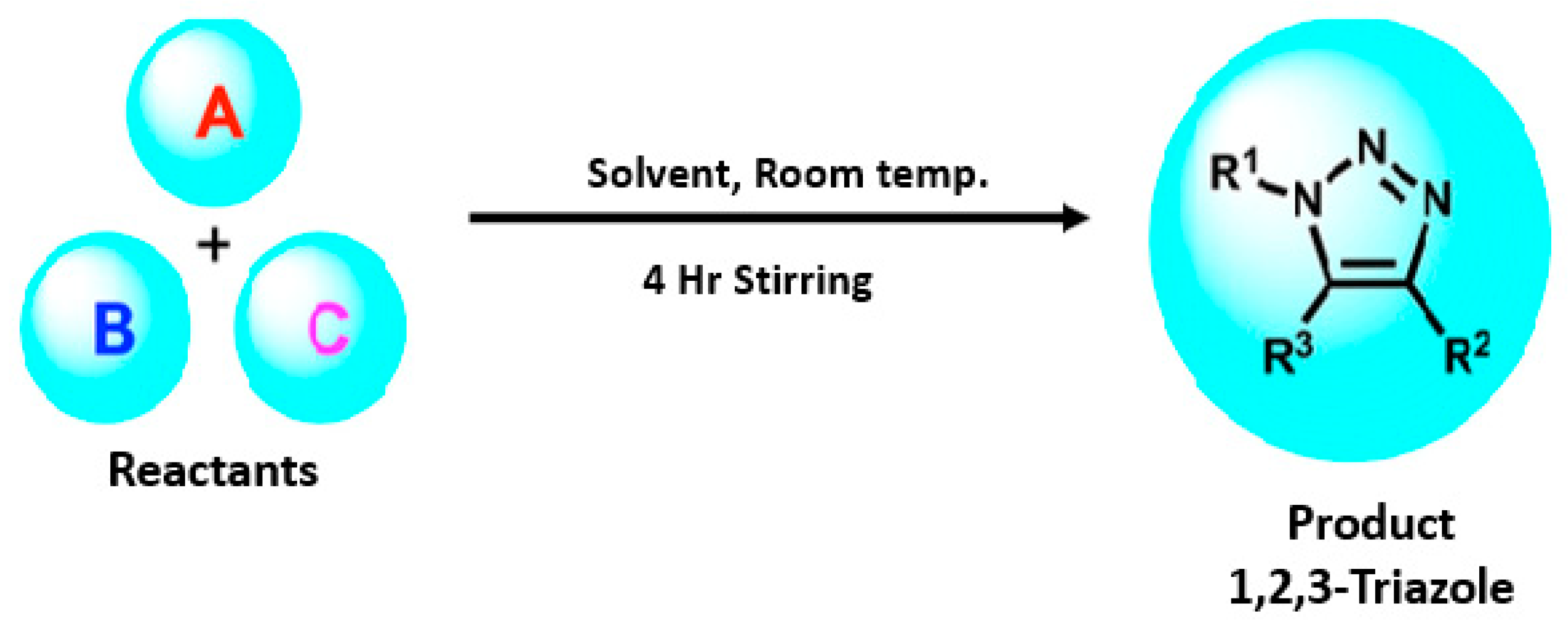
Designing Click One-Pot Synthesis and Antidiabetic Studies of 1,2,3-Triazole Derivatives
Abstract
1. Introduction
1.1. Target Selection
1.2. Dataset Collection
1.3. Lead Identification and Analogue Design
2. Results and Discussion
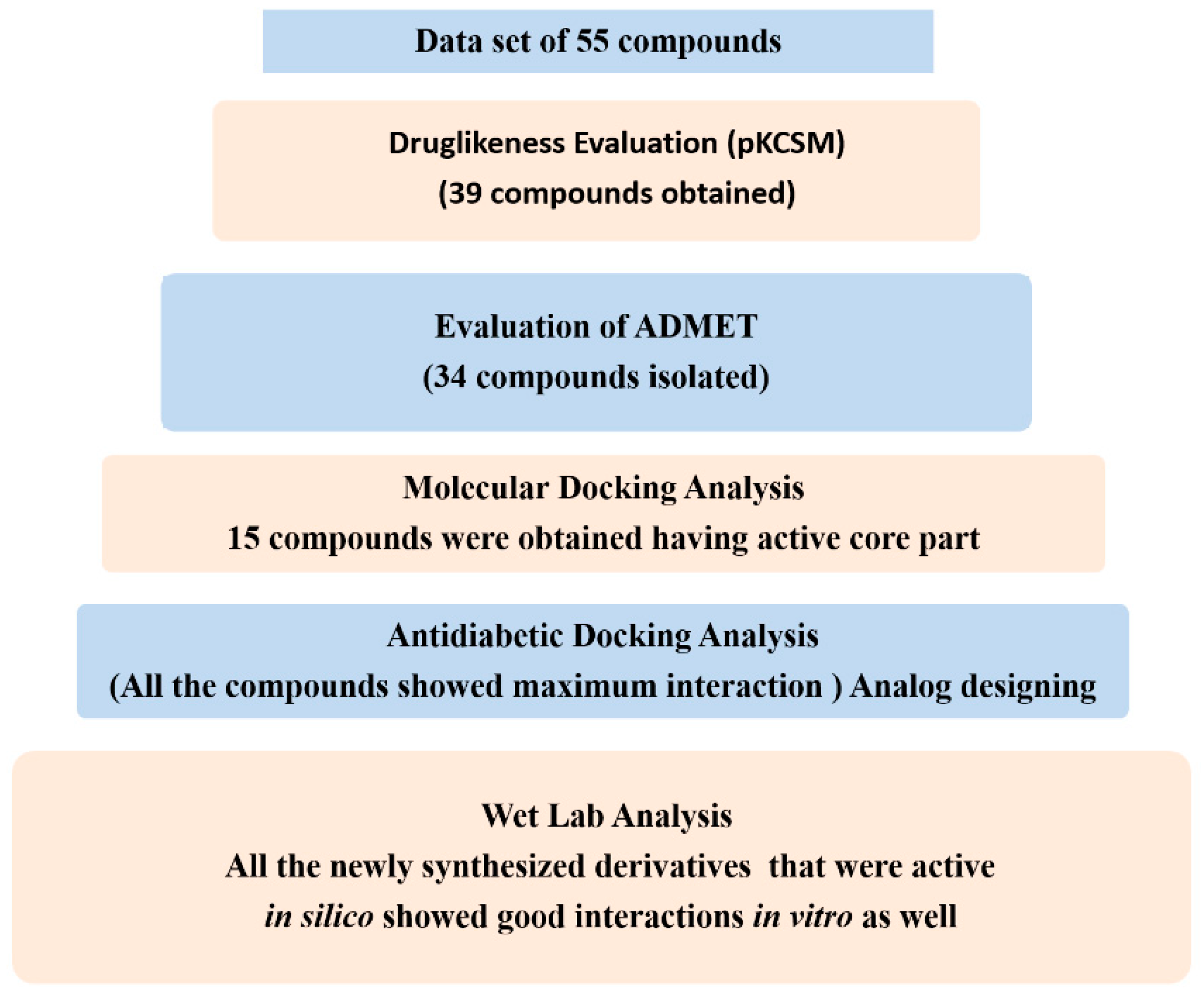
2.1. Virtual Screening for Antidiabetic Compounds
2.2. Drug-Likeness and ADMET Properties
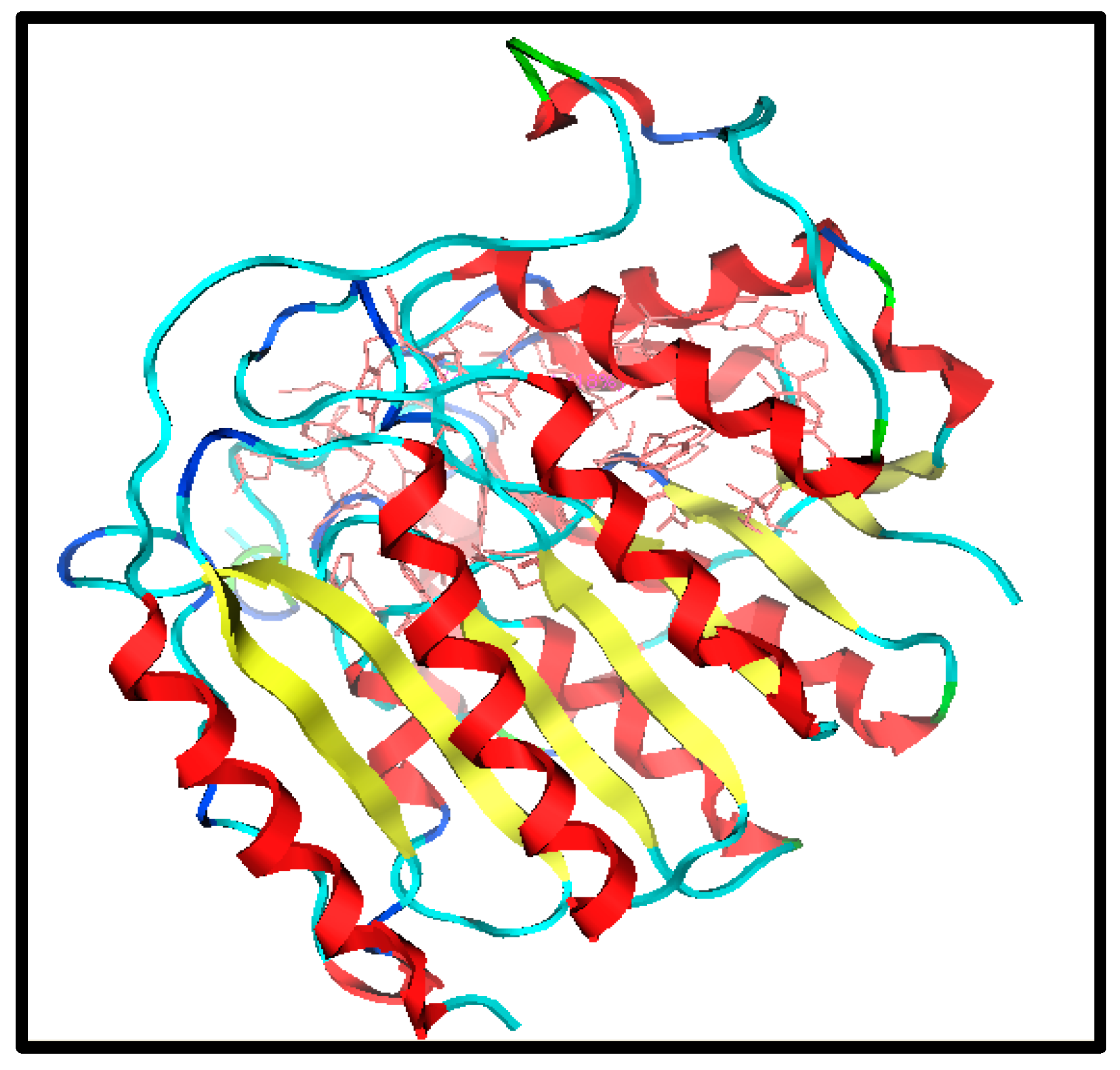
2.3. Molecular Docking Analysis
2.4. In Vitro Antidiabetic Assay
3. Materials and Methods
3.1. Materials
3.2. Experimental Equipment
3.3. Docking Procedure
3.4. Synthesis of the Hits Identified
General Procedure for the Synthesis
3.5. In Vitro Analysis
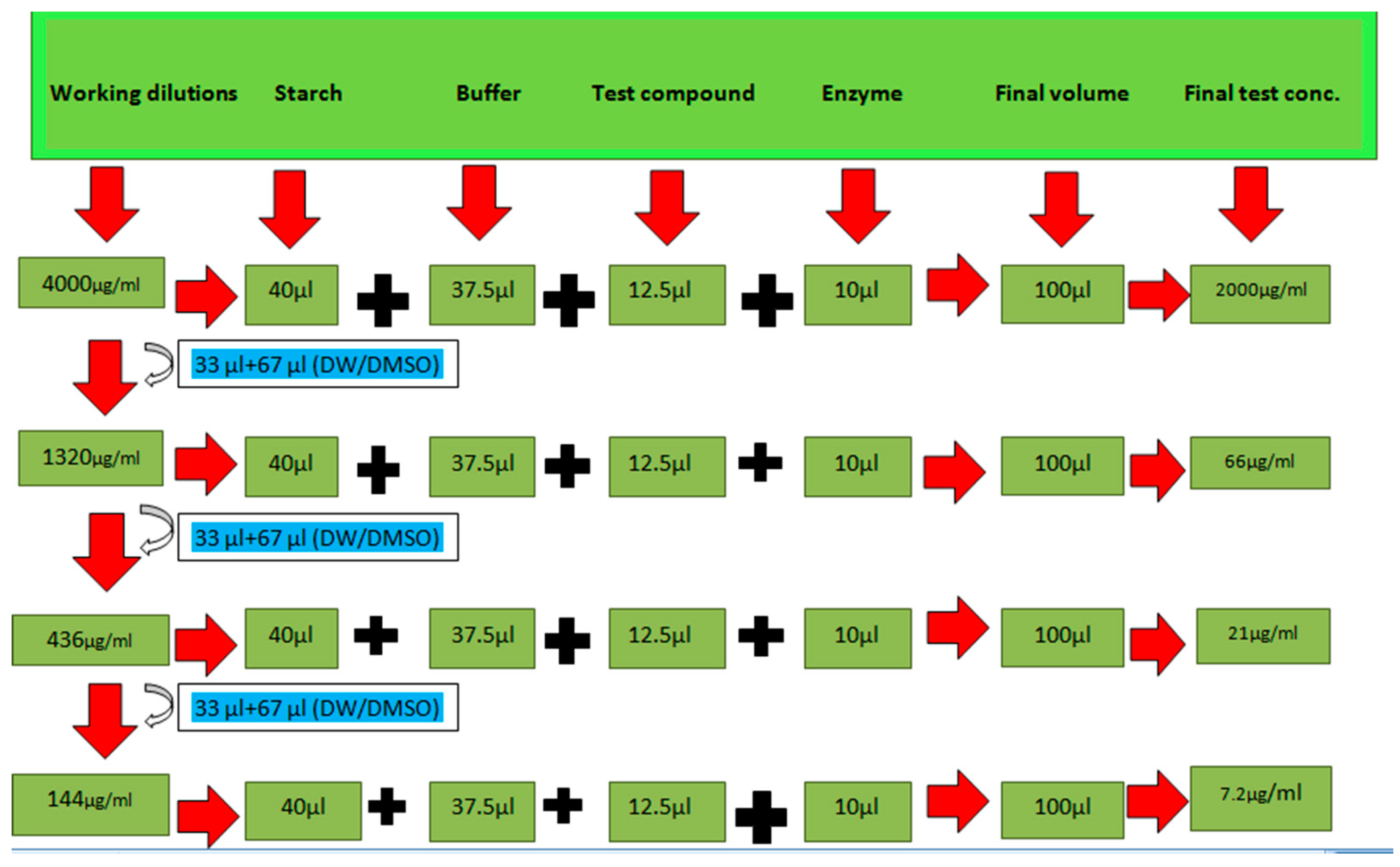
3.5.1. α-Amylase Inhibition Assay
3.5.2. Alpha-Glucosidase Inhibition Assay
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Razzaq, A.S.; Nah, J.R. In vitro, evaluation of antioxidant and antibacterial activities of new 1,2,3-triazole derivatives containing 1, 2, 4-triaozle ring. Sys. Rev. Pharm. 2021, 12, 8–13. [Google Scholar]
- Ren, L.; Yu, S.; Li, J.; Li, L. Pilot study on the effects of operating parameters on membrane fouling during ultrafiltration of alkali/surfactant/polymer flooding wastewater: Optimization and modeling. RSC Adv. 2019, 9, 11111–11122. [Google Scholar] [CrossRef] [PubMed]
- Deswal, L.; Kumar, A. Synthesis and antidiabetic evaluation of benzimidazole-tethered 1,2,3-triazoles. Arch. Der Pharm. 2020, 353, 2000090. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zheng, J.; Xu, W.; Pan, Y.; Chen, C.; Wei, D.; Ni, W. A New Series of Cytotoxic Pyrazoline Derivatives as Potential Anticancer Agents that Induce Cell Cycle. Molecules 2017, 22, 1635. [Google Scholar] [CrossRef]
