Preparation and Performance Evaluation of a Temperature and Salt Resistant Hydrophobic Associative Weak Polymer Gel System
Abstract
1. Introduction
2. Results and Discussion
2.1. Preparation and Structural Characterization of the Polymer and the Crosslinking Agent
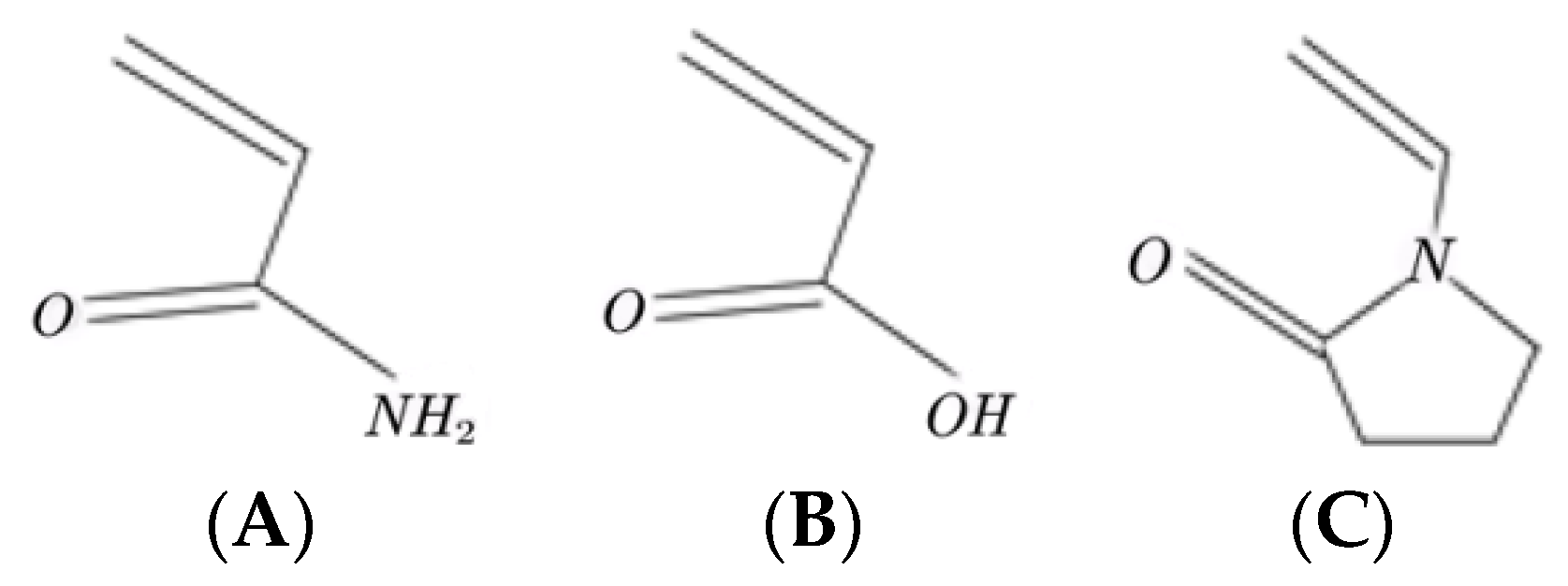
2.1.1. Selection of Monomers for the Synthesis of the Hydrophilic Main Chain Units
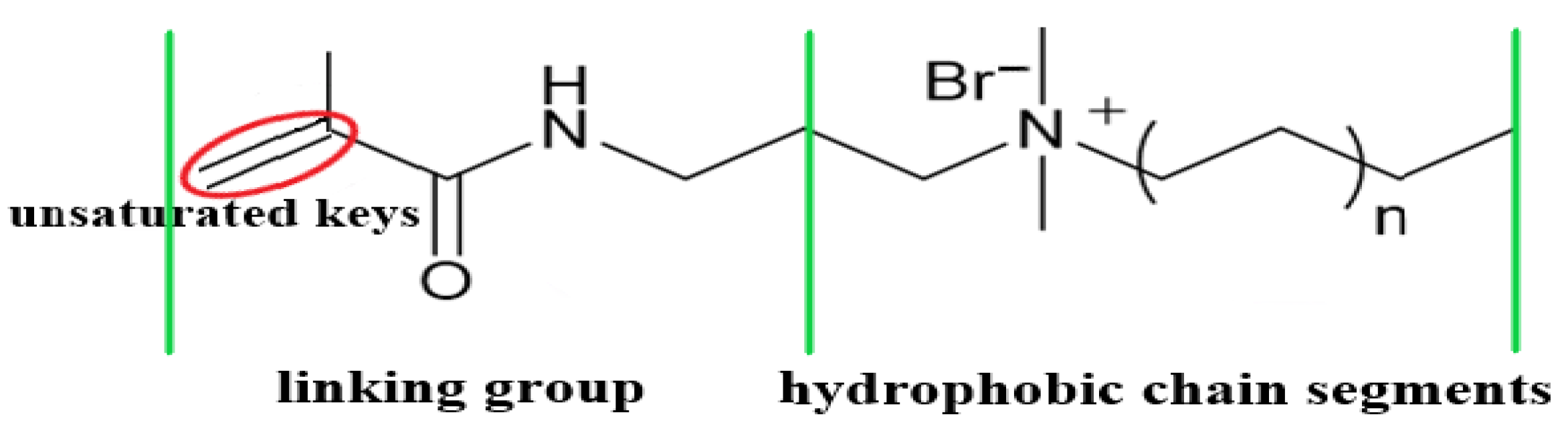
2.1.2. Design and Preparation of the Hydrophobic Functional Monomer
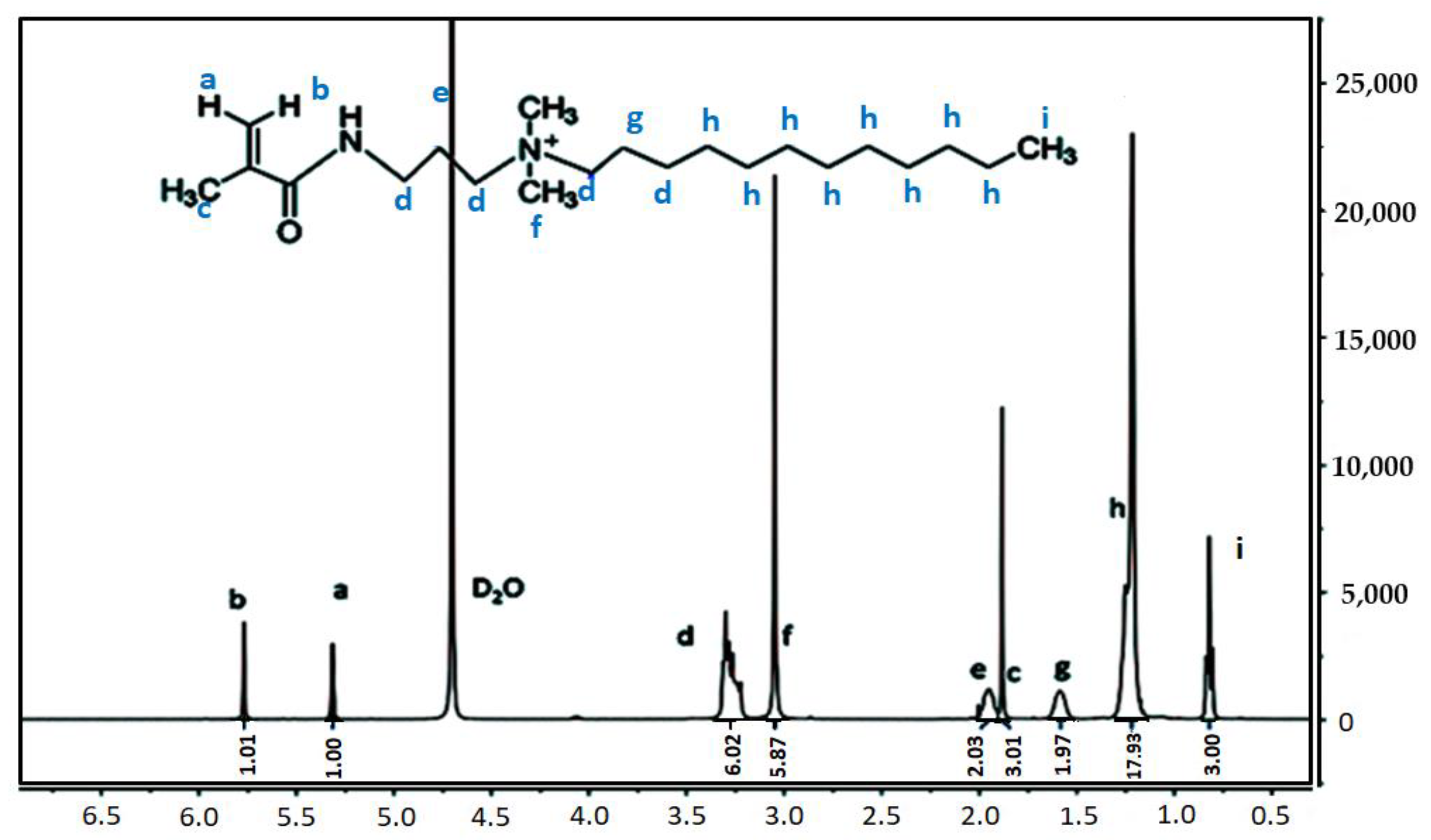
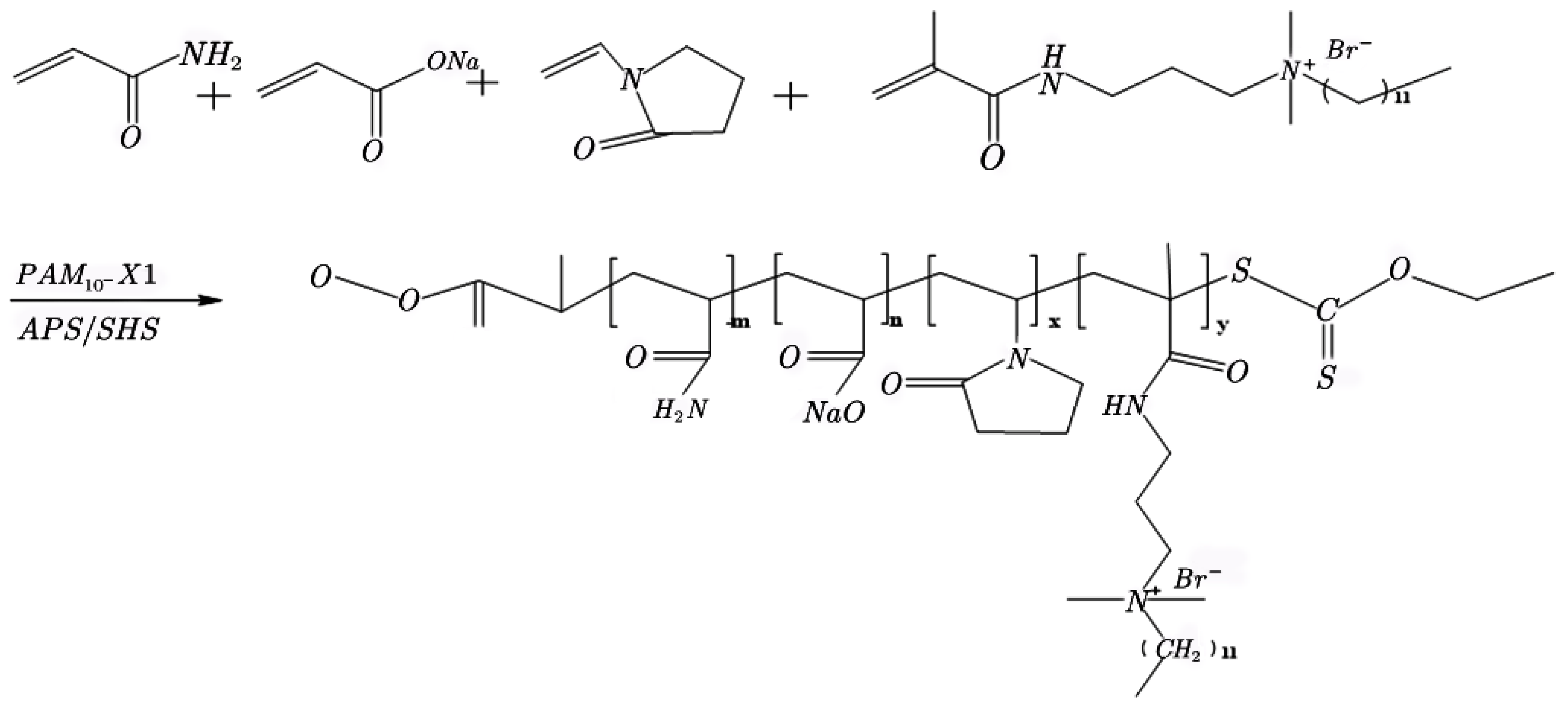
2.1.3. Preparation of the Hydrophobic associative Polymers
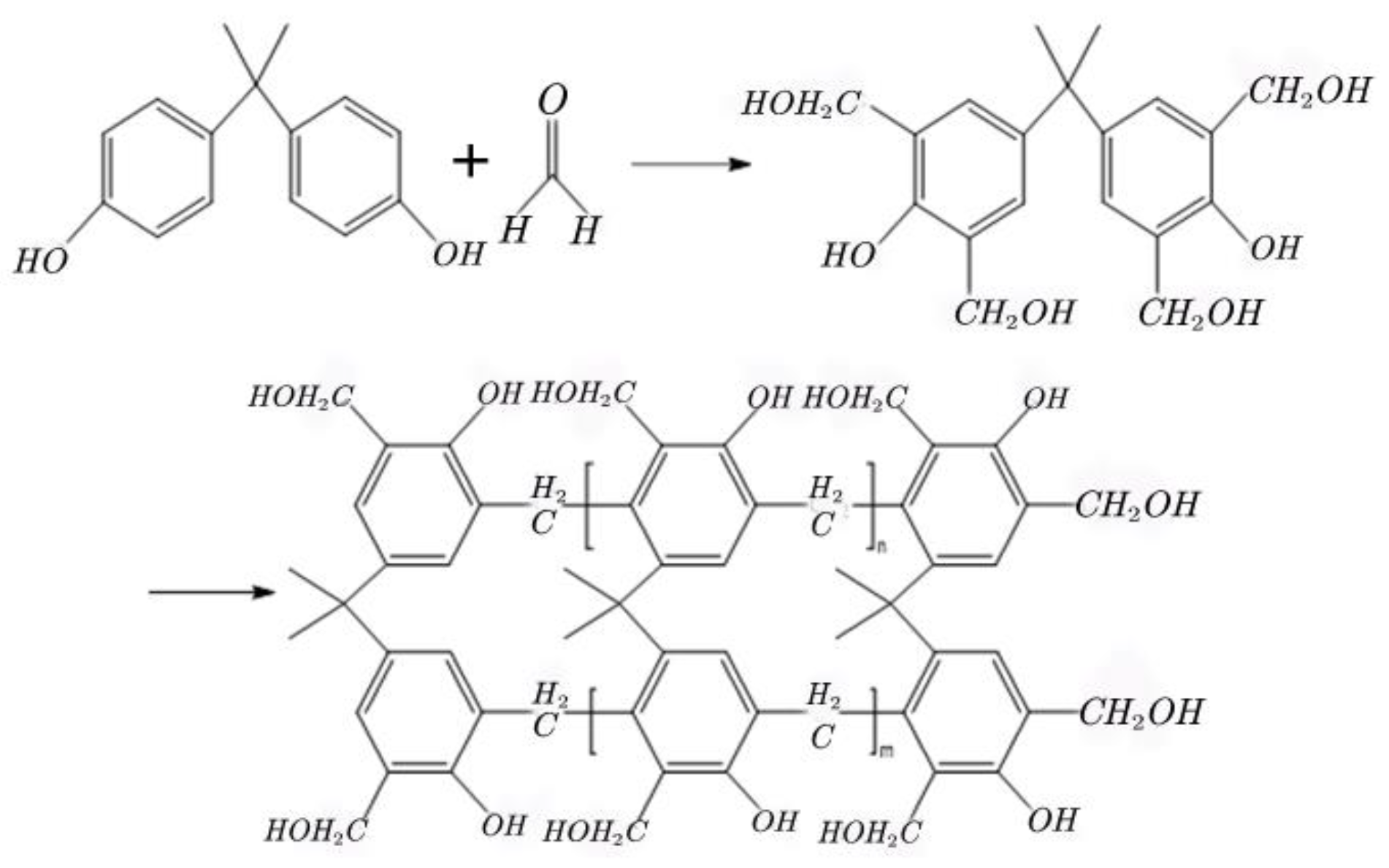
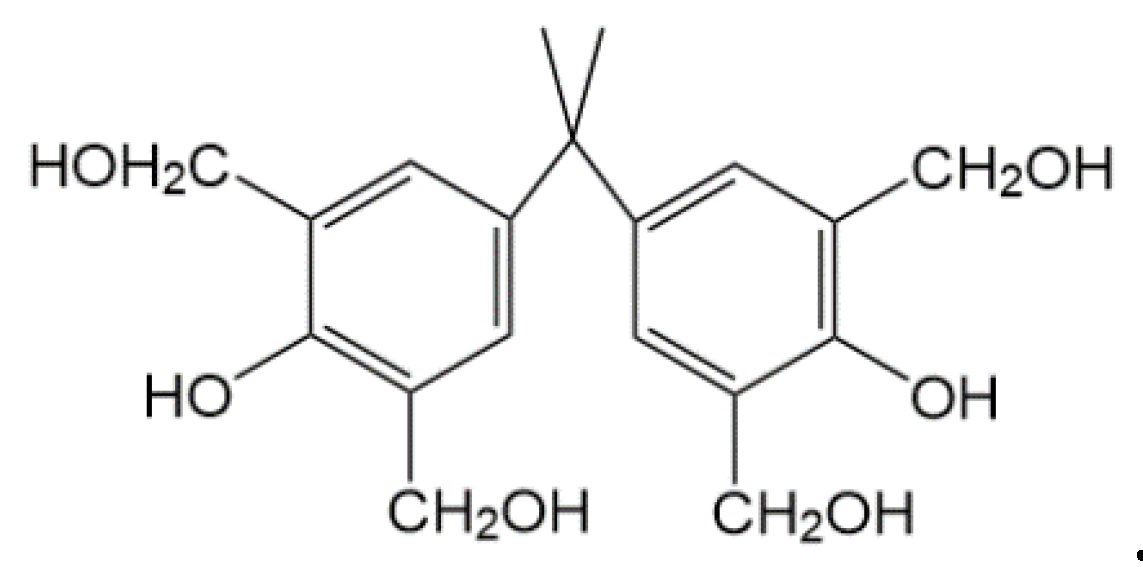
2.1.4. Preparation of the Phenolic Resin Crosslinker
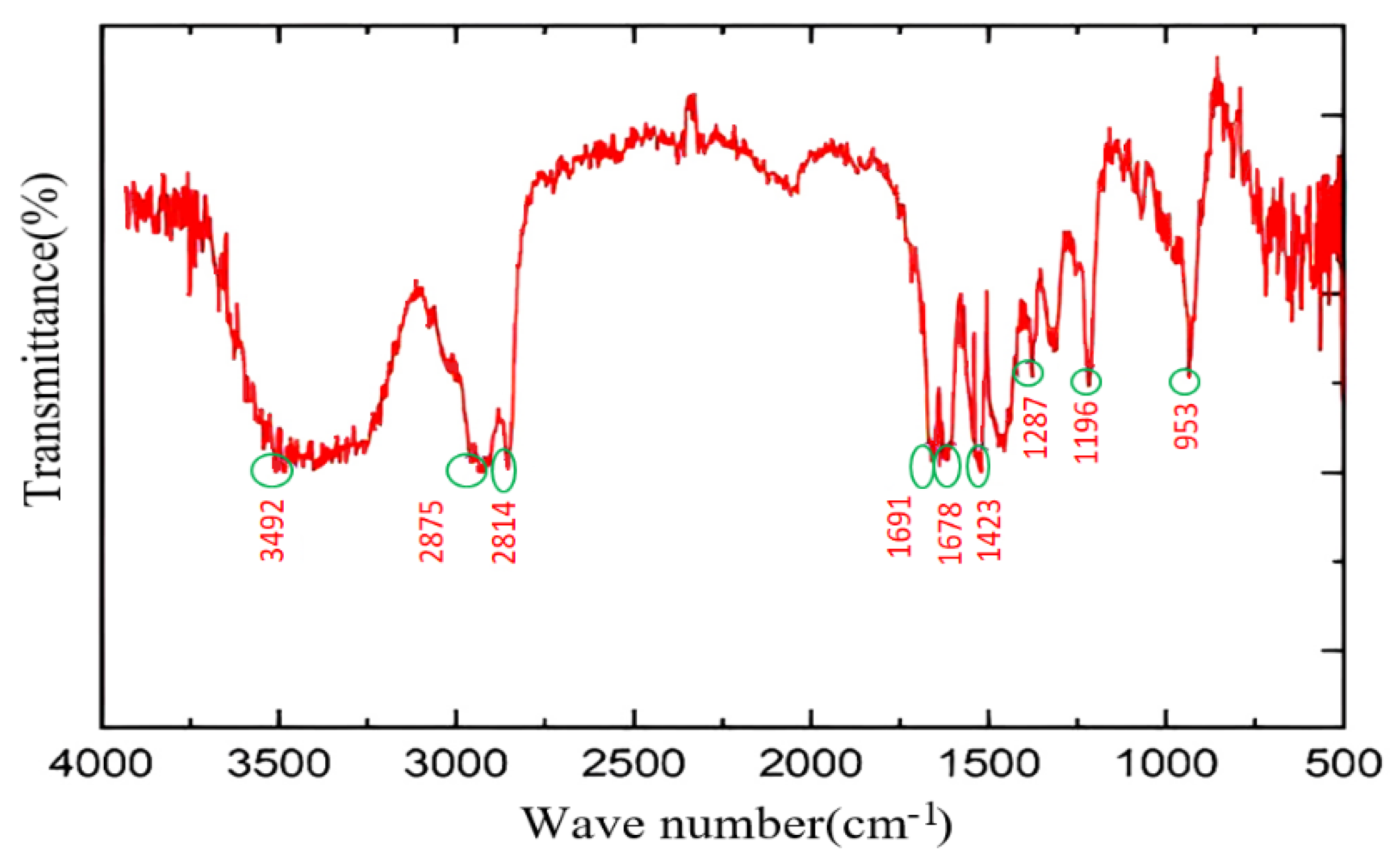
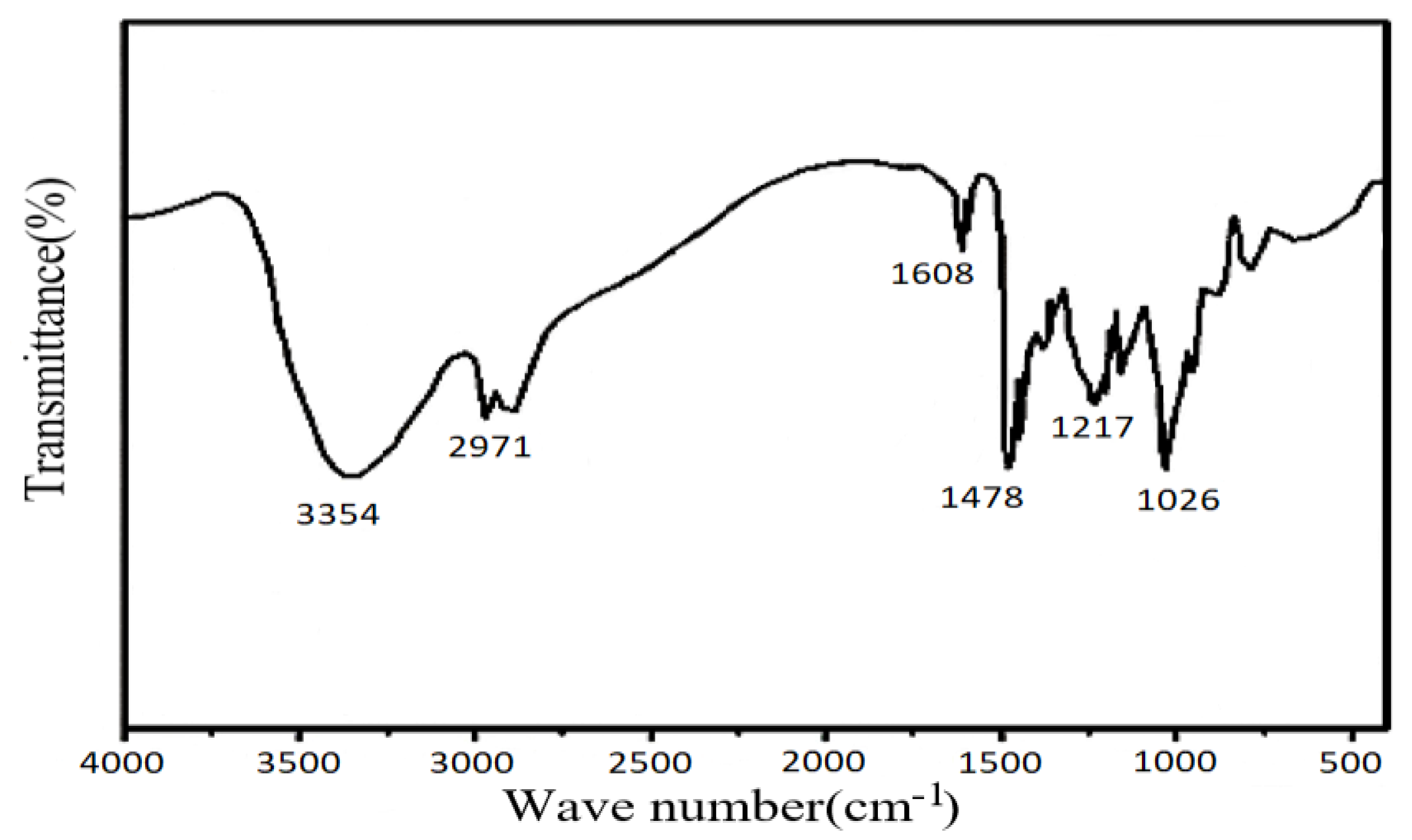
2.1.5. Structural Characterization of the Crosslinker
2.1.6. Crosslinking Mechanism of the Hydrophobically Associating Polymer and the Phenolic Resin
2.2. Performance Evaluation of the Hydrophobic Associative Polymer Gels
2.2.1. Influence of the Additive Concentration on the Gel Properties
2.2.2. Effect of Polymer and Crosslinker Concentration on Gel Properties
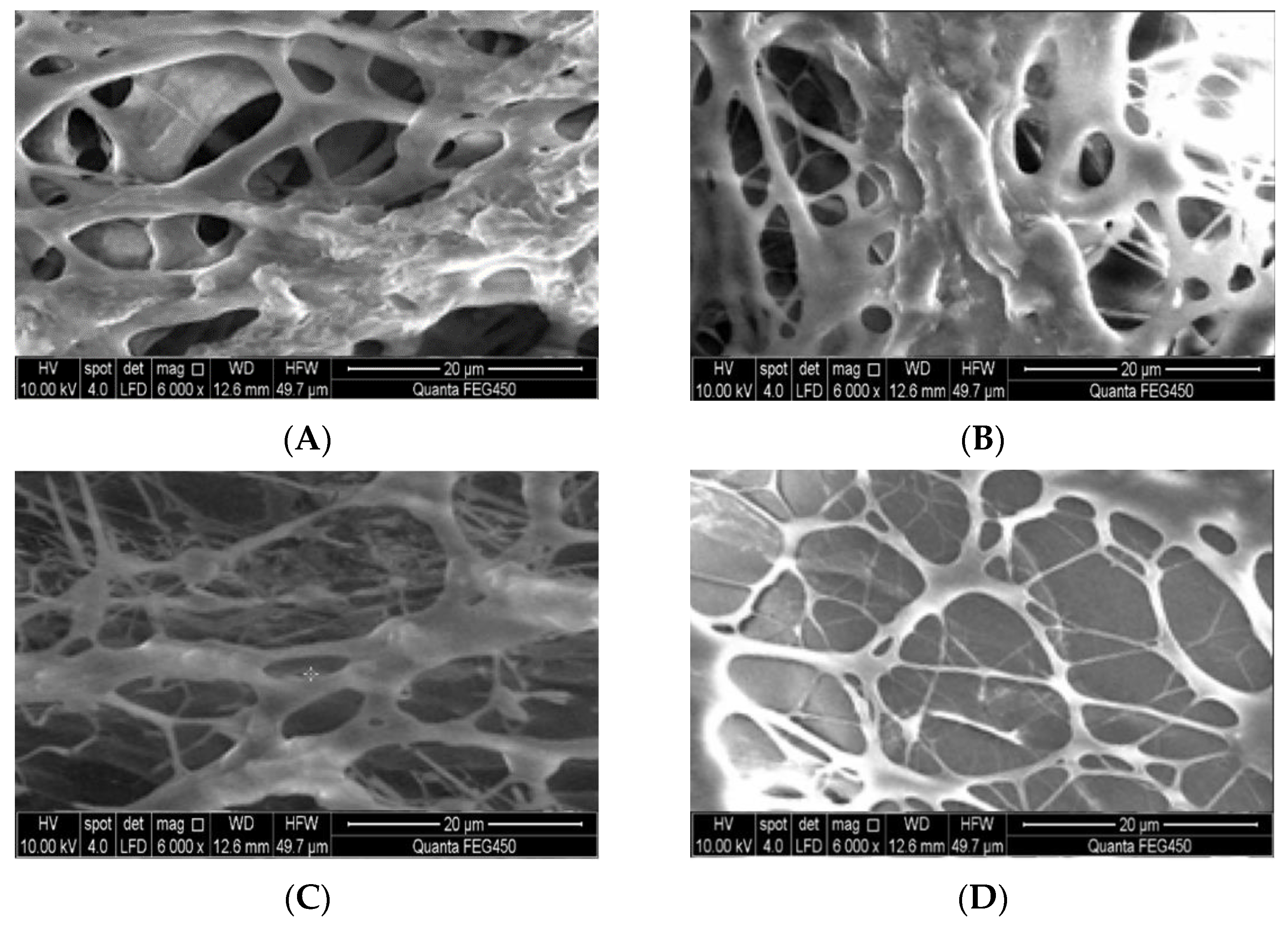
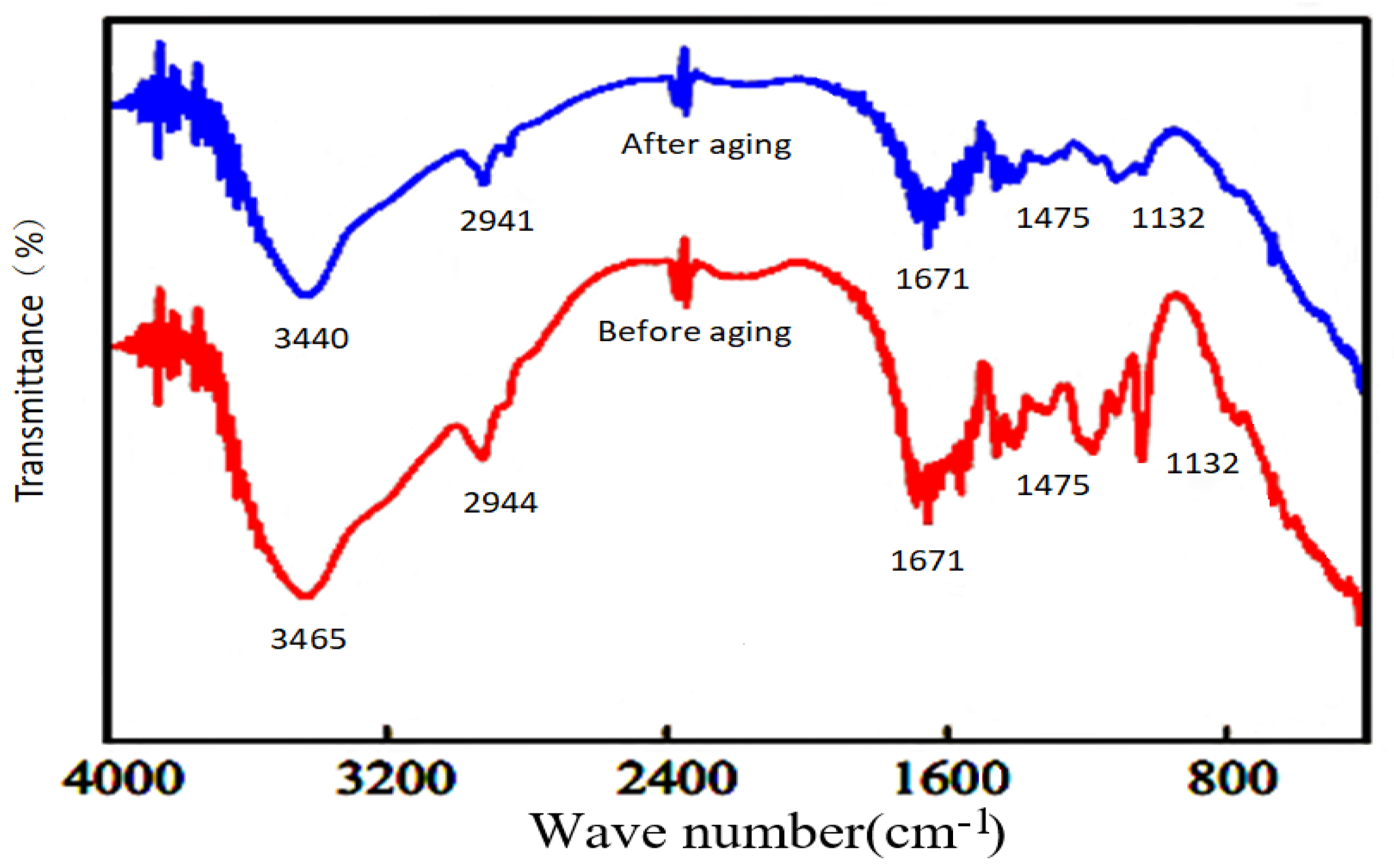
2.2.3. Stability of the Hydrophobic Associative Polymers
2.3. Evaluation of the Performance of the Hydrophobic Associative Polymer Gel
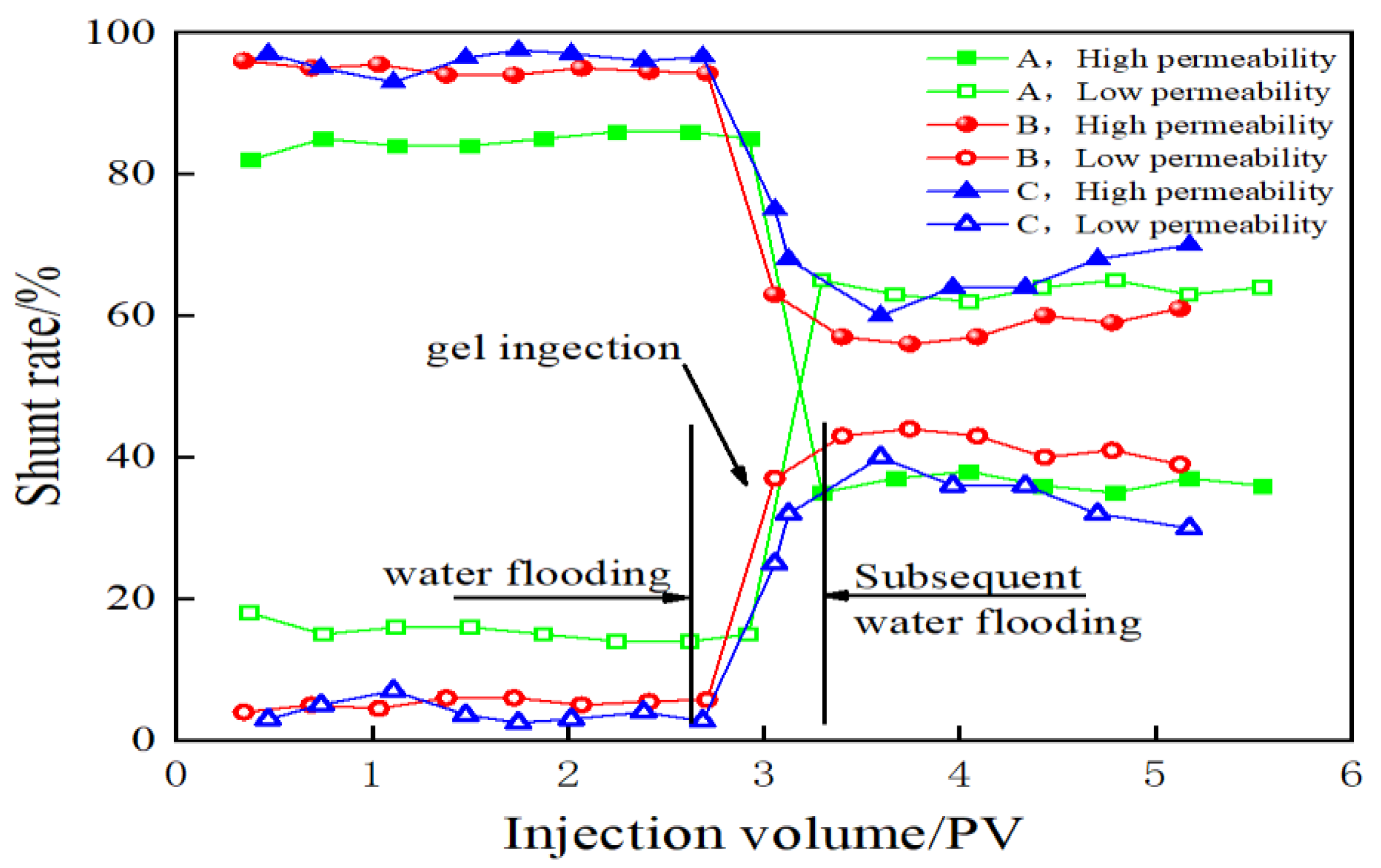
