Abstract
Cannabidiol (CBD) is a major phytocannabinoid present in Cannabis sativa (Linneo, 1753). This naturally occurring secondary metabolite does not induce intoxication or exhibit the characteristic profile of drugs of abuse from cannabis like Δ9-tetrahydrocannabinol (∆9-THC) does. In contrast to ∆9-THC, our knowledge of the neuro-molecular mechanisms of CBD is limited, and its pharmacology, which appears to be complex, has not yet been fully elucidated. The study of the pharmacological effects of CBD has grown exponentially in recent years, making it necessary to generate frequently updated reports on this important metabolite. In this article, a rationalized integration of the mechanisms of action of CBD on molecular targets and pharmacological implications in animal models and human diseases, such as epilepsy, pain, neuropsychiatric disorders, Alzheimer’s disease, and inflammatory diseases, are presented. We identify around 56 different molecular targets for CBD, including enzymes and ion channels/metabotropic receptors involved in neurologic conditions. Herein, we compiled the knowledge found in the scientific literature on the multiple mechanisms of actions of CBD. The in vitro and in vivo findings are essential for fully understanding the polypharmacological nature of this natural product.
1. Introduction
The herbaceous plant, Cannabis sativa (Linneo, 1753), has been known and used for thousands of years for recreational, religious, and medicinal purposes. The most ancient record of the use of this plant as a medicine is found in the world’s oldest pharmacopeia, the Pen-ts’ao ching (China, 2737 BC) [1,2]. This plant contains many secondary metabolites including terpenoids, flavonoids, alkaloids, lignans, fatty acids, and a large number of components (approximately 120) with a hybrid biogenetic origin involving mevalonate and polyketide pathways, which are exclusive to the plant and are named phytocannabinoids (Figure 1) [3,4,5]. The two major phytocannabinoids isolated from C. Sativa L. are: (-)-trans-Δ9-tetrahydrocannabinol (Δ9-THC) and (-)-cannabidiol (CBD), (Figure 2) both of which have been studied for their therapeutic potential [6,7]. Δ9-THC was discovered in 1964 and is recognized as the primary psychoactive compound responsible for the behavioral effects induced by the consumption of C. sativa preparations, such as marijuana or hashish [8]. It has been demonstrated that Δ9-THC shares behavioral effects like those of other drugs of abuse in drug reinforcement animal models [9]. Moreover, Cannabis-related use disorder is acknowledged in the Diagnostic and Statistical Manual of Mental Disorders (DSM 5). The study of this molecule led to the further discovery of an important neuromodulatory system widely distributed throughout the brain and body, identified as the endocannabinoid system.
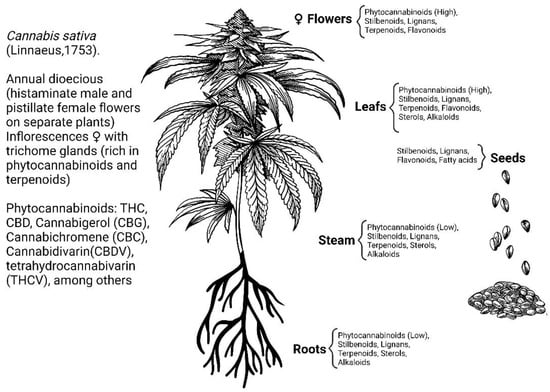
Figure 1.
Secondary metabolites of Cannabis sativa L. [5].
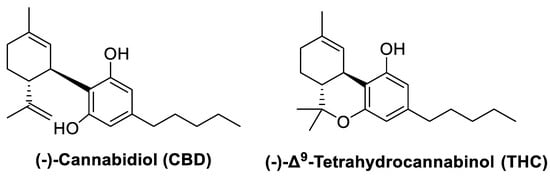
Figure 2.
Chemical structures of cannabidiol and tetrahydrocannabinol.
The endocannabinoid system is composed of (1) a network of cannabinoid receptors (CBR) expressed throughout the body identified as cannabinoid receptor type 1 and type 2 (CB1R and CB2R); (2) the endogenous endocannabinoid ligands, such as anandamide (AEA) and 2-arachidonoylglycerol (2-AG); and (3) enzymes required for the synthesis and degradation of the endogenous cannabinoids including fatty acid amide hydrolase (FAAH) and monoacylglycerol lipase (MAGL) [10]. Δ9-THC is a high-affinity partial agonist of both CB1R and CB2R receptors [11]. Currently, the FDA has approved three synthetic formulations of Δ9-THC and a Δ9-THC derivative with clinical application (Table 1) [12]. Side effects of these drugs are related to intoxication and include sedation, confusion, dysphoria, and paranoia, among others [13]. Unlike Δ9-THC, the second major phytocannabinoid, CBD, does not induce intoxication, and it does not exhibit the characteristic profile of a drug of abuse, although it has some psychoactive properties potentially relevant to its therapeutics usefulness. For this reason, CBD seems to be a more promising therapeutic compound than Δ9-THC. However, in contrast to this last one, our knowledge of the neuro-molecular mechanisms of CBD is limited, and its complex pharmacology has not been fully elucidated yet.
CBD is a phenolic monoterpene that was first isolated in 1940 from Mexican marijuana (Cannabis sativa L.) by Adams, Hunt, and Clark [14] and from the resin of Cannabis indica (syn. Cannabis sativa L.) by Jacob and Todd [15]. Later, in 1963, Mechoulam and Shvo [16] established the structure of CBD from Lebanese hashish. CBD is a hydrophobic drug, with absorption into the bloodstream occurring in the intestine. CBD is rapidly absorbed by adipose tissue and other organs and passes through the blood–brain barrier into the central nervous system (CNS). Unfortunately, the bioavailability of CBD is low when it is orally administered (6–19%); thus, the administration of 10 mg of CBD reaches the maximum concentration in plasma ~3 μg/L at 2.8 h [17]. The administration of CBD by different routes, across a wide range of doses, does not induce serious side effects or toxicity in humans or other species [18]. Repeated administration over a month to healthy volunteers (daily doses ranging from 10 to 400 mg) did not induce any significant abnormalities in neurological, psychiatric, or clinical exams [19]. Thus, it has been concluded that CBD possesses a desirable safety profile [20]. While the CBD pharmacokinetic profile has been well established, its pharmacodynamics have been more complicated to elucidate [21].
CBD exerts a broad-spectrum pharmacological effect on several conditions such as pain, inflammation, epilepsy, and anxiety, among others, supporting the therapeutic potential use of CBD in the treatment of diverse neurodegenerative diseases and neuropsychiatric conditions [22,23]. In rodent models, CBD has been shown to have anxiolytic [24,25], anti-depressive [26,27], and anti-inflammatory effects [28], among others. The study of the pharmacological effects of CBD has been growing in recent years, and, therefore, new and exciting findings are discovered gradually. The mechanisms of action for CBD to exert its effects are complex. CBD does not seem to interact with specific CBR [29], but it has been reported that it interacts with around 56 molecular targets, including ionotropic receptors, nuclear receptors, metabotropic receptors, and enzymes, among others [21]. Due to the broad spectrum of possible medical applications of CBD, many naturally occurring and synthetic CBD derivatives have been described in the scientific literature and numerous patents for its potential clinical use [30]. Recently, the FDA approved the use of pure CBD (Table 1) [12,31,32,33] for the treatment of medically refractory seizures in patients with Dravet syndrome (DS) or Lennox–Gastaut syndrome (LGS), conditions more prevalent in children. There are currently ongoing clinical trials for the use of CBD in other conditions such as autism [34,35], schizophrenia [36], and other diseases. Due to its multi-targeted properties, CBD has been shown to have enormous therapeutic potential while lacking psychotropic adverse events [37,38]. This work aims to review and integrate the different mechanisms of action of CBD on its molecular targets and its pharmacological implications in animal models and humans.

Table 1.
FDA-approved cannabis-based pharmaceutical-grade drugs developed and marketed to 2023 [12,31,32,33].
Table 1.
FDA-approved cannabis-based pharmaceutical-grade drugs developed and marketed to 2023 [12,31,32,33].
| Drug Name | Basic Formulation | Indication |
|---|---|---|
| Marinol®/Syndros® | Dronabinol (synthetic Δ9-THC) | Appetite stimulation. Antiemetic associated with chemotherapy |
| Cesamet® | Nabilone (synthetic Δ9-THC derivative) | Antiemetic associated with chemotherapy |
| Epidiolex® | CBD Plant-derived | Dravet syndrome and Lennox–Gastaut Seizures associated with tuberous sclerosis complex (TSC) |
| Sativex® Not FDA-approved Registered for commercial distribution in Europe and Canada | CBD: Δ9-THC 1:1 Plant-derived | Pain relief Spasticity related with multiple sclerosis (MS) Tourette syndrome (frequency and severity of motor and vocal tics) |
2. Molecular Targets Reported for Cannabidiol
CBD is a complex multi-target molecule, meaning that it can exert different pharmacological effects by interacting with highly diverse molecular targets. CBD behaves as an agonist (Table 2), inverse agonist, or antagonist (Table 3) on different receptors. CBD can also behave as an allosteric negative (NAM) or positive (PAM) modulator. CBD also exerts an effect on several enzymes, both neuro-enzymes and hepatic ones. CBD’s effects have been reported to vary across concentrations and doses in vitro and in vivo models (Figure 3). First, we review the effects of CBD on ion channels, followed by the effects of CBD on metabotropic receptors, concluding with the effects of CBD on neuro-enzymes that metabolize and regulate neurotransmission systems. Because of its relevance, the literature about the effects of CBD on hepatic enzymes is also briefly considered. This section will be divided by subheadings and provide a concise and precise description of the experimental results, their interpretation, as well as the experimental conclusions that can be drawn.

Table 2.
CBD such as an agonist or PAM of receptors in the nervous system.
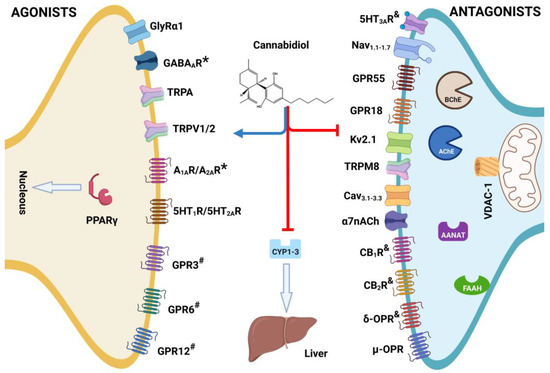
Figure 3.
Structure of cannabidiol (CBD) and its role such as agonist or * = positive allosteric modulator (PAM), antagonist, # = inverse agonist, and & = negative allosteric modulator (NAM) on multi-target receptors and enzymes associates with developed of diseases in vivo. Created with BioRender.com.
2.1. Ligand-Gated Ion Channels
Glycine receptors (GlyRs) are ligand-gated chloride ion channels that mediate fast inhibitory neurotransmission [53]. GlyRs are expressed in the spinal cord and the brain stem where they are mainly involved in motor control, sensorial processing, and pain perception [54]. GlyRs are pentameric membrane proteins composed of four isoforms of the α subunits (α1–4) and a single isoform of the β subunit [55]. It has been demonstrated that high-range concentrations of CBD directly activate the strychnine-sensitive GlyRs, while at low micromolar concentration range, CBD exerts a positive allosteric modulation (PAM) of GlyRs [56]. GlyRα3 is highly expressed in the superficial layer of the spinal dorsal horn and is involved in the antinociceptive process. It has been reported that CBD can suppress persistent inflammatory and neuropathic pain by targeting these receptors in animal rodent models for pain [40].
GABA-A receptors (GABA-ARs) are ligand-gated ion channels that mobilize chlorine anion, producing hyperpolarization of cells and a subsequent reduction of neuronal activity through the actions of the amino acid GABA, which is the major inhibitory neurotransmitter in the mammalian brain. GABA-ARs are formed by combinations of 19 subunits: 6 alpha, 3 beta, 3 gamma, 3 rho, and one each of delta, epsilon, pi, or theta, thus generating a wide variety of isoforms. GABA-AR exhibit multiple allosteric binding sites. Pharmacological interactions with GABA-AR are extremely complex [57]. It has been described that in GABA-AR transfected into Xenopus laevis oocytes, CBD is capable of increasing GABA-AR mediated currents in a dose-dependent manner, with the β subunit being the main binding site for it. Therefore, CBD is a PAM of GABA-AR in micromolar concentration ranges [58]. This mechanism of action seems to be related with the anxiolytic and anticonvulsant effects exerted by CBD [41].
The only ionotropic serotonin receptors are 5-HT3 ionotropic serotonin receptors (5-HT3Rs). They are ligand-gated cation channels located in the CNS and PNS [59,60]. The 5-HT3R is expressed in regions that are involved in the integration of the vomiting reflex, bradycardia, hypotension, pain transmission, analgesia, mood disorders, and control of anxiety. Meanwhile, peripheral receptors participate in the regulation of sensory transmission and autonomic functions [61,62,63]. CBD has been reported to act as a negative allosteric modulator (NAM) for 5-HT3R and a non-competitive inhibitor of the function of human and mice 5-HT3R expressed in HEK293 cells [63] and Xenopus laevis oocytes [64].
Five classes of nicotinic receptors nAchR subunits (α, β, y, ε, δ,) lead to different functional isoforms of the homopentameric receptor or heteropentameric receptors [65]. Homomeric α7 nicotinic acetylcholine receptors (α7-nAChR) are pentameric calcium (Ca2+) channels highly expressed in the nervous system and spinal cord. In the brain, α7-nAChR are distributed post- and presynaptic throughout the cortex, thalamus, and hippocampus. Both excitatory and inhibitory synaptic transmission can be modulated by these receptors [66]. In humans, a reduction in α7-nAChR expression has been associated with an increase in seizure susceptibility [67]. α7-nAChR agonists have pro-cognitive effects, and its modulation has been proposed to be relevant for the treatment of Alzheimer’s disease (AD) or cognitive symptoms of schizophrenia, among others [68]. CBD can inhibit α7nAChR in a dose-dependent manner and induce reduction of acetylcholine-evoked currents amplitude in in vitro BOSC-23 cells and in ex vivo patch clamp assays using rat hippocampal slices, while Δ9-THC does not affect this channel. Therefore, CBD acts as a NAM in the closed and desensitized states of these channels [69,70].
Voltage-gated sodium channel (VGSC or Nav) genes are relevant to epilepsy in humans. These Nav are transmembrane ion channels that allow the passage of sodium ions (Na+) along their electrochemical gradient [71]. After the activation of these channels, Na+ flow into the intracellular environment, with an opening of the channel for a few milliseconds, subsequently leading to its inactivation and closing of the channel, preventing a further flow of sodium ions [71]. The passage of Na+ through Nav channels generates transient sodium currents that produce action potentials in cardiac muscle, skeletal muscle, and neurons [72]. Mutations in the VGSC SCN1 gene led to the loss of function of the Nav1.1 channel, causing the development of DS. In a mouse model of Scn8a-associated epilepsy (encoding Nav1.6), CBD has shown efficacyin reducing seizures frequency at a dose of 320–360 mg/kg [73]. Similarly, it is suggested that refractory epilepsies may be associated with mutations in the SCN2A gene [74]. Nav channels have been the most studied targets of CBD since, in clinical studies, CBD (20 mg/kg/day) efficacy has been shown against drug-resistant seizures in DS [72]. The inhibitory mechanism of CBD is associated with the inhibition of the Nav channels on both rest and inactivate states, which suggests that CBD does not inhibit direct interaction with a specific binding site [72]. Similarly, it has been shown in vitro that CBD had no specificity for any of Nav channels (1.1–1.7), mNav1.6, or bacterial homomeric Nav channel (NaChBac) and voltage-gated potassium channel subunit Kv2.1 [71,72].
