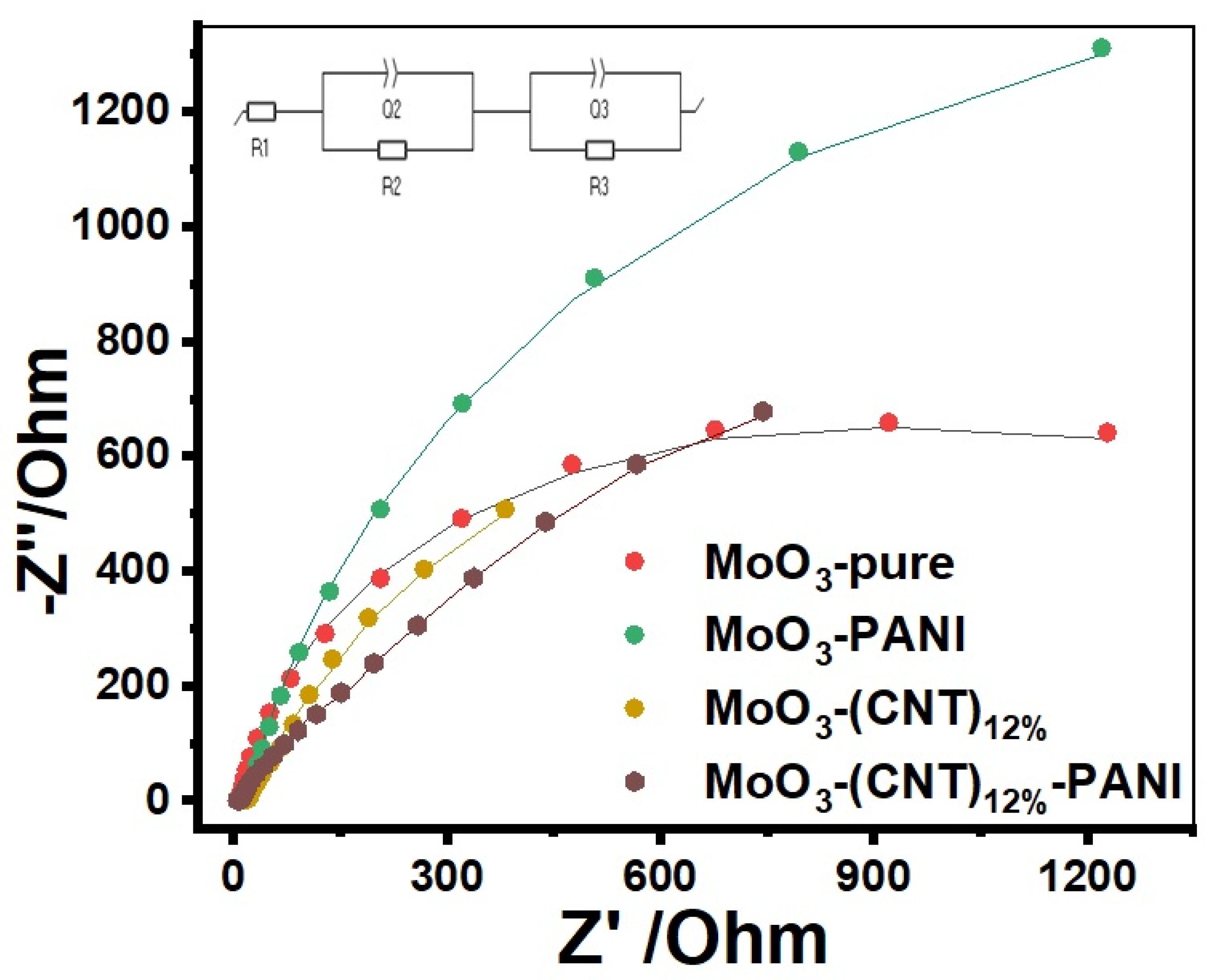
2. Results and Discussion
3. Materials and Methods
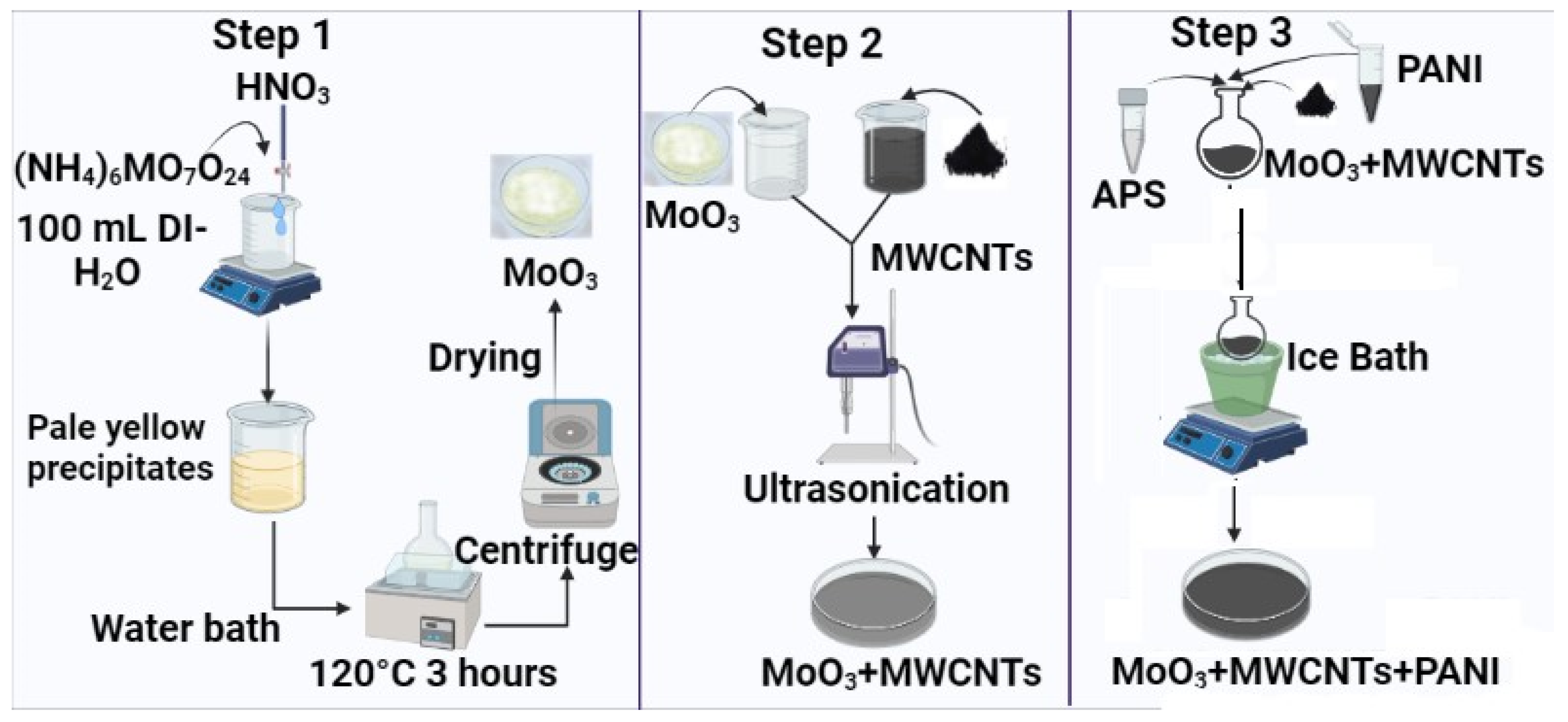
3.1. Materials
3.2. Synthesis of α-MoO3
3.3. Synthesis of α-MoO3-MWCNTs-PANI
3.4. Physiochemical Characterization
3.5. Electrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Asen, P.; Shahrokhian, S.; zad, A.I. Ternary nanostructures of Cr2O3/graphene oxide/conducting polymers for supercapacitor application. J. Electroanal. Chem. 2018, 823, 505–516. [Google Scholar] [CrossRef]
- Mann, M.E.; Bradley, R.S.; Hughes, M.K. Global-scale temperature patterns and climate forcing over the past six centuries. Nature 1998, 392, 779–787. [Google Scholar] [CrossRef]
- Ould Amrouche, S.; Rekioua, D.; Rekioua, T.; Bacha, S. Overview of energy storage in renewable energy systems. Int. J. Hydrogen Energy 2016, 41, 20914–20927. [Google Scholar] [CrossRef]
- Bhojane, P.; Shirage, P.M. Facile preparation of hexagonal WO3 nanopillars and its reduced graphene oxide nanocomposites for high-performance supercapacitor. J. Energy Storage 2022, 55, 105649. [Google Scholar] [CrossRef]
- Ibrahim, H.; Ilinca, A.; Perron, J. Energy storage systems—Characteristics and comparisons. Renew. Sustain. Energy Rev. 2008, 12, 1221–1250. [Google Scholar] [CrossRef]
- Das, C.K.; Bass, O.; Kothapalli, G.; Mahmoud, T.S.; Habibi, D. Overview of energy storage systems in distribution networks: Placement, sizing, operation, and power quality. Renew. Sustain. Energy Rev. 2018, 91, 1205–1230. [Google Scholar] [CrossRef]
- Zhu, W.; Kamali, A.R. Green preparation of nanostructured β-MoO3/hexagonal-shaped MoS2/graphene with enhanced lithium-ion storage performance. J. Alloy. Compd. 2023, 932, 167724. [Google Scholar] [CrossRef]
- Sheng, D.; Zhang, M.; Wang, X.; Zhou, S.; Fu, S.; Liu, X.; Zhang, Q. Carbon nanotubes embedded in α-MoO3 nanoribbons for enhanced lithium-ion storage. J. Mater. Sci. Mater. Electron. 2022, 33, 11743–11752. [Google Scholar] [CrossRef]
- Ding, J.; Sheng, R.; Zhang, Y.; Huang, Y.; Cheng, W.; Liu, Z.; Wang, X.; Guo, Y.; Wang, J.; Jia, D.; et al. Fe2O3/MoO3@NG Heterostructure Enables High Pseudocapacitance and Fast Electrochemical Reaction Kinetics for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2022, 14, 37747–37758. [Google Scholar] [CrossRef]
