Scissor-like Au4Cu2 Cluster with Phosphorescent Mechanochromism and Thermochromism
Abstract
1. Introduction
2. Results and Discussion
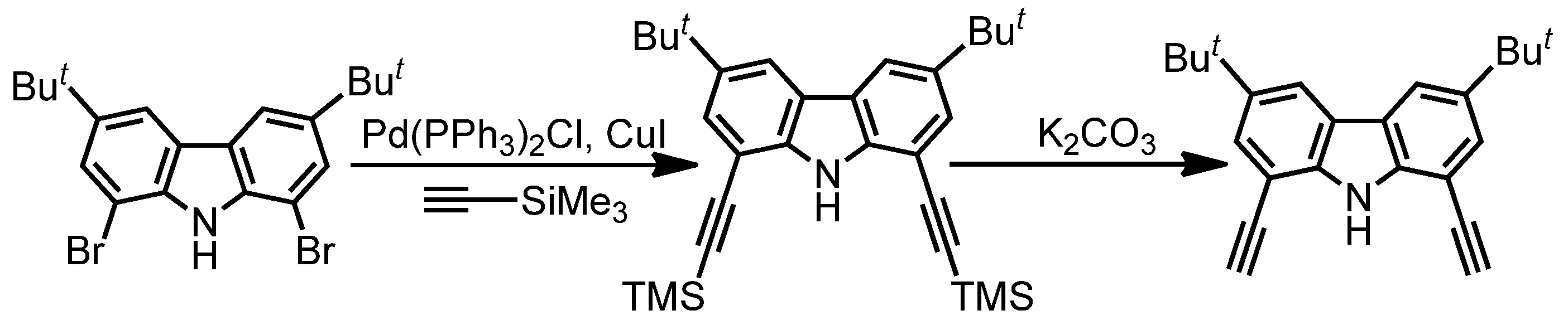
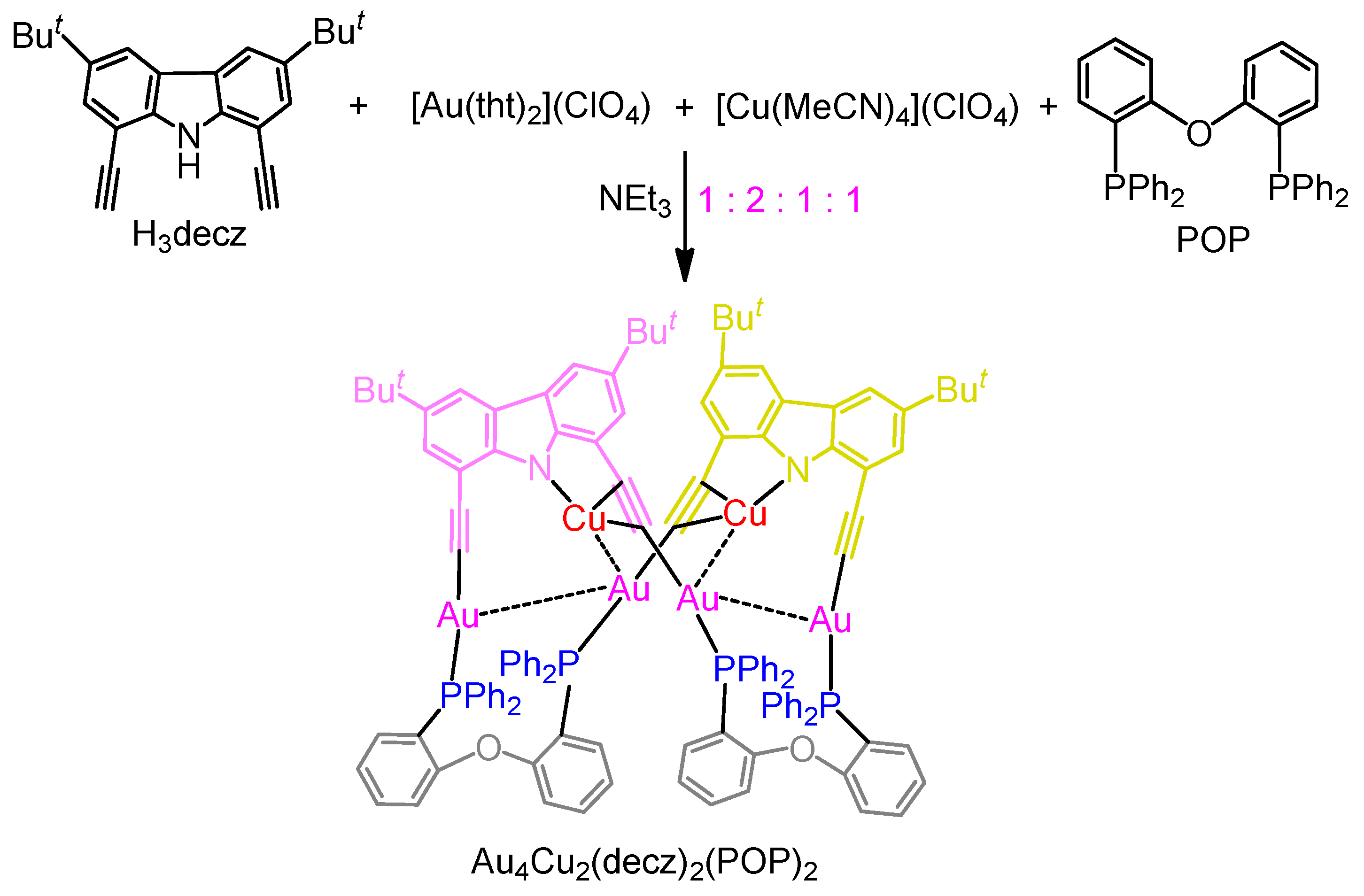
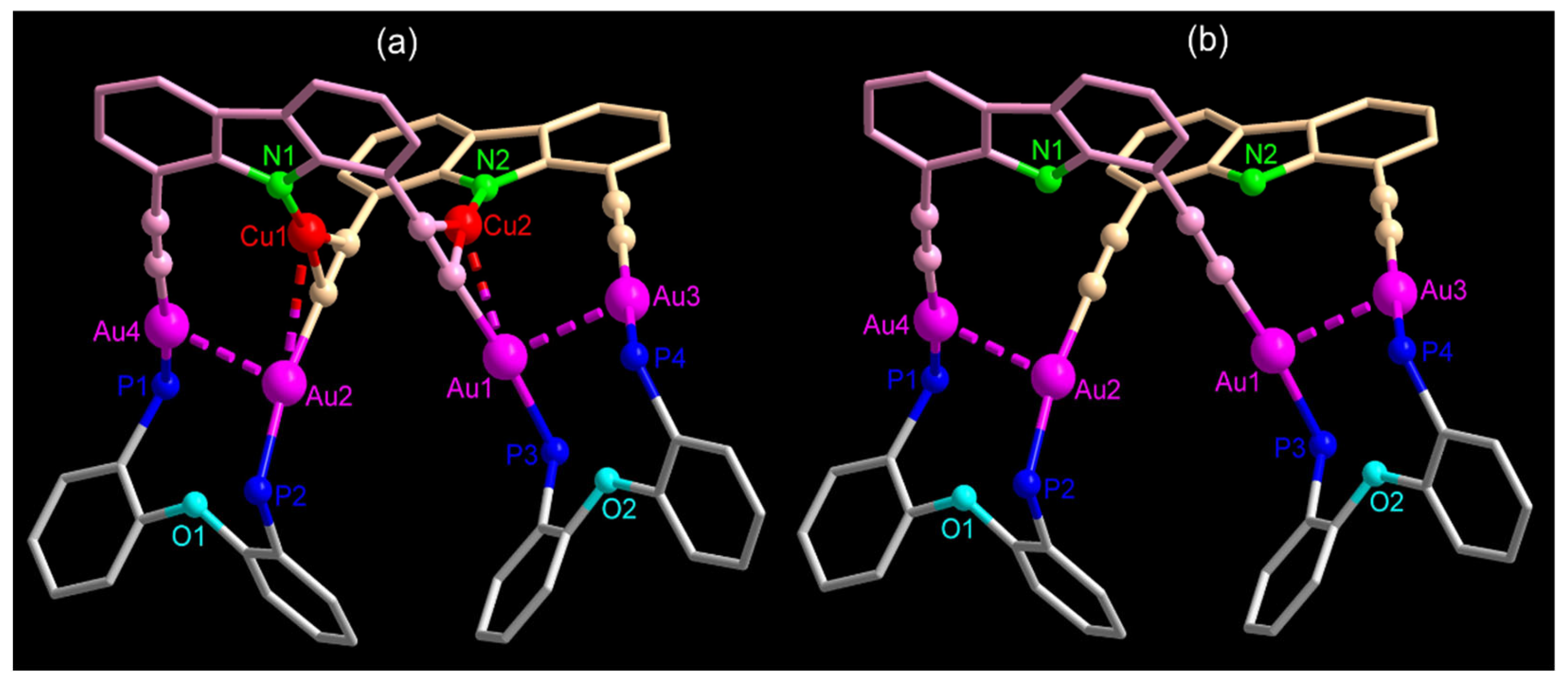
2.1. Synthesis and Characterization
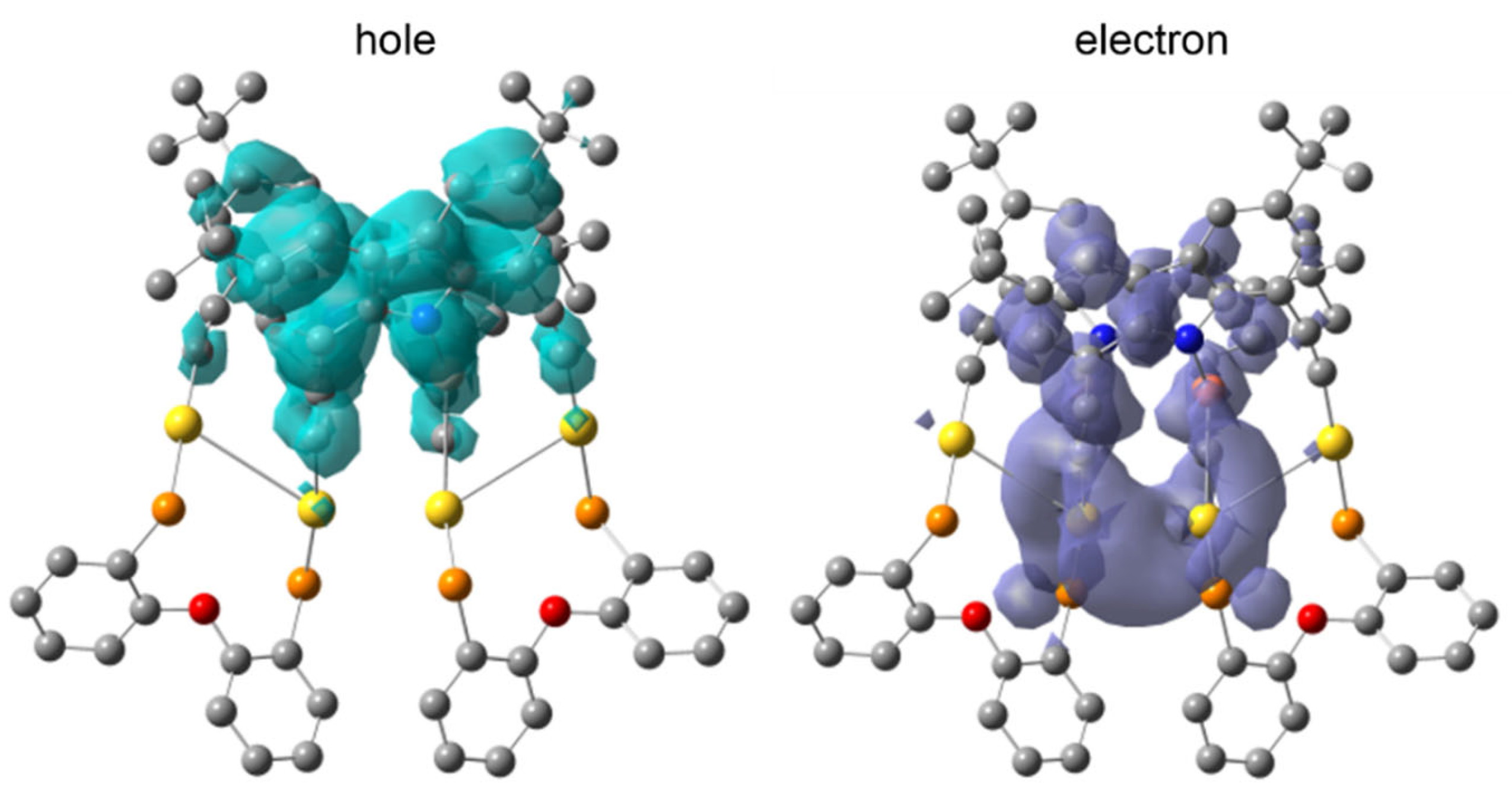
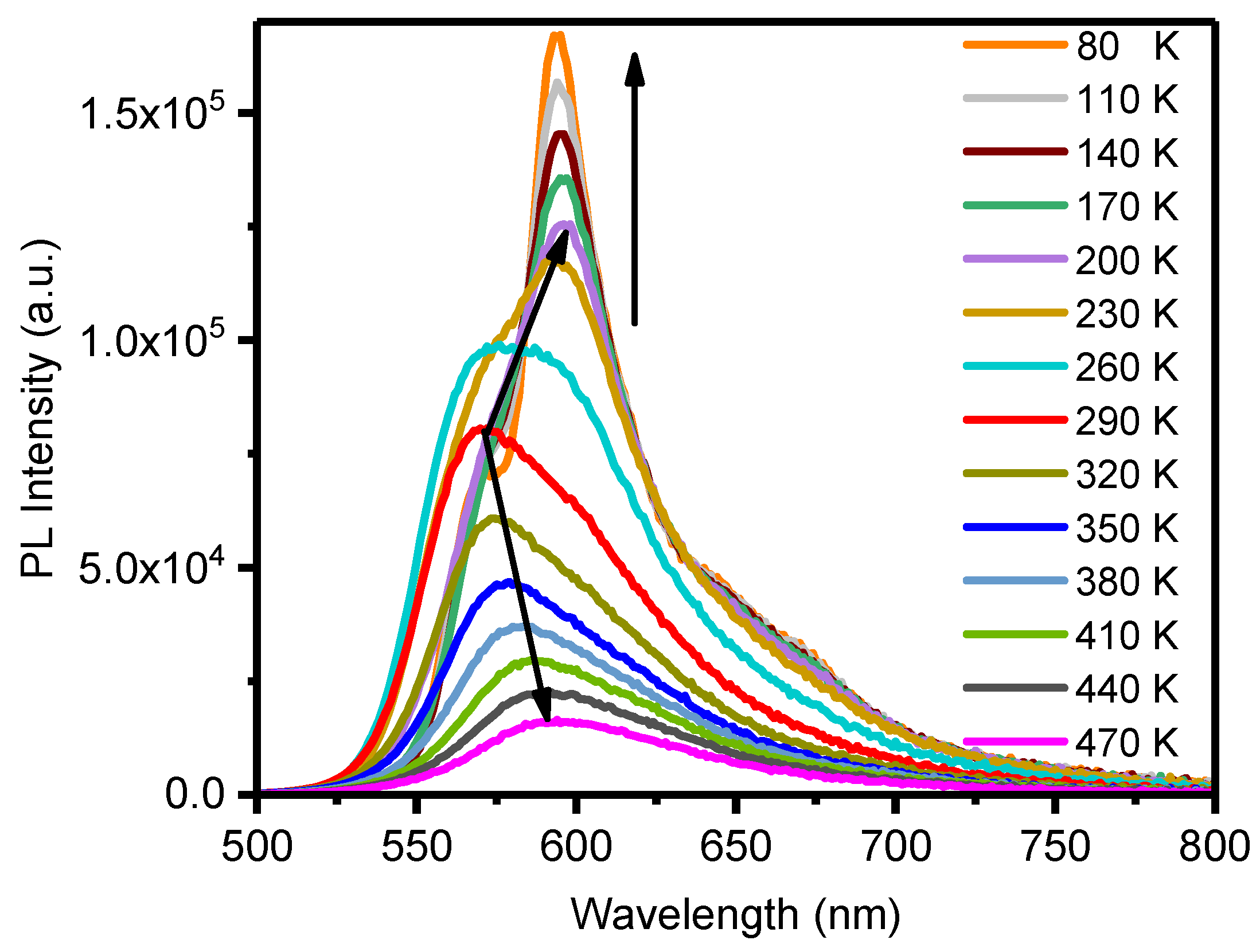
2.2. Photophysical Properties and Computational Studies
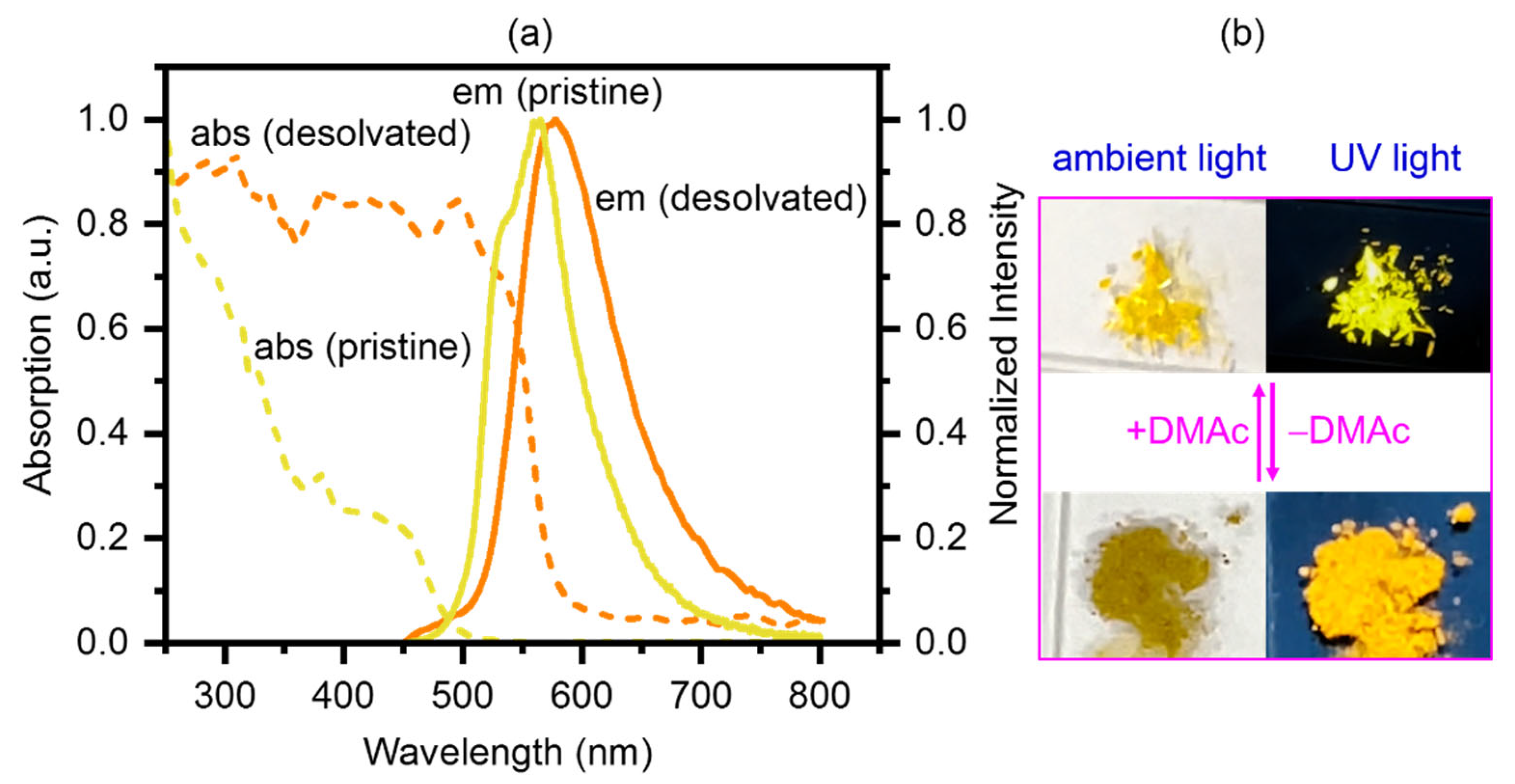
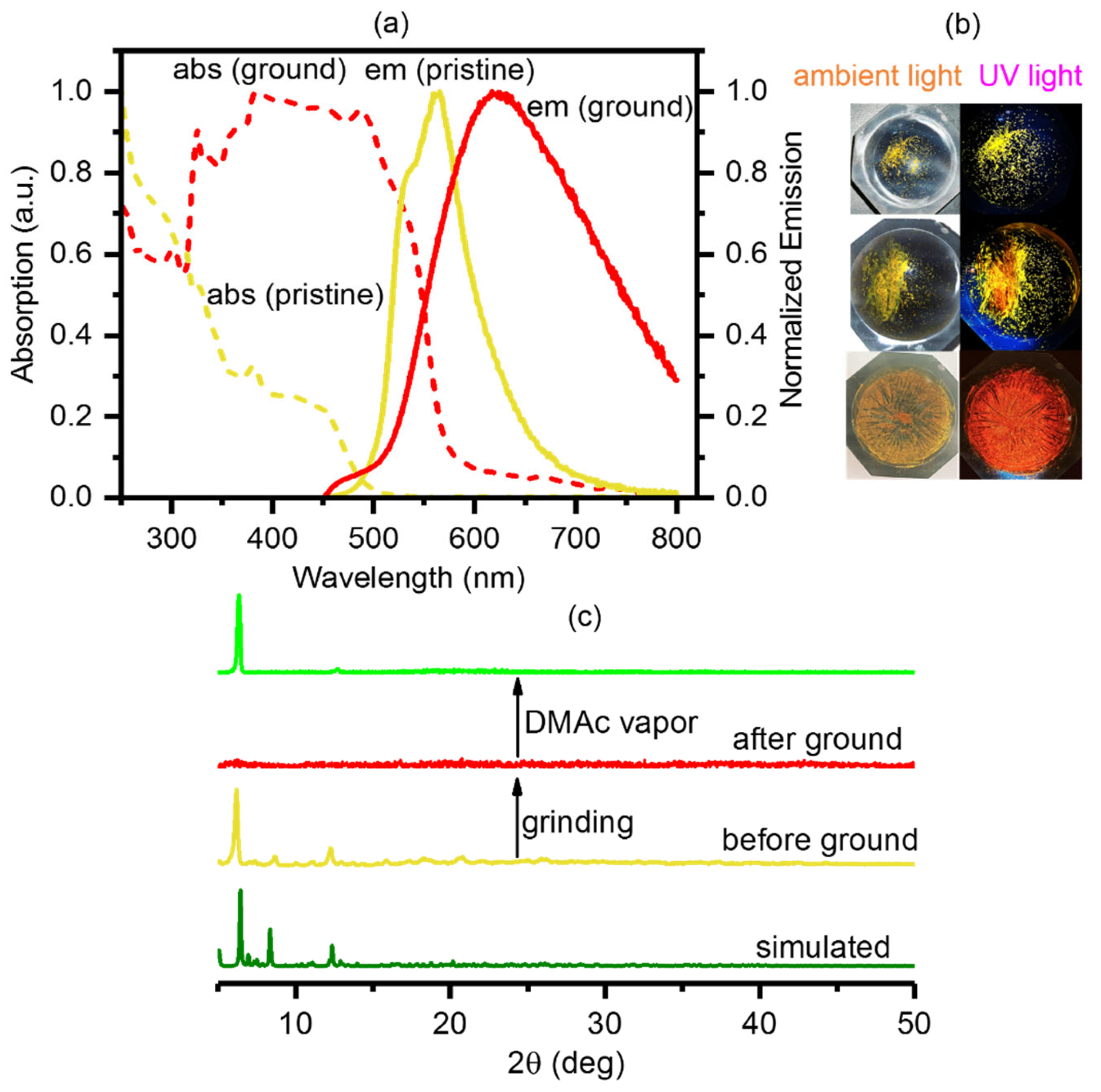
2.3. Stimuli-Responsive Properties
3. Experimental
3.1. Synthesis of Ligand H3decz
3.2. Synthesis of Au4Cu2(decz)2(POP)2
3.3. Physical Measurements
3.4. Crystal Structural Determination
3.5. Computational Method
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Sagara, Y.; Yamane, S.; Mitani, M.; Weder, C.; Kato, T. Mechanoresponsive Luminescent Molecular Assemblies: An Emerging Class of Materials. Adv. Mater. 2016, 28, 1073. [Google Scholar] [CrossRef] [PubMed]
- Chi, Z.; Zhang, X.; Xu, B.; Zhou, X.; Ma, C.; Zhang, Y.; Liu, S.; Xu, J. Recent advances in organic mechanofluorochromic materials. Chem. Soc. Rev. 2012, 41, 3878. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Fan, H.T.; Zang, S.Q.; Li, H.Y.; Wang, L.Y. Metal-containing crystalline luminescent thermochromic materials. Coord. Chem. Rev. 2018, 377, 307. [Google Scholar] [CrossRef]
