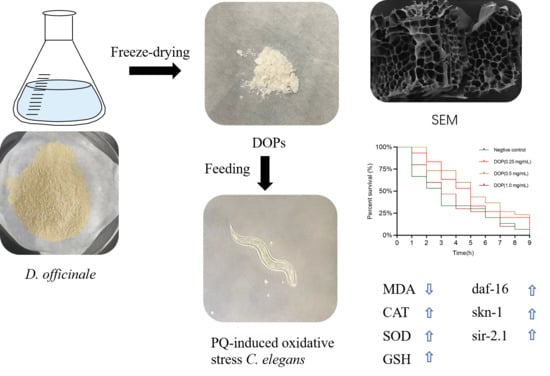
Insights into the Oxidative Stress Alleviation Potential of Enzymatically Prepared Dendrobium officinale Polysaccharides
Abstract
1. Introduction
2. Results and Discussion
2.1. Single-Factor Experimental Analysis
2.2. BBD Analysis
2.3. In Vitro Antioxidant Activity of Different Concentrations of DOPs
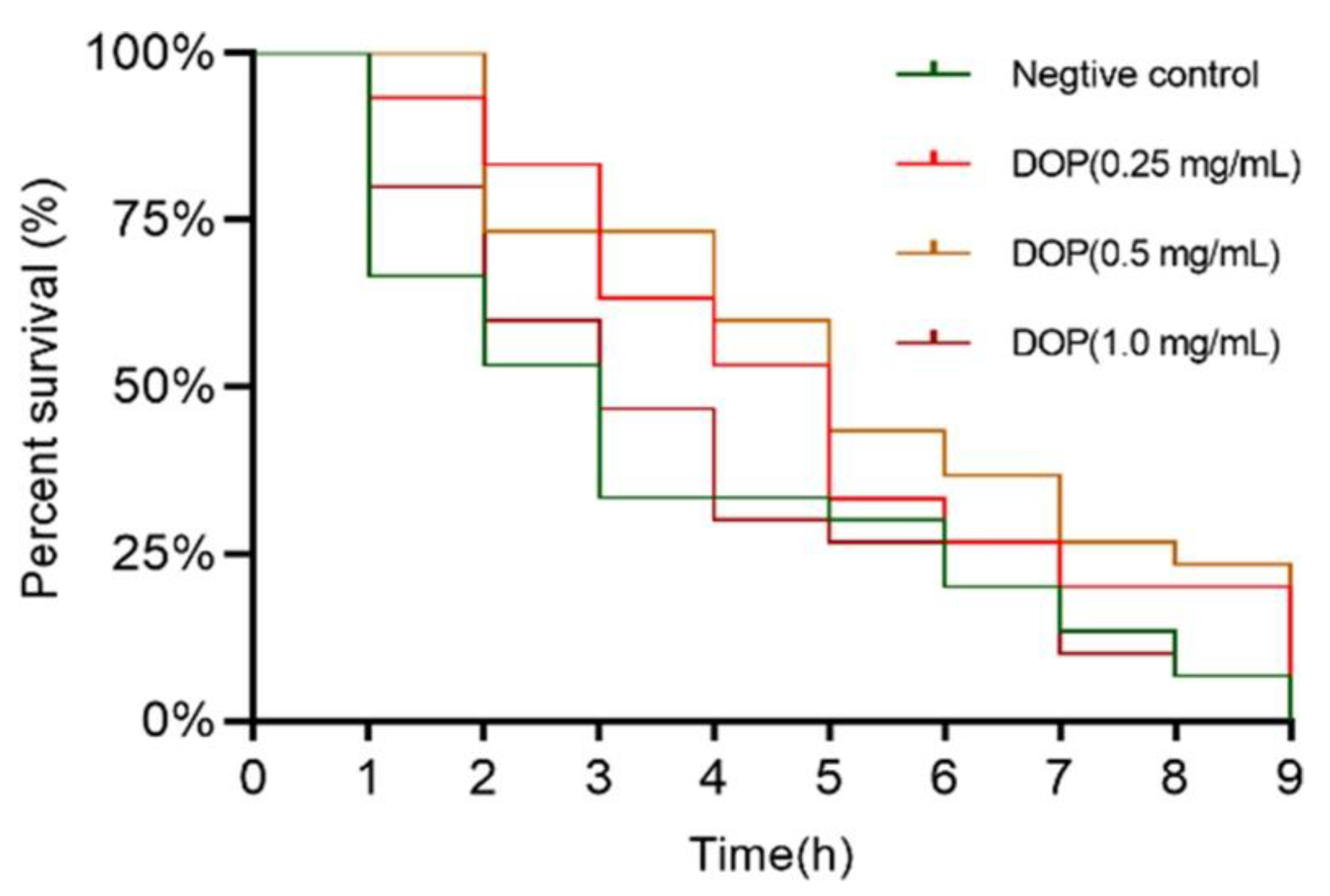
2.4. Effect of DOPs on the Longevity of Oxidative Stress Worms
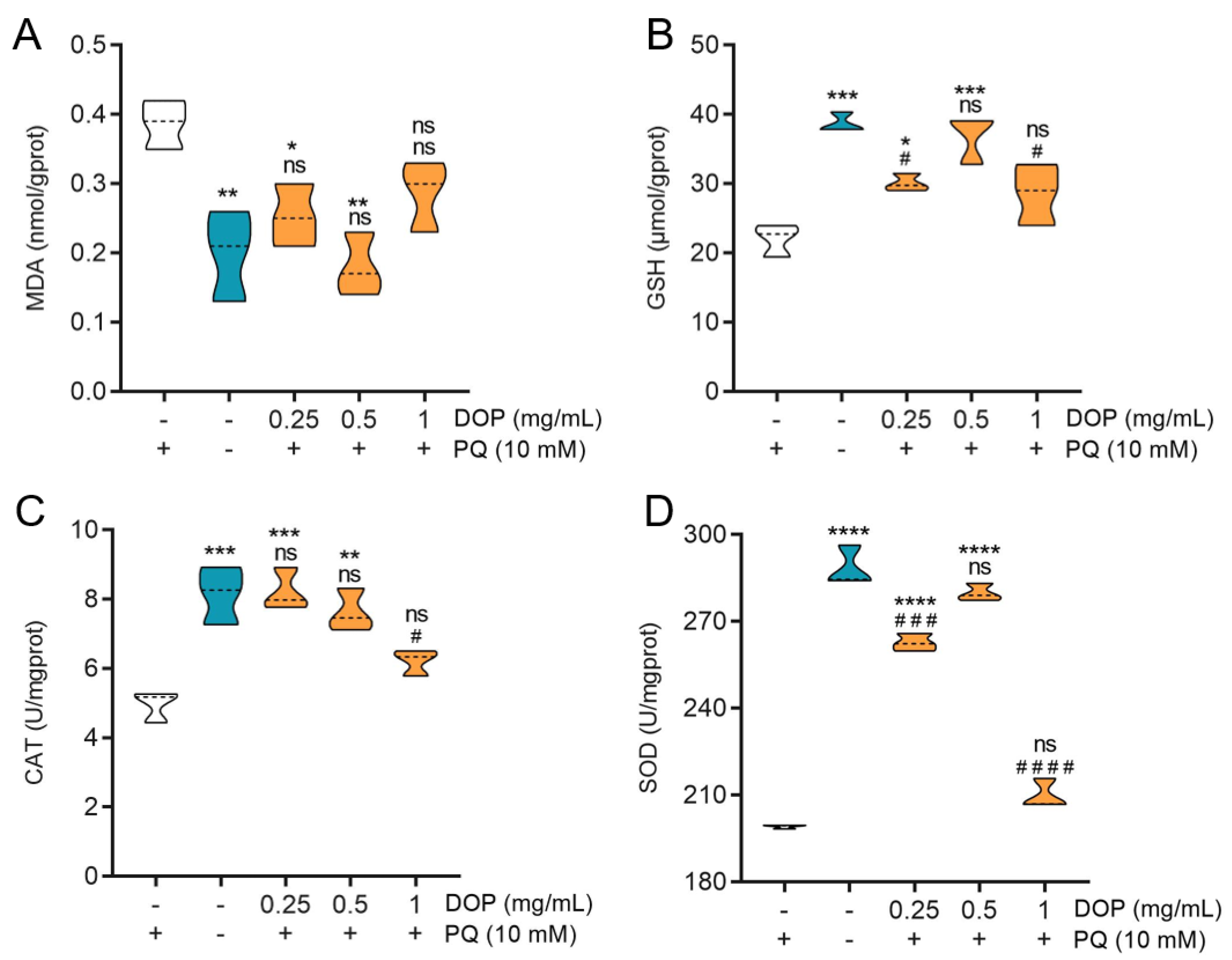
2.5. Effect of DOPs on Biochemical Indicators
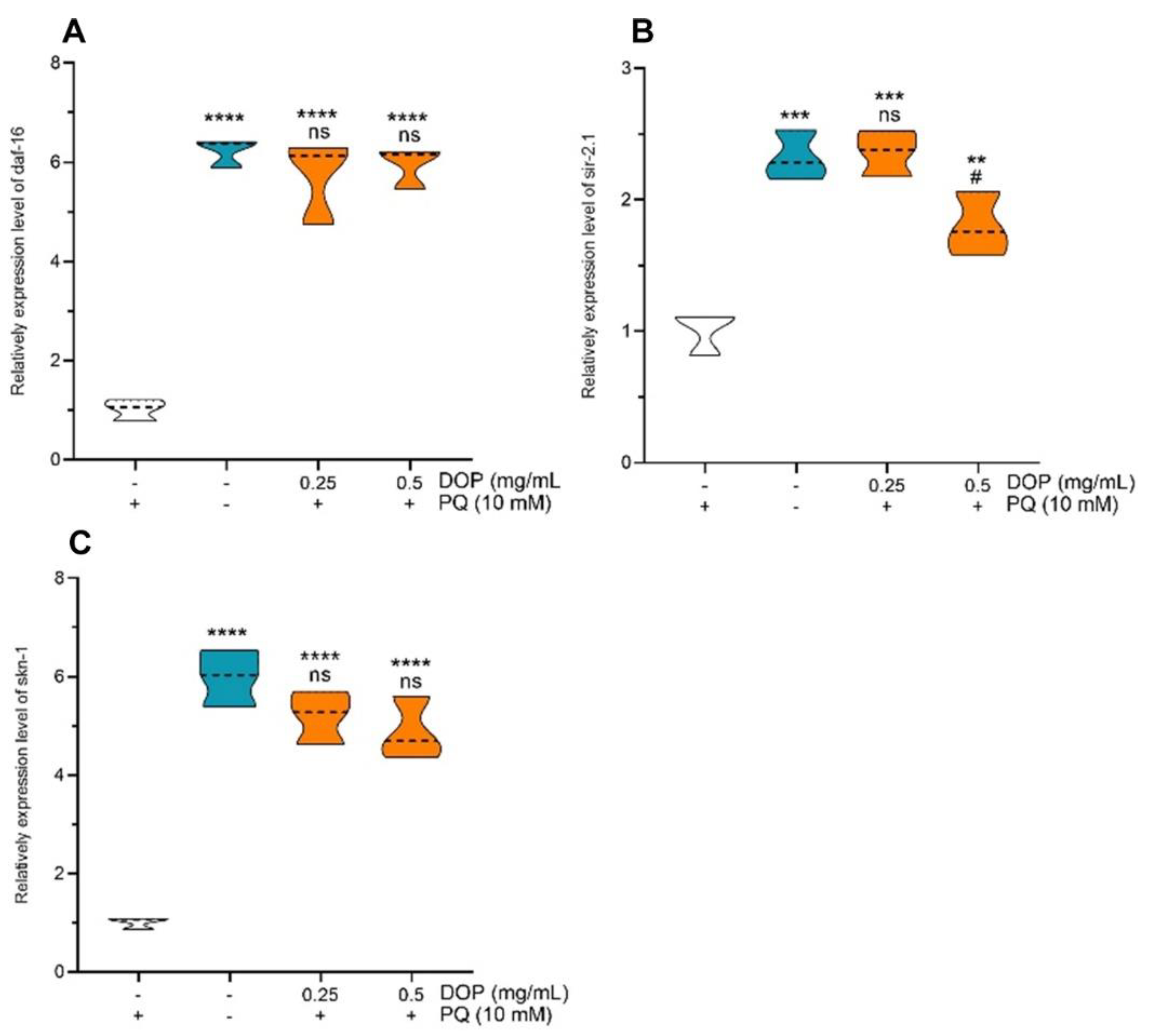
2.6. Effects of DOPs on the Expression of Stress-Related Genes
3. Materials and Methods
3.1. Materials and Chemicals
3.2. Single-Factor Experiment and Response Surface Analysis Optimization
3.3. Antioxidant Activity Assays of DOPs In Vitro
3.3.1. ABTS+ Scavenging Activity
3.3.2. H2O2 Scavenging Activity
3.3.3. Assay of Ferrous Metal Ions’ Chelating Activity
3.4. The Lifespan Assay of C. elegans under Oxidative Stress Conditions
3.5. Oxidative Stress and Antioxidant Biomarker Biochemical Measurement
3.6. Measurement of Oxidative Stress-Related Genes’ Expression
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Grattagliano, I.; Palmieri, V.O.; Portincasa, P.; Moschetta, A.; Palasciano, G. Oxidative Stress-Induced Risk Factors Associated with the Metabolic Syndrome: A Unifying Hypothesis. J. Nutr. Biochem. 2008, 19, 491–504. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Xiao, H.; Liu, Y.; Yang, Y.; Wang, Y.; Xu, S.; Huang, S.; Hou, S.; Liang, J. Polysaccharides from Dendrobium Officinale Ameliorate Colitis-Induced Lung Injury via Inhibiting Inflammation and Oxidative Stress. Chem. Biol. Interact. 2021, 347, 109615. [Google Scholar] [CrossRef]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative Stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative Stress in Cancer. Cancer Cell. 2020, 38, 167–197. [Google Scholar] [CrossRef]
- Santos, M.A.; Franco, F.N.; Caldeira, C.A.; de Araújo, G.R.; Vieira, A.; Chaves, M.M.; Lara, R.C. Antioxidant Effect of Resveratrol: Change in MAPK Cell Signaling Pathway during the Aging Process. Arch. Gerontol. Geriatr. 2021, 92, 104266. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.-S.; Yao, Y.-F.; Wang, L.-F.; Li, W.-J. Polysaccharides as Antioxidants and Prooxidants in Managing the Double-Edged Sword of Reactive Oxygen Species. Biomed. Pharm. 2023, 159, 114221. [Google Scholar] [CrossRef] [PubMed]