- Faizan, M.; Hussain, S.; Islam, M.; Kim, J.Y.; Han, D.; Bae, J.H.; Vikraman, D.; Ali, B.; Abbas, S.; Kim, H.S.; et al. MoO3@MoS2 Core-Shell Structured Hybrid Anode Materials for Lithium-Ion Batteries. Nanomaterials 2022, 12, 2008. [Google Scholar] [CrossRef]
- Chen, F.; Wang, J.; Huang, L.; Bao, H.; Shi, Y. Ordered Mesoporous Crystalline Mo-Doped WO2 Materials with High Tap Density as Anode Material for Lithium Ion Batteries. Chem. Mater. 2016, 28, 608–617. [Google Scholar] [CrossRef]
- Abbas, S.M.; Ahmad, N.; Ata-ur-Rehman; Rana, U.A.; Khan, S.U.-D.; Hussain, S.; Nam, K.-W. High rate capability and long cycle stability of Cr2O3 anode with CNTs for lithium ion batteries. Electrochim. Acta 2016, 212, 260–269. [Google Scholar] [CrossRef]
- Guo, C.; Liu, Z.; Han, K.; Zhang, L.; Ding, X.; Wang, X.; Mai, L. Nano-Sized Niobium Tungsten Oxide Anode for Advanced Fast-Charge Lithium-Ion Batteries. Small 2022, 18, 2107365. [Google Scholar] [CrossRef]
- Dong, X.; Zhang, Y.; Cui, C.; Zeng, S.; Fu, C.; Wang, L. Study on the binary transition metal oxide Mn2V2O7 structures for high performance lithium-ion batteries. J. Alloys Compd. 2022, 907, 164518. [Google Scholar] [CrossRef]
- Cho, J.S. Large Scale Process for Low Crystalline MoO3-Carbon Composite Microspheres Prepared by One-Step Spray Pyrolysis for Anodes in Lithium-Ion Batteries. Nanomaterials 2019, 9, 539. [Google Scholar] [CrossRef]
- Zhong, X.; Huan, H.; Liu, X.; Yu, Y. Facile synthesis of porous germanium-iron bimetal oxide nanowires as anode materials for lithium-ion batteries. Nano Res. 2018, 11, 3702–3709. [Google Scholar] [CrossRef]
- Zhu, L.; Han, T.; Ding, Y.; Long, J.; Lin, X.; Liu, J. A metal–organic-framework derived NiFe2O4@NiCo-LDH nanocube as high-performance lithium-ion battery anode under different temperatures. Appl. Surf. Sci. 2022, 599, 153953. [Google Scholar] [CrossRef]
- Xiao, Y.; Jiang, M.; Cao, M. Developing WO3 as high-performance anode material for lithium-ion batteries. Mater. Lett. 2021, 285, 129129. [Google Scholar] [CrossRef]
- Chu, Y.; Xiong, S. Mixed transition-metal oxides@carbon core-shell nanostructures derived from heterometallic clusters for enhanced lithium storage. Chin. Chem. Lett. 2022, 33, 486–490. [Google Scholar] [CrossRef]