T-type voltage-gated calcium channels (VGCCs) are a family of channels highly expressed in neurons where they modulate neuronal excitability and, in other tissues, low-threshold calcium spiking or cardiac pacemaker activity. These channels have been associated with the regulation of epilepsy, sleep, and pain; however, the mechanism underlaying these effects are unclear. The endogenous cannabinoid AEA has been suggested as an endogenous inhibitor of these channels through a non-CB receptor-mediated mechanism [75]. In an in vitro model with HEK 293 cells expressing VGCC channels, it has been proposed that CBD is an inhibitor of the cation currents recorded by patch clamp for CaV3.1, CaV3.2, and CaV3.3 channels [75].
Voltage-dependent anion-selective channel protein 1 (VDAC1) is a mitochondrial channel expressed in the outer membrane, where it has an important role in the control of cellular energy and metabolism, by acting as a regulator of metabolite transfer between the cell cytosol and mitochondria. CBD has been proposed as a direct inhibitor of the conductance of this channel by altering cytosolic calcium homeostasis, mitochondrial morphology, and function, as well as viability, which is associated with the strong immunosuppressive response and its anticancer effects [76].
2.2. Transient Receptor Potential Channels (TRP)
TRP channels are present in mammals and expressed in multiple body tissues, being of great importance in peripheral neurons to transmit nerve impulses produced by chemical and physical stimuli to the brain [77]. Such stimuli can be mechanical stress, heat, variation in pH, osmotic pressure, and compounds derived from plants that lead to the activation of these receptors and the consequent mobilization of cations such as K+, Na+, Mg2+, and Ca2+ [78,79]. TRP channels are classified into six families with 27 different channels, being canonical (TRPC), ankyrin (TRPA), polycystin (TRPP), mucolipin (TRPML), melastatin (TRPM), and vanilloid (TRPV) [46]. Six of these channels, TRPV1, TRPV2, TRPV3, TRPV4, TRPA1, and TRPM8, are of importance because they are activated or inhibited by cannabinoids and have been called ionotropic CBR [79]. These six channels are involved in important physiological processes such as immune function, regulation of neurotransmitter release, temperature sensation, and pain [80].
Transient receptors potential vanilloid (TRPV), and especially type 1 (TRPV1), is the most studied of the TRP channels, which was discovered to be activated by vanilloid agonists such as capsaicin, which subsequently leads to channel desensitization and a quiescent analgesic effect [46,81], which earned its discoverer the 2021 Nobel Prize in Medicine. CBD is a full, but not potent, agonist of this type of channel [46,77,81]. This mechanism of action is related to the anxiolytic, anti-hyperalgesic, and anti-inflammatory effects of CBD in animal models [62]. In addition, CBD activates the phagocytic capacity of microglia concentration-dependent (0.1–10 µM) by mobilizing Ca2+ dependent on TRPV1 and TRPV2. The latter has been proposed as an advantage in favoring the clearance of β-amyloid and reducing problems in patients with AD [45]. TRPV2 shares a sequence identity of 50% and similar desensitization with TRPV1. However, this receptor is insensitive to capsaicin and acts as a heat sensor: activating above 52 °C, it is associated with chronic pain and inflammation [46]. It has recently been suggested that CBD activates TRPV2 by binding in a small hydrophobic gap between the S5 and S6 helices of adjacent subunits, which have not been identified in other TRP channels [82,83]. TRPV3 is also a warm temperature sensor that activates in the range of 33–39 °C. This channel is widely expressed in the brain, skin, and tongue. It has been described that CBD produces a similar response in TRPV3 to that of its agonist carvacrol since it activates the channel but subsequently desensitizes it [44]. However, the response of CBD to TRPV3 is lower than for TRPV1 and TRPV2. It has been suggested that differences in sequence homology at the putative CBD binding site could be responsible for the low response [46]. TRPV4 is also a warm temperature sensor in the range of 25–34 °C. This channel is present in the skin where it has an important role in barrier functions and nociception. CBD has a poor response compared to the three previously mentioned TRPV channels [44,46,81].
Transient receptors potential ankyrin type 1 (TRPA1) is a sensor of low temperatures (<17 °C). It is present in the PNS, where it plays a role as a response sensor to cold, as well as hypersensitivity to cold and hyperalgesia to cold, so this receptor is important for detection of inflammatory and pain stimuli [80]. The canonical agonists of TRPA1 are isothiocyanates that are present in onions, mustard, and garlic [80]. CBD has been shown a more potent agonist than allyl isothiocyanate [44,46,81]. It was previously demonstrated in dissociated vagal afferent neurons that CBD generated its response to increasing intracellular calcium through TRPA1 [84].
Transient receptors potential melastatin 8 (TPRM8) is a temperature sensor that is activated at ~27 °C and is expressed in sensory neuron subpopulations of the PNS, where it has been found to act in the development of neuropathic pain and migraines [85]. TRPM8 agonists include icilin, eucalyptol, and menthol, which have been suggested to activate the channel at different sites [46,80]. However, CBD is an effective TPRM8 antagonist regardless of the type of agonist used [46,80].

Table 3.
CBD such as an antagonist, NAM, or inverse agonist of receptors in the nervous system.
Table 3.
CBD such as an antagonist, NAM, or inverse agonist of receptors in the nervous system.
| Receptor Type | Activity | IC50 (µM) | Disease Model | Tissue Expression | Cited |
|---|---|---|---|---|---|
| 5-HT3A | NAM | 0.6 | LiCl-induced nausea in rats. | CNS and PNS. | [62,86] |
| α7nAChR | Antagonist | 11.3 | Inflammation in mice. | PNS, CNS (cortical, thalamic, and hippocampal regions), and skeletal neuromuscular junction. | [70,87] |
| NaV1.1–1.7 | Antagonist | 1.9–3.8 | Drug-resistant seizures in DS models. | CNS and peripheral neurons. | [43,72] |
| Kv2.1 | Antagonist | 3.0 | Epilepsy in humans and microcephaly induced in zebrafish. | Hippocampal and cortical pyramidal neurons. | [72,88,89] |
| GPR3 | Inverse agonist | 1 | - | - | [90] |
| GPR6 | Inverse agonist | 0.1 | - | - | [90] |
| GPR12 | Inverse agonist | 10 | - | - | [90] |
| CaV3.1 | Antagonist | 0.82 | - | Widespread expression in neuronal and other tissue. | [75] |
| CaV3.2 | Antagonist | 0.78 | - | Widespread expression in neuronal and other tissue. | [75] |
| CaV3.3 | Antagonist | 3.7 | - | Widespread expression in neuronal and other tissue. | [75] |
| TRPM8 | Antagonist | 0.06 | Rat behavioral model of headache and hind paw and cutaneous facial allodynia. | Sensory neuron subpopulations of the PNS, and circuits related to migraine pathogenesis. | [85,91] |
| CB1R | NAM | 0.2 ** | Seizures in cobalt-epileptic rats. | Amygdala, olfactory bulb, cerebellum, hippocampus, basal ganglia, and neocortex. | [43,92] |
| CB2R | NAM | 0.24 ** | - | Cells of the immune and hematopoietic system. | [43,92] |
| µ-OPR | Antagonist | 8–12 | Drug abuse, mood disorders, and pain perception models. | Amygdala, spinal cord, substantia nigra, hypothalamic nuclei, hippocampus, and dorsal root ganglia. | [43] |
| δ-OPR | NAM | - | Drug abuse, mood disorders, and pain perception models. | Amygdala, spinal cord, substantia nigra, hypothalamic nuclei, hippocampus, and dorsal root ganglia. | [43] |
| GPR55 | Antagonist | 0.44 | Epilepsy in mouse model of DS. | Excitatory neurons of dentate gyrus in hippocampus. | [43] |
CNS = Central nervous system; PNS = Peripheral nervous system; DS = Dravet syndrome; LiCl = Lithium chloride; NAM = Negative allosteric modulator. ** Ki for human CB1 and CB2.
2.3. Metabotropic Receptors
Cannabinoid receptors (CB1R and CB2R) share 44% of their molecular structure, and both are coupled to Gi/o protein, which negatively modulates adenylyl cyclase. However, they differ in their specificity, function, and their pattern of distribution, as well as in cellular expression. CB1R is highly expressed in the brain; meanwhile, CB2R is found predominantly in immune cells, although recent evidence demonstrates that CB2R is expressed in microglia and also in neurons in the brain [93,94,95,96,97,98]. These receptors mediate the physiological actions of the endocannabinoids and the behavioral effects of the phytocannabinoid Δ9-THC [99]. It has been reported that CBD has a weak binding affinity for CBR (Ki ≥ 10 µM) [100], but it seems that CBD is capable of modulating some of its actions. It has been reported that CBD behaves as a non-competitive NAM of CB1R and CB2R, reducing the efficacy and potency of Δ9–THC and other cannabinoid receptors agonists, such as synthetic cannabinoids, CP–55,940 and WIN55,212 and of the endocannabinoids, AEA and 2-AG [101,102,103,104,105].
Serotonin receptors are classified into seven families (5-HT1-7R) with at least 14 distinct receptor subtypes. Except for the ligand-gated ion channel 5-HT3R, all serotonin receptors are classical seven-transmembrane GPCRs that mediate their effects on different secondary messenger enzymes via activation of distinct G-proteins [106]. Serotonin receptors are widely expressed in multiple brain regions, and specific neurons can express several different serotonin receptors. They play a role in many physiological processes, including thermoregulation, respiration, circadian rhythm (sleep-wake cycle), vascular function, emesis, cognition, and regulation of emotion [107]. Evidence has shown that CBD interacts with 5-HTRs, in particular, with 5-HT1AR and 5-HT2AR [108]. The 5-HT1AR is located presynaptically and postsynaptically and therefore can act as autoreceptor and heteroreceptor, where they exert their effects through Gαi/o proteins to inhibit adenylyl cyclase. Studies have shown that CBD acts as an agonist with modest affinity at the human 5-HT1AR [108]. One of the most conclusive pieces of evidence of the pharmacological effects of CBD is its anxiolytic property, because CBD produces the anxiolytic response through a wide range of concentrations. One of the proposed mechanisms is the interaction with the 5HT1AR [24,108,109,110,111]. Anxiolytic effects of CBD are induced with a bell-shaped dose–response curve when administered directly into the dorsolateral periaqueductal gray, an effect mediated by the 5HT1AR [24]. On the dorsal raphe nucleus, CBD acts as an indirect agonist of the somatodendritic 5-HT1A autoreceptors, contributing to the anti-emetic effect [112]. The 5-HT2AR is expressed mainly in the cerebral cortex, olfactory bulb, and brainstem nuclei. These receptors are coupled via Gq and are known as presynaptic and postsynaptic on serotoninergic terminals. CBD acts as a partial antagonist of 5-HT2A [108].
Adenosine produces its physiological response by activating four G-protein coupled receptors (A1, A2A, A2B, and A3 receptors), and they are found widely distributed in most of the body tissues, participating in a large variety of pathophysiological responses, such as vasodilation, pain, and inflammation [113]. It has been demonstrated that CBD activates the A1AR; this mechanism is related to the capacity of CBD to suppress ischemia-induced ventricular arrhythmias [49].
Opioidergic compounds interact with opioid receptors (μ, δ, and κ receptors) and play an important role in diverse physiological and pathophysiological processes, including analgesia, respiratory depression, and psychiatric illness. They are also expressed in the cardiovascular and immune system [114]. CBD behaves as PAM at and δOPR, and it is capable of accelerating µOPR mu agonist dissociation from the binding site, thus reducing its activity (demonstrated by kinetic binding studies) [115,116].
GPR55 is a receptor that is commonly expressed in association with CBR in the brain, PNS, and other tissues such the immune system cells and microglia [117]. This receptor has been associated with different diseases such as vascular functions, motor coordination, metabolic disorders, bone physiology, pain, and cancer. Its endogenous ligand is lysophosphatidylinositol (LPI). Once activated, GPR55 can interact downstream with Gαq/11, Gα12, Gα13, or Gα12/13, depending on the tissue or cell type [118]. CBD is an antagonist of GPR55 since it can block the effect of CP55940 in cells transfected with GPR55 in vitro [118]; CBD also acts in other tissues [119,120]. GPR18 has shown low sequence homology concerning CB1R, CB2R, and GPR55 receptors; its endogenous agonist is N-arachidonoyl glycine (NAGly). GPR18 has been described in various tissues such as lymphoid tissue, brain, lungs, ovary, and testis, where it has been associated with sperm physiology, metabolism, and with diseases such as cancer, intraocular pressure, and pain [121]. CBD acts as an antagonist of GPR18, shown to inhibit the migration of BV-2 microglia and transfected HEK293-GPR18 cells induced by NAGly and ∆9-THC [122]. GPR3, GPR6, and GPR12 are three receptors with about 60% molecular sequence similarity to the CB1 and CB2 receptors. These three receptors are expressed in the reproductive system and the brain and constitutively activate adenylate cyclase through Gαs proteins. These receptors are involved in the formation of synaptic contacts as well as in differentiation and neuronal growth [123]. The GPR3 is expressed in the nervous system: in dorsal root ganglia neurons, hippocampus, amygdala, cortex, and habenula [123]. Additionally, GPR3 is expressed in the ovary, testis, skin, adipose tissue, heart, liver, breast, and eye. GPR3 has been shown to prevent apoptosis in neurons and is associated with the development of neuropathic pain, emotional disorders, and morphine-induced antinociception [124]. Similarly, GPR12 is expressed in the limbic system and associated with emotion, behavior, and memory [123]. CBD has been shown to act as an inverse agonist for all three receptors: GPR3, GPR6, and GPR12 [125]. Although, CBD showed a weak to moderate response to GPR3 [124].
2.4. Nuclear Receptor: Peroxisome Proliferator-Activated Receptor γ (PPARγ)
PPARγ has been identified in adipose tissue and macrophages and has been involved in glucose energy metabolism and lipid storage [52]. In general, PPARγ ligands have shown anti-inflammatory activity, and CBD acts as an agonist of this receptor [50,52,126,127]. CBD has been shown to activate PPARγ in multiple sclerosis (MS) models [51]; also, CBD prevents neurodegeneration in a rat model of AD by reducing pro-inflammatory molecules and stimulating hippocampal neurogenesis [50]. CBD also reduces VCAM-1 level and the permeability produced by ischemia in a model of the blood–brain barrier [127].
2.5. Enzymes
Between the neuro-enzymes that have been shown interaction with CBD, we can highlight (Table 4) acetylcholinesterase (AChE), butyrylcholinesterase (BChE), fatty acid amide hydrolase (FAAH), and arylalkylamine N-acetyltransferase (AANAT). Current medications to treat patients with AD are based on blocking cholinesterase enzymes. However, these drugs have been shown to have side effects such as vomiting and nausea, as well as limitations by not being able to control neuroinflammation, oxidative stress, and amyloidogenesis [128]. CBD and Δ9-THC can inhibit AChE, while CBD only inhibits BChE.
FAAH is a membrane protein belonging to the serine hydrolases family; this enzyme is part of the endocannabinoid system, and its main role is terminating the signaling of bioactive lipids known as fatty acid amides (FAAs) present in the CNS and peripheral tissues; this includes hydrolysis of the AEA [129]. Inhibition of FAAH activity leads to an increase in the concentration of AEA, which, when interacting with its receptors, increases neuronal transmission to reduce pain, neuroinflammation, anxiety, and depression and counteracts nicotine addiction [129]. CBD is reported to inhibit the activity of the FAAH enzyme [91,100], although this inhibition is moderate [130].