- Romdhane, M.B.; Haddar, A.; Ghazala, I.; Jeddou, K.B.; Helbert, C.B.; Ellouz-Chaabouni, S. Optimization of Polysaccharides Extraction from Watermelon Rinds: Structure, Functional and Biological Activities. Food Chem. 2017, 216, 355–364. [Google Scholar] [CrossRef]
- Imre, B.; García, L.; Puglia, D.; Vilaplana, F. Reactive Compatibilization of Plant Polysaccharides and Biobased Polymers: Review on Current Strategies, Expectations and Reality. Carbohydr. Polym. 2019, 209, 20–37. [Google Scholar] [CrossRef]
- Wang, J.; Hu, S.; Nie, S.; Yu, Q.; Xie, M. Reviews on Mechanisms of In Vitro Antioxidant Activity of Polysaccharides. Oxidative Med. Cell. Longev. 2016, 2016, 5692852. [Google Scholar] [CrossRef]
- Mu, S.; Yang, W.; Huang, G. Antioxidant Activities and Mechanisms of Polysaccharides. Chem. Biol. Drug. Des. 2021, 97, 628–632. [Google Scholar] [CrossRef]
- Moreno-Arriola, E.; Cárdenas-Rodríguez, N.; Coballase-Urrutia, E.; Pedraza-Chaverri, J.; Carmona-Aparicio, L.; Ortega-Cuellar, D. Caenorhabditis Elegans: A Useful Model for Studying Metabolic Disorders in Which Oxidative Stress Is a Contributing Factor. Oxid. Med. Cell. Longev. 2014, 2014, 705253. [Google Scholar] [CrossRef] [PubMed]
- Racz, P.I.; Wildwater, M.; Rooseboom, M.; Kerkhof, E.; Pieters, R.; Yebra-Pimentel, E.S.; Dirks, R.P.; Spaink, H.P.; Smulders, C.; Whale, G.F. Application of Caenorhabditis Elegans (Nematode) and Danio Rerio Embryo (Zebrafish) as Model Systems to Screen for Developmental and Reproductive Toxicity of Piperazine Compounds. Toxicol. Vitr. 2017, 44, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Ayuda-Durán, B.; González-Manzano, S.; González-Paramás, A.M.; Santos-Buelga, C. Caernohabditis Elegans as a Model Organism to Evaluate the Antioxidant Effects of Phytochemicals. Molecules 2020, 25, 3194. [Google Scholar] [CrossRef] [PubMed]
- C. Elegans Sequencing Consortium. Genome Sequence of the Nematode C. elegans: A Platform for Investigating Biology. Science 1998, 282, 2012–2018. [Google Scholar] [CrossRef]
- Lant, B.; Storey, K.B. An Overview of Stress Response and Hypometabolic Strategies in Caenorhabditis Elegans: Conserved and Contrasting Signals with the Mammalian System. Int. J. Biol. Sci. 2010, 6, 9–50. [Google Scholar] [CrossRef]
- Altintas, O.; Park, S.; Lee, S.-J.V. The Role of Insulin/IGF-1 Signaling in the Longevity of Model Invertebrates, C. Elegans and D. Melanogaster. BMB Rep. 2016, 49, 81–92. [Google Scholar] [CrossRef]