- Wang, Q.; Kang, L.; Xing, Z.; Nie, C.; Hong, H.; Zhou, X.; Yun, Q.; Ju, Z.; Chen, B. Prussian Blue Analogue-Derived ZnO/ZnFe2O4 Core-Shell Nanospheres as High-Performance Anodes for Lithium-Ion and Potassium-Ion Batteries. Batter. Supercaps 2022, 6, e202200411. [Google Scholar]
- Wang, W.; Xiong, F.; Zhu, S.; Chen, J.; Xie, J.; An, Q. Defect engineering in molybdenum-based electrode materials for energy storage. Escience 2022, 2, 278–294. [Google Scholar] [CrossRef]
- Cheng, X.; Li, Y.; Sang, L.; Ma, J.; Shi, H.; Liu, X.; Lu, J.; Zhang, Y. Boosting the electrochemical performance of MoO3 anode for long-life lithium ion batteries: Dominated by an ultrathin TiO2 passivation layer. Electrochim. Acta 2018, 269, 241–249. [Google Scholar] [CrossRef]
- Li, Z.; Ma, J.; Zhang, B.; Song, C.; Wang, D. Crystal phase-and morphology-controlled synthesis of MoO3 materials. Crystengcomm 2017, 19, 1479–1485. [Google Scholar] [CrossRef]
- Li, J.; Liu, X. Preparation and characterization of α-MoO3 nanobelt and its application in supercapacitor. Mater. Lett. 2013, 112, 39–42. [Google Scholar] [CrossRef]
- Ren, H.; Sun, S.; Cui, J.; Li, X. Synthesis, Functional Modifications, and Diversified Applications of Molybdenum Oxides Micro-/Nanocrystals: A Review. Cryst. Growth Des. 2018, 18, 6326–6369. [Google Scholar] [CrossRef]
- Tang, K.; Farooqi, S.A.; Wang, X.; Yan, C. Recent Progress on Molybdenum Oxides for Rechargeable Batteries. ChemSusChem 2019, 12, 755–771. [Google Scholar] [CrossRef]
- Ding, J.; Abbas, S.A.; Hanmandlu, C.; Lin, L.; Lai, C.-S.; Wang, P.-C.; Li, L.-J.; Chu, C.-W.; Chang, C.-C. Facile synthesis of carbon/MoO3 nanocomposites as stable battery anodes. J. Power Sources 2017, 348, 270–280. [Google Scholar] [CrossRef]
- Li, T.; Beidaghi, M.; Xiao, X.; Huang, L.; Hu, Z.; Sun, W.; Chen, X.; Gogotsi, Y.; Zhou, J. Ethanol reduced molybdenum trioxide for Li-ion capacitors. Nano Energy 2016, 26, 100–107. [Google Scholar] [CrossRef]
- Li, Y.; Sun, H.; Cheng, X.; Zhang, Y.; Zhao, K. In-situ TEM experiments and first-principles studies on the electrochemical and mechanical behaviors of α-MoO3 in Li-ion batteries. Nano Energy 2016, 27, 95–102. [Google Scholar] [CrossRef]
