Construction of S-Scheme CuS/Bi5O7I Heterojunction for Boosted Photocatalytic Disinfection with Visible Light Exposure
Abstract
:1. Introduction
2. Results and Discussion
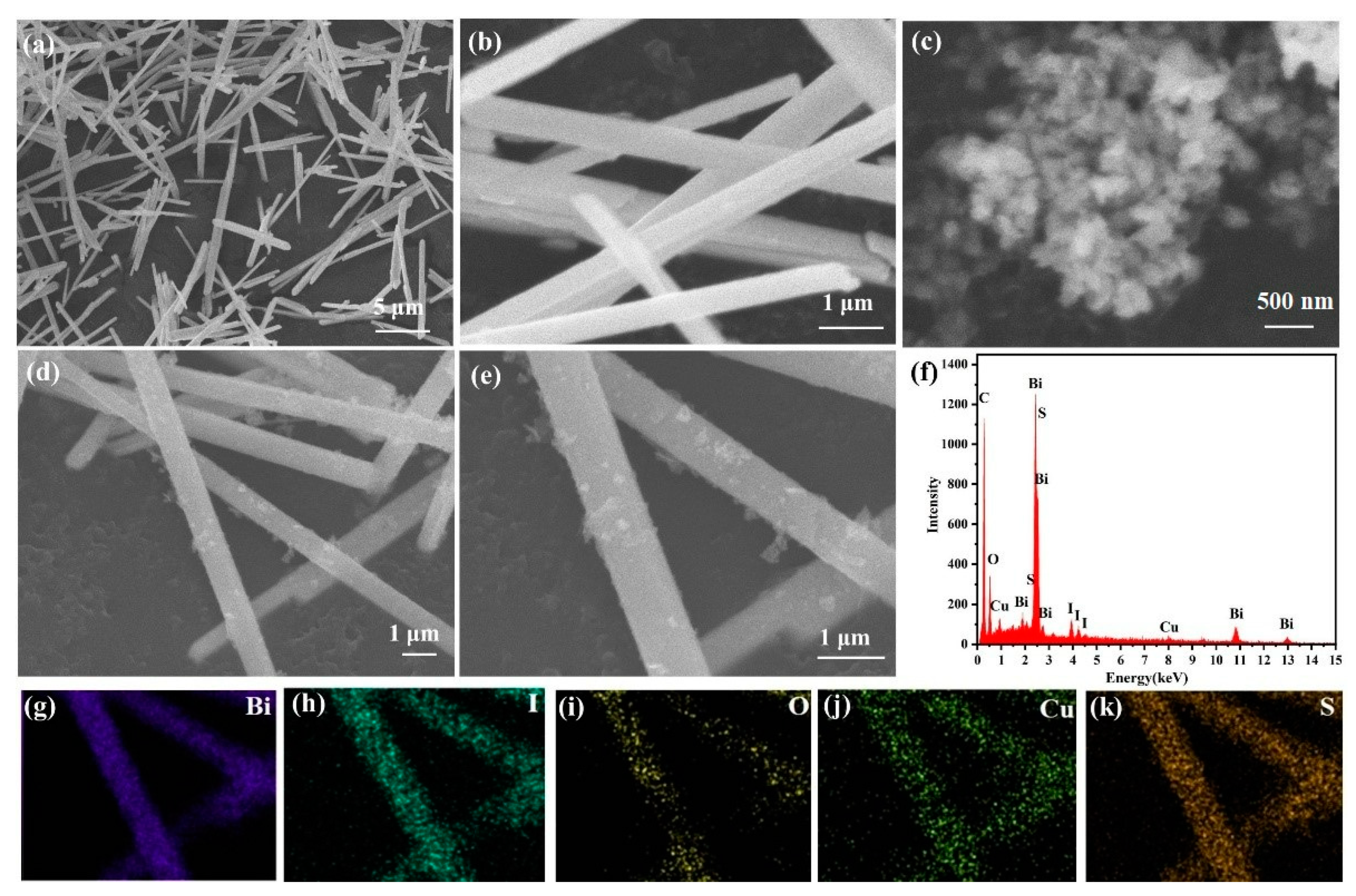
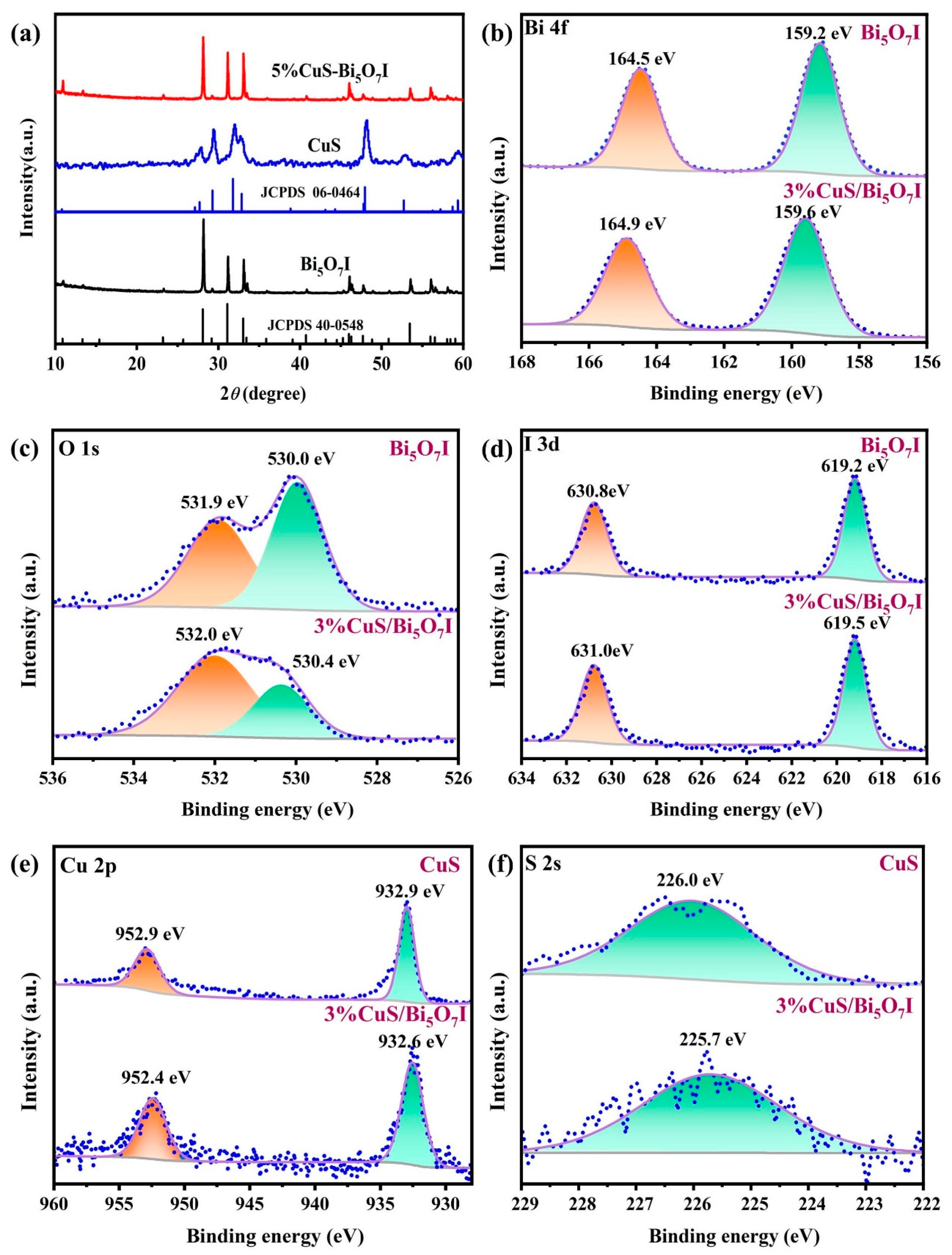
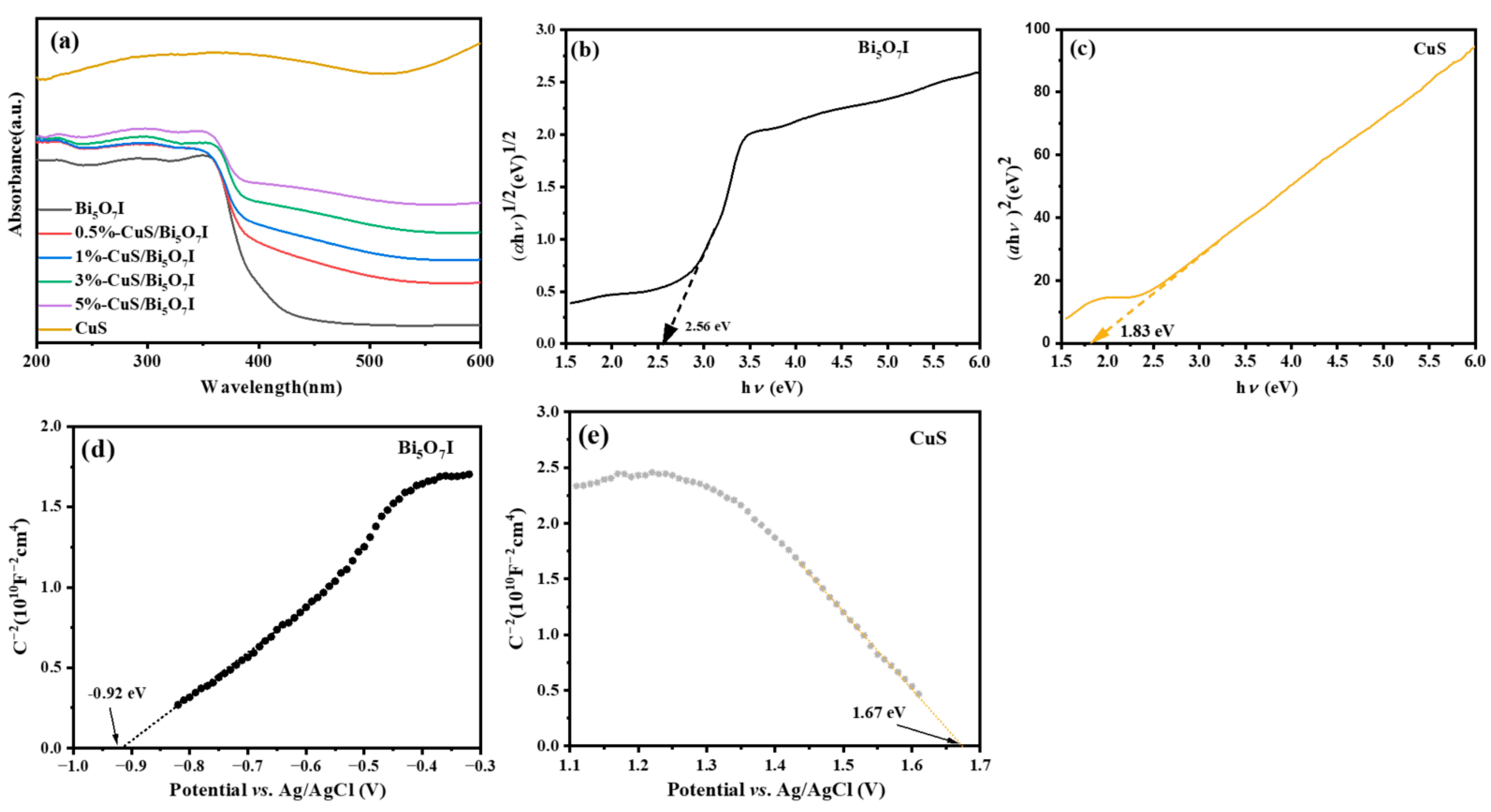
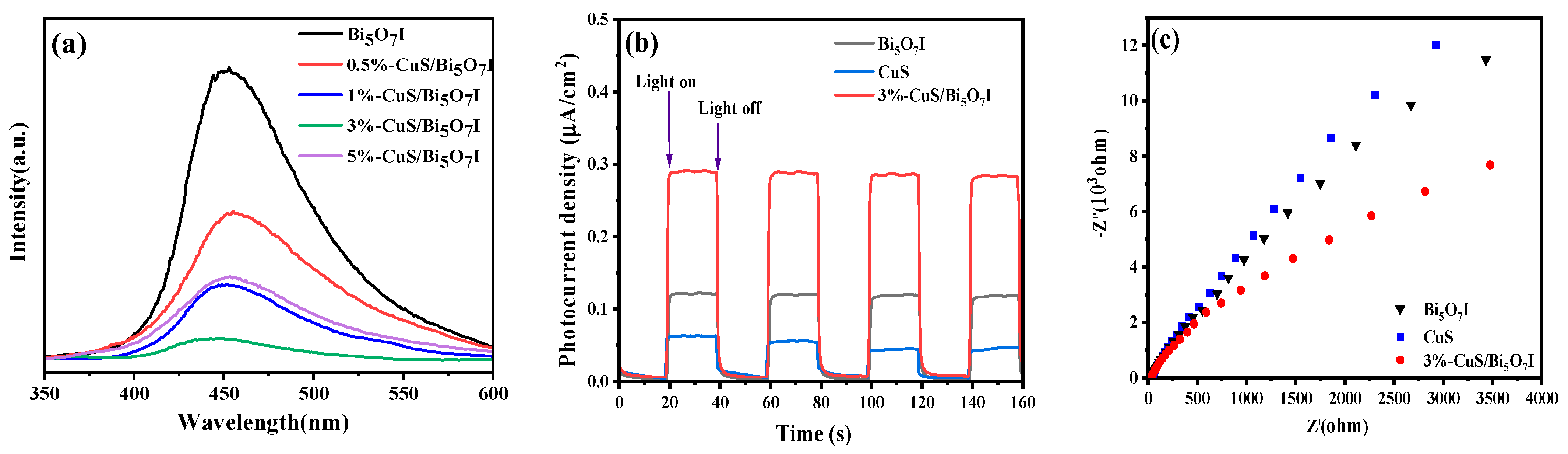
2.1. Characterization
2.2. Photocatalytic Disinfection Activity
2.3. Inactivation Process of E. coli
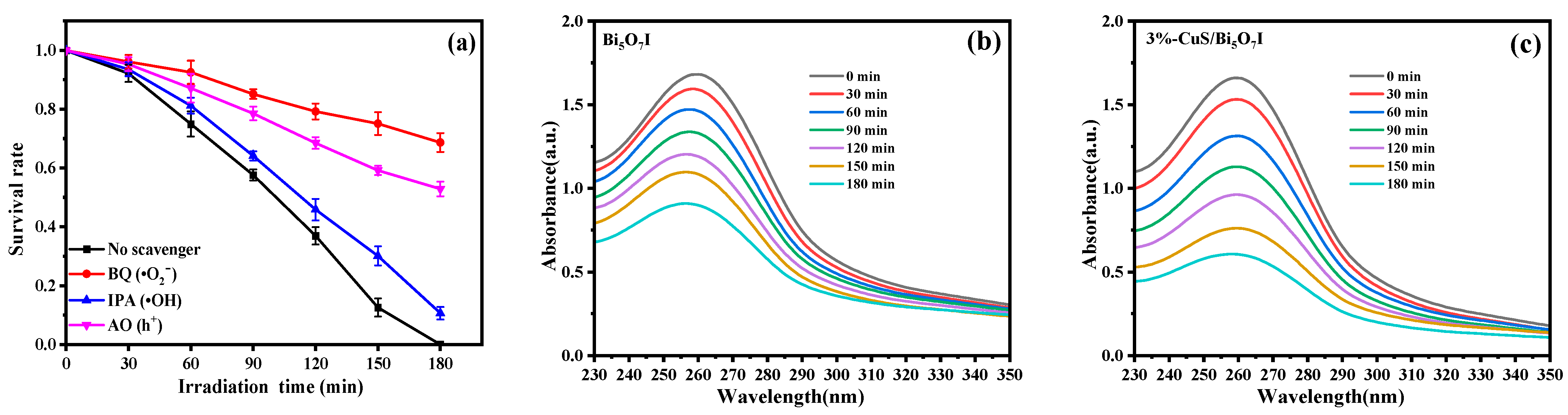
2.3.1. Investigation of Active Species
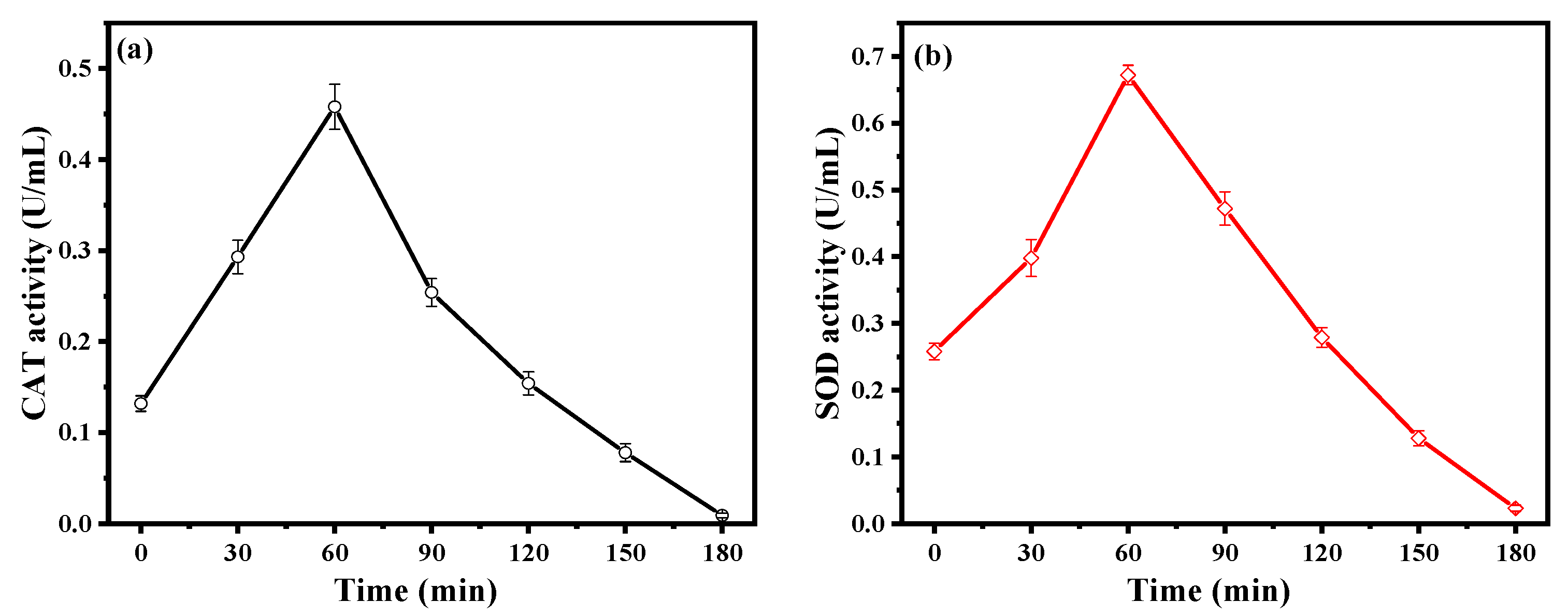
2.3.2. Activities of the Antioxidant Enzymes
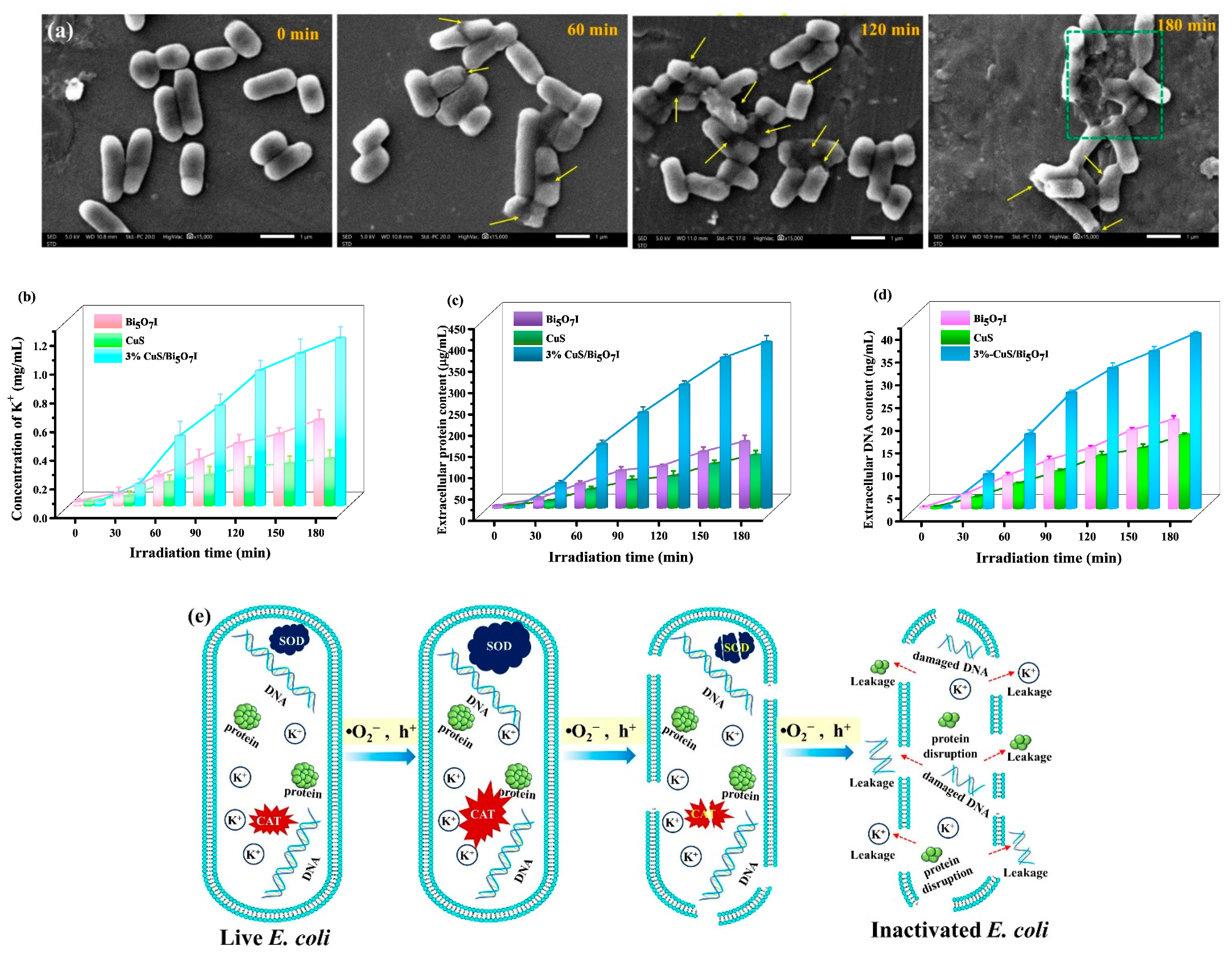
2.3.3. Microstructure Changes of E. coli Cell
2.3.4. Leakage of Intracellular Components
2.4. Mechanism of Improved Photocatalytic Activity with CuS/Bi5O7I Heterojunction
3. Experimental Section
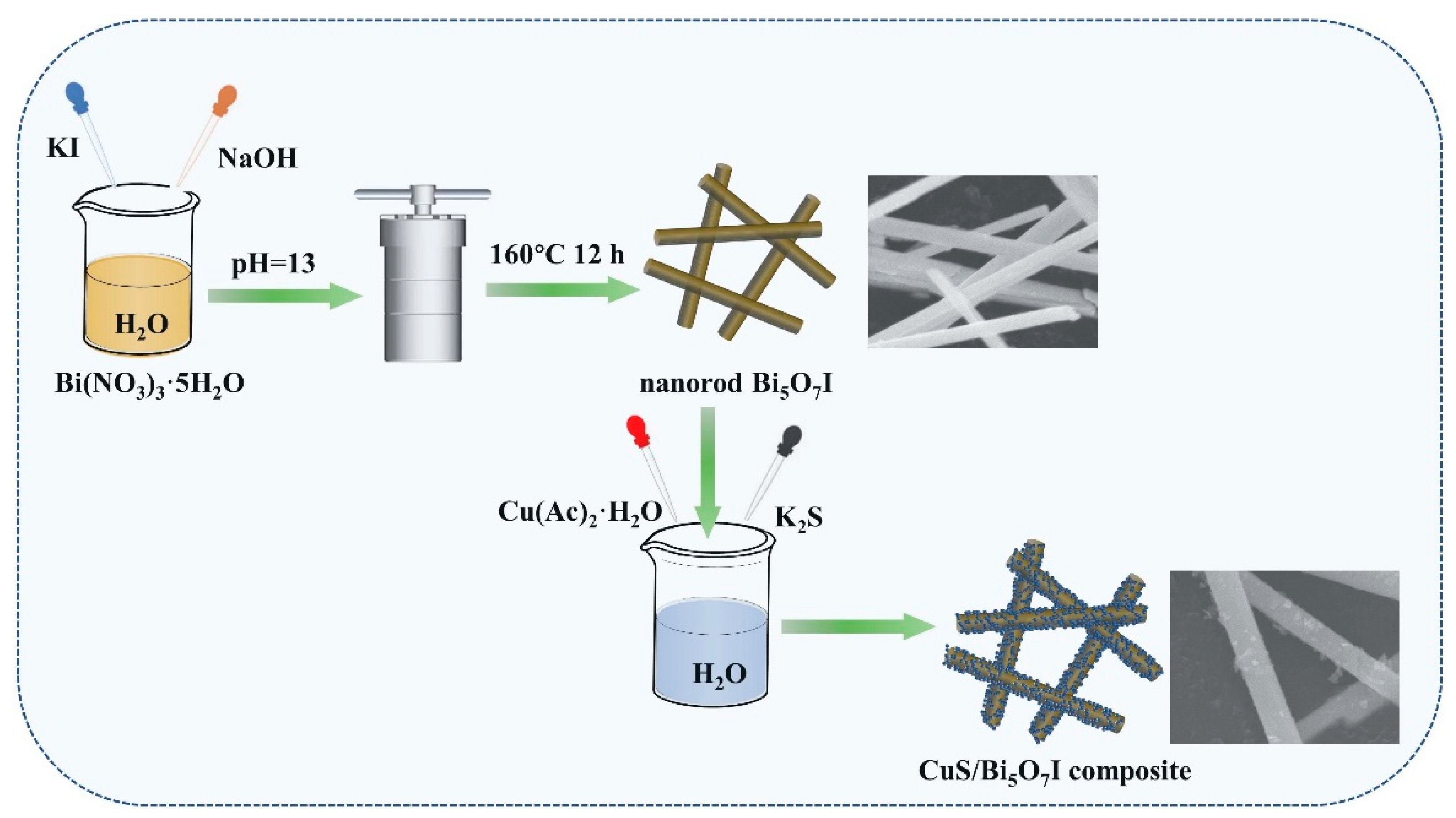
3.1. Preparation of Photocatalysts
3.2. Characterization
3.3. Photoelectrochemical Measurement
3.4. Determination of Photocatalytic Disinfection Performance
3.5. Fluorescence Microscopy Assays of Live/Dead E. coli
3.6. Microstructure Observation of E. coli
3.7. Antioxidant Enzymes Assay
3.8. Leakage of Intracellular Components
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Matsunaga, T.; Tomoda, R.; Nakajima, T.; Wake, H. Photoelectrochemical sterilization of microbial cells by semiconductor powders. Fems. Microbiol. Lett. 1985, 29, 211–214. [Google Scholar] [CrossRef]
- Kumar, A.; Hasija, V.; Sudhaik, A.; Raizada, P.; Nguyen, V.H.; Le, Q.V.; Singh, P.; Nguyen, D.C.; Thakur, S.; Hussain, C.M. The practicality and prospects for disinfection control by photocatalysis during and post-pandemic: A critical review. Environ. Res. 2022, 209, 112814. [Google Scholar] [CrossRef] [PubMed]