- Das, D.; Arur, S. Conserved Insulin Signaling in the Regulation of Oocyte Growth, Development, and Maturation. Mol. Reprod. Dev. 2017, 84, 444–459. [Google Scholar] [CrossRef]
- Loboda, A.; Damulewicz, M.; Pyza, E.; Jozkowicz, A.; Dulak, J. Role of Nrf2/HO-1 System in Development, Oxidative Stress Response and Diseases: An Evolutionarily Conserved Mechanism. Cell. Mol. Life Sci. 2016, 73, 3221–3247. [Google Scholar] [CrossRef]
- Fuse, Y.; Kobayashi, M. Conservation of the Keap1-Nrf2 System: An Evolutionary Journey through Stressful Space and Time. Molecules 2017, 22, 436. [Google Scholar] [CrossRef]
- Oliveira, R.P.; Porter Abate, J.; Dilks, K.; Landis, J.; Ashraf, J.; Murphy, C.T.; Blackwell, T.K. Condition-Adapted Stress and Longevity Gene Regulation by Caenorhabditis Elegans SKN-1/Nrf. Aging Cell 2009, 8, 524–541. [Google Scholar] [CrossRef]
- Chávez, V.; Mohri-Shiomi, A.; Maadani, A.; Vega, L.A.; Garsin, D.A. Oxidative Stress Enzymes are Required for DAF-16-Mediated Immunity due to Generation of Reactive Oxygen Species by Caenorhabditis Elegans. Genetics 2007, 176, 1567–1577. [Google Scholar] [CrossRef] [PubMed]
- Kampkötter, A.; Nkwonkam, C.G.; Zurawski, R.F.; Timpel, C.; Chovolou, Y.; Wätjen, W.; Kahl, R. Investigations of Protective Effects of the Flavonoids Quercetin and Rutin on Stress Resistance in the Model Organism Caenorhabditis Elegans. Toxicology 2007, 234, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Kampkötter, A.; Pielarski, T.; Rohrig, R.; Timpel, C.; Chovolou, Y.; Wätjen, W.; Kahl, R. The Ginkgo Biloba Extract EGb761 Reduces Stress Sensitivity, ROS Accumulation and Expression of Catalase and Glutathione S-Transferase 4 in Caenorhabditis Elegans. Pharm. Res. 2007, 55, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Trapika, I.G.S.C.; Tang, S.Y.S.; Cho, J.-L.; Qi, Y.; Li, C.G.; Li, Y.; Yao, M.; Yang, D.; Liu, B.; et al. Mechanisms and Active Compounds Polysaccharides and Bibenzyls of Medicinal Dendrobiums for Diabetes Management. Front. Nutr. 2021, 8, 811870. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Li, Y.; Tao, S.; Wei, G.; Huang, Y.; Chen, D.; Wu, C. Purification, Characterization and Biological Activity of Polysaccharides from Dendrobium Officinale. Molecules 2016, 21, 701. [Google Scholar] [CrossRef]
- He, Y.; Li, L.; Chang, H.; Cai, B.; Gao, H.; Chen, G.; Hou, W.; Jappar, Z.; Yan, Y. Research Progress on Extraction, Purification, Structure and Biological Activity of Dendrobium Officinale Polysaccharides. Front. Nutr. 2022, 9, 965073. [Google Scholar] [CrossRef]
