Synthesis of High Specific Surface Lithium-Ion Sieve Templated by Bacterial Cellulose for Selective Adsorption of Li+
Abstract
1. Introduction
2. Results and Discussion
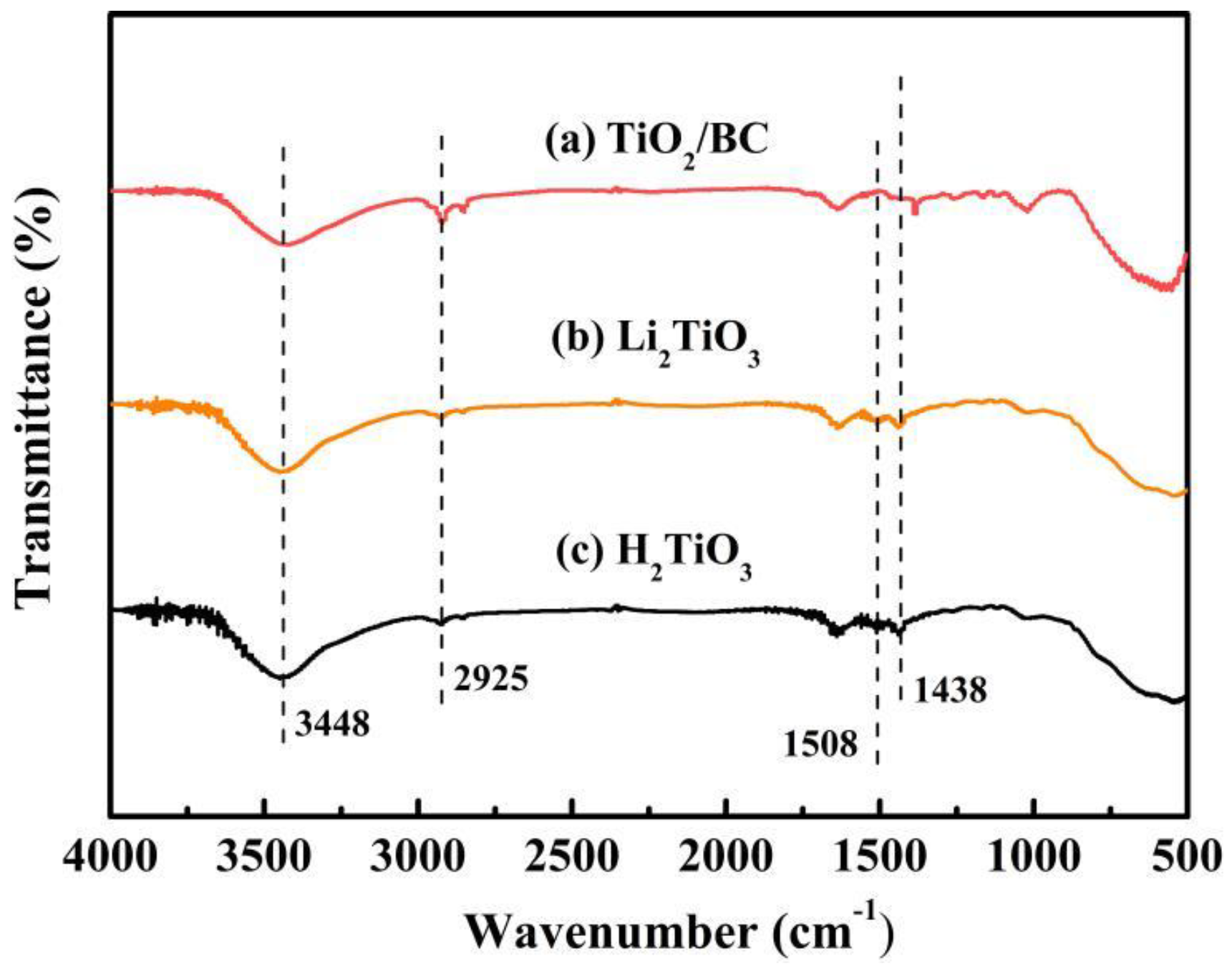
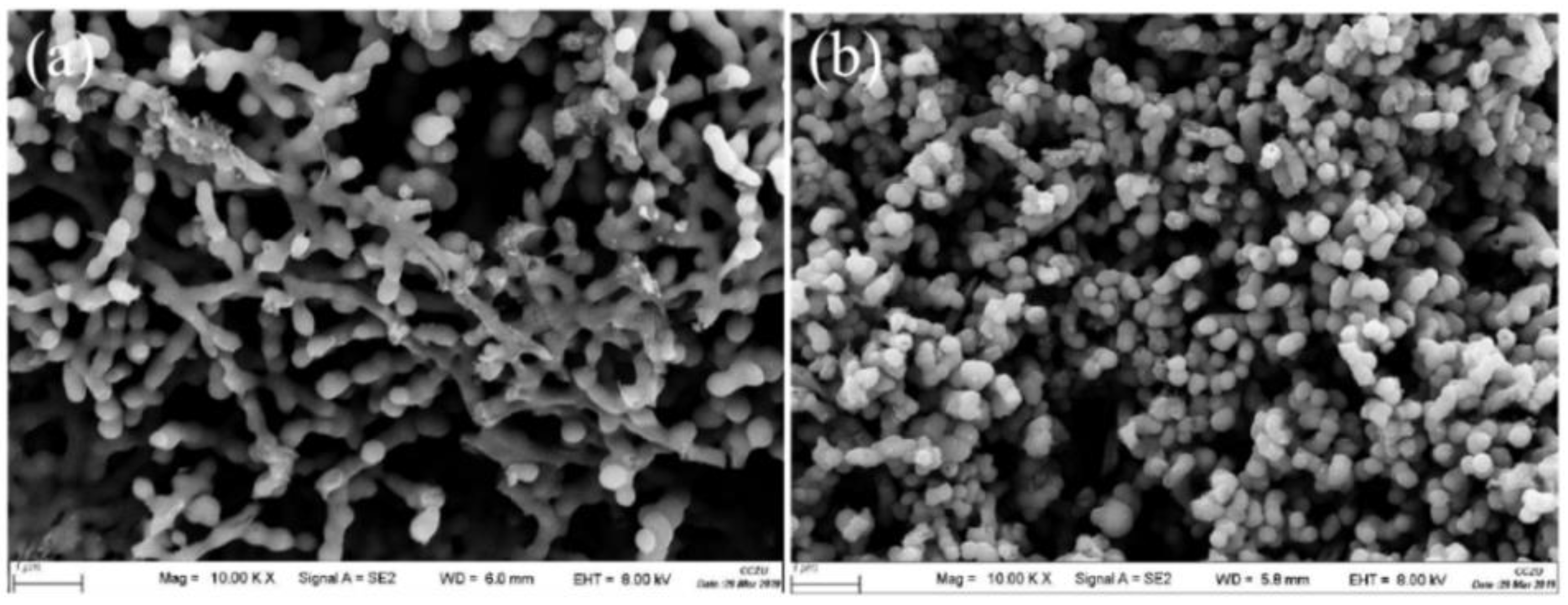
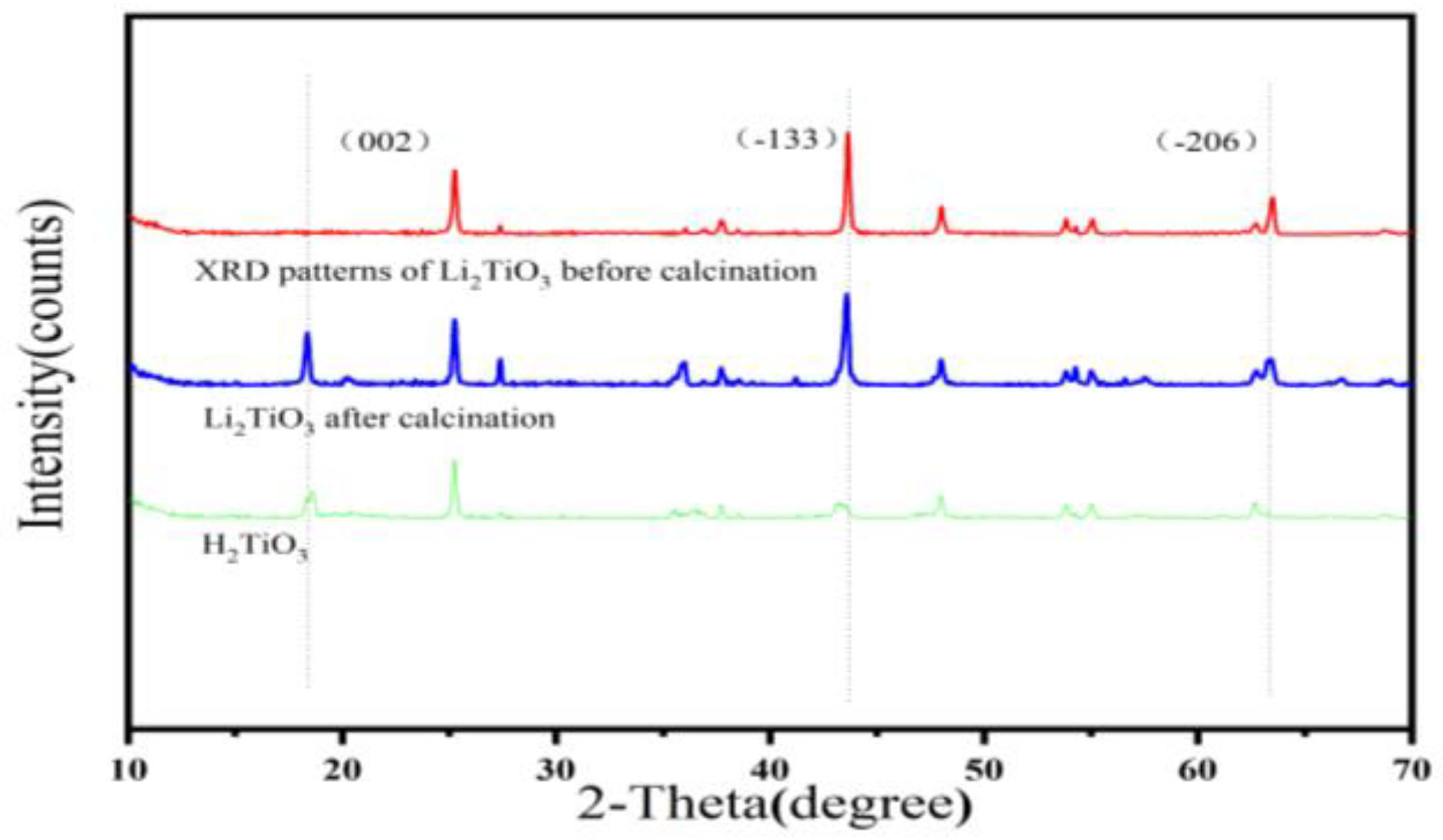
Characterizations of Li2TiO3 and H2TiO3
3. Experiment
3.1. Sample Materials and Reagents
3.2. Instruments
3.3. Static Adsorption Experiment
3.4. Synthesis of the Li2TiO3
3.5. Synthesis of the H2TiO3
4. Adsorption Performance of H2TiO3
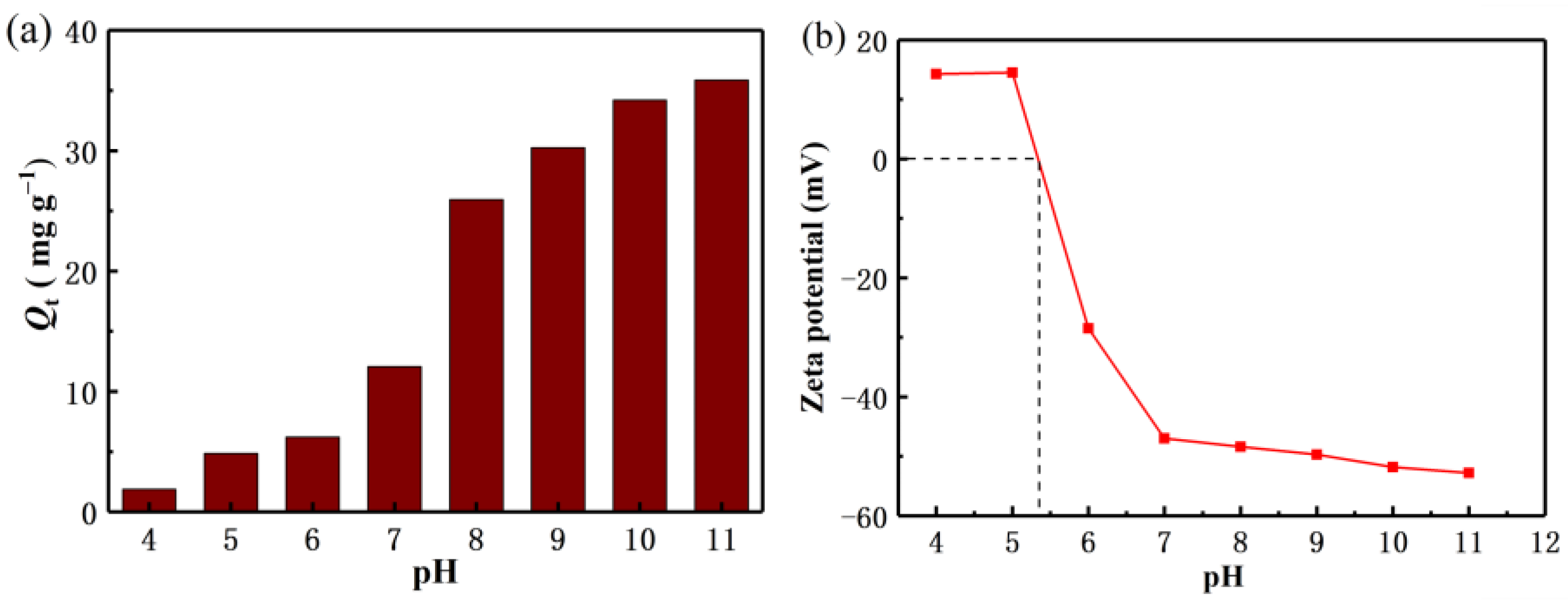
4.1. Effect of pH
4.2. Adsorption Kinetics
4.3. Adsorption Isotherms
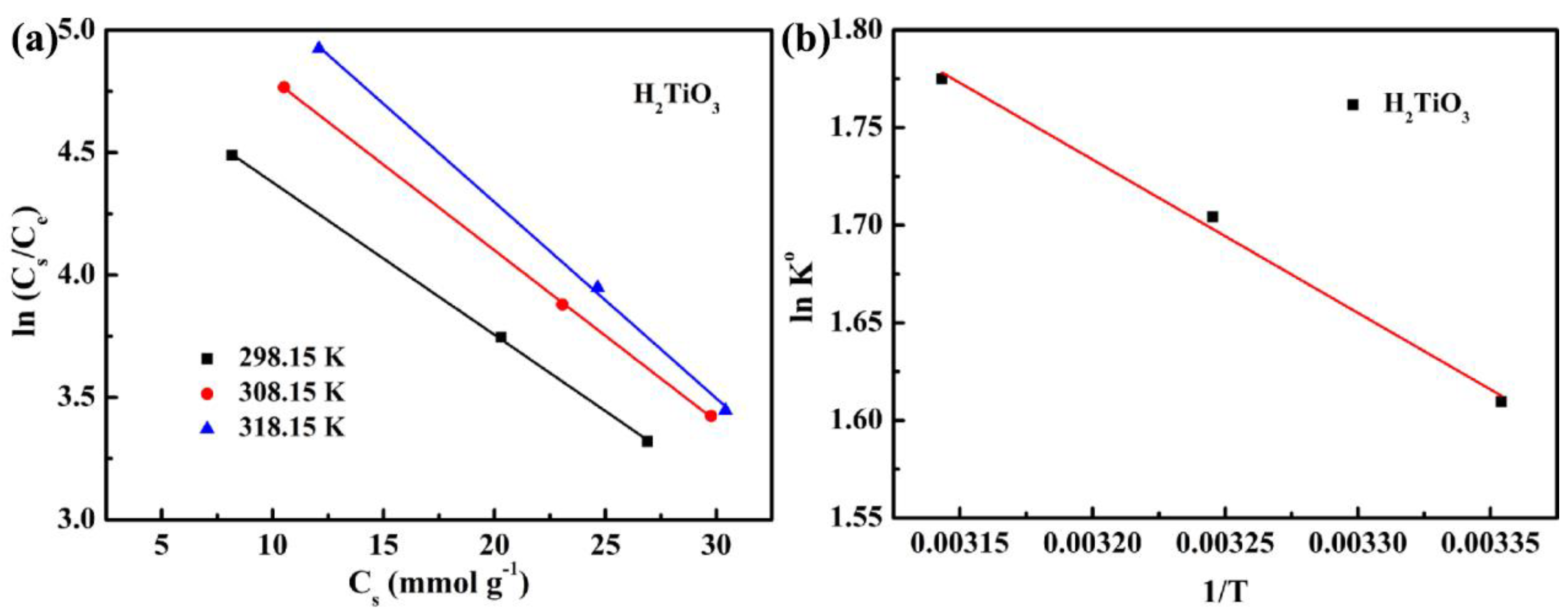
4.4. Effect of Temperature
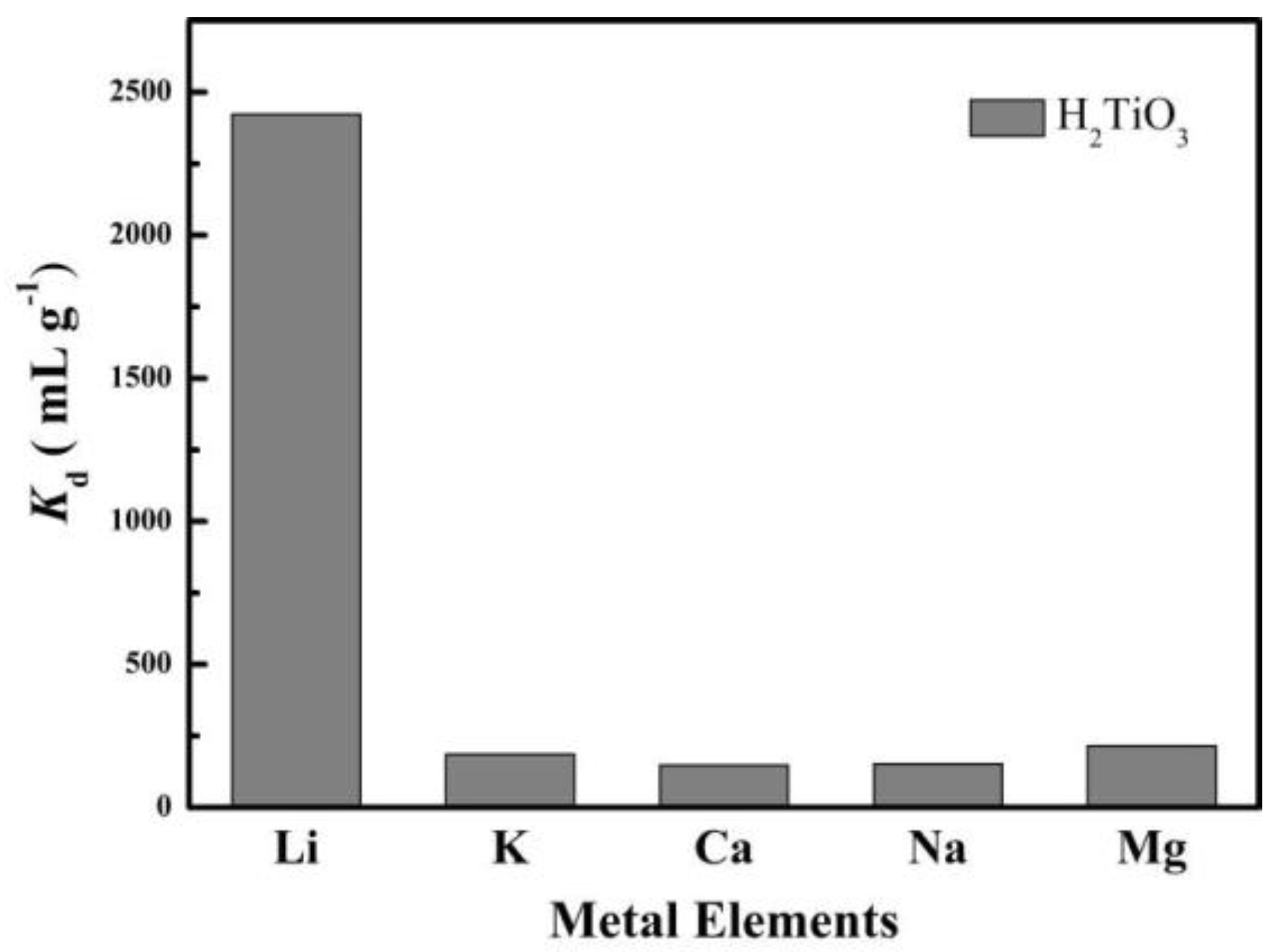
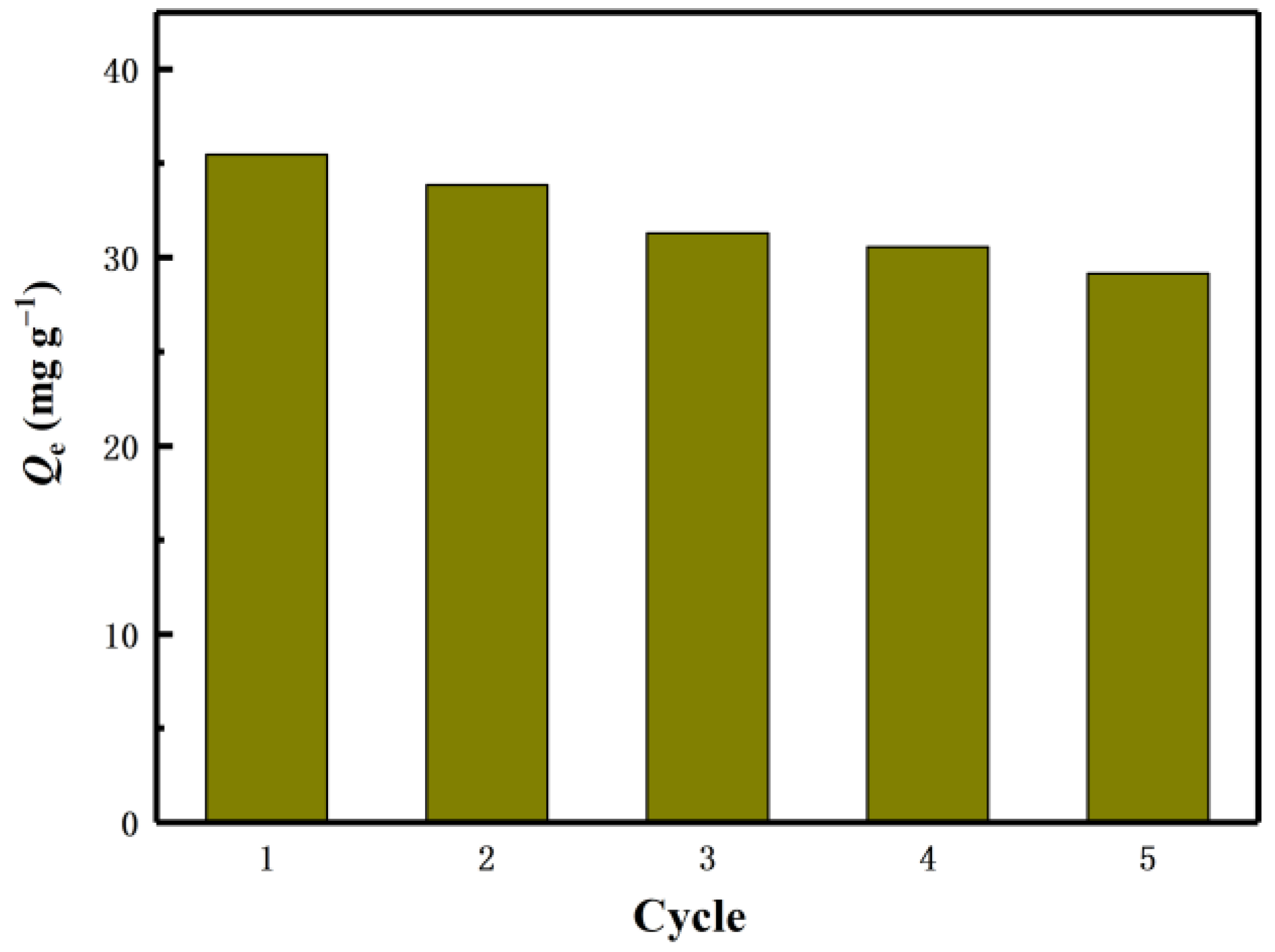
4.5. Selective and Reusability Tests
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chitrakar, R.; Makita, Y.; Ooi, K.; Sonoda, A. Lithium recovery from salt lake brine by H2TiO3. Dalton Trans. 2014, 43, 8933–8939. [Google Scholar] [CrossRef] [PubMed]
- Ciolacu, D.E.; Darie, R.N. Nanocomposites Based on Cellulose, Hemicelluloses, and Lignin. In Nanomaterials and Nanocomposites: Zero- to Three-Dimensional Materials and Their Composites; Wiley-VCH: Weinheim, Germany, 2016. [Google Scholar]