- Sciences, M.; Faculty, M.; Hospital, S.G.; Faculty, M.; Hospital, S.G.; Faculty, M.; Hospital, S.G.; Faculty, M.; Hospital, S.G.; Faculty, M.; et al. Signs and Symptoms of Depression in Children and Adolescents with Type 1 Diabetes Mellitus: A Case Report. Int. J. Health Med. Sci. 2022, 5, 150–153. [Google Scholar]
- de Vos, W.M.; de Keizer, A.; Stuart, M.A.C.; Kleijn, J.M. Thin polymer films as sacrificial layers for easier cleaning. Colloids Surf. A Physicochem. Eng. Asp. 2010, 358, 6–12. [Google Scholar] [CrossRef]
- Da, M.; Doł, A.; Siedzielnik, M.; Biernacki, K.; Ciupak, O. Novel 1,2,3-Triazole Derivatives as Mimics of Steroidal System—Synthesis, Crystal Structures Determination, Hirshfeld Surfaces Analysis and Molecular Docking. Molecules 2021, 26, 4059. [Google Scholar]
- Bozorov, K.; Zhao, J.; Aisa, H.A. 1,2,3-Triazole-containing hybrids as leads in medicinal chemistry: A recent overview. Bioorg. Med. Chem. 2019, 27, 3511–3531. [Google Scholar] [CrossRef]
- Andrade-cetto, A. Inhibition of gluconeogenesis by Malmea depressa root. J. Ethnopharmacol. 2011, 137, 930–933. [Google Scholar] [CrossRef]
- Tseng, P.; Ande, C.; Moremen, K.W.; Crich, D. Influence of Side Chain Conformation on the Activity of Glycosidase Inhibitors. Angew. Chem. 2023, 135, e202217809. [Google Scholar] [CrossRef]
- Rajasekaran, P.; Ande, C.; Vankar, Y.D. Synthesis of (5,6 & 6,6)-oxa-oxa annulated sugars as glycosidase inhibitors from 2-formyl galactal using iodocyclization as a key step. Arkivoc 2022, 6, 5–23. [Google Scholar]
- Creary, X.; Anderson, A.; Brophy, C.; Crowell, F.; Funk, Z. Method for Assigning Structure of 1,2,3-Triazoles. J. Org. Chem. 2012, 77, 8756–8761. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Wahab, B.F.; Farahat, A.A.; Kariuki, B.M. (E)-1-(5-Methyl-1-(4-nitrophenyl)-1H-1,2,3-triazol-4-yl)ethan-1-one Oxime. Molbank 2023, 1, M1593. [Google Scholar] [CrossRef]
- Chen, G.; Seukep, A.J.; Guo, M. Recent Advances in Molecular Docking for the Research and Discovery of Potential Marine Drugs. Mar. Drugs 2020, 18, 545. [Google Scholar] [CrossRef]
