Evaluation of the Anticancer Activity and Mechanism Studies of Glycyrrhetic Acid Derivatives toward HeLa Cells
Abstract
1. Introduction
2. Results and Discussion
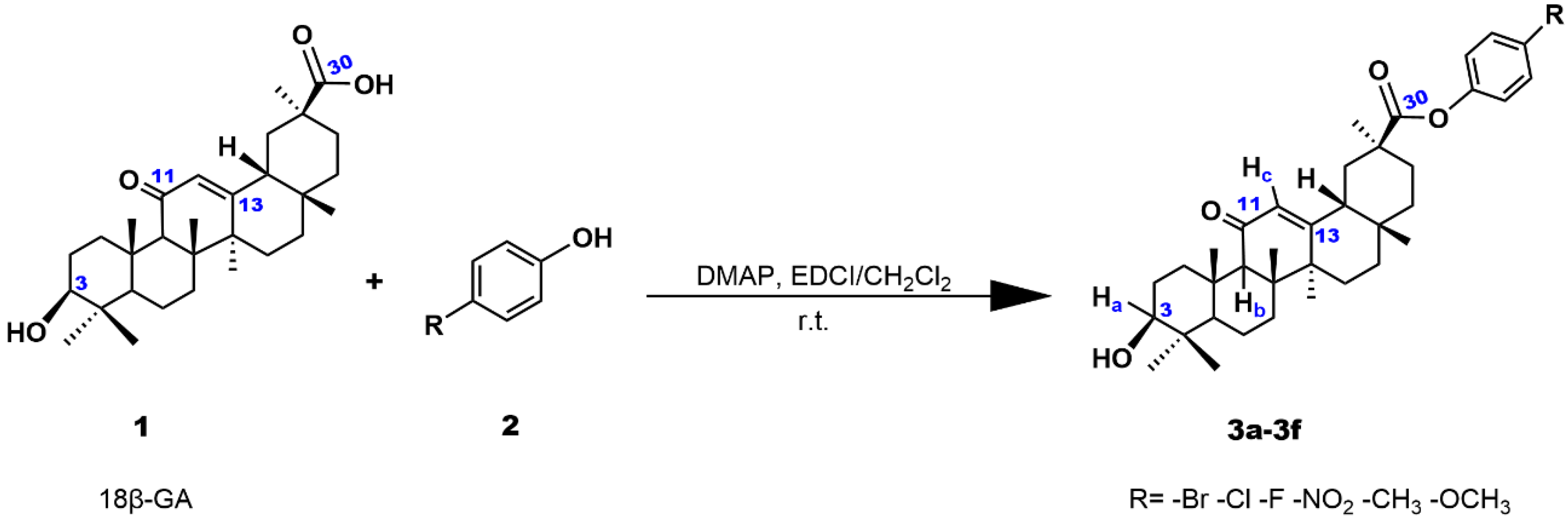
2.1. Chemistry
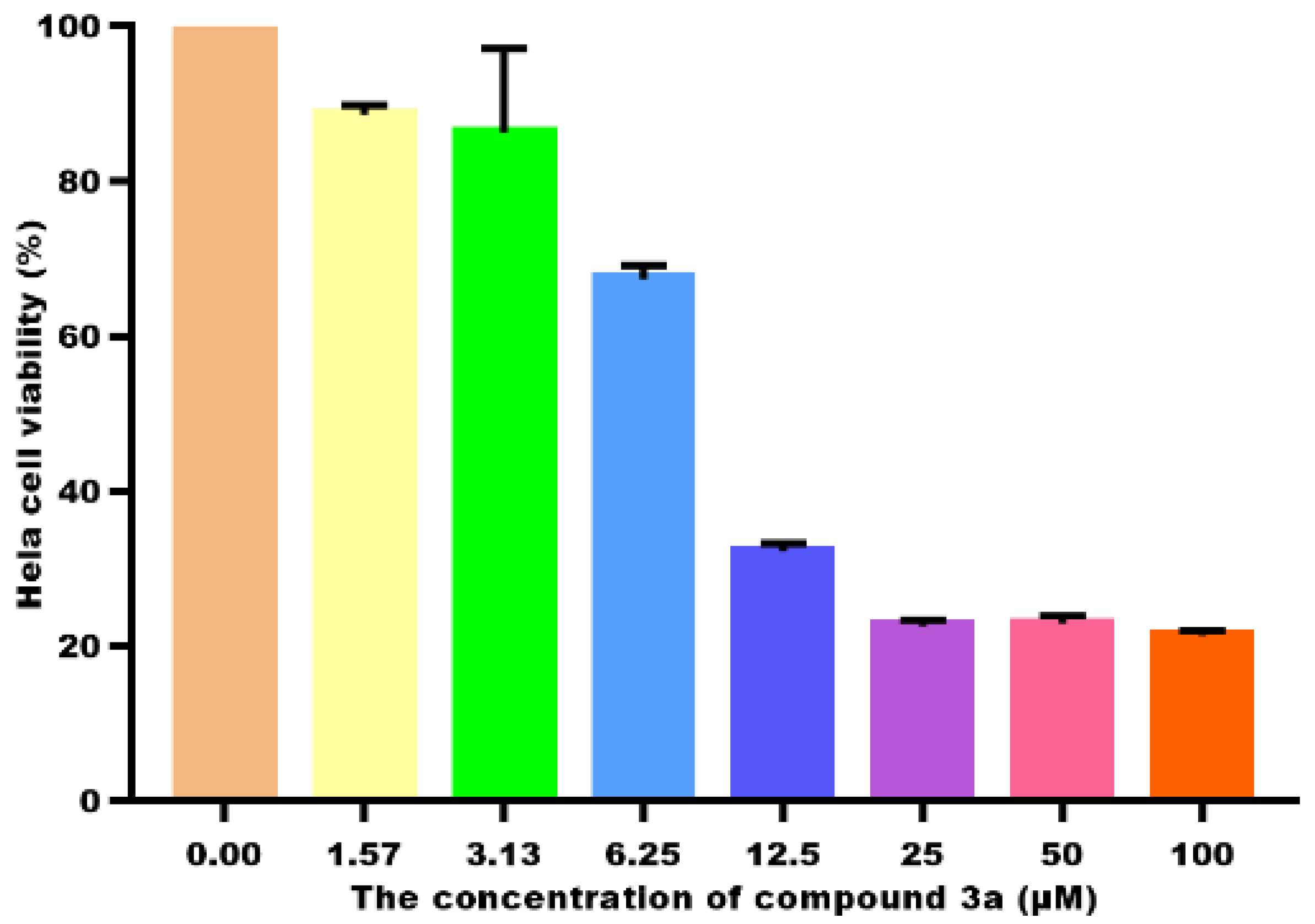
2.2. Cell Viability and IC50 Determination
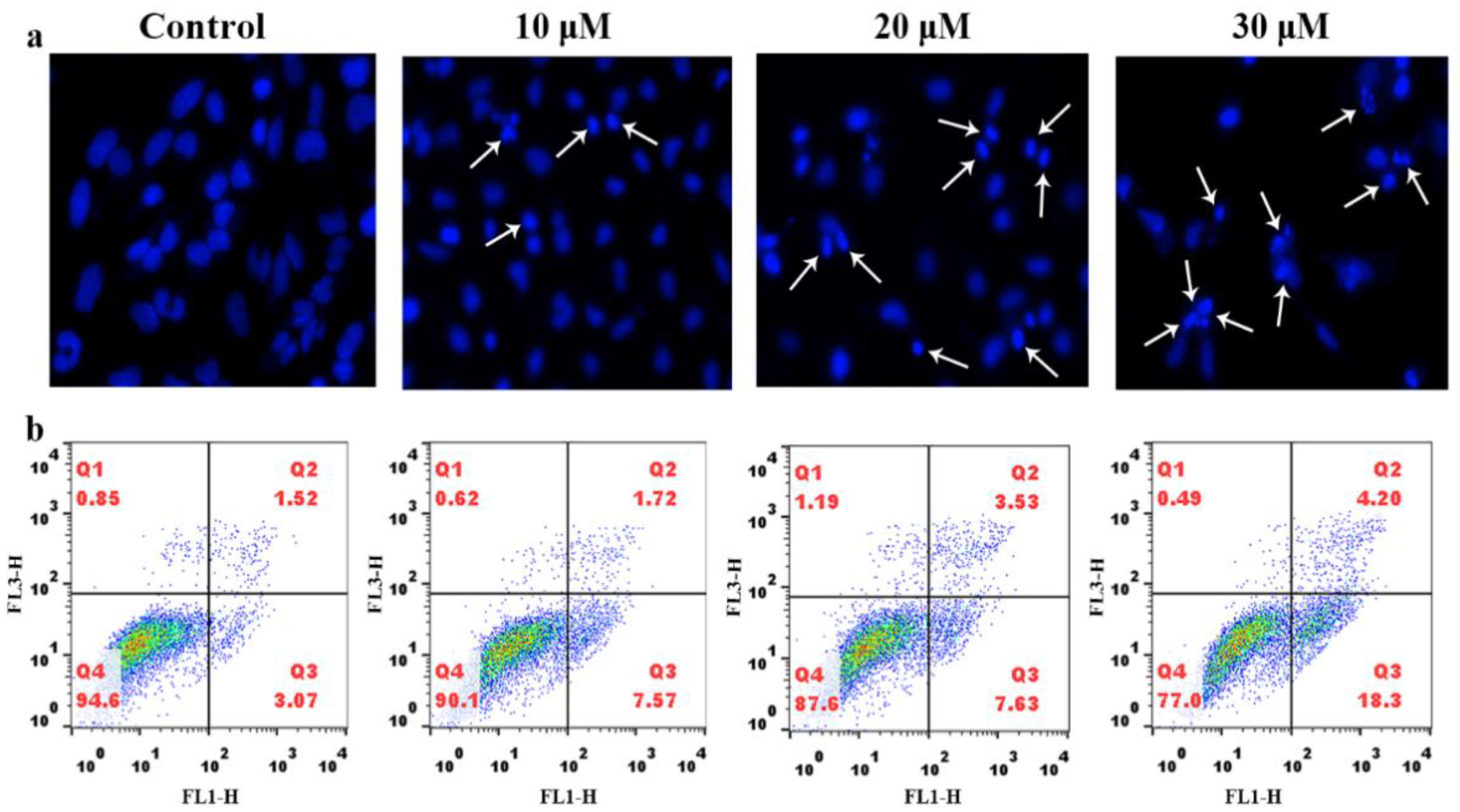
2.3. Apoptosis Studies
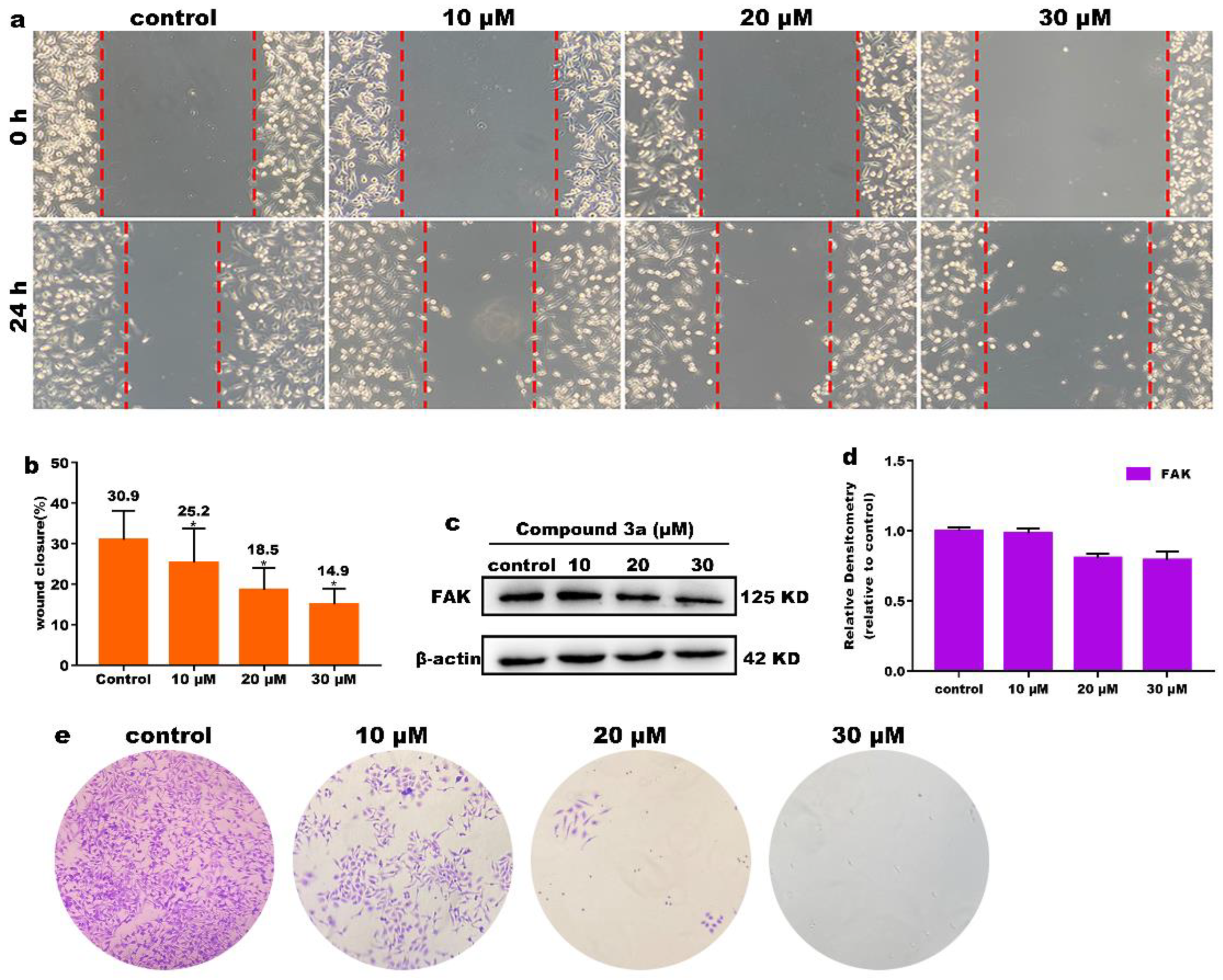
2.4. Inhibition of Cell Migration and Cloning
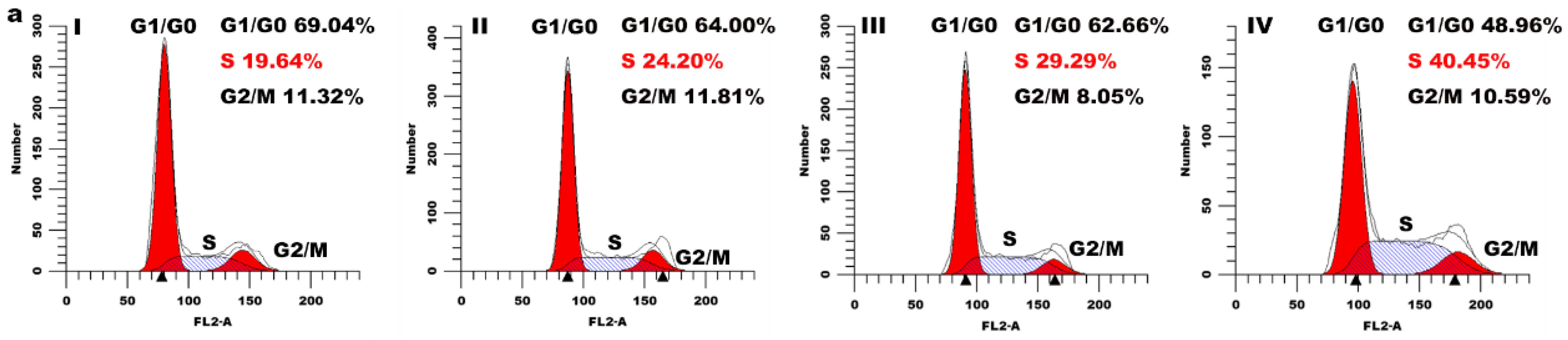
2.5. Cell Cycle Arrest Assay
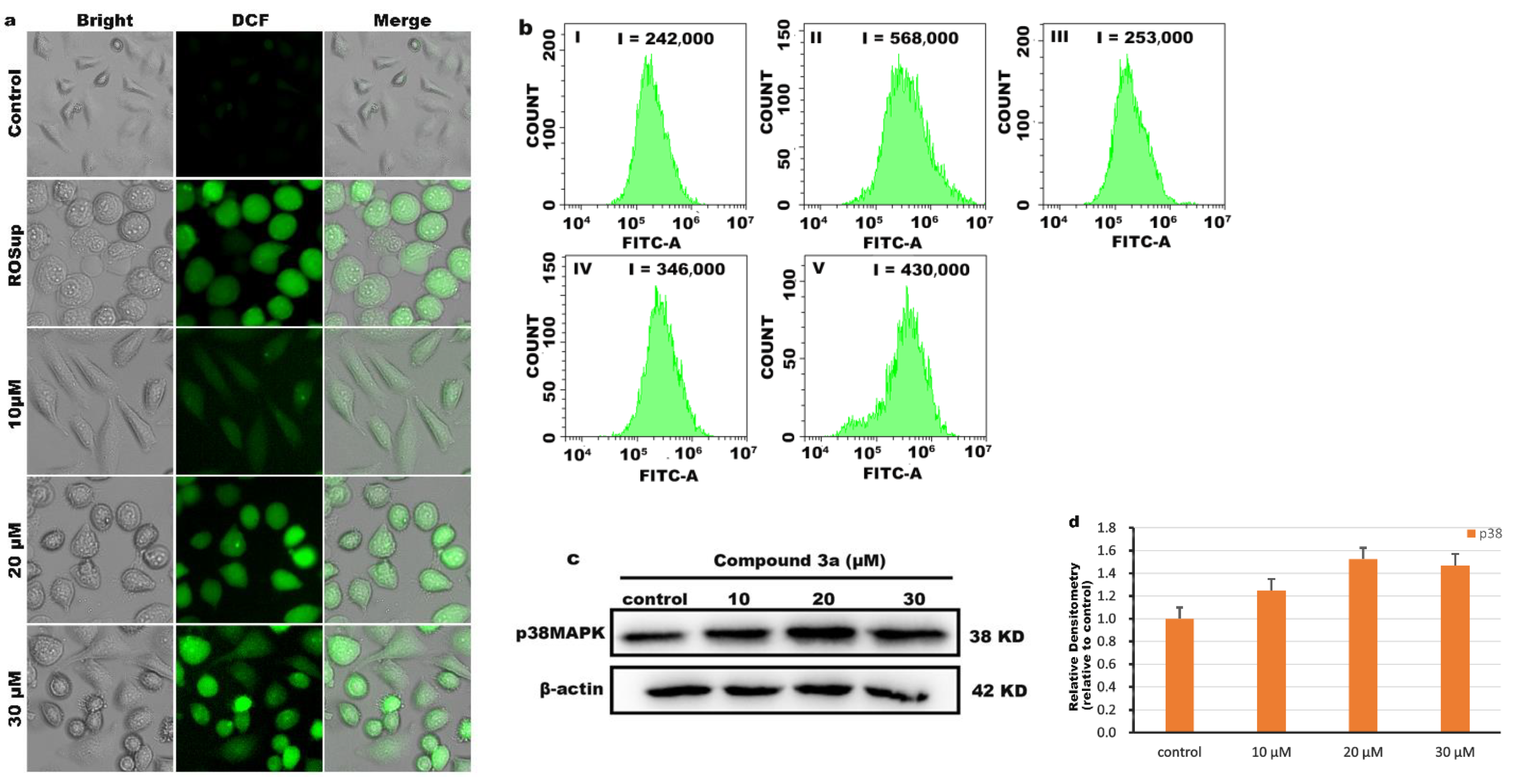
2.6. Determination of Intracellular Reactive Oxygen Species (ROS)
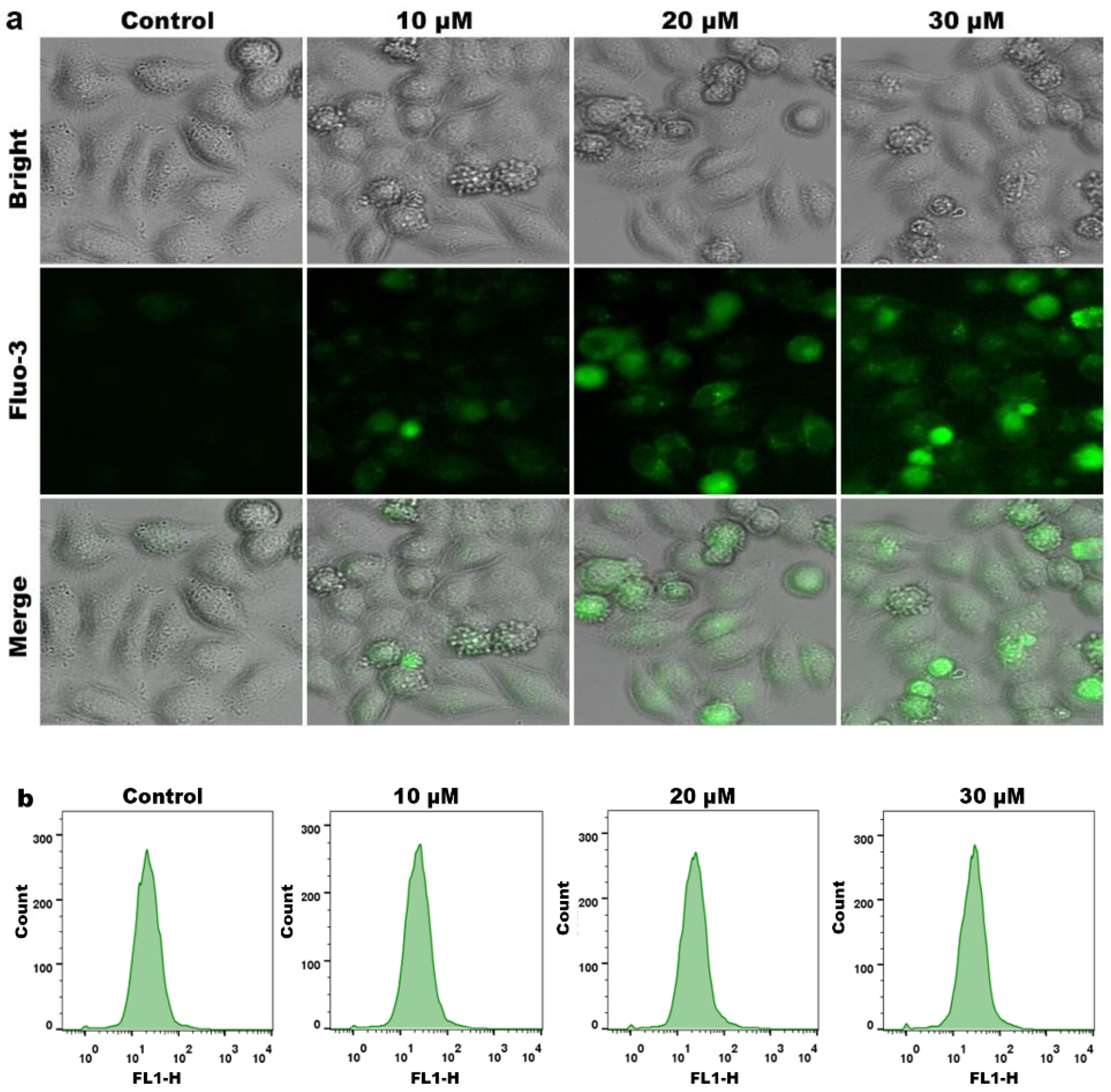
2.7. Effects of Compound on Ca2+ Levels
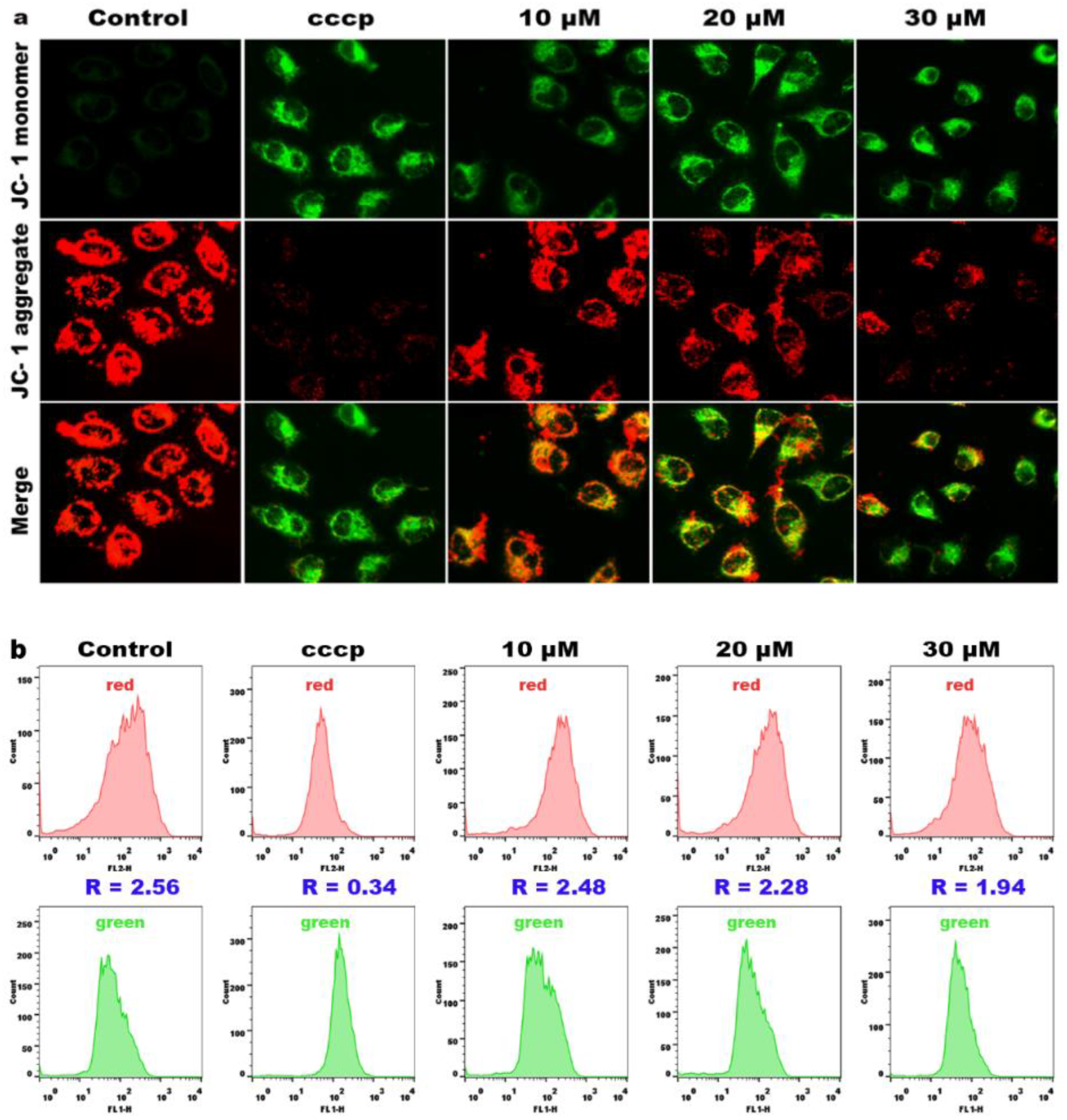
2.8. Effects on Mitochondrial Membrane Potential
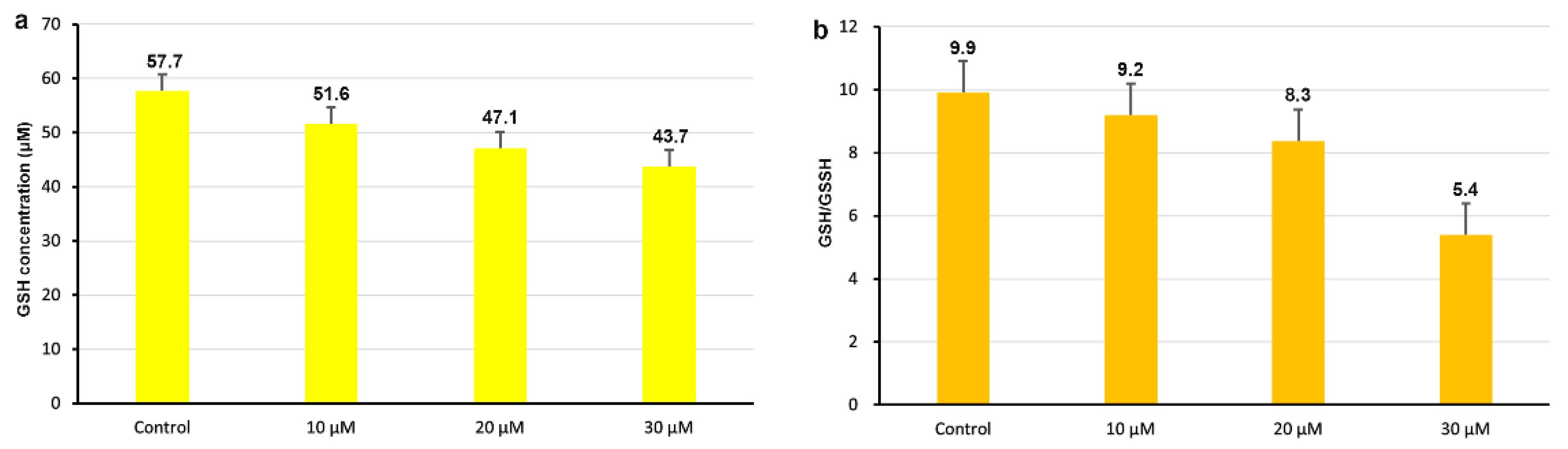
2.9. Analysis of Intracellular GSH Content
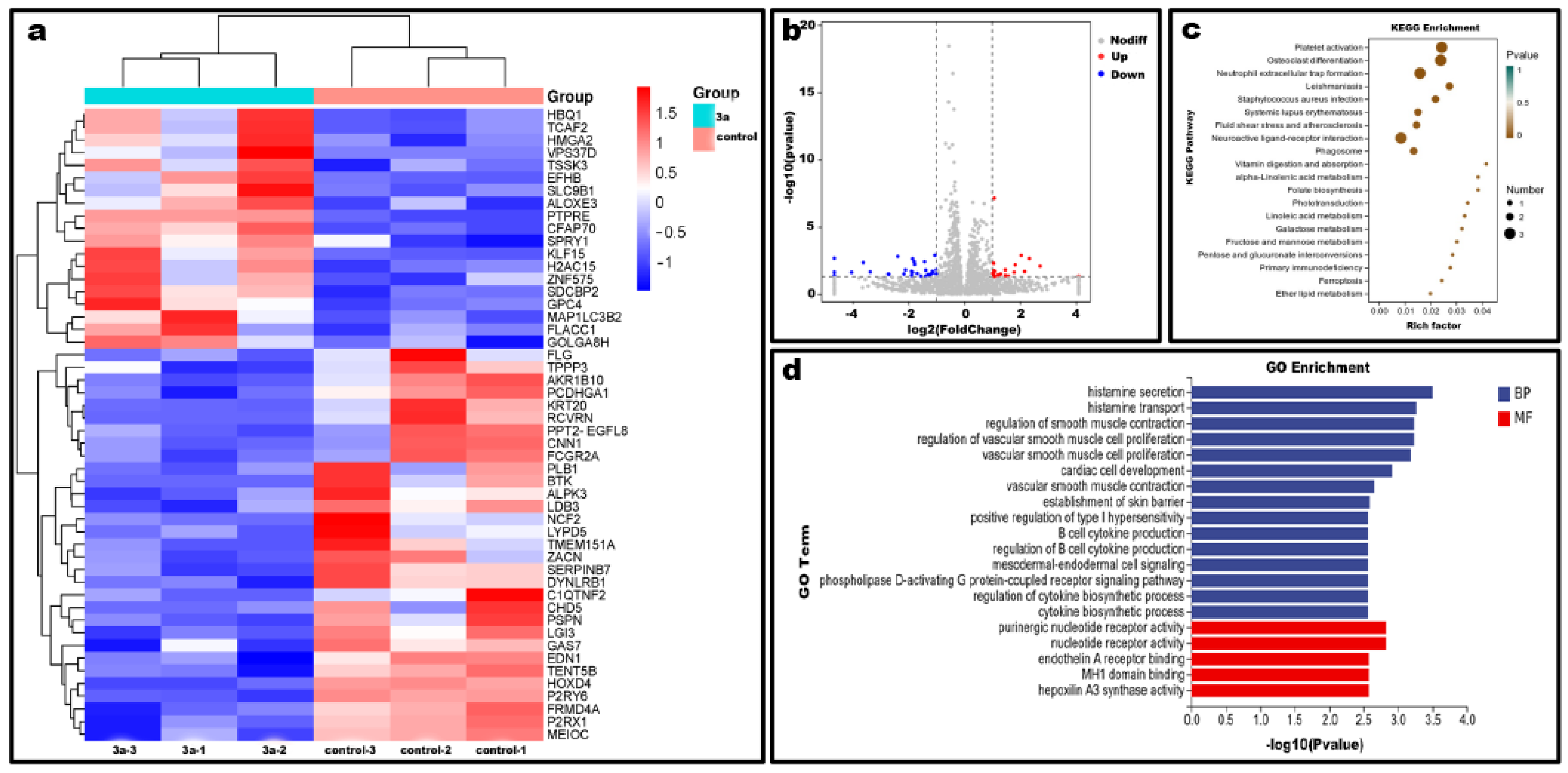
2.10. Differential Gene Expression Level Analysis
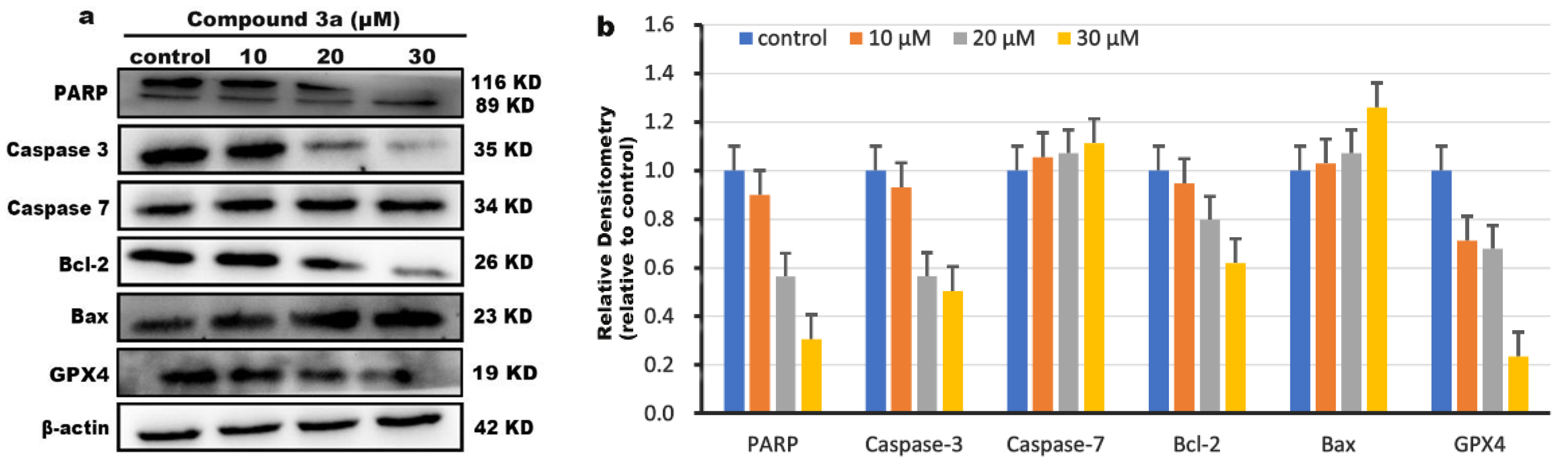
2.11. Western Blot Detection
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Compounds
3.3. Purity Determination of the Compound
3.4. Cell Viability Assay
3.5. DAPI Studies Apoptotic Morphology
3.6. Apoptosis Was Detected Using Flow Cytometry
3.7. Wound Healing Migration Assay
3.8. Colony Formation Assay
3.9. Cell Cycle Arrest Assay
3.10. Determination of Intracellular Reactive Oxygen Species (ROS)
3.11. Effects of Compound on Ca2+ Levels
3.12. Effects on Mitochondrial Membrane Potential
3.13. Detection of Intracellular GSH Levels
3.14. Transcriptome Analysis
3.15. Western Blot Detection
3.16. Statistical Analysis
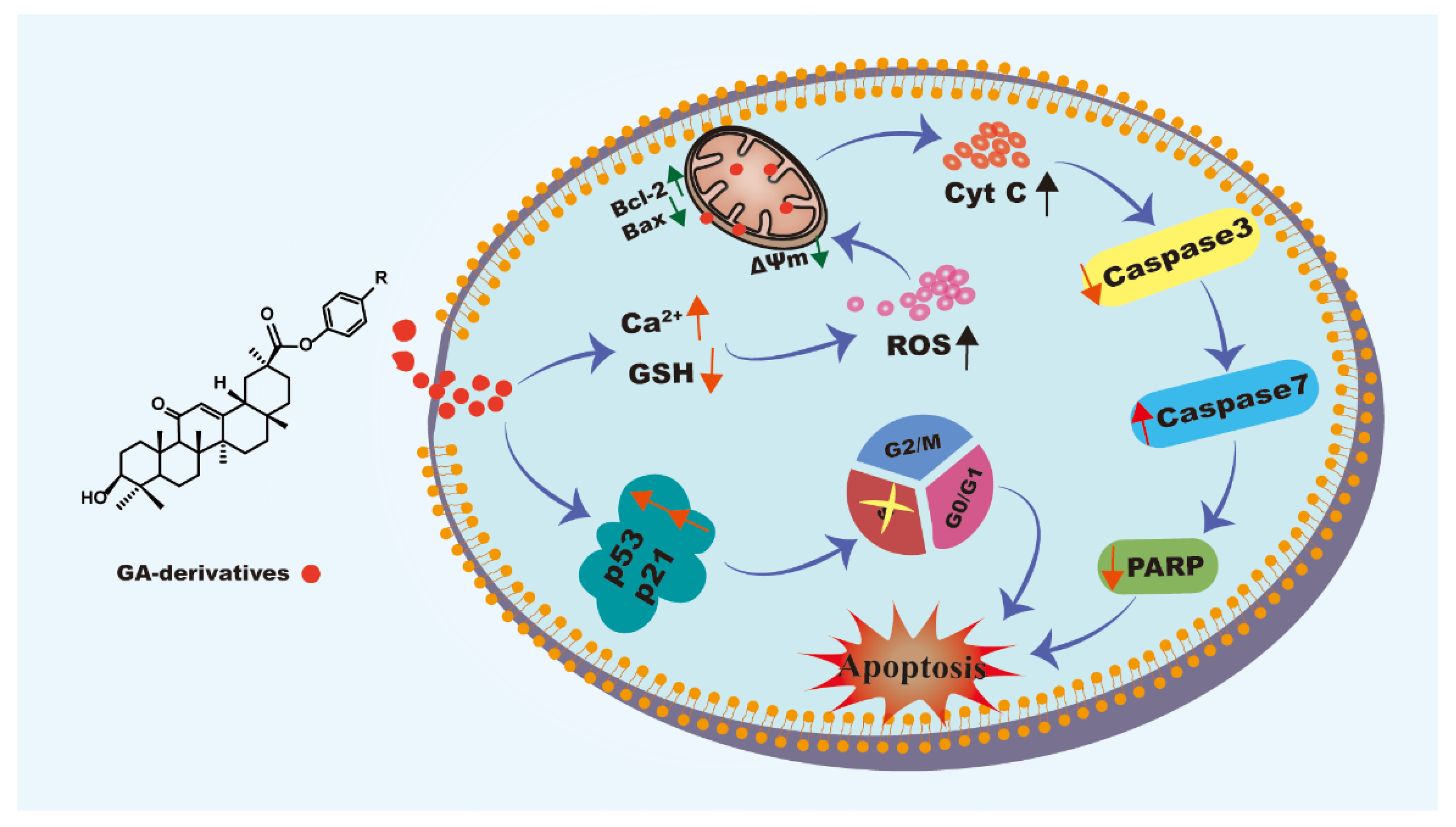