2.4. Direction of Liquid Flow Parallel to the Double Pipes
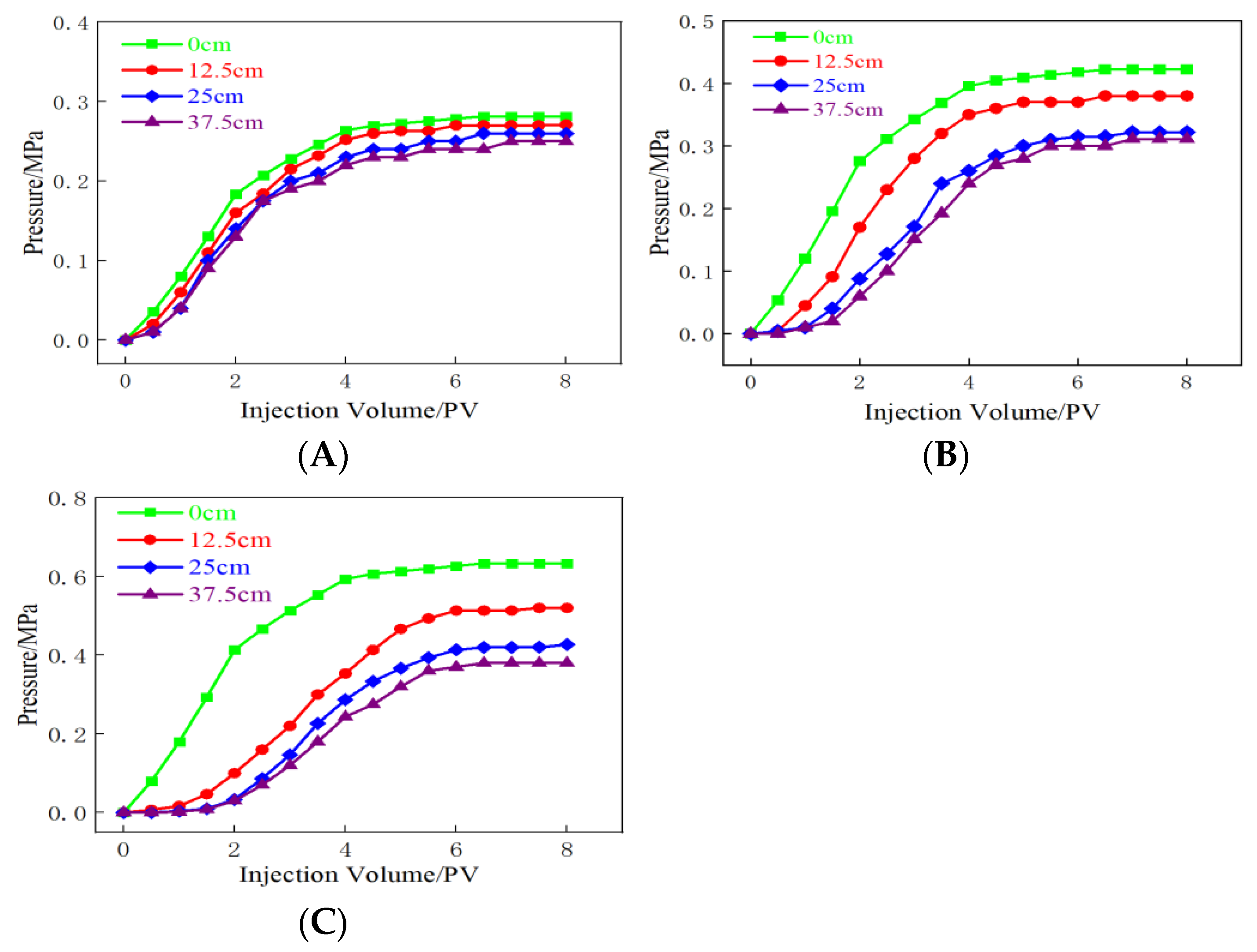
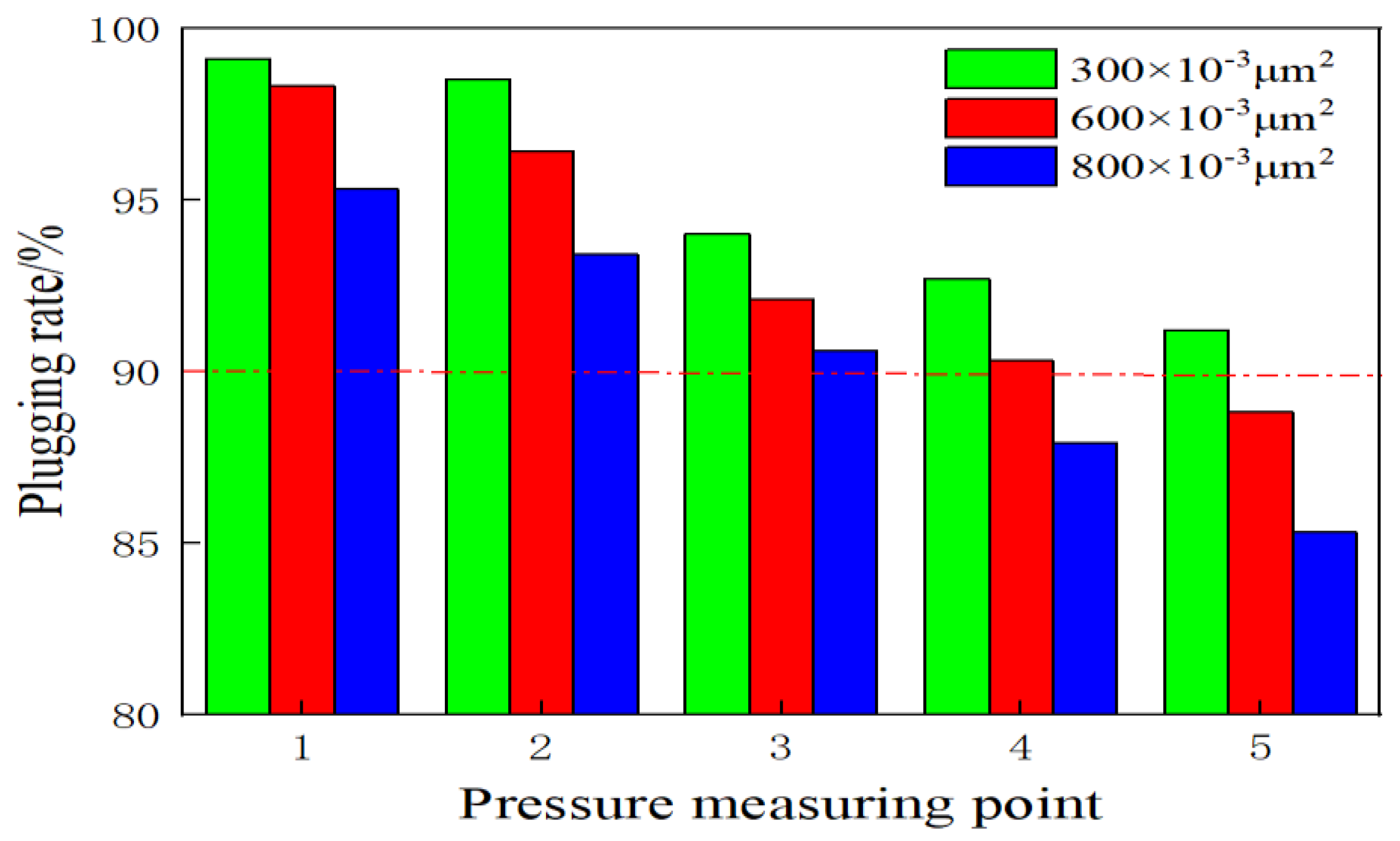
2.5. Gel Plugging Performance
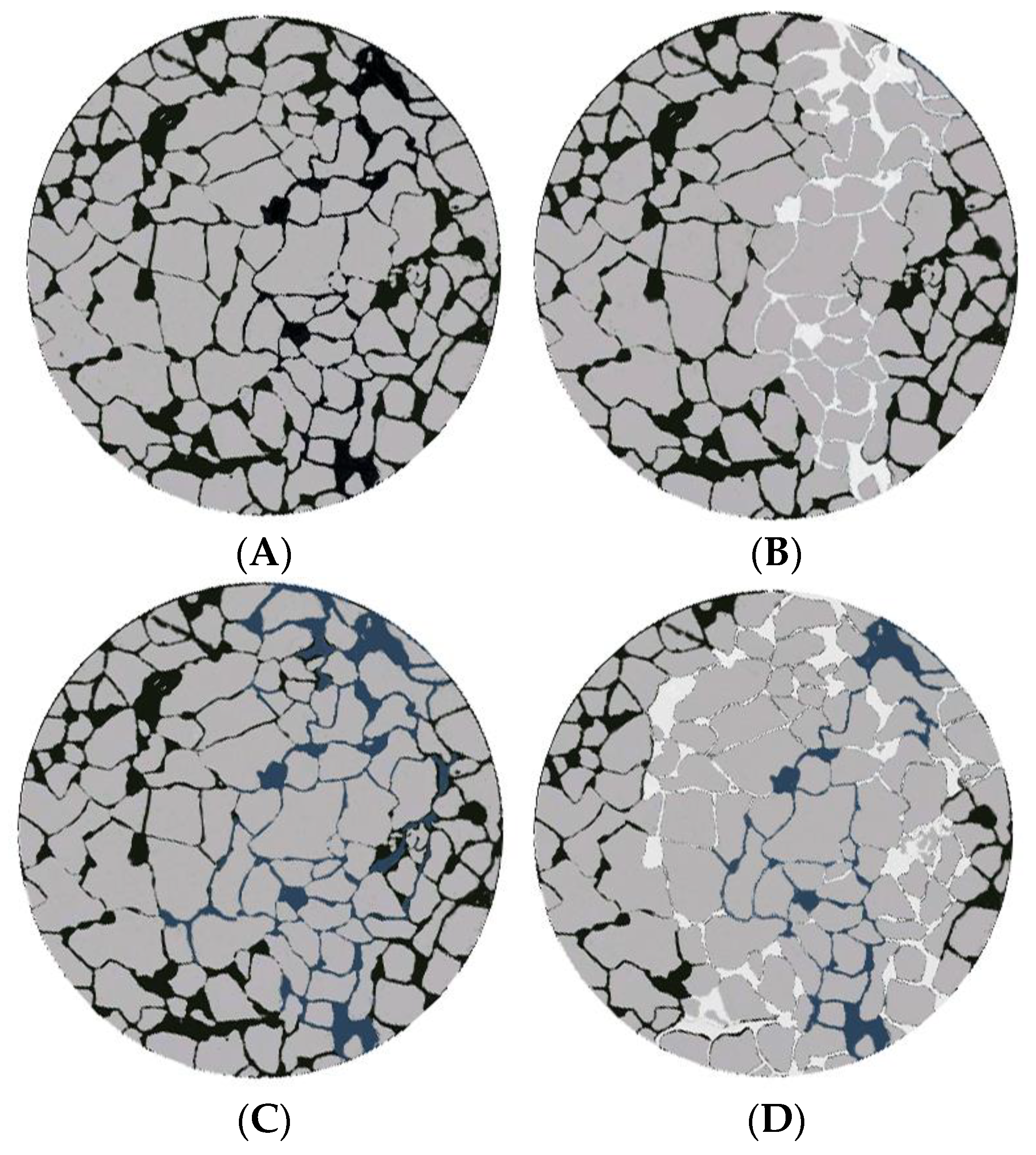
2.6. Micropore-Throat Migration and Plugging Characteristics of the Gel System
3. Materials and Methods
3.1. Reagents and Instruments
3.2. Experimental Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Li, Y.; Li, Y.; Peng, Y.; Yu, Y. Water shutoff and profile control in China over 60 years. Oil Drill. Prod. Technol. 2019, 41, 773–787. [Google Scholar]
- Wang, X.; Guo, J.; Chen, J. Research Progress of Deep Profile Control Technology and Its Application. Oilfield Chem. 2020, 37, 738–744. [Google Scholar]
- Xu, B.; Wang, Y. Profile control performance and field application of preformed particle gel in low-permeability fractured reservoir. J. Pet. Explor. Prod. Technol. 2020, 11, 477–482. [Google Scholar] [CrossRef]
- Wang, G.; Chen, T.; Zhang, R.; Song, X. Preparation and performance of a novel hydrophobic associating polymer with excellent temperature-resistance and salt-tolerance. Petrochem. Technol. 2020, 49, 657–663. [Google Scholar]
- Zhu, Y.; Guo, Y.; Xu, H.; Pang, X.; Li, H. Preparation and Performance Evaluation of Hydrophobically Associating Polymer with TemperatureResistance and Salt Tolerance. Oilfield Chem. 2021, 38, 317–323. [Google Scholar]