- Kobayashi, A.; Kato, M. Stimuli-responsive Luminescent Copper(I) Complexes for Intelligent Emissive Devices. Chem. Lett. 2017, 46, 154. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, J.-Y.; Qiao, D.; Chen, Z.-N. Phosphorescent mechanochromism through the contraction of Ag12Cu2 clusters in tetradecanuclear copper–silver acetylide complexes. J. Mater. Chem. C 2017, 5, 8782. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, L.-Y.; Wang, J.-Y.; Dai, F.-R.; Chen, Z.-N. Two-step phosphorescent mechanochromism due to intramolecular deformation. J. Mater. Chem. C 2020, 8, 715. [Google Scholar] [CrossRef]
- Jin, M.; Ito, H. Solid-state luminescence of Au(I) complexes with external stimuli-responsive properties. J. Photochem. Photobiol. C Photochem. Rev. 2022, 51, 100478. [Google Scholar] [CrossRef]
- López-de-Luzuriaga, J.M.; Monge, M.; Olmos, M.E. Luminescent aryl–group eleven metal complexes. Dalton Trans. 2017, 46, 2046. [Google Scholar] [CrossRef]
- Mirzadeh, N.; Privér, S.H.; Blake, A.J.; Schmidbaur, H.; Bhargava, S.K. Innovative Molecular Design Strategies in Materials Science Following the Aurophilicity Concept. Chem. Rev. 2020, 120, 7551. [Google Scholar] [CrossRef]
- Huitorel, B.; Utrera-Melero, R.; Massuyeau, F.; Mevelec, J.-Y.; Baptiste, B.; Polian, A.; Gacoin, T.; Martineau-Corcosd, C.; Perruchas, S. Luminescence mechanochromism of copper iodide clusters: A rational investigation. Dalton Trans. 2019, 48, 7899. [Google Scholar] [CrossRef]