- Zeng, Q.; Ko, C.-H.; Siu, W.-S.; Li, L.-F.; Han, X.-Q.; Yang, L.; Lau, C.B.-S.; Hu, J.-M.; Leung, P.-C. Polysaccharides of Dendrobium Officinale Kimura & Migo Protect Gastric Mucosal Cell against Oxidative Damage-Induced Apoptosis In Vitro and In Vivo. J. Ethnopharmacol. 2017, 208, 214–224. [Google Scholar] [CrossRef]
- Fan, H.; Meng, Q.; Xiao, T.; Zhang, L. Partial Characterization and Antioxidant Activities of Polysaccharides Sequentially Extracted from Dendrobium Officinale. J. Food Meas. Charact. 2018, 12, 1054–1064. [Google Scholar] [CrossRef]
- Pan, L.-H.; Wang, J.; Ye, X.-Q.; Zha, X.-Q.; Luo, J.-P. Enzyme-Assisted Extraction of Polysaccharides from Dendrobium Chrysotoxum and Its Functional Properties and Immunomodulatory Activity. LWT–Food Sci. Technol. 2015, 60, 1149–1154. [Google Scholar] [CrossRef]
- He, L.; Yan, X.; Liang, J.; Li, S.; He, H.; Xiong, Q.; Lai, X.; Hou, S.; Huang, S. Comparison of Different Extraction Methods for Polysaccharides from Dendrobium Officinale Stem. Carbohydr. Polym. 2018, 198, 101–108. [Google Scholar] [CrossRef]
- Shang, H.; Chen, S.; Li, R.; Zhou, H.; Wu, H.; Song, H. Influences of Extraction Methods on Physicochemical Characteristics and Activities of Astragalus Cicer L. Polysaccharides. Process. Biochem. 2018, 73, 220–227. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, Z.; Zhou, H.; Yu, C.; Han, Z.; Shao, S.; Hu, X.; Wei, X.; Wang, Y. Effects of Extraction Methods on Physicochemical Properties and Hypoglycemic Activities of Polysaccharides from Coarse Green Tea. Glycoconj. J. 2020, 37, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Ma, X.; Jiang, P.; Hu, L.; Zhi, Z.; Chen, J.; Ding, T.; Ye, X.; Liu, D. Characterization of Pectin from Grapefruit Peel: A Comparison of Ultrasound-Assisted and Conventional Heating Extractions. Food Hydrocoll. 2016, 61, 730–739. [Google Scholar] [CrossRef]
- Pawlaczyk-Graja, I.; Balicki, S.; Wilk, K.A. Effect of Various Extraction Methods on the Structure of Polyphenolic-Polysaccharide Conjugates from Fragaria Vesca L. Leaf. Int. J. Biol. Macromol. 2019, 130, 664–674. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.; Xu, W.; Wang, H.-X.; Huang, F.; Wang, L.-M. Natural Polysaccharides Experience Physiochemical and Functional Changes during Preparation: A Review. Carbohydr. Polym. 2020, 234, 115896. [Google Scholar] [CrossRef]