- Yao, E.; Yu, G.; Li, B.; Zhao, L.; Li, Y.; Bai, H.; Zhou, F. High-Temperature-Resistant, Low-Concentration Water-Controlling Composite Cross-Linked Polyacrylamide Weak Gel System Prepared from Oilfield Sewage. ACS Omega 2022, 7, 12570–12579. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Dai, C.; Gao, M.; Wang, X.; Liu, S.; Jin, X.; Li, T.; Zhao, M. Development of the Gemini Gel-Forming Surfactant with Ultra-High Temperature Resistance to 200 degrees C. Gels 2022, 8, 600. [Google Scholar] [CrossRef]
- Wang, C.; Zhong, L.; Liu, Y.; Han, Y.; Zhao, P.; Yuan, Y.; Han, X. Characteristics of Weak-Gel Flooding and Its Application in LD10-1 Oilfield. ACS Omega 2020, 5, 24935–24945. [Google Scholar] [CrossRef]
- Li, X.; Wei, J.; Zhao, L.; Ni, J.; Fu, L.; Wang, J. Experimental Study on Profile Control of Polymer and Weak Gel Molecules in Porous Media. Gels 2022, 8, 467. [Google Scholar] [CrossRef]
- Zhang, C.; Qu, G.; Song, G. Formulation Development of High Strength Gel System and Evaluation on Profile Control Performance for High Salinity and Low Permeability Fractured Reservoir. Int. J. Anal. Chem. 2017, 2017, 2319457. [Google Scholar] [CrossRef]
- Xie, K.; Cao, W.; Lu, X.; Song, K.; Liu, Y.; Zhang, Y.; Liu, J.; Lv, J.; Wang, W.; Na, R. Influence of water dilution on performance of chromium polymer weak gel in porous medium. J. Dispers. Sci. Technol. 2019, 41, 1549–1558. [Google Scholar] [CrossRef]
- Li, Z.; Deng, Z.; Guo, X.; Zhang, L.; Zhi, Y. Study of the impact of solidifier inorganic components on the performance of a solidifiable gel plugging fluid. J. Nat. Gas Sci. Eng. 2015, 23, 450–457. [Google Scholar] [CrossRef]