- Gao, A.; Sun, Z.; Li, S.; Hou, X.; Li, H.; Wu, Q.; Xi, X. The mechanism of manganese dissolution on Li1.6Mn1.6O4 ion sieves with HCl. Dalton Trans. 2018, 47, 3864–3871. [Google Scholar] [CrossRef] [PubMed]
- Grágeda, M.; González, A.; Grágeda, M.; Ushak, S. Purification of brines by chemical precipitation and ion-exchange processes for obtaining battery-grade lithium compounds. Int. J. Energy Res. 2018, 42, 2386–2399. [Google Scholar] [CrossRef]
- Guo, X.; Cao, X.; Huang, G.; Tian, Q.; Sun, H. Recovery of lithium from the effluent obtained in the process of spent lithium-ion batteries recycling. J. Environ. Manag. 2017, 198, 84–89. [Google Scholar] [CrossRef]
- He, L.; Xu, W.; Song, Y.; Luo, Y.; Liu, X.; Zhao, Z. New Insights into the Application of Lithium-Ion Battery Materials: Selective Extraction of Lithium from Brines via a Rocking-Chair Lithium-Ion Battery System. Glob. Chall. 2018, 2, 1700079. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-B.; Yang, Y.; Chen, L.-H.; Wang, Y.; Huang, S.-Z.; Tao, J.-W.; Ma, X.-T.; Hasan, T.; Li, Y.; Xu, Y.; et al. Hierarchical TiO2/C nanocomposite monoliths with a robust scaffolding architecture, mesopore–macropore network and TiO2–C heterostructure for high-performance lithium ion batteries. Nanoscale 2016, 8, 10928–10937. [Google Scholar] [CrossRef] [PubMed]
