Chemical Characterization of Trichoderma spp. Extracts with Antifungal Activity against Cocoa Pathogens
Abstract
1. Introduction
2. Results and Discussion
2.1. Antifungal Activity
2.2. Chemical Screening
2.3. GC-MS Metabolite Profile
3. Materials and Methods
3.1. Standards and Chemicals
3.2. Fungal Strains
3.3. Extraction Procedure
3.4. Antifungal Assay
3.5. Chemical Screening
3.6. Gas Chromatography–Mass Spectrometry (GC-MS) Analysis
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Armengot, L.; Ferrari, L.; Milz, J.; Velásquez, F.; Hohmann, P.; Schneider, M. Cacao Agroforestry Systems Do Not Increase Pest and Disease Incidence Compared with Monocultures under Good Cultural Management Practices. Crop. Prot. 2020, 130, 105047. [Google Scholar] [CrossRef]
- International Cocoa Organization (ICCO) Production of Cocoa Beans. Available online: https://www.icco.org/statistics (accessed on 16 February 2023).
- Anzules-Toala, V.; Pazmiño-Bonilla, E.; Alvarado-Huamán, L.; Borjas-Ventura, R.; Castro-Cepero, V.; Julca-Otiniano, A. Control of Cacao (Theobroma cacao) Diseases in Santo Domingo de Los Tsachilas, Ecuador. Agron. Mesoam. 2022, 33, 45939. [Google Scholar] [CrossRef]
- Orea, M.D.D.; Romero-Cortes, T.; Lopez-Perez, P.A.; Perez Espana, V.H.; Ramirez-Lepe, M.; Cuervo-Parra, J.A. Current Status of Cocoa Frosty Pod Rot Caused by Moniliophthora roreri and a Phylogenetic Analysis. Plant Pathol. J. (Faisalabad) 2017, 16, 41–53. [Google Scholar] [CrossRef]
- Meraz-Pérez, I.M.; Carvalho, M.R.; Sena, K.F.; Soares, Y.J.B.; Junior, A.S.E.; Lopes, U.V.; dos Santos Filho, L.P.; Araújo, S.A.; Soares, V.L.F.; Pirovani, C.P.; et al. The Moniliophthora perniciosa—Cacao Pod Pathosystem: Structural and Activated Defense Strategies against Disease Establishment. Physiol. Mol. Plant Pathol. 2021, 115, 101656. [Google Scholar] [CrossRef]
- Maridueña-Zavala, M.G.; Feijoo, M.I.J.; Cevallos-Cevallos, J.M. Pathogenicity of Moniliophthora roreri Isolates from Selected Morphology Groups in Harvested Cacao Pods and in Vitro Sensitivity to Compost Tea. Bionatura 2021, 6, 1569–1574. [Google Scholar] [CrossRef]
- Maridueña-Zavala, M.G.; Villavicencio-Vásquez, M.E.; Cevallos-Cevallos, J.M.; Peralta, E.L. Molecular and Morphological Characterization of Moniliophthora roreri Isolates from Cacao in Ecuador. Can. J. Plant Pathol. 2016, 38, 460–469. [Google Scholar] [CrossRef]
- Maridueña-Zavala, M.G.; Freire-Peñaherrera, A.; Espinoza-Lozano, R.F.; Villavicencio-Vasquez, M.; Jimenez-Feijoo, M.; Cevallos-Cevallos, J.M. Genetic Characterization of Moniliophthora perniciosa from Ecuador and in Vitro Sensitivity to Compost Tea. Eur. J. Plant Pathol. 2019, 154, 943–959. [Google Scholar] [CrossRef]
- Espinoza-Lozano, F.; Amaya-Márquez, D.; Pinto, C.M.; Villavicencio-Vásquez, M.; Sosa Del Castillo, D.; Pérez-Martínez, S. Multiple Introductions of Moniliophthora roreri from the Amazon to the Pacific Region in Ecuador and Shared High Azoxystrobin Sensitivity. Agronomy 2022, 12, 1119. [Google Scholar] [CrossRef]
- ten Hoopen, G.M.; Krauss, U. Biological Control of Cacao Diseases. In Cacao Diseases: A History of Old Enemies and New Encounters; Bailey, B.A., Meinhardt, L.W., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 511–566. [Google Scholar]