- Mehdar, Y.T.H.; Aljohani, F.S.; Said, M.A.; Ramli, Y. Greener pastures in evaluating antidiabetic drug for a quinoxaline Derivative: Synthesis, Characterization, Molecular Docking, in vitro and HSA/DFT/XRD studies. Arab. J. Chem. 2022, 15, 103851. [Google Scholar] [CrossRef]
- Attique, S.A.; Hassan, M.; Usman, M.; Atif, R.M.; Mahboob, S.; Al-ghanim, K.A.; Bilal, M. A Molecular Docking Approach to Evaluate the Pharmacological Properties of Natural and Synthetic Treatment Candidates for Use against Hypertension. Int. J. Environ. Res. Public Health 2019, 16, 923. [Google Scholar] [CrossRef] [PubMed]
- Sadegh, M.; Mohammadi-khanaposhtani, M.; Kiani, M.; Rashidi, P.; Zabihi, E.; Pourbagher, R.; Rahimi, R. Bioorganic Chemistry Biscoumarin-1,2,3-triazole hybrids as novel anti-diabetic agents: Design, synthesis, in vitro α-glucosidase inhibition, kinetic, and docking studies. Bioorg. Chem. 2019, 92, 103206. [Google Scholar] [CrossRef]
- Al-Ghulikah, H.; Ghabi, A.; Mtiraoui, H.; Jeanneau, E.; Msaddek, M. Synthesis of new 1,2,3-triazole linked benzimidazolidinone: Single crystal X-ray structure, biological activities evaluation and molecular docking studies. Arab. J. Chem. 2023, 16, 104566. [Google Scholar] [CrossRef]
- Iraji, A.; Brojeni, D.S.; Mojtabavi, S.; Faramarzi, M.A. Cyanoacetohydrazide linked to 1,2,3-triazole derivatives: A new class of α-glucosidase inhibitors. Sci. Rep. 2022, 12, 8647. [Google Scholar] [CrossRef]
- Klenam, F.; Dzidzor, C.; Amengor, K.; Brobbey, A.; Nii, J.; Amaning, C.; Peprah, P.; Kingsley, B.; Okon, I.; Kwame, F.; et al. Synthesis, molecular docking studies and ADME prediction of some new triazoles as potential antimalarial agents. Sci. Afr. 2021, 14, e00998. [Google Scholar] [CrossRef]