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Gao, C.; Dai, F.J.; Cui, H.W.; Tang, F.; Liu, T.; Liu, M.Y.; Qiu, W.W.; Tang, J. Synthesis of novel heterocyclic ring-fused 18β-glycyrrhetinic acid derivatives with antitumor and antimetastatic activity. Chem. Biol. Drug Des. 2014, 84, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Wu, G.R.; Zhang, X.Y.; Yan, M.M.; Zhao, R.; Xue, N.N.; Fang, K.; Wang, H.; Chen, M.; Guo, W.B.; et al. An overview of structurally modified glycyrrhetinic acid derivatives as antitumor agents. Molecules 2017, 22, 924. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sheng, R.; Fan, J.; Guo, R. A review on structure-activity relationships of glycyrrhetinic acid derivatives with diverse bioactivities. Mini. Rev. Med. Chem. 2022, 22, 2024–2066. [Google Scholar] [PubMed]
- Zhou, F.; Wu, G.R.; Cai, D.S.; Xu, B.; Yan, M.M.; Ma, T.; Guo, W.B.; Zhang, W.X.; Huang, X.M.; Jia, X.H.; et al. Synthesis and biological activity of glycyrrhetinic acid derivatives as antitumor agents. Eur. J. Med. Chem. 2019, 178, 623–635. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, S.; Csuk, R. Synthesis and antitumor activity of glycyrrhetinic acid derivatives. Bioorg. Med. Chem. 2010, 18, 7458–7474. [Google Scholar] [CrossRef] [PubMed]
- Alho, D.P.S.; Salvador, J.A.R.; Cascante, M.; Marin, S. Synthesis and antiproliferative activity of novel heterocyclic glycyrrhetinic acid derivatives. Molecules 2019, 24, 766. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Gong, P.; Wang, Y.T.; Xie, X.R.; Ma, Z.S.; Xu, Q.H.; Liu, D.; Jing, Y.K.; Zhao, L.X. Synthesis and antitumor effects of novel 18β-glycyrrhetinic acid derivatives featuring an exocyclic α, β-unsaturated carbonyl moiety in ring A. Bioorg. Chem. 2020, 103, 104187. [Google Scholar] [CrossRef]
- Hussain, H.; Ali, I.; Wang, D.j.; Hakkim, F.L.; Westermann, B.; Ahmed, I.; Ashour, A.M.; Khan, A.; Hussain, A.; Green, I.R.; et al. Glycyrrhetinic acid: A promising scaffold for the discovery of anticancer agents. Expert Opin. Drug Discov. 2021, 16, 1497–1516. [Google Scholar] [CrossRef]