- di Cologna, N.M.D.; Gómez-Mendoza, D.P.; Zanoelo, F.F.; Giannesi, G.C.; Guimarães, N.C.A.; Moreira, L.R.S.; Filho, E.X.F.; Ricart, C.A.O. Exploring Trichoderma and Aspergillus Secretomes: Proteomics Approaches for the Identification of Enzymes of Biotechnological Interest. Enzym. Microb Technol. 2018, 109, 1–10. [Google Scholar] [CrossRef]
- Adnan, M.; Islam, W.; Shabbir, A.; Khan, K.A.; Ghramh, H.A.; Huang, Z.; Chen, H.Y.H.; Lu, G. Plant Defense against Fungal Pathogens by Antagonistic Fungi with Trichoderma in Focus. Microb. Pathog. 2019, 129, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, F.V.; Musumeci, M.A. Trichoderma as Biological Control Agent: Scope and Prospects to Improve Efficacy. World J. Microbiol. Biotechnol. 2021, 37, 90. [Google Scholar] [CrossRef]
- Vinale, F.; Strakowska, J.; Mazzei, P.; Piccolo, A.; Marra, R.; Lombardi, N.; Manganiello, G.; Pascale, A.; Woo, S.L.; Lorito, M. Cremenolide, a New Antifungal, 10-Member Lactone from Trichoderma Cremeum with Plant Growth Promotion Activity. Nat. Prod. Res. 2016, 30, 2575–2581. [Google Scholar] [CrossRef] [PubMed]
- Guzmán-Guzmán, P.; Kumar, A.; Santos-Villalobos, S.D.L.; Parra-Cota, F.I.; Orozco-Mosqueda, M.D.C.; Fadiji, A.E.; Hyder, S.; Babalola, O.O.; Santoyo, G. Trichoderma Species: Our Best Fungal Allies in the Biocontrol of Plant Diseases—A Review. Plants 2023, 12, 432. [Google Scholar] [CrossRef] [PubMed]
- Salwan, R.; Sharma, A.; Kaur, R.; Sharma, R.; Sharma, V. The Riddles of Trichoderma Induced Plant Immunity. Biol. Control 2022, 174, 105037. [Google Scholar] [CrossRef]
- Verma, M.; Brar, S.K.; Tyagi, R.D.; Surampalli, R.Y.; Valéro, J.R. Antagonistic Fungi, Trichoderma Spp.: Panoply of Biological Control. Biochem. Eng. J. 2007, 37, 1–20. [Google Scholar] [CrossRef]
- Ahluwalia, V.; Kumar, J.; Rana, V.S.; Sati, O.P.; Walia, S. Comparative Evaluation of Two Trichoderma harzianum Strains for Major Secondary Metabolite Production and Antifungal Activity. Nat. Prod. Res. 2015, 29, 914–920. [Google Scholar] [CrossRef]
- Rukachaisirikul, V.; Chinpha, S.; Phongpaichit, S.; Saikhwan, N.; Sakayaroj, J.; Preedanon, S. Sesquiterpene and Monoterpene Derivatives from the Soil-Derived Fungus Trichoderma reesei PSU-SPSF013. Phytochem. Lett. 2019, 30, 124–129. [Google Scholar] [CrossRef]
- Dashtban, M.; Kepka, G.; Seiboth, B.; Qin, W. Xylitol Production by Genetically Engineered Trichoderma reesei Strains Using Barley Straw as Feedstock. Appl. Biochem. Biotechnol. 2013, 169, 554–569. [Google Scholar] [CrossRef]
- Reyes-Figueroa, O.; Ortiz-García, C.F.; Torres-de la Cruz, M.; Lagunes-Espinoza, L.D.; Valdovinos-Ponce, G. Trichoderma Del Agroecosistema Cacao Con Potencial de Biocontrol Sobre Moniliophthora roreri. Rev. Chapingo Ser. Cienc. For. Ambiente 2016, 22, 149–163. [Google Scholar] [CrossRef]
- Mejia, L.; Rojas, E.I.; Maynard, Z.; Van Bael, S.; Arnold, A.E.; Hebbar, P.; Samuels, G.J.; Robbins, N.; Herre, E.A. Endophytic Fungi as Biocontrol Agents of Theobroma cacao Pathogens. Biol. Control 2008, 46, 4–14. [Google Scholar] [CrossRef]
- Krauss, U.; Soberanis, W. Effect of Fertilization and Biocontrol Application Frequency on Cocoa Pod Diseases. Biol. Control 2002, 24, 82–89. [Google Scholar] [CrossRef]
- Leiva, S.; Oliva, M.; Hernández, E.; Chuquibala, B.; Rubio, K.; García, F.; de la Cruz, M.T. Assessment of the Potential of Trichoderma Spp. Strains Native to Bagua (Amazonas, Peru) in the Biocontrol of Frosty Pod Rot (Moniliophthora roreri). Agronomy 2020, 10, 1376. [Google Scholar] [CrossRef]