- Shang, H.; Zhou, H.; Duan, M.; Li, R.; Wu, H.; Lou, Y. Extraction Condition Optimization and Effects of Drying Methods on Physicochemical Properties and Antioxidant Activities of Polysaccharides from Comfrey (Symphytum officinale L.) Root. Int. J. Biol. Macromol. 2018, 112, 889–899. [Google Scholar] [CrossRef]
- Xiao, B.; Chen, S.; Huang, Q.; Tan, J.; Zeng, J.; Yao, J.; Feng, T.; Wang, G.; Zhang, Y. The Lipid Lowering and Antioxidative Stress Potential of Polysaccharide from Auricularia Auricula Prepared by Enzymatic Method. Int. J. Biol. Macromol. 2021, 187, 651–663. [Google Scholar] [CrossRef]
- Ahmadi Gavlighi, H.; Tabarsa, M.; You, S.; Surayot, U.; Ghaderi-Ghahfarokhi, M. Extraction, Characterization and Immunomodulatory Property of Pectic Polysaccharide from Pomegranate Peels: Enzymatic vs Conventional Approach. Int. J. Biol. Macromol. 2018, 116, 698–706. [Google Scholar] [CrossRef]
- Alboofetileh, M.; Rezaei, M.; Tabarsa, M. Enzyme-Assisted Extraction of Nizamuddinia Zanardinii for the Recovery of Sulfated Polysaccharides with Anticancer and Immune-Enhancing Activities. J. Appl. Phycol. 2019, 31, 1391–1402. [Google Scholar] [CrossRef]
- Huang, F.; Liu, H.; Zhang, R.; Dong, L.; Liu, L.; Ma, Y.; Jia, X.; Wang, G.; Zhang, M. Physicochemical Properties and Prebiotic Activities of Polysaccharides from Longan Pulp Based on Different Extraction Techniques. Carbohydr. Polym. 2019, 206, 344–351. [Google Scholar] [CrossRef]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Hawkins Byrne, D. Comparison of ABTS, DPPH, FRAP, and ORAC Assays for Estimating Antioxidant Activity from Guava Fruit Extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Chun-Hui, L.; Chang-Hai, W.; Zhi-Liang, X.; Yi, W. Isolation, Chemical Characterization and Antioxidant Activities of Two Polysaccharides from the Gel and the Skin of Aloe Barbadensis Miller Irrigated with Sea Water. Process. Biochem. 2007, 42, 961–970. [Google Scholar] [CrossRef]
- Kohgo, Y.; Ikuta, K.; Ohtake, T.; Torimoto, Y.; Kato, J. Body Iron Metabolism and Pathophysiology of Iron Overload. Int. J. Hematol. 2008, 88, 7–15. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, X.; Sun, X.; Han, R.; Yu, N.; Liang, J.; Zhou, A. Comparison of the Antioxidant Activities and Polysaccharide Characterization of Fresh and Dry Dendrobium Officinale Kimura et Migo. Molecules 2022, 27, 6654. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhang, X.; Liang, X.; Wu, K.; Cao, Y.; Ma, T.; Guo, S.; Chen, P.; Yu, S.; Ruan, Q.; et al. Defensing against Oxidative Stress in Caenorhabditis Elegans of a Polysaccharide LFP-05S from Lycii Fructus. Carbohydr. Polym. 2022, 289, 119433. [Google Scholar] [CrossRef]