- Conejo-Rodríguez, V.; Peñas-Defrutos, M.N. Espinet, P. 4-Pyridylisocyanide gold(I) and gold(I)-plus-silver(I) luminescent and mechanochromic materials: The silver role. Dalton Trans. 2019, 48, 10412. [Google Scholar] [CrossRef]
- Chu, A.; Hau, F.K.-W.; Yao, L.-Y.; Yam, V.W.-W. Decanuclear Gold(I) Sulfido Pseudopolymorphs Displaying Stimuli-Responsive RGBY Luminescence Changes. ACS Mater. Lett. 2019, 1, 277. [Google Scholar] [CrossRef]
- Zhang, D.; Suzuki, S.; Naota, T. Rapid Luminescent Enhancement Triggered by One-shot Needle stick stimulus Using a Liquescent Gold(I) Salt. Angew. Chem. Int. Ed. 2021, 60, 19701. [Google Scholar] [CrossRef]
- Liu, Q.; Xie, M.; Chang, X.; Gao, Q.; Chen, Y.; Lu, W. Correlating thermochromic and mechanochromic phosphorescence with polymorphs of a complex gold(I) double salt with infinite aurophilicity. Chem. Commun. 2018, 54, 12844. [Google Scholar] [CrossRef]
- Xie, P.; Wang, J.-Y.; Huang, Y.-Z.; Wu, X.-M.; Chen, Z.-N. Heteroctanuclear Au4Ag4 Cluster Complexes of 4,5-Diethynylacridin-9-One with Luminescent Mechanochromism. Molecules 2022, 27, 2127. [Google Scholar] [CrossRef]
- Wang, X.-Y.; Yin, Y.; Yin, J.; Chen, Z.; Liu, S.H. Persistent room-temperature phosphorescence or high-contrast phosphorescent mechanochromism: Polymorphism-dependent different emission characteristics from a single gold(I) complex. Dalton Trans. 2022, 50, 7744. [Google Scholar] [CrossRef]