- Jang, E.; Jang, Y.; Chung, E. Lithium recovery from shale gas produced water using solvent extraction. Appl. Geochem. 2017, 78, 343–350. [Google Scholar] [CrossRef]
- Jiang, J.H. Property of LiMn0.5Ti0.75O3 Type Ion-Exchangers and Extraction for Lithium. Adv. Mater. Res. 2012, 549, 118–121. [Google Scholar]
- Li, J.; Zou, T.; Liu, X.; Wang, D.; Ding, X. The Metallogenetic Regularities of Lithium Deposits in China. Acta Geol. Sin. Engl. Ed. 2015, 89, 652–670. [Google Scholar]
- Li, N.; Lu, D.; Zhang, J.; Wang, L. Yolk-shell structured composite for fast and selective lithium ion sieving. J. Colloid Interface Sci. 2018, 520, 33–40. [Google Scholar] [CrossRef]
- Li, Z.; Qin, J.; Yin, X.; Li, J.; Qiang, L.; Qin, Z. Direct sulfation of bacterial cellulose with a ClSO3H/DMF complex and structure characterization of the sulfates. Polym. Adv. Technol. 2014, 25, 168–172. [Google Scholar]
- Ma, L.; Xi, X.; Wang, K.; Zhao, L. Adsorption of Li by a lithium ion-sieve using a buffer system and application for the recovery of Li from a spent lithium-ion battery. Res. Chem. Intermed. 2018, 44, 6721–6739. [Google Scholar] [CrossRef]
