Therapeutic Potential of Albumin Nanoparticles Encapsulated Visnagin in MDA-MB-468 Triple-Negative Breast Cancer Cells
Abstract
1. Introduction
2. Results and Discussion
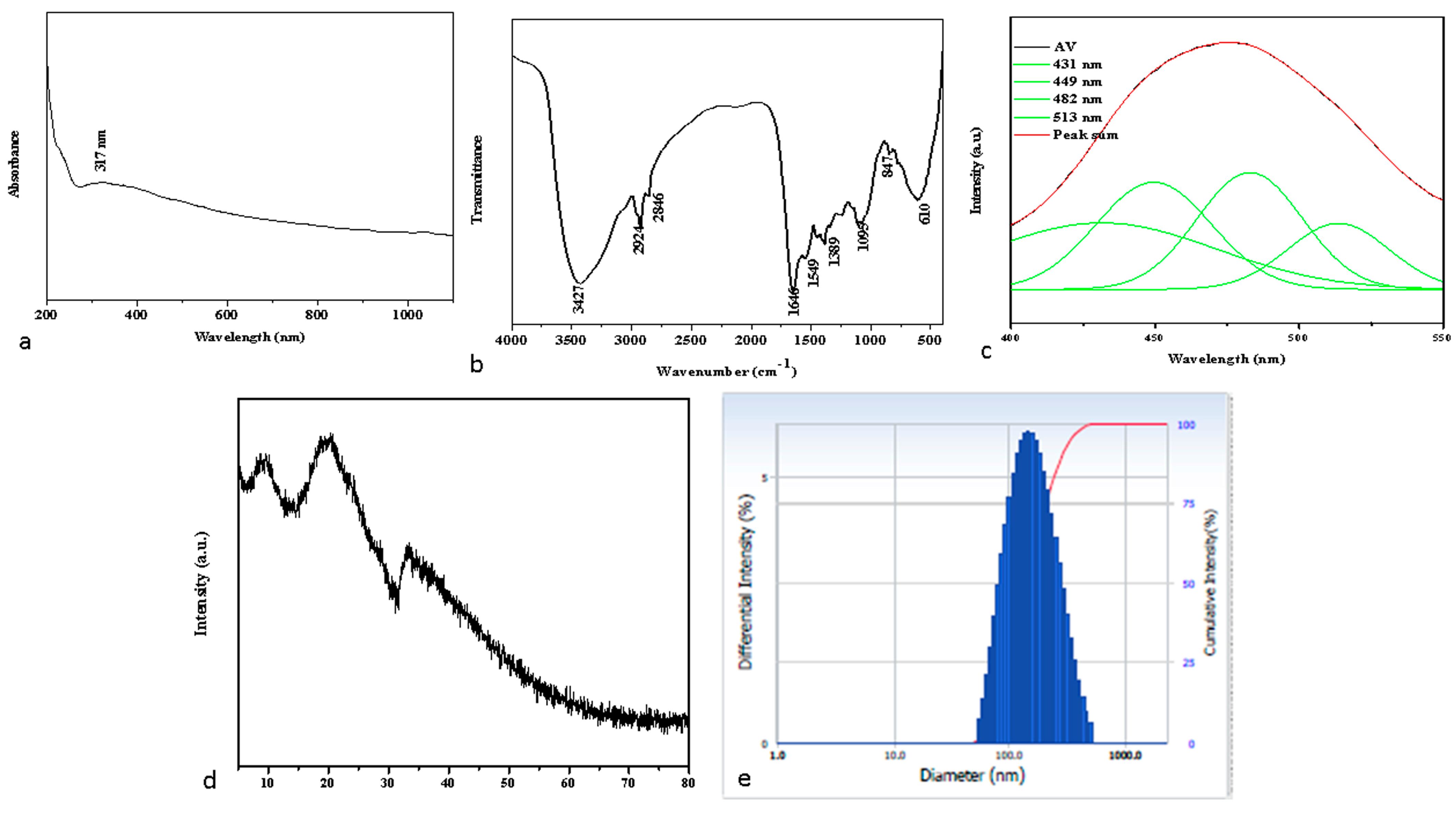
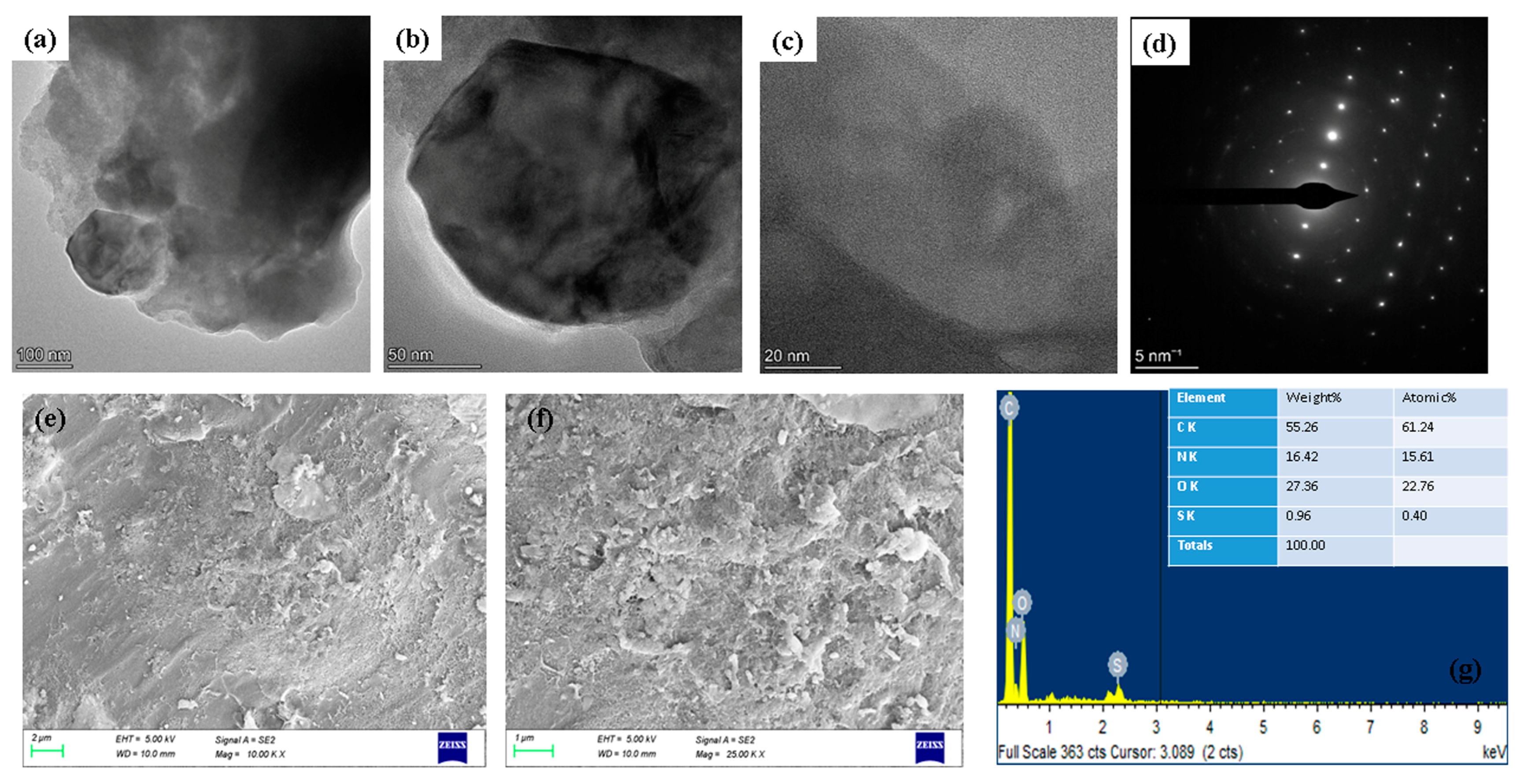
2.1. Spectral Characterization of Albumin-Binding Nanoparticles
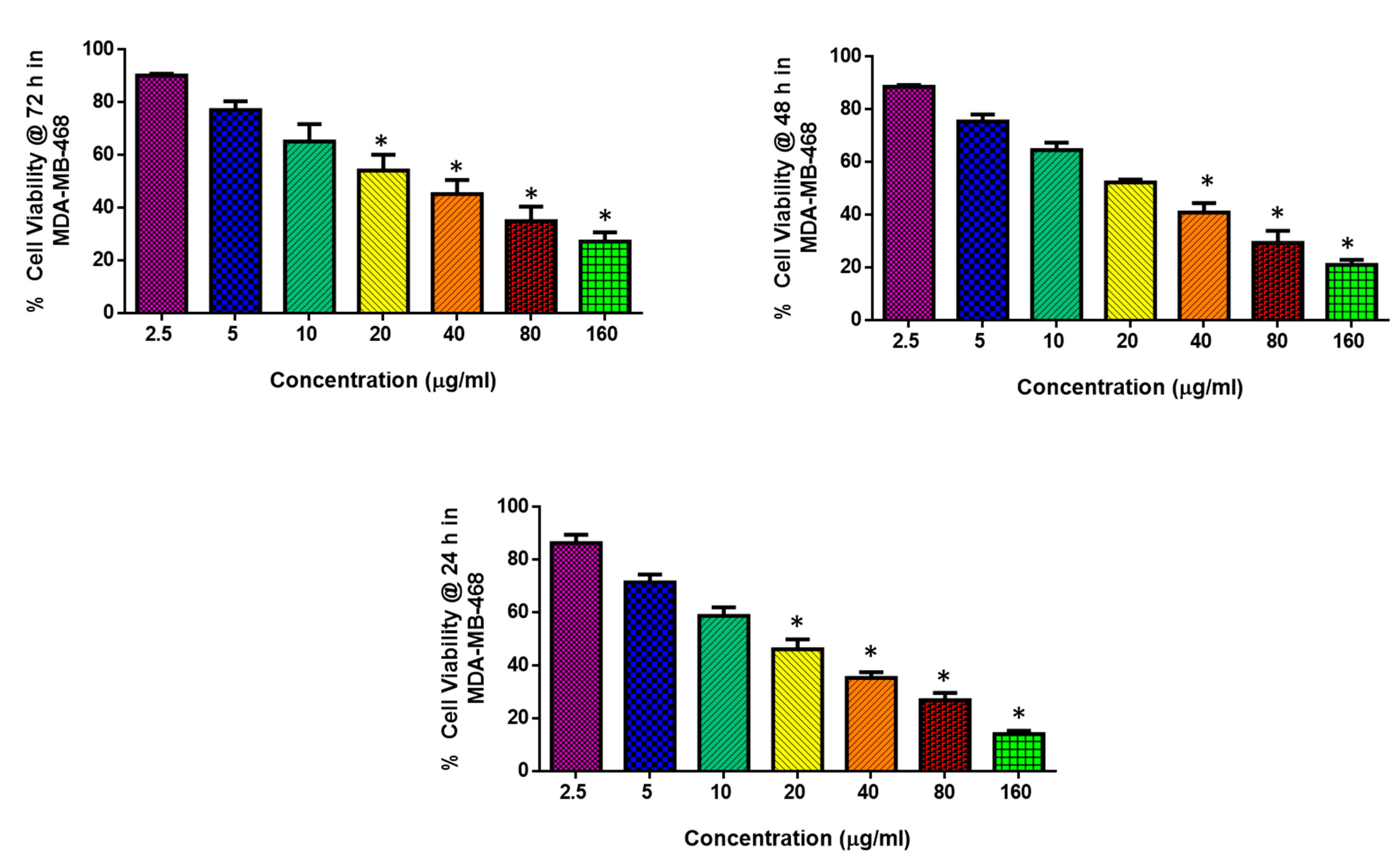
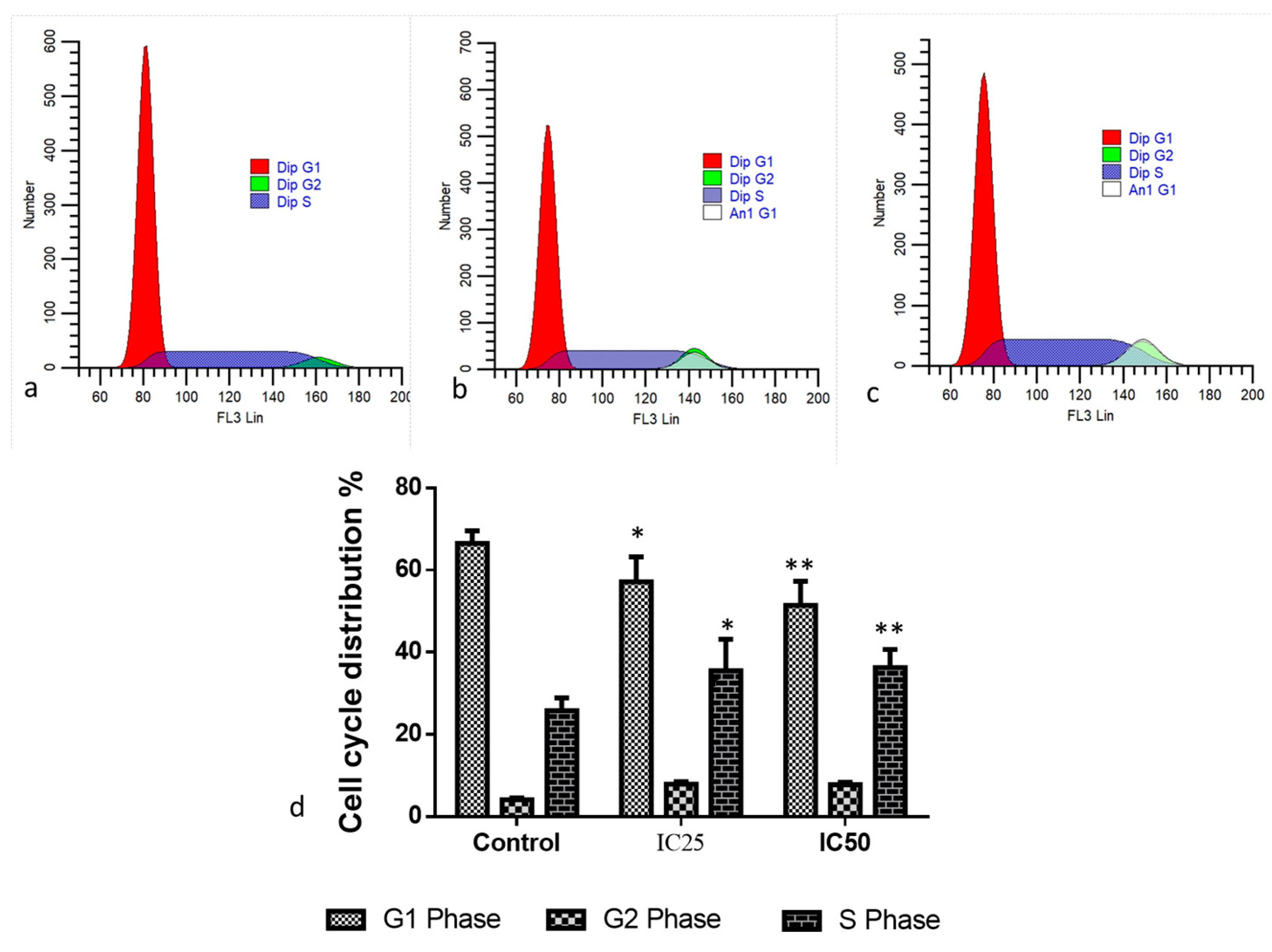
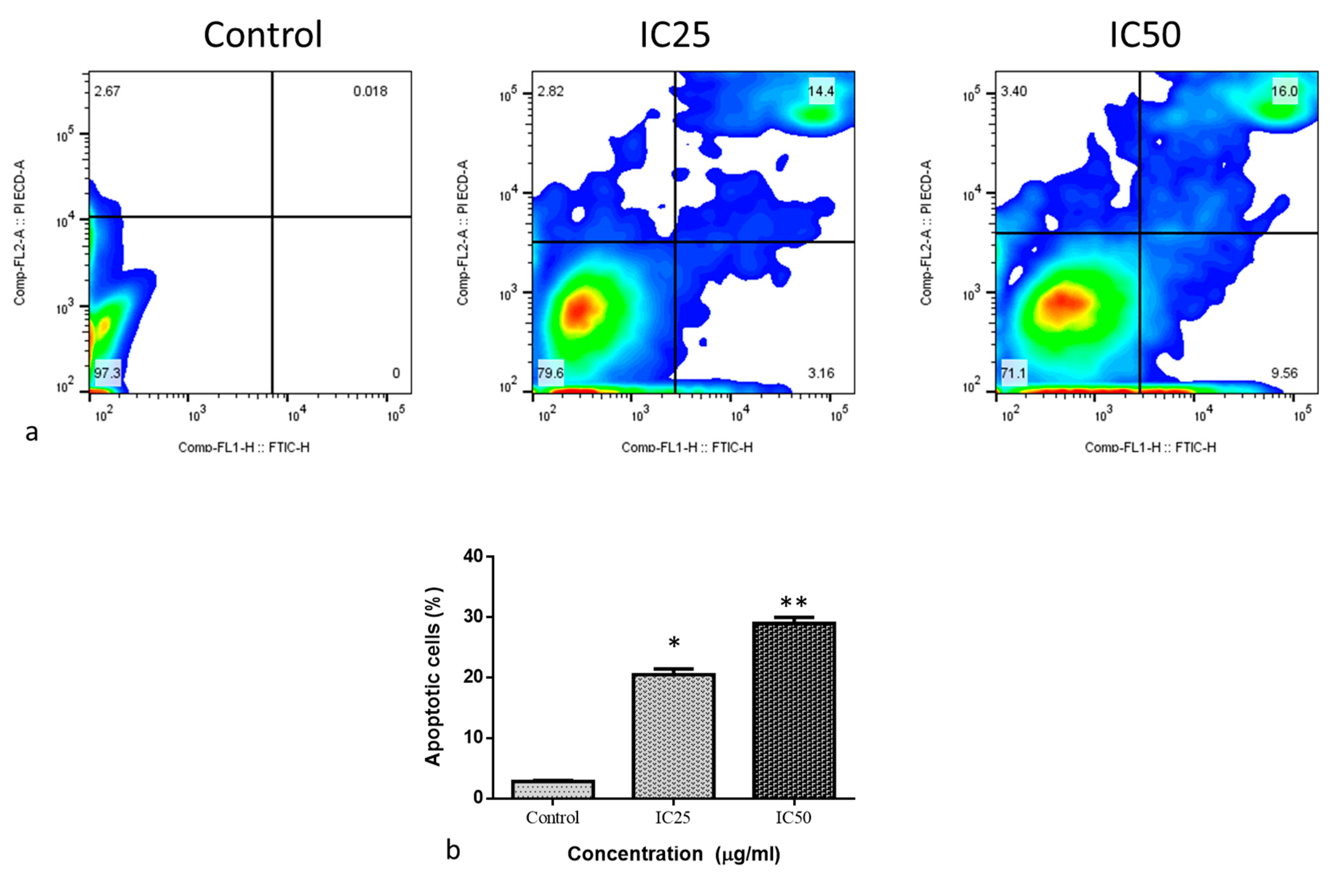
2.2. Cytotoxic and Apoptotic Effect of Albumin-Binding Nanoparticles in MDA-MB-468 Cells
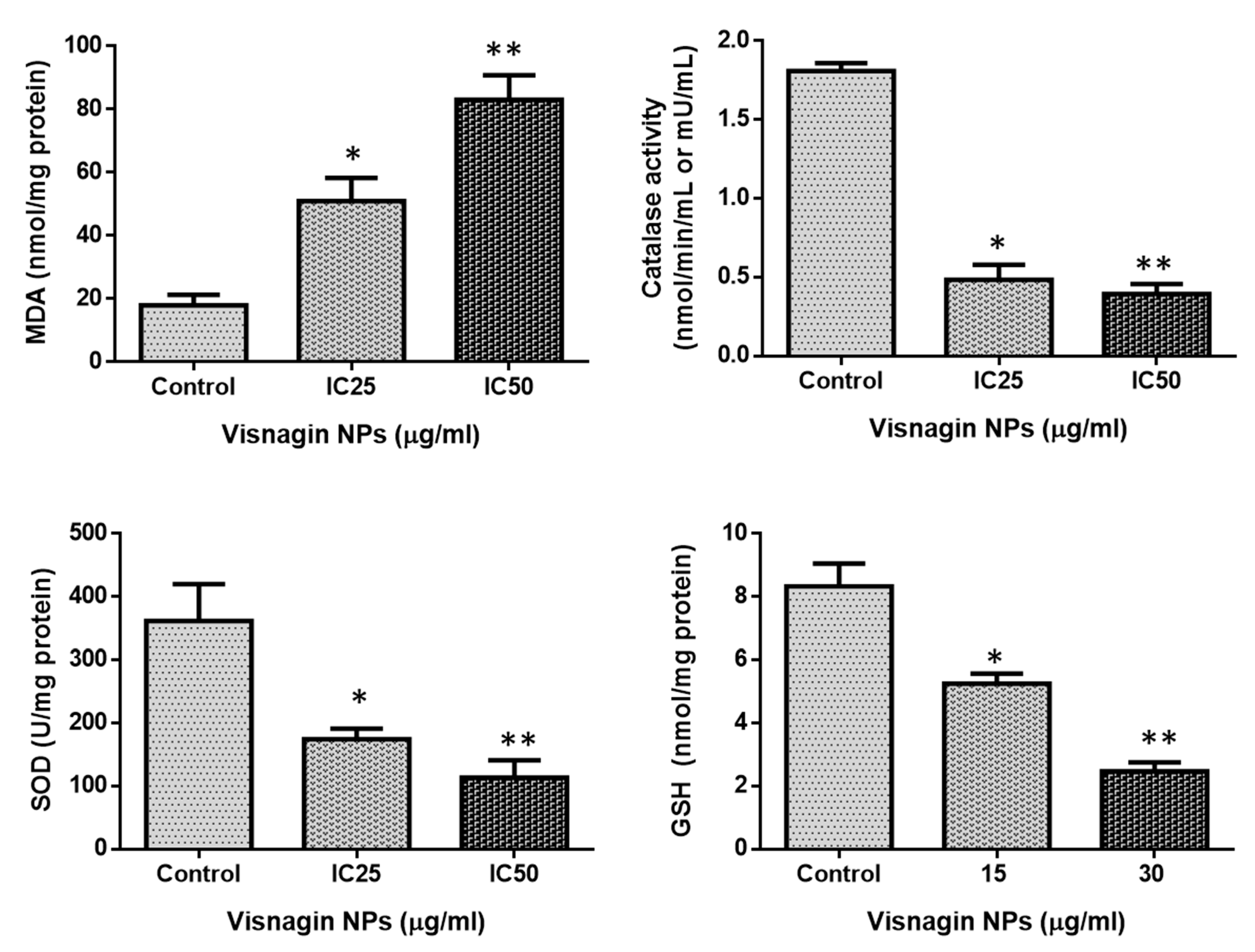
2.3. Effect of Albumin Visnagin NPs on Antioxidant Enzymes in MDA-MB-468 Cells
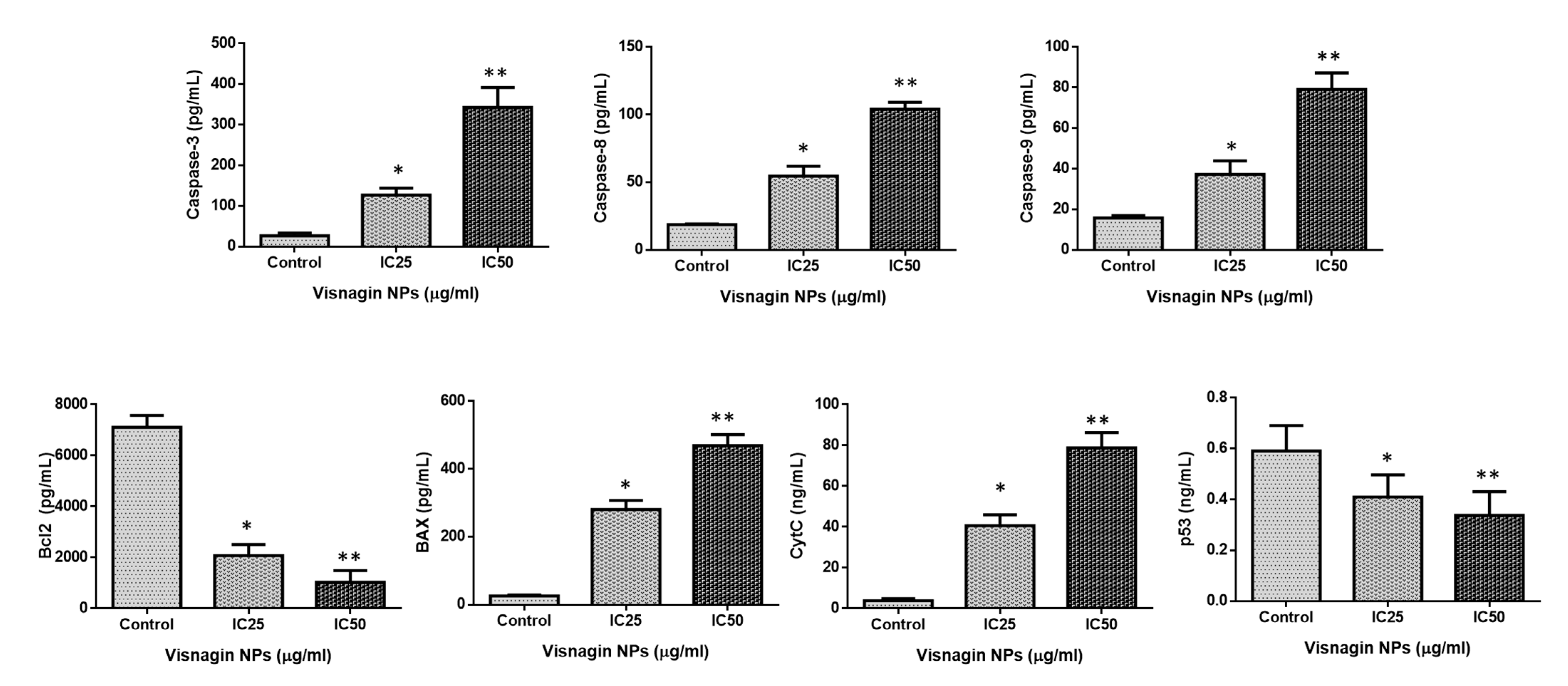
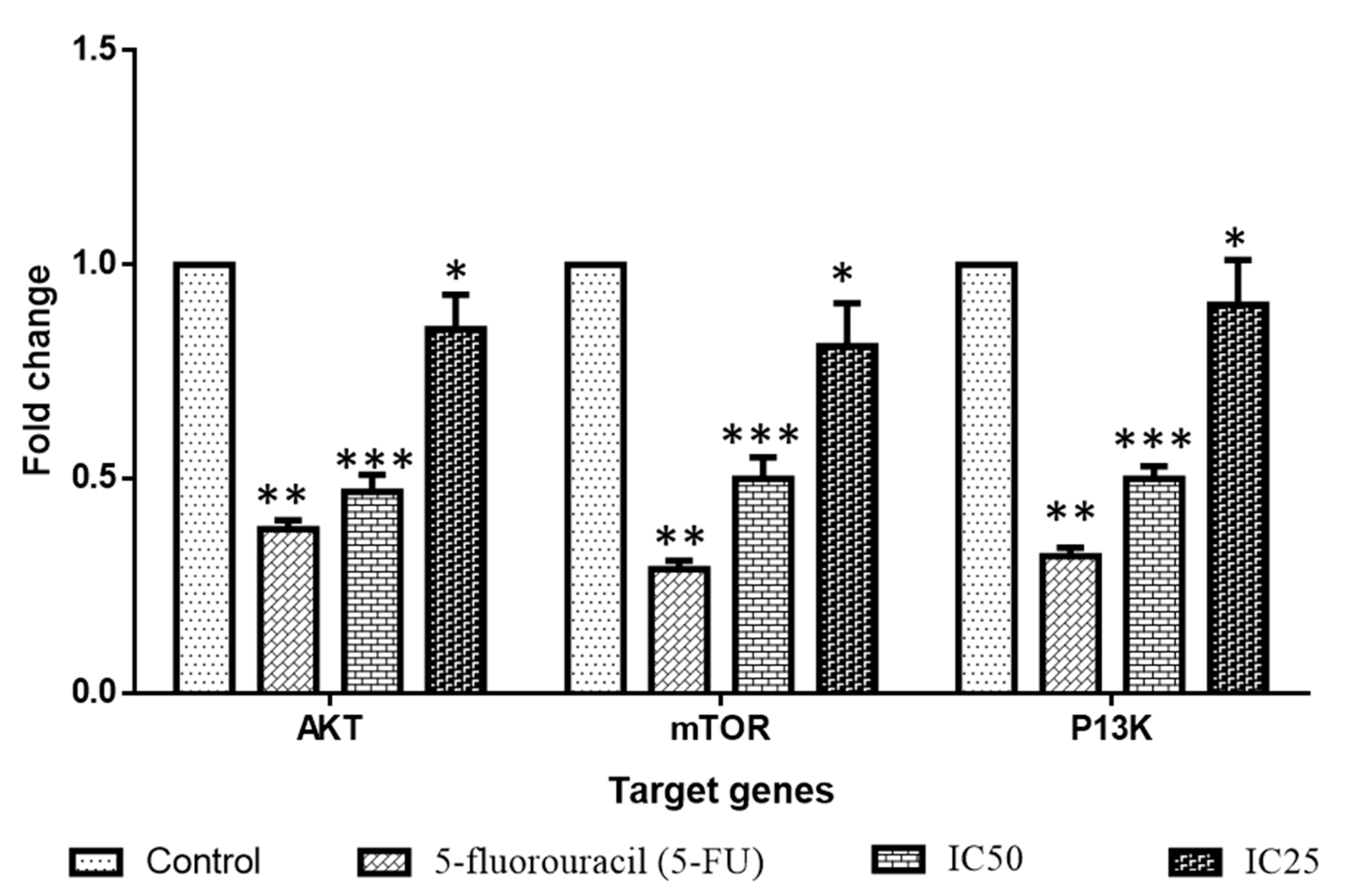
2.4. Effect of Albumin Visnagin NPs on Expression of Pro and Anti-Apoptotic Proteins in MDA-MB-468 Cells
3. Materials and Methods
3.1. Chemicals
3.2. Synthesis of Albumin Visnagin NPs
3.3. Characterization Analysis of Albumin Visnagin NPs
3.4. Cell Culture Chemicals
3.5. Cell Lines
3.6. Cell Viability Assay
3.7. Determination of Cell Apoptosis by AO/EtBr Dual Staining
3.8. Cell Cycle Analysis by PI Staining
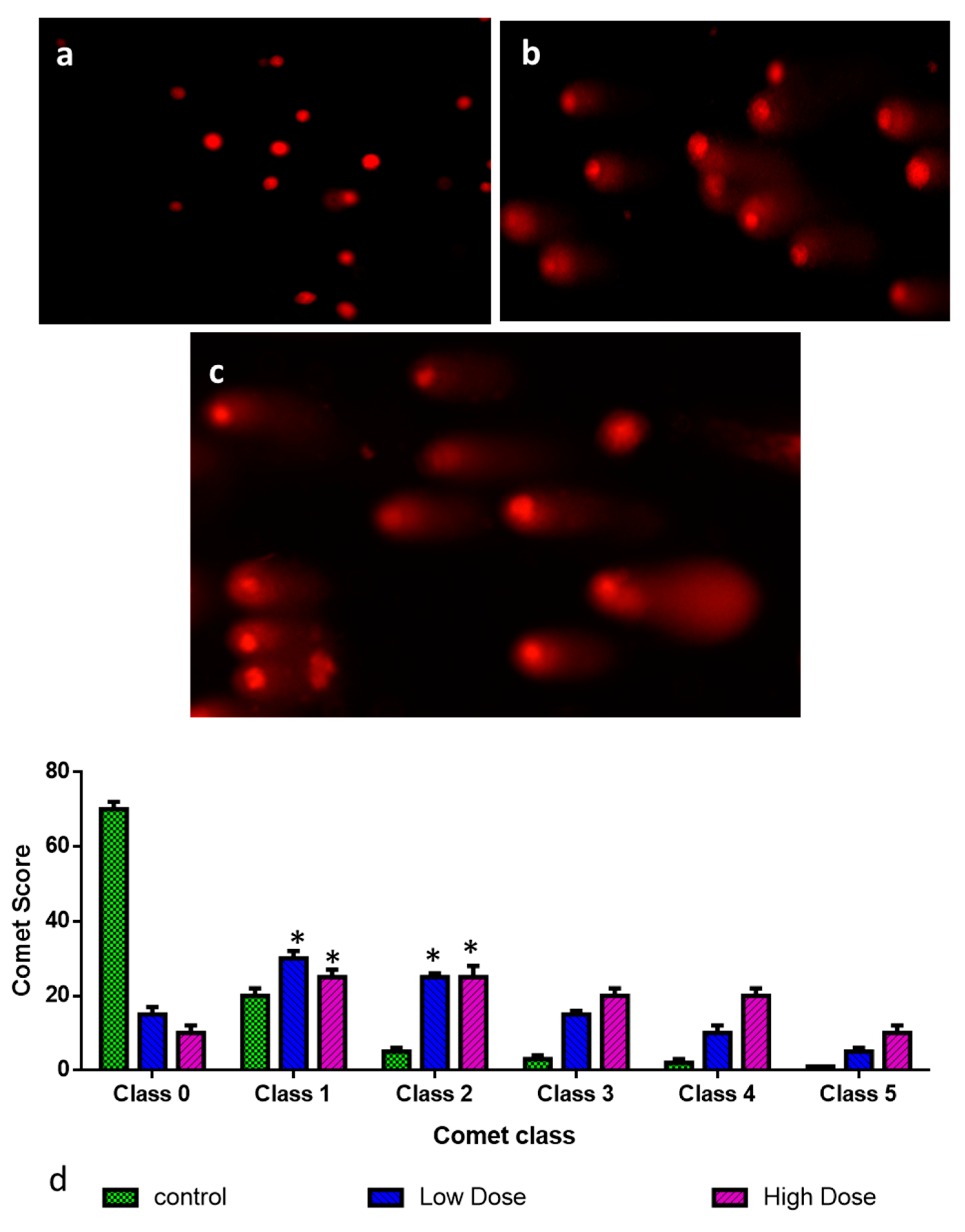
3.9. Assessment of DNA Damage by Comet Assay
3.10. Determination of Cell Apoptosis by Annexin V-FITC/PI Staining
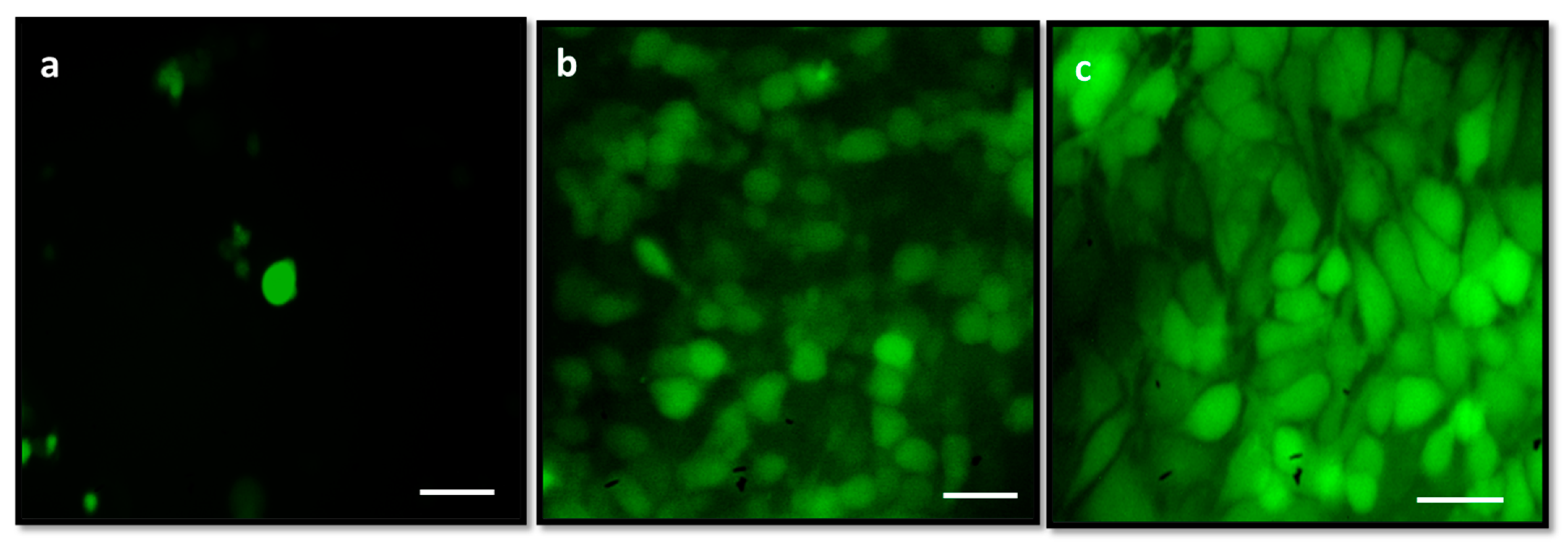
3.11. Analysis of Reactive Oxygen Species (ROS) by DCFH-DA Staining
3.12. Analysis of Oxidative Stress Parameters
3.13. Measurement of Caspase-3, -8, -9, Cyt-C, p53, Bax, and Bcl-2 by ELISA
3.14. Quantitative Real-Time PCR Analysis
3.15. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Han, Y.; Fan, S.; Qin, T.; Yang, J.; Sun, Y.; Lu, Y.; Mao, J.; Li, L. Role of autophagy in breast cancer and breast cancer stem cells (Review). Int. J. Oncol. 2018, 52, 1057–1070. [Google Scholar] [CrossRef] [PubMed]
