Abstract
Quinazolines are a privileged class of nitrogen-containing heterocycles, widely present in a variety of natural products and synthetic chemicals with a broad spectrum of biological and medicinal activities. Owing to their pharmaceutical applications and promising biological value, a variety of synthetic methodologies have been reported for these scaffolds. From the perspective of green and sustainable chemistry, transition-metal-free synthesis provides an alternative method for accessing several biologically active heterocycles. In this review, we summarize the recent progress achieved in the transition-metal-free synthesis of quinazolines and we cover the literature from 2015 to 2022. This aspect is present alongside the advantages, limitations, mechanistic rationalization, and future perspectives associated with the synthetic methodologies.
1. Introduction
Nitrogen heterocycles are found in a large number of biologically active natural products as well as synthetic compounds [1,2]. Amongst them, quinazolines are an attractive class of heterocycles and serve as building blocks in making advanced intermediates and active pharmaceutical ingredients (APIs) for the pharmaceutical industry [3,4]. They have been found to possess a diverse range of pharmacological properties including anti-inflammatory [5], anticancer [6], anticonvulsant [7], anti-hypertensive [8], anti-HIV [9], anti-tubercular [10], antibacterial [11], antifungal [12], antiviral [13], antimalarial [14], anti-plasmodial [15], α-adrenergic blocker [16], antioxidant [17], and antitumor activities [18]. Additionally, quinazoline derivatives have been applied as photochemotherapeutic agents [19], JAK2, DNA-gyrase, PDE5, and EGFR tyrosine kinase inhibitors [20,21,22,23], and CB2 receptor agonists [24]. Quinazolines have been used in regimens for a wide range of physical ailments such as hematological cancer, liver metastasis (Cediranib), breast cancer (Afatinib), high blood sugar and hypertension (Prazosin), benign prostatic hyperplasia (Afluzosin), and prostate enlargement and high blood pressure (Doxazosin) [25,26,27,28]. These fused N-heterocyclic cores are present in lung cancer drugs and therapeutic agents for pneumonia and post-traumatic stress or anxiety disorders [29,30,31,32]. Some quinazoline-based drugs are illustrated in Figure 1.
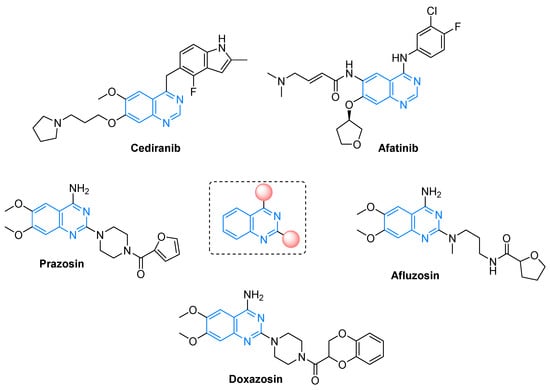
Figure 1.
Structure of quinazoline-based drugs.
Moreover, quinazolines are employed as fragments in the functionalized materials [33] and also serve as useful intermediates in fine chemicals [34]. Owing to this biologically active legacy, quinazolines have emerged as synthetic targets in the last few decades. The first example of the synthesis of quinazolines from cyanogen and anthranilic acid was reported by Griess in 1869 [35]. Consequently, several protocols have been developed since then [36,37,38,39]. Recently, transition-metal-free reactions have received considerable attention and are recognized as an indispensable tool in organic synthesis, as they are not only cost-effective but also environmentally friendly. The metal-free methodology compliments metal-mediated reactions by eliminating expensive metal complexes and toxic metal contamination [40]. There are two classical strategies for the construction of the quinazoline skeleton under metal-free conditions: (i) substituted quinazolines can be prepared from easily available ortho-functionalized anilines such as 2-aminobenzylamines and 2-aminobenzoketones; (ii) using N-arylamidines as a substrate, quinazoline derivatives have been developed, in which pre-activation at the ortho-position of the N-aryl ring is not necessary (Figure 2).
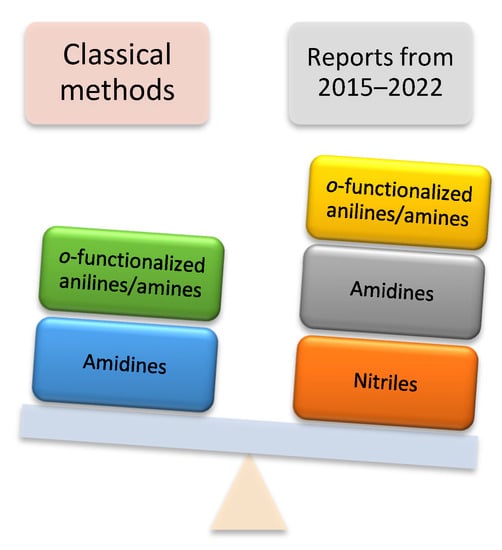
Figure 2.
Substrates scope in classical methods vs. reports between 2015–2022.
These methods mainly involve: (A) condensation of 2-aminobenzylamines with aldehydes followed by oxidation with oxidants such as MnO2, DDQ, and sodium hypochlorite [41,42,43]; a reaction between 2-aminobenzylamines with imines in the presence of K2S2O8 as oxidant [44], (B) oxidative cyclization of 2-aminobenzophenones with benzylamines [45]; two-component oxidative decarboxylative amination reaction of 2-aminobenzophenones with α-amino acid catalyzed by iodine/TBHP [46]; one-pot three-component reaction of 2-aminobenzophenones, aldehyde, and NH4OAc in mixtures of maltose-dimethylurea (DMU)-NH4Cl [47]; KI-catalyzed three-component reaction of 2-aminobenzophenones, methylarenes, and NH4OAc [48]; NS/TBHP-system-catalyzed reaction with NH4OAc as a nitrogen source and dimethylacetamide (DMA) as a one carbon synthon [49], (C) intramolecular oxidative cyclization of N-arylated amidines [50], and (D) microwave-promoted synthesis of quinazolines from N-arylamidines and aldehydes [51] (Figure 3). Despite the efficacy of these protocols, they also suffer from inevitable disadvantages such as the use of excessive quantities of dangerous peroxide reagents, toxic solvents, toxic oxidants such as NaOCl, longer reaction times, elevated temperatures, and requiring strong and hazardous oxidizing agents. Consequently, rapid growth has been witnessed in the last few years regarding the metal-free synthesis of quinazolines, and the methods are compiled in this review. Several reviews have been presented on the synthesis and biological activity of quinazolines [52,53]. This review primarily focuses on the recent progress achieved in the transition-metal-free synthesis of quinazolines and covers the literature from 2015 to 2022. In addition, highlighting the advances made so far, we also point out the limitations associated with them. Furthermore, the article is organized based on the substrates used in a chronological order.
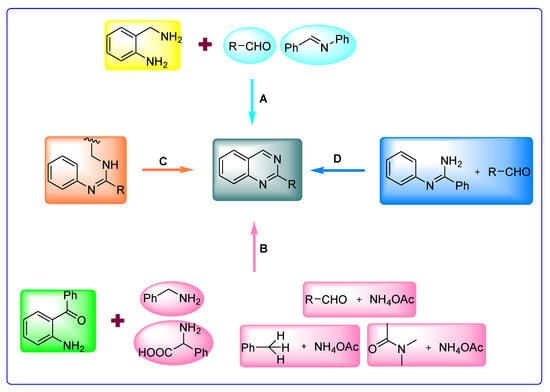
Figure 3.
Classical strategies for the synthesis of quinazolines.
2. Synthesis of Quinazolines
2.1. From O-Functionalized Anilines/Amines
In the recent years, transition-metal-free intra- or intermolecular oxidative C(sp3)-H amination has upsurged as a significant approach for the direct C-N bond formation [54]. Moreover, cost-effectiveness, step economy, and less toxicity are the benefits associated with this strategy. Liu et al. (2015) reported a rarely explored carbon source viz., tertiary amines for the C-N bond formation in quinazolines via an iodine-catalyzed C(sp3)-H amination [55]. The tandem reaction of 2-aminobenzophenone 1 and N-methylamines 2 (carbon source) in the presence of iodine as a catalyst, ammonium chloride as the nitrogen source, utilizing molecular oxygen as an oxidant in DMSO at 120 °C for 12 h furnished quinazolines 3 in 20–98% yields (Scheme 1). Under the optimized conditions, aromatic amines displayed greater reactivity than aliphatic amines. The major advantages of this approach involve: peroxide-free, broad substrate scope, operationally simple, oxygen as the green oxidant, metal-free, and easy scalability up to 10 mmol with 90% yield.
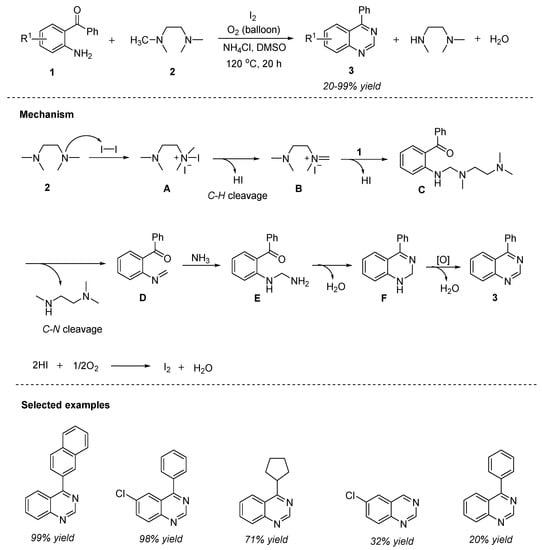
Scheme 1.
Iodine-catalyzed aerobic oxidative synthesis of quinazolines.
The plausible reaction mechanism depicted in Scheme 1 suggested that the initial reaction of tetramethylethylenediamine 2 (TMEDA) with iodine generated an aminium iodide A, which then gives an iminium iodide B by the removal of molecular hydrogen iodide. Nucleophilic addition of 1 to B forms an intermediate C by the simultaneous removal of another HI. Later, intermediate E was formed by the nucleophilic addition of ammonia to D. In the last step, E undergoes a tandem condensation–oxidation to provide the desired product 3. Simultaneously, the I2/I catalytic cycle is completed in the form of iodine regeneration via the oxidation of in situ generated HI by oxygen.
Likewise, Bhanage et al. (2016) disclosed a green and sustainable method for the construction of 2-arylquinazolines 6 from 2-aminobenzylamine 4 and N-mono substituted benzylamines 5 in the presence of molecular iodine at 80 °C for 5 h under O2 balloon (Scheme 2) [56]. The optimized reaction conditions worked well for a variety of substrates including electron-donating and electron-withdrawing groups and provided quinazolines 6 in 56–90% yields. Additive-free, solvent-free, transition-metal-free, utilizing oxygen as the green oxidant, shorter reaction times, and simple operation conditions are the major benefits of this approach.
