CdSSe Nano-Flowers for Ultrasensitive Raman Detection of Antibiotics
Abstract
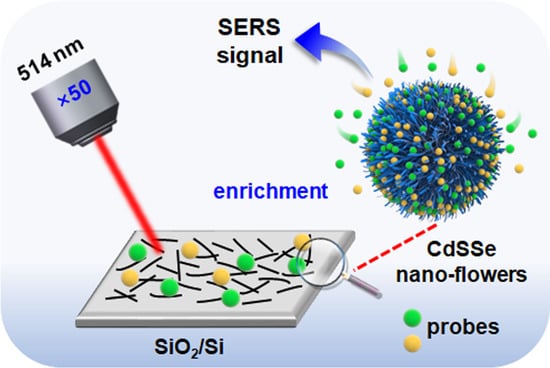
1. Introduction
2. Results and Discussion
2.1. Characterizations of CdSSe NWs and NFs
2.2. SERS Property of CdSSe NFs
2.3. SERS Reproducibility and Uniformity
2.4. SERS Detection of Antibiotics
3. Materials and Methods
3.1. Materials
3.2. Preparation of CdSSe NWs and CdSSe NFs
3.3. Characterization
3.4. Preparation of CdSSe NFs SERS Substrate and Raman Measurement
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lan, L.; Yao, Y.; Ping, J.; Ying, Y. Recent advances in nanomaterial-based biosensors for antibiotics detection. Biosens. Bioelectron. 2017, 91, 504–514. [Google Scholar] [CrossRef]
- Anderson, A.D.; Nelson, J.M.; Rossiter, S.; Angulo, F.J. Public Health Consequences of Use of Antimicrobial Agents in Food Animals in the United States. Microb. Drug Resist. 2003, 9, 373–379. [Google Scholar] [CrossRef]
- Lu, X.; Li, Y.; Thunders, M.; Matthew, C.; Wang, X.; Ai, X.; Zhou, X.; Qiu, J. Effect of enrofloxacin on the proteome of earthworms. Sci. Total Environ. 2018, 616–617, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhou, R.; Yao, X.; Zhao, K.; Deng, A.; Li, J. Sensitive detection of enrofloxacin using an electrochemiluminescence immunosensor based on gold-functionalized C60 and Au@BSA nanoflowers. New J. Chem. 2018, 42, 14142–14148. [Google Scholar] [CrossRef]
- Wang, B.; Lv, X.-L.; Feng, D.; Xie, L.-H.; Zhang, J.; Li, M.; Xie, Y.; Li, J.-R.; Zhou, H.-C. Highly Stable Zr(IV)-Based Metal–Organic Frameworks for the Detection and Removal of Antibiotics and Organic Explosives in Water. J. Am. Chem. Soc. 2016, 138, 6204–6216. [Google Scholar] [CrossRef]
- Luo, Q.; Yu, N.; Shi, C.; Wang, X.; Wu, J. Surface plasmon resonance sensor for antibiotics detection based on photo-initiated polymerization molecularly imprinted array. Talanta 2016, 161, 797–803. [Google Scholar] [CrossRef]
- Etayash, H.; Khan, M.F.; Kaur, K.; Thundat, T. Microfluidic cantilever detects bacteria and measures their susceptibility to antibiotics in small confined volumes. Nat. Commun. 2016, 7, 12947. [Google Scholar] [CrossRef]
- Karasinski, J.; White, L.; Zhang, Y.; Wang, E.; Andreescu, S.; Sadik, O.A.; Lavine, B.K.; Vora, M. Detection and identification of bacteria using antibiotic susceptibility and a multi-array electrochemical sensor with pattern recognition. Biosens. Bioelectron. 2007, 22, 2643–2649. [Google Scholar] [CrossRef]
- Santos, S.; Ramos, F. Analytical Strategies for the Detection and Quantification of Antibiotic Eesidues in Aquaculture Fishes: A Review. Trends Food Sci. Technol. 2016, 52, 16–30. [Google Scholar] [CrossRef]
- Yu, F.; Yu, S.; Yu, L.; Li, Y.; Wu, Y.; Zhang, H.; Qu, L.; Harrington, P.D.B. Determination of residual enrofloxacin in food samples by a sensitive method of chemiluminescence enzyme immunoassay. Food Chem. 2014, 149, 71–75. [Google Scholar] [CrossRef]
- Zhang, X.-H.; Deng, Y.; Zhao, M.-Z.; Zhou, Y.-L.; Zhang, X.-X. Highly-sensitive detection of eight typical fluoroquinolone antibiotics by capillary electrophoresis-mass spectroscopy coupled with immunoaffinity extraction. RSC Adv. 2018, 8, 4063–4071. [Google Scholar] [CrossRef]