- Park, M.J.; Nisola, G.M.; Vivas, E.L.; Limjuco, L.A.; Lawagon, C.P.; Gil Seo, J.; Kim, H.; Shon, H.K.; Chung, W.-J. Mixed matrix nanofiber as a flow-through membrane adsorber for continuous Li+ recovery from seawater. J. Membr. Sci. 2016, 510, 141–154. [Google Scholar] [CrossRef]
- Qian, W.U.; Liu, X.F.; Zheng, M.P.; Nie, Z.; Yu, J.J. Present situation, existing problems and countermeasures of development of salt lake lithium resources in China. Mod. Chem. Ind. 2017, 37, 1–5. [Google Scholar]
- Romero, V.C.E.; Tagliazucchi, M.; Flexer, V.; Calvo, E.J. Sustainable Electrochemical Extraction of Lithium from Natural Brine for Renewable Energy Storage. J. Electrochem. Soc. 2018, 165, A2294–A2302. [Google Scholar] [CrossRef]
- Sasaki, K.; Yu, Q. Synthesis of a Biotemplated Lithium Ion-Sieve Derived from Fungally Formed Birnessite. In Advances in the Environmental Biogeochemistry of Manganese Oxides; American Chemical Society: Washington, DC, USA, 2015; pp. 169–183. [Google Scholar] [CrossRef]
- Song, J.F.; Long, D.N.; Li, X.M.; He, T. Lithium extraction from Chinese salt-lake brines: Opportunities, challenges, and future outlook. Environ. Sci. Water Res. Technol. 2017, 3, 593–597. [Google Scholar] [CrossRef]