- Cheng, H.; Zheng, X.; Wu, Y.; Zhang, J.; Zhao, X.; Li, C. Experimental and Numerical Investigation on Oil Displacement Mechanism of Weak Gel in Waterflood Reservoirs. Gels 2022, 8, 309. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Lu, X.; Jin, Y.; Zhang, C.; Liu, K.; Hu, X. Preparation and Characterization of Hydrophobic-Associated Microspheres for Deep Profile Control in Offshore Oilfields. Int. J. Polym. Sci. 2018, 2018, 6362518. [Google Scholar] [CrossRef]
- Liu, J.; Wang, S.; He, L.; Hu, P.; Gao, Y. Preparation and properties of nano-silica hybrid hydrophobic associated polyacrylamide for polymer flooding. J. Pet. Sci. Eng. 2022, 208, 109434. [Google Scholar] [CrossRef]
- Jiang, G.; Jiang, Q.; Sun, Y.; Liu, P.; Zhang, Z.; Ni, X.; Yang, L.; Wang, C. Supramolecular-Structure-Associating Weak Gel of Wormlike Micelles of Erucoylamidopropyl Hydroxy Sulfobetaine and Hydrophobically Modified Polymers. Energy Fuels 2017, 31, 4780–4790. [Google Scholar] [CrossRef]
- Xie, K.; Lu, X.; Li, Q.; Jiang, W.; Yu, Q. Analysis of Reservoir Applicability of Hydrophobically Associating Polymer. SPE J. 2016, 21, 1–9. [Google Scholar] [CrossRef]
- Cao, G.; Wu, J.; Bai, Y.; Zhang, N.; Xing, P.; Xu, Q.; Li, D.; Cong, X.; Liu, J. Research on the Formulation System of Weak Gel and the Influencing Factors of Gel Formation after Polymer Flooding in Y1 Block. Processes 2022, 10, 1405. [Google Scholar] [CrossRef]