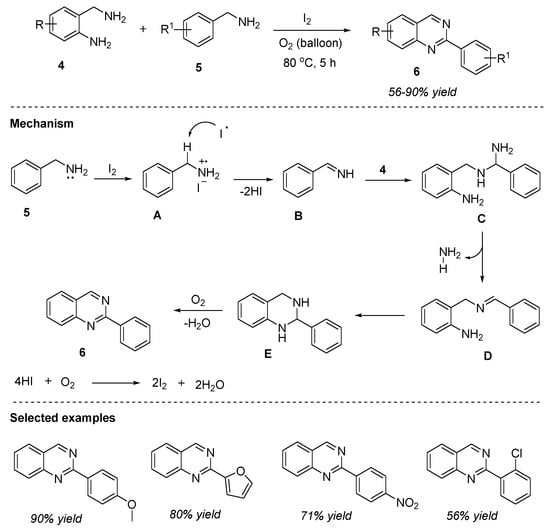
Scheme 2.
Iodine-catalyzed oxidation for the synthesis of quinazolines.
An insight into the reaction mechanism as shown in Scheme 2 suggested that the generation of the radical intermediate initiated the reaction, followed by the formation of imine B. Later, imine B reacted with 2-aminobenzylamine 4 to form intermediates C, D, and E successively. In the last step, E undergoes dehydrogenative aromatization to afford the desired product 6. Simultaneously, iodine is regenerated by the reaction of HI with oxygen.
In the same year, Sen et al. (2016) developed the facile synthesis of quinazolines through the o-iodoxybenzoicacid (IBX)-mediated reaction of 2-aminobenzylamine 4 and aldehydes 7 in acetonitrile at room temperature for 6 h (Scheme 3) [57]. Various aryl, heteroaryl, and alkyl aldehydes 7 could be transformed to the corresponding 2-substitued quinazolines 8 in 55–94% yields.
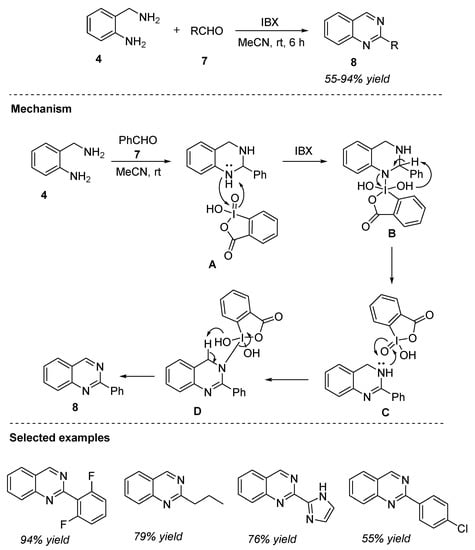
Scheme 3.
IBX-mediated reaction for the synthesis of quinazolines.
The plausible mechanism proposed by the authors is presented in Scheme 3. The initial attack of nitrogen in intermediate A on the iodine center of IBX, followed by reduction generated the dihydroquinazoline C. Similarly, attack by the nitrogen in dihydroquinazoline C on IBX afforded quinazoline 8 via an intermediate D.
Hypervalent iodine reagents provide a metal-free reaction condition which is essential in the field of medicinal chemistry for the synthesis of pharmacologically important heterocycles. One such example is iodobenzene diacetate (PIDA). Less toxic effect and easy availability made the oxidant be used widely in chemical transformations [58]. Das et al. (2017) disclosed an interesting approach for the synthesis of 2-substitued quinazolines 13 by the reaction of 2-aminobenzylamine 4 with a variety of aldehydes 9, nitriles 10, aliphatic/aryl amines 11, and aliphatic/aryl alcohols 12 in the presence of PIDA as the key reagent, dichloromethane as the solvent, at room temperature for 0.7–1.3 h (Scheme 4) [59]. In the case of alcohols 12 and nitriles 10, catalytic amounts of molecular iodine and zinc chloride, respectively, were employed to facilitate the reaction. A diverse range of aliphatic, aryl, or heteroaryl groups afforded 2-substitued quinazolines 15 in 76–98% yields. The major advantages of this strategy included: (a) readily available starting materials, (b) broad substrate scope, (c) employing a common oxidant for different substrates, (d) mild reaction conditions, (e) absence or presence of additives, and (f) product-selective reaction with no side products.
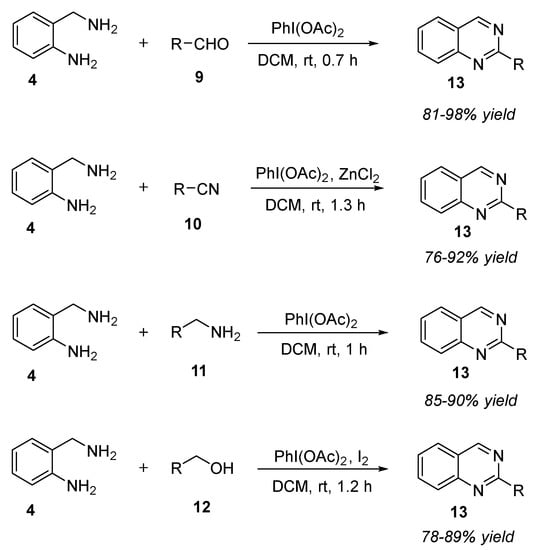
Scheme 4.
PIDA-mediated reaction for the synthesis of quinazolines.
The proposed mechanism as depicted in Scheme 5 suggested that the reaction between 2-aminobenzylamine 4 and aldehyde 9 resulted in the generation of intermediate A. A upon reaction with PIDA led to the formation of intermediate B. Elimination followed by the attack with another molecule of PIDA afforded the desired product via intermediates C and D. Oxidation of arylamine 11 and arylalcohol 12 derivatives in the presence of PIDA and PIDA/I2, respectively, generated the corresponding aldehydes 9. Aldehydes 9 then underwent a similar course of mechanism to furnish quinazoline 13. In the case of nitriles 10, coordination of ZnCl2 with the cyano group [60] provided the intermediate E, which then liberates ammonia in the presence of ZnCl2 to form intermediate C. Quinazoline 13 is formed in the subsequent steps by following a similar mechanism as detailed earlier.
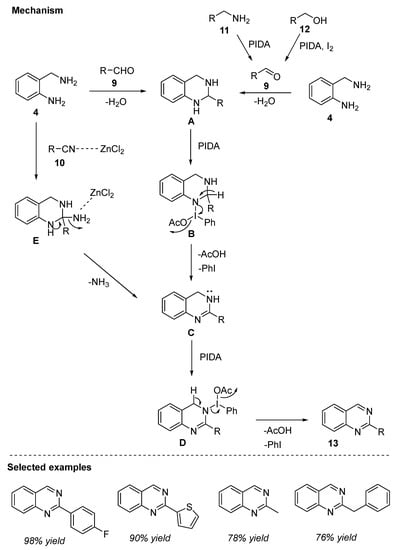
Scheme 5.
PIDA-mediated reaction mechanism for the synthesis of quinazolines.
Molecular iodine, a mild Lewis acidic reagent, is known to be an established catalyst in organic reactions. It performs several synthetic transformations such as Michael addition, iodination, oxidation, Prins-related reactions, protection–deprotection of functional groups, and functionalization of alcohols [61]. In spite of the recently reported transition-metal-free protocols for the functionalization of benzylic sp3 C-H bonds, there remains a need to develop new strategies for the synthesis of 2-arylquinazolines by overcoming the limitations of previous reports. In this connection, Bhanage et al. (2018) documented a green and sustainable approach for the synthesis of quinazolines via benzylic sp3 C-H bond amination [62]. The reaction of 2-aminobenzophenones 1 with aryl amines 5 catalyzed by molecular iodine under O2 atmosphere at 130 °C for 3–8 h afforded 2-arylquinazolines 15 in 68–92% yields (Scheme 6). Furthermore, the reaction of 2-aminobenzyl alcohol 14 and benzylamine 5 in the presence of molecular iodine under O2 at 130 °C for 15 h delivered 2-arylquinazolines 15 in 49–68% yields (Scheme 6). Broad substrate scope, use of oxygen as the green oxidant, additive- and solvent-free conditions, and lack of aqueous workup are the major benefits of this protocol. Aliphatic and unsaturated amines failed to form the desired product, and this remains the only limitation associated with this strategy.
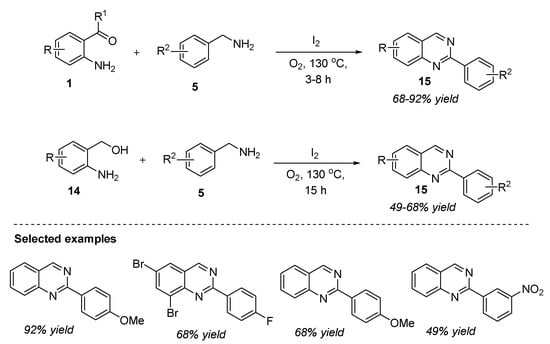
Scheme 6.
Molecular-iodine-catalyzed synthesis of quinazolines.
In the same year, Das et al. (2018) developed an oxidant-, base-, and metal-free approach for the synthesis of quinazolines by the reaction of 2-aminobenzylamine 4 with oxalic acid dihydrate or malonic acid in 1,4-dioxane at 120 °C for 6 h via an in situ condensation and oxidation process [63]. Quinazoline 16 and 2-methylquinazoline 17 were obtained in 75–85% yields (Scheme 7). Utilizing oxalic acid dihydrate as the in situ C1 source, easy handling, atom economy, and greener reaction conditions are the merits of this protocol.
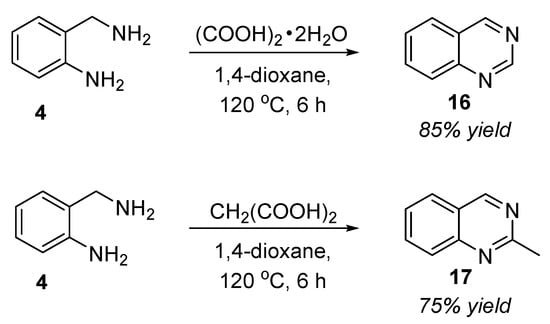
Scheme 7.
Oxalic-acid-dihydrate/Malonic-acid-mediated reaction for the synthesis of quinazolines.
In contrast to the above-mentioned strategy, Cho et al. (2018) reported a sustainable methodology for the synthesis of 2-arylquinazolines 19 from 2-aminobenzylamines 4 and α,α,α-trihalotoluenes 18 in the presence of sodium hydroxide and molecular oxygen in water at 100 °C for 16–24 h (Scheme 8) [64]. A variety of electron-donating and electron-withdrawing groups on the substrates delivered quinazolines 19 in 43–81% yields under optimized reaction conditions. The important uses of this synthesis method include: readily available substrates, green and renewable solvent, isolation of products by simple crystallization, formation of benign by-product (NaCl or NaBr), chemical oxidant or additive-free conditions, and avoiding huge solvent-consuming work-up procedures.