- Akram, M.; Iqbal, M.; Daniyal, M.; Khan, A.U. Awareness and current knowledge of breast cancer. Biol. Res. 2017, 50, 33. [Google Scholar] [CrossRef]
- Silvestri, M.; Cristaudo, A.; Morrone, A.; Messina, C.; Bennardo, L.; Nisticò, S.P.; Mariano, M.; Cameli, N. Emerging skin toxicities in patients with breast cancer treated with new cyclin-dependent kinase 4/6 inhibitors: A systematic review. Drug Saf. 2021, 44, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Danhier, F. To exploit the tumor microenvironment: Since the EPR effect fails in the clinic, what is the future of nanomedicine? J. Control. Release 2016, 244, 108–121. [Google Scholar] [CrossRef]
- Indira, K.; Mudali, U.K.; Nishimura, T.; Rajendran, N. A review on TiO2 nanotubes: Influence of anodization parameters, formation mechanism, properties, corrosion behavior, and biomedical applications. J. Bio-Tribo-Corros. 2015, 1, 28. [Google Scholar] [CrossRef]
- Hu, C.-M.J.; Fang, R.H.; Luk, B.T.; Zhang, L. Nanoparticle-detained toxins for safe and effective vaccination. Nat. Nanotechnol. 2013, 8, 933–938. [Google Scholar] [CrossRef]
- An, F.-F.; Zhang, X.-H. Strategies for Preparing Albumin-based Nanoparticles for Multifunctional Bioimaging and Drug Delivery. Theranostics 2017, 7, 3667. [Google Scholar] [CrossRef] [PubMed]
- Spada, A.; Emami, J.; Tuszynski, J.A.; Lavasanifar, A. The Uniqueness of Albumin as a Carrier in Nanodrug Delivery. Mol. Pharm. 2021, 18, 1862–1894. [Google Scholar] [CrossRef]
- Hoogenboezem, E.N.; Duvall, C.L. Harnessing albumin as a carrier for cancer therapies. Adv. Drug Deliv. Rev. 2018, 130, 73–89. [Google Scholar] [CrossRef]