- Bastos, C.N. Isolate of Trichoderma Brevicompactum for the Control of Cocoa Witches Broom Disease: Preliminary Results. Agrotrópica 2012, 24, 21–26. [Google Scholar] [CrossRef]
- Gonzalez, M.F.; Magdama, F.; Galarza, L.; Sosa, D.; Romero, C. Evaluation of the Sensitivity and Synergistic Effect of Trichoderma reesei and Mancozeb to Inhibit under In Vitro Conditions the Growth of Fusarium oxysporum. Commun. Integr. Biol. 2020, 13, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Galarza, L.; Akagi, Y.; Takao, K.; Kim, C.S.; Maekawa, N.; Itai, A.; Peralta, E.; Santos, E.; Kodama, M. Characterization of Trichoderma Species Isolated in Ecuador and Their Antagonistic Activities against Phytopathogenic Fungi from Ecuador and Japan. J. Gen. Plant Pathol. 2015, 81, 201–210. [Google Scholar] [CrossRef]
- Aneja, M.; Gianfagna, T.J.; Hebbar, P.K. Trichoderma harzianum Produces Nonanoic Acid, an Inhibitor of Spore Germination and Mycelial Growth of Two Cacao Pathogens. Physiol. Mol. Plant Pathol. 2005, 67, 304–307. [Google Scholar] [CrossRef]
- Evans, H.C.; Holmes, K.A.; Thomas, S.E. Endophytes and Mycoparasites Associated with an Indigenous Forest Tree, Theobroma Gileri, in Ecuador and a Preliminary Assessment of Their Potential as Biocontrol Agents of Cocoa Diseases. Mycol. Prog. 2003, 2, 149–160. [Google Scholar] [CrossRef]
- Villavicencio-Vásquez, M.; Espinoza-Lozano, R.F.; Pérez-Martínez, S.; Sosa, D.; Castillo, D. Hongos Endófitos Foliares Como Candidatos a Biocontroladores Contra Moniliophthora Spp. de Theobroma cacao (Malvaceae) En Ecuador. Acta Biol. Colomb. 2018, 23, 235–241. [Google Scholar] [CrossRef]
- Serrano, L.; Sosa Moreno, A.; Sosa Del Castillo, D.; Bonilla, J.; Romero, C.A.; Galarza, L.L.; Coronel–león, J.R. Biosurfactants Synthesized by Endophytic Bacillus Strains as Control of Moniliophthora perniciosa and Moniliophthora roreri. Sci. Agric. 2021, 78, e20200172. [Google Scholar] [CrossRef]
- Vargas Inciarte, L.; Fuenmayor Arrieta, Y.; Luzardo Méndez, M.; da Costa Jardin, M.; Vera, A.; Carmona, D.; Homen Pereira, M.; da Costa Jardin, P.; San Blas, E. Use of Different Trichoderma Species in Cherry Type Tomatoes (Solanum lycopersicun L.) Against Fusarium oxysporum Wilt in Tropical Greenhouses. Agron. Costarric. 2019, 43, 85–100. [Google Scholar] [CrossRef]
- Villavicencio, M.; Schuller, L.; Espinosa, F.; Noceda, C.; Sosa, D.; Pérez-Martínez, S. Foliar Endophytic Fungi of Theobroma cacao Stimulate More than Inhibit Moniliophthora Spp. Growth and Behave More as an Endophytes than Pathogens. AgriRxiv 2020, 2020, 1–33. [Google Scholar] [CrossRef]
- Moïse, N.A.; Severin, T.N.; Christelle, S.E.; Tibo, A.A.; Lambert, S.M.; Duplex, W.J. Efficacy of Trichoderma harzianum (Edtm) and Trichoderma Aureoviride (T4) as Potential Bio-Control Agent of Taro Leaf Blight Caused by Phytophthora Colocasiae. Int. J. Appl. Microbiol. Biotechnol. Res. 2018, 6, 115–126. [Google Scholar]