- Habibi-Yangjeh, A.; Asadzadeh-Khaneghah, S.; Feizpoor, S.; Rouhi, A. Review on heterogeneous photocatalytic disinfection of waterborne, airborne, and foodborne viruses: Can we win against pathogenic viruses? J. Colloid Interf. Sci. 2020, 580, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Meng, J.; Jing, S.; Wang, K.; Ban, C.; Feng, Y.; Duan, Y.; Ma, J.; Gan, L.; Zhou, X. Origin of bismuth-rich strategy in bismuth oxyhalide photocatalysts. Energy Environ. Mater. 2022, e12432. [Google Scholar] [CrossRef]
- Han, Q. Advances in preparation methods of bismuth-based photocatalysts. Chem. Eng. J. 2021, 414, 127877. [Google Scholar] [CrossRef]
- Zhang, W.; Peng, Y.; Yang, Y.; Zhang, L.; Bian, Z.; Wang, H. Bismuth-rich strategy intensifies the molecular oxygen activation and internal electrical field for the photocatalytic degradation of tetracycline hydrochloride. Chem. Eng. J. 2022, 430, 132963. [Google Scholar] [CrossRef]
- Yang, J.; Xu, L.; Liu, C.; Xie, T. Preparation and photocatalytic activity of porous Bi5O7I nanosheets. Appl. Surf. Sci. 2014, 319, 265–271. [Google Scholar] [CrossRef]
- Cao, C.-S.; Wang, J.; Yu, X.; Zhang, Y.; Zhu, L. Photodegradation of seven bisphenol analogues by Bi5O7I/UiO-67 heterojunction: Relationship between the chemical structures and removal efficiency. Appl. Catal. B Environ. 2020, 277, 119222. [Google Scholar] [CrossRef]
- Bai, Y.; Ye, L.; Chen, T.; Wang, L.; Shi, X.; Zhang, X.; Chen, D. Facet-dependent photocatalytic N2 fixation of bismuth-rich Bi5O7I nanosheets. ACS Appl. Mater. Interf. 2016, 8, 27661–27668. [Google Scholar] [CrossRef]
- Lan, M.; Zheng, N.; Dong, X.; Hua, C.; Ma, H.; Zhang, X. Bismuth-rich bismuth oxyiodide microspheres with abundant oxygen vacancies as an efficient photocatalyst for nitrogen fixation. Dalton Trans. 2020, 49, 9123–9129. [Google Scholar] [CrossRef]
- Ding, C.; Ye, L.; Zhao, Q.; Zhong, Z.; Liu, K.; Xie, H.; Bao, K.; Zhang, X. Huang, synthesis of BixOyIz from molecular precursor and selective photoreduction of CO2 into CO. J. CO2 Util. 2016, 14, 135–142. [Google Scholar] [CrossRef]