- Csuk, R.; Schwarz, S.; Kluge, R.; Ströhl, D. Synthesis and biological activity of some antitumor active derivatives from glycyrrhetinic acid. Eur. J. Med. Chem. 2010, 45, 5718–5723. [Google Scholar] [CrossRef]
- Tatsuzaki, J.; Taniguchi, M.; Bastow, K.F.; Nakagawa-Goto, K.; Morris-Natschke, S.L.; Itokawa, H.; Baba, K.; Lee, K.H. Anti-tumor agents 255: Novel glycyrrhetinic acid-dehydrozingerone conjugates as cytotoxic agents. Bioorg. Med. Chem. 2007, 15, 6193–6199. [Google Scholar] [CrossRef]
- Wang, R.; Li, Y.; Huai, X.D.; Zheng, Q.X.; Wang, W.; Li, H.J.; Huai, Q.Y. Design and preparation of derivatives of oleanolic and glycyrrhetinic acids with cytotoxic properties. Drug Des. Dev. Ther. 2018, 12, 1321–1336. [Google Scholar] [CrossRef] [PubMed]
- Csuk, R.; Schwarz, S.; Siewert, B.; Kluge, R.; Ströhl, D. Conversions at C-30 of glycyrrhetinic acid and their impact on antitumor activity. Arch. Pharm. 2012, 345, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wong, H.K.; Feng, Y.B.; Zhang, Z.J. 18beta-glycyrrhetinic acid induces apoptosis in pituitary adenoma cells via ROS/MAPKs-mediated pathway. J. Neurooncol. 2014, 116, 221–230. [Google Scholar] [CrossRef]
- Wang, H.; Ge, X.H.; Qu, H.H.; Wang, N.; Zhou, J.W.; Xu, W.J.; Xie, J.J.; Zhou, Y.P.; Shi, L.Q.; Qin, Z.K.; et al. Glycyrrhizic acid inhibits proliferation of gastric cancer cells by inducing cell cycle arrest and apoptosis. Cancer Manag. Res. 2020, 12, 2853–2861. [Google Scholar] [CrossRef]
- Zhang, Z.; Feng, Y.; Li, Z.Y.; Cao, X.Z. Antiproliferative and apoptotic activity of glycyrrhizinic acid in MCF-7 human breast cancer cells and evaluation of its effect on cell cycle, cell migration and m-TOR/PI3K/Akt signalling pathway. Arch. Med. Sci. 2019, 15, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.C.; Kuo, C.L.; Lu, K.W.; Lin, J.J.; Yang, J.L.; Wu, R.S.; Wu, P.P.; Chung, J.G. 18α-Glycyrrhetinic acid induces apoptosis of HL-60 human leukemia cells through caspases- and mitochondria-dependent signaling pathways. Molecules 2016, 21, 872. [Google Scholar] [CrossRef]