- Hu, Z.; Tao, Y.; Tao, X.; Su, Q.; Cai, J.; Qin, C.; Ding, W.; Li, C. Sesquiterpenes with Phytopathogenic Fungi Inhibitory Activities from Fungus Trichoderma Virens from Litchi Chinensis Sonn. J. Agric. Food Chem. 2019, 67, 10646–10652. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho, R.B.F.; De Almeida, A.A.C.; Campelo, N.B.; Lellis, D.R.O.D.; Nunes, L.C.C. Nerolidol and Its Pharmacological Application in Treating Neurodegenerative Diseases: A Review. Recent Pat. Biotechnol. 2018, 12, 158–168. [Google Scholar] [CrossRef]
- Afoulous, S.; Ferhout, H.; Raoelison, E.G.; Valentin, A.; Moukarzel, B.; Couderc, F.; Bouajila, J. Chemical Composition and Anticancer, Antiinflammatory, Antioxidant and Antimalarial Activities of Leaves Essential Oil of Cedrelopsis Grevei. Food Chem. Toxicol. 2013, 56, 352–362. [Google Scholar] [CrossRef] [PubMed]
- Nuñez, Y.O.; Salabarria, I.S.; Collado, I.G.; Hernández-Galán, R. The Antifungal Activity of Widdrol and Its Biotransformation by Colletotrichum gloeosporioides (Penz.) Penz. & Sacc. and Botrytis cinerea Pers.: Fr. J. Agric. Food Chem. 2006, 54, 7517–7521. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Yap, M.; Behringer, G.; Hung, R.; Bennett, J.W. Volatile Organic Compounds Emitted by Trichoderma Species Mediate Plant Growth. Fungal Biol. Biotechnol. 2016, 3, 7. [Google Scholar] [CrossRef]
- Dahham, S.; Tabana, Y.; Iqbal, M.; Ahamed, M.; Ezzat, M.; Majid, A.; Majid, A. The Anticancer, Antioxidant and Antimicrobial Properties of the Sesquiterpene β-Caryophyllene from the Essential Oil of Aquilaria Crassna. Molecules 2015, 20, 11808–11829. [Google Scholar] [CrossRef]
- Morath, S.U.; Hung, R.; Bennett, J.W. Fungal Volatile Organic Compounds: A Review with Emphasis on Their Biotechnological Potential. Fungal Biol. Rev. 2012, 26, 73–83. [Google Scholar] [CrossRef]
- Pinheiro, E.A.A.; Carvalho, J.M.; dos Santos, D.C.P.; Feitosa, A.O.; Marinho, P.S.B.; Guilhon, G.M.S.P.; Santos, L.S.; de Souza, A.L.D.; Marinho, A.M.R.; Pinheiro, E.A.A.; et al. Chemical Constituents of Aspergillus Sp. EJC08 Isolated as Endophyte from Bauhinia Guianensis and Their Antimicrobial Activity. Acad. Bras. Ciências 2013, 85, 1247–1253. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Wang, X.; Xu, D.; Fu, X.; Zhang, X.; Lai, D.; Zhou, L.; Zhang, G. Sorbicillinoids from Fungi and Their Bioactivities. Molecules 2016, 21, 715. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Gu, R.; Lin, F.; Xiao, H. Sorbicillinoids Hyperproduction without Affecting the Cellulosic Enzyme Production in Trichoderma reesei JNTR5. Biotechnol. Biofuels Bioprod. 2022, 15, 85. [Google Scholar] [CrossRef]
- Ngo, M.; Nguyen, M.; Han, J.; Park, M.; Kim, H.; Choi, G. In Vitro and in Vivo Antifungal Activity of Sorbicillinoids Produced by Trichoderma longibrachiatum Men. J. Fungi 2021, 7, 428. [Google Scholar] [CrossRef] [PubMed]
- Kotan, R.; Kordali, S.; Cakir, A. Screening of Antibacterial Activities of Twenty-One Oxygenated Monoterpenes. Z. Nat. C 2007, 62, 507–513. [Google Scholar] [CrossRef]
- Pohl, C.; Kock, J.; Thibane, V. Antifungal Free Fatty Acids: A Review. Sci. Against Microb. Pathog. Commun. Curr. Res. Technol. Adv. 2011, 3, 61–71. [Google Scholar]
- Stracquadanio, C.; Quiles, J.M.; Meca, G.; Cacciola, S.O. Antifungal Activity of Bioactive Metabolites Produced by Trichoderma and Trichoderma in Liquid Medium. J. Fungi 2020, 6, 263. [Google Scholar] [CrossRef]
- Gajera, H.P.; Hirpara, D.G.; Savaliya, D.D.; Golakiya, B.A. Extracellular Metabolomics of Trichoderma Biocontroller for Antifungal Action to Restrain Rhizoctonia solani Kuhn in Cotton. Physiol. Mol. Plant Pathol. 2020, 112, 101547. [Google Scholar] [CrossRef]