- Li, Q.; Li, Y.-L.; Li, B.; Hao, Y.-J.; Wang, X.-J.; Liu, R.-H.; Ling, Y.; Liu, X.; Li, F.-T. Construction of adjustable dominant {314} facet of Bi5O7I and facet-oxygen vacancy coupling dependent adsorption and photocatalytic activity. Appl. Catal. B Environ. 2021, 289, 120041. [Google Scholar] [CrossRef]
- Lin, J.; Hu, Z.; Li, H.; Qu, J.; Zhang, M.; Liang, W.; Hu, S. Ultrathin nanotubes of Bi5O7I with a reduced band gap as a high-performance photocatalyst. Inorg. Chem. 2019, 58, 9833–9843. [Google Scholar] [CrossRef]
- Ju, P.; Hao, L.; Zhang, Y.; Sun, J.; Dou, K.; Lu, Z.; Liao, D.; Zhai, X.; Sun, C. In-situ topotactic construction of novel rod-like Bi2S3/Bi5O7I p-n heterojunctions with highly enhanced photocatalytic activities. J. Mater. Sci. Technol. 2023, 135, 126–141. [Google Scholar] [CrossRef]
- Huang, W.; Xiao, X.; Lu, M.; Xiao, Y. In-situ fabrication of novel BiOCl/Bi5O7I 2D/3D heterostructures with enhanced photocatalytic activity. J. Alloys Compd. 2022, 895, 162669. [Google Scholar] [CrossRef]
- Guan, Y.; Wu, J.; Liu, Q.; Wang, H.; Liu, G.; He, P.; Qi, X. Study of sheetlike BiOI/rodlike Bi5O7I composite photocatalyst by in situ crystallization of BiOI with pH-dependence for Hg0 removal. Energy Fuels 2021, 35, 11415–11426. [Google Scholar] [CrossRef]
- Li, J.; Liu, E.; Ma, Y.; Hu, X.; Wan, J.; Sun, L.; Fan, J. Synthesis of MoS2/g-C3N4 nanosheets as 2D heterojunction photocatalysts with enhanced visible light activity. Appl. Surf. Sci. 2016, 364, 694–702. [Google Scholar] [CrossRef]
- Zhu, Y.; Xu, J.; Chen, M. Synthesis of La2Ti2O7/Bi5O7I photocatalysts with improved photocatalytic activity for degradation of CIP under visible light. Sep. Purif. Technol. 2022, 282, 120004. [Google Scholar] [CrossRef]
- Fu, J.; Xu, Q.; Low, J.; Jiang, C.; Yu, J. Ultrathin 2D/2D WO3/g-C3N4 step-scheme H2-production photocatalyst. Appl. Catal. B Environ. 2019, 243, 556–565. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, J.; Yu, H.; Yu, J. Emerging S-scheme photocatalyst. Adv. Mater. 2022, 34, e2107668. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, L.; Cheng, B.; Fan, J.; Yu, J. S-scheme Heterojunction photocatalyst. Chem 2020, 6, 1543–1559. [Google Scholar] [CrossRef]