- Jin, L.; Huang, R.; Huang, X.; Zhang, B.; Ji, M.; Wang, H. Discovery of 18β-glycyrrhetinic acid conjugated aminobenzothiazole derivatives as Hsp90-Cdc37 interaction disruptors that inhibit cell migration and reverse drug resistance. Bioorg. Med. Chem. 2018, 26, 1759–1775. [Google Scholar] [CrossRef]
- Sharma, G.; Kar, S.; Palit, S.; Das, P.K. 18β-glycyrrhetinic acid induces apoptosis through modulation of Akt/FOXO3a/Bim pathway in human breast cancer MCF-7 cells. J. Cell Physiol. 2012, 227, 1923–1931. [Google Scholar] [CrossRef]
- Indo, H.P.; Davidson, M.; Yen, H.C.; Suenaga, S.; Tomita, K.; Nishii, T.; Higuchi, M.; Koga, Y.; Ozawa, T.; Majima, H.J. Evidence of ROS generation by mitochondria in cells with impaired electron transport chain and mitochondrial DNA damage. Mitochondrion 2007, 7, 106–118. [Google Scholar] [CrossRef]
- Parida, P.K.; Sau, A.; Ghosh, T.; Jana, K.; Biswas, K.; Raha, S.; Misra, A.K. Synthesis and evaluation of triazole linked glycosylated 18β-glycyrrhetinic acid derivatives as anticancer agents. Bioorg. Med. Chem. Lett. 2014, 24, 3865–3868. [Google Scholar] [CrossRef]
- Zheng, Q.X.; Wang, R.; Xu, Y.; He, C.X.; Zhao, C.Y.; Wang, Z.F.; Zhang, R.; Dehaen, W.; Li, H.J.; Huai, Q.Y. Design, preparation and studies regarding cytotoxic properties of glycyrrhetinic acid derivatives. Biol. Pharm. Bull. 2020, 43, 102–109. [Google Scholar] [CrossRef]
- Cai, D.; Zhang, Z.H.; Chen, Y.; Zhang, Y.Y.; Sun, Y.Q.; Gong, Y.X. Exploring new structural features of the 18β-glycyrrhetinic acid scaffold for the inhibition of anaplastic lymphoma kinase. Molecules 2019, 24, 3631. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.J.; Ding, J.X.; Xu, W.G.; Sun, T.M.; Xiao, H.H.; Zhuang, X.L.; Chen, X.S. Receptor and microenvironment dual-recognizable nanogel for targeted chemotherapy of highly metastatic malignancy. Nano. Lett. 2017, 17, 4526–4533. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.L.; Ma, R.; Wang, Z.G.; Yiu, S.M.; Zhu, G.Y. Heterodinuclear Pt(IV)-Ru(II) anticancer prodrugs to combat both drug resistance and tumor metastasis. Chem. Commun. 2016, 52, 10735–10738. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.Y.; Deng, R.; Huang, Y.H.; Yang, X.; Jiang, N.; Zhou, J.; Lin, C.S.; Chen, S.L.; Wu, L.Y.; Cui, Q.; et al. DGKA interacts with SRC/FAK to promote the metastasis of non-small cell lung cancer. Sci. China Life Sci. 2022, 532, 215585. [Google Scholar] [CrossRef]
- Ying, X.X.; Huang, A.L.; Xing, Y.J.; Lan, L.P.; Yi, Z.F.; He, P.Q. Lycorine inhibits breast cancer growth and metastasis via inducing apoptosis and blocking Src/FAK-involved pathway. Sci. China Life Sci. 2017, 60, 417–428. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.Y.; Yang, Y.; Ming, M.; Liu, B. Mitochondrial ROS generation for regulation of autophagic pathways in cancer. Biochem. Biophys. Res. Commun. 2011, 414, 5–8. [Google Scholar] [CrossRef] [PubMed]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signaling pathways by reactive oxygen species. Biochim. Biophys. Acta 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- Suski, J.M.; Lebiedzinska, M.; Bonora, M.; Pinton, P.; Duszynski, J.; Wieckowski, M.R. Relation between mitochondrial membrane potential and ROS formation. Methods Mol. Biol. 2012, 810, 183–205. [Google Scholar]
- Zhang, Z.D.; Yang, Y.J.; Liu, X.W.; Qin, Z.; Li, S.H.; Li, J.Y. Aspirin eugenol ester ameliorates paraquat-induced oxidative damage through ROS/p38-MAPK-mediated mitochondrial apoptosis pathway. Toxicology 2021, 453, 152721. [Google Scholar] [CrossRef]