- Cai, L.; Rao, P.M.; Zheng, X. Morphology-controlled flame synthesis of single, branched, and flower-like alpha-MoO3 nanobelt arrays. Nano Lett. 2011, 11, 872–877. [Google Scholar] [CrossRef]
- Zhu, J.; Ding, Y.; Ma, Z.; Tang, W.; Chen, X.; Lu, Y. Recent Progress on Nanostructured Transition Metal Oxides As Anode Materials for Lithium-Ion Batteries. J. Electron. Mater. 2022, 51, 3391–3417. [Google Scholar] [CrossRef]
- Yan, D.; Luo, X.; Zhang, H.; Zhu, G.; Chen, L.; Chen, G.; Xu, H.; Yu, A. Single-crystalline α-MoO3 microbelts derived from a bio-templating method for superior lithium storage application. J. Alloys Compd. 2016, 688, 481–486. [Google Scholar] [CrossRef]
- Yen, J.-Z.; Yang, Y.-C.; Tuan, H.-Y. Interface engineering of high entropy Oxide@Polyaniline heterojunction enables highly stable and excellent lithium ion storage performance. Chem. Eng. J. 2022, 450, 137924. [Google Scholar] [CrossRef]
- Yuan, L.; Wang, J.; Chew, S.Y.; Chen, J.; Guo, Z.P.; Zhao, L.; Konstantinov, K.; Liu, H.K. Synthesis and characterization of SnO2–polypyrrole composite for lithium-ion battery. J. Power Sources 2007, 174, 1183–1187. [Google Scholar] [CrossRef]
- Bai, Z.; Zhang, Y.; Zhang, Y.; Guo, C.; Tang, B.; Sun, D. MOFs-derived porous Mn2O3 as high-performance anode material for Li-ion battery. J. Mater. Chem. A 2015, 3, 5266–5269. [Google Scholar] [CrossRef]
- Li, B.; Li, X.; Li, W.; Wang, Y.; Uchaker, E.; Pei, Y.; Cao, X.; Li, S.; Huang, B.; Cao, G. Mesoporous Tungsten Trioxide Polyaniline Nanocomposite as an Anode Material for High-Performance Lithium-Ion Batteries. Chemnanomat 2016, 2, 281–289. [Google Scholar] [CrossRef]
- Cai, J.-J.; Zuo, P.-J.; Cheng, X.-Q.; Xu, Y.-H.; Yin, G.-P. Nano-silicon/polyaniline composite for lithium storage. Electrochem. Commun. 2010, 12, 1572–1575. [Google Scholar] [CrossRef]
- Feng, M.; Tian, J.; Xie, H.; Kang, Y.; Shan, Z. Nano-silicon/polyaniline composites with an enhanced reversible capacity as anode materials for lithium ion batteries. J. Solid State Electrochem. 2015, 19, 1773–1782. [Google Scholar] [CrossRef]
- Han, F.; Li, D.; Li, W.-C.; Lei, C.; Sun, Q.; Lu, A.-H. Nanoengineered Polypyrrole-Coated Fe2O3@C Multifunctional Composites with an Improved Cycle Stability as Lithium-Ion Anodes. Adv. Funct. Mater. 2013, 23, 1692–1700. [Google Scholar] [CrossRef]