- Song, S.T.; Wu, S.X.; Peng, Y.S.; Zheng, X.F.; Lian, Q. Study on Synthesis and Properties of Spinel Structure Li1+xMn2−xO4 for Lithium Ion-Sieve Precursor. Appl. Mech. Mater. 2013, 437, 560–563. [Google Scholar]
- Wallington, T.J.; Alonso, E.; Everson, M.P.; Field, F.R.; Gruber, P.W.; Keoleian, G.A.; Kesler, S.E.; Kirchain, R.E.; Medina, P.A.; Kolinski Morris, E.K.; et al. Sustainable Mobility: Lithium, Rare Earth Elements, and Electric Vehicles. In Proceedings of the FISITA 2012 World Automotive Congress: Volume 3: Future Automotive Powertrains (I); Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Wang, S.; Chen, X.; Zhang, Y.; Zheng, S. Lithium adsorption from brine by iron-doped titanium lithium ion sieves. Particuology 2018, 41, 40–47. [Google Scholar] [CrossRef]
- Wang, S.; Li, P.; Cui, W.; Zhang, H.; Wang, H.; Zheng, S.; Zhang, Y. Hydrothermal synthesis of lithium-enriched β-Li2TiO3 with an ion-sieve application: Excellent lithium adsorption. RSC Adv. 2016, 6, 102608–102616. [Google Scholar] [CrossRef]
- Wei, S.; Wei, Y.; Chen, T.; Liu, C.; Tang, Y. Porous lithium ion sieves nanofibers: General synthesis strategy and highly selective recovery of lithium from brine water. Chem. Eng. J. 2020, 379, 122407. [Google Scholar] [CrossRef]