- Wang, Y.-W.; Yan, J.-J.; Hu, S.-N.; Young, D.J.; Li, H.-X.; Ren, Z.-G. A Photoluminescent Ag10Cu6 Cluster Stablized by a PNNP Ligand and Phenylacetylides Selectively and Reversibly Senses Ammonia in Air and Water. Chem.-Asian J. 2021, 16, 2681. [Google Scholar] [CrossRef]
- Tao, Y.-H.; Wang, Y.-W.; Hu, S.-N.; Young, D.J.; Lu, C.; Li, H.-X.; Ren, Z.-G. A photoluminescent Au(I)/Ag(I)/PNN coordination complex for relatively rapid and reversible alcohol sensing. Dalton Trans. 2021, 50, 6773. [Google Scholar] [CrossRef]
- Seki, T.; Tokodai, N.; Omagari, S.; Nakanishi, T.; Hasegawa, Y.; Iwasa, T.; Taketsugu, T.; Ito, H. Luminescent Mechanochromic 9-Anthryl Gold(I) Isocyanide Complex with an Emission Maximum at 900 nm after Mechanical Stimulation. J. Am. Chem. Soc. 2017, 139, 6514. [Google Scholar] [CrossRef]
- Dong, Y.; Zhang, J.; Li, A.; Gong, J.; He, B.; Xu, S.; Yin, J.; Liu, S.H.; Tang, B.Z. Structure-tuned and thermodynamically controlled mechanochromic self-recovery of AIE-active Au(I) complexes. J. Mater. Chem. C 2020, 8, 894. [Google Scholar] [CrossRef]
- Yang, Z.C.; Song, K.Y.; Zhou, P.K.; Zong, L.L.; Li, H.H.; Chen, Z.R.; Jiang, R. Sensitive luminescence mechanochromism and unique luminescence thermochromism tuned by bending the P-O-P skeleton in the diphosphonium/iodocuprate(I) hybrid. CrystEngComm. 2022, 24, 4940. [Google Scholar] [CrossRef]