Hepatic enzymes that include cytochromes P450 (CYP) are part of a large family of hemeprotein liver enzymes classified into families or subfamilies depending on their amino acid sequence homology and are the enzymes responsible for the first step in the metabolism and biotransformation of endogenous substrates, chemicals, and drugs [131]. It has been described that CBD inhibits cytochrome P450-mediated drug metabolism; for instance, CBD increases the plasma half-life of drugs such as hexobarbital, which is metabolized by CYP2C9 in patients. CBD has recently been reported to inhibit the catalytic activity of the liver enzymes CYP1A1-2, CYP1B1, CYP1B6, CYP2C9, CYP2C19, CYP2D6, CYP2E1, CYP3A4-5, CYP3A7, UGT1A9, and UGT2B7 in in vitro models [132,133,134,135,136,137,138,139].

Table 4.
Neuro and hepatic enzymes inhibited for CBD.
Table 4.
Neuro and hepatic enzymes inhibited for CBD.
| Enzyme Type | Activity | IC50 (µM) | Cited |
|---|---|---|---|
| AChE | Antagonist | 48.1 | [128] |
| BChE | Antagonist | 36.8 | [128] |
| FAAH | Antagonist | 15.2–27.5 | [91,100] |
| AANAT | Antagonist | <1.0 | [140] |
| CYP1A2 | Antagonist | <1.0 | [132] |
| CYP2B6 | Antagonist | 1.0 | [132] |
| CYP2E1 | Antagonist | 1.0 | [132] |
| CYP3A4 | Antagonist | <1.0 | [132] |
3. Therapeutic Evidence Involving CBD
Dose-dependent biphasic effects of several cannabinoids (phytocannabinoids, synthetic and endogenous) have been reported in several responses, such as motor activity [141,142,143], feeding behavior [144,145], sexual behavior [146,147], and anxiety responses [148,149], among others. Similarly, CBD has a biphasic profile on anxiety [150,151]. This should be considered in order to understand the implication of this drug in therapeutics. CBD emerges as a promising therapeutic drug associated with its multi-target activity, which, like other natural products, presents a complex polypharmacology. The high acceptance by people of the use of natural products or their derivatives may be a possible factor to obtain better results in treatment with cannabinoids associated with inflammatory diseases and for chronic diseases requiring very long-term treatments [152]. CBD is a promising molecule in therapeutics of different conditions. Because its consumption is safe and does not induce intoxication, the industry sells CBD mostly as an oil. However, CBD modifies the metabolism of other pharmacological treatments. Considering that CBD is used more as an adjuvant in the treatment of chronic conditions, such as MS or epilepsy, warning of possible pharmacological interactions among treatments should be considered. Regarding the therapeutic role that CBD has demonstrated, we will try to integrate into this discussion the results in vivo, in vitro, and in clinical trials, and then we will discuss the relationship between the activation and inhibition of the multiple receptors identified as targets of CBD (Table 5).

Table 5.
Targets of CBD on neurological diseases.
3.1. CBD Multi-Targets Promoting Anticonvulsant and Antiepileptic Effects
The modulation of ionic currents of ion channels such as GABAAR [41], Nav [71,72], and less documented Cav [75] might be important in the anticonvulsant and antiepileptic effect of CBD. First, the combined effect of CBD with clobazam (CBD acts as PAM on GABAAR) [41] has an important role in the suppression of common epileptic events by maintaining homeostasis over brain excitation and its importance for treating drug-resistant epilepsy caused by mutations that alter the channel [170]. Secondly, the target of CBD to treat DS is the non-specific inhibitory effect on the mutated sodium channels Nav1.1 and Nav1.2 [73,74]; although, it is also capable of inhibiting other sodium channels such as Nav1.3, Nav1.4, Nav1.5, Nav1.6, and Nav1.7 [71,72]. However, a very important factor that enhances the inhibition of GABAA and sodium channels by CBD is its inhibitory effect on CYP1, CYP2, and CYP3 liver enzymes, such as CYP3A4 and CYP2C19 enzymes, which are necessary for the degradation of anticonvulsant drugs such as clobazam and its metabolite N-desmethylclobazam, leading to an increase in their Tmax values and plasma half-life [41]. In addition, the antiepileptic effect of CBD is associated with the inhibition of the GPR55 receptor, thus increasing the release of neurotransmitters as shown in an epilepsy mouse model [169] and by reducing the expression of the CB1 receptor [163], although their mechanism of action is not clearly defined for these two receptors (Figure 4).
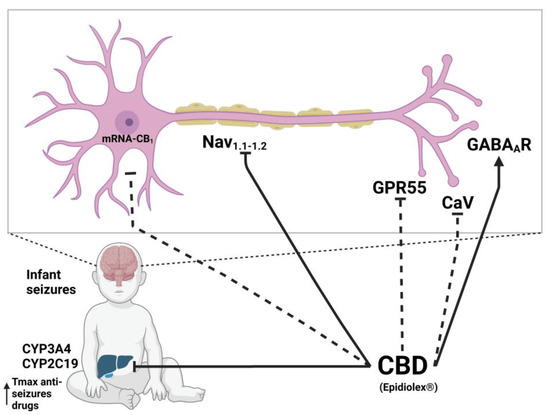
Figure 4.
Molecular mechanism of CBD on epilepsy. Dotted lines refer to mechanisms recently suggested as probable based on in vitro experiments, which require verification on in vivo models. Created with Biorender.com.
3.2. CBD Multi-Targets Implicated in Inflammatory and Immunosuppressive Process
Anti-inflammatory and immunosuppressive effects of cannabis have been known since 3000 years ago, when it was used as an antipyretic and to treat rheumatism [190]. Recently, in vitro and in vivo models have suggested that CBD seems to produce these effects by acting directly on the cells and tissues of the immune system [32,152], where CBD acts as an agonist of TRPV1 in vitro activating phagocytosis in microglia [45] and in vivo decreasing inflammation in both inflammatory bowel disease and allergic contact dermatitis models [175,176]. CBD is an agonist of A2AR, showing reduction of inflammation in a murine model of acute lung injury and inflammatory response induced for lipopolysaccharide (LPS) [174]. Additionally, in human sebocytes, CBD induces an anti-inflammatory pathway dependent on A2AR cAMP-TRIB3 and the subsequent inhibition of NFkB [180].
CBD also induces activation on the PPARγ acting in the inhibition of keratinocyte proliferation avoiding inflammatory response in psoriasis [177]. However, at the same time CBD can act as an antagonist of CB2R [176,177], it can also attenuate M2 polarization (anti-inflammatory response) of microglia via CB2R, as revealed in a CB2R knockout mice model [179]. CBD is also known for its low inhibition to δ-OPR and µ-OPR receptors, both receptors widely known for their immunosuppressive role [178]; CBD can also modulate the activity of the α7nAChR, which is known to have rapid activation to immunosuppress synthesis and secretion of TLR4-induced TNF in mast cells [87]. Additionally, CBD antagonizes GPR18, which is activated by the association of N-arachidonoyl glycine (NAGly) and Δ9-THC on a BV-2 microglia model; thus, CBD reduces the microglial migration and cytokine expression [122,191]; CBD also inhibits the mitochondrial channel VDAC1, which has been suggested to be the therapeutic target responsible for the antiproliferative and immunosuppressive properties of CBD [76]. More experimental information is still required to specify how CBD acts through the aforementioned receptors, as well as other receptors, such as the A1AR, TRPV2, and TRPA, to better understand its anti-inflammatory effect.
3.3. CBD Multi-Targets Engage in Antinociceptive and Analgesic Properties
Preclinical research has shown that depending on the dose and route of administration, CBD alone or in combination with other drugs has an antinociceptive effect in pain models [161,162]. In sub-chronic pain models in vivo, low doses of CBD (3 mg/Kg) produced an anxiolytic and analgesic effects [163]. In a rodent model, administration of CBD into periaqueductal gray (PAG) showed a dose-dependent analgesic effect in a tail-flick test, and this effect was blocked by the use of A1AR, CB1R, and TRPA1 antagonists [166], concluding that CBD might produce antinociceptive effects at the supraspinal level, most likely interacting to several targets involved in the control of pain, including TRPA1 channels and inactivation on the endocannabinoid system [91]. Another study found that CBD reduced hyperalgesia to thermal and mechanical stimuli in two different models of persistent pain. Anti-hyperalgesic effect of CBD (20 mg/kg) was prevented by the vanilloid antagonist capsazepine (10 mg/kg, i.p.), suggesting that TRPV1 are involved in its effect either through in vitro [100] or in vivo assays [165]. Moreover, acute or chronic treatment with CBD decreases the hyperalgesia and allodynia in Parkinson’s model [166]. CBD, together with a TRPV1 antagonist, reduces L-DOPA-induced dyskinesia in a model of Parkinson’s disease by acting on CB1R and PPARγ and reducing the expression of the inflammatory marker’s cyclooxygenase-2 (COX-2) and NFκB [126]. Systemic and intrathecal administration of CBD can reduce neuropathic pain; this effect was significantly reduced in mice lacking the α3 subunits of GlyR. This result suggests that GlyR mediates the CBD analgesic effect in neuropathic pain models [40]. The antiallodynic effects of CBD are mediated predominantly through TRPV1 since capsazepine fully prevented CBD analgesia [167]. In studies using Paclitaxel chemotherapeutic-induced neuropathic pain, CBD decreased mechanical and thermal allodynia in mice, an effect reversed by the 5HT1AR antagonist [164]. The 5HT1AR also mediates the CBD anti-allodynic effect in diabetic (streptozotocin-induced) neuropathic pain, since the specific receptor antagonist prevented the observed effect [168]. It is remarkable that in the latest two studies here reported [164,168], the effects were not prevented with CBR antagonists. This is interesting since it suggests that the effects of CBD are also dependent on the emotionally expression state of the animal since the models change the basal physiological levels.
3.4. CBD Multi-Targets with Antidepressant and Anxiolytic Consequences
Regarding neuropsychiatric disorders, some of the effects are well described, but the characterization of how CBD can exert them throughout the molecular target or targets is still a matter of debate. Experimental demonstrations are required as well as the exploration of other potential targets. The anxiolytic effects of CBD have been extensively demonstrated in clinical and preclinical studies (for reviews see [192,193,194,195]). The mechanism of action throughout CBD that can reduce anxiety seems to be complex and dose dependent. Accumulated evidence indicates that acute administration of CBD produces a typical ‘bell-shaped’ dose–response curve, being anxiolytic just at low-moderate doses but not at high doses [160]. The anxiolytic effects seem to be mediated by the ability of CBD to act as a 5-HT1AR agonist, since the reduction in anxiety-like parameters induced by CBD is blocked by a specific 5-HT1AR antagonist [110,111,154,159]. It must be taken into consideration that while in vitro studies suggest CBD acts as a direct 5-HT1A receptor agonist [108], in vivo studies are more consistent with CBD acting as a PAM, hence facilitating 5-HT1AR signaling [112]. On the other hand, it has been proposed that the lack of anxiolytic effect of the high doses of CBD is related to the activation of TRPV1 receptors, since blocking TRPV1 centrally allows for high doses of CBD to be effective [160]. Another proposed mechanism is by the indirect potentiation of AEA transmission since CBD can inhibit the FAAH enzyme [155] (Figure 5). This could be related to a recent study that demonstrated that CB1R (but not CB2R or GPR55-KO mice models) is relevant in modulating the anxiolytic actions of CBD using knockout mice for these receptors [158]. It is possible that these mechanisms and other ones can be acting in conjunction, since CBD can interact with other receptors involved in the regulation of anxiety such as GABAAR.
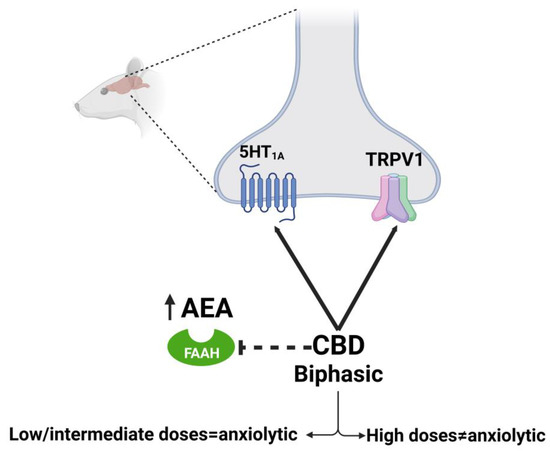
Figure 5.
Molecular mechanism of CBD on anxiety described in rat model. Dotted lines refer to hypothesized mechanisms. Created with Biorender.com.
CBD has a dose-dependent antidepressant-like profile in different preclinical models (for review [196]). Although CBD can modulate several molecular targets involved in the neurobiology of depression, only two of these mechanisms have so far been explored in vivo. The involvement of 5-HT1AR has been consistently found [153,156,157] since the effective antidepressant dose was blocked by the pre-treatment of selective 5-HT1AR antagonists. Supporting this idea, the depletion of serotonin blocked the antidepressant effect of CBD [197]. The other mechanism explored is the involvement of CBR. As it was discussed previously, CBD has a low affinity for CB1R, but it is capable of increasing AEA levels by inhibiting its reuptake [100]. Since pretreatment with a CB1R antagonist also blocked some anti-depressant CBD effects [156], it is proposed that the increases in AEA levels, which in turn activate the CB1R, explain the results. Further supporting this proposal, pharmacological increases in AEA promote antidepressant-like effects in preclinical studies [198]. Evidence indicates that, depending on the behavioral test and treatment duration, 5-HT1AR or CB1R signaling would prevail to mediate CBD anti-depressant effects [196].
3.5. CBD Multi-Targets in Antipsycothic Disorders
The antipsychotic capability of CBD has been demonstrated in several clinical studies, as well as in preclinical models (for review see: [174,199,200]). However, the specific pharmacological mechanisms underlying the antipsychotic action of CBD are not entirely understood. Two opposite lines of thought are possible. On one side, despite its low affinity for CB1R, CBD is capable of antagonizing CB1R agonists at reasonably low concentrations [105]. Other studies suggest that CBD acts as a NAM of CB1R [101]. In any case, these actions will result in a reduction of the endocannabinoid-mediated transmission. Conversely, CBD induces an enhancement of the endocannabinoid tonus by inhibiting AEA uptake and metabolism of the FAAH enzyme [100,173,174]. In support of the antipsychotic effect of CBD mediated by antagonism of the CB1R, the most important argument is that the acute administration of THC (an exogenous CB1 agonist) can transiently exacerbate psychosis in patients with schizophrenia [201] and induce transient psychotic-like symptoms in healthy volunteers [202,203]. This latest effect can be blocked by pre-treatment with CBD [204]. Arguing against this possibility, however, a CB1 antagonist failed to show any antipsychotic effect on patients with schizophrenia [205]. An important correlation to mention about the antipsychotic effects of CBD is the increase in serum of AEA in patients who received CBD and who showed a reduction in psychotic symptoms [130].
3.6. CBD Multi-Targets Implicated in Anti-Addictive Action
Systemic evidence has demonstrated that CBD has no potential for abuse since it lacks rewarding effects and did not induce withdrawal-related signs after repeated administration [206,207]. However, the “anti-addictive” actions of CBD have been described in some substance use disorders [172]. In a recent paper, the possible mechanisms underlying the “anti-addictive” potential of CBD involve dopaminergic, opioidergic, endocannabinoid, serotonergic, and glutamatergic systems [172]. However, from a pharmacological approach, the involvement of the 5-HT1AR in the effects of CBD on drug-induced reward is the only one that has been demonstrated. The intra-dorsal raphe injection of a 5-HT1AR antagonist abolished the CBD-mediated inhibition of the reward-facilitating effect of morphine measured in the intracranial self-stimulation (ICSS) paradigm [171], blocked the effects on ethanol self-administration, in combination with naltrexone, [206] and attenuated CBD-mediated reduction of cocaine self-administration [208].