- Larsen, M.T.; Kuhlmann, M.; Hvam, M.L.; Howard, K.A. Albumin-based drug delivery: Harnessing nature to cure disease. Mol. Cell. Ther. 2016, 4, 3. [Google Scholar] [CrossRef]
- Prajapati, R.; Garcia-Garrido, E.; Somoza, A. Albumin-Based Nanoparticles for the Delivery of Doxorubicin in Breast Cancer. Cancers 2021, 13, 3011. [Google Scholar] [CrossRef] [PubMed]
- Khalil, N.; Bishr, M.; Desouky, S.; Salama, O. Ammi visnaga L., a Potential Medicinal Plant: A Review. Molecules 2020, 25, 301. [Google Scholar] [CrossRef] [PubMed]
- Aydoğmuş-Öztürk, F.; Jahan, H.; Beyazit, N.; Günaydın, K.; Choudhary, M.I. The anticancer activity of visnagin, isolated from Ammi visnaga L., against the human malignant melanoma cell lines, HT 144. Mol. Biol. Rep. 2019, 46, 1709–1714. [Google Scholar] [CrossRef] [PubMed]
- El-Nakkady, S.S.; Roaiah, H.F.; El-Serwy, W.S.; Soliman, A.; El-Moez, S.A.; Abdel-Rahman, A.A.H. Antitumor and antimicrobial activities of some hetero aromatic benzofurans derived from naturally occurring visnagin. Acta Pol. Pharm. Drug Res. 2012, 69, 645–655. [Google Scholar]
- Abu-Hashem, A.A.; El-Shazly, M. Synthesis, reactions and biological activities of furochromones: A review. Eur. J. Med. Chem. 2015, 90, 633–665. [Google Scholar] [CrossRef]
- Khalil, N.; Bishr, M.; El-Degwy, M.; Abdelhady, M.; Amin, M.; Salama, O. Assessment of conventional solvent extraction vs. supercritical fluid extraction of khella (Ammi visnaga L.) furanochromones and their cytotoxicity. Molecules 2021, 26, 1290. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ni, Y. New insight into protein–nanomaterial interactions with UV-visible spectroscopy and chemometrics: Human serum albumin and silver nanoparticles. Analyst 2014, 139, 416–424. [Google Scholar] [CrossRef]