- Kong, A.N.; Yu, R.; Chen, C.; Mandlekar, S.; Primiano, T. Signal transduction events elicited by natural products: Role of MAPK and caspase pathways in homeostatic response and induction of apoptosis. Arch. Pharm. Res. 2000, 23, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Dolado, I.; Swat, A.; Ajenjo, N.; De Vita, G.; Cuadrado, A.; Nebreda, A.R. p38alpha MAP kinase as a sensor of reactive oxygen species in tumorigenesis. Cancer Cell 2007, 11, 191–205. [Google Scholar] [CrossRef] [PubMed]
- Kerkhofs, M.; Bittremieux, M.; Morciano, G.; Giorgi, C.; Pinton, P.; Parys, J.B.; Bultynck, G. Emerging molecular mechanisms in chemotherapy: Ca2+ signaling at the mitochondria-associated endoplasmic reticulum membranes. Cell Death Dis. 2018, 9, 334. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.L.; Shen, W.Y.; Chen, Z.F.; Zhao, L.F.; Qin, Q.P.; Yu, Y.C.; Liang, H. Oxoaporphine metal complexes (CoII, NiII, ZnII) with high antitumor activity by inducing mitochondria-mediated apoptosis and S-phase arrest in HepG2. Sci. Rep. 2017, 7, 46056. [Google Scholar] [CrossRef]
- Green, D.R.; Kroemer, G. The pathophysiology of mitochondrial cell death. Science 2004, 305, 626–629. [Google Scholar] [CrossRef]
- Sinha, K.; Das, J.; Pal, P.B.; Sil, P.C. Oxidative stress: The mitochondria-dependent and mitochondria-independent pathways of apoptosis. Arch. Toxicol. 2013, 87, 1157–1180. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Yu, T.; Kidachi, Y.; Akitaya, T.; Yoshida, K.; Kamiie, K.; Noshita, T.; Umetsu, H.; Ryoyama, K. Selective toxicity of glycyrrhetinic acid against tumorigenic r/m HM-SFME-1 cells is potentially attributed to downregulation of glutathione. Biochimie 2011, 93, 1172–1178. [Google Scholar] [CrossRef]
- Jiang, J.; Yin, L.; Li, J.Y.; Li, Q.; Shi, D.; Feng, L.; Liu, Y.; Jiang, W.D.; Wu, P.; Zhao, Y.; et al. Glutamate attenuates lipopolysaccharide-induced oxidative damage and mRNA expression changes of tight junction and defensin proteins, inflammatory and apoptosis response signaling molecules in the intestine of fish. Fish Shellfish Immunol. 2017, 70, 473–484. [Google Scholar] [CrossRef]