3.7. CBD Multi-Targets Implicated in Alzheimer’s Disease
CBD has neuroprotective properties against the neurotoxic effects of amyloid beta peptide (Aβ) in cell culture and cognitive behavioral models of neurodegeneration that mimic AD symptoms seen in patients [209]. First, CBD seems to activate the PPARγ and at the same time modulate the GPR3 and GPR6 as an inverse agonist. CBD has been shown to favor the activation of PPARγ, reducing inflammation in astrocytes and increasing the survival and neurogenesis of the rat and mouse hippocampus produced by neurotoxicity of Aβ [50,186,189]. The reduction of Aβ expression is also found in in vitro assays on human neuroblastoma SH-SY5Y [187]. Moreover, an overexpression of GPR3 in the brains of AD patients has been reported. While the genetic deletion of Gpr3 gene alleviates the cognitive deficits in AD mice models, CBD acts as inverse agonist on GPR3 [184,185,188]. On the other hand, GPR6 is repressed by Aβ, and its expression is restored by complement protein C1q-induced neuroprotection against Aβ in the hippocampal region in a mouse model [183]. CBD modulates the Kv2.1 channel, which in the presence of an inflammatory and oxidative environment produced by the accumulation of Aβ, causes the Kv2.1 oxidation, which contributes to cognitive impairment in a mice model of AD [181,182]. In this case, CBD must protect the Kv2.1 channel from oxidative damage due to its antioxidant properties. Currently, AChE and BChE enzymes are one of the main targets for AD therapies, these enzymes are inhibited in vitro by CBD [128]. However, the human serum concentration of CBD for the maximum approved dose for Epidiolex (20 mg/kg/day) is estimated to be between 0.3–3.2 μM in the brain [78]. It is likely that at this dose, CBD has no effect on the enzymes AChE and BChE associated with the development of AD.
CBD has a favorable safety profile in humans [18,20], in rats [210], and in dogs [211]. A recent pharmacovigilance study concluded that CBD is a safe compound that causes only rare adverse reactions [212]. However, like any other drug, it is not innocuous; the adverse effects reported in humans so far could vary in severity and include somnolence, fatigue, gastrointestinal ailments, such as diarrhea, vomiting, and changes in appetite/weight, among others [213]. Some of these adverse effects have been addressed experimentally: for example, drowsiness, a common side effect for CBD, in a driving simulation test, indicated that its deleterious effects are minimal, with slightly more collisions and slower brake reaction times [214]. Certain toxicity has been described in preclinical in vitro and in vivo toxicology studies [213]. For instance, CBD-rich cannabis extract causes hepatotoxicity in mice [215]. However, the translation of those results to be applicable to humans has not been bluntly stated, and this seems to be related with the doses range utilized. For example, it has been described that CBD administered between 8–10 µM induces the senescence of human Sertoli cells in vitro, by reducing the transcription of genes involved in cell division, but this concentration is above the average serum concentration of CBD in adults who consumed 1500 mg of daily CBD in a week (0.9 to 2.3 µM)[216]. Finally, CBD action on hepatic enzymes needs to be consider since it can modify the pharmacokinetics of other drugs. A pharmacovigilance study concluded that CBD–drug interactions must be highly considered, since CBD is indicated in people with ongoing treatments, some of them as aggressive as chemotherapy [212].
4. Conclusions
In this review, we identified around 56 different neurological molecular targets of CBD including, enzymes, ion channels, ionotropic, and metabotropic receptors. It is crucial to understand CBD’s mechanism of actions in order to ensure the safe use of this secondary metabolite as a therapeutic agent. In this work, we analyzed the multi-target nature of CBD and integrated the information available from in vitro and in vivo studies; we found that there is a little correlation between in vivo, in vitro studies, and clinical trials. The natural product CBD has proven to be a safe agent, because even at the highest doses used to treat neuropsychiatric diseases, it does not reach the serum concentrations necessary to produce long-term toxicity.
Most of the research groups dedicated to cannabinoids are focused on psychoactive cannabinoids, which has made CBD and others phytocannabinoids research an emergent field, and therefore there is room for opportunities to fill in the gap on CBD’s pharmacology. Future studies could be directed on the research of CBD derivatives to enhance specificity on particular targets and in the identification of molecular targets to differentiate, for example, between the effect of CBD on sleep and anxiolytic effect and the anti-emetic and its hypotensive role. Currently there are several clinical trials on the therapeutic effect of CBD in patients with Parkinson’s disease (NCT02818777 and NCT03582137).
Author Contributions
All the authors have contributed equally to the survey of the literature and published sources and compilation and analysis of the references. J.C.-A. and A.C.-A. prepared the initial draft of the manuscript. F.L. and S.J.C. revised the sections on chemistry and pharmacology and reviewed the English wording of the final document. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by grant numbers 5P20GM109091 from the National Institute of General Medical Sciences (NIGMS), a component of the National Institutes of Health (NIH); its contents are solely the responsibility of the authors and do not necessarily represent the official view of the NIGMS or the NIH.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing not applicable.
Acknowledgments
F.L. thanks the College of Pharmacy, University of South Carolina, for start-up funds.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
2-AG = 2-arachidonoylglycerol; 5-HT1–7R = 5-HT1–7 serotonin receptor; A1–3R = adenosine receptors; AANAT = arylalkylamine N-acetyltransferase; AChE = acetylcholinesterase; AD = Alzheimer disease; AD = Alzheimer’s disease; AEA = anandamide; AIDS = acquired immunodeficiency syndrome; BChE = butyrylcholinesterase; cAMP = cyclic adenosine monophosphate; CB1 = cannabinoid receptor type 1; CB2 = cannabinoid receptor type 2; CBD = cannabidiol; CNS = central nervous system; COX-2 = cyclooxygenase-2; CYP = cytochromes P450; DS = Dravet syndrome; FAAH = fatty acid amide hydrolase; GABA-AR = GABA-A receptor; GlyR = glycine receptor; GPCRs or GPRs = G-protein-coupled receptors; HIV = human immunodeficiency virus; Kv2.1 = voltage-gated potassium channel; LiCl = lithium chloride; LPS = lipopolysaccharide; MAGL = monoacylglycerol lipase; MS = multiple sclerosis; NaChBac = bacterial homomeric Nav channel; NAGly = N-arachidonoyl glycine; NAM = negative allosteric modulator; NFκB = nuclear factor kappa B; PAG = periaqueductal gray; PAM = positive allosteric modulator; PNS = peripheric nervous system; PPARγ = peroxisome proliferator-activated receptor γ; THC = Δ9-tetrahydrocannabinol; TLR4 = toll-like receptor 4; TNF = tumor necrosis factor; TPRM8 = transient receptors potential melastatin 8; TRP = transient receptor potential channels; TRPA1 = transient receptors potential ankyrin type 1; TRPV = transient receptors potential vanilloid; VCAM-1 = vascular cell adhesion molecule–1; VDAC1 = voltage-dependent anion-selective channel protein 1; VGCC = T-type voltage-gated calcium channels; VGSC or Nav = voltage-gated sodium channel; α7-nAChR = α7 nicotinic acetylcholine receptors; δ-OPR = opioid receptor delta; µ-OPR = opioid receptor mu.
References
- Bonini, S.A.; Premoli, M.; Tambaro, S.; Kumar, A.; Maccarinelli, G.; Memo, M.; Mastinu, A. Cannabis Sativa: A Comprehensive Ethnopharmacological Review of a Medicinal Plant with a Long History. J. Ethnopharmacol. 2018, 227, 300–315. [Google Scholar] [CrossRef]
- Zuardi, A.W. History of Cannabis as a Medicine: A Review. Braz. J. Psychiatry 2006, 28, 153–157. [Google Scholar] [CrossRef]
- Andre, C.M.; Hausman, J.F.; Guerriero, G. Cannabis Sativa: The Plant of the Thousand and One Molecules. Front. Plant Sci. 2016, 7, 19. [Google Scholar] [CrossRef] [PubMed]
- Hanuš, L.O.; Meyer, S.M.; Muñoz, E.; Taglialatela-Scafati, O.; Appendino, G. Phytocannabinoids: A Unified Critical Inventory. Nat. Prod. Rep. 2016, 33, 1357–1392. [Google Scholar] [CrossRef]
- Odieka, A.E.; Obuzor, G.U.; Oyedeji, O.O.; Gondwe, M.; Hosu, Y.S.; Oyedeji, A.O. The Medicinal Natural Products of Cannabis sativa Linn.: A Review. Molecules 2022, 27, 1689. [Google Scholar] [CrossRef] [PubMed]
- ElSohly, M.A.; Slade, D. Chemical Constituents of Marijuana: The Complex Mixture of Natural Cannabinoids. Life Sci. 2005, 78, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Reekie, T.A.; Scott, M.P.; Kassiou, M. The Evolving Science of Phytocannabinoids. Nat. Rev. Chem. 2017, 2, 0101. [Google Scholar] [CrossRef]
- Mechoulam, R.; Gaoni, Y. A Total Synthesis of Dl-Δ1-Tetrahydrocannabinol, the Active Constituent of Hashish. J. Am. Chem. Soc. 1965, 87, 3273–3275. [Google Scholar] [CrossRef]
- Koob, G.F.; Arends, M.A.; Le Moal, M. What Is Addiction? In Drugs Addiction and the Brain, 1st ed.; Academic Press: Oxford, UK, 2014; Volume 1, pp. 1–27. [Google Scholar] [CrossRef]
- Lu, H.C.; Mackie, K. Review of the Endocannabinoid System. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2021, 6, 607–615. [Google Scholar] [CrossRef]
- Pertwee, R.G. The Diverse CB1 and CB2 Receptor Pharmacology of Three Plant Cannabinoids: Δ9-Tetrahydrocannabinol, Cannabidiol and Δ9-Tetrahydrocannabivarin. Br. J. Pharmacol. 2008, 153, 199–215. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Available online: https://www.fda.gov/news-events/public-health-focus/fda-and-cannabis-research-and-drug-approval-process (accessed on 3 March 2023).
- Bajtel, Á.; Kiss, T.; Tóth, B.; Kiss, S.; Hegyi, P.; Vörhendi, N.; Csupor-Löffler, B.; Gede, N.; Hohmann, J.; Csupor, D. The Safety of Dronabinol and Nabilone: A Systematic Review and Meta-Analysis of Clinical Trials. Pharmaceuticals 2022, 15, 100. [Google Scholar] [CrossRef]
- Adams, R.; Hunt, M. Structure of Cannabidiol, a Product Isolated from the Marihuana Extract of Minnesota Wild Hemp. I. J. Am. Chem. Soc. 1940, 62, 196–200. [Google Scholar] [CrossRef]
- Jacob, A.; Todd, A.R. Cannabidiol and Cannabol, Constituents of Cannabis Indica Resin. Nature 1940, 145, 350. [Google Scholar] [CrossRef]
- Mechoulam, R.; Shvo, Y. Hashish—I: The Structure of Cannabidiol. Tetrahedron 1963, 19, 2073–2078. [Google Scholar] [CrossRef] [PubMed]
- Millar, S.A.; Stone, N.L.; Yates, A.S.; O’Sullivan, S.E. A Systematic Review on the Pharmacokinetics of Cannabidiol in Humans. Front. Pharmacol. 2018, 9, 1365. [Google Scholar] [CrossRef] [PubMed]
- Machado Bergamaschi, M.; Helena Costa Queiroz, R.; Waldo Zuardi, A.; Alexandre, S.; Crippa, J. Safety and Side Effects of Cannabidiol, a Cannabis sativa Constituent. Curr. Drug Saf. 2011, 6, 237–249. [Google Scholar] [CrossRef]
- Cunha, J.M.; Carlini, E.A.; Pereira, A.E.; Ramos, O.L.; Pimentel, C.; Gagliardi, R.; Sanvito, W.L.; Lander, N.; Mechoulam, R. Chronic Administration of Cannabidiol to Healthy Volunteers and Epileptic Patients. Pharmacology 1980, 21, 175–185. [Google Scholar] [CrossRef]
- Iffland, K.; Grotenhermen, F. An Update on Safety and Side Effects of Cannabidiol: A Review of Clinical Data and Relevant Animal Studies. Cannabis Cannabinoid Res. 2017, 2, 139–154. [Google Scholar] [CrossRef]
- Elsaid, S.; Le Foll, B. The Complexity of Pharmacology of Cannabidiol (CBD) and Its Implications in the Treatment of Brain Disorders. Neuropsychopharmacology 2020, 45, 229–230. [Google Scholar] [CrossRef]
- Campos, A.C.; Fogaça, M.V.; Scarante, F.F.; Joca, S.R.L.; Sales, A.J.; Gomes, F.V.; Sonego, A.B.; Rodrigues, N.S.; Galve-Roperh, I.; Guimarães, F.S. Plastic and Neuroprotective Mechanisms Involved in the Therapeutic Effects of Cannabidiol in Psychiatric Disorders. Front. Pharmacol. 2017, 8, 269. [Google Scholar] [CrossRef] [PubMed]