- Flauzino, J.; Nalepa, M.; Chronopoulos, D.; Šedajová, V.; Panáček, D.; Jakubec, P.; Kührová, P.; Pykal, M.; Banáš, P.; Panáček, A.; et al. Click and Detect: Versatile Ampicillin Aptasensor Enabled by Click Chemistry on a Graphene–Alkyne Derivative. Small 2023, 2207216. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Liu, L.; Jia, Z.; Shu, Y. Determination of enrofloxacin and ciprofloxacin in foods of animal origin by capillary electrophoresis with field amplified sample stacking–sweeping technique. Food Chem. 2015, 176, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Sureshkumar, V.; Sarathchandra, G. A HPTLC-Fluorescent Densitometry Assay for Simultaneous Detection of Enrofloxacin and Ciprofloxacin in Broiler Chicken Tissues. Food Anal. Methods 2018, 11, 1076–1085. [Google Scholar] [CrossRef]
- Chen, J.; Xu, F.; Jiang, H.; Hou, Y.; Rao, Q.; Guo, P.; Ding, S. A novel quantum dot-based fluoroimmunoassay method for detection of Enrofloxacin residue in chicken muscle tissue. Food Chem. 2009, 113, 1197–1201. [Google Scholar] [CrossRef]
- Chen, Q.; Andersson, A.; Mecklenburg, M.; Xie, B. A biosensing strategy for the rapid detection and classification of antibiotic resistance. Biosens. Bioelectron. 2015, 73, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wang, L.; Li, C.; Li, X.; Li, G. Reliable and Rapid Detection and Quantification of Enrofloxacin Using a Ratiometric SERS Aptasensor. Molecules 2022, 27, 8764. [Google Scholar] [CrossRef]
- Nie, S.; Emory, S.R. Probing Single Molecules and Single Nanoparticles by Surface-Enhanced Raman Scattering. Science 1997, 275, 1102–1106. [Google Scholar] [CrossRef]
- Liang, C.; Lu, Z.-A.; Zheng, M.; Chen, M.; Zhang, Y.; Zhang, B.; Zhang, J.; Xu, P. Band Structure Engineering within Two-Dimensional Borocarbonitride Nanosheets for Surface-Enhanced Raman Scattering. Nano Lett. 2022, 22, 6590–6598. [Google Scholar] [CrossRef]
- Fei, J.; Wu, L.; Zhang, Y.; Zong, S.; Wang, Z.; Cui, Y. Pharmacokinetics-on-a-Chip Using Label-Free SERS Technique for Programmable Dual-Drug Analysis. ACS Sens. 2017, 2, 773–780. [Google Scholar] [CrossRef]
- Cialla, D.; Pollok, S.; Steinbrücker, C.; Weber, K.; Popp, J. SERS-Based Detection of Biomolecules. Nanophotonics 2014, 3, 383–411. [Google Scholar] [CrossRef]
- Wang, K.; Guo, Z.; Li, Y.; Guo, Y.; Liu, H.; Zhang, W.; Zou, Z.; Zhang, Y.; Liu, Z. Few-Layer NbTe2 Nanosheets as Substrates for Surface-Enhanced Raman Scattering Analysis. ACS Appl. Nano Mater. 2020, 3, 11363–11371. [Google Scholar] [CrossRef]
- Wang, W.; Sang, Q.; Yang, M.; Du, J.; Yang, l.; Jiang, X.; Han, X.; Zhao, B. Detection of Several Quinolone Antibiotic Residues in Water Based on Ag-TiO2 SERS Strategy. Sci. Total Environ. 2020, 702, 134956. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Deng, M.; Rao, G.; Yan, Y.; Wu, C.; Jiao, Y.; Deng, A.; Yan, C.; Huang, J.; Wu, S.; et al. High-Performance SERS Substrate Based on Hierarchical 3D Cu Nanocrystals with Efficient Morphology Control. Small 2018, 14, e1802477. [Google Scholar] [CrossRef]
- Han, X.X.; Ji, W.; Zhao, B.; Ozaki, Y. Semiconductor-enhanced Raman scattering: Active nanomaterials and applications. Nanoscale 2017, 9, 4847–4861. [Google Scholar] [CrossRef]
- Liu, M.; Liu, W.; Zhang, W.; Duan, P.; Shafi, M.; Zhang, C.; Hu, X.; Wang, G.; Zhang, W. π-Conjugated Small Organic Molecule-Modified 2D MoS2 with a Charge-Localization Effect Enabling Direct and Sensitive SERS Detection. ACS Appl. Mater. Interfaces 2022, 14, 56975–56985. [Google Scholar] [CrossRef]