- Rani, K. Fourier Transform Infrared Spectroscopy (FTIR) spectral analysis of BSA nanoparticles (BSA NPs) and egg albumin nanoparticles (EA NPs). Res. J. Chem. Sci. 2016, 6, 29–36. [Google Scholar]
- Kudłacik-Kramarczyk, S.; Drabczyk, A.; Głąb, M.; Gajda, P.; Czopek, A.; Zagórska, A.; Jaromin, A.; Gubernator, J.; Makara, A.; Tyliszczak, B. The Development of the Innovative Synthesis Methodology of Albumin Nanoparticles Supported by Their Physicochemical, Cytotoxic and Hemolytic Evaluation. Materials 2021, 14, 4386. [Google Scholar] [CrossRef]
- Ramamurthy, N.; Kannan, S. Fourier transform infrared spectroscopic analysis of a plant (Calotropis gigantea Linn) from an industrial village, Cuddalore dt, Tamilnadu, India. Rom. J. Biophys. 2007, 17, 269–276. [Google Scholar]
- Solanki, R.; Patel, K.; Patel, S. Bovine serum albumin nanoparticles for the efficient delivery of berberine: Preparation, characterization and in vitro biological studies. Colloids Surf. A Physicochem. Eng. Asp. 2021, 608, 125501. [Google Scholar] [CrossRef]
- Beltagy, A.M.; Beltagy, D.M. Chemical composition of Ammi visnaga L. and new cytotoxic activity of its constituents khellin and visnagin. J. Pharm. Sci. Res. 2015, 7, 285. [Google Scholar]
- Hassan, H.A.; El-Kholy, W.M.; El-Sawi, M.R.F.; Galal, N.A.; Ramadan, M.F. Myrtle (Myrtus communis) leaf extract suppresses hepatotoxicity induced by monosodium glutamate and acrylamide through obstructing apoptosis, DNA fragmentation, and cell cycle arrest. Environ. Sci. Pollut. Res. 2020, 27, 23188–23198. [Google Scholar] [CrossRef]
- Qi, Q.; Wang, Q.; Wang, Z.; Gao, W.; Gong, X.; Wang, L. Visnagin inhibits cervical cancer cells proliferation through the induction of apoptosis and modulation of PI3K/AKT/mTOR and MAPK signaling pathway. Arab. J. Chem. 2022, 15, 103684. [Google Scholar] [CrossRef]