- Huang, X.H.; Tu, J.P.; Zhang, C.Q.; Chen, X.T.; Yuan, Y.F.; Wu, H.M. Spherical NiO-C composite for anode material of lithium ion batteries. Electrochim. Acta 2007, 52, 4177–4181. [Google Scholar] [CrossRef]
- Jo, M.S.; Ghosh, S.; Jeong, S.M.; Kang, Y.C.; Cho, J.S. Coral-Like Yolk-Shell-Structured Nickel Oxide/Carbon Composite Microspheres for High-Performance Li-Ion Storage Anodes. Nano-Micro Lett. 2019, 11, 3. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Liu, Z.; Liu, B.; Wang, S.; Xiong, C. Fabrication of cactus-like CNT/SiO2/MoO3 ternary composites for superior lithium storage. Energy 2021, 217, 119386. [Google Scholar] [CrossRef]
- Lee, H.-J.; Shim, H.-W.; Kim, J.-C.; Kim, D.-W. Mo-MoO3-graphene nanocomposites as anode materials for lithium-ion batteries: Scalable, facile preparation and characterization. Electrochim. Acta 2017, 251, 81–90. [Google Scholar] [CrossRef]
- Klinbumrung, A.; Thongtem, T.; Thongtem, S. Characterization of Orthorhombicα-MoO3 Microplates Produced by a Microwave Plasma Process. J. Nanomater. 2012, 2012, 930763. [Google Scholar] [CrossRef]
- Saraf, M.; Shuck, C.E.; Norouzi, N.; Matthews, K.; Inman, A.; Zhang, T.; Pomerantseva, E.; Gogotsi, Y. Free-Standing α-MoO MXene Hybrid Electrode in Water-in-Salt Electrolytes. Energy Environ. Mater. 2023, 1, e12516. [Google Scholar] [CrossRef]
- Sharma, R.; Sarkar, A.; Jha, R.; Sharma, A.K.; Bhushan, M.; Bhardwaj, R. Synthesis & material properties of α-MoO3 nanoparticles. Mater. Today Proc. 2022, 48, 683–686. [Google Scholar]
- Chiang, T.H.; Yeh, H.C. A novel synthesis of α-MoO3 nanobelts and the characterization. J. Alloys Compd. 2014, 585, 535–541. [Google Scholar] [CrossRef]
- Jain, V.M.; Shah, D.V.; Patel, K.K.; Doshi, Y. Surfactant free synthesis and characterization of α-MoO3 nanoplates: A feasibility study to remove methylene blue from aqueous medium. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1126, 012052. [Google Scholar] [CrossRef]