- Yam, V.W.-W.; Cheng, E.C.-C. Photochemistry and Photophysics of Coordination Compounds II. Photochemistry and Photophysics of Coordination Compounds: Gold; Springer: Berlin/Heidelberg, Germany, 2007; Volume 281, pp. 269–309. [Google Scholar]
- Pujadas, M.; Rodríguez, L. Luminescent Phosphine Gold(I) Alkynyl Complexes. Highlights from 2010 to 2018. Coord. Chem. Rev. 2020, 408, 213179. [Google Scholar] [CrossRef]
- Zhang, Q.-C.; Xiao, H.; Zhang, X.; Xu, L.-J.; Chen, Z.-N. Luminescent oligonuclear metal complexes and the use in organic light-emitting diodes. Coord. Chem. Rev. 2019, 378, 121. [Google Scholar] [CrossRef]
- Xu, L.-J.; Wang, J.-Y.; Zhu, X.-F.; Zeng, X.-C.; Chen, Z.-N. Phosphorescent Cationic Au4Ag2 Alkynyl Cluster Complexes for Efficient Solution-Processed Organic Light-Emitting Diodes. Adv. Funct. Mater. 2015, 25, 3033. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, J.-Y.; Huang, Y.-Z.; Yang, M.; Chen, Z.-N. Silver(I) nanoclusters of carbazole-1,8-bis(acetylide): From visible to near-infrared emission. Chem. Commun. 2019, 55, 6281. [Google Scholar] [CrossRef]
- Huang, Y.-Z.; Shi, L.-X.; Wang, J.-Y.; Su, H.-F.; Chen, Z.-N. Elaborate Design of Ag8Au10 Cluster [2]Catenane Phosphors for High-Efficiency Light-Emitting Devices. ACS Appl. Mater. Interfaces 2020, 12, 57264. [Google Scholar] [CrossRef]