- Rieger, A.M.; Nelson, K.L.; Konowalchuk, J.D.; Barreda, D.R. Modified annexin V/propidium iodide apoptosis assay for accurate assessment of cell death. J. Vis. Exp. JoVE 2011, 50, e2597. [Google Scholar]
- Banerjee, M.; Chattopadhyay, S.; Choudhuri, T.; Bera, R.; Kumar, S.; Chakraborty, B.; Mukherjee, S.K. Cytotoxicity and cell cycle arrest induced by andrographolide lead to programmed cell death of MDA-MB-231 breast cancer cell line. J. Biomed. Sci. 2016, 23, 40. [Google Scholar] [CrossRef]
- Al-Dulimi, A.G.; Al-Saffar, A.Z.; Sulaiman, G.M.; Khalil, K.A.; Khashan, K.S.; Al-Shmgani, H.S.; Ahmed, E.M. Immobilization of l-asparaginase on gold nanoparticles for novel drug delivery approach as anti-cancer agent against human breast carcinoma cells. J. Mater. Res. Technol. 2020, 9, 15394–15411. [Google Scholar] [CrossRef]
- Kamaludin, R.; Froemming, G.A.; Ibahim, M.J.; Narimah, A.H.H. Cell Apoptotic Determination by Annexin V-FITC Assay in Apigenin and Rutin-Induced Cell Death in Breast Cancer Cells. Open Conf. Proc. J. 2013, 4, 205. [Google Scholar] [CrossRef]
- Khazaei, S.; Esa, N.M.; Ramachandran, V.; Hamid, R.A.; Pandurangan, A.K.; Etemad, A.; Ismail, P. In vitro antiproliferative and apoptosis inducing effect of Allium atroviolaceum bulb extract on breast, cervical, and liver cancer cells. Front. Pharmacol. 2017, 8, 5. [Google Scholar] [CrossRef]
- Liou, G.Y.; Storz, P. Reactive oxygen species in cancer. Free. Radic. Res. 2010, 44, 479–496. [Google Scholar] [CrossRef]