- Kargozarfard, Z.; Riazi, M.; Ayatollahi, S.; Shahnazar, S. Performance of polyacrylamide/Cr(III) gel polymer in oil recovery from heterogeneous porous media: An experimental study. Korean J. Chem. Eng. 2016, 33, 3350–3358. [Google Scholar] [CrossRef]
- Ge, J.; Wu, Q.; Ding, L.; Guo, H.; Zhao, A. Preparation and rheological Evaluation of a thixotropic polymer gel for water shutoff in fractured tight reservoirs. J. Pet. Sci. Eng. 2022, 208, 109542. [Google Scholar] [CrossRef]
- Li, X.J.; Hou, J.R.; Yue, X.A.; Song, X.W. Effects of shear and absorption on in-depth profile control and oil displacement of weak gels. J. China Univ. Pet. 2007, 31, 147–151. [Google Scholar]
- Zhang, S.; Guo, J.; Gu, Y.; Zhao, Q.; Yang, R.; Yang, Y. Polyacrylamide gel formed by Cr(III) and phenolic resin for water control in high-temperature reservoirs. J. Pet. Sci. Eng. 2020, 194, 107423. [Google Scholar] [CrossRef]
- Cui, C.; Zhou, Z.; He, Z. Enhance oil recovery in low permeability reservoirs: Optimization and evaluation of ultra-high molecular weight HPAM/phenolic weak gel system. J. Pet. Sci. Eng. 2021, 195, 107908. [Google Scholar] [CrossRef]
- Liang, K.; Han, P.; Chen, Q.; Su, X.; Feng, Y. Comparative study on enhancing oil recovery under high temperature and high salinity: Polysaccharides versus synthetic polymer. ACS Omega 2019, 4, 10620–10628. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Yin, X.; Cao, R.; Zeng, P.; Wang, J.; Wu, D.; Luo, X.; Zhu, Y.; Zheng, Z.; Feng, Y. In situ crosslinked weak gels with ultralong and tunable gelation times for improving oil recovery. Chem. Eng. J. 2022, 432, 134350. [Google Scholar] [CrossRef]