- Xiao, H.; Zhang, X.; Wang, J.-Y.; Zhang, L.-Y.; Zhang, Q.-C.; Chen, Z.-N. Enhancing Phosphorescence through Rigidifying the Conformation to Achieve High-Efficiency OLEDs by Modified PEDOT. ACS Appl. Mater. Interfaces 2019, 11, 45853. [Google Scholar] [CrossRef]
- Bennington, M.S.; Feltham, H.L.C.; Buxton, Z.J.; Whitea, N.G.; Brooker, S. Tuneable reversible redox of cobalt(iii) carbazole complexes. Dalton Trans. 2017, 46, 4696. [Google Scholar] [CrossRef]
- Krause, L.; Herbst-Irmer, R.; Sheldrick, G.M.; Stalke, D. Comparison of silver and molybdenum microfocus X-ray sources for single-crystal structure determination. J. Appl. Crystallogr. 2015, 48, 3. [Google Scholar] [CrossRef]
- Hubschle, C.B.; Sheldrick, G.M.; Dittrich, B. ShelXle: A Qt graphical user interface for SHELXL. J. Appl. Crystallogr. 2011, 44, 1281. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision, A.03; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Adamo, C.; Barone, V. Toward reliable density functional methods without adjustable parameters: The PBE0 model. J. Chem. Phys. 1999, 110, 6158. [Google Scholar] [CrossRef]
- Ernzerhof, M.; Scuseria, G.E. Assessment of the Perdew-Burke-Ernzerhof exchange-correlation functional. J. Chem. Phys. 1999, 110, 5029. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parameterization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Bauernschmitt, R.; Ahlrichs, R. Treatment of Electronic Excitations within the Adiabatic Approximation of Time Dependent Density Functional Theory. Chem. Phys. Lett. 1996, 256, 454. [Google Scholar] [CrossRef]
- Casida, M.E.; Jamorski, C.; Casida, K.C.; Salahub, D.R. Molecular Excitation Energies to High-lying Bound States from Timedependent Density-functional Response Theory: Characterization and Correction of the Time-dependent Local Density Approximation Ionization Threshold. J. Chem. Phys. 1998, 108, 4439. [Google Scholar] [CrossRef]
- Stratmann, R.E.; Scuseria, G.E.; Frisch, M.J. An Efficient Implementation of Time-dependent Density-functional Theory for the Calculation of Excitation Energies of Large Molecules. J. Chem. Phys. 1998, 109, 8218. [Google Scholar] [CrossRef]
- Scalmani, G.; Frisch, M.J. Continuous surface charge polarizable continuum models of solvation. I. General formalism. J. Chem. Phys. 2010, 132, 114110. [Google Scholar] [CrossRef]
- Andrae, D.; Häussermann, U.; Dolg, M.; Stoll, H.; Preuss, H. Energy-adjusted Ab initio Pseudopotentials for the Second and Third Row Transition Elements. Theor. Chim. Acta 1990, 77, 123. [Google Scholar] [CrossRef]
- Schwerdtfeger, P.; Dolg, M.; Schwarz, W.H.E.; Bowmaker, G.A.; Boyd, P.D.W. Relativistic effects in gold chemistry. I. Diatomic gold compounds. J. Chem. Phys. 1989, 91, 1762. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A Multifunctional Wavefunction Analyzer. J. Comp. Chem. 2012, 33, 580. [Google Scholar] [CrossRef]