- Zhang, B.; Shi, H.; Yan, Y.; Liu, C.; Hu, X.; Liu, E.; Fan, J. A novel S-scheme 1D/2D Bi2S3/g-C3N4 heterojunctions with enhanced H2 evolution activity. Colloid Surf. A 2021, 608, 125598. [Google Scholar] [CrossRef]
- Lai, C.; Zhang, M.; Li, B.; Huang, D.; Zeng, G.; Qin, L.; Liu, X.; Yi, H.; Cheng, M.; Li, L.; et al. Fabrication of CuS/BiVO4 (0 4 0) binary heterojunction photocatalysts with enhanced photocatalytic activity for Ciprofloxacin degradation and mechanism insight. Chem. Eng. J. 2019, 358, 891–902. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Z.; Dong, Z.; Wu, Y.; Zhu, X.; Cheng, Z.; Liu, Y.; Wang, Y.; Zheng, Z.; Cao, X.; et al. CuS/TiO2 nanotube arrays heterojunction for the photoreduction of uranium (VI). J. Solid State Chem. 2021, 303, 122499. [Google Scholar] [CrossRef]
- Ni, L.; Wang, T.; Wang, H.; Wang, Y. An anaerobic-applicable Bi2MoO6/CuS heterojunction modified photocatalytic membrane for biofouling control in anammox MBRs: Generation and contribution of reactive species. Chem. Eng. J. 2022, 429, 132457. [Google Scholar] [CrossRef]
- Majhi, D.; Bhoi, Y.P.; Samal, P.K.; Mishra, B.G. Morphology controlled synthesis and photocatalytic study of novel CuS-Bi2O2CO3 heterojunction system for chlorpyrifos degradation under visible light illumination. Appl. Surf. Sci. 2018, 455, 891–902. [Google Scholar] [CrossRef]
- Wang, J.; Cao, C.; Wang, Y.; Wang, Y.; Sun, B.; Zhu, L. In situ preparation of p-n BiOI@Bi5O7I heterojunction for enhanced PFOA photocatalytic degradation under simulated solar light irradiation. Chem. Eng. J. 2020, 391, 123530. [Google Scholar] [CrossRef]
- Han, X.; Wang, S.; Huang, H.; Zhang, Y. Hydroxyl radicals and sulfate radicals synergistically boosting the photocatalytic and mineralization ability of 1D-2D Bi5O7I/NiFe-LDH heterojunction. Appl. Surf. Sci. 2021, 540, 148237. [Google Scholar] [CrossRef]