- Bai, Y.; Xiong, C.; Wei, F.; Li, J.; Shu, Y.; Liu, D. Gelation study on a hydrophobically associating polymer/polyethylenimine gel system for water shut-off treatment. Energy Fuels 2015, 29, 447–458. [Google Scholar] [CrossRef]
- Du, D.J.; Pu, W.F.; Tan, X.; Liu, R. Experimental study of secondary crosslinking core-shell hyperbranched associative polymer gel and its profile control performance in low-temperature fractured conglomerate reservoir. J. Pet. Sci. Eng. 2019, 179, 912–920. [Google Scholar] [CrossRef]
- Di, Q.; Zhang, J.; Hua, S.; Chen, H.; Gu, C. Visualization experiments on polymer-weak gel profile control and displacement by NMR technique. Pet. Explor. Dev. 2017, 44, 294–298. [Google Scholar] [CrossRef]
- Wang, J.; Kang, B.; Zhang, L.; Darowska, B.J.; Xu, P. Macroscopic Profile Modification and Microscopic Displacement Mechanism of Weak Gel Flowing in Porous Media. J. Chem. 2016, 2016, 2379362. [Google Scholar] [CrossRef]
- Zhang, J. Study of crosslinking and transport blocking properties gel system in porous media. Pet. Geol. Recovery Effic. 2012, 19, 54–56. [Google Scholar]


| No. | Polymer Concentration (mg/L) | Crosslinking Agent Concentration (mg/L) | Additive Concentration (mg/L) | Viscosity of Gel System at Different Times (mPa·s) | 90 Days Viscosity Retention (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Inital | 5 Days | 10 Days | 30 Days | 60 Days | 90 Days | |||||