- Ibeas Bih, C.; Chen, T.; Nunn, A.V.W.; Bazelot, M.; Dallas, M.; Whalley, B.J. Molecular Targets of Cannabidiol in Neurological Disorders. Neurotherapeutics 2015, 12, 699–730. [Google Scholar] [CrossRef] [PubMed]
- Campos, A.C.; Guimarães, F.S. Involvement of 5HT1A Receptors in the Anxiolytic-like Effects of Cannabidiol Injected into the Dorsolateral Periaqueductal Gray of Rats. Psychopharmacology 2008, 199, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Wright, M.; Di Ciano, P.; Brands, B. Use of Cannabidiol for the Treatment of Anxiety: A Short Synthesis of Pre-Clinical and Clinical Evidence. Cannabis Cannabinoid Res. 2020, 5, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Schiavon, A.P.; Bonato, J.M.; Milani, H.; Guimarães, F.S.; Weffort de Oliveira, R.M. Influence of Single and Repeated Cannabidiol Administration on Emotional Behavior and Markers of Cell Proliferation and Neurogenesis in Non-Stressed Mice. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2016, 64, 27–34. [Google Scholar] [CrossRef]
- Sales, A.J.; Guimarães, F.S.; Joca, S.R.L. CBD Modulates DNA Methylation in the Prefrontal Cortex and Hippocampus of Mice Exposed to Forced Swim. Behav. Brain Res. 2020, 388, 112627. [Google Scholar] [CrossRef]
- Atalay, S.; Jarocka-karpowicz, I.; Skrzydlewskas, E. Antioxidative and Anti-Inflammatory Properties of Cannabidiol. Antioxidants 2019, 9, 21. [Google Scholar] [CrossRef]
- Pertwee, R.G. Pharmacology of Cannabinoid Receptor Ligands. Curr. Med. Chem. 1999, 6, 635–664. [Google Scholar] [CrossRef]
- Noreen, N.; Muhammad, F.; Akhtar, B.; Azam, F.; Anwar, M.I. Is Cannabidiol a Promising Substance for New Drug Development? A Review of Its Potential Therapeutic Applications. Crit. Rev. Eukaryot. Gene Expr. 2018, 28, 73–86. [Google Scholar] [CrossRef]
- Namdar, D.; Anis, O.; Poulin, P.; Koltai, H. Chronological Review and Rational and Future Prospects of Cannabis-Based Drug Development. Molecules 2020, 25, 4821. [Google Scholar] [CrossRef]
- Martini, S.; Gemma, A.; Ferrari, M.; Cosentino, M.; Marino, F. Effects of Cannabidiol on Innate Immunity: Experimental Evidence and Clinical Relevance. Int. J. Mol. Sci. 2023, 24, 3125. [Google Scholar] [CrossRef]
- Abu-Sawwa, R.; Scutt, B.; Park, Y. Emerging Use of Epidiolex (Cannabidiol) in Epilepsy. J. Pediatr. Pharmacol. Ther. 2020, 25, 485–499. [Google Scholar] [CrossRef]
- Pretzsch, C.M.; Voinescu, B.; Mendez, M.A.; Wichers, R.; Ajram, L.; Ivin, G.; Heasman, M.; Williams, S.; Murphy, D.G.M.; Daly, E.; et al. The Effect of Cannabidiol (CBD) on Low-Frequency Activity and Functional Connectivity in the Brain of Adults with and without Autism Spectrum Disorder (ASD). J. Psychopharmacol. 2019, 33, 1141–1148. [Google Scholar] [CrossRef] [PubMed]
- Aran, A.; Harel, M.; Cassuto, H.; Polyansky, L.; Schnapp, A.; Wattad, N.; Shmueli, D.; Golan, D.; Castellanos, F.X. Cannabinoid Treatment for Autism: A Proof-of-Concept Randomized Trial. Mol. Autism 2021, 12, 6. [Google Scholar] [CrossRef] [PubMed]
- McGuire, P.; Robson, P.; Cubala, W.J.; Vasile, D.; Morrison, P.D.; Barron, R.; Taylor, A.; Wright, S. Cannabidiol (CBD) as an Adjunctive Therapy in Schizophrenia: A Multicenter Randomized Controlled Trial. Am. J. Psychiatry 2018, 175, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Izzo, A.A.; Borrelli, F.; Capasso, R.; Di Marzo, V.; Mechoulam, R. Non-Psychotropic Plant Cannabinoids: New Therapeutic Opportunities from an Ancient Herb. Trends Pharmacol. Sci. 2009, 30, 515–527. [Google Scholar] [CrossRef]
- Pisanti, S.; Malfitano, A.M.; Ciaglia, E.; Lamberti, A.; Ranieri, R.; Cuomo, G.; Abate, M.; Faggiana, G.; Proto, M.C.; Fiore, D.; et al. Cannabidiol: State of the Art and New Challenges for Therapeutic Applications. Pharmacol. Ther. 2017, 175, 133–150. [Google Scholar] [CrossRef]
- Imlach, W.L. New Approaches to Target Glycinergic Neurotransmission for the Treatment of Chronic Pain. Pharmacol. Res. 2017, 116, 93–99. [Google Scholar] [CrossRef]
- Xiong, W.; Cui, T.; Cheng, K.; Yang, F.; Chen, S.R.; Willenbring, D.; Guan, Y.; Pan, H.L.; Ren, K.; Xu, Y.; et al. Cannabinoids Suppress Inflammatory and Neuropathic Pain by Targeting A3 Glycine Receptors. J. Exp. Med. 2012, 209, 1121–1134. [Google Scholar] [CrossRef]
- Anderson, L.L.; Absalom, N.L.; Abelev, S.V.; Low, I.K.; Doohan, P.T.; Martin, L.J.; Chebib, M.; McGregor, I.S.; Arnold, J.C. Coadministered Cannabidiol and Clobazam: Preclinical Evidence for Both Pharmacodynamic and Pharmacokinetic Interactions. Epilepsia 2019, 60, 2224–2234. [Google Scholar] [CrossRef]
- Raol, Y.S.H.; Lund, I.V.; Bandyopadhyay, S.; Zhang, G.; Roberts, D.S.; Wolfe, J.H.; Russek, S.J.; Brooks-Kayal, A.R. Enhancing GABAA Receptor A1 Subunit Levels in Hippocampal Dentate Gyrus Inhibits Epilepsy Development in an Animal Model of Temporal Lobe Epilepsy. J. Neurosci. 2006, 26, 11342–11346. [Google Scholar] [CrossRef]
- Senn, L.; Cannazza, G.; Biagini, G. Receptors and Channels Possibly Mediating the Effects of Phytocannabinoids on Seizures and Epilepsy. Pharmaceuticals 2020, 13, 174. [Google Scholar] [CrossRef] [PubMed]
- De Petrocellis, L.; Orlando, P.; Moriello, A.S.; Aviello, G.; Stott, C.; Izzo, A.A.; di Marzo, V. Cannabinoid Actions at TRPV Channels: Effects on TRPV3 and TRPV4 and Their Potential Relevance to Gastrointestinal Inflammation. Acta Physiol. 2012, 204, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.; Eldeeb, K.; Millns, P.J.; Bennett, A.J.; Alexander, S.P.H.; Kendall, D.A. Cannabidiol Enhances Microglial Phagocytosis via Transient Receptor Potential (TRP) Channel Activation. Br. J. Pharmacol. 2014, 171, 2426–2439. [Google Scholar] [CrossRef]
- Muller, C.; Reggio, P.H. An Analysis of the Putative CBD Binding Site in the Ionotropic Cannabinoid Receptors. Front. Cell. Neurosci. 2020, 14, 615811. [Google Scholar] [CrossRef]
- De Petrocellis, L.; Ligresti, A.; Moriello, A.S.; Allarà, M.; Bisogno, T.; Petrosino, S.; Stott, C.G.; Di Marzo, V. Effects of Cannabinoids and Cannabinoid-Enriched Cannabis Extracts on TRP Channels and Endocannabinoid Metabolic Enzymes. Br. J. Pharmacol. 2011, 163, 1479–1494. [Google Scholar] [CrossRef]
- Dunwiddie, T.V.; Masino, S.A. The Role and Regulation of Adenosine in the Central Nervous System. Annu. Rev. Neurosci. 2001, 24, 31–55. [Google Scholar] [CrossRef]
- Gonca, E.; Darici, F. The Effect of Cannabidiol on Ischemia/Reperfusion-Induced Ventricular Arrhythmias: The Role of Adenosine A1 Receptors. J. Cardiovasc. Pharmacol. Ther. 2015, 20, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Esposito, G.; Scuderi, C.; Valenza, M.; Togna, G.I.; Latina, V.; de Filippis, D.; Cipriano, M.; Carratù, M.R.; Iuvone, T.; Steardo, L. Cannabidiol Reduces Aβ-Induced Neuroinflammation and Promotes Hippocampal Neurogenesis through PPARγ Involvement. PLoS ONE 2011, 6, e28668. [Google Scholar] [CrossRef]
- Giacoppo, S.; Pollastro, F.; Grassi, G.; Bramanti, P.; Mazzon, E. Target Regulation of PI3K/Akt/MTOR Pathway by Cannabidiol in Treatment of Experimental Multiple Sclerosis. Fitoterapia 2017, 116, 77–84. [Google Scholar] [CrossRef]
- O’Sullivan, S.E.; Sun, Y.; Bennett, A.J.; Randall, M.D.; Kendall, D.A. Time-Dependent Vascular Actions of Cannabidiol in the Rat Aorta. Eur. J. Pharmacol. 2009, 612, 61–68. [Google Scholar] [CrossRef]
- Lynch, J.W. Molecular Structure and Function of the Glycine Receptor Chloride Channel. Physiol. Rev. 2004, 84, 1051–1095. [Google Scholar] [CrossRef]
- Avila, A.; Nguyen, L.; Rigo, J.M. Glycine Receptors and Brain Development. Front. Cell. Neurosci. 2013, 7, 184. [Google Scholar] [CrossRef] [PubMed]
- Laube, B.; Maksay, G.; Schemm, R.; Betz, H. Modulation of Glycine Receptor Function: A Novel Approach for Therapeutic Intervention at Inhibitory Synapses? Trends Pharmacol. Sci. 2002, 23, 519–527. [Google Scholar] [CrossRef]
- Ahrens, J.; Demir, R.; Leuwer, M.; De La Roche, J.; Krampfl, K.; Foadi, N.; Karst, M.; Haeseler, G. The Nonpsychotropic Cannabinoid Cannabidiol Modulates and Directly Activates Alpha-1 and Alpha-1-Beta Glycine Receptor Function. Pharmacology 2009, 83, 217–222. [Google Scholar] [CrossRef]
- Ghit, A.; Assal, D.; Al-Shami, A.S.; Hussein, D.E.E. GABAA Receptors: Structure, Function, Pharmacology, and Related Disorders. J. Genet. Eng. Biotechnol. 2021, 19, 123. [Google Scholar] [CrossRef] [PubMed]
- Bakas, T.; van Nieuwenhuijzen, P.S.; Devenish, S.O.; McGregor, I.S.; Arnold, J.C.; Chebib, M. The Direct Actions of Cannabidiol and 2-Arachidonoyl Glycerol at GABAA Receptors. Pharmacol. Res. 2017, 119, 358–370. [Google Scholar] [CrossRef]
- Al Kury, L.T.; Mahgoub, M.; Howarth, F.C.; Oz, M. Natural Negative Allosteric Modulators of 5-HT3 Receptors. Molecules 2018, 23, 3186. [Google Scholar] [CrossRef]
- Yakel, J.L.; Jackson, M.B. 5-HT3 Receptors Mediate Rapid Responses in Cultured Hippocampus and a Clonal Cell Line. Neuron 1988, 1, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Färber, L.; Haus, U.; Späth, M.; Drechsler, S. Physiology and Pathophysiology of the 5-HT3 Receptor. Scand. J. Rheumatol. 2004, 33, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Kossakowski, R.; Schlicker, E.; Toczek, M.; Weresa, J.; Malinowska, B. Cannabidiol Affects the Bezold-Jarisch Reflex via TRPV1 and 5-HT3 Receptors and Has Peripheral Sympathomimetic Effects in Spontaneously Hypertensive and Normotensive Rats. Front. Pharmacol. 2019, 10, 500. [Google Scholar] [CrossRef]
- Xiong, W.; Koo, B.N.; Morton, R.; Zhang, L. Psychotropic and Nonpsychotropic Cannabis Derivatives Inhibit Human H5-HT3A Receptors through a Receptor Desensitization-Dependent Mechanism. Neuroscience 2011, 184, 28–37. [Google Scholar] [CrossRef]
- Yang, K.H.; Galadari, S.; Isaev, D.; Petroianu, G.; Shippenberg, T.S.; Oz, M. The Nonpsychoactive Cannabinoid Cannabidiol Inhibits 5- Hydroxytryptamine3A Receptor-Mediated Currents in Xenopus laevis Oocytes. J. Pharmacol. Exp. Ther. 2010, 333, 547–554. [Google Scholar] [CrossRef]
- Karlin, A. Emerging Structure of the Nicotinic Acetylcholine Receptors. Nat. Rev. Neurosci. 2002, 3, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, E.X.; Pereira, E.F.R.; Alkondon, M.; Rogers, S.W. Mammalian Nicotinic Acetylcholine Receptors: From Structure to Function. Physiol. Rev. 2009, 89, 73–120. [Google Scholar] [CrossRef] [PubMed]
- Schmiedhofer, P.; Vogel, F.D.; Koniuszewski, F.; Ernst, M. Cys-Loop Receptors on Cannabinoids: All High? Front. Physiol. 2022, 13, 2349. [Google Scholar] [CrossRef]
- Bertrand, D.; Lee, C.H.L.; Flood, D.; Marger, F.; Donnelly-Roberts, D. Therapeutic Potential of A7 Nicotinic Acetylcholine Receptors. Pharmacol. Rev. 2015, 67, 1025–1073. [Google Scholar] [CrossRef] [PubMed]
- Chrestia, J.F.; Esandi, M.D.C.; Bouzat, C. Cannabidiol as a Modulator of A7 Nicotinic Receptors. Cell. Mol. Life Sci. 2022, 79, 564. [Google Scholar] [CrossRef]
- Mahgoub, M.; Keun-Hang, S.Y.; Sydorenko, V.; Ashoor, A.; Kabbani, N.; Al Kury, L.; Sadek, B.; Howarth, C.F.; Isaev, D.; Galadari, S.; et al. Effects of Cannabidiol on the Function of A7-Nicotinic Acetylcholine Receptors. Eur. J. Pharmacol. 2013, 720, 310–319. [Google Scholar] [CrossRef]
- Watkins, A.R. Cannabinoid Interactions with Ion Channels and Receptors. Channels 2019, 13, 162–167. [Google Scholar] [CrossRef]
- Ghovanloo, M.R.; Shuart, N.G.; Mezeyova, J.; Dean, R.A.; Ruben, P.C.; Goodchild, S.J. Inhibitory Effects of Cannabidiol on Voltage-Dependent Sodium Currents. J. Biol. Chem. 2019, 293, 16546–16558. [Google Scholar] [CrossRef]
- Shapiro, L.; Escayg, A.; Wong, J.C. Cannabidiol Increases Seizure Resistance and Improves Behavior in an Scn8a Mouse Model. Front. Pharmacol. 2022, 13, 815950. [Google Scholar] [CrossRef]
- Mason, E.R.; Cummins, T.R. Differential Inhibition of Human Nav1.2 Resurgent and Persistent Sodium Currents by Cannabidiol and GS967. Int. J. Mol. Sci. 2020, 21, 2454. [Google Scholar] [CrossRef]
- Ross, H.R.; Napier, I.; Connor, M. Inhibition of Recombinant Human T-Type Calcium Channels by Δ9-Tetrahydrocannabinol and Cannabidiol. J. Biol. Chem. 2008, 283, 16124–16134. [Google Scholar] [CrossRef] [PubMed]