- Xu, C.; Yan, K.; Wang, P.; Zhou, X.; Zhang, T.; Fu, Y.; Yan, Q. CuBi2O4 and rGO co-modified 3D hierarchical flower-like Bi5O7I nanoflakes as Z-scheme heterojunction for enhanced photocatalytic performance. Sep. Purif. Technol. 2021, 257, 117935. [Google Scholar] [CrossRef]
- Dong, Z.; Pan, J.; Wang, B.; Jiang, Z.; Zhao, C.; Wang, J.; Song, C.; Zheng, Y.; Cui, C.; Li, C. The p-n-type Bi5O7I-modified porous C3N4 nano-heterojunction for enhanced visible light photocatalysis. J. Alloys Compd. 2018, 747, 788–795. [Google Scholar] [CrossRef]
- Das, K.; Majhi, D.; Bhoi, Y.P.; Mishra, B.G. Combustion synthesis, characterization and photocatalytic application of CuS/Bi4Ti3O12 p-n heterojunction materials towards efficient degradation of 2-methyl-4-chlorophenoxyacetic acid herbicide under visible light. Chem. Eng. J. 2019, 362, 588–599. [Google Scholar] [CrossRef]
- Shi, H.; Wan, J.; Dong, X.; Xi, J.; Zhang, L.; Wang, W.; Zhang, X.; Shi, Y.; Tang, Z. Ag bridged step-scheme MoS2/Bi4O5Br2 heterojunction for enhanced visible light driven photocatalytic disinfection activity. Appl. Surf. Sci. 2023, 607, 155056. [Google Scholar] [CrossRef]
- Miao, Z.; Wang, Q.; Zhang, Y.; Meng, L.; Wang, X. In situ construction of S-scheme AgBr/BiOBr heterojunction with surface oxygen vacancy for boosting photocatalytic CO2 reduction with H2O. Appl. Catal. B Environ. 2022, 301, 120802. [Google Scholar] [CrossRef]
- Xia, C.; Lu, R.; Han, Q. Synthesis of Bi4O5I2/Bi5O7I heterojunction at weak acidic solution with preferentially growing facets and high photocatalytic activity. Opt. Mater. 2022, 134, 113184. [Google Scholar] [CrossRef]
- Pang, J.; Han, Q.; Liu, W.; Shen, Z.; Wang, X.; Zhu, J. Two basic bismuth nitrates: [Bi6O6(OH)2](NO3)4·2H2O with superior photodegradation activity for rhodamine B and [Bi6O5(OH)3](NO3)5·3H2O with ultrahigh adsorption capacity for methyl orange. Appl. Surf. Sci. 2017, 422, 283–294. [Google Scholar] [CrossRef]