- Derivatives, B.; Peng, Z. Synthesis, In Vitro α-Glucosidase Inhibitory Activity and Molecular Docking Studies of Novel Benzothiazole-Triazole Derivatives. Molecules 2017, 22, 1555. [Google Scholar] [CrossRef]
- Pires, D.E.V.; Blundell, T.L.; Ascher, D.B. pkCSM: Predicting small-molecule pharmacokinetic and toxicity properties using graph-based signatures. J. Med. Chem. 2015, 58, 4066–4072. [Google Scholar] [CrossRef] [PubMed]
- Al-Radadi, N.S.; Zayed, E.M.; Mohamed, G.G.; Abd El Salam, H.A. Synthesis, Spectroscopic Characterization, Molecular Docking, and Evaluation of Antibacterial Potential of Transition Metal Complexes Obtained Using Triazole Chelating Ligand. J. Chem. 2020, 2020, 1–12. [Google Scholar] [CrossRef]
- Ahmed, M.N.; Yasin, K.A.; Ayub, K.; Mahmood, T.; Tahir, M.N.; Khan, B.A.; Hafeez, M.; Ahmed, M.; Ul-Haq, I. Click one pot synthesis, spectral analyses, crystal structures, DFT studies and brine shrimp cytotoxicity assay of two newly synthesized 1,4,5-trisubstituted 1,2,3-triazoles. J. Mol. Struct. 2016, 1106, 430–439. [Google Scholar] [CrossRef]
- Al-asri, J.; Wolber, G. Discovery of novel α-amylase inhibitors using structure-based drug design. J. Cheminformatics 2014, 6, P50. [Google Scholar] [CrossRef]
- Saeed, A.; Channar, P.A.; Larik, F.A.; Jabeen, F.; Saeed, S.; Flörke, U.; Ismail, H.; Dilshad, E.; Mirza, B. Department of Chemistry Quaid-i-Azam University-45320, Islamabad, Pakistan Cardiovascular and Metabolic Research Unit, Laurentian University 935 Ramsey Lake Road, Department Chemie, Fakultät für Naturwissenschaften, Department of Bioinformatics and Biosciences, Capital University of Science and Technology. Inorg. Chim. Acta 2017, 464, 204–213. [Google Scholar] [CrossRef]


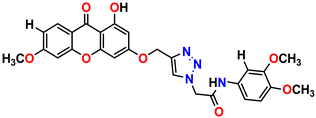 Compound 1 KS-1, 15.90 ± 0.91 µM | 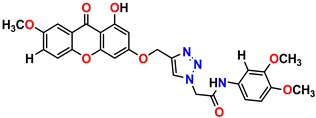 Compound 2 KS-2 > 100 µM |