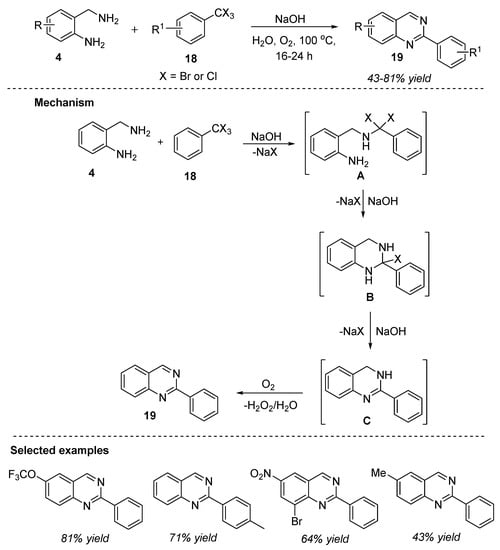
Scheme 8.
Base-promoted reaction for the synthesis of quinazolines.
The reaction mechanism outlined in Scheme 8 suggest that the initial base-promoted intermolecular substitution of α,α,α-trihalotoluene 18 by 2-aminobenzylamine 4 led to the generation of intermediate A. Later, base-mediated intramolecular substitution followed by elimination formed the intermediate C, which upon oxidation afforded the desired product, quinazoline 19.
Palakodety et al. (2019) reported the metal-free protocol for the construction of N,4-disubstituted quinazolin-2-amine 21 from 2-aminobenzophenone 1, phenylisothiocyanate 33, and ammonium acetate as the nitrogen source in the presence of iodine in dimethylsulfoxide at 50 °C for 2–3 h (Scheme 9) [65]. Under the optimized conditions, a wide range of electron-donating and electron-withdrawing groups present on isothiocyanate 20 and 2-aminobenzophenone 1 offered the corresponding N,4-disubstitued quinazolin-2-amines 21 in 87–95% yields. Moreover, meta and ortho-substituted 2-aminoaryl ketones 1 did not tolerate the established conditions due to the steric effect and this remains a drawback to this strategy. Readily available inexpensive substrates, operational simplicity, shorter reaction times, and excellent yields are the key benefits of this methodology.
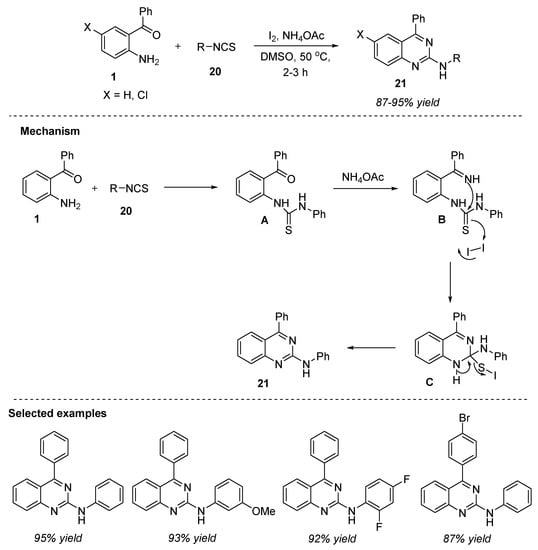
Scheme 9.
I2-catalyzed oxidative synthesis of quinazolines.
The plausible mechanism is shown in Scheme 9. Initially, 2-aminobenzophenone 1 undergoes condensation with phenylisothiocyanate 20 to afford the thiourea intermediate A. Then, A reacts with ammonium acetate to form intermediate B, which upon intramolecular attack of the imine NH group onto the thiocarbonyl carbon with concomitant trapping of S anion resulted in the generation of cyclized product C. Aromatization of C delivered the desired product 21 with subsequent removal of S and hydrogen iodide.
Hydrogen peroxide has emerged as an ideal oxidant for liquid-phase green chemistry owing to its non-toxic, atom efficient, and environmentally benign properties [66]. Furthermore, hydrogen peroxide oxidant usually does not require temperatures over 100 °C to carry out oxidation transformations. Phan et al. (2020) presented hydrogen-peroxide-mediated one-pot three-component reaction of 2-aminoaryl ketones 1, aldehydes 22, and ammonium acetate as the nitrogen source for the efficient synthesis of 2,4-substituted quinazolines 23. The transformation proceeded readily in the presence of hydrogen peroxide as an oxidant and DMSO as the solvent under air at 60 °C for 6 h (Scheme 10) [67]. The highlighted benefits of this approach are: readily available and atom-efficient H2O2, mild reaction conditions, transition-metal-free conditions, and broad substrate scope. Under the optimized conditions, various 2-aminobenzophenones 1 coupled with aromatic, heteroaromatic, and aliphatic aldehydes 22 and delivered the corresponding products 23 in 61–89% yields. This is the first report utilizing hydrogen peroxide as the green oxidant for the synthesis of 2,4-substituted quinazolines 23.
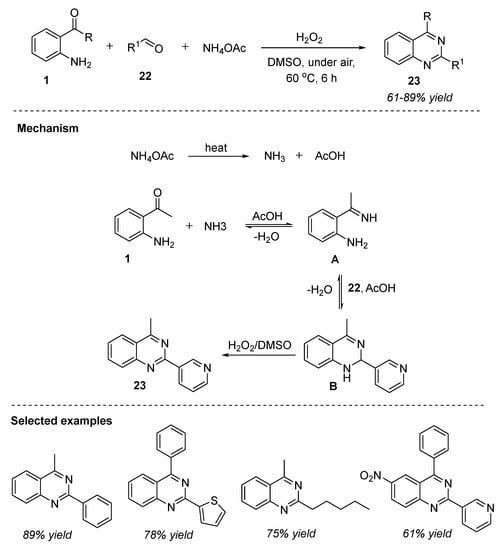
Scheme 10.
H2O2-mediated reaction for the synthesis of quinazolines.
The proposed pathway for the transformation was shown in Scheme 10. In the first step, ammonium acetate was decomposed to ammonia and acetic acid upon heating. Later, condensation between 2-aminoacetophenone 1 with NH3 generated an imine intermediate A. Subsequent condensation between the amino group of A with aldehyde 22 followed by nucleophilic cyclization provided the dihydroquinazoline intermediate B. In the presence of H2O2 and DMSO, intermediate B was rapidly oxidized to furnish the final product, quinazoline 23.
Hexamethyldisilazane (HMDS) is a commercially available stable reagent employed for the silylation reactions to increase the silylating power by utilizing several catalysts [68]. Wang et al. (2020) developed an efficient and mild synthetic route for the facile synthesis of substituted quinazolines. The reaction between 2-aminobenzophenones 1 and benzaldehydes 22 in the presence of catalytic trimethylsilyl trifluoromethanesulfonate (TMSOTf) and HMDS in which gaseous ammonia was formed in situ under neat, and microwave irradiation at 150 °C for 0.5 h, delivered substituted quinazolines 24 in 81–94% yields (Scheme 11) [69]. An array of aromatic benzaldehydes 22 substituted with electron-donating and electron-withdrawing groups and heterocyclic carbaldehydes were well tolerated to afford the corresponding quinazolines 24. Moreover, the structures of quinazolines 24 were also confirmed by single-crystal X-ray crystallography. Absence of unnecessary waste due to conventional reaction conditions viz., microwave irradiation, shorter reaction times, gram-scale synthesis with 90–91% yields, broad substrate scope, and good to excellent yields are the merits of this protocol.
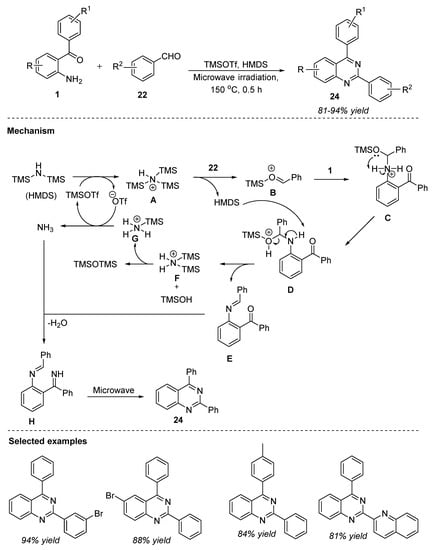
Scheme 11.
TMSOTf-HMDS-catalyzed synthesis of quinazolines.
The plausible mechanism, as shown in Scheme 11, indicated that in the initial step, the TMSOTf and HMDS system could form a complex A with the liberation of the triflate anion. The complex A reacts with benzaldehyde 22 to generate an oxonium intermediate B. Intermediate C was then formed by the intermolecular addition of 2-aminobenzophenone 1 with B. Deprotonation of intermediate D mediated by HMDS resulted in intermediate E. Simultaneous reaction between complex F and TMSOH led to the silylated product TMSOTMS ether and complex G, which upon further transformation, liberated gaseous ammonia by triflate anion. At the same time, TMSOTf was regenerated. Later, intermediate E underwent imination followed by oxidation with in situ ammonia, producing intermediate H. Finally, microwave irradiation of H furnished the desired product quinazoline 24.
Among the biological cascade systems, chemoenzymatic synthesis methods have drawn considerable attention by the integration of biocatalysis with the versatile reactivity of chemocatalysis [70]. Noteworthy advantages of the chemoenzymatic cascade process include: cost-effectiveness, simple operation conditions, improved selectivity, omitting isolation and purification of intermediates, lower waste generation, and higher reaction yields. In this connection, Zhu et al. (2022) reported the first example of the chemoenzymatic synthesis of quinazolines [71]. The condensation reaction of 2-aminobenzylamine 4 and benzyl alcohol 25 in the presence of the HLADH-SBFA catalytic system and maintaining the pH 9.0 by CHES buffer, under air at 30 °C for 24 h, furnished 2-phenylquinazoline 26 in 73% yields (Scheme 12). In this reaction, synthetic bridged flavin analog (SBFA) performed regeneration of both NAD(P)+ and the cofactor during the alcohol dehydrogenase (ADH)-catalyzed process of alcohol to aldehyde. HLADH was used as a biocatalyst and SBFA served as a bifunctional chemocatalyst. Mild reaction conditions, employing bifunctional organocatalyst SBFA, and utilizing O2 as an external oxidant, serve as a green and sustainable alternatives in this strategy.
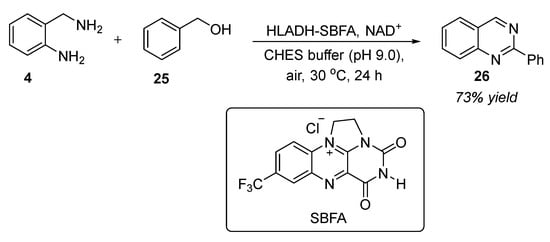
Scheme 12.