- Uranga, C.C.; Beld, J.; Mrse, A.; Córdova-Guerrero, I.; Burkart, M.D.; Hernández-Martínez, R. Fatty Acid Esters Produced by Lasiodiplodia Theobromae Function as Growth Regulators in Tobacco Seedlings. Biochem. Biophys. Res. Commun. 2016, 472, 339–345. [Google Scholar] [CrossRef]
- Gregori, R.; Borsetti, F.; Neri, F.; Mari, M.; Bertolini, P. Effects of Potassium Sorbate on Postharvest Brown Rot of Stone Fruit. J. Food Prot. 2008, 71, 1626–1631. [Google Scholar] [CrossRef]
- Liu, Q.; Ouyang, S.P.; Chung, A.; Wu, Q.; Chen, G.Q. Microbial Production of R-3-Hydroxybutyric Acid by Recombinant E. Coli Harboring Genes of PhbA, PhbB, and TesB. Appl. Microbiol. Biotechnol. 2007, 76, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Marra, R.; Nicoletti, R.; Pagano, E.; DellaGreca, M.; Salvatore, M.M.; Borrelli, F.; Lombardi, N.; Vinale, F.; Woo, S.L.; Andolfi, A. Inhibitory Effect of Trichodermanone C, a Sorbicillinoid Produced by Trichoderma citrinoviride Associated to the Green Alga Cladophora Sp., on Nitrite Production in LPS-Stimulated Macrophages. Nat. Prod. Res. 2019, 33, 3389–3397. [Google Scholar] [CrossRef]
- Wongsuk, T.; Pumeesat, P.; Luplertlop, N. Fungal Quorum Sensing Molecules: Role in Fungal Morphogenesis and Pathogenicity. J. Basic Microbiol. 2016, 56, 440–447. [Google Scholar] [CrossRef]
- Brilhante, R.S.N.; Caetano, P.; de Lima, R.A.C.; Marques, F.J.D.F.; Castelo-Branco, D.D.S.C.M.; de Melo, C.V.S.; Guedes, G.M.D.M.; de Oliveira, J.S.; de Camargo, Z.P.; Moreira, J.L.B.; et al. Terpinen-4-Ol, Tyrosol, and β-Lapachone as Potential Antifungals against Dimorphic Fungi. Braz. J. Microbiol. 2016, 47, 917–924. [Google Scholar] [CrossRef]
- Li, M.F.; Li, G.H.; Zhang, K.Q. Non-Volatile Metabolites from Trichoderma Spp. Metabolites 2019, 9, 58. [Google Scholar] [CrossRef] [PubMed]
- Pelo, S.P.; Adebo, O.A.; Green, E. Chemotaxonomic Profiling of Fungal Endophytes of Solanum Mauritianum (Alien Weed) Using Gas Chromatography High Resolution Time-of-Flight Mass Spectrometry (GC-HRTOF-MS). Metabolomics 2021, 17, 43. [Google Scholar] [CrossRef]
- Al-Marzoqi, A.H.; Hameed, I.H.; Idan, S.A. Analysis of Bioactive Chemical Components of Two Medicinal Plants (Coriandrum Sativum and Melia Azedarach) Leaves Using Gas Chromatography-Mass Spectrometry (GC-MS). Afr. J. Biotechnol. 2015, 14, 2812–2830. [Google Scholar] [CrossRef]
- Walters, D.; Raynor, L.; Mitchell, A.; Walker, R.; Walker, K. Antifungal Activities of Four Fatty Acids against Plant Pathogenic Fungi. Mycopathologia 2004, 157, 87–90. [Google Scholar] [CrossRef] [PubMed]
- Maridueña-Zavala, M.G.; Quevedo, A.; Aguaguiña, K.; Serrano, L.; Sosa, D. Colección de Cultivos Microbianos CIBE (CCM-CIBE): Una Colección Para La Investigación Microbial Culture Collection from CIBE (CCM-CIBE): A Collection for Research. Rev. Bionatura 2021, 6, 1664–1668. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and Direct Sequencing of Fungal Ribosomal RNA Genes for Phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar] [CrossRef]
- Liu, J.; Liu, G. Analysis of Secondary Metabolites from Plant Endophytic Fungi. In Plant Pathogenic Fungi and Oomycetes. Methods in Molecular Biology; Ma, W., Wolpert, T., Eds.; Humana Press: New York, NY, USA, 2018; Volume 1848, pp. 25–38. [Google Scholar]