- Rimmerman, N.; Ben-Hail, D.; Porat, Z.; Juknat, A.; Kozela, E.; Daniels, M.P.; Connelly, P.S.; Leishman, E.; Bradshaw, H.B.; Shoshan-Barmatz, V.; et al. Direct Modulation of the Outer Mitochondrial Membrane Channel, Voltage-Dependent Anion Channel 1 (VDAC1) by Cannabidiol: A Novel Mechanism for Cannabinoid-Induced Cell Death. Cell Death Dis. 2013, 4, e949. [Google Scholar] [CrossRef] [PubMed]
- Iannotti, F.A.; Hill, C.L.; Leo, A.; Alhusaini, A.; Soubrane, C.; Mazzarella, E.; Russo, E.; Whalley, B.J.; Di Marzo, V.; Stephens, G.J. Nonpsychotropic Plant Cannabinoids, Cannabidivarin (CBDV) and Cannabidiol (CBD), Activate and Desensitize Transient Receptor Potential Vanilloid 1 (TRPV1) Channels in Vitro: Potential for the Treatment of Neuronal Hyperexcitability. ACS Chem. Neurosci. 2014, 5, 1131–1141. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Rossi, E.; Saubamea, B.; Chasseigneaux, S.; Cochois, V.; Choublier, N.; Smirnova, M.; Glacial, F.; Perrière, N.; Bourdoulous, S.; et al. Cannabidiol Increases Proliferation, Migration, Tubulogenesis, and Integrity of Human Brain Endothelial Cells through TRPV2 Activation. Mol. Pharm. 2019, 16, 1312–1326. [Google Scholar] [CrossRef]
- Muller, C.; Morales, P.; Reggio, P.H. Cannabinoid Ligands Targeting TRP Channels. Front. Mol. Neurosci. 2019, 11, 487. [Google Scholar] [CrossRef]
- Turri, M.; Teatini, F.; Donato, F.; Zanette, G.; Tugnoli, V.; Deotto, L.; Bonetti, B.; Squintani, G. Pain Modulation after Oromucosal Cannabinoid Spray (SATIVEX®) in Patients with Multiple Sclerosis: A Study with Quantitative Sensory Testing and Laser-Evoked Potentials. Medicines 2018, 5, 59. [Google Scholar] [CrossRef]
- Chianese, G.; Lopatriello, A.; Schiano-Moriello, A.; Caprioglio, D.; Mattoteia, D.; Benetti, E.; Ciceri, D.; Arnoldi, L.; de Combarieu, E.; Vitale, R.M.; et al. Cannabitwinol, a Dimeric Phytocannabinoid from Hemp, Cannabis sativa L., Is a Selective Thermo-TRP Modulator. J. Nat. Prod. 2020, 83, 2727–2736. [Google Scholar] [CrossRef]
- Pumroy, R.A.; Samanta, A.; Liu, Y.; Hughes, T.E.T.; Zhao, S.; Yudin, Y.; Rohacs, T.; Han, S.; Moiseenkova-Bell, V.Y. Molecular Mechanism of TRPV2 Channel Modulation by Cannabidiol. eLife 2019, 8, e48792. [Google Scholar] [CrossRef]
- Qin, N.; Neeper, M.P.; Liu, Y.; Hutchinson, T.L.; Lubin, M.L.; Flores, C.M. TRPV2 Is Activated by Cannabidiol and Mediates CGRP Release in Cultured Rat Dorsal Root Ganglion Neurons. J. Neurosci. 2008, 28, 6231–6238. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, C.W.; Ragozzino, F.J.; Lindberg, J.E.M.; Peterson, B.A.; Lugo, J.M.; McLaughlin, R.J.; Peters, J.H. Cannabidiol Activation of Vagal Afferent Neurons Requires TRPA1. J. Neurophysiol. 2020, 28, 6231–6238. [Google Scholar] [CrossRef]
- Liu, Y.; Mikrani, R.; He, Y.; Faran Ashraf Baig, M.M.; Abbas, M.; Naveed, M.; Tang, M.; Zhang, Q.; Li, C.; Zhou, X. TRPM8 Channels: A Review of Distribution and Clinical Role. Eur. J. Pharmacol. 2020, 882, 173312. [Google Scholar] [CrossRef] [PubMed]
- Limebeer, C.L.; Rock, E.M.; Sharkey, K.A.; Parker, L.A. Cognition and Behavior Nausea-Induced 5-HT Release in the Interoceptive Insular Cortex and Regulation by Monoacylglycerol Lipase (MAGL) Inhibition and Cannabidiol. Eneuro 2018, 5, ENEURO.0256-18. [Google Scholar] [CrossRef] [PubMed]
- Guzmán-Mejía, F.; López-Rubalcava, C.; González-Espinosa, C. Stimulation of NAchRα7 Receptor Inhibits TNF Synthesis and Secretion in Response to LPS Treatment of Mast Cells by Targeting ERK1/2 and TACE Activation. J. Neuroimmune Pharmacol. 2018, 13, 39–52. [Google Scholar] [CrossRef]
- Speca, D.J.; Ogata, G.; Mandikian, D.; Bishop, H.I.; Wiler, S.W.; Eum, K.; Wenzel, H.J.; Doisy, E.T.; Matt, L.; Campi, K.L.; et al. Deletion of the Kv2.1 Delayed Rectifier Potassium Channel Leads to Neuronal and Behavioral Hyperexcitability. Genes, Brain Behav. 2014, 13, 394–408. [Google Scholar] [CrossRef]
- Srivastava, S.; Cohen, J.S.; Vernon, H.; Barañano, K.; McClellan, R.; Jamal, L.; Naidu, S.B.; Fatemi, A. Clinical Whole Exome Sequencing in Child Neurology Practice. Ann. Neurol. 2014, 76, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Laun, A.S.; Shrader, S.H.; Song, Z.H. Novel Inverse Agonists for the Orphan G Protein-Coupled Receptor 6. Heliyon 2018, 4, e00933. [Google Scholar] [CrossRef]
- Maione, S.; Piscitelli, F.; Gatta, L.; Vita, D.; De Petrocellis, L.; Palazzo, E.; De Novellis, V.; Di Marzo, V. Non-Psychoactive Cannabinoids Modulate the Descending Pathway of Antinociception in Anaesthetized Rats through Several Mechanisms of Action. Br. J. Pharmacol. 2011, 162, 584–596. [Google Scholar] [CrossRef]
- Zagzoog, A.; Mohamed, K.A.; Kim, H.J.J.; Kim, E.D.; Frank, C.S.; Black, T.; Jadhav, P.D.; Holbrook, L.A.; Laprairie, R.B. In Vitro and in Vivo Pharmacological Activity of Minor Cannabinoids Isolated from Cannabis Sativa. Sci. Rep. 2020, 10, 20405. [Google Scholar] [CrossRef]
- Joshi, N.; Onaivi, E.S. Endocannabinoid System Components: Overview and Tissue Distribution. Adv. Exp. Med. Biol. 2019, 1162, 1–12. [Google Scholar] [CrossRef]
- Kendall, D.A.; Yudowski, G.A. Cannabinoid Receptors in the Central Nervous System: Their Signaling and Roles in Disease. Front. Cell. Neurosci. 2017, 10, 294. [Google Scholar] [CrossRef]
- Onaivi, E.S.; Ishiguro, H.; Gong, J.P.; Patel, S.; Meozzi, P.A.; Myers, L.; Perchuk, A.; Mora, Z.; Tagliaferro, P.A.; Gardner, E.; et al. Brain Neuronal CB2 Cannabinoid Receptors in Drug Abuse and Depression: From Mice to Human Subjects. PLoS ONE 2008, 3, e1640. [Google Scholar] [CrossRef]
- Stumpf, A.; Parthier, D.; Sammons, R.P.; Stempel, A.V.; Breustedt, J.; Rost, B.R.; Schmitz, D. Cannabinoid Type 2 Receptors Mediate a Cell Type-Specific Self-Inhibition in Cortical Neurons. Neuropharmacology 2018, 139, 217–225. [Google Scholar] [CrossRef]
- Zou, S.; Kumar, U. Cannabinoid Receptors and the Endocannabinoid System: Signaling and Function in the Central Nervous System. Int. J. Mol. Sci. 2018, 19, 833. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.R.; Canseco-Alba, A.; Zhang, H.Y.; Tagliaferro, P.; Chung, M.; Dennis, E.; Sanabria, B.; Schanz, N.; Escosteguy-Neto, J.C.; Ishiguro, H.; et al. Cannabinoid Type 2 Receptors in Dopamine Neurons Inhibits Psychomotor Behaviors, Alters Anxiety, Depression and Alcohol Preference. Sci. Rep. 2017, 7, 17410. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.R.; Canseco-Alba, A.; Liang, Y.; Ishiguro, H.; Onaivi, E.S. Low Basal CB2R in Dopamine Neurons and Microglia Influences Cannabinoid Tetrad Effects. Int. J. Mol. Sci. 2020, 21, 9763. [Google Scholar] [CrossRef]
- Bisogno, T.; Hanuš, L.; De Petrocellis, L.; Tchilibon, S.; Ponde, D.E.; Brandi, I.; Moriello, A.S.; Davis, J.B.; Mechoulam, R.; Di Marzo, V. Molecular Targets for Cannabidiol and Its Synthetic Analogues: Effect on Vanilloid VR1 Receptors and on the Cellular Uptake and Enzymatic Hydrolysis of Anandamide. Br. J. Pharmacol. 2001, 134, 845–852. [Google Scholar] [CrossRef]
- Laprairie, R.B.; Bagher, A.M.; Kelly, M.E.M.; Denovan-Wright, E.M. Cannabidiol Is a Negative Allosteric Modulator of the Cannabinoid CB1 Receptor. Br. J. Pharmacol. 2015, 172, 4790–4805. [Google Scholar] [CrossRef]
- Martínez-Pinilla, E.; Varani, K.; Reyes-Resina, I.; Angelats, E.; Vincenzi, F.; Ferreiro-Vera, C.; Oyarzabal, J.; Canela, E.I.; Lanciego, J.L.; Nadal, X.; et al. Binding and Signaling Studies Disclose a Potential Allosteric Site for Cannabidiol in Cannabinoid CB2 Receptors. Front. Pharmacol. 2017, 8, 744. [Google Scholar] [CrossRef] [PubMed]
- Morales, P.; Goya, P.; Jagerovic, N.; Hernandez-Folgado, L. Allosteric Modulators of the CB1 Cannabinoid Receptor: A Structural Update Review. Cannabis Cannabinoid Res. 2016, 1, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Pertwee, R.G.; Ross, R.A.; Craib, S.J.; Thomas, A. (−)-Cannabidiol Antagonizes Cannabinoid Receptor Agonists and Noradrenaline in the Mouse Vas Deferens. Eur. J. Pharmacol. 2002, 456, 99–106. [Google Scholar] [CrossRef]
- Thomas, A.; Baillie, G.L.; Phillips, A.M.; Razdan, R.K.; Ross, R.A.; Pertwee, R.G. Cannabidiol Displays Unexpectedly High Potency as an Antagonist of CB1 and CB2 Receptor Agonists In Vitro. Br. J. Pharmacol. 2007, 150, 613–623. [Google Scholar] [CrossRef]
- Hoyer, D.; Hannon, J.P.; Martin, G.R. Molecular, Pharmacological and Functional Diversity of 5-HT Receptors. Pharmacol. Biochem. Behav. 2002, 71, 533–554. [Google Scholar] [CrossRef]
- Żmudzka, E.; Sałaciak, K.; Sapa, J.; Pytka, K. Serotonin Receptors in Depression and Anxiety: Insights from Animal Studies. Life Sci. 2018, 210, 106–124. [Google Scholar] [CrossRef] [PubMed]
- Russo, E.B.; Burnett, A.; Hall, B.; Parker, K.K. Agonistic Properties of Cannabidiol at 5-HT1a Receptors. Neurochem. Res. 2005, 30, 1037–1043. [Google Scholar] [CrossRef] [PubMed]
- Soares, V.D.P.; Campos, A.C.; de Bortoli, V.C.; Zangrossi, H.; Guimarães, F.S.; Zuardi, A.W. Intra-Dorsal Periaqueductal Gray Administration of Cannabidiol Blocks Panic-like Response by Activating 5-HT1A Receptors. Behav. Brain Res. 2010, 213, 225–229. [Google Scholar] [CrossRef]
- Fogaça, M.V.; Reis, F.M.C.V.; Campos, A.C.; Guimarães, F.S. Effects of Intra-Prelimbic Prefrontal Cortex Injection of Cannabidiol on Anxiety-like Behavior: Involvement of 5HT1A Receptors and Previous Stressful Experience. Eur. Neuropsychopharmacol. 2014, 24, 410–419. [Google Scholar] [CrossRef]
- Gomes, F.V.; Resstel, L.B.M.; Guimarães, F.S. The Anxiolytic-like Effects of Cannabidiol Injected into the Bed Nucleus of the Stria Terminalis Are Mediated by 5-HT1A Receptors. Psychopharmacology 2011, 213, 465–473. [Google Scholar] [CrossRef]
- Rock, E.M.; Bolognini, D.; Limebeer, C.L.; Cascio, M.G.; Anavi-Goffer, S.; Fletcher, P.J.; Mechoulam, R.; Pertwee, R.G.; Parker, L.A. Cannabidiol, a Non-Psychotropic Component of Cannabis, Attenuates Vomiting and Nausea-like Behaviour via Indirect Agonism of 5-HT1A Somatodendritic Autoreceptors in the Dorsal Raphe Nucleus. Br. J. Pharmacol. 2012, 165, 2620–2634. [Google Scholar] [CrossRef] [PubMed]
- Borea, P.A.; Gessi, S.; Merighi, S.; Vincenzi, F.; Varani, K. Pharmacology of Adenosine Receptors: The State of the Art. Physiol. Rev. 2018, 98, 1591–1625. [Google Scholar] [CrossRef]
- Liang, X.; Liu, R.; Chen, C.; Ji, F.; Li, T. Opioid System Modulates the Immune Function: A Review. Transl. Perioper. Pain Med. 2016, 1, 5. [Google Scholar]
- Bartuzi, D.; Kaczor, A.A.; Matosiuk, D. Activation and Allosteric Modulation of Human μ Opioid Receptor in Molecular Dynamics. J. Chem. Inf. Model. 2015, 55, 2421–2434. [Google Scholar] [CrossRef]
- Kathmann, M.; Flau, K.; Redmer, A.; Tränkle, C.; Schlicker, E. Cannabidiol Is an Allosteric Modulator at Mu- and Delta-Opioid Receptors. Naunyn. Schmiedebergs. Arch. Pharmacol. 2006, 372, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Cruz, S.L.; Sánchez-Miranda, E.; Castillo-Arellano, J.I.; Cervantes-Villagrana, R.D.; Ibarra-Sánchez, A.; González-Espinosa, C. Anandamide Inhibits FcεRI-Dependent Degranulation and Cytokine Synthesis in Mast Cells through CB2 and GPR55 Receptor Activation. Possible Involvement of CB2-GPR55 Heteromers. Int. Immunopharmacol. 2018, 64, 298–307. [Google Scholar] [CrossRef]
- Ryberg, E.; Larsson, N.; Sjögren, S.; Hjorth, S.; Hermansson, N.O.; Leonova, J.; Elebring, T.; Nilsson, K.; Drmota, T.; Greasley, P.J. The Orphan Receptor GPR55 Is a Novel Cannabinoid Receptor. Br. J. Pharmacol. 2007, 152, 1092–1101. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Fichna, J.; Schicho, R.; Saur, D.; Bashashati, M.; MacKie, K.; Li, Y.; Zimmer, A.; Göke, B.; Sharkey, K.A.; et al. A Role for O-1602 and G Protein-Coupled Receptor GPR55 in the Control of Colonic Motility in Mice. Neuropharmacology 2013, 71, 255–263. [Google Scholar] [CrossRef]
- Whyte, L.S.; Ryberg, E.; Sims, N.A.; Ridge, S.A.; Mackie, K.; Greasley, P.J.; Ross, R.A.; Rogers, M.J. The Putative Cannabinoid Receptor GPR55 Affects Osteoclast Function in Vitro and Bone Mass In Vivo. Proc. Natl. Acad. Sci. USA 2009, 106, 16511–16516. [Google Scholar] [CrossRef] [PubMed]
- Morales, P.; Lago-Fernandez, A.; Hurst, D.P.; Sotudeh, N.; Brailoiu, E.; Reggio, P.H.; Abood, M.E.; Jagerovic, N. Therapeutic Exploitation of GPR18: Beyond the Cannabinoids? J. Med. Chem. 2020, 63, 14216–14227. [Google Scholar] [CrossRef]