| A1 | 2000 | 3000 | 1500 | 15.32 | 922 | 987 | 1198 | 922 | 851 | 86.2 |
| A2 | 2000 | 3000 | 2000 | 11.05 | 1225 | 1582 | 1802 | 1465 | 1239 | 85.3 |
| A3 | 2000 | 3000 | 2500 | 18.56 | 1363 | 1790 | 2115 | 1610 | 1278 | 78.4 |
| No. | Polymer Concentration (mg/L) | Crosslinking Agent Concentration (mg/L) | Additive Concentration (mg/L) | Viscosity of Gel System at Different Times (mPa·s) | 90 Days Viscosity Retention (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Inital | 5 Days | 10 Days | 30 Days | 60 Days | 90 Days | |||||
| B1 | 1800 | 2200 | 2000 | 13.08 | 805 | 1292 | 1448 | 774 | 659 | 66.6 |
| B2 | 1800 | 2700 | 2000 | 11.28 | 1014 | 1473 | 1673 | 1273 | 814 | 65.7 |
| B3 | 1800 | 3600 | 2000 | 10.60 | 1000 | 1462 | 1617 | 1129 | 893 | 73.6 |
| B4 | 2000 | 2400 | 2000 | 11.72 | 1258 | 1482 | 1696 | 1109 | 1059 | 80.6 |
| B5 | 2000 | 3000 | 2000 | 11.05 | 1226 | 1584 | 1803 | 1465 | 1239 | 85.3 |
| B6 | 2000 | 4000 | 2000 | 10.10 | 1547 | 1868 | 2177 | 1519 | 1207 | 72.8 |
| B7 | 2250 | 2700 | 2000 | 15.80 | 1001 | 1222 | 1432 | 1099 | 864 | 77.1 |
| B8 | 2250 | 3400 | 2000 | 16.30 | 1383 | 1749 | 1926 | 1406 | 1140 | 75.4 |
| B9 | 2250 | 4500 | 2000 | 18.10 | 1213 | 1700 | 1866 | 1343 | 977 | 69.2 |
| Conditions | Permeability Ratio | Permeability (10−3 μm2) | Flow Rate of High Permeability Layer% | Flow Rate of Low Permeability Layer% | Increase of Flow Rate of Low Permeability Layer % | |||