- Kjer, J.; Debbab, A.; Aly, A.H.; Proksch, P. Methods for Isolation of Marine-Derived Endophytic Fungi and Their Bioactive Secondary Products. Nat. Protoc. 2010, 5, 479–490. [Google Scholar] [CrossRef]
- Guerrero-Rodríguez, E.; Solís-Gaona, S.; Hernández-Castillo, F.D.; Flores-Olivas, A.; Sandoval-López, V.; Jasso-Cantú, V. Actividad Biológica in Vitro de Extractos de Flourensia cernua D.C. en Patógenos de Postcosecha: Alternaria alternata (Fr.:Fr.) Keissl., Colletotrichum gloeosporioides (Penz.) Penz. y Sacc. y Penicillium digitatum (Pers.:Fr.) Sacc. Rev. Mex. Fitopatol. 2007, 25, 48–53. [Google Scholar]
- Jakšić, D.; Kocsubé, S.; Bencsik, O.; Kecskeméti, A.; Szekeres, A.; Jelić, D.; Kopjar, N.; Vágvölgyi, C.; Varga, J.; Šegvić Klarić, M. Fumonisin Production and Toxic Capacity in Airborne Black Aspergilli. Toxicol. Vitr. 2018, 53, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Ezziyyani, M.; Pérez Sánchez, C.; Emilia Requena, M.; Rubio, L.; Emilia Candela, M.; Candela, C.M.E. Biocontrol Por Streptomyces Rochei-Ziyani-, de La Podredumbre Del Pimiento (Capsicum annuum L.) Causada Por Phytophthora Capsici. An. Biol. 2004, 26, 69–78. [Google Scholar]
- Tiwari, P.; Kumar, B.; Kaur, M.; Kaur, G.; Kaur, H. Phytochemical Screening and Extraction: A Review. Int. Pharm. Sci. 2011, 1, 98–106. [Google Scholar]
- Arispe Vazquez, J.L.; Sanchez Arizpe, A.; Galindo Cepeda, M.E.; Vazquez Badillo, M.E.; Oyervides Garcia, A.; Rodriguez Guerra, R. Antagonism of Trichoderma Spp. in Fungi Associated with Damage of Diatraea saccharalis Fabricius. (Lepidoptera: Crambidae) in Corn. Boletín Micológico 2019, 34, 17–24. [Google Scholar] [CrossRef]
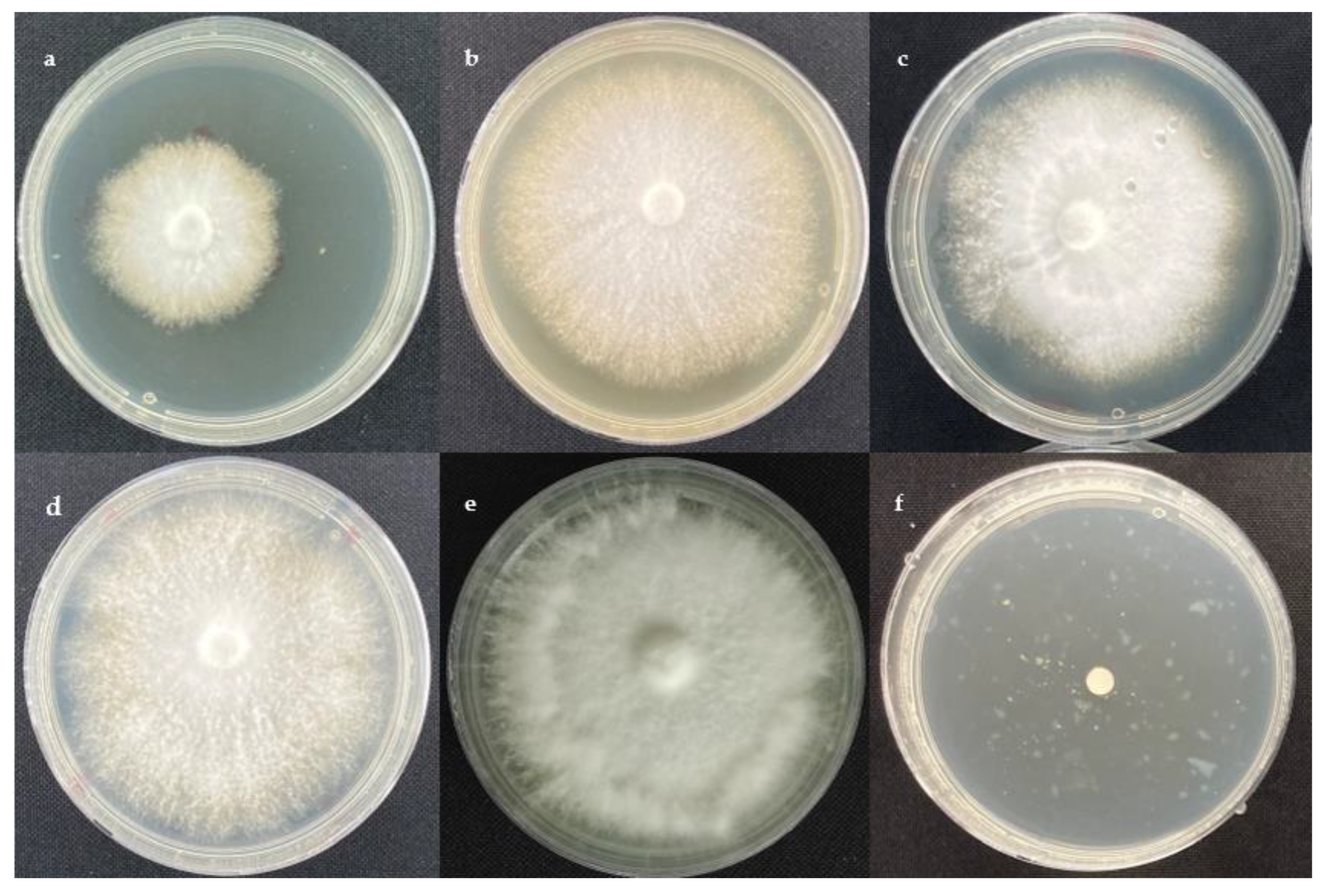
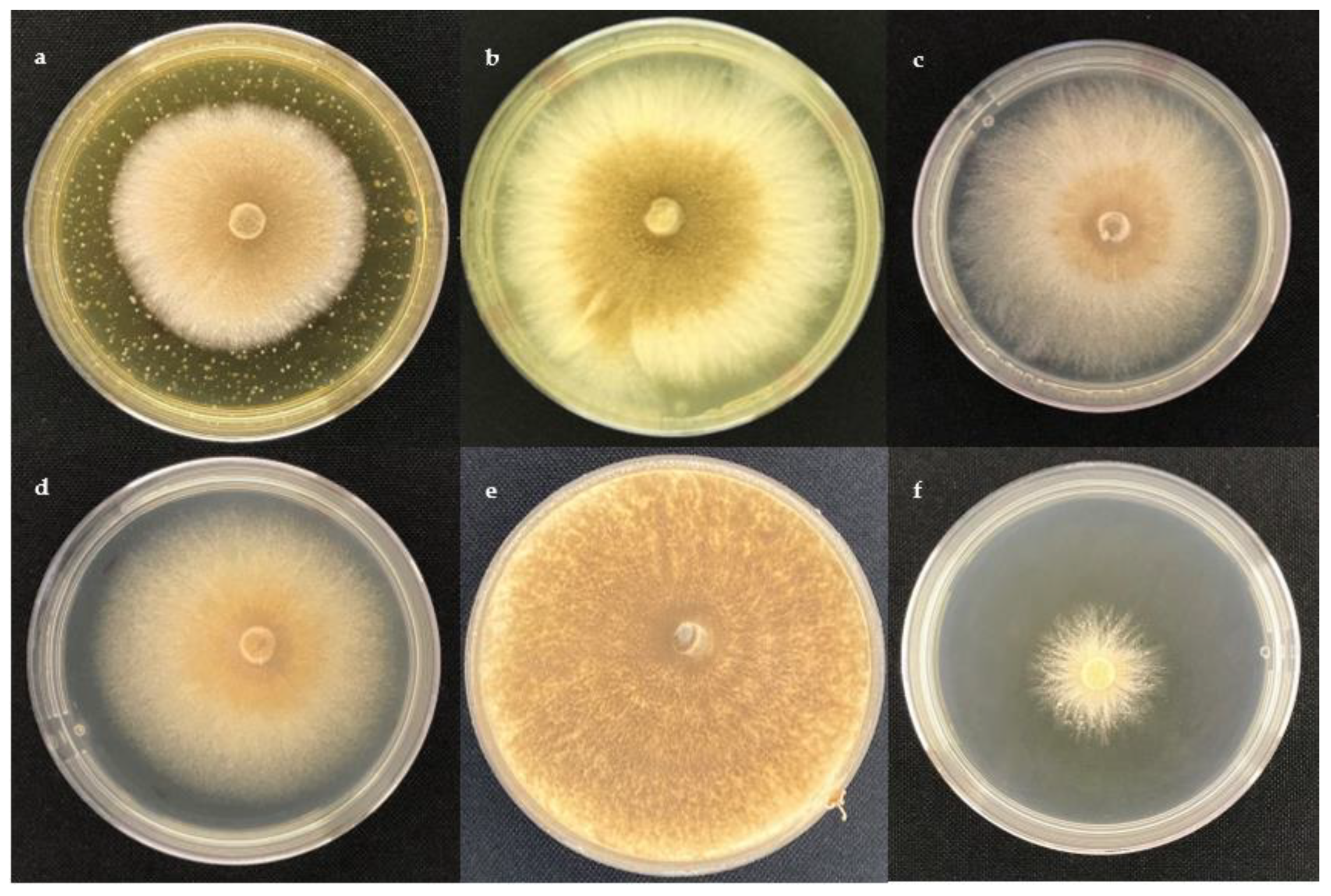
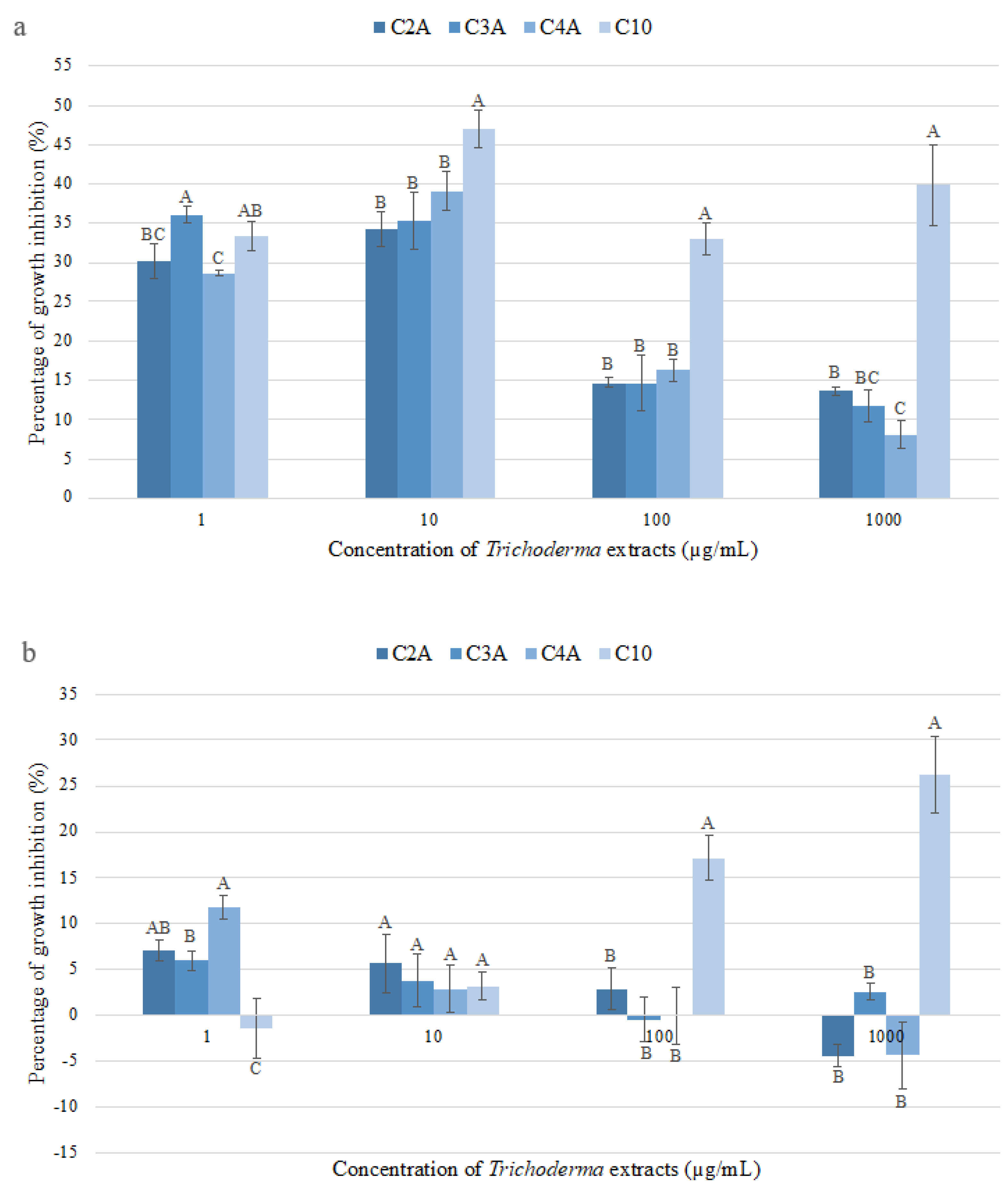
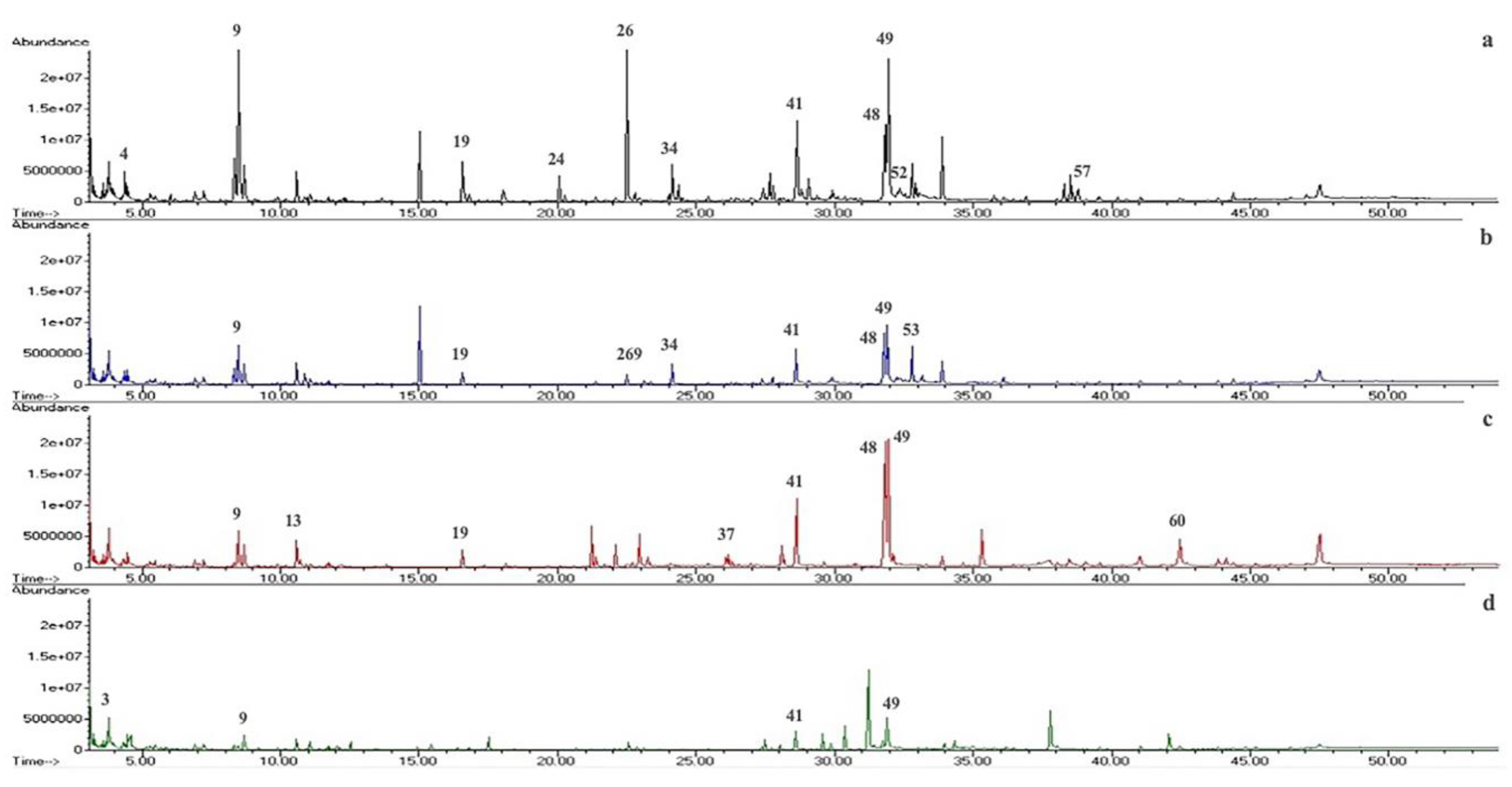
| Secondary Metabolites | Test | C2A | C3A | C4A | C10 |
|---|---|---|---|---|---|
| Alkaloids | Dragendorff | – | – | + | – |
| Mayer | ++ | – | – | – | |
| Wagner | +++ | – | – | – | |
| Lactones | Baljet | ++ | ++ | ++ | ++ |
| Quinones | Borntrager | ++ | – | – | +++ |
| Triterpenes and sterols | Liebermann-Burchard | + | + | + | + |
| Resins | Resins | – | – | – | – |
| Reducing sugars | Fehling | +++ | +++ | +++ | +++ |
| Saponins | Foam | – | – | – | – |
| Amino acids | Ninhydrin | – | – | – | – |
| Flavonoids | Shinoda | – | – | – | + |
| Anthocyanidins | – | – | – | + | |
| Catechins | + | + | – | + |
| Peak | Compounds | Peak Area (%) (1),(2) | Retention Index (Estimated) (3) | Retention Index (Reference) (4) | |||
|---|---|---|---|---|---|---|---|
| C2A | C3A | C4A | C10 | ||||
| 1 | Ethyl Valerate | 0.54 ± 0.06 | 0.95 ± 0.02 | - | - | 901.23 | 884 |
| 2 | 3-Methylcyclohexanol | 0.80 ± 0.19 | - | - | - | 912.21 | 969 |
| 3 | Butyl Isobutyrate | - | 1.22 ± 0.04 | 0.76 ± 0.04 | 1.37 ± 0.01 | 912.66 | 920 |
| 4 | Ethyl 3-Hydroxybutyrate | 1.22 ± 0.11 | - | - | - | 931.88 | 947 |
| 5 | 3-Hydroxybutyric acid | - | - | - | 2.84 ± 0.48 | 942.31 | 938 |
| 6 | 1,1-Diethoxyacetone | 0.05 ± 0.01 | - | - | - | 996.06 | 941 |
| 7 | 4-sec-Butoxy-2-butanone | - | - | 0.07 ± 0.01 | - | 1018.54 | 964 |
| 8 | Sorbic Acid | - | - | - | 0.20 ± 0.02 | 1052.53 | 990 |
| 9 | Phenylethyl Alcohol | 8.90 ± 1.06 | 5.42 ± 0.08 | 3.12 ± 0.13 | 0.83 ± 0.01 | 1104.39 | 1136 |