- Navas, I.; Vinodkumar, R.; Lethy, K.J.; Detty, A.P.; Ganesan, V.; Sathe, V.; Mahadevan Pillai, V.P. Growth and characterization of molybdenum oxide nanorods by RF magnetron sputtering and subsequent annealing. J. Phys. D Appl. Phys. 2009, 42, 175305. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, D.; Liang, X.; Sun, A. Ultrasonic-assisted preparation of metastable hexagonal MoO3 nanorods and their transformation to microbelts. Ultrason. Sonochem. 2011, 18, 288–292. [Google Scholar] [CrossRef]
- Sen, S.K.; Dutta, S.; Khan, M.R.; Manir, M.S.; Dutta, S.; Al Mortuza, A.; Razia, S.; Hakim, M.A. Characterization and Antibacterial Activity Study of Hydrothermally Synthesized h-MoO3 Nanorods and α-MoO3 Nanoplates. Bionanoscience 2019, 9, 873–882. [Google Scholar] [CrossRef]
- Shahsank, M.; Bhojya Naik, H.S.; Sumedha, H.N.; Nagaraju, G. Implementing an in-situ carbon formation of MoO3 nanoparticles for high performance lithium-ion battery. Ceram. Int. 2021, 47, 10261–10267. [Google Scholar] [CrossRef]
- Zhou, J.; Lin, N.; Wang, L.; Zhang, K.; Zhu, Y.; Qian, Y. Synthesis of hexagonal MoO3 nanorods and a study of their electrochemical performance as anode materials for lithium-ion batteries. J. Mater. Chem. A 2015, 3, 7463–7468. [Google Scholar] [CrossRef]
- Cao, L.; Li, Y.; Wu, J.; Li, W.; Huang, J.; Feng, Y.; Yao, C.; Li, J.; Wang, R.; Kang, Q.; et al. Facile synthesis of carbon coated MoO3 nanorods decorated with WO2 nanoparticles as stable anodes for lithium-ion batteries. J. Alloys Compd. 2018, 744, 672–678. [Google Scholar] [CrossRef]
- Liu, C.-L.; Wang, Y.; Zhang, C.; Li, X.-S.; Dong, W.-S. In situ synthesis of α-MoO3/graphene composites as anode materials for lithium ion battery. Mater. Chem. Phys. 2014, 143, 1111–1118. [Google Scholar] [CrossRef]
- Besenhard, J.; Heydecke, J.; Fritz, H. Characteristics of molybdenum oxide and chromium oxide cathodes in primary and secondary organic electrolyte lithium batteries I. Morphology, structure and their changes during discharge and cycling. Solid State Ion. 1982, 6, 215–224. [Google Scholar] [CrossRef]
- Oh, S.H.; Park, S.M.; Kang, D.-W.; Kang, Y.C.; Cho, J.S. Fibrous network of highly integrated carbon nanotubes/MoO3 composite bundles anchored with MoO3 nanoplates for superior lithium ion battery anodes. J. Ind. Eng. Chem. 2020, 83, 438–448. [Google Scholar] [CrossRef]