- Wen, Z.; Mou, Y.; Song, Z.; Xie, L.; Wang, Y.; Jing, C. Adsorption Materials for Lithium Ion from Brine Resources and Their Performances Progress in Chemistry. Prog. Chem. 2017, 29, 231. [Google Scholar]
- Xu, X.; Chen, Y.; Wan, P.; Gasem, K.; Wang, K.; He, T.; Adidharma, H.; Fan, M. Extraction of lithium with functionalized lithium ion-sieves. Prog. Mater. Sci. 2016, 84, 276–313. [Google Scholar] [CrossRef]
- Yang, S.S.; Zhou, M.L.; Wu, J.Q.; Shen, J.N.; Gao, C.J. Development and Adsorption Properties for a Novel Lithium Ion-Sieve. Mater. Sci. Forum 2016, 852, 691–697. [Google Scholar]
- Yang, Y.; Xu, S.; He, Y. Lithium recycling and cathode material regeneration from acid leach liquor of spent lithium-ion battery via facile co-extraction and co-precipitation processes. Waste Manag. 2017, 64, 219–227. [Google Scholar] [CrossRef]
- Yao, W.R.; Xu, Q.H. Research Progress in Nanocellulose Preparation. Adv. Mater. Res. 2014, 988, 101–105. [Google Scholar]
- Yu, C.L.; Yanagisawa, K.; Kamiya, S.; Kozawa, T.; Ueda, T. Monoclinic Li2TiO3 nano-particles via hydrothermal reaction: Processing and structure. Ceram. Int. 2014, 40, 1901–1908. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, Y.; Zhang, F.; Li, Z.; Yan, Y. Dual-template docking oriented ionic imprinted bilayer mesoporous films with efficient recovery of neodymium and dysprosium. J. Hazard. Mater. 2018, 353, 496–504. [Google Scholar] [CrossRef]