| λabs/nm (ε/M−1 cm−1) | λem (nm)/τem (μs)/Φem (%) | ||
|---|---|---|---|
| CH2Cl2 | CH2Cl2 a | Solid b | PMMA Film c |
| 290 (39680), 342 (17540), 400 (12200), 450 (11200) | 576/2.6/3.2 | 565/14.5/50.2 580/6.0/1.2 (desolvated) | 530/19.3/7.9 |
| 580/1.9/3.6 | 623/5.3/12.6 (ground) | 529/16.7/5.6 (ground) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, X.-M.; Wang, J.-Y.; Huang, Y.-Z.; Chen, Z.-N. Scissor-like Au4Cu2 Cluster with Phosphorescent Mechanochromism and Thermochromism. Molecules 2023, 28, 3247. https://doi.org/10.3390/molecules28073247
Wu X-M, Wang J-Y, Huang Y-Z, Chen Z-N. Scissor-like Au4Cu2 Cluster with Phosphorescent Mechanochromism and Thermochromism. Molecules. 2023; 28(7):3247. https://doi.org/10.3390/molecules28073247
Chicago/Turabian StyleWu, Xue-Meng, Jin-Yun Wang, Ya-Zi Huang, and Zhong-Ning Chen. 2023. "Scissor-like Au4Cu2 Cluster with Phosphorescent Mechanochromism and Thermochromism" Molecules 28, no. 7: 3247. https://doi.org/10.3390/molecules28073247
APA StyleWu, X.-M., Wang, J.-Y., Huang, Y.-Z., & Chen, Z.-N. (2023). Scissor-like Au4Cu2 Cluster with Phosphorescent Mechanochromism and Thermochromism. Molecules, 28(7), 3247. https://doi.org/10.3390/molecules28073247