- Lu, C.; Wang, L.; Yang, D.; Jin, Z.; Wang, X.; Xu, J.; Li, Z.; Shi, W.; Guan, W.; Huang, W. Boosted tetracycline and Cr(VI) simultaneous cleanup over Z-Scheme BiPO4/CuBi2O4 p-n heterojunction with 0D/1D trepang-like structure under simulated sunlight irradiation. J. Alloys Compd. 2022, 919, 165849. [Google Scholar] [CrossRef]
- Li, J.; Yang, R.; Hu, D.; Xu, Y.; Ma, Z. Efficient bacterial inactivation with S-doped g-C3N4 nanosheets under visible light irradiation. Environ. Sci. Pollut. R 2022, 29, 34637–34650. [Google Scholar] [CrossRef]
- Du, J.; Xu, Z.; Li, H.; Yang, H.; Xu, S.; Wang, J.; Jia, Y.; Ma, S.; Zhan, S. Ag3PO4/g-C3N4 Z-scheme composites with enhanced visible-light-driven disinfection and organic pollutants degradation: Uncovering the mechanism. Appl. Surf. Sci. 2021, 541, 148487. [Google Scholar] [CrossRef]
- Shi, H.; Xie, Y.; Wang, W.; Zhang, L.; Zhang, X.; Shi, Y.; Fan, J.; Tang, Z. In-situ construction of step-scheme MoS2/Bi4O5Br2 heterojunction with improved photocatalytic activity of Rhodamine B degradation and disinfection. J. Colloid Interf. Sci. 2022, 623, 500–512. [Google Scholar] [CrossRef]
- Qi, Z.; Li, G.; Wang, M.; Chen, C.; Xu, Z.; An, T. Photoelectrocatalytic inactivation mechanism of E. coli DH5alpha (TET) and synergistic degradation of corresponding antibiotics in water. Water Res. 2022, 215, 118240. [Google Scholar] [CrossRef]
- Ming, J.; Sun, X.; Ma, Q.; Liu, N.; Zhang, C.; Kawazoe, N.; Chen, G.; Yang, Y. Advanced photocatalytic sterilization for recalcitrant Enterococcus sp. contaminated water by newly developed Z-scheme Bi2WO6 based composites under solar light. Chemosphere 2023, 310, 136912. [Google Scholar] [CrossRef] [PubMed]
- Diao, M.; Qi, D.; Xu, M.; Lu, Z.; Lv, F.; Bie, X.; Zhang, C.; Zhao, H. Antibacterial activity and mechanism of monolauroyl-galactosylglycerol against Bacillus cereus. Food Control 2018, 85, 339–344. [Google Scholar] [CrossRef]
- Guan, G.; Zhang, L.; Zhu, J.; Wu, H.; Li, W.; Sun, Q. Antibacterial properties and mechanism of biopolymer-based films functionalized by CuO/ZnO nanoparticles against Escherichia coli and Staphylococcus aureus. J. Hazard Mater. 2021, 402, 123542. [Google Scholar] [CrossRef] [PubMed]