- Li, M.-Y.; Wang, Y.; Liu, C.-L.; Gao, H.; Dong, W.-S. Iron oxide/carbon microsphere lithium-ion battery electrode with high capacity and good cycling stability. Electrochim. Acta 2012, 67, 187–193. [Google Scholar] [CrossRef]
- Tao, T.; Glushenkov, A.M.; Zhang, C.; Zhang, H.; Zhou, D.; Guo, Z.; Liu, H.K.; Chen, Q.; Hu, H.; Chen, Y. MoO3 nanoparticles dispersed uniformly in carbon matrix: A high capacity composite anode for Li-ion batteries. J. Mater. Chem. 2011, 21, 9350. [Google Scholar] [CrossRef]
- Liu, Z.; Sun, L.; Yang, W.; Yang, J.; Han, S.; Chen, D.; Liu, Y.; Liu, X. The synergic effects of Na and K co-doping on the crystal structure and electrochemical properties of Li4Ti5O12 as anode material for lithium ion battery. Solid State Sci. 2015, 44, 39–44. [Google Scholar] [CrossRef]
- Nadimicherla, R.; Zha, R.; Wei, L.; Guo, X. Single crystalline flowerlike α-MoO3 nanorods and their application as anode material for lithium-ion batteries. J. Alloys Compd. 2016, 687, 79–86. [Google Scholar] [CrossRef]
- Francis, M.K.; Bhargav, P.B.; Ramesh, A.; Ahmed, N.; Balaji, C. Electrochemical performance analysis of NiMoO4/α-MoO3 composite as anode material for high capacity lithium-ion batteries. Appl. Phys. A 2022, 128, 132. [Google Scholar] [CrossRef]
- Deng, Z.; Zhang, Z.; Lai, Y.; Liu, J.; Li, J.; Liu, Y. Electrochemical Impedance Spectroscopy Study of a Lithium/Sulfur Battery: Modeling and Analysis of Capacity Fading. J. Electrochem. Soc. 2013, 160, A553–A558. [Google Scholar] [CrossRef]
- Kim, J.H.; Kwak, E.; Oh, K.Y. Degradation pathways dependency of a lithium iron phosphate battery on temperature and compressive force. Int. J. Energy Res. 2020, 6888–6906. [Google Scholar] [CrossRef]
- Mishra, P.; Yavas, D.; Bastawros, A.F.; Hebert, K.R. Electrochemical impedance spectroscopy analysis of corrosion product layer formation on pipeline steel. Electrochimica Acta 2020, 346, 136232. [Google Scholar] [CrossRef]
- Xu, C.; Du, H.; Li, B.; Kang, F.; Zeng, Y. Capacitive Behavior and Charge Storage Mechanism of Manganese Dioxide in Aqueous Solution Containing Bivalent Cations. J. Electrochem. Soc. 2009, 156, A73. [Google Scholar] [CrossRef]
- Mohan, V.M.; Chen, W.; Murakami, K. Synthesis, structure and electrochemical properties of polyaniline/MoO3 nanobelt composite for lithium battery. Mater. Res. Bull. 2013, 48, 603–608. [Google Scholar] [CrossRef]