HLADH-SBFA-catalyzed synthesis of quinazolines.
Another interesting strategy was put forth by Ogawa et al. (2022) for the synthesis of 2-substituted quinazolines by the 4,6-dihydoxysalicylic-acid-catalyzed oxidative condensation. The organocatalytic oxidation of 2-aminobenzylamines 4 and benzylamines 5 employing 4,6-dihydoxysalicylic acid as the catalyst and a catalytic amount of Lewis acid, BF3.Et2O in DMSO under O2 atmosphere at 90 °C for 48 h, afforded quinazoline derivatives 27 in 17–81% yields with an excellent environmental (E)-factor (Scheme 13) [72]. Broad substrate scope, gram-scale synthesis, clean reaction system, and easy isolation are the noteworthy uses of this approach.
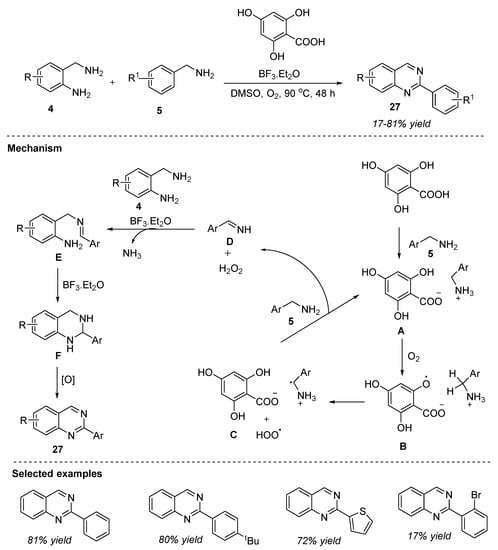
Scheme 13.
4,6-Dihydoxysalicylic-acid-catalyzed synthesis of quinazolines.
The organocatalytic oxidative reaction mechanism was depicted in Scheme 13. In the first step, benzylamine 5 reacted with 4,6-dihydrosalicyclic acid to form salt A which generated aryloxy radical B and HOO• via hydrogen abstraction by O2. In the next step, intramolecular hydrogen abstraction of the benzyl group led to the formation of radical cation C [73], which then gave imine D under the action of HOO•. Imine D then underwent an amino group exchange reaction with 2-aminobenzylamine 4 in the presence of BF3.Et2O to generate intermediate E. Later, F was formed by the intramolecular cyclization of E, enhanced by BF3.Et2O. In the last step, oxidative aromatization of F delivered the desired product, 2-arylquinazoline 27.
Visible-light photocatalysis has received considerable attention owing to its ability to carry out redox catalysis via a single electron transfer process under mild conditions. Le et al. (2022) synthesized quinazolines by the condensation of 2-aminobenzylamines 4 and α-ketoacids 28 followed by blue-light LED irradiation at room temperature. The reaction of 2-aminobenzylamines 4 and α-ketoacids 28 in the presence of an organic dye photocatalyst, Rose Bengal, in DMF under air in blue-light LED irradiation at room temperature for 16 h gave the corresponding quinazolines 29 in 26–88% yields (Scheme 14) [74]. Transition-metal-free, additive-free, external-oxidizing-agent-free, and simple and mild reaction conditions made this a green and environmentally friendly process. A variety of electron-donating, electron-withdrawing, electrically neutral, and heterocyclic substituents on α-ketoacids worked well under the optimized conditions.
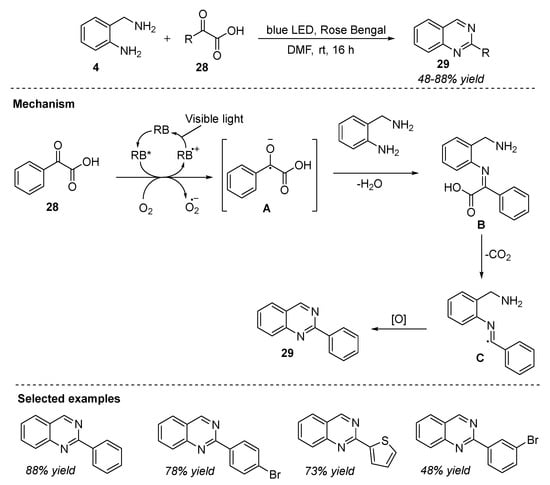
Scheme 14.
Visible-light-photocatalyzed synthesis of quinazolines.
The reaction mechanism proposed by the authors was shown in Scheme 14. Initially, benzoylformic acid 28 reacted with excited Rose Bengal under blue-light irradiation to generate anion intermediate A, which then dehydrates with 2-aminobenzylamine 4 to give another intermediate B. Then, B undergoes decarboxylation to yield free radical intermediate C, followed by oxidation and cyclization which furnished quinazoline 29.
2.2. From Amidines and Azirines
Molecular iodine is a less toxic and inexpensive oxidant widely used for the oxidative C-X (X = C, N, O, or S) bond construction from C-H bonds for the synthesis of heterocyclic compounds. In this relation, Chang et al. (2016) reported an I2/KI-promoted oxidative C-C bond formation reaction from C(sp3)-H and C(sp2)-H bonds from N,N’-disubstituted amidines 30 in the presence of potassium carbonate in DMSO at 100 °C for 4 h to provide quinazolines 31 in 39–99% yields (Scheme 15) [75]. The reaction proceeded smoothly with both electron-donating and electron-withdrawing groups on the N’-phenyl rings and gave the corresponding products 31. Gram-scale synthesis with a 61% yield, readily prepared starting precursors, and broad substrate scope are the merits of this protocol.
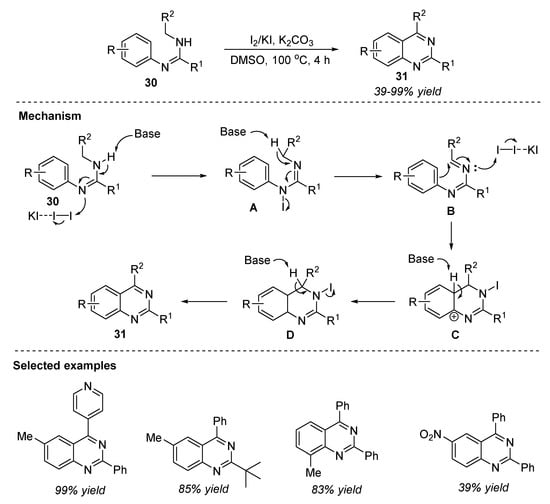
Scheme 15.
I2/KI-promoted oxidative synthesis of quinazolines.
The mechanism of oxidative cyclization is depicted in Scheme 15. Initially, the oxidation of N-aryl amidine 30 by molecular iodine in the presence of base K2CO3 led to an imine intermediate B. Next, iodine-mediated iodocyclization of B generated a N-iodo species C with a new C–C bond. Base-promoted deprotonation followed by elimination of one molecule of HI, delivered the quinazoline 31.
In the same year, Tang et al. (2016) disclosed a fast catalytic synthesis of multi-substituted quinazolines from amidines via visible-light-mediated oxidative C(sp3)-C(sp2) bond formation. Metal-free oxidative coupling of amidine 30 in the presence of Rose Bengal as a photoredox organocatalyst, CBr4 as an oxidant, and K2CO3 as the base, in DMSO as the solvent at 100 °C under 18 W fluorescent lamp as the visible-light source provided quinazolines 32 in 30–96% yields (Scheme 16) [76]. The major advantages of this reaction include: lower catalyst loading, improved efficacy of reaction, and tolerating a wide range of functional groups in substrates.
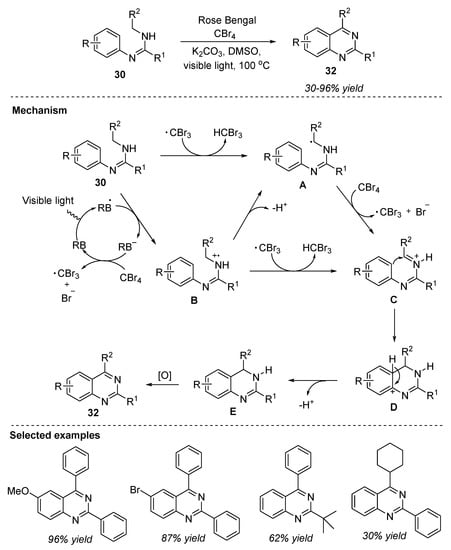
Scheme 16.
Visible-light-mediated oxidative synthesis of quinazolines.
The plausible reaction mechanism was demonstrated in more than one pathway (Scheme 16) [77]. In the absence of Rose Bengal, CBr4 was cracked into CBr3 radical and Br radical under visible-light irradiation [78]. CBr3 radical abstracted a hydrogen atom from another molecule of benzimidamide to yield α-amino radical intermediate A, which then entered the radical chain process with CBr4 to give iminium ion C. Intramolecular Friedel-Craft reaction of C generated an intermediate D, which upon dehydrogenation followed by aromatization delivered the desired product 32. In another pathway, quenching of visible-light-excited Rose Bengal (RB*) by benzimidamide led to the formation of Rose Bengal radical anion (RB•−) and radical cation B. The photoredox cycle was completed by the formation of a superoxide radical anion by the transfer of an electron to CBr4 and regeneration of Rose Bengal. Furthermore, the radical cation B gave up a hydrogen atom to the radical anion to generate CBr3 radical, bromide anion, and radical intermediate A. The CBr3 radical abstracted a hydrogen atom from radical cation B and formed iminium ion C, in another pathway.
An interesting approach was developed by Levin et al. (2022) for the construction of quinazoline scaffolds by the selective cleavage of the N-N bond of the indazole core 33 [79]. This chlorodiazirine-34-mediated reaction provides unified access to quinazolines 35 in the presence of sodium carbonate and methyl tert-butyl ether (MtBE) at 60 °C for 12 h (Scheme 17). Indazoles 33 were tolerant towards a wide range of alkyl substitutions. Moreover, free alcohol, thiophene, and halogen groups substituted in various positions also gave quinazolines 35 in 30–80% yields. The two major limitations associated with this strategy include: (a) substrates with less solubility in refluxing MtBE displayed poor reactivity, and (b) nucleophilicity of diazole was deactivated due to inductive withdrawal and precludes productive reactivity.
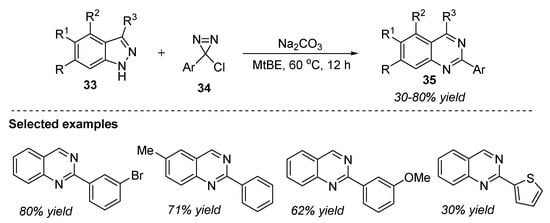
Scheme 17.