- Xia, D.; Liu, H.; Xu, B.; Wang, Y.; Liao, Y.; Huang, Y.; Ye, L.; He, C.; Wong, P.K.; Qiu, R. Single Ag atom engineered 3D-MnO2 porous hollow microspheres for rapid photothermocatalytic inactivation of E. coli under solar light. Appl. Catal. B Environ. 2019, 245, 177–189. [Google Scholar] [CrossRef]
- Xiao, K.; Wang, T.; Sun, M.; Hanif, A.; Gu, Q.; Tian, B.; Jiang, Z.; Wang, B.; Sun, H.; Shang, J.; et al. Photocatalytic Bacterial inactivation by a rape pollen-MoS2 Biohybrid catalyst: Synergetic effects and inactivation mechanisms. Environ. Sci. Technol. 2020, 54, 537–549. [Google Scholar] [CrossRef]
- Shi, W.; Li, M.; Huang, X.; Ren, H.; Guo, F.; Tang, Y.; Lu, C. Construction of CuBi2O4/Bi2MoO6 p-n heterojunction with nanosheets-on-microrods structure for improved photocatalytic activity towards broad-spectrum antibiotics degradation. Chem. Eng. J. 2020, 394, 125009. [Google Scholar] [CrossRef]
- Fan, H.-T.; Wu, Z.; Liu, K.-C.; Liu, W.-S. Fabrication of 3D CuS@ZnIn2S4 hierarchical nanocages with 2D/2D nanosheet subunits p-n heterojunctions for improved photocatalytic hydrogen evolution. Chem. Eng. J. 2022, 433, 134474. [Google Scholar] [CrossRef]
- Li, S.; Cai, M.; Liu, Y.; Zhang, J.; Wang, C.; Zang, S.; Li, Y.; Zhang, P.; Li, X. In situ construction of a C3N5 nanosheet/Bi2WO6 nanodot S-scheme heterojunction with enhanced structural defects for the efficient photocatalytic removal of tetracycline and Cr(vi). Inorg. Chem. Front. 2022, 9, 2479–2497. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, Y.; Zhang, Z.; Dong, Z.; Xu, J. 2D/2D CsPbBr3/BiOCl heterojunction with an S-scheme charge transfer for boosting the photocatalytic conversion of CO2. Inorg. Chem. 2022, 61, 10557–10566. [Google Scholar] [CrossRef]
- Yang, H.; He, D.; Liu, C.; Zhang, T.; Qu, J.; Jin, D.; Zhang, K.; Lv, Y.; Zhang, Z.; Zhang, Y.N. Visible-light-driven photocatalytic disinfection by S-scheme alpha-Fe2O3/g-C3N4 heterojunction: Bactericidal performance and mechanism insight. Chemosphere 2022, 287, 132072. [Google Scholar] [CrossRef]