- Sepand, M.R.; Ghahremani, M.H.; Razavi-Azarkhiavi, K.; Aghsami, M.; Rajabi, J.; Keshavarz-Bahaghighat, H.; Soodi, M. Ellagic acid confers protection against gentamicin-induced oxidative damage, mitochondrial dysfunction and apoptosis-related nephrotoxicity. J. Pharm. Pharmacol. 2016, 68, 1222–1232. [Google Scholar] [CrossRef]
- You, B.R.; Park, W.H. Auranofin induces mesothelioma cell death through oxidative stress and GSH depletion. Oncol. Rep. 2016, 35, 46–551. [Google Scholar] [CrossRef]
- Chota, A.; George, B.P.; Abrahamse, H. Interactions of multidomain pro-apoptotic and anti-apoptotic proteins in cancer cell death. Oncotarget 2021, 12, 1615–1626. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Kim, C.N.; Yang, J.; Jemmerson, R.; Wang, X. Induction of apoptotic program in cell-free extracts: Requirement for dATP and cytochrome c. Cell 1996, 86, 147–157. [Google Scholar] [CrossRef]
- Xu, P.; Wang, Y.; Deng, Z.; Tan, Z.B.; Pei, X.J. MicroRNA-15a promotes prostate cancer cell ferroptosis by inhibiting GPX4 expression. Oncol. Lett. 2022, 23, 67. [Google Scholar] [CrossRef] [PubMed]
- Georgieva, A.; Popov, G.; Shkondrov, A.; Toshkova, R.; Krasteva, I.; Kondeva-Burdina, M.; Manov, V. Antiproliferative and antitumor activity of saponins from Astragalus glycyphyllos on myeloid Graffi tumor. J. Ethnopharmacol. 2021, 267, 113519. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.M.; Cheng, K.C.; Huang, J.Y.; Wang, S.Y.; Lin, Y.N.; Tseng, Y.T.; Hsieh, C.W.; Wung, B.S. Sulforaphane inhibits blue light-induced inflammation and apoptosis by upregulating the SIRT1/PGC-1α/Nrf2 pathway and autophagy in retinal pigment epithelial cells. Toxicol. Appl. Pharmacol. 2021, 421, 115545. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Zhang, H.W.; Tian, L.; Yang, L.L.; Zhou, Y.; Zhang, Y.Y.; Liu, Y.J.; Xing, D.G. Evaluation of anticancer effects in vitro of new iridium(III) complexes targeting the mitochondria. J. Inorg. Biochem. 2021, 221, 111465. [Google Scholar] [CrossRef] [PubMed]