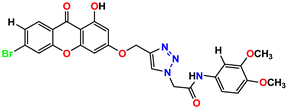 Compound 3 KS-03, 11.82 ± 0.82 | 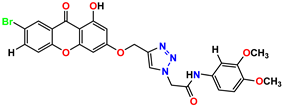 Compound 4 KS-04, 29.84 ± 3.47 |
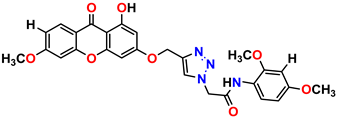 Compound 5 KS-05, 2.06 ± 0.16 | 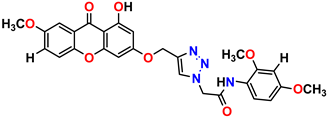 Compound 6 KS-06, 8.31 ± 0.88 |
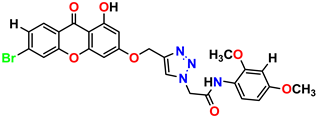 Compound 7 KS-07, 2.78 ± 0.22 | 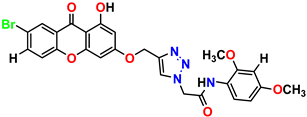 Compound 8 KS-08, 3.07 ± 0.56 |
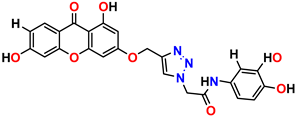 Compound 9 KS-09, 6.13 ± 0.09 | 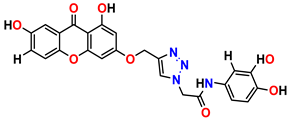 Compound 10 KS-10, 7.06 ± 0.89 |
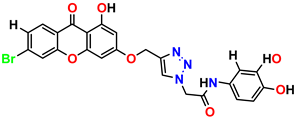 Compound 11 KS-11, 5.23 ± 0.53 | 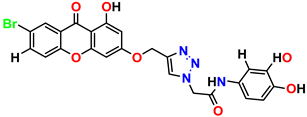 Compound 12 KS-12, 3.17 ± 0.61 |
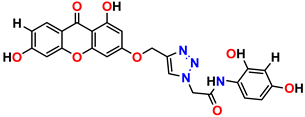 Compound 13 KS-13, 17.61 ± 1.68 |  Compound 14 KS-14, 15.62 ± 1.13 |
 Compound 15 KS-15, 5.87 ± 0.76 | 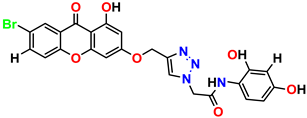 Compound 16 KS-16, 5.88 ± 0.32 |
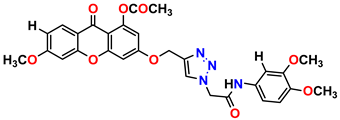 Compound 17 KS-17, 98.63 ± 4.12 | 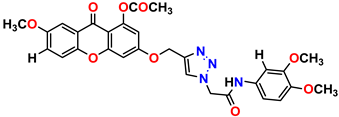 Compound 18 KS-18, >100 |
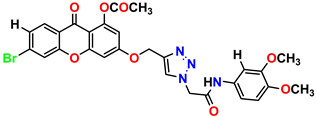 Compound 19 KS-19, 26.11 ± 2.95 | 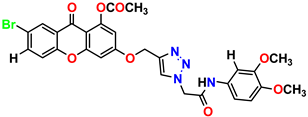 Compound 20 KS-20, 32.33 ± 0.82 |
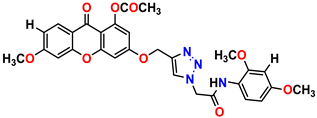 Compound 21 KS-21, 33.16 ± 2.51 |  Compound 22 KS-22, 42.60 ± 0.09 |
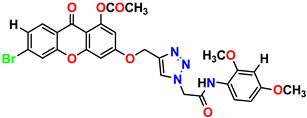 Compound 23 KS-23, 12.78 ± 0.01 | 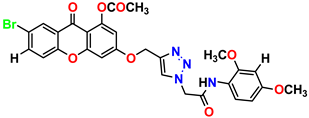 Compound 24 KS-24, 12.13 ± 1.84 |
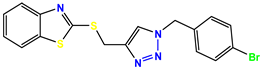 Compound 25 KS-25, 28.7 | 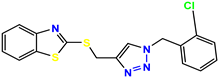 Compound 26 KS-26, >100 |
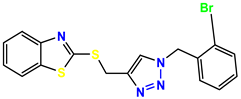 Compound 27, KS-27, 37.4 | 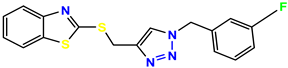 Compound 28, KS-28, 61.1 |
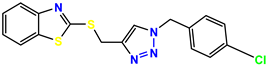 Compound 29 KS-29, 27.4 | 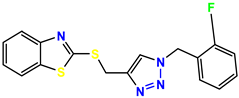 Compound 30 KS-30, >100 |