- Huang, Z.-F.; Song, J.; Wang, X.; Pan, L.; Li, K.; Zhang, X.; Wang, L.; Zou, J.-J. Switching charge transfer of C3N4/W18O49 from type-II to Z-scheme by interfacial band bending for highly efficient photocatalytic hydrogen evolution. Nano Energy 2017, 40, 308–316. [Google Scholar] [CrossRef]
- Chen, Q.; Lan, X.; Chen, K.; Ren, Q.; Shi, J. Construction of WO3/CsPbBr3 S-scheme heterojunction via electrostatic Self-assembly for efficient and Long-Period photocatalytic CO2 reduction. J. Colloid Interf. Sci. 2022, 616, 253–260. [Google Scholar] [CrossRef]
- Luo, J.; Zhou, X.; Yang, F.; Ning, X.; Zhan, L.; Wu, Z.; Zhou, X. Generating a captivating S-scheme CuBi2O4/CoV2O6 heterojunction with boosted charge spatial separation for efficiently removing tetracycline antibiotic from wastewater. J. Clean. Prod. 2022, 357, 131992. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Z.; Guo, W.; Zhang, K.; Wang, N.; Li, Z.; Li, J. Construction of S-Scheme CuS/Bi5O7I Heterojunction for Boosted Photocatalytic Disinfection with Visible Light Exposure. Molecules 2023, 28, 3084. https://doi.org/10.3390/molecules28073084
Ma Z, Guo W, Zhang K, Wang N, Li Z, Li J. Construction of S-Scheme CuS/Bi5O7I Heterojunction for Boosted Photocatalytic Disinfection with Visible Light Exposure. Molecules. 2023; 28(7):3084. https://doi.org/10.3390/molecules28073084
Chicago/Turabian StyleMa, Zhanqiang, Wei Guo, Kaiyue Zhang, Nan Wang, Ziyue Li, and Juan Li. 2023. "Construction of S-Scheme CuS/Bi5O7I Heterojunction for Boosted Photocatalytic Disinfection with Visible Light Exposure" Molecules 28, no. 7: 3084. https://doi.org/10.3390/molecules28073084
APA StyleMa, Z., Guo, W., Zhang, K., Wang, N., Li, Z., & Li, J. (2023). Construction of S-Scheme CuS/Bi5O7I Heterojunction for Boosted Photocatalytic Disinfection with Visible Light Exposure. Molecules, 28(7), 3084. https://doi.org/10.3390/molecules28073084