- Castello, P.R.; Drechsel, D.A.; Patel, M. Mitochondria are a Major Source of Paraquat-Induced Reactive Oxygen Species Production in the Brain. J. Biol. Chem. 2007, 282, 14186–14193. [Google Scholar] [CrossRef]
- Kim, J.; Takahashi, M.; Shimizu, T.; Shirasawa, T.; Kajita, M.; Kanayama, A.; Miyamoto, Y. Effects of a Potent Antioxidant, Platinum Nanoparticle, on the Lifespan of Caenorhabditis Elegans. Mech. Ageing Dev. 2008, 129, 322–331. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.-F.; Ding, L.; Du, C.-X.; Wu, X. Effect of Short-Term Water Deficit Stress on Antioxidative Systems in Cucumber Seedling Roots. Bot. Stud. 2014, 55, 46. [Google Scholar] [CrossRef]
- Irigaray, P.; Caccamo, D.; Belpomme, D. Oxidative Stress in Electrohypersensitivity Self-reporting Patients: Results of a Prospective In Vivo Investigation with Comprehensive Molecular Analysis. Int. J. Mol. Med. 2018, 42, 1885–1898. [Google Scholar] [CrossRef]
- Kenyon, C. The First Long-Lived Mutants: Discovery of the Insulin/IGF-1 Pathway for Ageing. Phil. Trans. R. Soc. B 2011, 366, 9–16. [Google Scholar] [CrossRef]
- Lin, K.; Hsin, H.; Libina, N.; Kenyon, C. Regulation of the Caenorhabditis Elegans Longevity Protein DAF-16 by Insulin/IGF-1 and Germline Signaling. Nat. Genet. 2001, 28, 139–145. [Google Scholar] [CrossRef]
- Robida-Stubbs, S.; Glover-Cutter, K.; Lamming, D.W.; Mizunuma, M.; Narasimhan, S.D.; Neumann-Haefelin, E.; Sabatini, D.M.; Blackwell, T.K. TOR Signaling and Rapamycin Influence Longevity by Regulating SKN-1/Nrf and DAF-16/FoxO. Cell Metab. 2012, 15, 713–724. [Google Scholar] [CrossRef] [PubMed]
- Accili, D.; Arden, K.C. FoxOs at the Crossroads of Cellular Metabolism, Differentiation, and Transformation. Cell 2004, 117, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Duangjan, C.; Rangsinth, P.; Gu, X.; Zhang, S.; Wink, M.; Tencomnao, T. Glochidion zeylanicum Leaf Extracts Exhibit Lifespan Extending and Oxidative Stress Resistance Properties in Caenorhabditis Elegans via DAF-16/FoxO and SKN-1/Nrf-2 Signaling Pathways. Phytomedicine 2019, 64, 153061. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, Y.; Zhang, W.; Sun, W.; Ji, X.; Zhang, S.; Qiao, K. Lentinan Extends Lifespan and Increases Oxidative Stress Resistance through DAF-16 and SKN-1 Pathways in Caenorhabditis Elegans. Int. J. Biol. Macromol. 2022, 202, 286–295. [Google Scholar] [CrossRef]