- Hasanzadeh, D.; Mahdavi, M.; Dehghan, G.; Charoudeh, H.N. Farnesiferol C induces cell cycle arrest and apoptosis mediated by oxidative stress in MCF-7 cell line. Toxicol. Rep. 2017, 4, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Sahu, A.; Varma, M.; Kachhawa, K. A prognostic study of MDA, SOD and catalase in breast Cancer patients. Int. J. Sci. Res. 2015, 4, 157–159. [Google Scholar]
- Parrish, A.B.; Freel, C.D.; Kornbluth, S. Cellular Mechanisms Controlling Caspase Activation and Function. Cold Spring Harb. Perspect. Biol. 2013, 5, a008672. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zong, W.-X. Cellular Apoptosis Assay of Breast Cancer. Breast Cancer Methods Mol. Biol. 2016, 1406, 139–149. [Google Scholar] [CrossRef]
- Porta, C.; Paglino, C.; Mosca, A. Targeting PI3K/Akt/mTOR Signaling in Cancer. Front. Oncol. 2014, 4, 64. [Google Scholar] [CrossRef]
- Tapia, O.; Riquelme, I.; Leal, P.; Sandoval, A.; Aedo, S.; Weber, H.; Letelier, P.; Bellolio, E.; Villaseca, M.; Garcia, P.; et al. The PI3K/AKT/mTOR pathway is activated in gastric cancer with potential prognostic and predictive significance. Virchows Arch. 2014, 465, 25–33. [Google Scholar] [CrossRef]
- Elderdery, A.Y.; Alzahrani, B.; Hamza, S.M.A.; Mostafa-Hedeab, G.; Mok, P.L.; Subbiah, S.K. Synthesis, Characterization, and Antiproliferative Effect of CuO-TiO2-Chitosan-Amygdalin Nanocomposites in Human Leukemic MOLT4 Cells. Bioinorg. Chem. Appl. 2022, 2022, 1473922. [Google Scholar] [CrossRef]
- Elderdery, A.Y.; Alzahrani, B.; Alabdulsalam, A.A.; Hamza, S.M.A.; Elkhalifa, A.M.E.; Alhamidi, A.H.; Alanazi, F.; Mohamedain, A.; Subbiah, S.K.; Mok, P.L. Structural, Optical, Antibacterial, and Anticancer Properties of Cerium Oxide Nanoparticles Prepared by Green Synthesis Using Morinda citrifolia Leaves Extract. Bioinorg. Chem. Appl. 2022, 2022, 6835625. [Google Scholar] [CrossRef]