| 10 | 5,8-Decadien-2-one,5,9-dimethyl-,(E) | - | - | 0.07 ± 0.01 | - | 1125.63 | 1204 |
| 11 | 4-Ethoxy-4-oxobutanoic Acid | 0.13 ± 0.03 | - | - | - | 1154.13 | 1141 |
| 12 | Diethyl Succinate | 0.11 ± 0.01 | 0.19 ± 0.04 | 0.11 ± 0.03 | 0.33 ± 0.00 | 1167.54 | 1151 |
| 13 | β-Fenchyl Alcohol | - | - | 0.69 ± 0.02 | - | 1186.38 | 1138 |
| 14 | 5-Hydroxy-4,4,6-trimethyl-7-oxabicyclo [4.1.0]heptan-2-one | - | - | 0.36 ± 0.03 | - | 1200.26 | 1298 |
| 15 | 4-Hydroxy-2,4,5-trimethyl-2,5-cyclohexadien-1-one | - | - | - | 0.58 ± 0.03 | 1235.51 | 1246 |
| 16 | 1,3-Dioxolane-2-ethanethioic acid, 2-methyl- | 0.10 ± 0.03 | - | - | - | 1239.61 | 1224 |
| 17 | Mevalonolactone | 0.14 ± 0.02 | - | - | - | 1247.20 | 1156 |
| 18 | 2,5-Dimethylhydroquinone | 0.04 ± 0.02 | 0.06 ± 0.00 | - | - | 1364.40 | 1348 |
| 19 | Tyrosol | 1.80 ± 0.16 | 1.73 ± 0.02 | 1.34 ± 0.07 | 0.19 ± 0.00 | 1405.59 | 1356 |
| 20 | 1,4-Cadinadiene | 0.41 ± 0.02 | - | - | - | 1415.20 | 1440 |
| 21 | Neoclovene | - | - | 0.13 ± 0.01 | - | 1437.69 | 1416 |
| 22 | Caryophyllene | 0.09 ± 0.01 | - | - | - | 1440.57 | 1494 |
| 23 | 7-Epi-cis-Sesquisabinene hydrate | 0.05 ± 0.02 | - | - | - | 1495.09 | 1523 |
| 24 | Nerolidol | 0.97 ± 0.07 | 0.11 ± 0.01 | - | - | 1547.13 | 1564 |
| 25 | 1-(3,3,6a-Trimethyl-1a,2,3,5,6a,6b-hexahydro-1H-6-oxa-cyclopropa[e]inden-5-yl)-ethanone | - | - | - | 0.25 ± 0.02 | 1573.29 | 1508 |
| 26 | Spiro[4.5]dec-8-en-7-ol, 1,8-dimethyl-4-(1-methylethyl)- | 6.78 ± 0.80 | 1.12 ± 0.07 | 0.22 ± 0.05 | - | 1652.04 | 1630 |
| 27 | Widdrol | 0.55 ± 0.04 | - | - | - | 1664.67 | 1651 |
| 28 | 4-Acoren-3-one | 0.30 ± 0.01 | - | - | - | 1672.24 | 1614 |
| 29 | 7-Phenylheptan-1-ol | - | - | - | 0.21 ± 0.00 | 1679.42 | 1633 |
| 30 | 6-Phenylhexanoic Acid | - | 0.48 ± 0.08 | - | - | 1679.55 | 1647 |
| 31 | N-Methyl-N-[4-(1-pyrrolidinyl)-2-butynyl]-2-aminoacetamide | 0.09 ± 0.02 | - | - | - | 1684.37 | 1770 |
| 32 | Alpha-Bisabolol oxide B | - | 0.32 ± 0.03 | - | - | 1690.13 | 1707 |
| 33 | Formic acid, 3,7,11-trimethyl-1,6,10-dodecatrien-3-yl ester | 0.09 ± 0.04 | - | - | - | 1690.35 | 1752 |
| 34 | 1-(hydroxymethyl)-2,5,5,8a-tetramethyldecahydro-2-naphthalenol | 1.70 ± 0.18 | 1.99 ± 0.02 | - | - | 1726.09 | 1825 |
| 35 | β-Santanol Acetate | 0.06 ± 0.01 | 0.16 ± 0.04 | - | - | 1795.41 | 1791 |
| 36 | 1,1,4,6-Tetramethyl-1a,2,3,4a,5,7,7a,7b-octahydrocyclopropa[e]azulene-4,5,6-triol | 0.07 ± 0.01 | - | - | - | 1805.00 | 1869 |
| 37 | Methyl 5,7-hexadecadiynoate | - | - | 0.32 ± 0.01 | - | 1828.37 | 1913 |
| 38 | Palmitelaidic Acid | - | 0.19 ± 0.03 | 0.53 ± 0.04 | 0.27 ± 0.03 | 1918.46 | 1976 |
| 39 | Artemisinin | 0.21 ± 0.05 | - | - | - | 1918.63 | 1903 |
| 40 | (S)-3-(4-Hydroxybenzyl)piperazine-2,5-dione | 0.06 ± 0.03 | - | - | - | 1932.11 | 2001 |
| 41 | Palmitic Acid | 4.67 ± 0.78 | 4.44 ± 0.04 | 5.93 ± 0.10 | - | 1942.18 | 1968 |
| 42 | 5α-Acetoxymethyl-4a,5,8,8α-tetrahydro-2,4aβ-dimethyl-1,4-naphthalindione | 1.41 ± 0.13 | - | - | - | 1963.71 | 1998 |
| 43 | Ethyl Palmitate | 0.10 ± 0.02 | - | - | - | 1975.09 | 1978 |
| 44 | 2,3-Dehydro-9-hydroxy-β-agarofuran | 0.26 ± 0.03 | - | - | - | 1979.63 | 2076 |
| 45 | Lactaropallidin | 0.36 ± 0.07 | 0.48 ± 0.02 | - | - | 2018.27 | 2003 |
| 46 | Methyl octadeca-6,9-diynoate | - | - | 0.10 ± 0.00 | - | 2039.21 | 2112 |