Chlorodiazirine-mediated reaction for the synthesis of quinazolines.
2.3. From Nitriles
Liu et al. (2017) reported a (2+2+2) modular synthesis of multi-substituted quinazolines by the direct reaction of two equivalents of nitriles 36 with aryldiazonium salts 37. The reaction of nitriles 36 and aryldiazonium tetrafluoroborate 37 at 110 °C for 3 h provided quinazolines 38 in 11–73% yields (Scheme 18) [80]. Under the optimized conditions, several aryldiazonium tetrafluoroborates 37 with electron-donating and electron-withdrawing groups at different positions in the ring were reacted smoothly to afford the corresponding products 38. Similarly, ortho- or para-substituted phenyldiazonium salts 37 gave quinazolines in good yields with the exception of nitro substituent. Moreover, aliphatic, aromatic, and heteroaromatic nitriles 36 served as suitable substrates for this annulation. Readily available substrates, shorter reaction times, one-pot, neat and metal-free conditions, gram-scale synthesis with 74% yields, flexibility in substitution patterns, atom-efficient, and easy handling procedures are the benefits of this strategy.
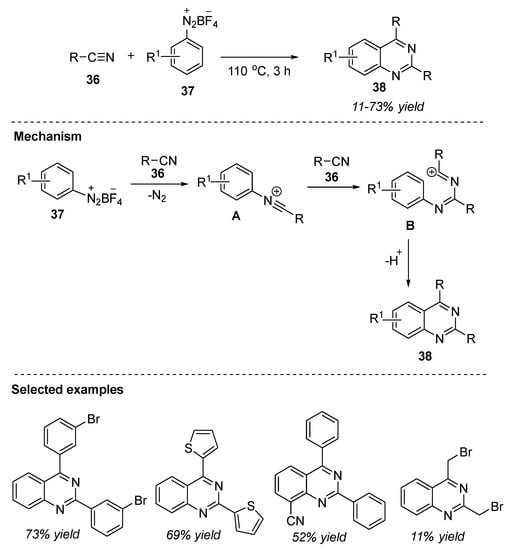
Scheme 18.
(2 + 2 + 2) Annulation reaction for the synthesis of quinazolines.
The authors proposed that the N-arylnitrilium ion A was generated in situ by the reaction of aryldiazonium salt 37 and nitrile 36. The N-arylnitrilium ion A undergoes a further reaction with another nitrile moiety 36 via tandem addition/electrophilic cyclization to furnish 2,4-disubstituted quinazolines 38 in an atom-economic way (Scheme 18).
North et al. (2017) reported a metal-free oxidative annulation reaction for the synthesis of 2-aminated quinazolines 41 by the reaction of 2-aminobenzophenone 1 with unsubstituted cyanamide/4-morpholinecarbonitrile 39/40 in the presence of p-toluene sulfonic acid (PTSA)/potassium tertiary butoxide (KOtBu) in DMF at 110 °C for 12 h (Scheme 19) [81]. The reaction proceeded with a variety of strong electron-withdrawing and unsubstituted aromatic rings to deliver quinazolines 41 in 75–81% yields. The plausible reaction mechanism for the construction of 2-aminoquinazolines 41 mediated by an acid or base is shown in Scheme 19. The initial step is the protonation of nitrogen in the cyano group of 39 by the weak acid PTSA resulting in the increased electrophilic nature of the cyanamide carbon [82]. In the next step, amine 1 attacks the electrophilic carbon generating the intermediate, amidine A. Simultaneous cyclization followed by elimination of the water molecule from intermediate A delivered the product 2-aminoquinazoline 41. On the other hand, base-mediated proton removal of amine 1 led to the formation of a strong nucleophile B which then reacts with the cyanamide carbon 39 followed by ring closure, resulting in the formation of the desired product 41.
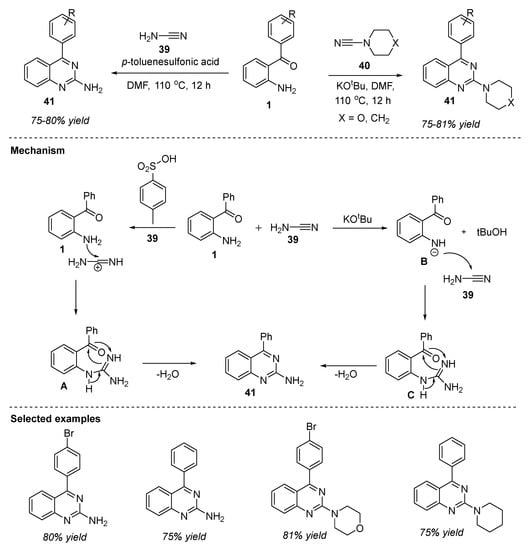
Scheme 19.
PTSA/KOtBu-mediated reaction for the synthesis of quinazolines.
In the recent years, reports have shown that the treatment of amines with nitriles in the presence of Lewis acids such as trimethylsilyl iodide (TMSI) led to the in situ generation of amidines [83]. In this regard, Pahari et al. (2018) disclosed the synthesis of 2,4-disubstituted quinazolines 43 in 72–78% yields by the reaction between nitriles 42 and 2-aminobenzophenone 1 in the presence of the TMSOTf catalyst under microwave irradiation at 100 °C for 10 min (Scheme 20) [84]. Nitriles 42 activated by the Lewis acid catalyst, TMSOTf, were used as the nitrogen source in this reaction. The significant features of this approach include: single-step, mild and solvent-free conditions, in situ generated amidines, broad substrate scope, one-pot, and shorter reaction times. The reaction mechanism as illustrated in Scheme 7 involves the activation of electron-rich nitriles 42 by coordination with TMSOTf. Later, the nucleophilic attack of amine 1 onto the activated nitrile A generated an amidine intermediate B which then attacks the carbonyl to deliver quinazoline 43 via aromatization.
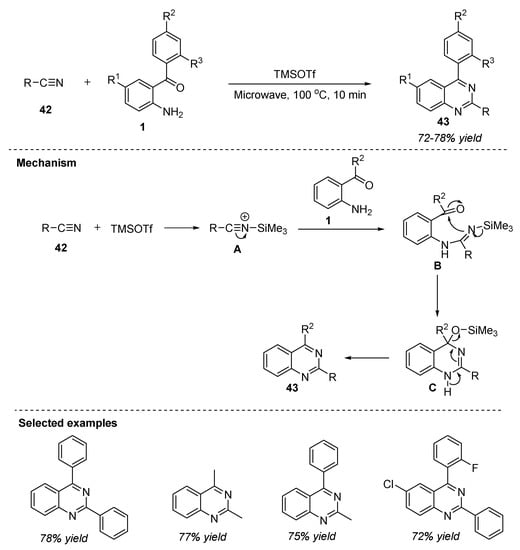
Scheme 20.
TMSOTf-catalyzed reaction for the synthesis of quinazolines.
Conjugated π-systems with arenes or heteroarenes have gained attention due to their applicability in materials science. On the other hand, the synthesis of π-conjugated molecules under transition-metal-free conditions remains a challenge because of intrinsic mechanistic limitations [85]. In this connection, Jiang et al. (2018) developed a transition-metal-free chemoselective synthesis of π-conjugated quinazoline-substituted ethenes 46 by the reaction of 2-ethynylanilines 45 and benzonitriles 44 in the presence of KOtBu and dimethylacetamide(DMA)/toluene (4:1) under air at 110 °C for 2 h (Scheme 21) [86]. Twenty-five derivatives were synthesized in moderate to high yields (31–77% yields). Interestingly, all the synthesized quinazolines 46 displayed a marked luminescence phenomenon. A variety of electron-donating and electron-withdrawing groups underwent the reaction and delivered the quinazolines 46 in a complete (Z)-configuration. Under optimized reaction conditions, unsubstituted and electron-donating-group-substituted benzonitriles 44 failed to give the desired product and this marks a major limitation in this approach.
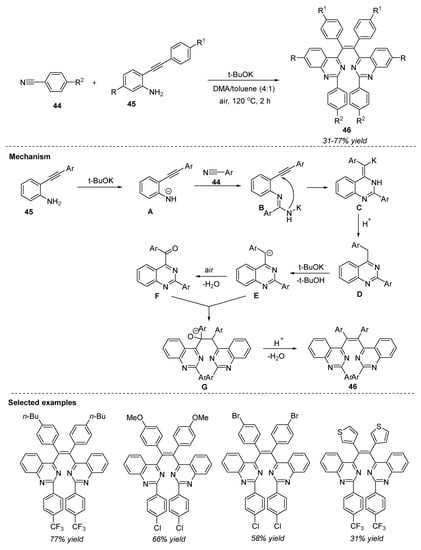
Scheme 21.
Metal-free chemoselective synthesis of π-conjugated quinazolines.
The mechanism involved the initial deprotonation of 2-ethynylanilines 45 in the presence of a base to generate the anion intermediate A, which attacks the cyano group 44 selectively and leads to the formation of amidine intermediate B. Later, intermediate C is formed by the intramolecular cyclization of alkynes and amidines, followed by protonation, to result in intermediate D. Subsequent deprotonation, oxidation followed by the attack of E afforded F. In the last step, the desired product 46 is formed with the elimination of one water molecule from G (Scheme 21). The benzene ring at the 2-position of quinazoline drives the stereoselectivity by forming a stereoregular product.
Palakodety et al. (2019) documented step economy and sustainable synthesis of substituted quinazolines 49 by utilizing the starting precursor 2-aminobenzonitrile 47, Grignard reagent, and phenylisothiocyanate 20 in the presence of an inexpensive base NaOH and water as a solvent at 80 °C for 1–2 h [87]. The reaction of 2-aminobenzonitrile 47 with aryl magnesium bromide resulted in the formation of ortho-aminoketimine 48 [88]. Later, the reaction of o-aminoketimine 48 with isothiocyanate 20 and NaOH in water provided N,4-substituted quinazolines 49 in 76–91% yields (Scheme 22). The noteworthy advantages of this approach include: broad substrate scope, step economy, operational simplicity, shorter reaction times, good functional group tolerance, easy workup procedure, inexpensive precursors, and using water as an environmentally benign solvent.
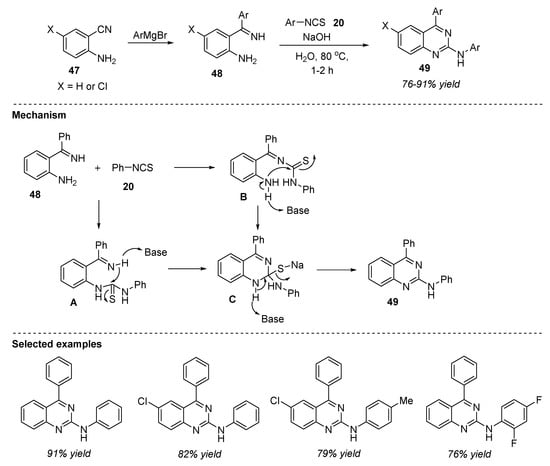
Scheme 22.