- Yin, Y.; Miao, P.; Zhang, Y.; Han, J.; Zhang, X.; Gong, Y.; Gu, L.; Xu, C.; Yao, T.; Xu, P.; et al. Significantly Increased Raman Enhancement on MoX2 (X = S, Se) Monolayers upon Phase Transition. Adv. Funct. Mater. 2017, 27, 1606694. [Google Scholar] [CrossRef]
- Duan, X.; Huang, Y.; Cui, Y.; Wang, J.; Lieber, C.M. Indium phosphide nanowires as building blocks for nanoscale electronic and optoelectronic devices. Nature 2001, 409, 66–69. [Google Scholar] [CrossRef]
- Gong, S.; Schwalb, W.; Wang, Y.; Chen, Y.; Tang, Y.; Si, J.; Shirinzadeh, B.; Cheng, W. A wearable and highly sensitive pressure sensor with ultrathin gold nanowires. Nat. Commun. 2014, 5, 3132. [Google Scholar] [CrossRef]
- Mohanty, P.; Yoon, I.; Kang, T.; Seo, K.; Varadwaj, K.S.K.; Choi, W.; Park, Q.-H.; Ahn, J.P.; Suh, Y.D.; Ihee, H.; et al. Simple Vapor-Phase Synthesis of Single-Crystalline Ag Nanowires and Single-Nanowire Surface-Enhanced Raman Scattering. J. Am. Chem. Soc. 2007, 129, 9576–9577. [Google Scholar] [CrossRef]
- Cui, H.; Li, S.; Deng, S.; Chen, H.; Wang, C. Flexible, Transparent, and Free-Standing Silicon Nanowire SERS Platform for in Situ Food Inspection. ACS Sens. 2017, 2, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Fang, Y.; Zhang, Z.; Zhu, L.; Sun, M. Nanowire-supported plasmonic waveguide for remote excitation of surface-enhanced Raman scattering. Light Sci. Appl. 2014, 3, e199. [Google Scholar] [CrossRef]
- Krebber, R.; Hoffend, F.-J.; Ruttmann, F. Simple and rapid determination of enrofloxacin and ciprofloxacin in edible tissues by turbulent flow chromatography/tandem mass spectrometry (TFC–MS/MS). Anal. Chim. Acta 2009, 637, 208–213. [Google Scholar] [CrossRef]
- Guo, S.; Li, Z.; Song, G.; Zou, B.; Wang, X.; Liu, R. Large-area photodetector with high-sensitivity and broadband spectral response based on composition-graded CdSSe nanowire-chip. J. Alloys Compd. 2015, 649, 793–800. [Google Scholar] [CrossRef]
- Day, R.W.; Mankin, M.N.; Gao, R.; No, Y.-S.; Kim, S.-K.; Bell, D.; Park, H.-G.; Lieber, C.M. Plateau–Rayleigh crystal growth of periodic shells on one-dimensional substrates. Nat. Nanotechnol. 2015, 10, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Dzhagan, V.; Milekhin, A.G.; Valakh, M.Y.; Pedetti, S.; Tessier, M.; Dubertret, B.; Zahn, D.R.T. Morphology-induced phonon spectra of CdSe/CdS nanoplatelets: Core/shell vs. core–crown. Nanoscale 2016, 8, 17204–17212. [Google Scholar] [CrossRef] [PubMed]
- Gong, K.; Kelley, D.F.; Kelley, A.M. Resonance Raman excitation profiles of CdS in pure CdS and CdSe/CdS core/shell quantum dots: CdS-localized excitons. J. Chem. Phys. 2017, 147, 224702. [Google Scholar] [CrossRef]
- Nien, Y.-T.; Zaman, B.; Ouyang, J.; Chen, I.-G.; Hwang, C.-S.; Yu, K. Raman scattering for the size of CdSe and CdS nanocrystals and comparison with other techniques. Mater. Lett. 2008, 62, 4522–4524. [Google Scholar] [CrossRef]
- Tang, B.; Zhou, J.; Sun, P.; Wang, X.; Bai, L.; Dan, J.; Yang, J.; Zhou, K.; Zhao, X.; Pennycook, S.J.; et al. Phase-Controlled Synthesis of Monolayer Ternary Telluride with a Random Local Displacement of Tellurium Atoms. Adv. Mater. 2019, 31, e1900862. [Google Scholar] [CrossRef]