- Zhu, M.Z. Study on extracting lithium in salt lake brine with solvent extraction method. Ind. Miner. Process. 2016, 32, 121. [Google Scholar]



| Sorbents | Qe,exp (mg g−1) | PFOKM | PSOKM | ||||
|---|---|---|---|---|---|---|---|
| Qe,c (mg g−1) | k1 (min−1) | R2 | Qe,c (mg g−1) | k2 × 10−2 (g mg−1 min−1) | R2 | ||
| H2TiO3 | 35.45 | 33.28 | 0.0172 | 0.975 | 36.29 | 0.068 | 0.991 |
| Sorbents | Langmuir Isotherm Model | Freundlich Isotherm Model | ||||
|---|---|---|---|---|---|---|
| Qm (mg g−1) | KL (L mg−1) | R2 | KF (mg g−1) | 1/n | R2 | |
| H2TiO3 | 38.96 | 0.003 | 0.998 | 2.01 | 0.38 | 0.948 |
| Sorbents | ∆H° (kJ mol−1) | ∆S° (J mol−1) | T (K) | K° | ∆G° (kJ mol−1) | R2 |
|---|---|---|---|---|---|---|
| H2TiO3 | 6.54 | 35.31 | 298.15 | 5.00 | −3.99 | 0.992 |
| 308.15 | 5.50 | −4.37 | ||||
| 318.15 | 5.90 | −4.70 |
| Cation | H2TiO3 | ||
|---|---|---|---|
| Cf (mg L−1) | Kd (mL g−1) | k | |
| Li+ | 14.609 | 2422.548 | |
| K+ | 48.059 | 40.388 | 0.017 |
| Ca2+ | 45.961 | 87.879 | 2.176 |
| Na+ | 47.634 | 49.670 | 0.565 |
| Mg2+ | 44.087 | 134.121 | 2.700 |
| Adsorbent | Adsorption Capacity | Reference Literature |
|---|---|---|
| Li1.6Mn1.6O4 | 44 mg g−1 | (Gao et al. 2018) [3] |