Base-catalyzed synthesis of quinazolines.
The plausible mechanism detailed by the authors is depicted in Scheme 22. In the first step, o-aminoketimine 48 reacts with phenylisothiocyanate 20 to generate the thiourea intermediate A. In the next step, the subsequent intramolecular nucleophilic attack of the amine or imine group on the thiocarbonyl carbon led to the formation of the cyclized product C via N-C bond formation. Aromatization followed by subsequent liberation of S and H2O offered the desired quinazoline 49.
3. Conclusions
Owing to the biologically active legacy, quinazolines have emerged as an important synthetic target in the last few decades. The transition-metal-free approaches in organic transformations are always more economical and complements metal-mediated reactions that suffer from drawbacks such as the possibility of toxic metal contamination in the final products, using expensive metal complexes, and the restricted applicability of products as pharmaceuticals, due to metal contamination. Consequently, rapid growth has been witnessed in the last few years in metal-free synthesis methods. Despite the efficacy of the reported protocols, they also suffer from inevitable disadvantages such as the use of excessive quantities of dangerous peroxide reagents, toxic solvents, toxic oxidants, longer reaction times, elevated temperatures, and requiring strong and hazardous oxidizing agents. Moreover, most of them are not solely focused on the development of an efficient approach with improved results viz., product yields, cost-effectiveness, atom-economy, and environmentally benign conditions. Hence, the development of novel synthetic strategies that proceed in a green reaction medium in the presence of benign reagents without the use of metal complexes, reagents, and hazardous organic solvents and also liberating minimum or benign waste are highly desirable.
This review highlights the recent progress achieved in the transition-metal-free synthesis of quinazolines and covers the literature from 2015 to 2022. It is presented alongside the advantages, limitations, and mechanistic rationalization associated with them. As discussed in the review, it is obvious that these strategies have noteworthy benefits such as readily available substrates, mild reaction conditions, broad substrate scopes, good to excellent yields, green conditions, gram-scale synthesis, etc. Furthermore, oxygen or air has been used instead of external toxic oxidants, and water as a green solvent made the strategies remarkable from a green and environmentally friendly point of view. In addition, employing microwave and visible-light irradiation techniques resulted in shorter reaction times and enhanced efficiency of reaction. Moreover, π-conjugated quinazolines displayed marked luminescence phenomena with excellent chemoselectivity. Furthermore, metal-free multicomponent reactions paved the way for the facile synthesis of substituted quinazolines. In spite of the merits achieved so far, some limitations such as prolonged reaction times, limited substrate scopes, and steric effects are also observed. Hence, metal-free methodologies should be developed in the near future, addressing these limitations which are efficient and environmentally benign. We believe the present review article will help researchers working on this fascinating area to construct quinazolines with immense applications in the field of medicinal chemistry.
Author Contributions
Conceptualization, R.T. and D.S.; methodology, R.T. and D.S.; writing—original draft preparation, R.T.; writing—review and editing, R.T. and D.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Research Foundation of Korea (NRF-2020R1A6A1A03043708 and NRF-2021R1A2C1012280).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
References
- Belenkii, L.I.; Gramenitskaya, V.N.; Evdokimenkova, Y.B. Advances in Heterocyclic Chemistry; Katritzky, A.R., Ed.; Elsevier: Amsterdam, The Netherlands, 2011; Volume 102, p. 1. [Google Scholar]
- Polshettiwar, V.; Varma, R.S. Greener and Sustainable Approaches to the Synthesis of Pharmaceutically Active Heterocycles. Curr. Opin. Drug Discov. Dev. 2007, 10, 723–737. [Google Scholar]
- Barker, A.J.; Gibson, K.H.; Grundy, W.; Godfrey, A.A.; Barlow, J.J.; Healy, M.P.; Woodburn, J.R.; Ashton, S.E.; Curry, B.J.; Scarlett, L.; et al. Studies Leading to the Identification of ZD1839 (iressa™): An Orally Active, Selective Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor Targeted to the Treatment of Cancer. Bioorg. Med. Chem. Lett. 2001, 11, 1911–1914. [Google Scholar] [CrossRef] [PubMed]
- Rewcastle, G.W.; Palmer, B.D.; Bridges, A.J.; Hollis Showalter, H.D.; Sun, L.; Nelson, J.; McMichael, A.; Kraker, A.J.; Fry, D.W.; Denny, W.A. Tyrosine Kinase Inhibitors. 9. Synthesis and Evaluation of Fused Tricyclic Quinazoline Analogues as ATP Site Inhibitors of the Tyrosine Kinase Activity of the Epidermal Growth Factor Receptor. J. Med. Chem. 1996, 39, 918–928. [Google Scholar] [CrossRef] [PubMed]
- Abbas, S.E.; Awadallah, F.M.; Ibrahin, N.A.; Said, E.G.; Kamel, G.M. New Quinazolinone-pyrimidine Hybrids: Synthesis, Anti-inflammatory, and Ulcerogenicity studies. Eur. J. Med. Chem. 2012, 53, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Arun, Y.; Bhaskar, G.; Balachandran, C.; Ignacimuthu, S.; Perumal, P.T. Facile One-pot Synthesis of Novel Dispirooxindole-Pyrrolidine Derivatives and their Antimicrobial and Anticancer Activity Against A549 Human Lung Adenocarcinoma Cancer Cell Line. Bioorg. Med. Chem. Lett. 2013, 23, 1839–1845. [Google Scholar] [CrossRef] [PubMed]
- Ugale, V.G.; Bari, S.B. Quinazolines: New Horizons in Anticonvulsant Therapy. Eur. J. Med. Chem. 2014, 80, 447–501. [Google Scholar] [CrossRef]
- Yen, M.-H.; Sheu, J.-R.; Peng, I.-H.; Lee, Y.-M.; Chern, J.-W. Pharmacological Activity of DC−015, a Novel Potent and Selective α1-Adrenoceptor Antagonist. J. Pharm. Pharmacol. 1996, 48, 90–95. [Google Scholar] [CrossRef]
- Machara, A.; Lux, V.; Kozisek, M.; Grantz-Saskova, K.; Stepanek, O.; Kotora, M.; Parkan, K.; Pavova, M.; Glass, B.; Sehr, P. Specific Inhibitors of HIV Capsid Assembly Binding to the C-Terminal Domain of the Capsid Protein: Evaluation of 2-Arylquinazolines as Potential Antiviral Compounds. J. Med. Chem. 2016, 59, 545–558. [Google Scholar] [CrossRef]
- Waisser, K.; Gregor, J.; Dostal, H.; Kunes, J.; Kubicova, L.; Klimesova, V.; Kaustova, J. Influence of the replacement of the oxo function with the thioxo group on the antimycobacterial activity of 3-aryl-6,8-dichloro-2H-1,3-benzoxazine-2,4(3H)-diones and 3-arylquinazoline-2,4(1H,3H)-diones. Farmaco 2001, 56, 803–807. [Google Scholar] [CrossRef] [PubMed]
- Waites, K.B.; Crabb, D.M.; Duffy, L.B.; Huband, M.D. In Vitro Antibacterial Activity of AZD0914 against Human Mycoplasmas and Ureaplasmas. Antimicrob. Agents Chemother. 2015, 59, 3627–3629. [Google Scholar] [CrossRef]
- Mohameda, M.S.; Kamel, M.M.; Kassem, E.M.M.; Abotaleb, N.; Abdelmoez, S.I.; Ahmeda, M.F. Novel 6,8-dibromo-4(3H)quinazolinone derivatives of anti-bacterial and anti-fungal activities. Eur. J. Med. Chem. 2010, 45, 3311–3319. [Google Scholar] [CrossRef]
- Velázquez, F.; Chelliah, M.; Clasby, M.; Guo, Z.; Howe, J.; Miller, R.; Neelamkavil, S.; Shah, U.; Soriano, A.; Xia, Y.; et al. Design and Synthesis of P2–P4 Macrocycles Containing a Unique Spirocyclic Proline: A New Class of HCV NS3/4A Inhibitors. ACS Med. Chem. Lett. 2016, 7, 1173–1178. [Google Scholar] [CrossRef] [PubMed]
- Kancharla, P.; Lu, W.; Salem, S.M.; Kelly, J.X.; Reynolds, K.A. Stereospecific Synthesis of 23-Hydroxyundecylprodiginines and Analogues and Conversion to Antimalarial Premarineosins via a Rieske Oxygenase Catalyzed Bicyclization. J. Org. Chem. 2014, 79, 11674–11689. [Google Scholar] [CrossRef] [PubMed]
- Kabri, Y.; Azas, N.; Dumetre, A.; Hutter, S.; Laget, M.; Verhaeghe, P.; Gellis, A.; Vanelle, P. Original Quinazoline Derivatives Displaying Antiplasmodial Properties. Eur. J. Med. Chem. 2010, 45, 616–622. [Google Scholar] [CrossRef]
- de Silva, J.F.M.; Walters, M.; Al-Damluji, S.; Ganellin, C.R. Molecular Features of the Prazosin Molecule Required for Activation of Transport-P. Bioorg. Med. Chem. 2008, 16, 7254–7263. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, P.; Kumari, P.; Kalal, B.L. Exploration of Antimicrobial and Antioxidant Potential of Newly Synthesized 2,3-disubstituted Quinazoline-4(3H)-ones. Bioorg. Med. Chem. Lett. 2011, 21, 4353–4357. [Google Scholar] [CrossRef]