| Compound | SGC-7901 | BEL-7402 | B16 | A549 | HeLa | LO2 |
|---|---|---|---|---|---|---|
| 3a | 48.4 ± 1.7 | 32.4 ± 3.6 | 47.4 ± 2.3 | 39.5 ± 8.5 | 11.4 ± 0.2 | 52.4 ± 3.0 |
| 3b | 44.9 ± 5.9 | 39.9 ± 4.5 | 44.4 ± 5.7 | 17.7 ± 2.0 | 24.0 ± 0.8 | 32.4 ± 7.4 |
| 3c | 43.5 ± 3.8 | >100 | 56.7 ± 4.5 | 45.3 ± 3.4 | 38.0 ± 6.4 | 74.2 ± 5.5 |
| 3d | 32.1 ± 4.4 | 26.4 ± 3.2 | 39.1 ± 4.7 | 26.9 ± 1.5 | 23.2 ± 0.89 | 78.7 ± 4.4 |
| 3e | 73.1 ± 5.6 | >100 | 43.4 ± 3.6 | 21.2±1.1 | 26.6 ± 1.5 | 36.7 ± 3.0 |
| 3f | 69.2 ± 13.3 | 41.6 ± 2.3 | 48.3 ± 1.2 | 32.7 ± 1.7 | 45.5 ± 2.1 | 71.7 ± 2.6 |
| GA | 75.9 ± 10.8 | 45.9 ± 3.6 | 57.6 ± 5.8 | 61.1 ± 12.5 | 62.9 ± 3.4 | 87.5 ± 5.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Xu, Y.; Yang, Y.; Yao, X.; Fu, Y.; Wang, Y.; Liu, Y.; Wang, X. Evaluation of the Anticancer Activity and Mechanism Studies of Glycyrrhetic Acid Derivatives toward HeLa Cells. Molecules 2023, 28, 3164. https://doi.org/10.3390/molecules28073164
Chen J, Xu Y, Yang Y, Yao X, Fu Y, Wang Y, Liu Y, Wang X. Evaluation of the Anticancer Activity and Mechanism Studies of Glycyrrhetic Acid Derivatives toward HeLa Cells. Molecules. 2023; 28(7):3164. https://doi.org/10.3390/molecules28073164
Chicago/Turabian StyleChen, Ju, Yunran Xu, Yan Yang, Xin Yao, Yuan Fu, Yi Wang, Yunjun Liu, and Xiuzhen Wang. 2023. "Evaluation of the Anticancer Activity and Mechanism Studies of Glycyrrhetic Acid Derivatives toward HeLa Cells" Molecules 28, no. 7: 3164. https://doi.org/10.3390/molecules28073164
APA StyleChen, J., Xu, Y., Yang, Y., Yao, X., Fu, Y., Wang, Y., Liu, Y., & Wang, X. (2023). Evaluation of the Anticancer Activity and Mechanism Studies of Glycyrrhetic Acid Derivatives toward HeLa Cells. Molecules, 28(7), 3164. https://doi.org/10.3390/molecules28073164