- Elderdery, A.Y.; Alzahrani, B.; Hamza, S.M.A.; Mostafa-Hedeab, G.; Mok, P.L.; Subbiah, S.K. Synthesis of Zinc Oxide (ZnO)-Titanium Dioxide (TiO2)-Chitosan-Farnesol Nanocomposites and Assessment of Their Anticancer Potential in Human Leukemic MOLT-4 Cell Line. Bioinorg. Chem. Appl. 2022, 2022, 5949086. [Google Scholar] [CrossRef]
- Sarathbabu, S.; Marimuthu, S.K.; Ghatak, S.; Vidyalakshmi, S.; Gurusubramanian, G.; Ghosh, S.K.; Subramanian, S.; Zhang, W.; Kumar, N.S. Induction of Apoptosis by Pierisin-6 in HPV Positive HeLa and HepG2 Cancer Cells is Mediated by the Caspase-3 Dependent Mitochondrial Pathway. Anti-Cancer Agents Med. Chem. 2019, 19, 337–346. [Google Scholar] [CrossRef]
- Sarathbabu, S.; Sarma, R.J.; Lalhruaitluanga, H.; Velmurugan, D.; Subramanian, S.; Kumar, N.S. In vitro DNA binding activity and molecular docking reveals pierisin-5 as an anti-proliferative agent against gastric cancer. J. Biomol. Struct. Dyn. 2020, 38, 3474–3488. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Liu, Y.; Yang, C. Evaluating In Vitro DNA Damage Using Comet Assay. J. Vis. Exp. 2017, 128, e56450. [Google Scholar] [CrossRef]
- Collins, A.R. The Comet Assay for DNA Damage and Repair: Principles, Applications, and Limitations. Mol. Biotechnol. 2004, 26, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Xue, X. Detection of Total Reactive Oxygen Species in Adherent Cells by 2’,7’-Dichlorodihydrofluorescein Diacetate Staining. J. Vis. Exp. 2020, 160, e60682. [Google Scholar] [CrossRef]
- Hussain, S.A.; Venkatesh, T. YBX1/lncRNA SBF2-AS1 interaction regulates proliferation and tamoxifen sensitivity via PI3K/AKT/MTOR signaling in breast cancer cells. Mol. Biol. Rep. 2023, 50, 3413–3428. [Google Scholar] [CrossRef]

| Genes | Primers | Sequence |