- Hasani, A.; Van Le, Q.; Tekalgne, M.; Guo, W.; Hong, S.H.; Choi, K.S.; Lee, T.H.; Jang, H.W.; Kim, S.Y. Tungsten Trioxide Doped with CdSe Quantum Dots for Smart Windows. ACS Appl. Mater. Interfaces 2018, 10, 43785–43791. [Google Scholar] [CrossRef]
- Li, H.; Xu, Q.; Wang, X.; Liu, W. Ultrasensitive Surface-Enhanced Raman Spectroscopy Detection Based on Amorphous Molybdenum Oxide Quantum Dots. Small 2018, 14, e1801523. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Wang, J.; Malfatti, L.; Carboni, D.; Senes, N.; Innocenzi, P. Highly Durable Graphene-Mediated Surface Enhanced Raman Scattering (G-SERS) Nanocomposites for Molecular Detection. Appl. Surf. Sci. 2018, 450, 451–460. [Google Scholar] [CrossRef]
- Kotak, K.; Schulte, A.; Hay, J.; Sugden, J. Photostability of Aniline Blue (CI 42755) and Methyl Blue (CI 42780). Dye. Pigment. 1997, 34, 159–167. [Google Scholar] [CrossRef]
- Fan, X.; Xu, P.; Zhou, D.; Sun, Y.; Li, Y.C.; Nguyen, M.A.T.; Terrones, M.; Mallouk, T.E. Fast and Efficient Preparation of Exfoliated 2H MoS2 Nanosheets by Sonication-Assisted Lithium Intercalation and Infrared Laser-Induced 1T to 2H Phase Reversion. Nano Lett. 2015, 15, 5956–5960. [Google Scholar] [CrossRef]
- Wang, D.; Yang, Z.; Li, F.; He, D. The microstructure and optical properties of crystallized hydrogenated silicon films prepared by very high frequency glow discharge. Appl. Surf. Sci. 2011, 257, 8350–8354. [Google Scholar] [CrossRef]
- Li, Y.; Chen, H.; Guo, Y.; Wang, K.; Zhang, Y.; Lan, P.; Guo, J.; Zhang, W.; Zhong, H.; Guo, Z.; et al. Lamellar hafnium ditelluride as an ultrasensitive surface-enhanced Raman scattering platform for label-free detection of uric acid. Photon. Res. 2021, 9, 1039–1047. [Google Scholar] [CrossRef]
- Wang, K.; Meng, Y.; Jiao, X.; Huang, W.; Fan, D.; Liu, T.C.-Y. Facile Synthesis of an Economic 3D Surface-Enhanced Raman Scattering Platform for Ultrasensitive Detection of Antibiotics. Food Anal. Methods 2020, 13, 1947–1955. [Google Scholar] [CrossRef]
- Li, J.; Yan, H.; Tan, X.; Lu, Z.; Han, H. Cauliflower-Inspired 3D SERS Substrate for Multiple Mycotoxins Detection. Anal. Chem. 2019, 91, 3885–3892. [Google Scholar] [CrossRef]
- Le Ru, E.C.; Blackie, E.; Meyer, M.; Etchegoin, P.G. Surface Enhanced Raman Scattering Enhancement Factors: A Comprehensive Study. J. Phys. Chem. C 2007, 111, 13794–13803. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, K.; Li, Y.; Wang, H.; Qian, Z.; Zhu, X.; Hussain, S.; Xie, L. CdSSe Nano-Flowers for Ultrasensitive Raman Detection of Antibiotics. Molecules 2023, 28, 2980. https://doi.org/10.3390/molecules28072980
Wang K, Li Y, Wang H, Qian Z, Zhu X, Hussain S, Xie L. CdSSe Nano-Flowers for Ultrasensitive Raman Detection of Antibiotics. Molecules. 2023; 28(7):2980. https://doi.org/10.3390/molecules28072980
Chicago/Turabian StyleWang, Kangkang, You Li, Honggang Wang, Ziyue Qian, Xiaokai Zhu, Sabir Hussain, and Liming Xie. 2023. "CdSSe Nano-Flowers for Ultrasensitive Raman Detection of Antibiotics" Molecules 28, no. 7: 2980. https://doi.org/10.3390/molecules28072980
APA StyleWang, K., Li, Y., Wang, H., Qian, Z., Zhu, X., Hussain, S., & Xie, L. (2023). CdSSe Nano-Flowers for Ultrasensitive Raman Detection of Antibiotics. Molecules, 28(7), 2980. https://doi.org/10.3390/molecules28072980