| Electrode Material | Initial Cycle Discharge (mAhg−1) | Reversible Capacity (mAhg−1) | Current Density (mAg−1) | Ref. |
|---|---|---|---|---|
| MoO3-(CNT)12%-PANI | 801 | 406 | 100 | This Work |
| MoO3-(CNT)12% | 961 | 517 | 100 | This work |
| α-MoO3 | 301 | 180 | 30 | [61] |
| α-MoO3 | 211 | 133 | 300 | [61] |
| MoO3 | 668 | 157 | 200 | [52] |
| MoO3 | 974 | 286 | 100 | [62] |
| MoO3-NiMoO4 | 1031 | 324 | 100 | [62] |
| α-MoO3-CNT | 583 | 194 | 500 | [8] |
| No | Samples | R1 (Ω) | R2 (Ω) | Q2 (Fsα−1) | α2 | R3 (Ω) | Q3 (Fsα−1) | α3 | χ2 |
|---|---|---|---|---|---|---|---|---|---|
| 1 | MoO3 | 7.11 | 9.92 | 1.21 × 10−3 | 0.474 | 1208 | 4.39 × 10−3 | 0.904 | 0.0077 |
| 2 | MoO3 PANI | 2.66 | 20.01 | 0.16 × 10−3 | 0.644 | 1201 | 5.25 × 10−3 | 0.882 | 0.00279 |
| 3 | MoO3 (CNT)12% | 3.07 | 22.20 | 0.11 × 10−3 | 0.678 | 339 | 0.0133 | 0.788 | 0.00796 |
| 4 | MoO3-(CNT)12% PANI | 5.16 | 45.40 | 7.65 × 10−5 | 0.722 | 696 | 4.64 × 10−3 | 0.754 | 0.00383 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiran, L.; Aydınol, M.K.; Ahmad, A.; Shah, S.S.; Bahtiyar, D.; Shahzad, M.I.; Eldin, S.M.; Bahajjaj, A.A.A. Flowers Like α-MoO3/CNTs/PANI Nanocomposites as Anode Materials for High-Performance Lithium Storage. Molecules 2023, 28, 3319. https://doi.org/10.3390/molecules28083319
Kiran L, Aydınol MK, Ahmad A, Shah SS, Bahtiyar D, Shahzad MI, Eldin SM, Bahajjaj AAA. Flowers Like α-MoO3/CNTs/PANI Nanocomposites as Anode Materials for High-Performance Lithium Storage. Molecules. 2023; 28(8):3319. https://doi.org/10.3390/molecules28083319
Chicago/Turabian StyleKiran, Laraib, Mehmet Kadri Aydınol, Awais Ahmad, Syed Sakhawat Shah, Doruk Bahtiyar, Muhammad Imran Shahzad, Sayed M. Eldin, and Aboud Ahmed Awadh Bahajjaj. 2023. "Flowers Like α-MoO3/CNTs/PANI Nanocomposites as Anode Materials for High-Performance Lithium Storage" Molecules 28, no. 8: 3319. https://doi.org/10.3390/molecules28083319
APA StyleKiran, L., Aydınol, M. K., Ahmad, A., Shah, S. S., Bahtiyar, D., Shahzad, M. I., Eldin, S. M., & Bahajjaj, A. A. A. (2023). Flowers Like α-MoO3/CNTs/PANI Nanocomposites as Anode Materials for High-Performance Lithium Storage. Molecules, 28(8), 3319. https://doi.org/10.3390/molecules28083319