- Noolvi, M.N.; Patel, H.M.; Bhardwaj, V.; Chauhan, A. Synthesis and in vitro antitumor activity of substituted quinazoline and quinoxaline derivatives: Search for anticancer agent. Eur. J. Med. Chem. 2011, 46, 2327–2346. [Google Scholar] [CrossRef]
- Barraja, P.; Caracausi, L.; Diana, P.; Montalbano, A.; Carbone, A.; Salvador, A.; Brun, P.; Castagliuolo, I.; Tisi, S.; Dall’Acqua, F.; et al. Pyrrolo [3,2-h]quinazolines as Photochemotherapeutic Agents. ChemMedChem 2011, 6, 1238–1248. [Google Scholar] [CrossRef]
- Boyapati, S.; Kulandaivelu, U.; Sangu, S.; Vanga, M.R. Synthesis, Antimicrobial Evaluation, and Docking Studies of Novel 4-Substituted Quinazoline Derivatives as DNA-gyrase Inhibitors. Arch. Pharm. 2010, 343, 570–576. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.H.; Khadka, D.B.; Cho, S.H.; Ju, H.K.; Lee, K.Y.; Han, H.J.; Lee, K.T.; Cho, W.J. Virtual screening and synthesis of quinazolines as novel JAK2 inhibitors. Bioorg. Med. Chem. 2011, 19, 968–977. [Google Scholar] [CrossRef]
- Kim, Y.H.; Choi, H.; Lee, J.; Hwang, I.C.; Moon, S.K.; Kim, S.J.; Lee, H.W.; Im, D.S.; Lee, S.S.; Ahn, S.K.; et al. Quinazolines as potent and highly selective PDE5 inhibitors as potential therapeutics for male erectile dysfunction. Bioorg. Med. Chem. Lett. 2008, 18, 6279–6282. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Lopez, O.; Conejo-Garcia, A.; Nunez, M.C.; Kimatrai, M.; Garcia-Rubino, M.E.; Morales, F.; Gomez-Perez, V.; Campos, J.M. Novel Substituted Quinazolines for Potent EGFR Tyrosine Kinase Inhibitors. Curr. Med. Chem. 2011, 18, 943–963. [Google Scholar] [CrossRef]
- Saari, R.; Törmä, J.-C.; Nevalainen, T. Microwave-assisted Synthesis of Quinoline, Isoquinoline, Quinoxaline and Quinazoline Derivatives as CB2 Receptor Agonists. Bioorg. Med. Chem. 2011, 19, 939–950. [Google Scholar] [CrossRef] [PubMed]
- Burris, H.A., III. Dual Kinase Inhibition in the Treatment of Breast Cancer: Initial Experience with the EGFR/ErbB-2 Inhibitor Lapatinib. Oncologist 2004, 9, 10–15. [Google Scholar] [CrossRef]
- Miller, V.A.; Hirsh, V.; Cadranel, J.; Chen, Y.-M.; Park, K.; Kim, S.-W.; Zhou, C.; Su, W.-C.; Wang, M.; Sun, Y.; et al. Afatinib versus Placebo for Patients with Advanced, Metastatic Non-small-cell Lung Cancer After Failure of Erlotinib, Gefitinib, or both, and one or two lines of Chemotherapy (LUX-Lung 1): A phase 2b/3 randomised trial. Lancet Oncol. 2012, 13, 528–538. [Google Scholar] [CrossRef]
- Goss, G.; Shepherd, F.A.; Laurie, S.; Gauthier, I.; Leighl, N.; Chen, E.; Feld, R.; Powers, J.; Seymour, L. A phase I and Pharmacokinetic Study of Daily Oral Cediranib, An Inhibitor of Vascular Endothelial Growth Factor Tyrosine Kinases, in Combination with Cisplatin and Gemcitabine in Patients with Advanced Non-small Cell Lung Cancer: A Study of the National Cancer Institute of Canada Clinical Trials Group. Eur. J. Cancer 2009, 45, 782–788. [Google Scholar]
- Ajani, O.O.; Audu, O.Y.; Aderohunmu, D.V.; Owolabi, F.E.; Olomieja, A.O. Undeniable Pharmacological Potentials of Quinazoline Motifs in Therapeutic Medicine. Am. J. Drug Discov. Dev. 2017, 7, 1–24. [Google Scholar] [CrossRef]
- Assiddiq, B.F.; Tan, K.Y.; Toy, W.; Chan, S.P.; Chong, P.K.; Pin, L.Y.P. EGFR S1166 Phosphorylation Induced by a Combination of EGF and Gefitinib Has a Potentially Negative Impact on Lung Cancer Cell Growth. J. Proteome Res. 2012, 11, 4110–4119. [Google Scholar] [CrossRef]
- Sos, M.L.; Koker, M.; Weir, B.A.; Heynck, S.; Rabinovsky, R.; Zander, T.; Seeger, J.M.; Weiss, J.; Fischer, F.; Frommolt, P.; et al. PTEN loss contributes to erlotinib resistance in EGFR-mutant lung cancer by activation of Akt and EGFR. Cancer Res. 2009, 69, 3256–3261. [Google Scholar] [CrossRef] [PubMed]
- Bolognesi, M.L.; Budriesi, R.; Chiarini, A.; Poggesi, E.; Leonardi, A.; Melchiorre, C. Design, Synthesis, and Biological Activity of Prazosin-related Antagonists. Role of the Piperazine and Furan Units of Prazosin on the Selectivity for Alpha1-adrenoreceptor Subtypes. J. Med. Chem. 1998, 41, 4844–4853. [Google Scholar] [CrossRef]
- Short, C.E.S.; Gilleece, Y.C.; Fisher, M.J.; Churchill, D.R. Trimetrexate and Folinic acid: A Valuable Salvage Option for Pneumocystis Jirovecii Pneumonia. AIDS 2009, 23, 1287–1290. [Google Scholar] [CrossRef]
- Wilson, J.N.; Liu, W.; Brown, A.S.; Landgraf, R. Binding-induced, Turn-on Fluorescence of the EGFR/ERBB Kinase Inhibitor, Lapatinib. Org. Biomol. Chem. 2015, 13, 5006–5011. [Google Scholar] [CrossRef]
- Zhang, Y.; Tortorella, M.D.; Liao, J.; Qin, X.; Chen, T.; Luo, J.; Guan, J.; Talley, J.J.; Tu, Z. Synthesis and Evaluation of Novel Erlotinib–NSAID Conjugates as More Comprehensive Anticancer Agents. ACS Med. Chem. Lett. 2015, 6, 1086–1090. [Google Scholar] [CrossRef] [PubMed]
- Griess, P. Ueber die Einwirkung des Cyans auf Anthranilsäure. Chem. Ber. 1869, 2, 415–418. [Google Scholar] [CrossRef]
- Wang, C.; Li, S.F.; Liu, H.X.; Jiang, Y.Y.; Fu, H. Copper-Catalyzed Synthesis of Quinazoline Derivatives via Ullmann-Type Coupling and Aerobic Oxidation. J. Org. Chem. 2010, 75, 7936–7938. [Google Scholar] [CrossRef]
- Han, B.; Yang, X.L.; Wang, C.; Bai, Y.W.; Pan, T.C.; Chen, X.; Yu, W. CuCl/DABCO/4-HO-TEMPO-Catalyzed Aerobic Oxidative Synthesis of 2-Substituted Quinazolines and 4H-3,1-Benzoxazines. J. Org. Chem. 2012, 77, 1136–1142. [Google Scholar] [CrossRef]
- Zhang, W.; Guo, F.; Wang, F.; Zhao, N.; Liu, L.; Li, J.; Wang, Z.H. Synthesis of Quinazolines via CuO Nanoparticles Catalyzed Aerobic Oxidative Coupling of Aromatic Alcohols and Amidines. Org. Biomol. Chem. 2014, 12, 5752–5756. [Google Scholar] [CrossRef]
- Zhao, D.; Shen, Q.; Li, J.X. Potassium Iodide-Catalyzed Three-Component Synthesis of 2-Arylquinazolines via Amination of Benzylic C—H Bonds of Methylarenes. Adv. Synth. Catal. 2015, 357, 339–344. [Google Scholar] [CrossRef]
- Roscales, S.; Csákӱ, A.G. Transition-Metal-free C–C bond Forming Reactions of Aryl, Alkenyl and Alkynylboronic acids and their Derivatives. Chem. Soc. Rev. 2014, 43, 8215–8225. [Google Scholar] [CrossRef] [PubMed]
- Eynde, J.J.V.; Godin, J.; Mayence, A.; Maquestiau, A.; Anders, E. A New and Convenient Method for the Preparation of 2-Substituted Quinazolines. Synthesis 1993, 1993, 867–869. [Google Scholar] [CrossRef]
- Peng, Y.-Y.; Zeng, Y.; Qiu, G.; Cai, L.; Pike, V.W. A Convenient One-pot Procedure for the Synthesis of 2-aryl Quinazolines using Active MnO2 as Oxidant. J. Heterocycl. Chem. 2010, 47, 1240–1245. [Google Scholar] [CrossRef]
- Maheswari, C.U.; Gadde, S.K.; Venkateshwar, M.; Kumar, R.A.; Kantam, M.L.; Reddy, K.R. Highly Efficient One-pot Synthesis of 2-substituted Quinazolines and 4H-benzo [d][1, 3] oxazines via Cross Dehydrogenative Coupling using Sodium Hypochlorite. Adv. Synth. Catal. 2010, 352, 341–346. [Google Scholar] [CrossRef]
- Laha, J.K.; Tummalapalli, K.S.S.; Jethava, K.P. Implications of Dynamic Imine Chemistry for the Sustainable Synthesis of Nitrogen Heterocycles via Transimination Followed by Intramolecular Cyclisation. Org. Biomol. Chem. 2016, 14, 2473–2479. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, D.; Yu, C.; Wan, C.; Wang, Z. A Simple and Efficient Approach to the Synthesis of 2-Phenylquinazolines via sp3 C−H Functionalization. Org. Lett. 2010, 12, 2841–2843. [Google Scholar] [CrossRef]
- Yan, Y.; Wang, Z. Metal-free Intramolecular Oxidative Decarboxylative Amination of Primary α-amino acids with Product Selectivity. Chem. Commun. 2011, 47, 9513–9515. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-H.; Zhang, X.-N.; Mo, L.-P.; Li, Y.-X.; Ma, F.-P. Catalyst-free Synthesis of Quinazoline Derivatives using Low Melting Sugar–Urea–Salt Mixture as a Solvent. Green Chem. 2012, 14, 1502–1506. [Google Scholar] [CrossRef]
- Alonso, R.; Caballero, A.; Campos, P.J.; Sampedro, D.; Rodriguez, M.A. An Efficient Synthesis of Quinazolines: A Theoretical and Experimental Study on the Photochemistry of Oxime Derivatives. Tetrahedron 2010, 66, 4469–4473. [Google Scholar] [CrossRef]
- Yan, Y.; Zhang, Y.; Feng, C.; Zha, Z.; Wang, Z. Selective Iodine-Catalyzed Intermolecular Oxidative Amination of C(sp3)—H Bonds with ortho-Carbonyl-Substituted Anilines to Give Quinazolines. Angew. Chem. Int. Ed. 2012, 51, 8077–8081. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.P.; Zhang, F.-H.; Long, Y.-Q. Solvent/Oxidant-Switchable Synthesis of Multisubstituted Quinazolines and Benzimidazoles via Metal-Free Selective Oxidative Annulation of Arylamidines. Org. Lett. 2014, 16, 2822–2825. [Google Scholar] [CrossRef]