- Tissenbaum, H.A.; Guarente, L. Increased Dosage of a Sir-2 Gene Extends Lifespan in Caenorhabditis Elegans. Nature 2001, 410, 227–230. [Google Scholar] [CrossRef]
- Yang, J.; Chen, H.; Nie, Q.; Huang, X.; Nie, S. Dendrobium Officinale Polysaccharide Ameliorates the Liver Metabolism Disorders of Type II Diabetic Rats. Int. J. Biol. Macromol. 2020, 164, 1939–1948. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, Q.; Jiang, W.; Yao, J.; Zeng, J.; Wang, W.; Zhang, Y. Comparison of the Chemical Composition and Antioxidant Stress Ability of Polysaccharides from Auricularia Auricula under Different Drying Methods. Food Funct. 2022, 13, 2938–2951. [Google Scholar] [CrossRef]
- Ye, C.-L.; Hu, W.-L.; Dai, D.-H. Extraction of Polysaccharides and the Antioxidant Activity from the Seeds of Plantago asiatica L. Int. J. Biol. Macromol. 2011, 49, 466–470. [Google Scholar] [CrossRef]
- Zhang, N.; Chen, H.; Ma, L.; Zhang, Y. Physical Modifications of Polysaccharide from Inonotus Obliquus and the Antioxidant Properties. Int. J. Biol. Macromol. 2013, 54, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Dan-Yang, M.; Jin, W.; Yan-Ping, L.; Xu, Y.; Shi, D.; Xing-Ming, M.; Kai-Zhong, D. Constituent and effects of polysaccharides isolated from Sophora moorcroftiana seeds on lifespan, reproduction, stress resistance, and antimicrobial capacity in Caenorhabditis elegans. Chin. J. Nat. Med. 2018, 16, 252–260. [Google Scholar]
- Li, W.; Li, Z.; Peng, M.-J.; Zhang, X.; Chen, Y.; Yang, Y.-Y.; Zhai, X.-X.; Liu, G.; Cao, Y. Oenothein B Boosts Antioxidant Capacity and Supports Metabolic Pathways That Regulate Antioxidant Defense in Caenorhabditis elegans. Food Funct. 2020, 11, 9157–9167. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Chen, Y.; Wang, G.; Feng, T.; Shen, M.; Xiao, B.; Gu, J.; Wang, W.; Li, J.; Zhang, Y. Evaluation of the Antioxidant Effects of Acid Hydrolysates from Auricularia Auricular Polysaccharides Using a Caenorhabditis Elegans Model. Food Funct. 2019, 10, 5531–5543. [Google Scholar] [CrossRef]

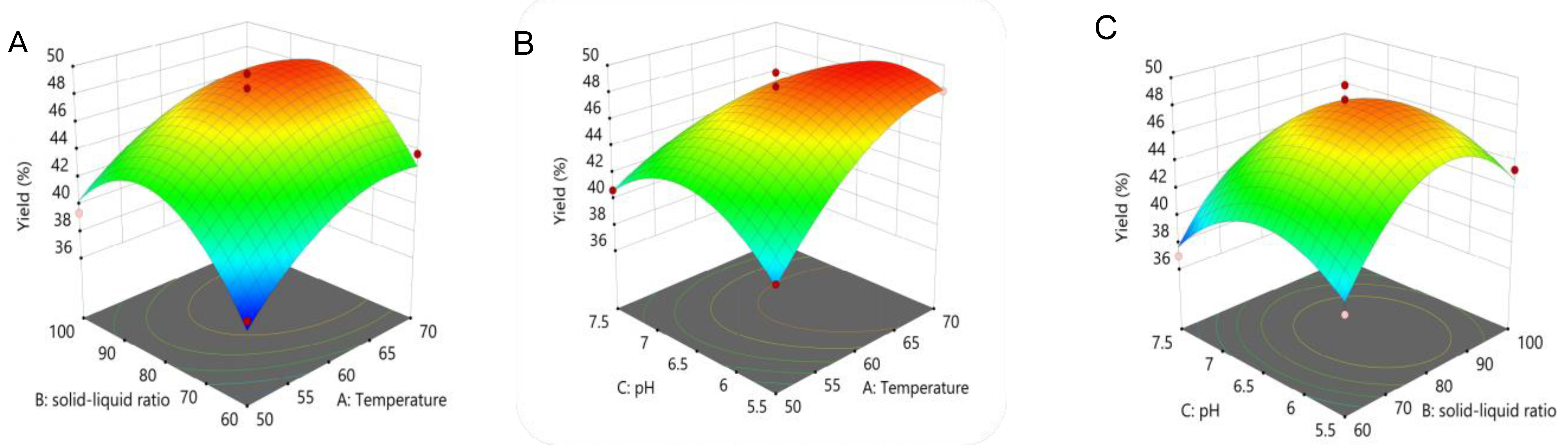

| A. Extraction Temperature (°C) | B. Liquid–Material Ratio (mL/g) | C. pH | Extraction Yield (Y) (%) | |
|---|---|---|---|---|
| 1 | 1 (70) | 0 (80) | 1 (7.5) | 41.67 |
| 2 | 1 | 0 | −1 (5.5) | 48.11 |
| 3 | 1 | 1 (100) | 0 (6.5) | 43.88 |
| 4 | 0 (60) | −1 (60) | 1 | 36.98 |
| 5 | −1 (50) | −1 | 0 | 37.24 |
| 6 | 0 | 0 | 0 | 48.43 |
| 7 | 0 | −1 | −1 | 38.41 |
| 8 | 0 | 0 | 0 | 46.74 |
| 9 | −1 | 0 | −1 | 39.13 |
| 10 | 0 | 1 | −1 | 43.41 |
| 11 | 0 | 0 | 0 | 47.92 |
| 12 | 1 | −1 | 0 | 43.75 |