|---|---|---|
| PI3K | Forward Reverse | 5′-AACACAGAAGACCAATACTC-3′ 5′-TTCGCCATCTACCACTAC-3′ |
| AKT | Forward Reverse | 5′-AGAAGCAGGAGGAGGAGGAG-3′ 5′-CCCAGCAGCTTCAGGTACTC-3′ |
| mTOR | Forward Reverse | 5′-AGGCCGCATTGTCTCTATCAA-3′ 5′-GCAGTAAATGCAGGTAGTCATCCA-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alsrhani, A.; Elderdery, A.Y.; Alzahrani, B.; Alzerwi, N.A.N.; Althobiti, M.M.; Rayzah, M.; Idrees, B.; Elkhalifa, A.M.E.; Subbiah, S.K.; Mok, P.L. Therapeutic Potential of Albumin Nanoparticles Encapsulated Visnagin in MDA-MB-468 Triple-Negative Breast Cancer Cells. Molecules 2023, 28, 3228. https://doi.org/10.3390/molecules28073228
Alsrhani A, Elderdery AY, Alzahrani B, Alzerwi NAN, Althobiti MM, Rayzah M, Idrees B, Elkhalifa AME, Subbiah SK, Mok PL. Therapeutic Potential of Albumin Nanoparticles Encapsulated Visnagin in MDA-MB-468 Triple-Negative Breast Cancer Cells. Molecules. 2023; 28(7):3228. https://doi.org/10.3390/molecules28073228
Chicago/Turabian StyleAlsrhani, Abdullah, Abozer Y. Elderdery, Badr Alzahrani, Nasser A. N. Alzerwi, Maryam Musleh Althobiti, Musaed Rayzah, Bandar Idrees, Ahmed M. E. Elkhalifa, Suresh K. Subbiah, and Pooi Ling Mok. 2023. "Therapeutic Potential of Albumin Nanoparticles Encapsulated Visnagin in MDA-MB-468 Triple-Negative Breast Cancer Cells" Molecules 28, no. 7: 3228. https://doi.org/10.3390/molecules28073228
APA StyleAlsrhani, A., Elderdery, A. Y., Alzahrani, B., Alzerwi, N. A. N., Althobiti, M. M., Rayzah, M., Idrees, B., Elkhalifa, A. M. E., Subbiah, S. K., & Mok, P. L. (2023). Therapeutic Potential of Albumin Nanoparticles Encapsulated Visnagin in MDA-MB-468 Triple-Negative Breast Cancer Cells. Molecules, 28(7), 3228. https://doi.org/10.3390/molecules28073228