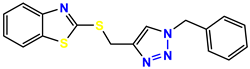 Compound 31 KS-31, >100 | 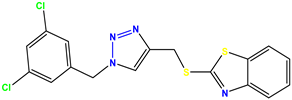 Compound 32 KS-32, 41.0 |
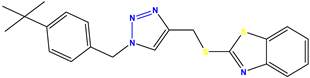 Compound 33 KS-33, 29.4 |  Compound 34 KS-34, 45.9 |
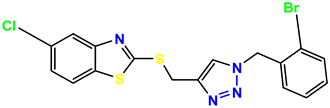 Compound 35 KS-35, 48.4 | 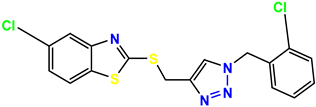 Compound 36 KS-36, 33.6 |
 Compound 37 KS-37, 28.2 | 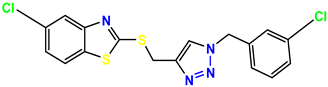 Compound 38, KS-38 |
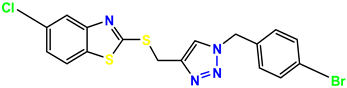 Compound 39 KS-39 | 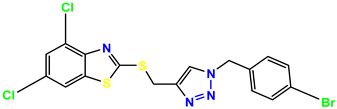 Compound 40 KS-40 |
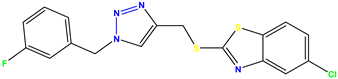 Compound 41 KS-41 | 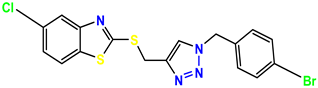 Compound 42 KS-42 |
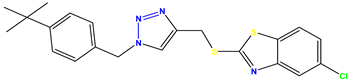 Compound 43 KS-43 |  Compound 44 KS-44 |
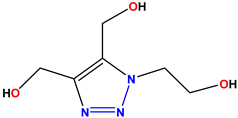 Compound 45 KS-45 | 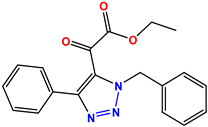 Compound 46 KS-46 |
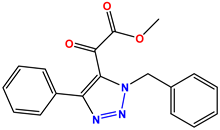 Compound 47 KS-47 |  Compound 48 KS-48 |
 Compound 49 KS-49 |  Compound 50 KS-50 |
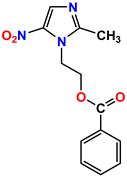 Compound 51 KS-51 | 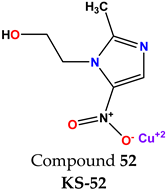 Compound 52 KS-52 |
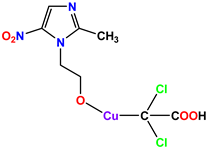 Compound 53 KS-53 |  Compound 54 KS-54 |
 Compound 55 KS-55 | 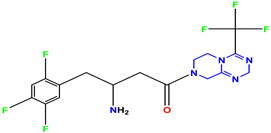 Standard 1 Sitagliptin |
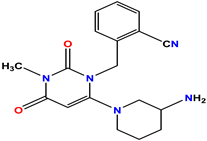 Standard 2 Alogliptin |  Standard 3 Metformin |
| Sr. No | Molecular Wt. g/mol | Log P | Rotatable Bonds | Acceptors | Donors | Surface Area (g/cm2) |
|---|---|---|---|---|---|---|
| 01 | 321.4 | 3.1 | 6 | 5 | 01 | 140.4 |
| 02 | 231.3 | 2.5 | 4 | 5 | 0 | 99.2 |
| 03 | 249.3 | 2.9 | 4 | 3 | 0 | 112.1 |
| 04 | 235.3 | 3.7 | 3 | 3 | 0 | 105.9 |
| Sr. No | Absorption mg/L | Distribution mg/L | Metabolism mg/L | Excretion mg/L | Toxicity Oral/Hepatotoxicity |
|---|---|---|---|---|---|
| 01 | 98.726 | 0.128 | Yes | 0.394 | No/No |