- Kumar, V.; Mohan, C.; Gupta, M.; Mahajan, M.P. A Catalyst- and Solvent-Free Selective Approach to Biologically Important Quinazolines and Benzo[g]quinazoline. Tetrahedron 2005, 61, 3533–3538. [Google Scholar] [CrossRef]
- Wang, D.; Gao, F. Quinazoline derivatives: Synthesis and bioactivities. Chem. Cent. J. 2013, 7, 95–110. [Google Scholar] [CrossRef]
- Faisal, M.; Saeed, A. Chemical Insights Into the Synthetic Chemistry of Quinazolines: Recent Advances. Front. Chem. 2021, 8, 594717–594740. [Google Scholar] [CrossRef]
- Zalatan, D.N.; Du Bois, J. Metal-Catalyzed Oxidations of C-H to C-N bonds. Top. Curr. Chem. 2010, 292, 347–378. [Google Scholar]
- Yan, Y.; Xu, Y.; Niu, B.; Xie, H.; Liu, Y. I2-Catalyzed Aerobic Oxidative C(sp3)-H Amination/C-N Cleavage of Tertiary Amine: Synthesis of Quinazolines and Quinazolinones. J. Org. Chem. 2015, 80, 5581–5587. [Google Scholar] [CrossRef]
- Tiwari, A.R.; Bhanage, B.M. Synthesis of Quinazolines from 2-aminobenzylamines with Benzylamines and N-substituted Benzylamines under Transition Metal-Free Conditions. Org. Biomol. Chem. 2016, 14, 10567–10571. [Google Scholar] [CrossRef]
- Hati, S.; Sen, S. Synthesis of Quinazolines and Dihydroquinazolines: O-Iodoxybenzoic Acid Mediated Tandem Reaction of o-Aminobenzylamine with Aldehydes. Synthesis 2016, 48, 1389–1398. [Google Scholar] [CrossRef]
- Xu, J.-H.; Jiang, Q.; Guo, C.-C. Phenyliodonium Diacetate Mediated Direct Synthesis of Benzonitriles from Styrenes through Oxidative Cleavage of C═C Bonds. J. Org. Chem. 2013, 78, 11881–11886. [Google Scholar] [CrossRef] [PubMed]
- Saha, M.; Mukherjee, P.; Das, A.R. A Facile and Versatile Protocol for the One-Pot PhI(OAc)2 Mediated Divergent Synthesis of Quinazolines from 2-Aminobenzylamine. Tet. Lett. 2017, 58, 2044–2049. [Google Scholar] [CrossRef]
- Vorona, S.; Artamonova, T.; Zevatskii, Y.; Myznikov, L. An Improved Protocol for the Preparation of 5-Substituted Tetrazoles from Organic Thiocyanates and Nitriles. Synthesis 2014, 46, 781–786. [Google Scholar]
- Tekale, S.U.; Kauthale, S.S.; Dake, S.A.; Sarda, S.R.; Pawar, R. Molecular Iodine: An Efficient and Versatile Reagent for Organic Synthesis. Curr. Org. Chem. 2012, 16, 1485–1501. [Google Scholar] [CrossRef]
- Deshmukh, D.S.; Bhanage, B.M. Molecular Iodine-Catalysed Benzylic sp3 C–H Bond Amination for the Synthesis of 2-Arylquinazolines from 2-Aminobenzaldehydes, 2-Aminobenzophenones and 2-Aminobenzyl Alcohols. Synlett 2018, 29, 979–985. [Google Scholar]
- Sharma, S.; Bhattacherjee, D.; Das, P. Oxalic/Malonic Acids as Carbon Building Block for Benzazoles, Quinazoline and Quinazolinones Synthesis. Org. Biomol. Chem. 2018, 16, 1337–1342. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, T.; Kim, D.I.; Cho, E.J. Base-Promoted Synthesis of 2-Aryl Quinazolines from 2-Aminobenzylamines in Water. J. Org. Chem. 2018, 83, 7423–7430. [Google Scholar] [CrossRef]
- Jatangi, N.; Palakodety, R.K. I2-Catalyzed Oxidative Synthesis of N,4-Disubstituted Quinazolines and Quinazoline Oxides. Org. Biomol. Chem. 2019, 17, 3714–3717. [Google Scholar] [CrossRef] [PubMed]
- Cousin, T.; Chatel, G.; Kardos, N.; Andrioletti, B.; Draye, M. Recent Trends in the Development of Sustainable Catalytic Systems for the Oxidative Cleavage of Cycloalkenes by Hydrogen Peroxide. Catal. Sci. Technol. 2019, 9, 5256–5278. [Google Scholar] [CrossRef]
- Trinh, K.H.; Nguyen, K.X.; Pham, P.H.; Nguyen, T.T.; Phan, A.N.Q.; Phan, N.T.S. Hydrogen Peroxide-Mediated Synthesis of 2,4-Substituted Quinazolines via One-Pot Three-Component Reactions Under Metal-Free Conditions. RSC Adv. 2020, 10, 29900–29909. [Google Scholar] [CrossRef]
- Bruynes, C.A.; Jurriens, T.K. Catalysts for silylations with 1,1,1,3,3,3-hexamethyldisilazane. J. Org. Chem. 1982, 47, 3966–3969. [Google Scholar] [CrossRef]
- Chan, C.-K.; Lai, C.-Y.; Wang, C.-C. TMSOTf-Catalyzed Synthesis of Substituted Quinazolines Using Hexamethyldisilazane as Nitrogen Source under Neat and Microwave Irradiation. Org. Biomol. Chem. 2020, 18, 7201–7212. [Google Scholar] [CrossRef]
- Rudroff, F.; Mihovilovic, M.D.; Gröger, H.; Snajdrova, R.; Iding, H.; Bornscheuer, U.T. Opportunities and Challenges for Combining Chemo and Biocatalysis. Nat. Catal. 2018, 1, 12–22. [Google Scholar] [CrossRef]
- Tan, Z.; Zhang, X.; Xu, M.; Fu, Y.; Zhuang, W.; Li, M.; Wu, X.; Ying, H.; Ouyang, P.; Zhu, C. Cooperative Chemoenzymatic Synthesis of N-heterocycles via Synergizing Bio with Organocatalysis. Sci Adv. 2022, 8, eadd1912. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Yamakawa, C.; Nishimura, R.; Dong, C.-P.; Kodama, S.; Nomoto, A.; Ueshima, M.; Ogawa, A. Metal-Free Synthesis of 2-Substituted Quinazolines via Green Oxidation of o-Aminobenzylamines: Practical Construction of N-Containing Heterocycles Based on a Salicylic Acid-Catalyzed Oxidation System. Front. Chem. 2011, 9, 822–841. [Google Scholar] [CrossRef]
- Salamone, M.; DiLabio, G.A.; Bietti, M. Hydrogen Atom Abstraction Selectivity in the Reactions of Alkylamines with the Benzyloxyl and Cumyloxyl Radicals. The Importance of Structure and of Substrate Radical Hydrogen Bonding. J. Am. Chem. Soc. 2011, 133, 16625–16634. [Google Scholar] [CrossRef]
- Yang, J.; Xie, Z.; Jin, L.; Chen, X.; Le, Z. Synthesis of Quinazoline by Decarboxylation of 2-Aminobenzylamine and α-Keto acid Under Visible Light Catalysis. Org. Biomol. Chem. 2022, 20, 3558–3563. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; Wang, B.; Hu, Z.; Zhou, Y.; Yu, W.; Chang, J. Synthesis of Quinazolines from N,N’-Disubstituted Amidines via I2/KI-Mediated Oxidative C-C Bond Formation. J. Org. Chem. 2016, 81, 9924–9930. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.-C.; Yang, P.; Tang, Y. Transition Metal-Free Visible Light-Driven Photoredox Oxidative Annulation of Arylamidines. J. Org. Chem. 2016, 81, 309–317. [Google Scholar] [CrossRef]
- Kee, C.W.; Chan, K.M.; Wong, M.W.; Tan, C.H. Selective Bromination of sp3 C–H Bonds by Organophotoredox Catalysis. Asian J. Org. Chem. 2014, 3, 536–544. [Google Scholar] [CrossRef]
- Franz, J.F.; Kraus, W.B.; Zeitler, K. No Photocatalyst Required–Versatile, Visible Light Mediated Transformations with Polyhalomethanes. Chem. Commun. 2015, 51, 8280–8283. [Google Scholar] [CrossRef]
- Hyland, E.E.; Kelly, P.Q.; McKillop, A.M.; Dherange, B.D.; Levin, M.D. Unified Access to Pyrimidines and Quinazolines Enabled by N−N Cleaving Carbon Atom Insertion. J. Am. Chem. Soc. 2022, 144, 19258–19264. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, M.; Liu, S.-T. Preparation of Quinazolines via a 2+2+2 Annulation from Aryldiazonium Salts and Nitriles. J. Org. Chem. 2017, 82, 8290–8295. [Google Scholar] [CrossRef]
- Pandya, A.N.; Villa, E.M.; North, E.J. A simple and efficient approach for the synthesis of 2 aminated quinazoline derivatives via metal free oxidative annulation. Tet. Lett. 2017, 58, 1276–1279. [Google Scholar] [CrossRef]
- Jalani, H.B.; Pandya, A.N.; Baraiya, A.B.; Kaila, J.C.; Pandya, D.H.; Sharma, J.A.; Sudarsanam, V.; Vasu, K.K. An efficient synthesis of 2-aminopyrroles from enaminone–amidine adduct and phenacyl/benzyl/heteroalkyl-halides. Tet. Lett. 2011, 52, 6331–6335. [Google Scholar] [CrossRef]
- Saikia, U.P.; Hussain, F.L.; Suri, M.; Pahari, P. Selective N-acetylation of aromatic amines using acetonitrile as acylating agent. Tet. Lett. 2016, 57, 1158–1160. [Google Scholar] [CrossRef]
- Saikia, U.P.; Borah, G.; Pahari, P. Lewis-Acid-Catalyzed Activation of Nitriles: A Microwave Assisted Solvent-Free Synthesis of 2,4-Disubstituted Quinazolines and 1,3-Diazaspiro [5.5]undec-1-enes. Eur. J. Org. Chem. 2018, 2018, 1211–1217. [Google Scholar] [CrossRef]
- Sun, C.-L.; Shi, Z.-J. Transition-Metal-Free Coupling Reactions. Chem. Rev. 2014, 114, 9219–9280. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, J.; Huang, Y.; Zhu, J.; Hu, R.; Wu, W.; Jiang, H. Facile Synthesis of π-Conjugated Quinazoline-Substituted Ethenes from 2-Ethynylanilines and Benzonitriles under Transition-Metal-Free Conditions. J. Org. Chem. 2018, 83, 10453–10464. [Google Scholar] [CrossRef]
- Jatangi, N.; Palakodety, R.K. Base-catalyzed synthesis of quinazolines in aqueous medium. Tet. Lett. 2019, 60, 151186. [Google Scholar] [CrossRef]
- Whyte, A.; Burton, K.I.; Zhang, J.; Lautens, M. Enantioselective Intramolecular Copper-Catalyzed Borylacylation. Angew. Chem. Int. Ed. 2018, 57, 13927–13930. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).