| RHBC-Mnx | 88.4mg g−1 | (Yu et al. 2020) [29] |
| Li2CO3 | 32.6 mg g−1 | (Chitrakar et al. 2014) [1] |
| β-Li2TiO3 | 30.4mg g−1 | (Wang et al. 2016) [22] |
| H2TiO3 | 35.45 mg g−1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Zheng, X.; Xu, T.; Zhang, Y.; Li, G.; Li, Z. Synthesis of High Specific Surface Lithium-Ion Sieve Templated by Bacterial Cellulose for Selective Adsorption of Li+. Molecules 2023, 28, 3191. https://doi.org/10.3390/molecules28073191
Zhang X, Zheng X, Xu T, Zhang Y, Li G, Li Z. Synthesis of High Specific Surface Lithium-Ion Sieve Templated by Bacterial Cellulose for Selective Adsorption of Li+. Molecules. 2023; 28(7):3191. https://doi.org/10.3390/molecules28073191
Chicago/Turabian StyleZhang, Xi, Xudong Zheng, Tongtong Xu, Yuzhe Zhang, Guomeng Li, and Zhongyu Li. 2023. "Synthesis of High Specific Surface Lithium-Ion Sieve Templated by Bacterial Cellulose for Selective Adsorption of Li+" Molecules 28, no. 7: 3191. https://doi.org/10.3390/molecules28073191
APA StyleZhang, X., Zheng, X., Xu, T., Zhang, Y., Li, G., & Li, Z. (2023). Synthesis of High Specific Surface Lithium-Ion Sieve Templated by Bacterial Cellulose for Selective Adsorption of Li+. Molecules, 28(7), 3191. https://doi.org/10.3390/molecules28073191