| 02 | 94.696 | 0.271 | Yes | 0.327 | 0.23/No |
| 03 | 97.12 | 0.088 | Yes | 0.272 | No/No |
| 04 | 94.539 | 0.191 | Yes | 0.28 | 0.8/No |
| Sr. No | Compounds | Hydrogen Bonding | Arene–pi Interactions | Binding Energy (S) | |
|---|---|---|---|---|---|
| 01 | K-1 | 2.74, 2.86 1.13, 2.64 2.59 | Tyr18, Ser39, Tyr24, Arg37, Asn136 | -- | −0.6467 |
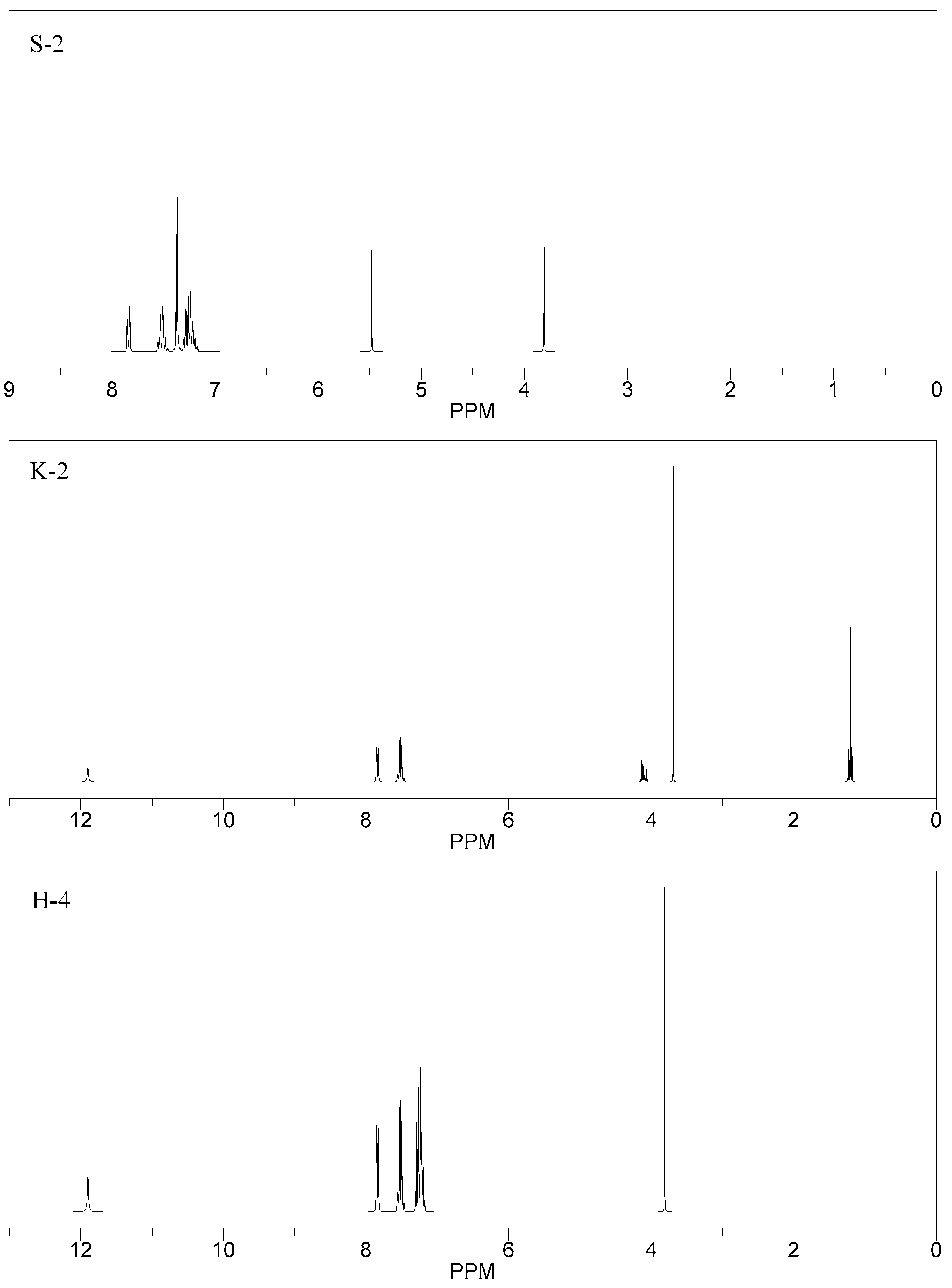
| 02 | S-2 | 2.48, 1.8, 2.52 | Lys36, Ser39 | Yes | −4.0720 |
| 03 | K-2 | 2.47, 2.02 | Lys33, Trp141 | -- | −3.0564 |
| 04 | H-4 | 2.32, 1.15 | Gyl151, Ser150 | yes | −2.5427 |
| Dose µg/mL | %Age Inhibition at Different Concentration | |||||||
|---|---|---|---|---|---|---|---|---|
| 800 | 400 | 200 | 100 | 50 | 25 | 12.5 | 6.25 | |
| K-1 | 87.012 | 81.11 | 74.89 | 64.24 | 55.81 | 42.21 | 37.23 | 23.11 |
| S-2 | 84.22 | 78.56 | 71.59 | 59.1 | 52.22 | 44.81 | 32.22 | 21.47 |
| K-2 | 81.89 | 73.45 | 58.23 | 48.56 | 39.76 | 29.34 | 20.42 | 12.78 |
| H-4 | 83.12 | 76.11 | 64.72 | 53.12 | 45.12 | 39.12 | 25.1 | 16.89 |
| %Age Inhibition at Different Concentrations | ||||||||
|---|---|---|---|---|---|---|---|---|
| Dose µg/mL | 800 | 400 | 200 | 100 | 50 | 25 | 12.5 | 6.25 |
| K-1 | 99.17 | 97.01 | 95.55 | 91.11 | 88.96 | 83.11 | 72.37 | 68.04 |
| S-2 | ±96.22 | ±95.01 | 93.81 | 90.24 | 86.02 | 72.41 | 69.23 | 65.10 |
| K-2 | 87.19 | 83.12 | 71.03 | 65.10 | 57.8 | 51.69 | 44.32 | 31.09 |
| H-4 | 89.12 | 81.01 | 75.00 | 67.11 | 55.21 | 49.11 | 40.81 | 41.09 |
| Samples | Reactants | Product | Yield | |||||
|---|---|---|---|---|---|---|---|---|
| A | B | C | R1 | R2 | R3 | |||
| K-1 | 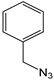 | 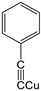 | 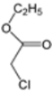 | 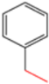 |  | 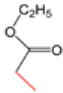 | 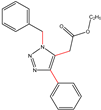 ethyl 2-(1-benzyl-4-phenyl-1 H-1,2,3-triazol-5-yl)acetate | 79% |