| 13 | −1 | 1 | 0 | 39.39 |
| 14 | 0 | 0 | 0 | 49.48 |
| 15 | 0 | 1 | 1 | 40.36 |
| 16 | −1 | 0 | 1 | 40.69 |
| 17 | 0 | 0 | 0 | 47.4 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 279.85 | 9 | 31.09 | 23.83 | 0.0002 |
| A | 54.92 | 1 | 54.92 | 42.08 | 0.0003 |
| B | 14.2 | 1 | 14.2 | 10.88 | 0.0131 |
| C | 10.95 | 1 | 10.95 | 8.39 | 0.0231 |
| AB | 1.02 | 1 | 1.02 | 0.7816 | 0.4060 |
| AC | 16 | 1 | 16 | 12.26 | 0.0100 |
| BC | 0.6561 | 1 | 0.6561 | 0.5027 | 0.5012 |
| A2 | 19.64 | 1 | 19.64 | 15.05 | 0.0061 |
| B2 | 95.78 | 1 | 95.78 | 73.39 | <0.0001 |
| C2 | 49.67 | 1 | 49.67 | 38.06 | 0.0005 |
| Residual | 9.14 | 7 | 1.31 | ||
| Lack of Fit | 4.81 | 3 | 1.6 | 1.48 | 0.3471 |
| Pure Error | 4.33 | 4 | 1.08 |
| Mean Survival Time (h) | Median Survival Time (h) | Max (h) | n | |
|---|---|---|---|---|
| Negative control | 3.57 ± 0.50 | 3 ± 0.52 | 9 | 42 |
| DOPs (0.25 mg/mL) | 4.93 ± 0.47 ** | 5 ± 0.57 ** | 10 | 38 |
| DOPs (0.5 mg/mL) | 5.37 ± 0.49 ** | 5 ± 0.54 ** | 10 | 35 |
| DOPs (1.0 mg/mL) | 3.88 ± 0.47 * | 3 ± 0.61 ns | 10 | 31 |
| Gene | Forward 5′ | Reverse 5′ |
|---|---|---|
| β-actin | CCAGGAATTGCTGATCGTATGCAGAA | TGGAGAGGGAAGCGAGGATAGA |
| daf-16 | GGAAGAACTCGATCCGTCACA | GATTCCTTCCTGGCTTTGCA |
| skn-1 | TTCGCCTTCTCTCGAGGATATC | AACGTCTGCAAATCACATTCGT |
| sir-2.1 | AAATCTTCCCAGGACAGTTCGTA | ATGGGCAACACGCATAGCA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, Y.; Zhang, X.; Lin, Y.; Sun, J.; Chen, S.; Wang, W.; Li, J. Insights into the Oxidative Stress Alleviation Potential of Enzymatically Prepared Dendrobium officinale Polysaccharides. Molecules 2023, 28, 3071. https://doi.org/10.3390/molecules28073071
Tang Y, Zhang X, Lin Y, Sun J, Chen S, Wang W, Li J. Insights into the Oxidative Stress Alleviation Potential of Enzymatically Prepared Dendrobium officinale Polysaccharides. Molecules. 2023; 28(7):3071. https://doi.org/10.3390/molecules28073071
Chicago/Turabian StyleTang, Yingqi, Xiong Zhang, Yudan Lin, Jiehan Sun, Shihao Chen, Weimin Wang, and Jia Li. 2023. "Insights into the Oxidative Stress Alleviation Potential of Enzymatically Prepared Dendrobium officinale Polysaccharides" Molecules 28, no. 7: 3071. https://doi.org/10.3390/molecules28073071
APA StyleTang, Y., Zhang, X., Lin, Y., Sun, J., Chen, S., Wang, W., & Li, J. (2023). Insights into the Oxidative Stress Alleviation Potential of Enzymatically Prepared Dendrobium officinale Polysaccharides. Molecules, 28(7), 3071. https://doi.org/10.3390/molecules28073071