| 47 | Hanphyllin | - | 0.18 ± 0.01 | - | - | 2044.11 | 2085 |
| 48 | Linoleic acid | 5.00 ± 1.71 | 6.51 ± 0.13 | 13.42 ± 0.15 | 1.70 ± 0.14 | 2109.95 | 2183 |
| 49 | Oleic acid | 9.76 ± 1.80 | 9.63 ± 0.13 | 11.45 ± 0.13 | 7.57 ± 0.38 | 2116.35 | 2175 |
| 50 | Ethyl Linoleate | - | - | - | 0.48 ± 0.20 | 2126.39 | 2193 |
| 51 | Cyclopropanecarboxylic acid, 2,6-di-t-butyl-4-methoxy-phenyl ester | 0.61 ± 0.04 | 1.32 ± 0.05 | - | - | 2134.17 | 2104 |
| 52 | Stearic acid | 1.58 ± 0.36 | - | - | - | 2139.50 | 2167 |
| 53 | Sorbicillin | 1.96 ± 0.08 | 4.14 ± 0.06 | - | - | 2164.42 | 2160 |
| 54 | 3-Ethyl-3-hydroxyandrostan-17-one | - | - | 0.25 ± 0.08 | - | 2171.72 | 2251 |
| 55 | Acetyloxyparthenin | - | - | - | 0.36 ± 0.07 | 2363.24 | 2284 |
| 56 | 6,8-dimethoxy-3-methyl-3-(3′-methylbut-2′-enyl)-1H-quinoline-2,4-dione | - | - | 2.48 ± 0.45 | - | 2456.98 | 2397 |
| 57 | S-[(E)-1,3-Diphenylbut-2-enyl] N,N-dimethylcarbamothioate | 0.92 ± 0.02 | - | - | - | 2525.64 | 2436 |
| 58 | 4a,7a-Epoxy-5H-cyclopenta[a]cyclopropa[f]cycloundecen-4(1H)-one, 1a,6,7,10,11,11a-hexahydro-7,10,11-trihydroxy-1,1,3,6,9-pentamethyl- | 0.05 ± 0.01 | 0.23 ± 0.02 | - | - | 2534.54 | 2591 |
| 59 | 1,3,5,7,9,11,13,15,17,19,21,23-Cyclotetracosadodecaene | 0.16 ± 0.02 | - | - | - | 2619.26 | 2664 |
| 60 | Phorbol | - | - | 0.40 ± 0.06 | - | 2793.65 | 2774 |
| 61 | 8,9-Benzodispiro[2.0.2.4]decane, 7-(3-methoxy-2-oxa-1-oxocyclopent-5-yl)-10-phenyl- | - | - | - | 0.61 ± 0.02 | 2952.57 | 2974 |
| 62 | 3-Hydroxyspirost-8-en-11-one | - | - | 0.11 ± 0.04 | - | 3130.95 | 3044 |
| 63 | 7,8-Epoxylanostan-11-ol, 3-acetoxy- | 0.10 ± 0.01 | - | - | - | 3139.42 | 3145 |
| Strain | Source | Location | DNA Region | Identity | Identify Code |
|---|---|---|---|---|---|
| C2A | Soil | Guayas, Ecuador | ITS1-5,8-ITS2 | Trichoderma reesei | CCMCIBE-H1103 |
| C3A | Soil | Guayas, Ecuador | ITS1-5,8-ITS2 | Trichoderma sp. | CCMCIBE-H1104 |
| C4A | Soil | Guayas, Ecuador | ITS1-5,8-ITS2 | Trichoderma harzianum | CCMCIBE-H1105 |
| C10 | Soil | Guayas, Ecuador | ITS1-5,8-ITS2 | Trichoderma spirale | CCMCIBE-H1106 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chóez-Guaranda, I.; Espinoza-Lozano, F.; Reyes-Araujo, D.; Romero, C.; Manzano, P.; Galarza, L.; Sosa, D. Chemical Characterization of Trichoderma spp. Extracts with Antifungal Activity against Cocoa Pathogens. Molecules 2023, 28, 3208. https://doi.org/10.3390/molecules28073208
Chóez-Guaranda I, Espinoza-Lozano F, Reyes-Araujo D, Romero C, Manzano P, Galarza L, Sosa D. Chemical Characterization of Trichoderma spp. Extracts with Antifungal Activity against Cocoa Pathogens. Molecules. 2023; 28(7):3208. https://doi.org/10.3390/molecules28073208
Chicago/Turabian StyleChóez-Guaranda, Ivan, Fernando Espinoza-Lozano, Dennys Reyes-Araujo, Christian Romero, Patricia Manzano, Luis Galarza, and Daynet Sosa. 2023. "Chemical Characterization of Trichoderma spp. Extracts with Antifungal Activity against Cocoa Pathogens" Molecules 28, no. 7: 3208. https://doi.org/10.3390/molecules28073208
APA StyleChóez-Guaranda, I., Espinoza-Lozano, F., Reyes-Araujo, D., Romero, C., Manzano, P., Galarza, L., & Sosa, D. (2023). Chemical Characterization of Trichoderma spp. Extracts with Antifungal Activity against Cocoa Pathogens. Molecules, 28(7), 3208. https://doi.org/10.3390/molecules28073208