|---|---|---|---|---|---|---|---|---|
| High Permeability Layer | Low Permeability Layer | Water Flooding | Sequent Water Flooding | Water Flooding | Sequent Water Flooding | |||
| 40,300.86 mg/L, 120 °C | 200 | 1000 | 5 | 96.84 | 67.2 | 3.16 | 32.8 | 29.64 |
| 40 | 800 | 20 | 94.64 | 58.6 | 5.36 | 41.4 | 36.04 | |
| 5 | 200 | 40 | 85.03 | 36.4 | 14.97 | 63.6 | 48.63 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhi, J.; Liu, Y.; Chen, J.; Bo, L.; Qu, G.; Jiang, N.; He, W. Preparation and Performance Evaluation of a Temperature and Salt Resistant Hydrophobic Associative Weak Polymer Gel System. Molecules 2023, 28, 3125. https://doi.org/10.3390/molecules28073125
Zhi J, Liu Y, Chen J, Bo L, Qu G, Jiang N, He W. Preparation and Performance Evaluation of a Temperature and Salt Resistant Hydrophobic Associative Weak Polymer Gel System. Molecules. 2023; 28(7):3125. https://doi.org/10.3390/molecules28073125
Chicago/Turabian StyleZhi, Jiqiang, Yikun Liu, Jinfeng Chen, Lifeng Bo, Guohui Qu, Nan Jiang, and Weizhong He. 2023. "Preparation and Performance Evaluation of a Temperature and Salt Resistant Hydrophobic Associative Weak Polymer Gel System" Molecules 28, no. 7: 3125. https://doi.org/10.3390/molecules28073125
APA StyleZhi, J., Liu, Y., Chen, J., Bo, L., Qu, G., Jiang, N., & He, W. (2023). Preparation and Performance Evaluation of a Temperature and Salt Resistant Hydrophobic Associative Weak Polymer Gel System. Molecules, 28(7), 3125. https://doi.org/10.3390/molecules28073125