- McHugh, D.; Hu, S.S.J.; Rimmerman, N.; Juknat, A.; Vogel, Z.; Walker, J.M.; Bradshaw, H.B. N-Arachidonoyl Glycine, an Abundant Endogenous Lipid, Potently Drives Directed Cellular Migration through GPR18, the Putative Abnormal Cannabidiol Receptor. BMC Neurosci. 2010, 11, 44. [Google Scholar] [CrossRef]
- Morales, P.; Isawi, I.; Reggio, P.H. Towards a Better Understanding of the Cannabinoid-Related Orphan Receptors GPR3, GPR6, and GPR12. Drug Metab. Rev. 2018, 50, 74–93. [Google Scholar] [CrossRef] [PubMed]
- Galiazzo, G.; De Silva, M.; Giancola, F.; Rinnovati, R.; Peli, A.; Chiocchetti, R. Cellular Distribution of Cannabinoid-Related Receptors TRPV1, PPAR-Gamma, GPR55 and GPR3 in the Equine Cervical Dorsal Root Ganglia. Equine Vet. J. 2022, 54, 788–798. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.J.; Laun, A.S.; Song, Z.H. Cannabidiol, a Novel Inverse Agonist for GPR12. Biochem. Biophys. Res. Commun. 2017, 493, 451–454. [Google Scholar] [CrossRef] [PubMed]
- Dos-Santos-Pereira, M.; da-Silva, C.A.; Guimarães, F.S.; Del-Bel, E. Co-Administration of Cannabidiol and Capsazepine Reduces L-DOPA-Induced Dyskinesia in Mice: Possible Mechanism of Action. Neurobiol. Dis. 2016, 94, 179–195. [Google Scholar] [CrossRef] [PubMed]
- Hind, W.H.; England, T.J.; O’Sullivan, S.E. Cannabidiol Protects an in Vitro Model of the Blood-Brain Barrier from Oxygen-Glucose Deprivation via PPARγ and 5-HT1A Receptors. Br. J. Pharmacol. 2016, 173, 815–825. [Google Scholar] [CrossRef]
- Mooko, T.; Bala, A.; Tripathy, S.; Kumar, C.S.; Mahadevappa, C.P.; Chaudhary, S.K.; Matsabisa, M.G. Cannabis sativa L. Flower and Bud Extracts Inhibited In Vitro Cholinesterases and b-Secretase Enzymes Activities: Possible Mechanisms of Cannabis Use in Alzheimer Disease. Endocr. Metab. Immune Disord. Drug Targets 2022, 22, 297–309. [Google Scholar] [CrossRef]
- Tripathi, R.K.P. A Perspective Review on Fatty Acid Amide Hydrolase (FAAH) Inhibitors as Potential Therapeutic Agents. Eur. J. Med. Chem. 2020, 188, 111953. [Google Scholar] [CrossRef]
- Leweke, F.M.; Piomelli, D.; Pahlisch, F.; Muhl, D.; Gerth, C.W.; Hoyer, C.; Klosterkötter, J.; Hellmich, M.; Koethe, D. Cannabidiol Enhances Anandamide Signaling and Alleviates Psychotic Symptoms of Schizophrenia. Transl. Psychiatry 2012, 2, e94. [Google Scholar] [CrossRef]
- Patel, R.; Barker, J.; Elshaer, A. Pharmaceutical Excipients and Drug Metabolism: A Mini-Review. Int. J. Mol. Sci. 2020, 21, 8224. [Google Scholar] [CrossRef]
- Nasrin, S.; Watson, C.J.W.; Perez-Paramo, Y.X.; Lazarus, P. Cannabinoid Metabolites as Inhibitors of Major Hepatic CYP450 Enzymes, with Implications for Cannabis-Drug Interactions. Drug Metab. Dispos. 2021, 49, 1070–1080. [Google Scholar] [CrossRef]
- Patsalos, P.N.; Szaflarski, J.P.; Gidal, B.; VanLandingham, K.; Critchley, D.; Morrison, G. Clinical Implications of Trials Investigating Drug-Drug Interactions between Cannabidiol and Enzyme Inducers or Inhibitors or Common Antiseizure Drugs. Epilepsia 2020, 61, 1854–1868. [Google Scholar] [CrossRef]
- Yamaori, S.; Kushihara, M.; Yamamoto, I.; Watanabe, K. Characterization of Major Phytocannabinoids, Cannabidiol and Cannabinol, as Isoform-Selective and Potent Inhibitors of Human CYP1 Enzymes. Biochem. Pharmacol. 2010, 79, 1691–1698. [Google Scholar] [CrossRef]
- Yamaori, S.; Koeda, K.; Kushihara, M.; Hada, Y.; Yamamoto, I.; Watanabe, K. Comparison in the in Vitro Inhibitory Effects of Major Phytocannabinoids and Polycyclic Aromatic Hydrocarbons Contained in Marijuana Smoke on Cytochrome P450 2C9 Activity. Drug Metab. Pharmacokinet. 2012, 27, 294–300. [Google Scholar] [CrossRef]
- Yamaori, S.; Okushima, Y.; Masuda, K.; Kushihara, M.; Katsu, T.; Narimatsu, S.; Yamamoto, I.; Watanabe, K. Structural Requirements for Potent Direct Inhibition of Human Cytochrome P450 1A1 by Cannabidiol: Role of Pentylresorcinol Moiety. Biol. Pharm. Bull. 2013, 36, 1197–1203. [Google Scholar] [CrossRef]
- Yamaori, S.; Okushima, Y.; Yamamoto, I.; Watanabe, K. Characterization of the Structural Determinants Required for Potent Mechanism-Based Inhibition of Human Cytochrome P450 1A1 by Cannabidiol. Chem. Biol. Interact. 2014, 215, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Yamaori, S.; Okamoto, Y.; Yamamoto, I.; Watanabe, K. Cannabidiol, a Major Phytocannabinoid, as a Potent Atypical Inhibitor for CYP2D6. Drug Metab. Dispos. 2011, 39, 2049–2056. [Google Scholar] [CrossRef] [PubMed]
- Yamaori, S.; Ebisawa, J.; Okushima, Y.; Yamamoto, I.; Watanabe, K. Potent Inhibition of Human Cytochrome P450 3A Isoforms by Cannabidiol: Role of Phenolic Hydroxyl Groups in the Resorcinol Moiety. Life Sci. 2011, 88, 730–736. [Google Scholar] [CrossRef] [PubMed]
- Koch, M.; Dehghani, F.; Habazettl, I.; Schomerus, C.; Korf, H.W. Cannabinoids Attenuate Norepinephrine-Induced Melatonin Biosynthesis in the Rat Pineal Gland by Reducing Arylalkylamine N-Acetyltransferase Activity without Involvement of Cannabinoid Receptors. J. Neurochem. 2006, 98, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Genn, R.F.; Tucci, S.; Marco, E.M.; Viveros, M.P.; File, S.E. Unconditioned and Conditioned Anxiogenic Effects of the Cannabinoid Receptor Agonist CP 55,940 in the Social Interaction Test. Pharmacol. Biochem. Behav. 2004, 77, 567–573. [Google Scholar] [CrossRef]
- Häring, M.; Kaiser, N.; Monory, K.; Lutz, B. Circuit Specific Functions of Cannabinoid CB1 Receptor in the Balance of Investigatory Drive and Exploration. PLoS ONE 2011, 6, e26617. [Google Scholar] [CrossRef]
- Lafenêtre, P.; Chaouloff, F.; Marsicano, G. Bidirectional Regulation of Novelty-Induced Behavioral Inhibition by the Endocannabinoid System. Neuropharmacology 2009, 57, 715–721. [Google Scholar] [CrossRef]
- Wiley, J.L.; Burston, J.J.; Leggett, D.C.; Alekseeva, O.O.; Razdan, R.K.; Mahadevan, A.; Martin, B.R. CB1 Cannabinoid Receptor-Mediated Modulation of Food Intake in Mice. Br. J. Pharmacol. 2005, 145, 293–300. [Google Scholar] [CrossRef]
- Bellocchio, L.; Lafentre, P.; Cannich, A.; Cota, D.; Puente, N.; Grandes, P.; Chaouloff, F.; Piazza, P.V.; Marsicano, G. Bimodal Control of Stimulated Food Intake by the Endocannabinoid System. Nat. Neurosci. 2010, 13, 281–283. [Google Scholar] [CrossRef]
- Rodríguez-Manzo, G.; Canseco-Alba, A. Biphasic Effects of Anandamide on Behavioural Responses: Emphasis on Copulatory Behaviour. Behav. Pharmacol. 2015, 26, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Canseco-Alba, A.; Rodríguez-Manzo, G. Intra-VTA Anandamide Infusion Produces Dose-Based Biphasic Effects on Male Rat Sexual Behavior Expression. Pharmacol. Biochem. Behav. 2016, 150–151, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Marco, E.M.; Pérez-Álvarez, L.; Borcel, E.; Rubio, M.; Guaza, C.; Ambrosio, E.; File, S.E.; Viveros, M.P. Involvement of 5-HT1A Receptors in Behavioural Effects of the Cannabinoid Receptor Agonist CP 55,940 in Male Rats. Behav. Pharmacol. 2004, 15, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Sulcova, E.; Mechoulam, R.; Fride, E. Biphasic Effects of Anandamide. Pharmacol. Biochem. Behav. 1998, 59, 347–352. [Google Scholar] [CrossRef]
- Mota Oliveira, M.; Ribeiro, A.M.; Pinto, C.; Santos, C. Cannabis, a Plant and Its Secret: Cannabidiol. Eur. Psychiatry 2015, 30, 1620. [Google Scholar] [CrossRef]
- Zuardi, A.W.; Rodrigues, N.P.; Silva, A.L.; Bernardo, S.A.; Hallak, J.E.C.; Guimarães, F.S.; Crippa, J.A.S. Inverted U-Shaped Dose-Response Curve of the Anxiolytic Effect of Cannabidiol during Public Speaking in Real Life. Front. Pharmacol. 2017, 8, 259. [Google Scholar] [CrossRef]
- Scheau, C.; Caruntu, C.; Badarau, I.A.; Scheau, A.E.; Docea, A.O.; Calina, D.; Caruntu, A. Cannabinoids and Inflammations of the Gut-Lung-Skin Barrier. J. Pers. Med. 2021, 11, 494. [Google Scholar] [CrossRef]
- Linge, R.; Jiménez-Sánchez, L.; Campa, L.; Pilar-Cuéllar, F.; Vidal, R.; Pazos, A.; Adell, A.; Díaz, Á. Cannabidiol Induces Rapid-Acting Antidepressant-like Effects and Enhances Cortical 5-HT/Glutamate Neurotransmission: Role of 5-HT1A Receptors. Neuropharmacology 2016, 103, 16–26. [Google Scholar] [CrossRef]
- Marinho, A.L.Z.; Vila-Verde, C.; Fogaça, M.V.; Guimarães, F.S. Effects of Intra-Infralimbic Prefrontal Cortex Injections of Cannabidiol in the Modulation of Emotional Behaviors in Rats: Contribution of 5HT1A Receptors and Stressful Experiences. Behav. Brain Res. 2015, 286, 49–56. [Google Scholar] [CrossRef]
- Patel, S.; Hill, M.N.; Cheer, J.F.; Wotjak, C.T.; Holmes, A. The Endocannabinoid System as a Target for Novel Anxiolytic Drugs. Neurosci. Biobehav. Rev. 2017, 76, 56–66. [Google Scholar] [CrossRef]
- Sartim, A.G.; Guimarães, F.S.; Joca, S.R.L. Antidepressant-like Effect of Cannabidiol Injection into the Ventral Medial Prefrontal Cortex—Possible Involvement of 5-HT1A and CB1 Receptors. Behav. Brain Res. 2016, 303, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Zanelati, T.V.; Biojone, C.; Moreira, F.A.; Guimarães, F.S.; Joca, S.R.L. Antidepressant-like Effects of Cannabidiol in Mice: Possible Involvement of 5-HT1A Receptors. Br. J. Pharmacol. 2010, 159, 122–128. [Google Scholar] [CrossRef]
- Austrich-Olivares, A.; García-Gutiérrez, M.S.; Illescas, L.; Gasparyan, A.; Manzanares, J. Cannabinoid CB1 Receptor Involvement in the Actions of CBD on Anxiety and Coping Behaviors in Mice. Pharmaceuticals 2022, 15, 473. [Google Scholar] [CrossRef] [PubMed]
- Campos, A.C.; Ferreira, F.R.; Guimarães, F.S. Cannabidiol Blocks Long-Lasting Behavioral Consequences of Predator Threat Stress: Possible Involvement of 5HT1A Receptors. J. Psychiatr. Res. 2012, 46, 1501–1510. [Google Scholar] [CrossRef] [PubMed]
- Campos, A.C.; Guimarães, F.S. Evidence for a Potential Role for TRPV1 Receptors in the Dorsolateral Periaqueductal Gray in the Attenuation of the Anxiolytic Effects of Cannabinoids. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2009, 33, 1517–1521. [Google Scholar] [CrossRef] [PubMed]
- Mlost, J.; Bryk, M.; Starowicz, K. Cannabidiol for Pain Treatment: Focus on Pharmacology and Mechanism of Action. Int. J. Mol. Sci. 2020, 21, 8870. [Google Scholar] [CrossRef]
- Schott, M. Insights on Cannabidiol’s Antiallodynic and Anxiolytic Mechanisms of Action in a Model of Neuropathic Pain. Pain Rep. 2019, 4, e774. [Google Scholar] [CrossRef]
- Silva-Cardoso, G.K.; Lazarini-Lopes, W.; Hallak, J.E.; Crippa, J.A.; Zuardi, A.W.; Garcia-Cairasco, N.; Leite-Panissi, C.R.A. Cannabidiol Effectively Reverses Mechanical and Thermal Allodynia, Hyperalgesia, and Anxious Behaviors in a Neuropathic Pain Model: Possible Role of CB1 and TRPV1 Receptors. Neuropharmacology 2021, 197, 108712. [Google Scholar] [CrossRef]
- Ward, S.J.; McAllister, S.D.; Kawamura, R.; Murase, R.; Neelakantan, H.; Walker, E.A. Cannabidiol Inhibits Paclitaxel-Induced Neuropathic Pain through 5-HT1A Receptors without Diminishing Nervous System Function or Chemotherapy Efficacy. Br. J. Pharmacol. 2014, 171, 636–645. [Google Scholar] [CrossRef]
- Costa, B.; Trovato, A.E.; Comelli, F.; Giagnoni, G.; Colleoni, M. The Non-Psychoactive Cannabis Constituent Cannabidiol Is an Orally Effective Therapeutic Agent in Rat Chronic Inflammatory and Neuropathic Pain. Eur. J. Pharmacol. 2007, 556, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Crivelaro do Nascimento, G.; Ferrari, D.P.; Guimaraes, F.S.; Del Bel, E.A.; Bortolanza, M.; Ferreira- Junior, N.C. Cannabidiol Increases the Nociceptive Threshold in a Preclinical Model of Parkinson’s Disease. Neuropharmacology 2020, 163, 107808. [Google Scholar] [CrossRef] [PubMed]
- De Gregorio, D.; McLaughlin, R.J.; Posa, L.; Ochoa-Sanchez, R.; Enns, J.; Lopez-Canul, M.; Aboud, M.; Maione, S.; Comai, S.; Gobbi, G. Cannabidiol Modulates Serotonergic Transmission and Reverses Both Allodynia and Anxiety-like Behavior in a Model of Neuropathic Pain. Pain 2019, 160, 136–150. [Google Scholar] [CrossRef] [PubMed]
- Jesus, C.H.A.; Redivo, D.D.B.; Gasparin, A.T.; Sotomaior, B.B.; de Carvalho, M.C.; Genaro, K.; Zuardi, A.W.; Hallak, J.E.C.; Crippa, J.A.; Zanoveli, J.M.; et al. Cannabidiol Attenuates Mechanical Allodynia in Streptozotocin-Induced Diabetic Rats via Serotonergic System Activation through 5-HT1A Receptors. Brain Res. 2019, 1715, 156–164. [Google Scholar] [CrossRef]