| S-2 |  | 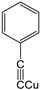 | 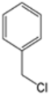 | 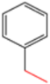 |  | 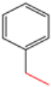 | 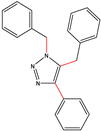 1,5-dibenzyl-4-phenyl-1H-56%1,2,3-triazole | 71% |
| K-2 | NaN3 |  |  | H |  | 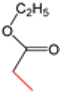 | 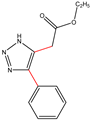 ethyl 2-(4-phenyl-1H-1,2,3-triazol-5-yl)acetate | 56% |
| H-4 | NaN3 |  | 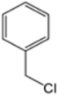 | H | 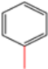 | 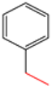 |  5-benzyl-4-phenyl-1H-1,2,3-triazole | 67% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shafique, K.; Farrukh, A.; Mahmood Ali, T.; Qasim, S.; Jafri, L.; Abd-Rabboh, H.S.M.; AL-Anazy, M.m.; Kalsoom, S. Designing Click One-Pot Synthesis and Antidiabetic Studies of 1,2,3-Triazole Derivatives. Molecules 2023, 28, 3104. https://doi.org/10.3390/molecules28073104
Shafique K, Farrukh A, Mahmood Ali T, Qasim S, Jafri L, Abd-Rabboh HSM, AL-Anazy Mm, Kalsoom S. Designing Click One-Pot Synthesis and Antidiabetic Studies of 1,2,3-Triazole Derivatives. Molecules. 2023; 28(7):3104. https://doi.org/10.3390/molecules28073104
Chicago/Turabian StyleShafique, Kainat, Aftab Farrukh, Tariq Mahmood Ali, Sumera Qasim, Laila Jafri, Hisham S. M. Abd-Rabboh, Murefah mana AL-Anazy, and Saima Kalsoom. 2023. "Designing Click One-Pot Synthesis and Antidiabetic Studies of 1,2,3-Triazole Derivatives" Molecules 28, no. 7: 3104. https://doi.org/10.3390/molecules28073104
APA StyleShafique, K., Farrukh, A., Mahmood Ali, T., Qasim, S., Jafri, L., Abd-Rabboh, H. S. M., AL-Anazy, M. m., & Kalsoom, S. (2023). Designing Click One-Pot Synthesis and Antidiabetic Studies of 1,2,3-Triazole Derivatives. Molecules, 28(7), 3104. https://doi.org/10.3390/molecules28073104