- Kaplan, J.S.; Stella, N.; Catterall, W.A.; Westenbroek, R.E. Cannabidiol Attenuates Seizures and Social Deficits in a Mouse Model of Dravet Syndrome. Proc. Natl. Acad. Sci. USA 2017, 114, 11229–11234. [Google Scholar] [CrossRef]
- Zhang, T.; Yang, Y.; Sima, X. No Association of GABRA1 Rs2279020 and GABRA6 Rs3219151 Polymorphisms with Risk of Epilepsy and Antiepileptic Drug Responsiveness in Asian and Arabic Populations: Evidence from a Meta-Analysis with Trial Sequential Analysis. Front. Neurol. 2022, 13, 2059. [Google Scholar] [CrossRef] [PubMed]
- Katsidoni, V.; Anagnostou, I.; Panagis, G. Cannabidiol Inhibits the Reward-Facilitating Effect of Morphine: Involvement of 5-HT1A Receptors in the Dorsal Raphe Nucleus. Addict. Biol. 2013, 18, 286–296. [Google Scholar] [CrossRef]
- Navarrete, F.; García-Gutiérrez, M.S.; Gasparyan, A.; Austrich-Olivares, A.; Manzanares, J. Role of Cannabidiol in the Therapeutic Intervention for Substance Use Disorders. Front. Pharmacol. 2021, 12, 1216. [Google Scholar] [CrossRef]
- Fowler, C.J.; Fowler, C.J. Transport of Endocannabinoids across the Plasma Membrane and within the Cell. FEBS J. 2013, 280, 1895–1904. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.; Ferraz-De-Paula, V.; Pinheiro, M.L.; Vitoretti, L.B.; Mariano-Souza, D.P.; Quinteiro-Filho, W.M.; Akamine, A.T.; Almeida, V.I.; Quevedo, J.; Dal-Pizzol, F.; et al. Cannabidiol, a Non-Psychotropic Plant-Derived Cannabinoid, Decreases Inflammation in a Murine Model of Acute Lung Injury: Role for the Adenosine A2A Receptor. Eur. J. Pharmacol. 2012, 678, 78–85. [Google Scholar] [CrossRef]
- Petrosino, S.; Verde, R.; Vaia, M.; Allará, M.; Iuvone, T.; Di Marzo, V. Anti-Inflammatory Properties of Cannabidiol, a Nonpsychotropic Cannabinoid, in Experimental Allergic Contact Dermatitis. J. Pharmacol. Exp. Ther. 2018, 365, 652–663. [Google Scholar] [CrossRef] [PubMed]
- Couch, D.G.; Tasker, C.; Theophilidou, E.; Lund, J.N.; O’Sullivan, S.E. Cannabidiol and Palmitoylethanolamide Are Anti-Inflammatory in the Acutely Inflamed Human Colon. Clin. Sci. 2017, 131, 2611–2626. [Google Scholar] [CrossRef] [PubMed]
- Harvey, B.S.; Sia, T.C.; Wattchow, D.A.; Smid, S.D. Interleukin 17A Evoked Mucosal Damage Is Attenuated by Cannabidiol and Anandamide in a Human Colonic Explant Model. Cytokine 2014, 65, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Madera-Salcedo, I.K.; Cruz, S.L.; Gonzalez-Espinosa, C. Morphine Prevents Lipopolysaccharide-Induced TNF Secretion in Mast Cells Blocking IκB Kinase Activation and SNAP-23 Phosphorylation: Correlation with the Formation of a -Arrestin/TRAF6 Complex. J. Immunol. 2013, 191, 3400–3409. [Google Scholar] [CrossRef] [PubMed]
- Mecha, M.; Feliú, A.; Carrillo-Salinas, F.J.; Rueda-Zubiaurre, A.; Ortega-Gutiérrez, S.; de Sola, R.G.; Guaza, C. Endocannabinoids Drive the Acquisition of an Alternative Phenotype in Microglia. Brain. Behav. Immun. 2015, 49, 233–245. [Google Scholar] [CrossRef]
- Oláh, A.; Tóth, B.I.; Borbíró, I.; Sugawara, K.; Szöllõsi, A.G.; Czifra, G.; Pál, B.; Ambrus, L.; Kloepper, J.; Camera, E.; et al. Cannabidiol Exerts Sebostatic and Antiinflammatory Effects on Human Sebocytes. J. Clin. Investig. 2014, 124, 3713–3724. [Google Scholar] [CrossRef]
- Wei, Y.; Shin, M.R.; Sesti, F. Oxidation of KCNB1 Channels in the Human Brain and in Mouse Model of Alzheimer’s Disease. Cell Death Dis. 2018, 9, 820. [Google Scholar] [CrossRef]
- Wu, X.; Hernandez-Enriquez, B.; Banas, M.; Xu, R.; Sesti, F. Molecular Mechanisms Underlying the Apoptotic Effect of KCNB1 K+ Channel Oxidation. J. Biol. Chem. 2013, 288, 4128–4134. [Google Scholar] [CrossRef]
- Benoit, M.E.; Hernandez, M.X.; Dinh, M.L.; Benavente, F.; Vasquez, O.; Tenner, A.J. C1q-Induced LRP1B and GPR6 Proteins Expressed Early in Alzheimer Disease Mouse Models, Are Essential for the C1q-Mediated Protection against Amyloid- β Neurotoxicity. J. Biol. Chem. 2013, 288, 654–665. [Google Scholar] [CrossRef]
- Huang, Y.; Skwarek-Maruszewska, A.; Horré, K.; Vandewyer, E.; Wolfs, L.; Snellinx, A.; Saito, T.; Radaelli, E.; Corthout, N.; Colombelli, J.; et al. Loss of GPR3 Reduces the Amyloid Plaque Burden and Improves Memory in Alzheimer’s Disease Mouse Models. Sci. Transl. Med. 2015, 7, 309ra164. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Guimarães, T.R.; Todd, N.; Ferguson, C.; Weiss, K.M.; Stauffer, F.R.; McDermott, B.; Hurtle, B.T.; Saito, T.; Saido, T.C.; et al. G Protein–Biased GPR3 Signaling Ameliorates Amyloid Pathology in a Preclinical Alzheimer’s Disease Mouse Model. Proc. Natl. Acad. Sci. USA 2022, 119, e2204828119. [Google Scholar] [CrossRef] [PubMed]
- Hughes, B.; Herron, C.E. Cannabidiol Reverses Deficits in Hippocampal LTP in a Model of Alzheimer’s Disease. Neurochem. Res. 2019, 44, 703–713. [Google Scholar] [CrossRef]
- Scuderi, C.; Steardo, L.; Esposito, G. Cannabidiol Promotes Amyloid Precursor Protein Ubiquitination and Reduction of Beta Amyloid Expression in SHSY5YAPP+ Cells Through PPARγ Involvement. Phyther. Res. 2014, 28, 1007–1013. [Google Scholar] [CrossRef] [PubMed]
- Thathiah, A.; Spittaels, K.; Hoffmann, M.; Staes, M.; Cohen, A.; Horré, K.; Vanbrabant, M.; Coun, F.; Baekelandt, V.; Delacourte, A.; et al. The Orphan G Protein-Coupled Receptor 3 Modulates Amyloid-Beta Peptide Generation in Neurons. Science 2009, 323, 946–951. [Google Scholar] [CrossRef]
- Vallée, A.; Lecarpentier, Y.; Guillevin, R.; Vallée, J.N. Effects of Cannabidiol Interactions with Wnt/β-Catenin Pathway and PPARγ on Oxidative Stress and Neuroinflammation in Alzheimer’s Disease. Acta Biochim. Biophys. Sin. 2017, 49, 853–866. [Google Scholar] [CrossRef]
- Rubin, V. Cannabis and Culture. In Anthropology; Mouton Publishers: The Hage, The Netherlands; Paris, France, 1975; p. 598. [Google Scholar]
- McHugh, D.; Roskowski, D.; Xie, S.; Bradshaw, H.B. Δ9-THC and N-Arachidonoyl Glycine Regulate BV-2 Microglial Morphology and Cytokine Release Plasticity: Implications for Signaling at GPR18. Front. Pharmacol. 2014, 4, 162. [Google Scholar] [CrossRef]
- Blessing, E.M.; Steenkamp, M.M.; Manzanares, J.; Marmar, C.R. Cannabidiol as a Potential Treatment for Anxiety Disorders. Neurotherapeutics 2015, 12, 825–836. [Google Scholar] [CrossRef]
- Lee, J.L.C.; Bertoglio, L.J.; Guimarães, F.S.; Stevenson, C.W. Cannabidiol Regulation of Emotion and Emotional Memory Processing: Relevance for Treating Anxiety-Related and Substance Abuse Disorders. Br. J. Pharmacol. 2017, 174, 3242–3256. [Google Scholar] [CrossRef]
- Shannon, S.; Lewis, N.; Lee, H.; Hughes, S. Cannabidiol in Anxiety and Sleep: A Large Case Series. Perm. J. 2019, 23, 18–41. [Google Scholar] [CrossRef]
- Skelley, J.W.; Deas, C.M.; Curren, Z.; Ennis, J. Use of Cannabidiol in Anxiety and Anxiety-Related Disorders. J. Am. Pharm. Assoc. 2020, 60, 253–261. [Google Scholar] [CrossRef]
- Silote, G.P.; Sartim, A.; Sales, A.; Eskelund, A.; Guimarães, F.S.; Wegener, G.; Joca, S. Emerging Evidence for the Antidepressant Effect of Cannabidiol and the Underlying Molecular Mechanisms. J. Chem. Neuroanat. 2019, 98, 104–116. [Google Scholar] [CrossRef]
- Sales, A.J.; Crestani, C.C.; Guimarães, F.S.; Joca, S.R.L. Antidepressant-like Effect Induced by Cannabidiol Is Dependent on Brain Serotonin Levels. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2018, 86, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Micale, V.; Di Marzo, V.; Sulcova, A.; Wotjak, C.T.; Drago, F. Endocannabinoid System and Mood Disorders: Priming a Target for New Therapies. Pharmacol. Ther. 2013, 138, 18–37. [Google Scholar] [CrossRef] [PubMed]
- Davies, C.; Bhattacharyya, S. Cannabidiol as a Potential Treatment for Psychosis. Ther. Adv. Psychopharmacol. 2019, 9, 204512531988191. [Google Scholar] [CrossRef]
- Gururajan, A.; Malone, D.T. Does Cannabidiol Have a Role in the Treatment of Schizophrenia? Schizophr. Res. 2016, 176, 281–290. [Google Scholar] [CrossRef]
- Sewell, R.A.; Skosnik, P.D.; Garcia-Sosa, I.; Ranganathan, M.; D’Souza, D.C. Behavioral, Cognitive and Psychophysiological Effects of Cannabinoids: Relevance to Psychosis and Schizophrenia. Braz. J. Psychiatry 2010, 32, 515–530. [Google Scholar] [CrossRef]
- D’Souza, D.C.; Perry, E.; MacDougall, L.; Ammerman, Y.; Cooper, T.; Wu, Y.T.; Braley, G.; Gueorguieva, R.; Krystal, J.H. The Psychotomimetic Effects of Intravenous Delta-9-Tetrahydrocannabinol in Healthy Individuals: Implications for Psychosis. Neuropsychopharmacology 2004, 29, 1558–1572. [Google Scholar] [CrossRef] [PubMed]
- Morrison, P.D.; Zois, V.; McKeown, D.A.; Lee, T.D.; Holt, D.W.; Powell, J.F.; Kapur, S.; Murray, R.M. The Acute Effects of Synthetic Intravenous Δ9-Tetrahydrocannabinol on Psychosis, Mood and Cognitive Functioning. Psychol. Med. 2009, 39, 1607–1616. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, S.; Morrison, P.D.; Fusar-Poli, P.; Martin-Santos, R.; Borgwardt, S.; Winton-Brown, T.; Nosarti, C.; O’Carroll, C.M.; Seal, M.; Allen, P.; et al. Opposite Effects of Δ-9-Tetrahydrocannabinol and Cannabidiol on Human Brain Function and Psychopathology. Neuropsychopharmacology 2009, 35, 764–774. [Google Scholar] [CrossRef]
- Meltzer, H.Y.; Arvanitis, L.; Bauer, D.; Rein, W. Placebo-Controlled Evaluation of Four Novel Compounds for the Treatment of Schizophrenia and Schizoaffective Disorder. Am. J. Psychiatry 2004, 161, 975–984. [Google Scholar] [CrossRef]
- Viudez-Martínez, A.; García-Gutiérrez, M.S.; Fraguas-Sánchez, A.I.; Torres-Suárez, A.I.; Manzanares, J. Effects of Cannabidiol plus Naltrexone on Motivation and Ethanol Consumption. Br. J. Pharmacol. 2018, 175, 3369–3378. [Google Scholar] [CrossRef] [PubMed]
- Viudez-Martínez, A.; García-Gutiérrez, M.S.; Medrano-Relinque, J.; Navarrón, C.M.; Navarrete, F.; Manzanares, J. Cannabidiol Does Not Display Drug Abuse Potential in Mice Behavior. Acta Pharmacol. Sin. 2018, 40, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Galaj, E.; Bi, G.H.; Yang, H.J.; Xi, Z.X. Cannabidiol Attenuates the Rewarding Effects of Cocaine in Rats by CB2, 5-HT1A and TRPV1 Receptor Mechanisms. Neuropharmacology 2020, 167, 107740. [Google Scholar] [CrossRef]
- Watt, G.; Shang, K.; Zieba, J.; Olaya, J.; Li, H.; Garner, B.; Karl, T. Chronic Treatment with 50 Mg/Kg Cannabidiol Improves Cognition and Moderately Reduces Aβ 40 Levels in 12-Month-Old Male AβPP Swe/PS1ΔE9 Transgenic Mice. J. Alzheimer’s Dis. 2020, 74, 937–950. [Google Scholar] [CrossRef]
- Polanska, H.H.; Petrlakova, K.; Papouskova, B.; Hendrych, M.; Samadian, A.; Storch, J.; Babula, P.; Masarik, M.; Vacek, J. Safety Assessment and Redox Status in Rats after Chronic Exposure to Cannabidiol and Cannabigerol. Toxicology 2023, 488, 153460. [Google Scholar] [CrossRef] [PubMed]
- Vaughn, D.; Kulpa, J.; Paulionis, L. Preliminary Investigation of the Safety of Escalating Cannabinoid Doses in Healthy Dogs. Front. Vet. Sci. 2020, 7, 51. [Google Scholar] [CrossRef]
- Ammendolia, I.; Mannucci, C.; Cardia, L.; Calapai, G.; Gangemi, S.; Esposito, E.; Calapai, F. Pharmacovigilance on Cannabidiol as an Antiepileptic Agent. Front. Pharmacol. 2023, 14, 1091978. [Google Scholar] [CrossRef]
- Huestis, M.A.; Solimini, R.; Pichini, S.; Pacifici, R.; Carlier, J.; Busardò, F.P. Cannabidiol Adverse Effects and Toxicity. Curr. Neuropharmacol. 2019, 17, 974–989. [Google Scholar] [CrossRef] [PubMed]
- Rudisill, T.M.; Innes, K.; Wen, S.; Haggerty, T.; Smith, G.S. The Effects of Cannabidiol on the Driving Performance of Healthy Adults: A Pilot RCT. AJPM Focus 2023, 2, 100053. [Google Scholar] [CrossRef] [PubMed]
- Ewing, L.E.; Skinner, C.M.; Quick, C.M.; Kennon-McGill, S.; McGill, M.R.; Walker, L.A.; ElSohly, M.A.; Gurley, B.J.; Koturbash, I. Hepatotoxicity of a Cannabidiol-Rich Cannabis Extract in the Mouse Model. Molecules 2019, 24, 1694. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, X.; Cournoyer, P.; Choudhuri, S.; Yu, X.; Guo, L.; Chen, S. Cannabidiol-Induced Transcriptomic Changes and Cellular Senescence in Human Sertoli Cells. Toxicol. Sci. 2023, 191, 227–238. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).